Introduction
Prostate carcinoma (PCa) is a common male malignancy
and a major cause of cancer-associated mortality worldwide
(1). An increased incidence of PCa
has been observed currently, due to improved life expectancy,
lifestyle changes and the existence of cancer screening programs
(2). Despite efforts made in the
treatment of PCa, the overall 5-year survival rate remains <30%
(3), which is mainly due to the lack
of radical treatment for metastatic tumors that is commonly
observed in patients with PCa at the time of first diagnosis
(4). Current knowledge of PCa
metastasis remains limited (5).
Investigation of the underlying molecular mechanism of PCa
metastasis is therefore required.
Transforming growth factor-β (TGF-β) is a
well-established signal transduction pathway in the human body. The
TGF-β signaling pathway is involved in numerous cellular
activities, including cell growth, proliferation, differentiation,
apoptosis and homeostasis in developing embryos and adult organisms
(6). The TGF-β signaling pathway has
a dual role in cancer development as it can inhibit cancer cell
proliferation in the early stages, but also promote tumor
metastasis through epithelial-mesenchymal transition in the later
stages (7–9). It has been reported that the TGF-β
signaling pathway can be regulated in several types of cancer (such
as breast cancer) by a considerable number of long noncoding RNAs
(lncRNAs) (10). lncRNAs represent a
group of non-protein-coding RNA transcripts that have pivotal roles
in cancer (11). In particular, it
has been demonstrated that the lncRNA MIR4435-2HG promotes lung
cancer by interacting with β-catenin signaling, which has been
shown to crosstalk with TGF-β (12).
However, its role in PCa remains unknown. The present study
demonstrated that MIR4435-2HG promoted PCa potential through the
TGF-β1 signaling pathway.
Materials and methods
Research subjects
A total of 68 patients with PCa who were admitted to
The First Affiliated Hospital of Guangzhou University of Chinese
Medicine between January 2011 and January 2013, and 62 healthy
volunteers, were enrolled in the present study. A 5-year follow-up
was performed on all patients with PCa following discharge. The
inclusion criteria were as follows: i) Patients with PCa that was
confirmed by pathological biopsy; and ii) patients who had not been
treated prior to admission. The exclusion criteria were as follows:
i) Patients with PCa who were diagnosed with other diseases; ii)
patients who had received any treatments prior to this study; iii)
patients who were transferred to other hospitals during treatment;
and iv) patients who succumbed to an unrelated cause or clinical
disorder during follow-up. All patients received surgical resection
and/or radiation and chemotherapy according to their condition. The
62 healthy volunteers received systemic physiological examinations
at the same hospital and all indexes were within the normal range.
Healthy volunteers with a previous history or family history of
malignancies were included. The 62 healthy volunteers were selected
to match the age distribution of the patient group. The age of the
68 patients with PCa was in the range of 40–76 years old with a
mean age of 56.5±5.8 years. According to the American Joint
Committee on Cancer (AJCC) staging system (13), 11, 19, 20 and 18 patients had stage
I, II, III and IV PCa, respectively. The age of the 62 controls was
in the range of 42–74 years old with a mean age of 57.0±6.2 years.
No significant differences in age or other basic clinical data,
including the body mass index and disease history, were found
between the patient and control groups. The present study was
approved by the Ethics Committee of The First Affiliated Hospital
of Guangzhou University of Chinese Medicine prior to patient
admission. Written informed consent was collected from all patients
with PCa and healthy volunteers.
Specimens and cell lines
Blood samples (5 ml) were extracted from the elbow
vein of all fasting participants on day 1 following admission.
Plasma samples were obtained using conventional methods and were
stored in liquid nitrogen prior to further experiments. The 22Rv1
human PCa cell line was used for all in vitro experiments
and was purchased from the American Type Culture Collection. Cells
were cultured in DMEM supplemented with 10% FBS and placed at 37°C
in a humidified incubator containing 5% CO2. For
experiments involving TGF-β1, cells were treated with exogenous
TGF-β1 (Sigma-Aldrich; Merck KGaA) at 5, 10 and 20 ng/ml at 37°C
for 24 h before use.
