Introduction
Breast cancer is the most commonly diagnosed
malignant tumor among Chinese women. In 2015, the estimated
incidence of breast cancer was 304,000 new cases, with 70,000
associated deaths, which accounted for 6.67% of all cases of
mortality in China (1). Adjuvant
chemotherapy improves survival in patients with early-stage breast
cancer (2); however, it is
associated with severe side effects and a negative impact on the
quality of life, which may outweigh treatment benefits (3,4).
Therefore, personalizing treatment based on tumor biology to reduce
unnecessary chemotherapy is the main method of optimizing
individualized patient care (5).
The recommendation of adjuvant chemotherapy is
routinely based on tumor burden (tumor size and lymph node status),
tumor biological characteristics (grade and immunohistochemical
markers) and patient factors (menopausal status, presence of
comorbid conditions and patient preference) (6). Along with the recognition of intrinsic
biological subtypes within the breast cancer spectrum, a novel
approach for the classification of patients for therapeutic
purposes has been adopted (7).
Patients were classified into four intrinsic subtypes: Luminal A,
luminal B, HER2 enriched and Basal-like subtypes. Because of
economic and technical reasons, a clinicopathological surrogate
definition was widely adopted in clinical setting. For hormonal
receptor (HR)+/HER2− patients, luminal A-like
subtype is defined as estrogen receptor (ER)-positive, with
progesterone receptor (PR) ≥20% and Ki-67 <14% based on
clinicopathological criteria, while other
HR+/HER2− patients are classified as luminal
B-like subtype (7). In general,
systemic therapy recommendations are based on subtype
classification (7). Therefore,
luminal A-like disease generally requires only endocrine therapy,
whereas chemotherapy is considered for most patients with breast
cancer of a luminal B-like subtype (7).
Previously, several multi-gene expression profiles
have also been developed to guide adjuvant chemotherapy decision
making for patients with HR+/HER2−
early-stage breast cancer (8,9). The
21-gene recurrence score (RS) has been tested using the reverse
transcription-PCR method on sections of fixed, paraffin-embedded
tumor tissue, which was first reported in 2004, to quantify the
likelihood of distant recurrence in tamoxifen-treated patients with
node-negative, ER-positive breast cancer (10). Further validation studies
demonstrated that it can predict the benefit of chemotherapy in
node-negative (11) and
node-positive patients (12).
Additionally, the latest prospective TAILORx and West German Study
Group Plan B trials have indicated the prognostic value and
chemotherapeutical predictive value of RS in patients with
HR+/HER2− breast cancer (8,9,13). Based on these findings, the 21-gene
RS has been recommended for use in patients with
HR+/HER2−, lymph node-negative breast cancer
by the National Comprehensive Cancer Network (NCCN) (14).
Utilization of the 21-gene RS can lead to a change
in chemotherapy recommendation for 20–70% of cases, which has
resulted in a 13–34% reduction in adjuvant chemotherapy usage
(15). Results from validation
studies of the 21-gene RS have indicated that RS and
clinicopathological parameters, including tumor size and tumor
grade, are independent prognostic factors based on multivariate cox
regression analysis (9,10,13,16–18). In
a clinical setting, treatment decisions for a specific patient
should be based on clinical and genomic risk factors. According to
the results of the MINDACT clinical trial, while some patients can
be classified into different risk groups using clinicopathological
characteristics and genomic assays, for patients with a discordant
risk of clinicopathological features and a 70-gene prognosis
signature, chemotherapy has no additional benefit (19). However, to the best of our knowledge,
there is a dearth of data for the 21-gene RS in this segment of
patients. Therefore, it is important to find out whether the
chemotherapy recommendation for patients with different luminal
subtypes will be affected by the 21-gene RS.
The aim of the present study was to assess the
influence of the 21-gene RS on the chemotherapy decision-making
process for patients with different luminal subtypes in a real
world population, particularly for patients with discordance in the
luminal and 21-gene RS risk categories (luminal A-like subtype with
high RS or luminal B-like subtype with low RS).
Materials and methods
Study population
The present study retrospectively reviewed data on
consecutive patients with breast cancer undergoing 21-gene RS
testing between January 2014 and December 2016 at Ruijin Hospital
(Shanghai, China). This included a total of 772 female patients
with ages ranging from 26 to 88 (mean ± standard deviation,
57.15±12.53). Male patients, and patients with special subtypes
(carcinomas other than invasive ductal carcinoma, including
invasive lobular carcinoma, carcinoma with medullary features,
metaplastic carcinoma and mucinous carcinoma), HER2-positive tumors
and patients with >3 lymph nodes involved were excluded from the
study. All medical records were retrieved from the Shanghai
Jiaotong University Breast Cancer database. Performance status and
comorbidities were evaluated using Charlson comorbidity index
(CCI), a weighted index that takes into account patient age, and
the number and seriousness of comorbid diseases (20). Patients with a higher CCI score have
a worse performance status. Luminal-like subtypes were defined
based on the 2013 St. Gallen Expert Consensus (21). The definition of luminal A-like tumor
was as follows: ER-positive, PR ≥20%, HER2-negative and Ki-67
<14%. Luminal B-like tumors can be divided into two groups:
Luminal B-like/HER2− and luminal
B-like/HER2+. Since HER2-positive patients were not
included in the present study, luminal B-like tumors in the study
were defined as ER-positive, HER2-negative and PR <20% or Ki-67
≥14%. Since the usage of the 21-gene RS in lymph node-positive
patients remains controversial, lymph node status was evaluated to
help distinguish the influence of the 21-gene RS on chemotherapy.
The study protocol was approved by the Ethic Committees of Ruijin
Hospital.
Evaluation of ER, PR, HER2 and Ki-67
index status
Tumors were classified histologically according to
the World Health Organization Classification of Tumors guidelines
(22). Tumor staging was assessed
according to American Joint Committee on Cancer (AJCC) Cancer
Staging Handbook (23). Histological
grade was evaluated according to Elston and Ellis scoring system
(24). The pathology and
immunohistochemistry (IHC) staining methods used in the present
study were previously described (25). Briefly, IHC staining was performed on
4 µm slices of formalin-fixed paraffin-embedded (FFPE) tissue
sections. Following dewaxing and antigen retrieval, the tissue
sections were incubated with the peroxidase-blocking solution
(ready-to-use, cat. no. S2023; Dako; Agilent Technologies, Inc.)
for 10 min at room temperature. IHC staining of ER, PR, HER2 and
Ki-67 was routinely carried out by using the Ventana BenchMark XT
system (Ventana Medical Systems, Inc.). All procedures were
performed automatically in the BenchMark. The following antibodies
were used for the IHC assay: ER (cat. no. IR657, clone 1D5; 1:100;
mouse monoclonal; Dako; Agilent Technologies, Inc.), PR (cat. no.
IR068, clone PR636, mouse monoclonal; 1:100; Dako; Agilent
Technologies, Inc.), HER2 (cat. no. 790-2991, clone 4B5, rabbit
monoclonal; 1:100; Roche Diagnostics) and Ki-67 (cat. no. IR626,
clone MIB-1, mouse monoclonal; 1:100; Dako; Agilent Technologies,
Inc.). The tissue sections were incubated with primary antibody of
ER, PR and Ki67 for 32 min at 42°C and of HER2 for 16 min at 42°C.
