Introduction
Lung cancer is one of the most common malignancies,
with high incidence and high mortality rates worldwide, and
non-small cell lung cancer (NSCLC) accounts for ~80–85% of the
total cases of lung cancer (1,2). In 2011
in the United States, the 5-year survival rate of lung cancer is
<20%, which is mainly due to the development of drug resistance
that occurs after 1 year of treatment (1,3,4). Cisplatin (DDP) is one of the most
commonly used chemotherapeutic drugs for clinical NSCLC, although
it is only 20–30% effective in patients with advanced non-surgical
NSCLC (5). One of the reasons for
this is the emergence of multidrug resistance (MDR) (5). The mechanism of MDR formation in tumor
cells is complex, although it predominantly includes drug
absorption reduction, an increase in metabolism, drug target
alterations and damage sustained to the tumor cell apoptosis
pathway. The most important mechanism of drug resistance is the
high expression of the ATP-binding cassette (ABC) transporters in
the efflux pump on the tumor cell membrane, particularly high
expression of P-glycoprotein (P-gp; ABCB1 gene-encoded),
MDR-associated protein 1 (MRP1; ABCC1 gene-encoded) and breast
cancer resistance protein (BCRP; ABCG2 gene-encoded) (6,7). The
chemotherapeutic drug binds to the transmembrane domain of the ABC
transporter on the tumor cell membrane, resulting in the activation
of the ATP-binding domain, which subsequently hydrolyzes ATP to ADP
with the concomitant release of energy, altering the morphology of
the ABC transporter under the action of Mg2+; thus, it
is transferred to the outside of the tumor cells prior to the
cytotoxic action of the drug, which reduces the drug concentration
in the tumor cells and causes drug resistance (8). Previous studies have revealed that the
WNT/β-catenin pathway is an important signal transduction pathway
regulating tumor cell DDP resistance (9,10).
Following activation of the WNT signaling pathway,
non-phosphorylated (activated) β-catenin is induced to enter the
nucleus, promoting the expression of downstream signaling
molecules, including ABCB1, ABCC1 and ABCG2, thereby promoting the
occurrence of DDP resistance in tumor cells (9,10).
Mineral dust-induced gene (MDIG) is a novel lung
cancer-associated oncogene that was identified in the alveolar
macrophages of coal miners in 2005, and was subsequently revealed
to be the same gene as myc-induced nuclear antigen 53 (Mina53) and
nuclear protein 52 (11–13). Previous studies have reported that
MDIG is a downstream target gene of c-myc, serving an important
regulatory role in the proliferation, growth, differentiation,
invasion and migration, and genomic stability of tumor cells
(11–16). However, whether MDIG is associated
with the DDP resistance of lung cancer has, to the best of our
knowledge, not yet been established.
In the present study, A549 (lung adenocarcinoma) and
A549/DDP (lung adenocarcinoma resistant to DDP) cell lines were
used, and the effects of MDIG on the expression levels of ABC
transporters and the WNT/β-catenin signaling pathway in A549 and
A549/DDP cells were investigated following MDIG silencing and MDIG
overexpression to elucidate the role of MDIG in DDP resistance of
lung adenocarcinoma and its molecular mechanism. The overall aim
was to identify novel treatments for the effective targeted therapy
of lung cancer.
Materials and methods
Cell lines and cell culture
The human lung adenocarcinoma cell line, A549, was
purchased from the Type Culture Collection of the Chinese Academy
of Sciences, and the lung adenocarcinoma cell line resistant to
DDP, A549/DDP, was purchased from the BeNa Culture Collection. A549
cells were cultured in HyClone™ RPMI-1640 culture medium (HyClone;
GE Healthcare Life Sciences) containing 10% HyClone™ FBS medium (GE
Healthcare Life Sciences) in a humidified environment with 5%
CO2 in a cell incubator (Thermo Fisher Scientific, Inc.)
at 37°C. A549/DDP cells were cultured in RPMI-1640 medium
containing DDP (final concentration, 1 µg/ml) (MedChemExpress) and
10% FBS to maintain their drug resistance with 5% CO2 in
a cell incubator at 37°C.
Lentiviral transfection
MDIG-overexpression lentiviral vector (LV-MDIG;
GenBank accession no. NM_032778) and control lentiviral vector
(Vector), and MDIG-silenced lentiviral vector [LV-MDIG-RNA
interference (RNAi) 1 sequence, 5′-GGGTGATTTGTTGTACTTT-3′;
LV-MDIG-RNAi 2 sequence, 5′-AACGATTCAGTTTCACCAA-3′] and control
lentiviral vector (LV-con sequence, 5′-TTCTCCGAACGTGTCACGT-3′) were
purchased from Shanghai GeneChem Co., Ltd. The medium used to
dilute the virus was purchased from Gibco (Thermo Fisher
Scientific, Inc.) and the reinforcing fluid was provided by
Shanghai GeneChem Co., Ltd. The lentiviral vector carrying green
fluorescent protein (GFP) gene was transferred to the target cell
together with the aforementioned vectors. Aliquots (2.5 ml,
5×104 cells/ml) of the target cells were inoculated into
a T12.5 flask (Corning Inc.) the day prior to transfection, and
cultured in a cell incubator at 37°C in a humidified environment
with 5% CO2. When the cell confluence reached 30–50%,
the cells were incubated with lentivirus according to the
counterstaining index of the target cells (multiplicity of
infection, A549-50 and A549/DDP-50, respectively). After 16 h, the
medium was replaced with 5 ml fresh medium, and subsequently cells
were cultured for a further 48 h. The cells were observed under an
Axio Observer A1 inverted fluorescence microscope (Zeiss GmbH), and
the transfection efficiency was expressed as a percentage of
GFP-positive cells identified using the GFP fluorescence module
(settings: Excitation, BP470/40; beam splitter, FT495; emission,
BP525/50). The stably transfected cells were continuously
exchanged, passaged and frozen, and subsequently used for further
experiments. All experiments were performed after fourth-generation
cells transfected with lentivirus.
