Introduction
Ovarian cancer is the leading cause of gynecological
cancer-related deaths in the United States (1). As the most common subtype of ovarian
cancer, high-grade serous ovarian cancer (SOC) is a highly lethal
tumor with a 5-year survival rate of <30% due to its severe
resistance to platinum-based chemotherapy (2). Over the past decades, the efficacy of
early diagnosis and treatment of SOC has improved (3), but little progress has been made in
terms of overall survival.
Oxidative stress and reactive oxygen species (ROS)
are closely associated with the occurrence and development of
cancers (4,5). Although a slight increase in the
intracellular ROS level is necessary for rapid proliferation of
cancer cells (6–8), excessive generation of intracellular
ROS may induce cell death by damaging cellular DNA, lipids and
proteins (9). At present, a number
of anticancer drugs, including chemotherapeutic agents (10) and molecular targeted drugs (11), which may effectively eliminate cancer
cells by inducing generation of ROS, have been applied in a
clinical setting. Based on previous research, it may be
hypothesized that enhancing the sensitivity of cancer cells to
oxidative stress may improve the therapeutic effect of oxidative
stress inducers.
X-box-binding protein 1 (XBP1) is an important
transcription factor in the unfolded protein response (UPR), which
is activated by endoplasmic reticulum (ER) stress. During ER
stress, XBP1 is subjected to unconventional splicing and converted
to an active form, termed spliced XBP1 (XBP1s) (12). XBP1 is widely expressed in adult
tissues, and it serves a key role in cardiac myogenesis and plasma
cell differentiation (13,14). In addition, XBP1s is crucial for
cancer cell survival under microenvironment stress conditions, such
as hypoxia (15). Overexpression of
XBP1 has been observed in cancer tissues; high expression of XBP1
may enhance cancer cell proliferation and migration and confer drug
resistance (16–18). Several studies have demonstrated that
blocking UPR signals may lead to tumor cell growth arrest and
apoptosis in a variety of malignant tumors (19,20),
which suggests that XBP1 may be a potential target for cancer
therapy. In addition, an association between XBP1 and oxidative
stress has been reported (21,22),
which indicates that XBP1 silencing may act synergistically with
ROS inducers in cancer treatment.
Cubillos-Ruiz et al (23) first reported that the
immunosuppressive effect of XBP1 in patients with ovarian cancer
was mediated by dendritic cell dysfunction. However, the number of
studies focusing on the expression of XBP1 in SOC is limited, and
little is known regarding its potential role in ovarian cancer
cells. To the best of our knowledge, the present study is the first
to demonstrate that XBP1 is overexpressed in SOC cells and it is
also the first to investigate the biological function of XBP1 in
ovarian cancer cells under conditions of oxidative stress. In
addition, the present study aimed to determine whether the
downregulation of XBP1 may increase the sensitivity of ovarian
cancer cells to oxidative stress, and whether the combination of
XBP1 silencing and oxidative stress inducers may exert synergistic
anti-SOC effects. The association between the downregulation of
XBP1 and the expression of p-p38 was also investigated.
Materials and methods
Cell culture
The human SOC cell lines A2780, HO8910 and SKOV3
were acquired from the Type Culture Collection of the Chinese
Academy of Sciences, and the normal ovarian epithelial cell line
HOSEpiC was purchased from ScienCell Research Laboratories, Inc.
Cells were cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum and 1% penicillin-streptomycin (all from Gibco; Thermo
Fisher Scientific, Inc.). 293T cells (The Cell Bank of Type Culture
Collection of the Chinese Academy of Sciences) were cultured in
DMEM high glucose medium (Gibco; Thermo Fisher Scientific, Inc.).
All cell lines were cultured at 37°C with 5% CO2.
Database analysis
The mRNA expression levels of XBP1 in 426 SOC and 88
normal ovarian tissue samples were analyzed using the Gene
Expression Profiling Interactive Analysis database (GEPIA;
gepia.cancer-pku.cn; version no. GEPIA2). The Student's t-test was
used to compare the mean values of two groups.
Establishment of stable cell
lines
Short hairpin RNA (shRNA) sequences, as shown in
Table I, were designed using the
Sigma-Aldrich RNAi Design Service. For stable transfection, XBP1
(shXBP1-2 and shXBP1-3) and negative control (shCtrl) shRNA were
embedded into the pLKO.1-puro vector (Addgene) at the AgeI
and EcoRI sites. Subsequently, the recombined pLKO.1-puro
vector and the packaging plasmid psPAX2 and pMD2.G (Addgene) were
co-transfected into 293T cells at a ratio of 4:3:1 using the
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C. At 48 h, the supernatant
containing lentiviral particles was collected, and the ovarian
cancer cell lines were infected with the lentiviruses using
polybrene (Sigma-Aldrich; Merck KGaA) at a concentration of 8 mg/ml
for 24 h at 37°C. Stably infected cells were screened using 2 µg/ml
puromycin (Sigma-Aldrich; Merck KGaA) for 3–5 days, and the
knockdown efficiency of the shRNA was determined by western
blotting and reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) analysis.
 | Table I.Sequences of primers and shRNAs. |
Table I.
Sequences of primers and shRNAs.
| Gene/target | Sequence
(5′→3′) |
|---|
| XBP1 | F:
ATGGATTCTGGCGGTATT |
|
| R:
AAAGGGAGGCTGGTAAGG |
| FTH1 | F:
TGAAGCTGCAGAACCAACGAGG |
|
| R:
GCACACTCCATTGCATTCAGCC |
| NQO1 | F:
CCTGCCATTCTGAAAGGCTGGT |
|
| R:
GTGGTGATGGAAAGCACTGCCT |
| HMOX1 | F:
CCAGGCAGAGAATGCTGAGTTC |
|
| R:
AAGACTGGGCTCTCCTTGTTGC |
| GAPDH | F:
GAAGGTGAAGGTCGGAGTC |
|
| R:
GAAGATGGTGATGGGATTTC |
| shXBP1-2 |
GACCCAGTCATGTTCTTCAAA |
| shXBP1-3 |
GAACAGCAAGTGGTAGATTTA |
RT-qPCR
Total RNA was extracted from cultured cells with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and reverse-transcribed into cDNA with the Prime
Script® RT Reagent kit (Takara Bio, Inc.) according to
the manufacturer's protocol. qPCR was performed in a 10-µl reaction
solution containing 20 ng cDNA, 0.4 µl forward primer, 0.4 µl
reverse primer and 5 µl 2X SYBR Premix Ex Taq buffer (Takara Bio,
Inc.). The PCR amplification was performed at 95°C for 5 min,
followed by 40 cycles of 95°C for 15 sec and 65°C for 40 sec, using
an ABI PRISM Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). All experiments were performed in triplicate and
normalized to GAPDH mRNA expression levels. mRNA levels were
calculated using the 2−ΔΔCq method (24). The primer sequences are presented in
Table I.