ELISA
Plasma levels of TGF-β1 were measured using a human
TGF-β1 ELISA kit (cat. no. ab108912; Abcam), according to the
manufacturer's instructions.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from plasma and 22Rv1 cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) to detect MIR4435-2HG. cDNA was synthesized using
Reverse Transcriptase AMV (Sigma-Aldrich; Merck KGaA) using the
following conditions: 5 min at 25°C, 25 min at 52°C and 10 min at
80°C. PCR reaction systems were prepared using the Applied
Biosystems PowerUp™ SYBR™ Green Master Mix (Thermo Fisher
Scientific, Inc.). The PCR reaction conditions were as follows: 1
min at 95°C, followed by 10 sec at 95°C, 30 sec at 55°C and 40 sec
at 72°C. The primers for MIR4435-2HG and the endogenous control
GAPDH were synthesized by Sangon Biotech Co., Ltd. and were
designed as follows: MIR4435-2HG forward, 5′-CGGAGCATGGAACTCGACA-3′
and reverse, 5′-CAAGTCTCACACATCCGGG-3′; and GAPDH forward,
5′-AAGGTGAAGGTCGGAGTCA-3′ and reverse, 5′-AATGAAGGGGTCATTGATG-3′.
The relative expression levels of MIR4435-2HG were normalized to
the GAPDH endogenous control and expressed as 2−ΔΔCq
(14).
Vectors and cell transfection
pcDNA3.1 vectors expressing MIR4435-2HG were
constructed by Sangon Biotech Co., Ltd. Lipofectamine™ 2000 reagent
(Thermo Fisher Scientific, Inc.) was used transfect 10 nM vectors
into 105 cells. Cells were incubated with the vectors
for 5 h and fresh culture medium was added. Cells were harvested 24
h following transfection for subsequent experiments. Control (C)
cells were untransfected cells and negative control (NC) cells were
cells transfected with empty vectors.
Transwell migration and invasion
assay
Cells were harvested for Transwell migration and
invasion assays only if the MIR4435-2HG overexpression rate was
>200% (assessed by RT-qPCR). Briefly, cell suspensions were
prepared in serum-free culture medium. In cases of TGF-β1
treatment, TGF-β1 (10 ng/ml) was added into the medium. Cell
density was normalized to 5×104 cells/ml, 0.1 ml cell
suspension was transferred into the upper chamber of the Transwell
(pore size, 8 µm), and DMEM supplemented with 10% FBS was added
into the lower chamber. After 3 h at 37°C and 5% CO2,
cells in the upper chamber were stained for 15 min with 0.5%
crystal violet (Sigma-Aldrich; Merck KGaA) at room temperature. For
the invasion assay, Matrigel (cat. no. 356234; EMD Millipore) was
used to coat the upper chamber at 37°C for 6 h and the steps
described for the migration assay were performed. Stained cells
were counted in five randomly-selected fields using a light
microscope (magnification, ×40).
Western blotting
22Rv1 cells were lysed with RIPA solution (Thermo
Fisher Scientific, Inc.) to extract the protein. Protein
concentrations were measured using a bicinchoninic acid assay
(Thermo Fisher Scientific, Inc.). Proteins were separated by 10%
SDS-PAGE (30 µg per lane) and transferred onto polyvinylidene
fluoride membranes. The membranes were blocked with 5% skimmed milk
dissolved in FBS for 2 h at room temperature. The membranes were
then incubated with the primary antibodies against TGF-β1 (1:1,600;
cat. no. ab92486; Abcam) and GAPDH (1:1,400; cat. no. ab9485;
Abcam) for 16 h at 4°C, followed by horseradish
peroxidase-conjugated anti-rabbit immunoglobulin G secondary
antibody (1:1,000; cat. no. MBS435036; MyBioSource) for 2 h at
24°C. Enhanced chemiluminescence reagent (Sigma-Aldrich; Merck
KGaA) was used to detect the signal on the membrane. Data were
analyzed via densitometry using Image J V1.34 software (National
Institutes of Health) and normalized to the expression of the
internal control (GAPDH).
Statistical analysis
Data are presented as the means ± standard deviation
of three independent replicates. Comparisons between patients with
PCa and healthy controls were performed by unpaired t-test.