Sections were incubated in secondary goat anti-mouse (cat. no.
P0447; 1:100; Dako; Agilent Technologies, Inc.) or goat anti-rabbit
(cat. no. P0448; 1:100; Dako; Agilent Technologies, Inc.)
antibodies for 30 min at room temperature. All histological and IHC
tumor slides were evaluated by two pathologists with a light
microscope at magnification of ×100. Positive staining for ER/PR
was defined as nuclear staining in ≥1% of the tumor cells. The
Ki-67 index was characterized as the proportion of cells with
positive nuclear staining among ≥1,000 tumor cells in the area
counted. Negative HER2 status was considered as a score of between
0 and 1+, using IHC, or a negative result upon fluorescence in
situ hybridization (FISH).
FISH was performed using the PathVysion HER-2 DNA
FISH kit, (Vysis, Inc.; Abbott Pharmaceutical) according to the
manufacturer's instructions. Acid pretreatment and protease
digestion were performed (Vysis paraffin pretreatment kit; Vysis,
Inc.), followed by standard saline citrate (SSC) and formamide
denaturation (72°C for 5 min). After dehydration, the HER2/CEP 17
probe mixer was added. Slides were incubated in a moist chamber
overnight at 37°C under a coverslip. On the following day, slides
were washed in a stringency buffer (SSC, NP40), air-dried in the
dark and incubated with 4,6 diamidino-2-phenylindole (DAPI) for
nuclear identification.
21-gene RS testing
The tests were performed on formalin-fixed,
paraffin-embedded tissues, as previously described (10,25). In
brief, fixed tissues were incubated for 5–10 h in 10%
neutral-buffered formalin prior to being alcohol-dehydrated and
embedded in paraffin. Hematoxylin and eosin-stained slides were
reviewed to assess the percentage of invasive breast cancer in the
overall area. RNA was extracted from two 10-µm unstained sections
from sufficient (invasive component ≥50%) invasive breast cancer
with RNeasy FFPE Kit (cat. no. 73504; Qiagen, Inc.) according to
the manufacturer's protocol. Total RNA content was measured, and
the absence of DNA contamination was verified. Reverse
transcription of the purified RNA was carried out with the
Omniscript RT kit (Qiagen, Inc.) at 65°C for 5 min and 37°C for 60
min. Probes for PCR were designed using Primer Express (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and Primer3 programs,
as previously reported (26). The
sequences of all probes are shown in Table SI. Gene-specific reverse
transcription was performed followed by standardized quantitative
PCR reactions in 96-well plates with TaqMan (DRR390A, Takara
Biotechnology Co., Ltd.) using Applied Biosystems (Thermo Fisher
Scientific, Inc.) 7500 Real-Time PCR system. The thermocycling
conditions of the PCR were as follows: 95°C for 10 min, 95°C for 20
sec, and 60°C for 45 sec (for 40 cycles). The expression of each
gene was measured in triplicate and normalized relative to a set of
5 reference genes. The RS values, ranging between 0 and 100, were
derived from the reference-normalized expression measurements of 16
cancer-associated genes (Ki-67, aurora kinase A, survivin (SURV),
cyclin B1, MYB proto-oncogene-like 2, growth factor receptor bound
protein 7, HER2, ER, PR, B-cell lymphoma 2, Cub and EGF Like Domain
Containing 2 protein, matrix metallopeptidase 11, cathepsin V,
glutathione S-transferase µ1, cluster of differentiation 68 and
BCL2-associated athanogene 1). These 16 cancer-related genes were
selected as these were consistently univariately associated with
clinical outcome in all three clinical association studies
(27–29), and the selected five reference genes
(actin β, GAPDH, glucuronidase β, ribosomal protein lateral stalk
subunit P0 and transferrin receptor) consistently had a low
variation in their expression and lacked any association with the
clinical outcome in each clinical study (10). The expression levels of each gene
were measured in triplicate. Patients were subsequently divided
into low-risk (RS <18), intermediate-risk (RS, 18–30) and
high-risk (RS >30) groups.
Adjuvant chemotherapy
recommendation
Adjuvant treatment decisions for each patient with
breast cancer were made by a multidisciplinary team comprising
breast surgeons, medical oncologists, pathologists, radiation
oncologists and specialized breast nurses. The recommended
chemotherapy regimens included EC [epirubicin, 90 mg/m2
intravenously (IV) on day 1; and cyclophosphamide, 600
mg/m2 IV on day 1, every 21 days for 4 cycles], EC-T
(epirubicin, 90 mg/m2 IV on day 1; and cyclophosphamide,
600 mg/m2 IV on day 1, every 21 days for 4 cycles;
followed by docetaxel, 80–100 mg/m2 IV on day 1, every
21 days for 4 cycles) and TC (docetaxel, 75 mg/m2 IV on
day 1; and cyclophosphamide, 600 mg/m2 IV day 1, every
21 days for 4 cycles).
Statistical analysis
The χ2 test was applied to evaluate the
distribution of each RS risk category and chemotherapy
recommendation among patients with different luminal subtypes and
lymph node statuses. Fisher's exact test was performed when
necessary (sample size <40 or minimum theoretical frequency
<5). Logistic regression was used in the multivariate analyses
to identify factors associated with chemotherapy recommendation.
Expression of gene was measured in triplicate. Continuous variables
were presented as mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Statistical analysis was performed using SPSS version 17.0 (SPSS,
Inc.).
Results
Patients and baseline
clinicopathological features
Between January 2014 and December 2016, 899 patients
underwent 21-gene RS testing at Ruijin Hospital. A total of 772
patients with HR+/HER2− and N0-1
invasive ductal carcinoma for whom 21-gene RS testing results were
available were included in the present study.
The baseline clinicopathological features of the
patients are shown in Table I. Among
the patients, 235 (30.44%) were <50 years of age, 244 (31.61%)
had T2 tumors and 173 (22.41%) were diagnosed with
N1 tumors. A total of 254 cases (32.90%) were classified
as luminal A-like subtype and 518 (67.10%) were classified as
luminal B-like subtype. The mean value of RS in the whole
population was 23.51±9.24, with 184 patients (23.83%) in the low
risk RS category, 430 patients (55.70%) in the intermediate risk RS
category and 158 patients (20.47%) in the high risk RS category
(data not shown).
 | Table I.Clinicopathological characteristics
of patients and chemotherapy recommendation. |
Table I.