Western blot analysis
The cell lines HOSEpiC, A2780, HO8910 and SKOV3 were
lysed on ice with cell lysis buffer IP (Beyotime Institute of
Biotechnology) containing protease inhibitors for total protein
extraction. Western blotting was performed as previously described
(3). Protein expression levels were
evaluated using enhanced chemiluminescence ECL reagent (Gene Tech
Co., Ltd.). Densitometric analysis was performed using ImageJ
software (version 1.8.0; National Institutes of Health) and
normalized to the internal control β-actin. The primary antibodies
used were as follows: XBP1 (1:1,000; rabbit polyclonal; cat. no.
ab198999; Abcam), HMOX1 (1:500; mouse monoclonal; cat. no.
sc-136960; Santa Cruz Biotechnology, Inc.), p-p38 (1:1,000; rabbit
monoclonal; cat. no. D3F9; Cell Signaling Technology, Inc.), p38
(1:1,000; rabbit monoclonal; cat. no. 3042S; Cell Signaling
Technology, Inc.), p53 (1:500; mouse monoclonal; cat. no. sc-126;
Santa Cruz Biotechnology, Inc.), Bcl2 (1:1,000, rabbit polyclonal;
cat. no. 12789-1-AP; ProteinTech Group, Inc.) and β-actin (1:5,000;
mouse monoclonal; cat. no. 60008-1-Ig; ProteinTech Group, Inc.).
The secondary antibodies were as follows: Anti-mouse IgG-HRP
(1:5,000; goat polyclonal; cat. no. sc-2005; Santa Cruz
Biotechnology, Inc.), anti-rabbit IgG-HRP (1:5,000; goat
polyclonal; cat. no. sc-2004; Santa Cruz Biotechnology, Inc.).
Hydrogen peroxide
(H2O2) treatment
A2780 cells were treated with 30 and 40 µM
H2O2 for 24 and 48 h according to the
different experimental purposes at 37°C. The HO8910 cells were
treated with 100 and 200 µM H2O2 for 24 and
48 h at 37°C.
Colony formation and cell
proliferation assays
For the colony formation assay, 1,000 cells/well
were seeded into 6-well plates and incubated for 10–14 days, until
colonies were visible. Cells were washed twice with PBS, fixed with
4% (w/v) paraformaldehyde (Sigma-Aldrich; Merck KGaA) for 10 min at
4°C and stained with 0.5% crystal violet solution for 30 min at
room temperature. The number of colonies containing >50 cells
was counted using an optical microscope. To measure cell
proliferation, 2×103 cells/well were seeded into 96-well
plates and incubated for 1, 2, 3, 4 and 5 days at 37°C; 10 µl Cell
Counting Kit-8 solution (Dojindo Molecular Technologies, Inc.) was
added into each well and incubated for 2 h at 37°C. The absorbance
of each well at 450 nm was determined using a Synergy H4 microplate
reader (Bio-Tek Instruments, Inc.).
Apoptosis assay
For apoptosis analysis, 3.0×105
cells/well were harvested from 6-well-plates, washed twice with
PBS, re-suspended in 500 µl 1X binding buffer and incubated with 5
µl Annexin V-FITC/PI (BD Biosciences) for 15 min at 4°C. Apoptotic
rates were evaluated using flow cytometry.
Immunofluorescence
A total of 4.0×104 cells/well were fixed
with 4% (w/v) paraformaldehyde for 30 min at 4°C and
auto-fluorescence was quenched with BSA. Subsequently, cells were
stained with primary antibodies against XBP1 (1:200; rabbit
polyclonal; cat. no. ab198999; Abcam) overnight at 4°C, followed by
incubation with secondary antibodies (Alexa Fluor-594; 1:1,000;
cat. no. R37117; Thermo Fisher Scientific, Inc.) for 1 h at 37°C.
The cells were mounted with ProLong® Gold Antifade
Reagent with DAPI (Thermo Fisher Scientific, Inc.) and visualized
using a LAS-AF-Lite multi-photon confocal microscope system (Leica
Microsystems, Inc.).
ROS detection
Intracellular ROS levels were determined by the
fluorescence intensity of the dichlorodihydrofluorescein diacetate
(DCFH-DA) probe. Cells were incubated with 10 mM DCFH-DA for 30 min
at 37°C. Following two washes with ice-cold PBS, the cells were
harvested for immediate detection by flow cytometry. The
fluorescence intensity of cells was also observed under a
multi-photon confocal microscope system.
Statistical analysis
The data are expressed as the mean ± standard error
of the mean of three independent experiments. Statistical analysis
was performed using SPSS 19.0 software (IBM Corp.). Student's
t-test was used to compare the mean values of two groups. One-way
analysis of variance was used to compare the mean values of
multiple groups with equal variances and Tukey's test was used to
perform multiple comparisons as a post hoc test. P<0.05 was
considered to indicate statistically significant differences.