Comparisons amongst patients and cell transfection groups were
analyzed by one-way ANOVA followed by Tukey's test. Correlations
between plasma levels of TGF-β1 and MIR4435-2HG in patients with
PCa and healthy controls were analyzed by Pearson's correlation
coefficient. Patients were divided into high (n=31) and low (n=37)
MIR4435-2HG plasma level groups based on Youden's index, an index
used to define an optimized cut-off value (15). Kaplan-Meier analysis and the log-rank
test were used to compare survival curves. The association between
MIR4435-2H plasma levels and clinical characteristics of patients
with PCa were analyzed by χ2 test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Plasma levels of MIR4435-2HG and
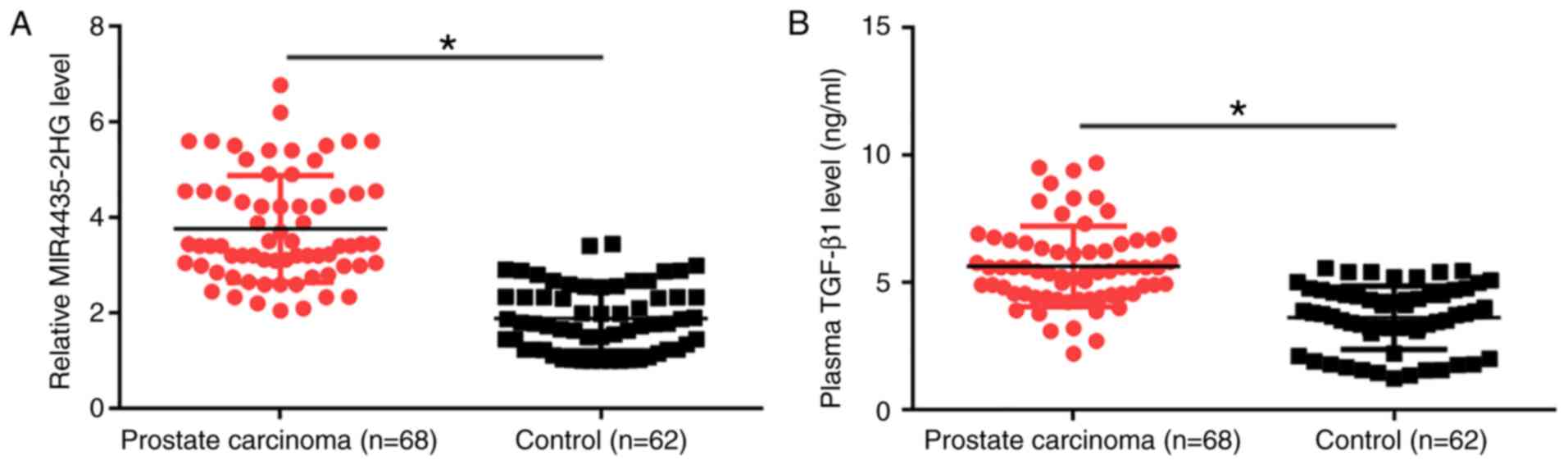
TGF-β1 are upregulated in patients with PCa
MIR4435-2HG and TGF-β1 plasma levels were measured
by RT-qPCR and ELISA, respectively. Plasma levels of MIR4435-2HG
(Fig. 1A) and TGF-β1 (Fig. 1B) were significantly upregulated in
patients with PCa compared with healthy controls (P<0.05). In
addition, MIR4435-2HG and TGF-β1 levels showed increasing trends
with clinical stages (I–IV, data not shown); however, these
associations were not significant. The associations between
MIR4435-2H levels and the clinical characteristics of patients with
PCa were analyzed by χ2 test. The results demonstrated
that the plasma levels of MIR4435-2HG were not associated with the
disease stage or age of patients (Table
I).
 | Table I.Correlation between MIR4435-2H levels
and clinical characteristics of patients with prostate
carcinoma. |
Table I.
Correlation between MIR4435-2H levels
and clinical characteristics of patients with prostate
carcinoma.