Cytotoxicity assay
Cytotoxicity was determined using the Cell Counting
kit-8 (CCK-8; MedChemExpress). The specific experimental procedures
were performed according to the manufacturer's protocol. A549 and
A549/DDP cells in the exponential growth phase, and the
MDIG-silenced and overexpressing cells following transfection with
the respective lentiviruses, were inoculated into 96-well plates
(100 µl/well) at ~5×103 cells/well, and wells containing
only RPMI-1640 medium were used as a blank control. Three replicate
wells were used for each experimental group, with an incubation
period of 24 h. Sterile saline (0.9%) was used to prepare and
dilute DDP, as described previously (17). Subsequently, different concentrations
of DDP (20, 40, 80, 100, 160, 200 and 320 µg/ml) (MedChemExpress)
were sequentially added, and the cells were incubated at 37°C in a
humidified atmosphere with 5% CO2 for 24 h.
Subsequently, 10 µl/well CCK-8 solution was added, and the cells
were incubated for a further 1–2 h at 37°C. The optical density
(OD) at 450 nm was measured using a microplate reader (Tecan
Infinite M200 PRO; Tecan Group, Ltd.). The percentage cell
viability was calculated as follows: Cell viability (%)=(the
average OD value of the experimental group-average OD value of the
blank control group)/(the average OD value of the negative control
group-the average OD value of the blank control group) ×100%. The
concentration was plotted on the abscissa and the corresponding
cell viability was plotted on the ordinate, then the curve was
plotted and, finally, the IC50 value was calculated for
DDP against the cells of each experimental group using GraphPad
Prism version 7.0 software (GraphPad Software, Inc.). The
IC50 value for DDP was defined as the value for which
cell viability (%) was decreased to 50% by DDP. The resistance
coefficient was determined by the ratio of the average
IC50 value of A549/DDP cells to the average
IC50 value of A549 cells. Each experiment was repeated
at least three times.
RNA isolation, cDNA synthesis and
reverse transcription- quantitative PCR (RT-qPCR)
Total RNA from each group of cells (including
MDIG-knockdown and MDIG-overexpressing A549 cells, and
DDP-resistant A549/DDP cells) was extracted using
TRIzol® reagent (Ambion; Thermo Fisher Scientific,
Inc.), and subsequently RNA quantification was performed on a
NanoQuant plate™ (Tecan Group, Ltd.). The cDNA was
reverse-transcribed and synthesized using the PrimeScript™ RT
reagent kit with gDNA Eraser (Perfect Real Time) (Takara
Biotechnology Co., Ltd.) according to the manufacturer's protocol:
35°C for 15 min, 85°C for 5 sec and gradually decreased to 4°C.
Actin was used as the internal reference gene. The PCR primers used
in this experiment were synthesized by Takara Biotechnology Co.,
Ltd., and the primer sequences are shown in Table I. RT-qPCR analysis was performed
using SYBR Premix Ex Taq™ II (Takara Biotechnology Co., Ltd.),
according to the manufacturer's protocol. The thermocycling
conditions for the PCR reaction were as follows: Pre-denaturation
at 95°C for 30 sec, followed by 95°C for 5 sec and 60°C for 30 sec
(40 cycles). The melting curve was established at 95°C for 5 sec,
60°C for 1 min, 95°C for 15 sec, and the cooling process was 50°C
for 50 sec. The cycle threshold value (Cq) was determined using a
LightCycler 480 machine (Roche Diagnostics), and, finally, the
2−ΔΔCq method (18) was
used to calculate the relative ratio of the gene of interest,
expressed as a percentage relative to the normal control group.
 | Table I.Primer sequences of genes used in the
present study. |
Table I.
Primer sequences of genes used in the
present study.
| Genes | Primer
sequences |
|---|
| MDIG | Forward:
5′-GCAACGATTCAGTTTCACCAACC-3′ |
|
| Reverse:
5′-ATGTACACATTCGAGCCAACCAAG-3′ |
| ABCB1 | Forward:
5′-CACATTTGGCAAAGCTGGAGA-3′ |
|
| Reverse:
5′-CATCATTGGCGAGCCTGGTA-3′ |
| ABCG2 | Forward:
5′-TGCCCAGGACTCAATGCAAC-3′ |
|
| Reverse:
5′-TCGATGCCCTGCTTTACCAAATA-3′ |
| ABCC1 | Forward:
5′-GTGATGGCGATGAAGACCAAGA-3′ |
|
| Reverse:
5′-GCCAGCTCCCAGGCATAAAG-3′ |
| Actin | Forward:
5′-CCTGGCACCCAGCCAAT-3′ |
|
| Reverse:
5′-GGGCCGGACTCGTCATAC-3′ |
Western blotting
The cells of each group were extracted with RIPA
lysate (Beijing Suolaibao Technology Co., Ltd.) containing 10% PMSF
and protease inhibitor cocktail (Roche Diagnostics GmbH) on ice.