Clinicopathological characteristics
of patients and chemotherapy recommendation.
|
|
| Chemotherapy
recommendation |
|
|---|
|
|
|
|
|
|---|
| Variable | Patients, n | No chemotherapy, n
(%) | Chemotherapy, n
(%) | P-value |
|---|
| Age, years |
|
|
| <0.001 |
|
<50 | 235 | 76 (32.34) | 159 (67.66) |
|
|
≥50 | 537 | 252 (46.93) | 285 (53.07) |
|
| Menopausal
status |
|
|
| <0.001 |
|
Premenopausal | 272 | 92 (33.82) | 180 (66.18) |
|
|
Postmenopausal | 500 | 236 (47.20) | 264 (52.80) |
|
| Charlson
comorbidity index |
|
|
| <0.001 |
| 0 | 204 | 65 (31.86) | 139 (68.14) |
|
|
1–2 | 328 | 112 (34.15) | 216 (65.85) |
|
| ≥3 | 240 | 151 (62.92) | 89 (37.08) |
|
| Surgery |
|
|
| 0.525 |
|
Mastectomy | 437 | 190 (43.48) | 247 (56.52) |
|
|
BCS | 335 | 138 (41.19) | 197 (58.81) |
|
| T size stage |
|
|
| <0.001 |
|
T1 | 528 | 255 (48.30) | 273 (51.70) |
|
|
T2 | 244 | 73 (29.92) | 171 (70.08) |
|
| N stage |
|
|
| <0.001 |
|
N0 | 599 | 302 (50.42) | 297 (49.58) |
|
|
N1 | 173 | 26 (15.03) | 147 (84.97) |
|
| Stage |
|
|
| <0.001 |
| I | 461 | 243 (52.71) | 218 (47.29) |
|
|
II–III | 311 | 85 (27.33) | 226 (72.67) |
|
| Grade |
|
|
| <0.001 |
| I | 96 | 71 (73.96) | 25 (26.04) |
|
| II | 489 | 230 (47.03) | 259 (52.97) |
|
|
III | 187 | 27 (14.44) | 160 (85.56) |
|
| ER |
|
|
| 0.102 |
|
<50% | 26 | 7 (26.92) | 19 (73.08) |
|
|
≥50% | 746 | 321 (43.03) | 425 (56.97) |
|
| PR |
|
|
| <0.001 |
|
<20% | 261 | 69 (26.44) | 192 (73.564) |
|
|
≥20% | 511 | 259 (50.68) | 252 (49.32) |
|
| Ki-67 |
|
|
| <0.001 |
|
<14% | 378 | 229 (60.58) | 149 (39.42) |
|
|
≥14% | 394 | 99 (25.13) | 295 (74.87) |
|
| Subtypes |
|
|
| <0.001 |
| Luminal
A-like | 254 | 176 (69.29) | 78 (30.71) |
|
| Luminal
B-like | 518 | 152 (29.34) | 366 (70.66) |
|
| RS categories |
|
|
| <0.001 |
|
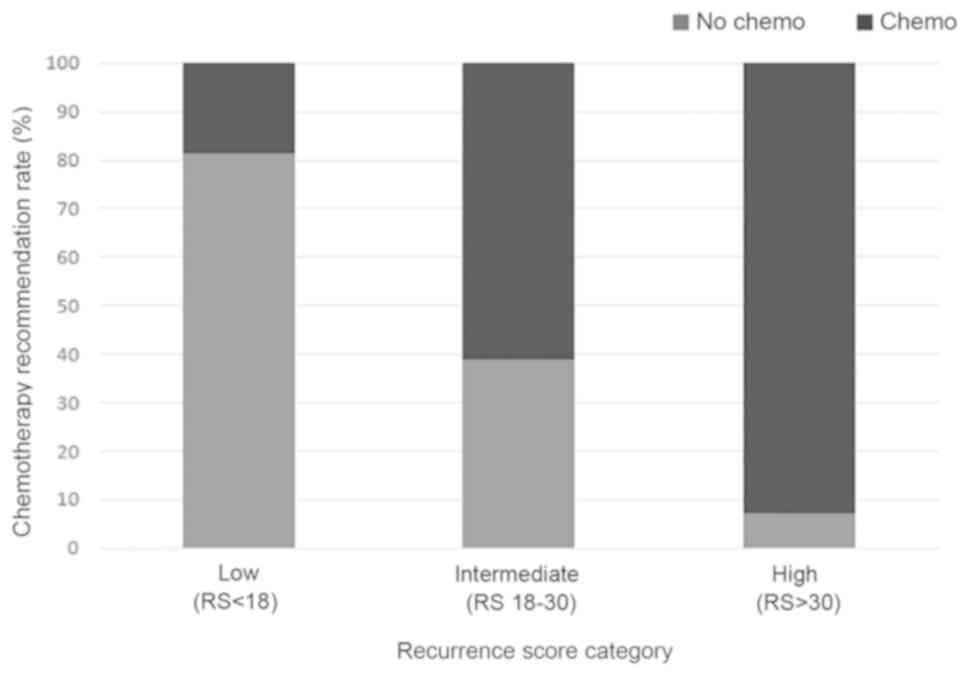
Low | 184 | 150 (81.52) | 34 (18.48) |
|
|
Intermediate | 430 | 167 (38.84) | 263 (61.16) |
|
|
High | 158 | 11
(6.96) | 147 (93.04) |
|
Association between
clinicopathological features and adjuvant chemotherapy
recommendation for the whole population
More than half of all patients (444/772 patients;
57.51%) were recommended to receive adjuvant chemotherapy, which
was the general recommendation for patients of younger age,
premenopausal status, with less comorbidities, larger tumor size,
lymph node involvement, higher tumor stage, higher tumor grade, low
PR expression, high Ki-67 level, luminal B-like subtype and higher
RS (all P<0.05; Table I). In the
multivariate analysis, lower CCI score, lymph node involvement,
higher tumor grade, lower PR level, higher Ki-67 level and higher
RS score were found to be independently associated with
chemotherapy being recommended (Table
II). Patients with high-risk RS were more likely to be
recommended for chemotherapy, compared with those with low-risk RS
[odds ratio (OR), 62.54; 95% CI, 25.58–152.92; P<0.001; Table II; Fig.
1].
 | Table II.Multivariate analysis for factors
influencing chemotherapy recommendation. |
Table II.
Multivariate analysis for factors
influencing chemotherapy recommendation.
| Variables | OR | 95% CI | P-value |
|---|
| Age, years |
|
| 0.151 |
|
≥50 | 1.00 |
|
|
|
<50 | 2.22 | 0.75–6.59 |
|
| Charlson
comorbidity index |
|
| <0.001 |
| 0 | 1.00 |
|
|
|
1–2 | 0.64 | 0.38–1.08 |
|
| ≥3 | 0.11 | 0.06–0.20 |
|
| T size stage |
|
| 0.201 |
|
T1 | 1.00 |
|
|
|
T2 | 1.37 | 0.85–2.22 |
|
| N stage |
|
| <0.001 |
|
N0 | 1.00 |
|
|
|
N1 | 16.09 | 8.49–30.50 |
|
| Grade |
|
| <0.001 |
| I | 1.00 |
|
|
| II | 2.36 | 1.23–4.55 |
|
|
III | 7.48 | 3.13–17.90 |
|
| ER |
|
| 0.574 |
|
≥50% | 1.00 |
|
|
|
<50% | 0.674 | 0.17–2.67 |
|
| PR |
|
| <0.001 |
|
≥20% | 1.00 |
|
|
|
<20% | 2.99 | 1.81–4.92 |
|
| Ki-67 |
|
| <0.001 |
|
≤14% | 1.00 |
|
|
|
>14% | 4.54 | 2.82–7.31 |
|
| Subtypes |
|
| 0.172 |
| Luminal
A-like | 1.00 |
|
|
| Luminal
B-like | 1.94 | 0.75–5.01 |
|
| RS categories |
|
| <0.001 |
|
Low | 1.00 |
|
|
|
Intermediate | 9.04 | 5.18–15.77 |
|
|
High | 62.54 | 25.58–152.92 |
|
When dividing RS into five intervals (0–10, 11–17,
18–25, 26–30 and >30), the proportion of patients for whom
chemotherapy was recommended had the tendency to increase along
with the 21-gene RS. For patients with an RS of 0–10 and 11–17, the
proportion of patients that were not recommended for chemotherapy
was 79.31 and 82.54%, respectively, whereas only 50.15% of patients
with a RS of 18–25 did not receive a chemotherapy recommendation.