Results
XBP1 expression is increased in
SOC
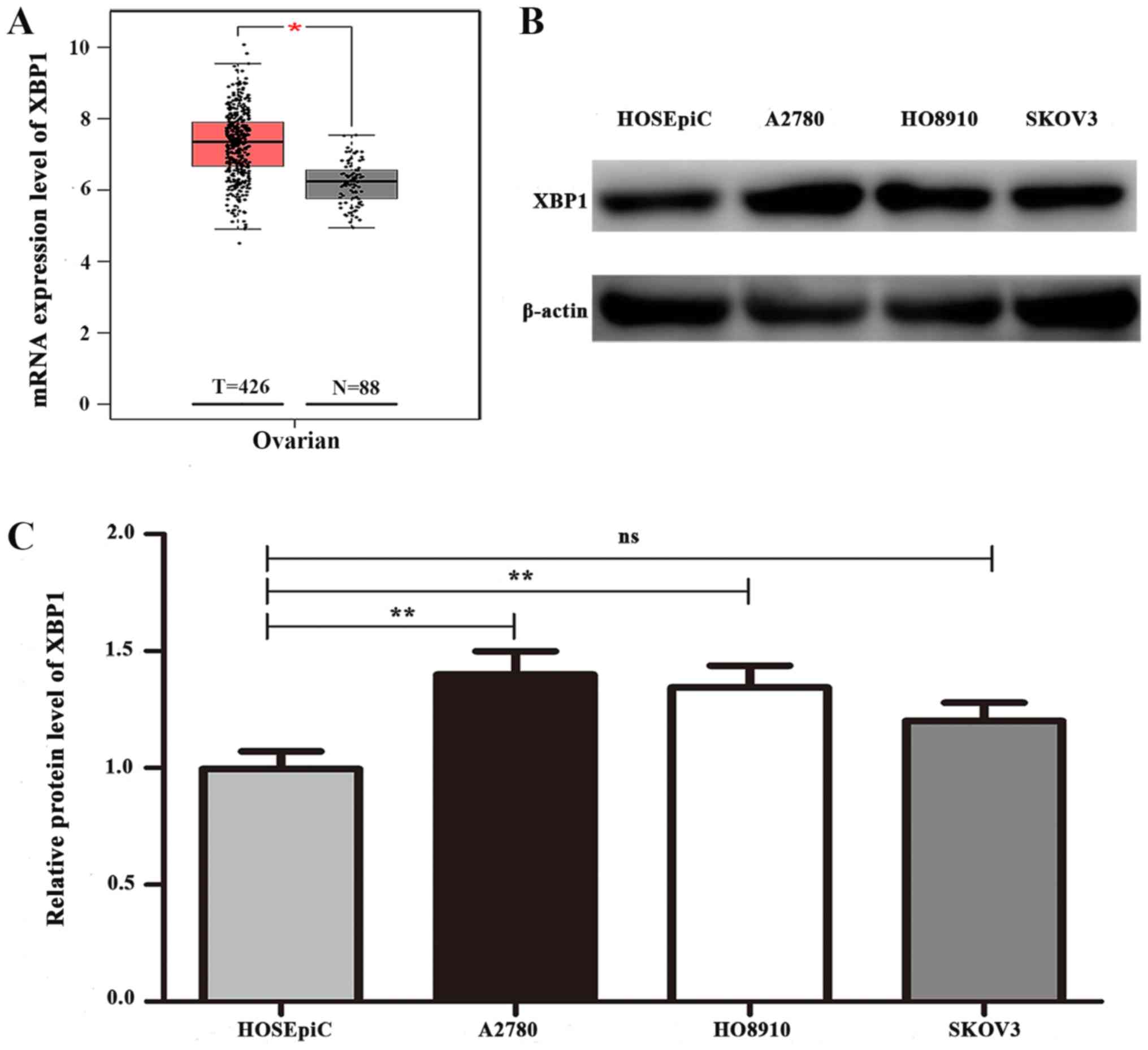
To investigate the role of XBP1 in SOC, the mRNA
expression levels of XBP1 in 426 SOC and 88 normal ovarian tissue
samples were analyzed using the GEPIA database. The results
revealed that the mRNA expression level of XBP1 in SOC tissues was
significantly higher compared with that in normal ovarian tissues
(Fig. 1A). For further verification,
the protein expression level of XBP1 was detected in three SOC cell
lines and the normal ovarian epithelial cell line HOSEpiC. Western
blotting revealed a higher expression level of XBP1 in the A2780
and HO8910 ovarian cancer cells compared with that in HOSEpiC
(Fig. 1B), whereas no increase in
XBP1 expression was observed in SKOV3 cells. Densitometric analysis
demonstrated significant differences in the protein expression
levels of XBP1 between HOSEpiC and A2780 and HO8910 cells
(P<0.05; Fig. 1C). Therefore,
A2780 and HO8910 cells were selected for further experiments
involving downregulation of XBP1 expression to investigate its
biological function in ovarian cancer.
XBP1 downregulation inhibits cell
proliferation
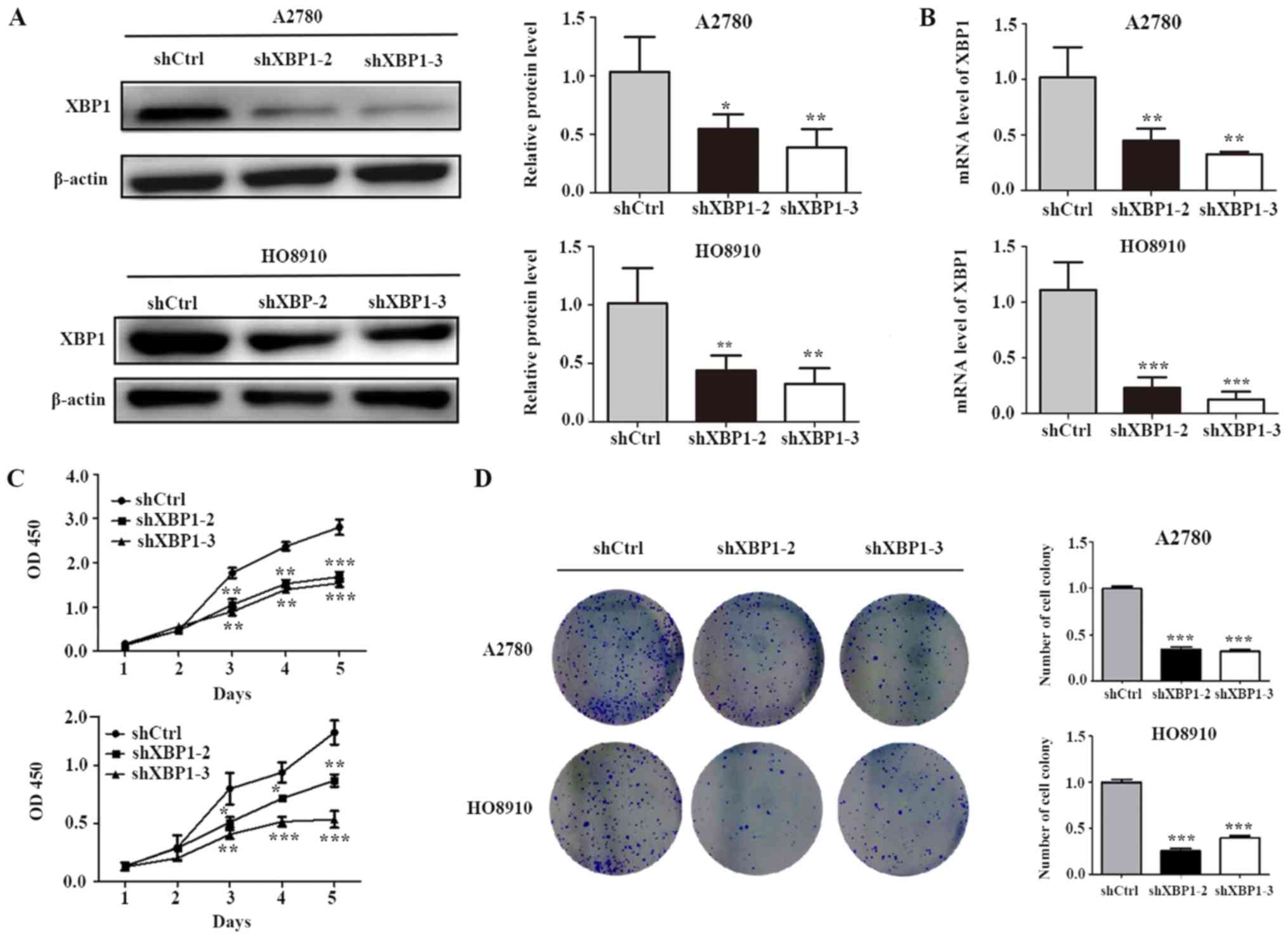
To investigate the potential functions of XBP1 in