Subsequently, total protein concentration was determined using a
Bicinchoninic Acid Protein Quantification kit (Thermo Fisher
Scientific, Inc.). The protein samples (30 µg/lane) were separated
using SDS-PAGE (8% gels; Bio-Rad Laboratories, Inc.), and
subsequently transferred onto PVDF membranes (0.45 µm) for 1.5–2.5
h, followed by blocking at room temperature for 2 h with 5% non-fat
dried milk. The membranes were washed 3 times with TBS with 0.1%
Tween-20 (TBST) for 15 min each time, and then incubated with the
primary antibodies of interest, as follows: Anti-Mina53 (cat. no.
ab173573, 1:1,000 dilution, Abcam), anti-ABCB1 (cat. no. 13342,
1:1,000 dilution; Cell Signaling Technology, Inc.), anti-ABCC1
(cat. no. 14685, 1:1,000 dilution; Cell Signaling Technology,
Inc.), anti-ABCG2 (cat. no. 42078, 1:1,000 dilution; Cell Signaling
Technology, Inc.), non-phospho (active) β-catenin (cat. no. 8814,
1:1,000 dilution; Cell Signaling Technology, Inc.), anti-WNT family
member 5A (WNT5A; cat. no. ab174963, 1:500 dilution; Abcam),
anti-WNT family member 3A (WNT3A; cat. no. ab28472, 1:1,000
dilution; Abcam) and anti-GAPDH (cat. no. 5174, 1:1,000 dilution;
Cell Signaling Technology, Inc.), overnight at 4°C. Following
washing of the membranes with TBST, they were incubated with
horseradish peroxidase-conjugated secondary antibody [anti-rabbit
immunoglobulin G (IgG); cat. no. 2306, 1:4,000 dilution; OriGene
Technologies, Inc.] at room temperature for 2 h. Immunoreactive
bands were detected with an ECL western blotting system (Clarity
Western ECL Substrate; Bio-Rad Laboratories, Inc.). ImageJ version
1.8.0 software (National Institutes of Health) was used to analyze
the gray value of each band, and to calculate the gray value
percentage of each protein band compared with the internal
reference (GAPDH). Each experiment was repeated at least three
times.
Statistical analysis
All experiments were repeated at least three times.
The data are expressed as the means ± SD. All data were tested for
normality and homogeneity of variance: Comparisons among groups
were performed using ANOVA, and the comparisons within groups were
performed using the Bonferroni method (when the variance was
uniform) and the Dunnett's T3 method (when the variance was not
uniform). All statistical analyses were performed using GraphPad
Prism version 7.0 software (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Construction of MDIG-knockdown and
MDIG-overexpressing A549 cells
Inverted fluorescence microscopy was used to observe
fourth-generation A549 cells transfected with lentivirus at
bright-field ×100 magnification, and subsequently, in the same
field of view, the cells were analyzed for GFP fluorescence at
GFP-field ×100 and ×400 magnification. All cell groups exhibited a
high cell viability state and high transfection efficiency
(>90%; Fig. 1), and the
efficiency of MDIG knockdown and overexpression was verified by
RT-qPCR and western blot analysis. The results revealed that,
compared with the normal A549 group and the LV-con group, the
LV-MDIG-RNAi 1 and the LV-MDIG-RNAi 2 groups exhibited a marked
reduction in the mRNA and protein expression levels of MDIG in the
A549 cells (P<0.01; Fig. 1A). By
contrast, compared with the A549 and vector groups, the LV-MDIG
group was observed to have increased mRNA and protein expression
levels of MDIG in the A549 cells (P<0.01; Fig. 1B). Taken together, these results
indicated that the processes of lentiviral transfection for
knockdown and overexpression were successful.