The proportion of chemotherapy recommended patients further
increased to 83.92% in RS 26–30 patients, and to 93.04% in patients
with an RS >30 (Fig. S1).
There were 3 patients who received capecitabine
alone, due to age and comorbidities. Among the 441 patients who
received standard intravenous chemotherapy, the chemotherapy
regimens and cycles recommended were associated with 21-gene RS.
Among patients in the low RS category, 94.1% received fewer cycles
of chemotherapy, and the regimens included either anthracycline or
taxane. On the other hand, among patients in the high RS category,
36.8% received more cycles of chemotherapy, for which both
anthracycline and taxane were used (P<0.001; Table SII).
Chemotherapy recommendation for
patients with luminal A-like subtype breast cancer
A total of 254 patients with luminal A-like breast
cancer were included in the present study. Based on the 21-gene RS
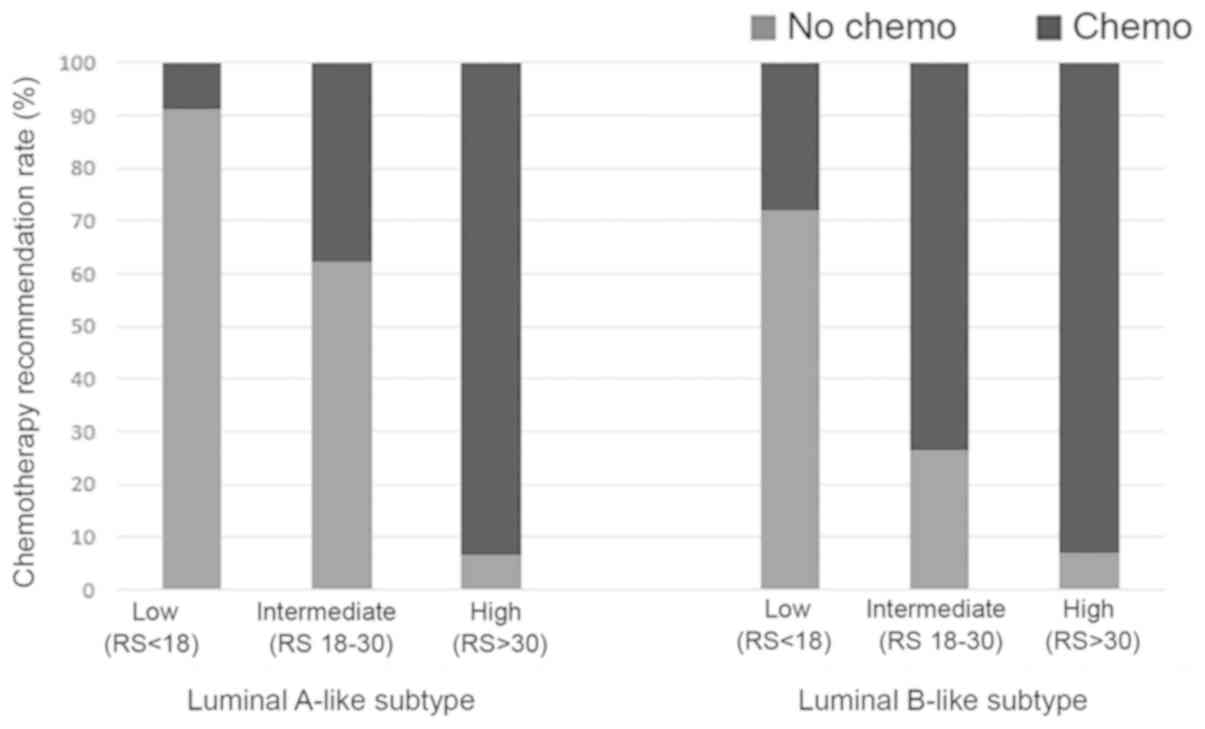
test results, 35.83% (91/254) of luminal A-like subtype patients
were classified in the low RS category, 58.27% (148/254) in the
intermediate RS category and 5.91% (15/254) in the high RS category
(Table III). Chemotherapy was
recommended for the majority of patients with a high RS (93.33%),
but was recommended for only 37.84% patients with an intermediate
RS and 8.79% of patients with a low RS (Table III).
 | Table III.Clinicopathological features and
chemotherapy recommendation in patients with luminal A-like subtype
breast cancer. |
Table III.
Clinicopathological features and
chemotherapy recommendation in patients with luminal A-like subtype
breast cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | No chemotherapy, n
(%) | Chemotherapy, n
(%) | P-value | OR | 95% CI | P-value |
|---|
| Age, years |
|
| <0.001 |
|
| 0.046 |
|
≥50 | 131 (79.39) | 34 (20.61) |
| 1.00 |
|
|
|
<50 | 45
(50.56) | 44 (49.44) |
| 5.98 | 1.04–34.99 |
|
| Charlson
comorbidity index |
|
| <0.001 |
|
| 0.019 |
| 0 | 39
(50.65) | 38 (49.35) |
| 1.00 |
|
|
|
1–2 | 67
(68.37) | 31 (31.63) |
| 1.53 | 0.28–8.37 |
|
| ≥3 | 70
(88.61) | 9
(11.39) |
| 0.25 | 0.03–2.08 |
|
| T size stage |
|
| 0.036 |
|
| 0.422 |
|
T1 | 139 (72.77) | 52 (27.23) |
| 1.00 |
|
|
|
T2 | 37
(58.73) | 26 (41.27) |
| 1.46 | 0.58–3.70 |
|
| N stage |
|
| <0.001 |
|
| <0.001 |
|
N0 | 160 (80.00) | 40 (20.00) |
| 1.00 |
|
|
|
N1 | 16
(29.63) | 38 (70.37) |
| 39.40 | 12.75–121.73 |
|
| Grade |
|
| <0.001 |
|
| 0.047 |
| I | 54
(83.08) | 11 (16.92) |
| 1.00 |
|
|
| II | 120 (67.04) | 59 (32.96) |
| 2.49 | 0.88–7.03 |
|
|
III | 2
(20.00) | 8
(80.00) |
| 14.21 | 1.48–136.37 |
|
| ER |
|
| 0.555 | – |
| 0.999 |
|
≥50% | 173 (68.92) | 78 (31.08) |
|
|
|
|
|
<50% | 3
(100.00) | 0
(0.00) |
|
|
|
|
| RS categories |
|
| <0.001 |
|
| <0.001 |
|
Low | 83
(91.21) | 8
(8.79) |
| 1.00 |
|
|
|
Intermediate | 92
(62.16) | 56 (37.84) |
| 7.93 | 2.64–23.83 |
|
|
High | 1
(6.67) | 14 (93.33) |
| 435.05 | 29.90–6331.06 |
|
For patients with breast cancer of the luminal
A-like subtype, chemotherapy was recommended for 30.71% (78/254).