SOC cells, shXBP1-2/shXBP1-3/shCtrl lentiviral infection was
performed in A2780 and HO8910 cells. To confirm that XBP1 was
effectively downregulated by shRNA, RT-qPCR and western blotting
were used to evaluate XBP1 mRNA and protein expression levels,
respectively. Western blot analysis revealed that the protein level
of XBP1 was significantly decreased by shXBP1-2 and shXBP1-3 in the
two cell lines (Fig. 2A), and the
results of qPCR demonstrated that shXBP1-2 and shXBP1-3
significantly decreased the mRNA level of XBP1 (Fig. 2B). Decreased levels of endogenous
XBP1 were associated with inhibition of cell proliferation
(Fig. 2C) and colony formation
(Fig. 2D).
XBP1 downregulation enhances cell
sensitivity to oxidative stress
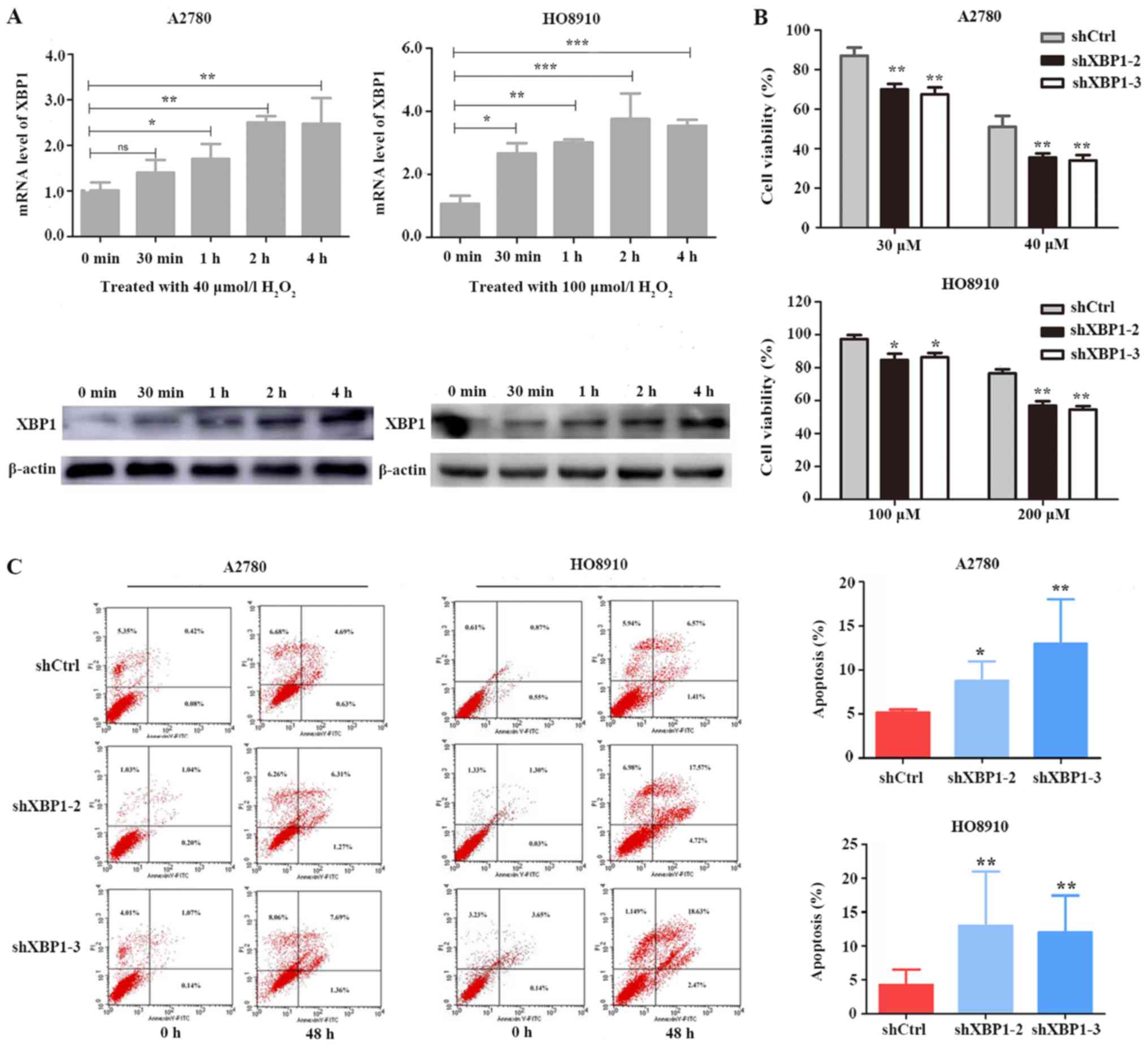
To determine whether XBP1 protects SOC cells against
oxidative stress, A2780 and HO8910 cells were treated with
H2O2. Under the oxidative stress induced by
H2O2, the mRNA and protein expression levels
of XBP1 gradually increased over time (Fig. 3A). A2780 cells were treated with 30
and 40 uM H2O2 and HO8910 cells were treated
with 100 and 200 uM H2O2 for 24 h. XBP1
downregulation significantly reduced the proliferative ability of
cells compared with shCtrl (Fig.