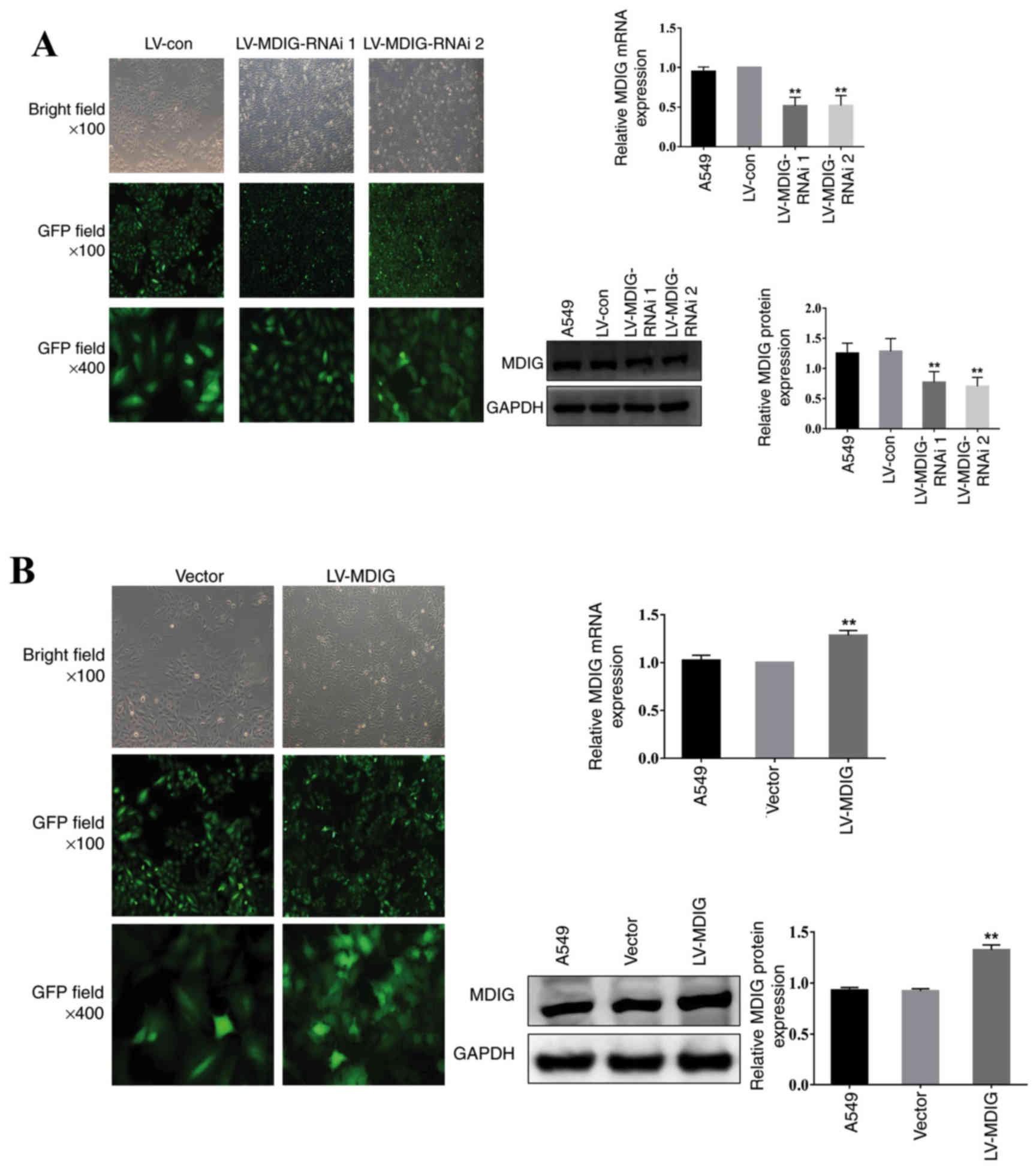 | Figure 1.Knockdown and overexpression of MDIG
in A549 cells. The transfection efficiency and cell morphology
(bright field, ×100 magnification; GFP field, ×100 magnification;
and GFP field, ×400 magnification) were observed under an inverted
fluorescence microscope, and the mRNA and protein expression levels
of MDIG in each group were examined using reverse
transcription-quantitative PCR and western blot analysis,
respectively. (A) Stably transfected MDIG-silenced A549 cells.
**P<0.01 vs. LV-con and A549 groups. (B) Stably transfected
MDIG-overexpressing A549 cells. The details of the groups
(LV-MDIG-RNAi 1, LV-MDIG, etc.) can be found in the Materials and
methods section. **P<0.01 vs. vector and A549 groups. Con,
control; GFP, green fluorescence protein; LV, lentiviral vector;
MDIG, mineral dust-induced gene; RNAi, RNA interference. |
Construction of MDIG-knockdown and
MDIG-overexpressing A549/DDP cells
As for A549 cells, fourth-generation A549/DDP cells
transfected with lentivirus were observed by inverted fluorescence
microscopy. All cells were shown to be in good condition, and the
transfection efficiency was high (Fig.
2). The RT-qPCR and western blotting experiments demonstrated
that the mRNA and protein expression levels of MDIG were lower in
the LV-MDIG-RNAi 1 and LV-MDIG-RNAi 2 groups compared with the
A549/DDP and the LV-con groups (P<0.01; Fig. 2A). Conversely, the mRNA and protein
expression levels of MDIG were higher in the LV-MDIG group compared
with those in the A549/DDP and vector groups (P<0.01; Fig. 2B). These results indicated that these
stably transfected cell lines could be used in subsequent
experiments.