Clinicopathological factors, including age, CCI score, tumor size,
lymph node status, tumor grade and RS category, were found to be
associated with chemotherapy recommendation upon univariate
analysis. In the multivariate analysis, chemotherapy recommendation
was found to be associated with a younger age (OR, 5.98; 95% CI,
1.04–34.99; P=0.046), lower CCI (≥3 vs. 0; OR, 0.25; 95% CI,
0.03–2.08; P=0.019), lymph node involvement (OR, 39.40; 95% CI,
12.75–121.73; P<0.001), grade III (OR, 14.21; 95% CI,
1.48–136.37; P=0.047) and the high-risk RS category (OR, 435.05;
95% CI, 29.90–6331.06; P<0.001) (Table III). Notably, the chemotherapy
recommendation rate in the high-risk RS category was 10 times
higher than that in the low risk RS category for patients with
luminal A-like subtype breast cancer (Fig. 2).
Chemotherapy recommendation for
patients with luminal B-like subtype breast cancer
Among the 518 patients with luminal B-like breast
cancer, 17.95% were categorized as low RS, 54.44% as intermediate
RS and 27.61% as high RS (Table
IV). In the group of patients with luminal B-like subtype
breast cancer, adjuvant chemotherapy was recommended for 93.01% of
patients in the high-risk RS category, whereas adjuvant
chemotherapy was recommended for only 27.96% of patients in the
low-risk RS category (Fig. 2). In
addition to age, CCI score, tumor size, lymph node status, tumor
grade and Ki-67 expression, RS was also associated with
chemotherapy recommendation upon univariate analysis (Table IV). Belonging to the high-risk RS
category was an important independent influencing factor for
chemotherapy decision compared with belonging to the low risk RS
category (OR, 57.20: 95% CI, 22.42–145.96; P<0.001).
 | Table IV.Clinicopathological features and
chemotherapy recommendation in patients with luminal B-like subtype
breast cancer. |
Table IV.
Clinicopathological features and
chemotherapy recommendation in patients with luminal B-like subtype
breast cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | No chemotherapy, n
(%) | Chemotherapy, n
(%) | P-value | OR | 95% CI | P-value |
|---|
| Age, years |
|
| 0.011 |
|
| 0.979 |
|
≥50 | 121 (32.53) | 251 (67.47) |
| 1.00 |
|
|
|
<50 | 31
(21.23) | 115 (78.77) |
| 1.02 | 0.25–4.22 |
|
| Charlson
comorbidity index |
|
| <0.001 |
|
| <0.001 |
| 0 | 26
(20.47) | 101 (79.53) |
| 1.00 |
|
|
|
1–2 | 45
(19.57) | 185 (80.43) |
| 1.00 | 0.50–1.99 |
|
| ≥3 | 81
(50.31) | 80
(49.69) |
| 0.17 | 0.08–0.35 |
|
| T stage |
|
| 0.001 |
|
| 0.281 |
|
T1 | 116 (34.42) | 221 (65.58) |
| 1.00 |
|
|
|
T2 | 36
(19.89) | 145 (80.11) |
| 1.38 | 0.77–2.45 |
|
| N stage |
|
| <0.001 |
|
| <0.001 |
|
N0 | 142 (35.59) | 257 (64.41) |
| 1.00 |
|
|
|
N1 | 10
(8.40) | 109 (91.60) |
| 10.60 | 4.59–24.51 |
|
| Grade |
|
| <0.001 |
|
| 0.001 |
| I | 17
(54.84) | 14
(45.16) |
| 1.00 |
|
|
| II | 110 (35.48) | 200 (64.52) |
| 2.42 | 0.95–6.17 |
|
|
III | 25
(14.12) | 152 (85.88) |
| 6.80 | 2.28–20.27 |
|
| ER |
|
| 0.198 |
|
| 0.859 |
|
≥50% | 148 (29.90) | 347 (70.10) |
| 1.00 |
|
|
|
<50% | 4
(17.39) | 19
(82.61) |
| 1.16 | 0.23–5.70 |
|
| PR |
|
| 0.143 |
|
| 0.112 |
|
≥20% | 83
(32.30) | 174 (67.70) |
| 1.00 |
|
|
|
<20% | 69
(26.44) | 192 (73.56) |
| 1.87 | 0.87–4.03 |
|
| Ki-67 |
|
| <0.001 |
|
| 0.031 |
|
≤14% | 53
(42.74) | 71
(57.26) |
| 1.00 |
|
|
|
>14% | 99
(25.13) | 295 (74.87) |
| 1.94 | 1.06–3.53 |
|
| RS categories |
|
| <0.001 |
|
| <0.001 |
|
Low | 67
(72.04) | 26
(27.96) |
| 1.00 |
|
|
|
Intermediate | 75
(26.60) | 207 (73.40) |
| 11.77 | 6.10–22.72 |
|
|
High | 10
(6.99) | 133 (93.01) |
| 57.20 | 22.42–145.96 |
|
Chemotherapy recommendation for
patients stratified by lymph node status
The association between clinicopathological features
and chemotherapy recommendation stratified by lymph node status was
further analyzed (Tables SIII and
SIV). Among lymph node-negative
patients, chemotherapy was recommended for 91.60% in the high-risk
RS category, whereas the percentage rose to 97.44% for patients
with node-positive breast cancer (Fig.
S2). Lower CCI score, higher Ki-67 level and higher risk RS
category were independent factors that influenced chemotherapy
recommendation for both node-negative and node-positive patients
according to multivariate analyses, whereas higher tumor grade and
lower PR level were associated with chemotherapy recommendation
only in node-negative patients (Tables
SIII and SIV). The high risk RS
category was found to be impact factor with highest OR value for
node-negative (OR, 84.04; 95% CI, 31.11–227.05; P<0.001) and
node-positive patients (OR, 20.83; 95% CI, 2.06–210.59;
P=0.002).
Chemotherapy recommendation for
patients with luminal A-like subtype breast cancer and a high RS or
patients with luminal B-like subtype breast cancer and a low
RS
A total of 15 patients with breast cancer with
luminal A-like subtype tumors were identified to have a high RS
(5.91%), whereas 93 patients with the luminal B-like subtype were
identified to have a low RS (17.95%). Chemotherapy was recommended
for 93.33% (14/15) of patients with luminal A-like subtype breast
cancer and a high RS, and for 27.96% (26/93) of patients with
luminal B-like subtype breast cancer and a low RS. For these 108
patients, age (P=0.026), CCI score (P=0.003), lymph node status
(P<0.001), luminal subtypes (P<0.001) and RS category
(P<0.001) were associated with chemotherapy decision according
to the univariate analysis (Table
V). In the multivariate analysis, lymph node involvement (OR,
55.04; 95% CI, 9.07–333.89; P<0.001), lower CCI (≥3 vs. 0; OR,
0.90; 95% CI, 0.05–16.43; P=0.034) and the high RS category (OR,
134.52; 95% CI, 10.39–1741.89; P<0.001) were found to be
independently associated with chemotherapy recommendation (Table VI).
 | Table V.Chemotherapy recommendation in
patients with breast cancer with luminal A-like subtype and low RS
or luminal B-like subtype and high RS tumors. |
Table V.