| Characteristics | Cases, n | High (n=31) | Low (n=37) | χ2
value | P-value |
|---|
| AJCC stage |
|
|
|
|
|
| I | 11 | 6 | 5 | 0.46 | 0.93 |
| II | 19 | 8 | 11 |
|
|
| III | 20 | 9 | 11 |
|
|
| IV | 18 | 8 | 10 |
|
|
| Age, years |
|
|
|
|
|
| ≥55 | 36 | 17 | 19 | 0.08 | 0.77 |
|
<55 | 32 | 14 | 18 |
|
|
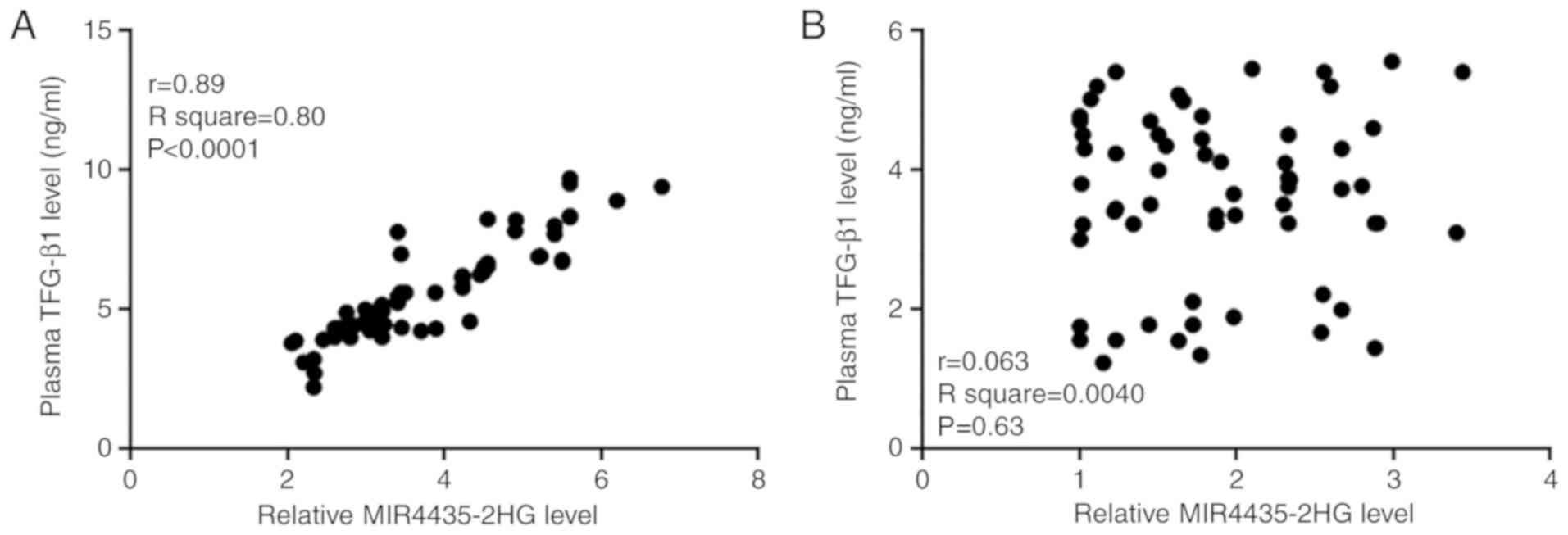
MIR4435-2HG and TGF-β1 are positively
correlated in patients with PCa
Correlations between TGF-β1 and MIR4435-2HG plasma
levels in patients and healthy controls were analyzed by Pearson's
correlation coefficient. As presented in Fig. 2A, MIR4435-2HG and TGF-β1 plasma
levels were positively correlated in patients with PCa. Conversely,
there was no correlation between MIR4435-2HG and TGF-β1 plasma
levels in healthy controls (Fig.
2B).