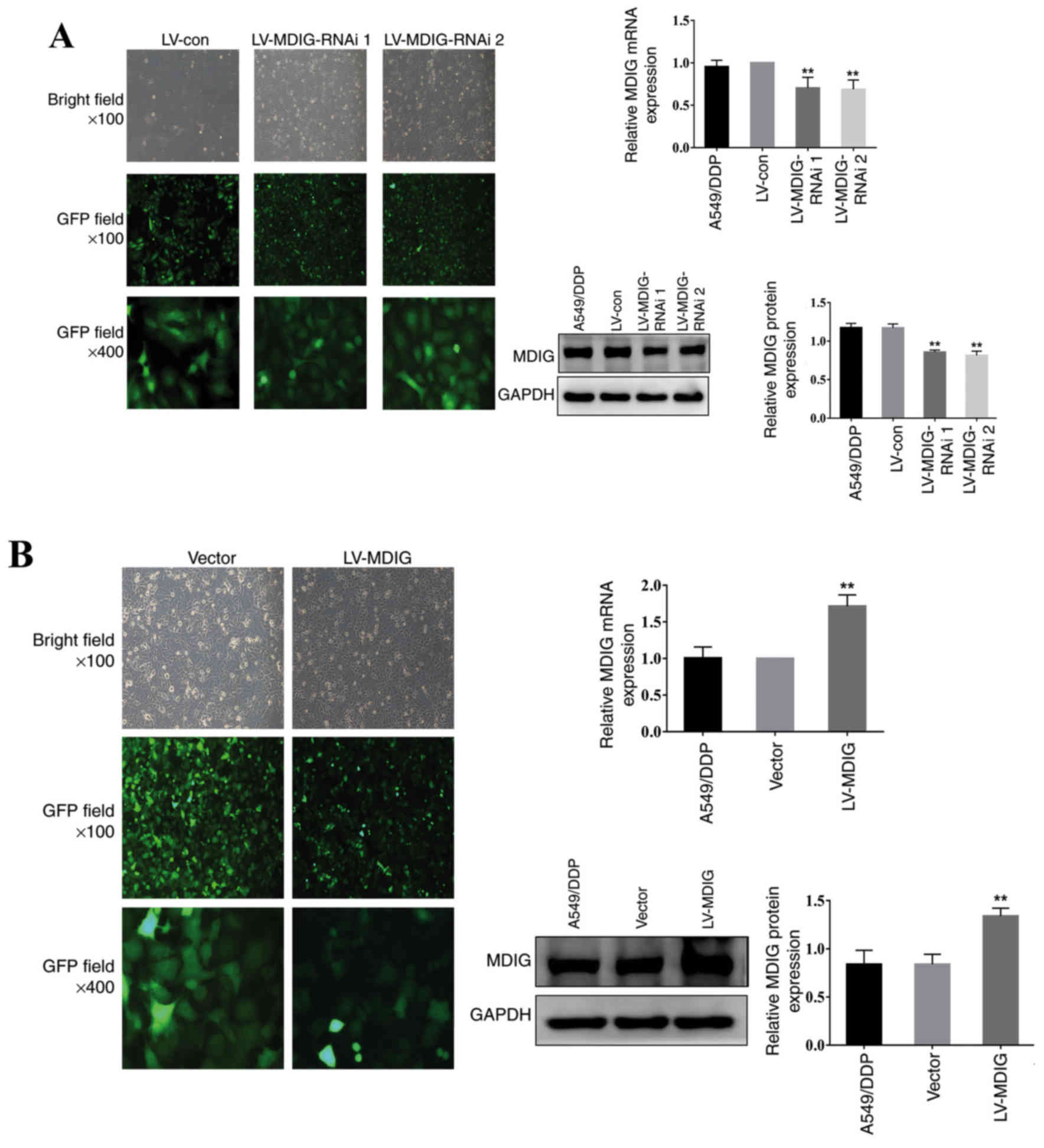 | Figure 2.Knockdown and overexpression of MDIG
in A549/DDP cells. The transfection efficiency and cell morphology
(bright-field, ×100 magnification; GFP field, ×100 magnification;
and GFP field, ×400 magnification) were observed under an inverted
fluorescence microscope, and the mRNA and protein expression levels
of MDIG in each group were examined using reverse
transcription-quantitative PCR and western blot analysis,
respectively. (A) Stably transfected MDIG-silenced A549/DDP cells.
**P<0.01 vs. LV-con and A549/DDP groups. (B) Stably transfected
MDIG-overexpressing A549/DDP cells. **P<0.01 vs. vector and
A549/DDP groups. The naming of the groups (LV-MDIG-RNAi 1, LV-MDIG,
etc.) is detailed in the Materials and methods section. Con,
control; DDP, cisplatin; GFP, green fluorescence protein; LV,
lentiviral vector; MDIG, mineral dust-induced gene; RNAi, RNA
interference. |
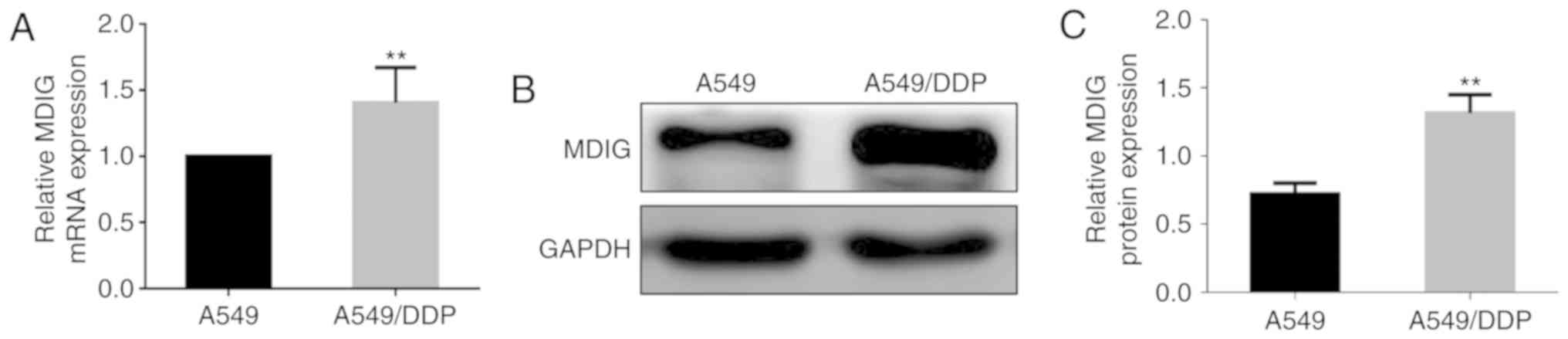
Basic expression levels of MDIG in the
A549 and A549/DDP cells, and the effect of DDP
In order to investigate the association between MDIG
and platinum-based drug resistance in tumor cells, RT-qPCR and
western blot analyses were used to detect the basic mRNA and