Chemotherapy recommendation in
patients with breast cancer with luminal A-like subtype and low RS
or luminal B-like subtype and high RS tumors.
| Variables | Patients, n | No chemotherapy, n
(%) | Chemotherapy, n
(%) | P-value |
|---|
| Age, years |
|
|
| 0.026 |
|
≥50 | 71 | 50 (70.42) | 21 (29.58) |
|
|
<50 | 37 | 18 (48.65) | 19 (51.35) |
|
| Charlson
comorbidity index |
|
|
| 0.003 |
| 0 | 30 | 16 (53.33) | 14 (46.67) |
|
|
1–2 | 37 | 18 (48.65) | 19 (51.35) |
|
| ≥3 | 41 | 34 (82.93) | 7
(17.07) |
|
| T size stage |
|
|
| 0.961 |
|
T1 | 78 | 49 (62.82) | 29 (37.18) |
|
|
T2 | 30 | 19 (63.33) | 11 (36.67) |
|
| N stage |
|
|
| <0.001 |
|
N0 | 88 | 64 (72.73) | 24 (27.27) |
|
|
N1 | 20 | 4
(20.00) | 16 (80.00) |
|
| Grade |
|
|
| 0.078 |
| I | 8 | 6
(75.00) | 2
(25.00) |
|
| II | 73 | 50 (68.49) | 23 (31.51) |
|
|
III | 27 | 12 (44.44) | 15 (55.56) |
|
| ER |
|
|
| 0.529 |
|
≥50% | 106 | 66 (62.26) | 40 (37.74) |
|
|
<50% | 2 | 2
(100.00) | 0
(0.00) |
|
| Subtypes |
|
|
| <0.001 |
| Luminal
A-like | 15 | 1
(6.67) | 14 (93.33) |
|
| Luminal
B-like | 93 | 67 (72.04) | 26 (27.96) |
|
| RS categories |
|
|
| <0.001 |
|
Low | 93 | 67 (72.04) | 26 (27.96) |
|
|
High | 15 | 1
(6.67) | 14 (93.33) |
|
 | Table VI.Multivariate analysis of chemotherapy
decision influencing factors in patients with luminal A-like
subtype and low RS or luminal B-like subtype and high RS
tumors. |
Table VI.
Multivariate analysis of chemotherapy
decision influencing factors in patients with luminal A-like
subtype and low RS or luminal B-like subtype and high RS
tumors.
| Variables | OR | 95% CI | P-value |
|---|
| Age, years |
|
| 0.096 |
|
≥50 | 1.00 |
|
|
|
<50 | 9.56 | 0.67–136.85 |
|
| Charlson
comorbidity index |
|
| 0.034 |
| 0 | 1.00 |
|
|
|
1–2 | 6.09 | 0.44–84.95 |
|
| ≥3 | 0.90 | 0.05–16.43 |
|
| N stage |
|
| <0.001 |
|
N0 | 1.00 |
|
|
|
N1 | 55.04 | 9.07–333.89 |
|
| Grade |
|
| 0.005 |
| I | 1.00 |
|
|
| II | 1.78 | 0.08–41.90 |
|
|
III | 20.78 | 0.81–531.84 |
|
| RS categories |
|
| <0.001 |
|
Low | 1.00 |
|
|
|
High | 134.52 | 10.39–1741.89 |
|
Discussion
The present study assessed the influence of the
21-gene RS on chemotherapy decision for patients with
HR+/HER2− breast cancer of different luminal
subtypes. The present study revealed that the 21-gene RS was
independently associated with adjuvant chemotherapy recommendation
in patients with breast cancer, regardless of their luminal subtype
and lymph node status. For patients with luminal A-like subtype
breast cancer with a high RS or luminal B-like subtype with a low
RS, high RS was identified to be the most important factor
influencing adjuvant chemotherapy decision when compared with low
RS (adjusted OR, 134.52).
Currently, two methods are used to determine breast
cancer subtypes: Multigene-based assays and IHC-based markers.
Based on a previous study conducted by Prat et al (30), the definition of the luminal A-like
subtype was updated as ER-positive, PR ≥20%, HER2-negative and
Ki-67 <14% (21). Additionally,
the 2013 St. Gallen expert consensus added a ‘recurrence risk of
‘low’ based on multi-gene-expression assay (if available)’ to the
definition of the luminal A-like subtype (21). In clinical trials (8,9,13), chemotherapy has been assigned
strictly based on the study design. For example, in a prospective
clinical trial of TAILORx for the 21-gene RS, patients with a score
of 0–10 were assigned to receive endocrine therapy alone, and those
with a score ≥26 were assigned to receive chemotherapy plus
endocrine therapy (8). However, in
the real world, treatment decisions are usually developed based on
a comprehensive consideration of all types of clinicopathological
characteristics. In the present study, adjuvant treatment decisions
for each patient with breast cancer were made by a
multidisciplinary team that took all-sided patient characteristics
into consideration, which may help assess the associations among
clinicopathological features, the 21-gene RS and chemotherapy
recommendation for HR+/HER2− patients.
The cutoff points were pre-specified prior to the
validation study of the RS (10),
which categorized patients into low-risk (RS <18),
intermediate-risk (RS ≥18 and <31) and high-risk (RS ≥31)
groups. Low-risk patients have a good prognosis and benefit little
from additional chemotherapy, whereas for high-risk patients,
chemotherapy is highly recommended. This cutoff point has been
widely used in subsequent validation studies of the 21-gene RS
(11,12,17). The
aforementioned cutoff points were also pre-specified in the present
study. Since the prospective clinical trials for the 21-gene RS
used different cutoff values, including RS <11 (≤11 in Plan B
study), 11–25 and RS >25, the present study also analyzed the
change in the chemotherapy recommended along with 21-gene RS
intervals. The results revealed that a similar proportion of
patients with RS 0–10 and 11–17 were spared from chemotherapy
(79.31 and 82.54%), whereas RS 26–30 and RS >30 patients had a
high proportion of chemotherapy recommendation (83.92 and
93.04%).
The recommendation of multi-gene profiles for
clinically low-risk patients may have certain challenges. The
70-gene prognosis signature has limited value in
HR+/HER2−, node-negative patients with low
clinical risk, since these patients have excellent outcomes even
with a genomic high-risk cancer and omitting chemotherapy (19,31).
However, to the best of our knowledge, there is a lack of data
regarding the role of the 21-gene RS in low clinical risk patients,
including patients with luminal A disease. In the present study, a
total of 15 patients with luminal A-like subtype breast cancer were
classified as high RS (5.91%). Despite the small percentage of high
RS patients with luminal A-like subtype breast cancer, 93.3% of
them were recommended to receive adjuvant chemotherapy. In
addition, the high RS category was revealed to be the most
influential factor for chemotherapy recommendation in patients with
luminal A-like breast cancer (OR, 435.05; P<0.001).
Although the 2013 St. Gallen Consensus recommends
the usage of multigene signatures, particularly in luminal B
disease, for the selection of patients who should receive adjuvant
chemotherapy (21), few studies have
focused on the role of the 21-gene RS in luminal B breast cancer
(32,33). In the prospective Plan B study, 12%
of luminal B (Ki-67 ≥20%) tumors were found to be of low risk (RS
≤11) and 48% were found to be of intermediate risk (RS, 12–25)
according to the 21-gene RS (34).