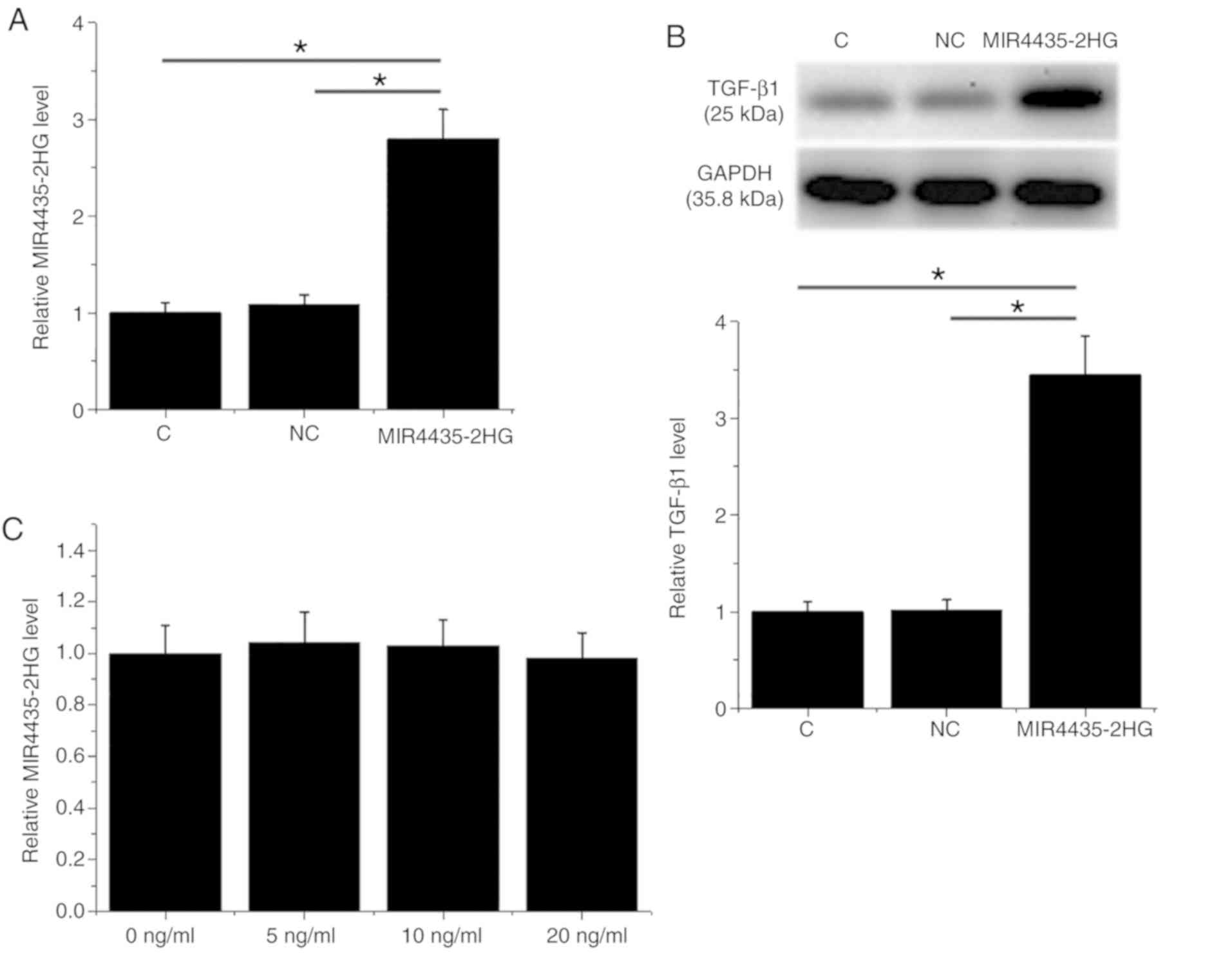
MIR4435-2HG overexpression induces
TGF-β1 upregulation in the 22Rv1 cell line
Following transfection, MIR4435-2HG overexpression
was confirmed in the 22Rv1 cell line (Fig. 3A; P<0.05). Furthermore,
MIR4435-2HG overexpression induced TGF-β1 upregulation (Fig. 3B; P<0.05) compared with the
negative control (NC) and control (C). However, treatment with
exogenous TGF-β1 (Sigma-Aldrich; Merck KGaA) at 5, 10 and 20 ng/ml
for 24 h had no effect on MIR4435-2HG expression level (Fig. 3C).
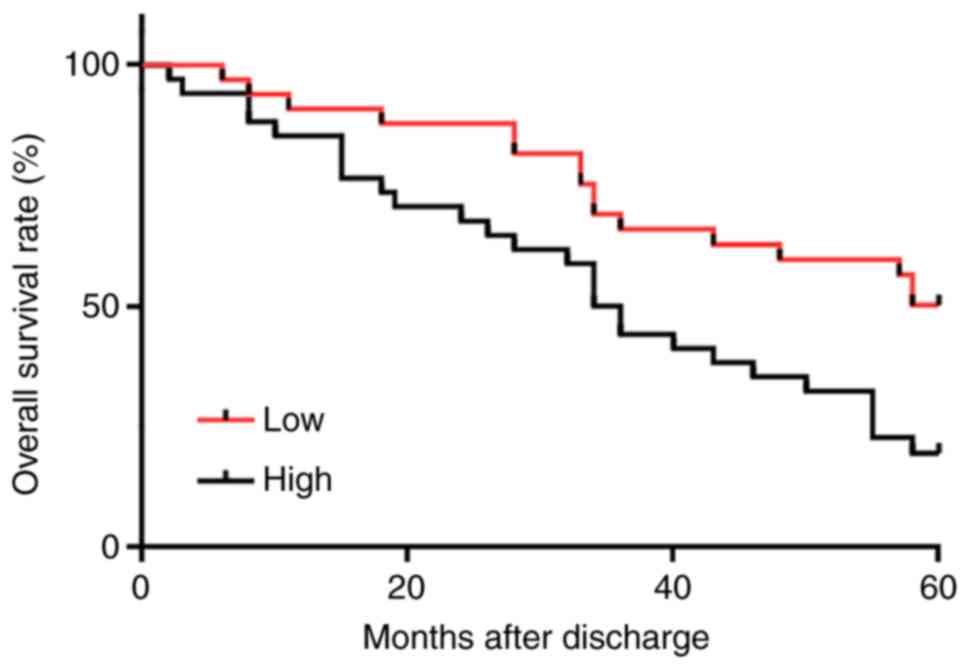
High plasma levels of MIR4435-2HG are
closely associated with poor survival in patients with PCa
Survival curves of patients with PCA were plotted
and compared using the Kaplan-Meier method and log-rank test,
respectively. As presented in Fig.