3B). Following treatment with H2O2 for 48
h, A2780 cells were treated with 20 uM H2O2
and HO8910 cells were treated with 100 uM
H2O2, the apoptotic rates were significantly
increased in the XBP1-knockdown groups compared with those in the
respective control groups (Fig. 3C).
These results suggested that downregulation of XBP1 may enhance the
sensitivity of ovarian cancer cells to oxidative stress.
XBP1 downregulation increases
intracellular ROS levels
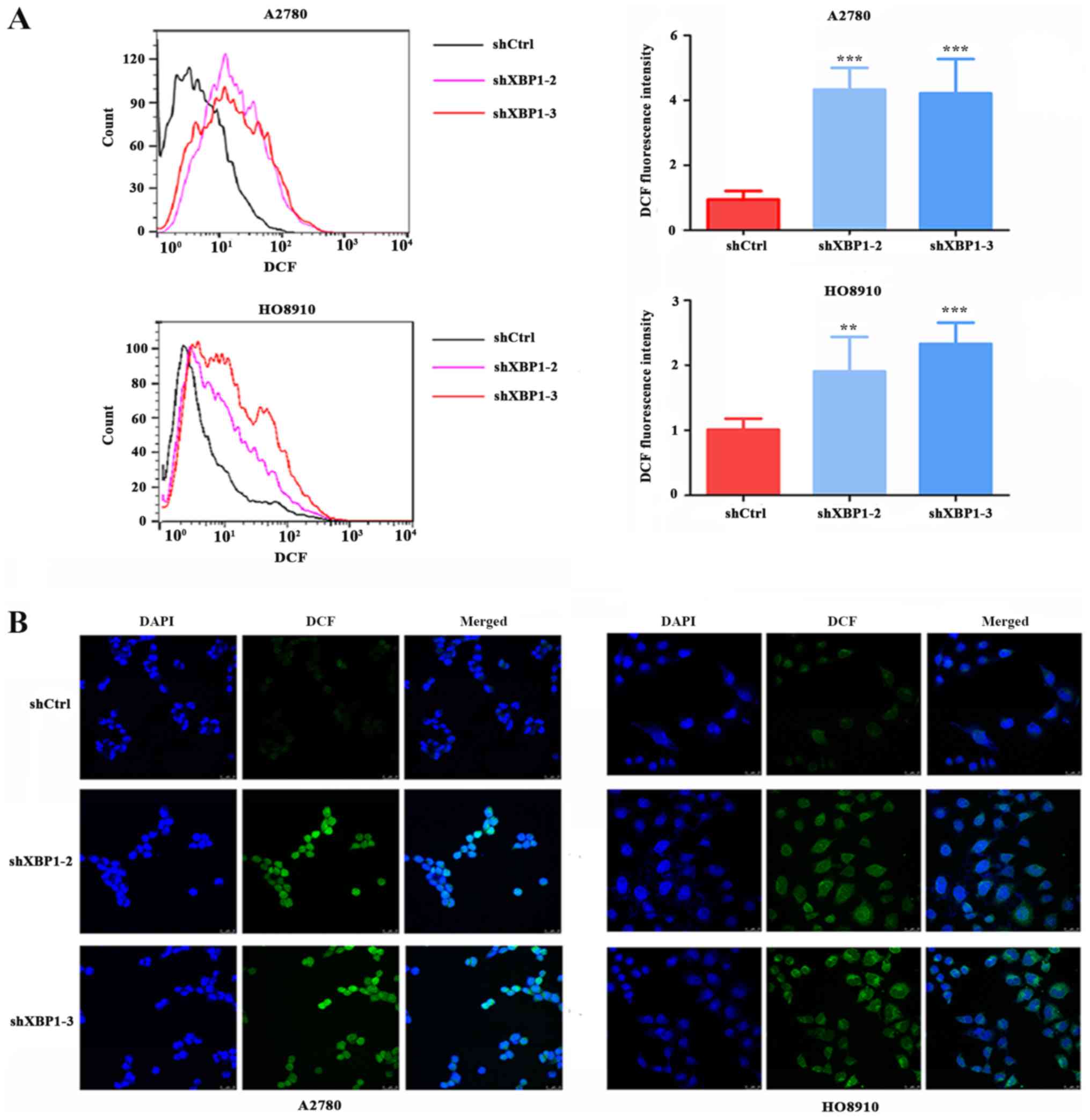
To investigate whether XBP1 is involved in the
regulation of the intracellular ROS level, the intracellular ROS
levels were measured in the XBP1-knockdown and control groups. The
downregulation of XBP1 resulted in a significant increase in
intracellular ROS levels in A2780 and HO8910 cells compared with
shCtrl. ROS levels, as detected by flow cytometry, were increased
in the A2780 and HO8910 cells of the knockdown group compared with
the shCtrl group (Fig. 4A). Cell
immunofluorescence was also performed to detect the intracellular
ROS level in A2780 and HO8910 cells, and the results were
consistent with those of flow cytometry (Fig. 4B).