Similarly, in the present study, 17.95% of luminal B-like tumors
were identified to be of low risk (RS <18) and 54.44% were
identified to be of intermediate risk (RS, 18–30). Additionally,
72.04% of low-risk RS patients with luminal B-like subtype breast
cancer were not recommended to receive adjuvant chemotherapy, which
indicated that physicians are more confident using the 21-gene RS
score to guide adjuvant chemotherapy decisions rather than routine
clinicopathological features.
Evidence for the utility value of the 21-gene RS in
node-positive disease is mostly derived from retrospective
analysis, making it less robust than that in the node-negative
population. As a result, recommendations of major cancer
associations currently differ, with the American Society of
Clinical Oncology recommending the 21-gene RS for node-negative
patients only, whereas the NCCN recommends the 21-gene RS for
patients with limited node positivity (1–3 involved nodes)
(14,31). However, the 21-gene RS remains one of
the few multigene assays that have been validated for prediction of
the likelihood of chemotherapy benefit for node-positive patients
(12). Additionally, in the
transATAC study, node-positive patients with a low RS exhibited
good prognosis without receiving chemotherapy (the rate of disease
recurrence at 9 years was 17% for RS <18) (17). In the Plan B study, disease-free
survival in pN1 patients with low RS reached 97% without
chemotherapy (97.9% for RS <12 and 97.2% for RS 12–25), which is
similar to that of pN0 patients (98.6% for RS <12 and
98.5% for RS 12–25) (9). In the
present study, 39.3% of node-positive patients with a low RS were
not recommended to receive chemotherapy. After adjusting for
clinicopathological factors, the 21-gene RS was found to be the
most important impact factor for chemotherapy recommendation for
node-positive patients (OR, 20.83; P=0.002).
In a clinical setting, patients may be classified
into different risk groups based on clinicopathological
characteristics and genomic assays. In the MINDACT clinical trial,
8.85% (592/6,693) of patients were of a low clinical risk and high
genomic risk, whereas 23.16% (1,550/6,693) of patients were of a
high clinical risk and low genomic risk (19). The results show that patients with
high clinical risk and low genomic risk receive little benefit from
additional chemotherapy (19).
However, to the best of our knowledge, for patients with a
discordant risk of the 21-gene RS and clinicopathological
characteristics, including luminal subtypes, there is no solid
evidence to guide the treatment decision. In the present study,
5.91% (15/254) of patients with luminal A-like breast cancer were
categorized as high RS, while chemotherapy was recommended for
93.33% of them; whereas 17.95% (93/518) of patients with luminal
B-like breast cancer were categorized as low RS, and chemotherapy
was recommended for only 27.96% of them. Multivariate analyses
demonstrated that the 21-gene RS is the most important impact
factor for chemotherapy recommendation for all patients, regardless
of luminal subtype or node status, with an adjusted OR of 62.54
(P<0.001).
The present study has several limitations. First, it
is a retrospective study. Patients were from a single-center and
enrollment bias may exist. Second, some N1 patients were
included. The prognostic and predictive value of the 21-gene RS for
node-positive patients was not validated through prospective
clinical trials (12). The updated
ASCO guidelines state that the 70-gene prognosis signature can be
applied to HR+/HER2− patients with one
positive node, but not to those with 2–3 positive nodes. Third,
various clinicopathological characteristics were taken into
consideration when the multidisciplinary team developed the
treatment decision, but some of these, including patient
performance status and comorbidities, were not discussed in the
present study. Additionally, it should be noted that in the present
study, IHC-based markers were used to determine breast cancer
subtypes rather than PAM50 multigene-based assays. Finally, there
was no long-term follow-up data in the study in order to analyze
whether chemotherapy can improve disease outcome for these luminal
subtypes and RS discordant cases. The findings may not be
generalized at present, since further research and studies have to
be conducted to reduce the limitations previously mentioned.
In conclusion, the present study demonstrated that
the luminal subtypes and 21-gene RS are associated with
chemotherapy recommendation for patients with
HR+/HER2− breast cancer. The RS was found to
be independently associated with adjuvant chemotherapy
recommendation in patients with breast cancer, regardless of
luminal subtype and lymph node status. For patients of the luminal
A-like subtype with a high RS or patients with luminal B-like
subtype breast cancer with a low RS, a high RS is the most
important independent factor that has an influence on the adjuvant
chemotherapy decision.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms Yidong Du
(Comprehensive Breast Health Center, Ruijin Hospital, Shanghai
Jiaotong University School of Medicine) for assisting with data
entry and Dr Yiwei Tong (Comprehensive Breast Health Center, Ruijin
Hospital, Shanghai Jiaotong University School of Medicine) for
assisting with language editing.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81772797).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC and KS designed and conceived the study. WW, LL
and XF performed the investigation. JH, WG, SZ, JW, OH, JH, YL, LZ
and WC acquired the clinical data of patients. WW wrote the
manuscript. XC and KS reviewed and edited the manuscript, and
provided funds. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study has been approved by the Ethic
Committees of Shanghai Ruijin Hospital. All procedures performed
involving human participants were in accordance with the ethical
standards of the institutional committee and with the 1964 Helsinki
Declaration and its later amendments or comparable ethical
standards. Informed consent was obtained from all individual
participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou
XN, Chen R, Gu XY, Wei WW and He J: Report of cancer epidemiology
in China, 2015. Zhonghua Zhong Liu Za Zhi. 41:19–28. 2019.(In
Chinese; Abstract available in Chinese from the publisher).
PubMed/NCBI
|
|
2
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Effects of chemotherapy and
hormonal therapy for early breast cancer on recurrence and 15-year
survival: An overview of randomized trials. Lancet. 365:1687–1717.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silberholz J, Bertsimas D and Vahdat L:
Clinical benefit, toxicity and cost of metastatic breast cancer
therapies: Systematic review and meta-analysis. Breast Cancer Res
Treat. 176:535–543. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alarid-Escudero F, Blaes AH and Kuntz KM:
Trade-offs between efficacy and cardiac toxicity of adjuvant
chemotherapy in early-stage breast cancer patients: Do competing
risks matter? Breast J. 23:401–409. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Abreu FB, Schwartz GN, Wells WA and
Tsongalis GJ: Personalized therapy for breast cancer. Clin Genet.
86:62–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coates AS, Winer EP, Goldhirsch A, Gelber
RD, Gnant M, Piccart-Gebhart M, Thürlimann B and Senn HJ; Panel
Members, : Tailoring therapies-Improving the management of early
breast cancer: St gallen international expert consensus on the
primary therapy of early breast cancer 2015. Ann Oncol.