4, patients with high MIR4435-2HG plasma levels exhibited a
significantly lower overall survival rate compared with patients
with a low MIR4435-2HG plasma level (χ2=7.238;
P=0.0071).
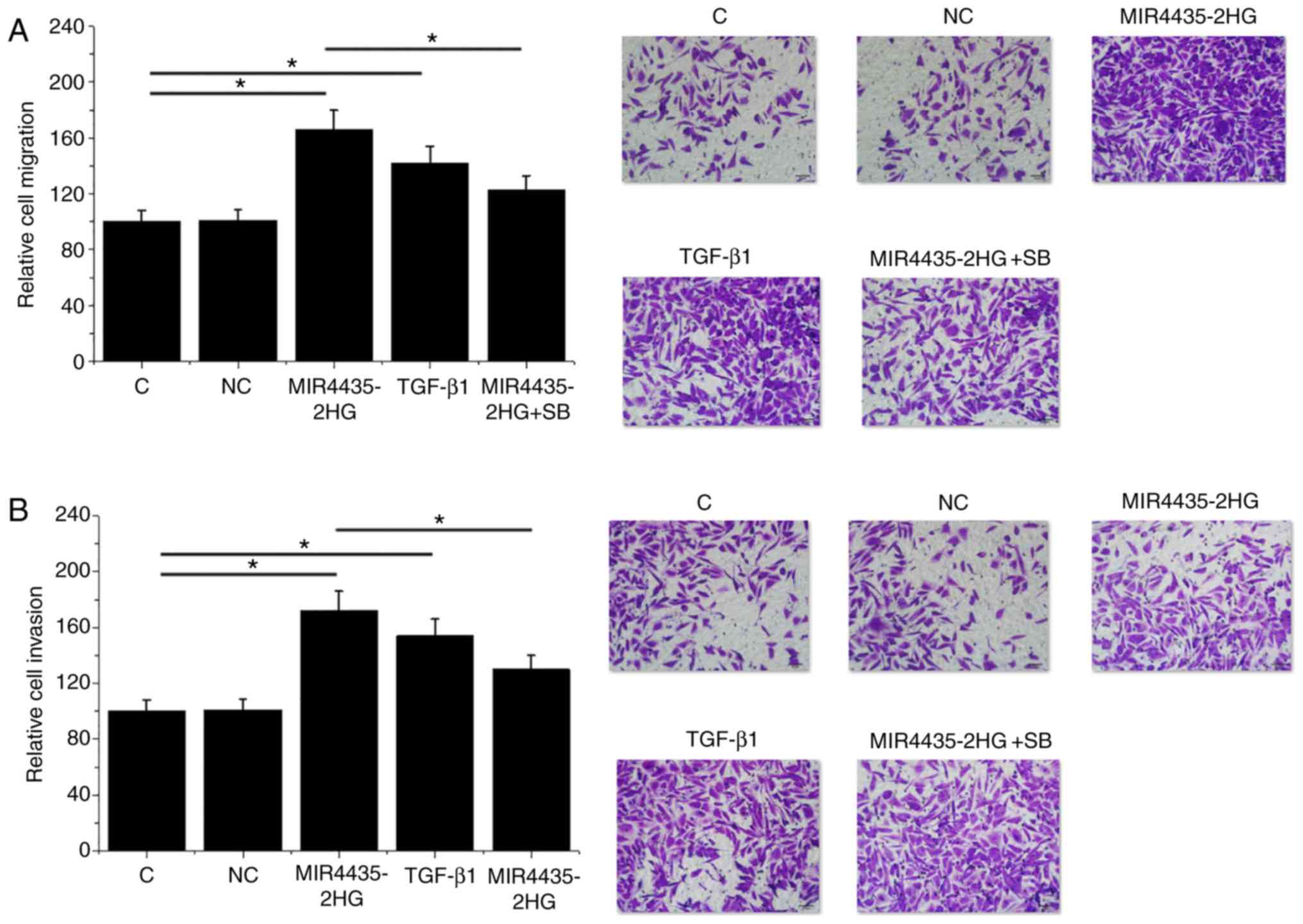
MIR4435-2HG overexpression promotes
22Rv1 cell migration and invasion, potentially through TGF-β1
MIR4435-2HG overexpression and treatment with
exogenous TGF-β1 (10 ng/ml) stimulated 22Rv1 cell migration
(Fig. 5A) and invasion (Fig. 5B; P<0.05) compared with the C and
NC groups. In addition, treatment with the TGF-β inhibitor SB431542
at 10 nM attenuated the stimulatory effects of MIR4435-2HG
overexpression on cell migration and invasion (P<0.05).