protein expression levels of MDIG in the A549 and A549/DDP cells
(Fig. 3). The results revealed that
the mRNA and protein expression levels of MDIG in A549/DDP cells
were significantly higher compared with those in A549 cells
(P<0.01), which suggested that MDIG expression was associated
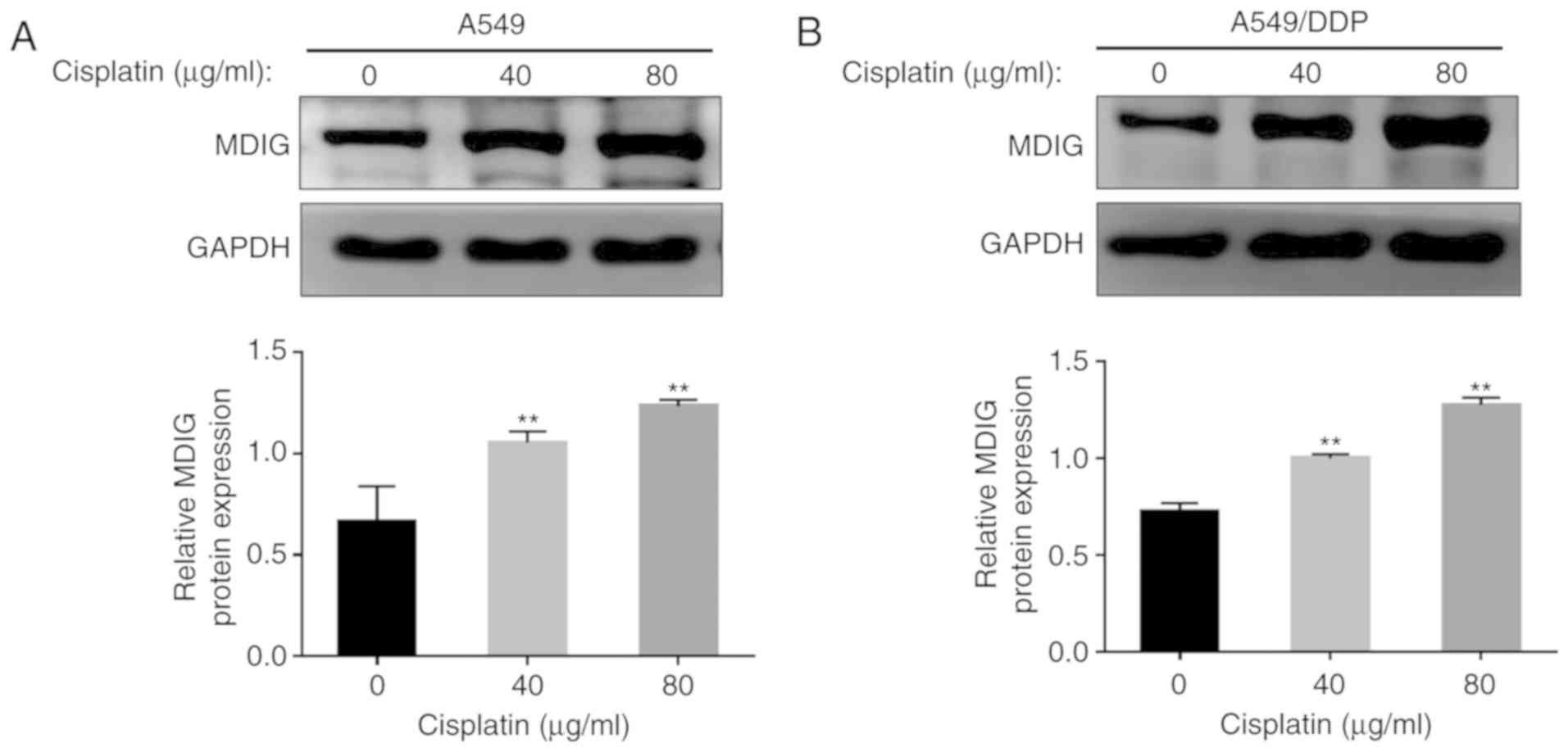
with DDP resistance. To further determine the association between
MDIG expression and DDP resistance, A549 and A549/DDP cells were
treated with different concentrations of DDP (0, 40 and 80 µg/ml),
and the protein expression levels of MDIG were revealed to increase
in a dose-dependent manner with increasing DDP concentrations in
the two cell lines (P<0.01; Fig.
4).
MDIG promotes resistance to DDP in
A549 and A549/DDP cells
In order to further investigate the effect of
MDIG on DDP resistance in lung adenocarcinoma cells, the
IC50 values for DDP in A549 and A549/DDP cells were
determined using a CCK-8 assay. The results demonstrated that the
IC50 value for DDP in the A549/DDP cells was
significantly higher compared with that in the A549 cells
(93.79±8.70 vs. 29.91±1.43 µg/ml; P<0.01), and the resistance
coefficient was 3.14 (Fig. 5A).