26:1533–1546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ; Panel members, : Strategies for
subtypes-dealing with the diversity of breast cancer: Highlights of
the st gallen international expert consensus on the primary therapy
of early breast cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sparano JA, Gray RJ, Makower DF, Pritchard
KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Perez EA, Olson JA
Jr, et al: Prospective validation of a 21-gene expression assay in
breast cancer. N Engl J Med. 373:2005–2014. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gluz O, Nitz UA, Christgen M, Kates RE,
Shak S, Clemens M, Kraemer S, Aktas B, Kuemmel S, Reimer T, et al:
West German Study Group Phase III PlanB trial: First prospective
outcome data for the 21-gene recurrence score assay and concordance
of prognostic markers by central and local pathology assessment. J
Clin Oncol. 34:2341–2349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paik S, Shak S, Tang G, Kim C, Baker J,
Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al: A
multigene assay to predict recurrence of tamoxifen-treated,
node-negative breast cancer. N Engl J Med. 351:2817–2826. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paik S, Tang G, Shak S, Kim C, Baker J,
Kim W, Cronin M, Baehner FL, Watson D, Bryant J, et al: Gene
expression and benefit of chemotherapy in women with node-negative,
estrogen receptor-positive breast cancer. J Clin Oncol.
24:3726–3734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Albain KS, Barlow WE, Shak S, Hortobagyi
GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL,
Davidson NE, et al: Prognostic and predictive value of the 21-gene
recurrence score assay in postmenopausal women with node-positive,
oestrogen-receptor-positive breast cancer on chemotherapy: A
retrospective analysis of a randomised trial. Lancet Oncol.
11:55–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sparano JA, Gray RJ, Makower DF, Pritchard
KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA
Jr, et al: Adjuvant chemotherapy guided by a 21-gene expression
assay in breast cancer. N Engl J Med. 379:111–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Network NCC, . NCCN Clinical Practice
Guidlines in Oncology. Breast Cancer. Version 2.2017. http://www.nccn.org/professionals/physician_gls/pdf/breast_blocks.pdfApril
6–2017
|
|
15
|
Hornberger J, Alvarado MD, Rebecca C,
Gutierrez HR, Yu TM and Gradishar WJ: Clinical validity/utility,
change in practice patterns, and economic implications of risk
stratifiers to predict outcomes for early-stage breast cancer: A
systematic review. J Natl Cancer Inst. 104:1068–1079. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Habel LA, Shak S, Jacobs MK, Capra A,
Alexander C, Pho M, Baker J, Walker M, Watson D, Hackett J, et al:
A population-based study of tumor gene expression and risk of
breast cancer death among lymph node-negative patients. Breast
Cancer Res. 8:R252006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dowsett M, Cuzick J, Wale C, Forbes J,
Mallon EA, Salter J, Quinn E, Dunbier A, Baum M, Buzdar A, et al:
Prediction of risk of distant recurrence using the 21-gene
recurrence score in node-negative and node-positive postmenopausal
patients with breast cancer treated with anastrozole or tamoxifen:
A TransATAC study. J Clin Oncol. 28:1829–1834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toi M, Iwata H, Yamanaka T, Masuda N, Ohno
S, Nakamura S, Nakayama T, Kashiwaba M, Kamigaki S and Kuroi K;
Japan Breast Cancer Research Group-Translational Research Group, :
Clinical significance of the 21-gene signature (Oncotype DX) in
hormone receptor-positive early stage primary breast cancer in the
Japanese population. Cancer. 116:3112–3118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Van der Hoeven JJ: 70-Gene signature as an
aid to treatment decisions in early-stage breast cancer. Ned
Tijdschr Geneeskd. 161:D13692017.(In Dutch). PubMed/NCBI
|
|
20
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: Development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ; Panel members, :
Personalizing the treatment of women with early breast cancer:
Highlights of the st gallen international expert consensus on the
primary therapy of early breast cancer 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lakhani SR, Ellis IO, Schnitt SJ, Tan PH
and van de Vijver MJ: WHO classification of tumours of the
breastWorld Health Organization Classification of Tumours. 4th.
International Agency for Research on Cancer; Lyon: pp. 60–61.
2012
|
|
23
|
Edge S, Byrd D and Compton C: AJCC Cancer
Staging Handbook7th. American Joint Committee on Cancer. Springer;
New York, NY: 2010, PubMed/NCBI
|
|
24
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu J, Fang Y, Lin L, Fei X, Gao W, Zhu S,
Zong Y, Chen X, Huang O, He J, et al: Distribution patterns of
21-gene recurrence score in 980 Chinese estrogen receptor-positive,
HER2-negative early breast cancer patients. Oncotarget.
8:38706–38716. 2017.PubMed/NCBI
|
|
26
|
Cronin M, Sangli C, Liu ML, Pho M, Dutta
D, Nguyen A, Jeong J, Wu J, Langone KC and Watson D: Analytical
validation of the Oncotype DX genomic diagnostic test for
recurrence prognosis and therapeutic response prediction in
node-negative, estrogen receptor-positive breast cancer. Clin Chem.
53:1084–1091. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Esteban J, Baker J, Cronin M, et al: Tumor
gene expression and prognosis in breast cancer: Multi-gene RT-PCR
assay of paraffin-embedded tissue. Proc Am Soc Clin Oncol.
22:8502003.
|
|
28
|
Paik S, Shak S, Tang G, et al: Multi-gene
RT-PCR assay for predicting recurrence in node negative breast
cancer patients: NSABP studies B-20 and B-14. Breast Cancer Res
Treat. 82:A162003.
|
|
29
|
Cobleigh MA, Bitterman P, Baker J, et al:
Tumor gene expression predicts distant disease-free survival (DDFS)
in breast cancer patients with 10 or more positive nodes: High
throughout RT-PCR assay of paraffin-embedded tumor tissues. Prog
Proc Am Soc Clin Oncol. 22:8502003.
|
|
30
|
Prat A, Cheang MC, Martín M, Parker JS,
Carrasco E, Caballero R, Tyldesley S, Gelmon K, Bernard PS, Nielsen
TO and Perou CM: Prognostic significance of progesterone
receptor-positive tumor cells within immunohistochemically defined
luminal A breast cancer. J Clin Oncol. 31:203–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Harris LN, Ismaila N, McShane LM, Andre F,
Collyar DE, Gonzalez-Angulo AM, Hammond EH, Kuderer NM, Liu MC,
Mennel RG, et al: Use of biomarkers to guide decisions on adjuvant
systemic therapy for women with early-stage invasive breast cancer:
American Society of Clinical Oncology Clinical Practice Guideline.
J Clin Oncol. 34:1134–1150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arima N, Nishimura R, Osako T, Okumura Y,
Nakano M, Fujisue M, Nishiyama Y and Toyozumi Y: Ki-67 index value
and progesterone receptor status can predict prognosis and suitable
treatment in node-negative breast cancer patients with estrogen
receptor-positive and HER2-negative tumors. Oncol Lett. 17:616–622.
2019.PubMed/NCBI
|
|
33
|
Zhu L, Ma N, Wang B, Zhou C, Yan Y, Wang
K, He J and Ren Y: Clinical analysis of 21-gene recurrence score
test in hormone receptor-positive early-stage breast cancer. Oncol
Lett. 17:5469–5480. 2019.PubMed/NCBI
|
|
34
|
Nitz U, Gluz O, Christgen M, Kates RE,
Clemens M, Malter W, Nuding B, Aktas B, Kuemmel S, Reimer T, et al:
Reducing chemotherapy use in clinically high-risk, genomically
low-risk pN0 and pN1 early breast cancer patients: Five-year data
from the prospective, randomised phase 3 West German Study Group
(WSG) PlanB trial. Breast Cancer Res Treat. 165:573–583. 2017.
View Article : Google Scholar : PubMed/NCBI
|