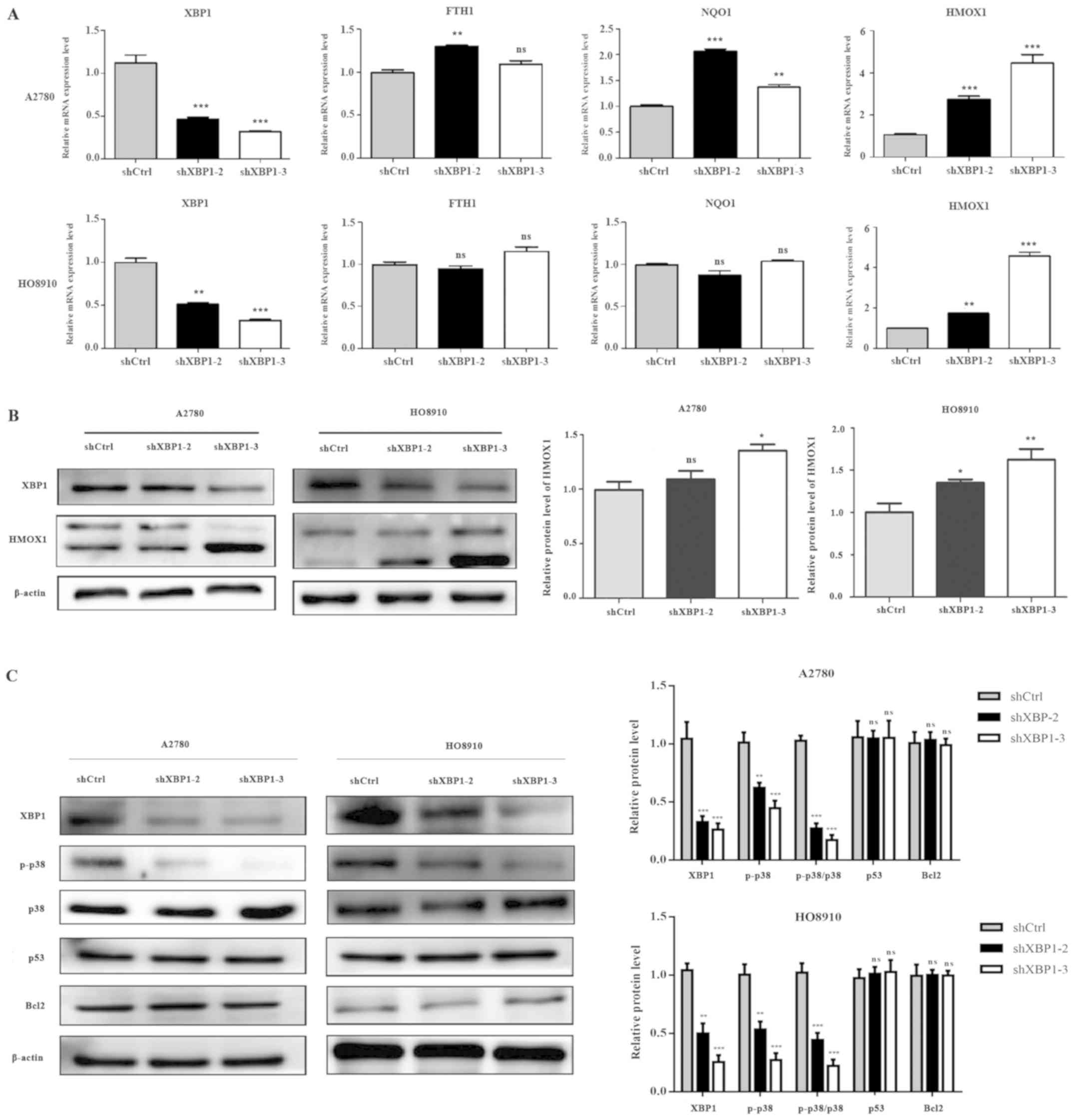
XBP1 downregulation induces changes in
heme oxygenase 1 (HMOX1) and phosphorylated (p-)p38 expression
To identify the underlying molecular mechanisms of
XBP1-dependent ROS sensitivity regulation in SOC cells, the mRNA
levels of the antioxidant genes ferritin heavy chain 1 (FTH1),
quinine oxidoreductase (NQO1) and HMOX1, which are regulated by
nuclear factor erythroid 2 like 2 (Nrf2), were evaluated by
RT-qPCR. The expression levels of these genes were increased to a
variable extent in A2780 cells of the knockdown group; HMOX1 was
also increased in HO8910 cells with downregulated XBP1 expression
(Fig. 5A). The results of western
blotting further confirmed that HMOX1 expression was increased in
the knockdown group (Fig. 5B). The
results indicated that the downregulation of XBP1 increased
antioxidant production in ovarian cancer. The expression levels of
the antioxidant genes FTH1, NQO1 and HMOX1 in the Nrf2 signaling
pathway were increased to varying degrees, and HMOX1 was
significantly upregulated following XBP1 knockdown. However, the
increased intracellular ROS levels induced by the downregulation of
XBP1 may stimulate the expression of these antioxidant genes in
response. Detection of apoptosis-related proteins demonstrated that
P53, Bcl2 and total p38 expression levels did not change in the
knockdown group, whereas the phosphorylation level of the stress
kinase p38 was decreased compared with shCtrl (Fig. 5C).
Discussion
To the best of our knowledge, the present study is
the first to demonstrate that XBP1 is overexpressed in A2780 and
HO8910 SOC cells, and that the downregulation of XBP1 in A2780 and
HO8910 cells may result in decreased cell viability. Oxidative
stress may stimulate the expression of XBP1 in SOC cells. In
addition, the downregulation of XBP1 may significantly enhance the
sensitivity of SOC cells to oxidative stress through the induction
of ROS generation. These results suggested that XBP1 may serve an
important role in promoting ovarian cancer cell proliferation and
exert protective effects in SOC cells against oxidative stress.
Downregulation of XBP1 markedly increased the expression of HMOX1
in A2780 and HO8910 cells; the expression levels of FTH1 and NQO1
were also increased in A2780 cells. The results obtained in the
current study indicated that the downregulation of XBP1 increased
antioxidant production. The increased intracellular ROS level
caused by the downregulation of XBP1 may stimulate the expression
of antioxidant genes. To further investigate the mechanism of XBP1
is involvement in the resistance of ovarian cancer cells to
oxidative stress, the expression levels of the stress-related
kinase p-p38 and the apoptosis-related proteins p53 and Bcl2 were
detected. The results demonstrated that the downregulation of XBP1
decreased the phosphorylation levels of the stress kinase p38, but
did not affect the expression of P53, Bcl2 and total p38.