Discussion
Tumor metastasis is a major challenge in the
treatment of PCa. The present study demonstrated that MIR4435-2HG,
which is characterized as an oncogenic lnRNA in lung cancer
(12), promotes the migration and
invasion of PCa cells in vitro. Furthermore, the effects of
MIR4435-2HG in PCa may be mediated by TGF-β1 upregulation.
The involvement of TGF-β in cancer biology has been
intensively explored (16). During
the development of PCa, the TGF-β signaling pathway promotes the
migration and invasion of cancer cells through interactions with
numerous downstream targets, including the PI3K/AKT/mTOR pathway
(17). Conversely, inhibition of
TGF-β signaling by inhibitors such as PMEPA1 leads to tumor
metastasis inhibition (18).
Similarly, the present study demonstrated that the TGF-β1 plasma
level was upregulated in patients with PCa compared with healthy
controls. In addition, treatment with exogenous TGF-β1 accelerated
the migration and invasion of PCa cells. These results suggested
that TGF-β1 may stimulate tumor metastasis in PCa.
The TGF-β signaling pathway may participate in
cancer biology by mediating the expression of lncRNAs (19). In addition, activation of TGF-β
signaling can be regulated by certain lncRNAs, such as cancer
susceptibility 9 and Angelman syndrome chromosome region (20). To the best of our knowledge, the
associations between TGF-β signaling and lncRNAs in PCa have been
poorly investigated. The present study demonstrated that
MIR4435-2HG was upregulated in patients with PCa. MIR4435-2HG may
therefore serve as an upstream activator of TGF-β1 signaling
pathway. In addition, TGF-β1 upregulation by MIR4435-2HG may be
involved in the regulation of PCa cell migration and invasion. The
results from this study enrich the understanding of PCa
pathogenesis. It has been reported that MIR4435-2HG can interact
with the β-catenin signaling pathway to promote lung cancer
(12), and that β-catenin can
interact with TGF-β signaling (21).
β-catenin may therefore be considered as a mediator between
MIR4435-2HG and TGF-β. However, β-catenin was not investigated in
this study, which represents a limitation. In addition, the present
study failed to detect secreted TGF-β1 in the cell culture medium,
which is another limitation. Future investigations will focus on
these points.
Tumor metastasis is a major cause of mortality in
patients with PCa. The present study demonstrated that the
circulating level of MIR4435-2HG may serve as a prognostic
biomarker for PCa. However, more clinical trials are needed to
further confirm this hypothesis. In addition, it is noteworthy that
the TGF-β inhibitor only partially attenuated the effects of
MIR4435-2HG on cancer cell migration and invasion. MIR4435-2HG may
therefore interact with other downstream effectors to regulate PCa
cell migration and invasion. The present study did not investigate
the role of the TGF-β inhibitor in regulating PCa cell behavior,
since previous studies had already revealed that TGF-β signaling
inhibition suppresses PCa by affecting cancer cell behavior,
including invasion and migration (18,22,23).
The present study did not investigate the genes
involved in the regulation of PCa cell migration and invasion
through MIR4435-2HG. Future studies will perform a deeper
analysis.