Based on this, following MDIG overexpression (LV-MDIG) and MDIG
knockdown (LV-MDIG-RNAi) in A549 and A549/DDP cells, the
IC50 values for DDP of each group of cells was
determined, revealing that, compared with the A549 and vector
groups, the IC50 value of the A549 cells overexpressing
MDIG was significantly increased (IC50 of LV-MDIG group,
176.90±13.40 µg/ml; IC50 of A549 group, 29.91±1.43
µg/ml; and IC50 value of vector group, 37.57±1.98 µg/ml;
P<0.01; Fig. 5B), whereas the
IC50 values of the A549 groups were significantly
decreased following MDIG knockdown (IC50 of LV-MDIG-RNAi
1 group, 10.84±1.44 µg/ml; IC50 of LV-MDIG-RNAi 2 group,
8.471±1.74 µg/ml; IC50 of A549 group, 29.91±1.43 µg/ml;
and IC50 of LV-con group, 26.67±1.059 µg/ml; P<0.01;
Fig. 5C). Furthermore, in the
MDIG-knockdown and MDIG-overexpressing A549/DDP cells, similar
results were obtained, i.e., the IC50 values of the
MDIG-overexpressing A549/DDP groups were significantly higher
compared with those of the A549/DDP and vector groups
(IC50 of LV-MDIG group, 262±25.51 µg/ml; IC50
of A549/DDP group, 93.79±8.70 µg/ml; and IC50 of vector
group, 114.7±7.379 µg/ml; P<0.01; Fig. 5D). Conversely, following MDIG
knockdown, the IC50 values were significantly lower
(IC50 of LV-MDIG-RNAi 1 group, 31.05±1.73 µg/ml;
IC50 of LV-MDIG-RNAi 2 group, 35.1±5.85 µg/ml;
IC50 of A549/DDP group, 93.79±8.70 µg/ml; and
IC50 of LV-con group, 104.8±21.13 µg/ml; P<0.01;
Fig. 5E).
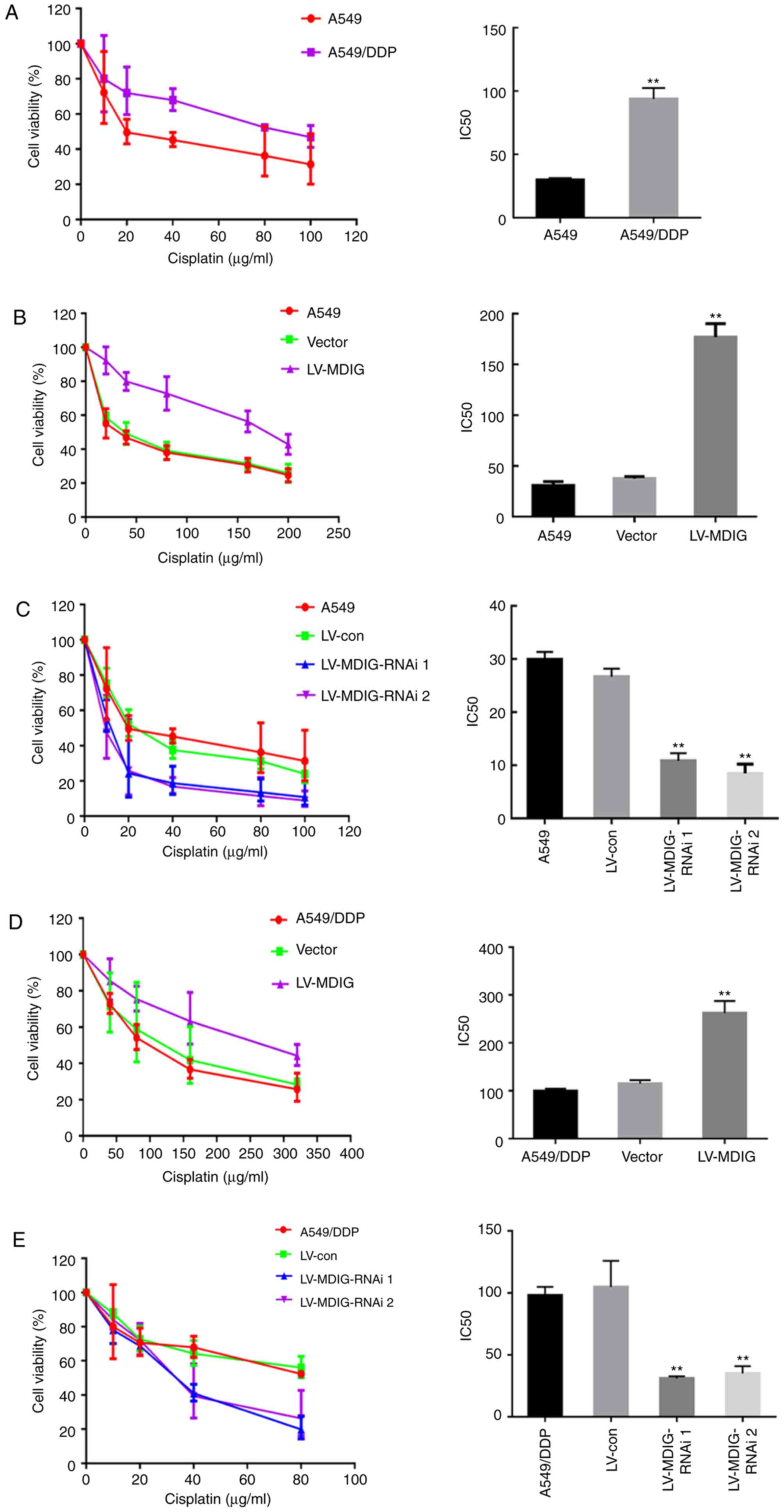 | Figure 5.MDIG is associated with DDP
resistance in A549 and A549/DDP cells. (A) A549 and A549/DDP cells
were treated with different concentrations of DDP for 48 h, cell
viability was determined by CCK-8 assay, and the IC50
values for DDP were calculated. **P<0.01 vs. A549 group. A CCK-8
assay was used to determine the cell viability of (B)
MDIG-overexpressing (**P<0.01 vs. A549 group), and (C)
MDIG-silenced A549 cells, and the IC50 values for DDP