ER stress and the UPR pathway serve an important
role in the occurrence and development of various tumors (25). Oncogene activation, hypoxia and
nutrient deprivation induce ER stress in cancer cells (26,27), and
the activation of UPR in a stress environment is crucial for cancer
cell growth and survival (28,29). The
initial activation of UPR may restore the normal function of ER
through various signaling pathways, and a variety of molecules,
such as HMOX1 and the MAPK family member p38, are involved in ER
stress and oxidative stress (30),
which indicates an association between the two. XBP-1 is a
transcription factor in the UPR pathway, and previous studies have
reported that XBP1 serves a protective role against oxidative
stress (21,31,32);
therefore, XBP1 may be a potential link between the ER stress and
oxidative stress pathways in cancer.
The imbalance between ROS and antioxidants is
associated with a variety of pathogenic conditions, including
cancer progression (33). A slight
increase in ROS levels is beneficial to the development and
progression of cancer, but excessive ROS production induces
oxidative damage to proteins, lipids, RNA, DNA and other biological
macromolecules in cancer cells, leading to cancer cell senescence
and death (9). Therefore, tumor
cells with increased endogenous ROS levels are likely to be more
vulnerable to exogenous ROS-inducing agents. To the best of our
knowledge, the present study was the first to reveal that XBP1
serves a role in controlling the intracellular ROS levels of SOC
cells. XBP1 knockdown significantly enhanced endogenous ROS levels
and increased sensitivity of SOC cells to oxidative stress, which
indicated that knockdown of XBP1 combined with a ROS inducer may be
applied in the treatment of ovarian cancer in the future. In the
present study, the knockdown of XBP1 increased the protein
expression levels of HMOX1. When cancer cells respond to oxidative
stress, HMOX1 may reduce intracellular ROS by upregulating its
expression. HMOX1 upregulation may occur due to the increased
intracellular ROS level following XBP1 downregulation.
In the present study, the expression levels of the
apoptosis-related proteins p53 and Bcl2 were not associated with
XBP1 in SOC cells, but the phosphorylation level of p38 was
associated with XBP1 expression. The p38 MAPK signaling pathway is
a key signal transduction cascade, which controls the balance
between cancer cell survival and death in response to
microenvironmental stress (34,35). The
complexity of this regulation may determine the cell fate, i.e.,
survival or death, depending largely on the type and intensity of
the stress, as well as the cell type (36). Accumulating evidence suggests that
the p38 signaling pathway serves a dual role in various types of
cancer. Wagner and Nebreda (37)
suggested that p38 is associated with apoptosis in hepatoma cells
and another study indicated that p38 is closely related to the
chemoresistance of colorectal cancer (38). This dual role poses a challenge to
the development of highly effective anticancer therapies targeting
the p38 MAPK pathway. The present study demonstrated that the
knockdown of XBP1 decreased the phosphorylation levels of the
stress kinase p38. It is necessary to further explore the
association between XBP1 and p38 and to elucidate the role of p38
in the development of SOC.
In conclusion, the results of the present study
demonstrated that XBP1 is overexpressed in SOC and that knockdown
of XBP1 significantly inhibited cell proliferation, which indicated
that XBP1 may be a crucial transcription factor for the survival of
ovarian cancer cells. In addition, XBP1 knockdown enhanced the
sensitivity of SOC cells to oxidative stress through upregulation
of endogenous ROS levels. In conclusion, XBP1 may be a candidate
molecular target for inhibition of SOC cell growth, and may act
synergistically with ROS inducers in anticancer treatment.
Acknowledgements
The authors would like to thank the Chinese Academy
of Sciences Committee (Shanghai, China) for providing the SOC cell
lines A2780 and HO8910.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. NSF-81572552, NSF-81772774
and NSF-81772808), the Cancer Research Program of National Cancer
Center (grant no. NCC 2017A01), and the Science and Technology
Commission of Shanghai Municipality (grant nos. 17411963500 and
17411951000).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The experiments were conceived and designed by LG
and RQL. GHZ, JYK, MMC, QM and ALZ performed the experiments. SHX
performed the statistical analysis. YCW, HZ, YiT and YuT analyzed
the data. GHZ and JYK wrote the manuscript. LG, SHX and RQL revised
the manuscript. All authors agreed to be accountable for all
aspects of this work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Markman M: Current standards of care for
chemotherapy of optimally cytoreduced advanced epithelial ovarian
cancer. Gynecol Oncol. 131:241–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang H, Zhong A, Sun J, Chen M, Xie S,
Zheng H, Wang Y, Yu Y, Guo L and Lu R: COPS5 inhibition arrests the
proliferation and growth of serous ovarian cancer cells via the
elevation of p27 level. Biochem Biophys Res Commun. 493:85–93.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coscia F, Lengyel E, Duraiswamy J,
Ashcroft B, Bassani-Sternberg M, Wierer M, Johnson A, Wroblewski K,
Montag A, Yamada SD, et al: Multi-level proteomics identifies CT45
as a chemosensitivity mediator and immunotherapy target in ovarian
cancer. Cell. 175:159–170.e16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gentric G, Kieffer Y, Mieulet V, Goundiam
O, Bonneau C, Nemati F, Hurbain I, Raposo G, Popova T, Stern MH, et
al: PML-Regulated mitochondrial metabolism enhances
chemosensitivity in human ovarian cancers. Cell Metab.
29:156–173.e10. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Macintyre G, Goranova TE, De Silva D,
Ennis D, Piskorz AM, Eldridge M, Sie D, Lewsley LA, Hanif A, Wilson
C, et al: Copy number signatures and mutational processes in
ovarian carcinoma. Nat Genet. 50:1262–1270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schumacker PT: Reactive oxygen species in
cancer cells: Live by the sword, die by the sword. Cancer Cell.
10:175–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Szatrowski TP and Nathan CF: Production of
large amounts of hydrogen peroxide by human tumor cells. Cancer
Res. 51:794–798. 1991.PubMed/NCBI
|
|
9
|
Zou Z, Chang H, Li H and Wang S: Induction
of reactive oxygen species: An emerging approach for cancer
therapy. Apoptosis. 22:1321–1335. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pluchino LA, Choudhary S and Wang HC:
Reactive oxygen species-mediated synergistic and preferential
induction of cell death and reduction of clonogenic resistance in
breast cancer cells by combined cisplatin and FK228. Cancer Lett.