In conclusion, the results from the present study
suggested that MIR4435-2HG may stimulate PCa cell migration and
invasion by promoting TGF-β signaling.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MC, HZ and HM designed the experiments. HZ, HM, XH
and WT performed the experiments. XL, JL, and CZ collected and
analyzed data. MC drafted the manuscript. All authors approved the
final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The First Affiliated Hospital of Guangzhou University of Chinese
Medicine prior to patient admission. Written informed consent was
collected from all patients with PCa and healthy volunteers.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Scher HI, Solo K, Valant J, Todd MB and
Mehra M: Prevalence of prostate cancer clinical states and
mortality in the United States: Estimates using a dynamic
progression model. PLoS One. 10:e01394402015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Angelis R, Sant M, Coleman MP,
Francisci S, Baili P, Pierannunzio D, Trama A, Visser O, Brenner H,
Ardanaz E, et al: Cancer survival in Europe 1999–2007 by country
and age: Results of EUROCARE-5-a population-based study. Lancet
Oncol. 15:23–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sweeney CJ, Chen YH, Carducci M, Liu G,
Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, et
al: Chemohormonal therapy in metastatic hormone-sensitive prostate
cancer. N Engl J Med. 373:737–746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herrera FG, Tawadros T and Berthold DR:
Bone metastases in prostate cancer: Pathophysiology, clinical
complications, actual treatment, and future directions. Bone
Cancer. Heymann D: 2nd. Elsevier; Amsterdam: pp. 657–663. 2015,
View Article : Google Scholar
|
|
6
|
Massagué J and Chen YG: Controlling
TGF-beta signaling. Genes Dev. 14:627–644. 2000.PubMed/NCBI
|
|
7
|
Akhurst RJ and Derynck R: TGF-beta
signaling in cancer-a double-edged sword. Trends Cell Biol.
11:S44–S51. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Derynck R, Muthusamy BP and Saeteurn KY:
Signaling pathway cooperation in TGF-β-induced
epithelial-mesenchymal transition. Curr Opin Cell Biol. 31:56–66.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Tian XJ, Zhang H, Teng Y, Li R,
Bai F, Elankumaran S and Xing J: TGF-β-induced
epithelial-to-mesenchymal transition proceeds through stepwise
activation of multiple feedback loops. Sci Signal. 7:ra912014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu W, Chen F, Cui X, Yang L, Chen J, Zhao
J, Huang D, Liu J, Yang L, Zeng J, et al: LncRNA NKILA suppresses
TGF-β-induced epithelial-mesenchymal transition by blocking NF-κB
signaling in breast cancer. Int J Cancer. 143:2213–2224. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qian H, Chen L, Huang J, Wang X, Ma S, Cui
F, Luo L, Ling L, Luo K and Zheng G: The lncRNA MIR4435-2HG
promotes lung cancer progression by activating β-catenin
signalling. J Mol Med (Berl). 96:753–764. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Edge SB and Compton CC: The American Joint
Committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hughes G: Youden's index and the weight of
evidence. Methods Inf Med. 54:198–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meulmeester E and Ten Dijke P: The dynamic
roles of TGF-β in cancer. J Pathol. 223:206–218. 2011. View Article : Google Scholar
|
|
17
|
Vo BT, Morton D Jr, Komaragiri S, Millena
AC, Leath C and Khan SA: TGF-β effects on prostate cancer cell
migration and invasion are mediated by PGE2 through activation of
PI3K/AKT/mTOR pathway. Endocrinology. 154:1768–1779. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fournier PG, Juárez P, Jiang G, Clines GA,
Niewolna M, Kim HS, Walton HW, Peng XH, Liu Y, Mohammad KS, et al:
The TGF-β signaling regulator PMEPA1 suppresses prostate cancer
metastases to bone. Cancer Cell. 27:809–821. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan YH, Ji CX, Xu B, Fan HY, Cheng ZJ and
Zhu XG: Long noncoding RNA activated by TGF-β in human cancers: A
meta-analysis. Clin Chim Acta. 468:10–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li C, Wan L, Liu Z, Xu G, Wang S, Su Z,
Zhang Y, Zhang C, Liu X, Lei Z and Zhang HT: Long non-coding RNA
XIST promotes TGF-β-induced epithelial-mesenchymal transition by
regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer.
Cancer Lett. 418:185–195. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishita M, Hashimoto MK, Ogata S, Laurent
MN, Ueno N, Shibuya H and Cho KW: Interaction between Wnt and
TGF-beta signalling pathways during formation of Spemann's
organizer. Nature. 403:781–785. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tuxhorn JA, McAlhany SJ, Yang F, Dang TD
and Rowley DR: Inhibition of transforming growth factor-beta
activity decreases angiogenesis in a human prostate cancer-reactive
stroma xenograft model. Cancer Res. 62:6021–6025. 2002.PubMed/NCBI
|
|
23
|
Paller C, Pu H, Begemann DE, Wade CA,
Hensley PJ and Kyprianou N: TGF-β receptor I inhibitor enhances
response to enzalutamide in a pre-clinical model of advanced
prostate cancer. Prostate. 79:31–43. 2019. View Article : Google Scholar : PubMed/NCBI
|