381:124–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao S, Xia M, Mao Y, Zhang Q, Donkor PO,
Qiu F and Kang N: Combined oridonin with cetuximab treatment shows
synergistic anticancer effects on laryngeal squamous cell
carcinoma: Involvement of inhibition of EGFR and activation of
reactive oxygen species-mediated JNK pathway. Int J Oncol.
49:2075–2087. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshida H, Matsui T, Yamamoto A, Okada T
and Mori K: XBP1 mRNA is induced by ATF6 and spliced by IRE1 in
response to ER stress to produce a highly active transcription
factor. Cell. 107:881–891. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reimold AM, Iwakoshi NN, Manis J,
Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D,
Grusby MJ, Alt F and Glimcher LH: Plasma cell differentiation
requires the transcription factor XBP-1. Nature. 412:300–307. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clauss IM, Gravallese EM, Darling JM,
Shapiro F, Glimcher MJ and Glimcher LH: In situ hybridization
studies suggest a role for the basic region-leucine zipper protein
hXBP-1 in exocrine gland and skeletal development during mouse
embryogenesis. Dev Dyn. 197:146–156. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Romero-Ramirez L, Cao H, Nelson D, Hammond
E, Lee AH, Yoshida H, Mori K, Glimcher LH, Denko NC, Giaccia AJ, et
al: XBP1 is essential for survival under hypoxic conditions and is
required for tumor growth. Cancer Res. 64:5943–5947. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen X, Iliopoulos D, Zhang Q, Tang Q,
Greenblatt MB, Hatziapostolou M, Lim E, Tam WL, Ni M, Chen Y, et
al: XBP1 promotes triple-negative breast cancer by controlling the
HIF1α pathway. Nature. 508:103–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li H, Chen X, Gao Y, Wu J, Zeng F and Song
F: XBP1 induces snail expression to promote
epithelial-to-mesenchymal transition and invasion of breast cancer
cells. Cell Signal. 27:82–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gomez BP, Riggins RB, Shajahan AN, Klimach
U, Wang A, Crawford AC, Zhu Y, Zwart A, Wang M and Clarke R: Human
X-box binding protein-1 confers both estrogen independence and
antiestrogen resistance in breast cancer cell lines. FASEB J.
21:4013–4027. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen L, Li Q, She T, Li H, Yue Y, Gao S,
Yan T, Liu S, Ma J and Wang Y: IRE1α-XBP1 signaling pathway, a
potential therapeutic target in multiple myeloma. Leuk Res.
49:7–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ojha R and Amaravadi RK: Targeting the
unfolded protein response in cancer. Pharmacol Res. 120:258–266.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Zhang X, Liang Y, Yu H, Chen X,
Zheng T, Zheng B, Wang L, Zhao L, Shi C and Zhao S: Targeting X
box-binding protein-1 (XBP1) enhances sensitivity of glioma cells
to oxidative stress. Neuropathol Appl Neurobiol. 37:395–405. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Adachi M, Zhao S, Hareyama M, Koong
AC, Luo D, Rando TA, Imai K and Shinomura Y: Preventing oxidative
stress: A new role for XBP1. Cell Death Differ. 16:847–857. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cubillos-Ruiz JR, Silberman PC, Rutkowski
MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE,
Gupta D, Holcomb K, et al: ER stress sensor XBP1 controls
anti-tumor immunity by disrupting dendritic cell homeostasis. Cell.
161:1527–1538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mohamed E, Cao Y and Rodriguez PC:
Endoplasmic reticulum stress regulates tumor growth and anti-tumor
immunity: A promising opportunity for cancer immunotherapy. Cancer
Immunol Immunother. 66:1069–1078. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blais JD, Addison CL, Edge R, Falls T,
Zhao H, Wary K, Koumenis C, Harding HP, Ron D, Holcik M and Bell
JC: Perk-dependent translational regulation promotes tumor cell
adaptation and angiogenesis in response to hypoxic stress. Mol Cell
Biol. 26:9517–9532. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de la Cadena SG, Hernandez-Fonseca K,
Camacho-Arroyo I and Massieu L: Glucose deprivation induces
reticulum stress by the PERK pathway and caspase-7- and
calpain-mediated caspase-12 activation. Apoptosis. 19:414–427.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rouschop KM, Dubois LJ, Keulers TG, van
den Beucken T, Lambin P, Bussink J, van der Kogel AJ, Koritzinsky M
and Wouters BG: PERK/eIF2α signaling protects therapy resistant
hypoxic cells through induction of glutathione synthesis and
protection against ROS. Proc Natl Acad Sci USA. 110:4622–4627.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schewe DM and Aguirre-Ghiso JA:
ATF6alpha-Rheb-mTOR signaling promotes survival of dormant tumor
cells in vivo. Proc Natl Acad Sci USA. 105:10519–10524. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsuzawa A, Nishitoh H, Tobiume K, Takeda
K and Ichijo H: Physiological roles of ASK1-mediated signal
transduction in oxidative stress- and endoplasmic reticulum
stress-induced apoptosis: Advanced findings from ASK1 knockout
mice. Antioxid Redox Signal. 4:415–425. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen TH, Chiang YH, Hou JN, Cheng CC,
Sofiyatun E, Chiu CH and Chen WJ: XBP1-mediated BiP/GRP78
upregulation copes with oxidative stress in mosquito cells during
dengue 2 virus infection. Biomed Res Int. 2017:35191582017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martin D, Li Y, Yang J, Wang G, Margariti
A, Jiang Z, Yu H, Zampetaki A, Hu Y, Xu Q and Zeng L: Unspliced
X-box-binding protein 1 (XBP1) protects endothelial cells from
oxidative stress through interaction with histone deacetylase 3. J
Biol Chem. 289:30625–30634. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matés JM, Pérez-Gómez C and Núñez de
Castro I: Antioxidant enzymes and human diseases. Clin Biochem.
32:595–603. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signalling. Biochem J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cuenda A and Rousseau S: p38 MAP-kinases
pathway regulation, function and role in human diseases. Biochim
Biophys Acta. 1773:1358–1375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Engelberg D: Stress-activated protein
kinases-tumor suppressors or tumor initiators. Semin Cancer Biol.
14:271–282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grossi V, Peserico A, Tezil T and Simone
C: p38α MAPK pathway: A key factor in colorectal cancer therapy and
chemoresistance. World J Gastroenterol. 20:9744–9758. 2014.
View Article : Google Scholar : PubMed/NCBI
|