were calculated. **P<0.01 vs. LV-con and A549 groups. A CCK-8
assay was used to determine the cell viability of (D)
MDIG-overexpressing (**P<0.01 vs. vector and A549/DDP groups)
and (E) MDIG-silenced A549/DDP cells, and the IC50
values for DDP were calculated (**P<0.01 vs. the LV-con and
A549/DDP groups). Con, control; DDP, cisplatin; LV, lentiviral
vector; MDIG, mineral dust-induced gene; RNAi, RNA
interference. |
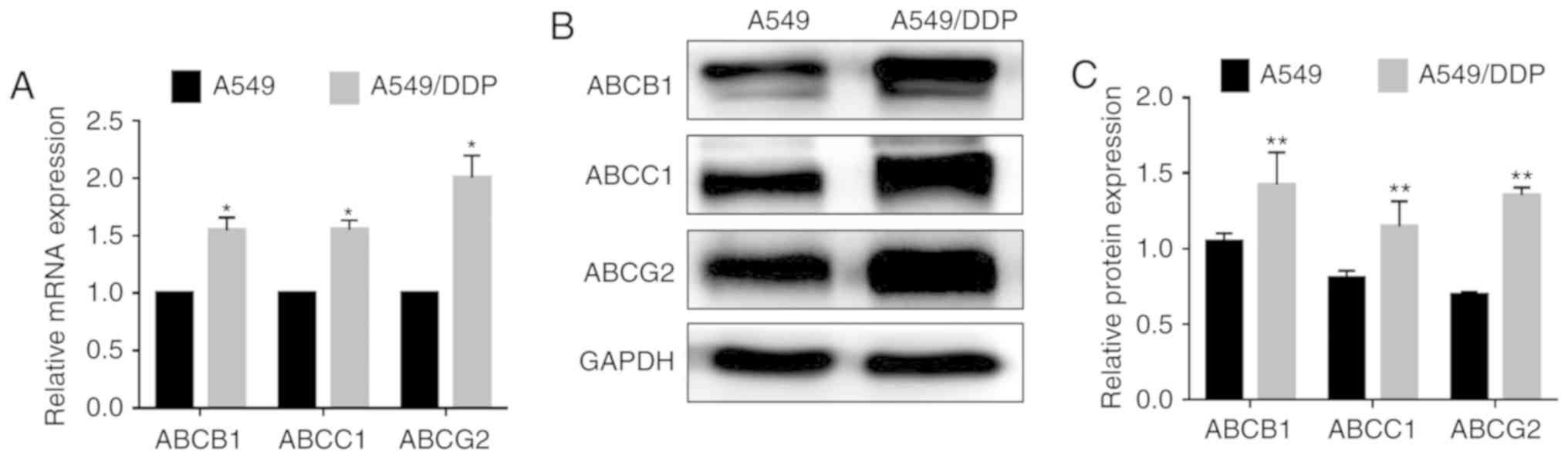
MDIG promotes ABC transporter
expression in A549 and A549/DDP cells
Subsequently, in order to investigate the molecular
mechanism of MDIG-promoted DDP resistance in lung adenocarcinoma
cells, the expression levels of ABC transporters in A549 and
A549/DDP cells were examined. As shown in Fig. 6, the basal mRNA and protein
expression levels of ABCB1, ABCC1 and ABCG2 were significantly
higher in A549/DDP cells compared with in A549 cells, indicating
that efflux pump ABC transporters are involved in DDP resistance in
lung adenocarcinoma cells. To further clarify whether MDIG affected
the resistance of lung adenocarcinoma to DDP by regulating the
expression levels of ABC transporters, MDIG was overexpressed (the
LV-MDIG group) or silenced (the LV-MDIG RNAi 1 and LV-MDIG RNAi 2
groups) in A549 and A549/DDP cells, and the mRNA and protein
expression levels of the ABC transporters in either of the cell
types was determined by RT-qPCR and western blot analysis,
respectively. The results revealed that the mRNA and protein
expression levels of the efflux pump transporters, ABCB1, ABCC1 and
ABCG2, in A549 and A549/DDP cells were significantly upregulated
following MDIG overexpression compared with the normal and vector
groups (P<0.05; Fig. 7).
Conversely, following MDIG knockdown, the mRNA and protein
expression levels of the ABC transporters were significantly lower
compared with the normal and the LV-con groups (P<0.01; Fig. 8).
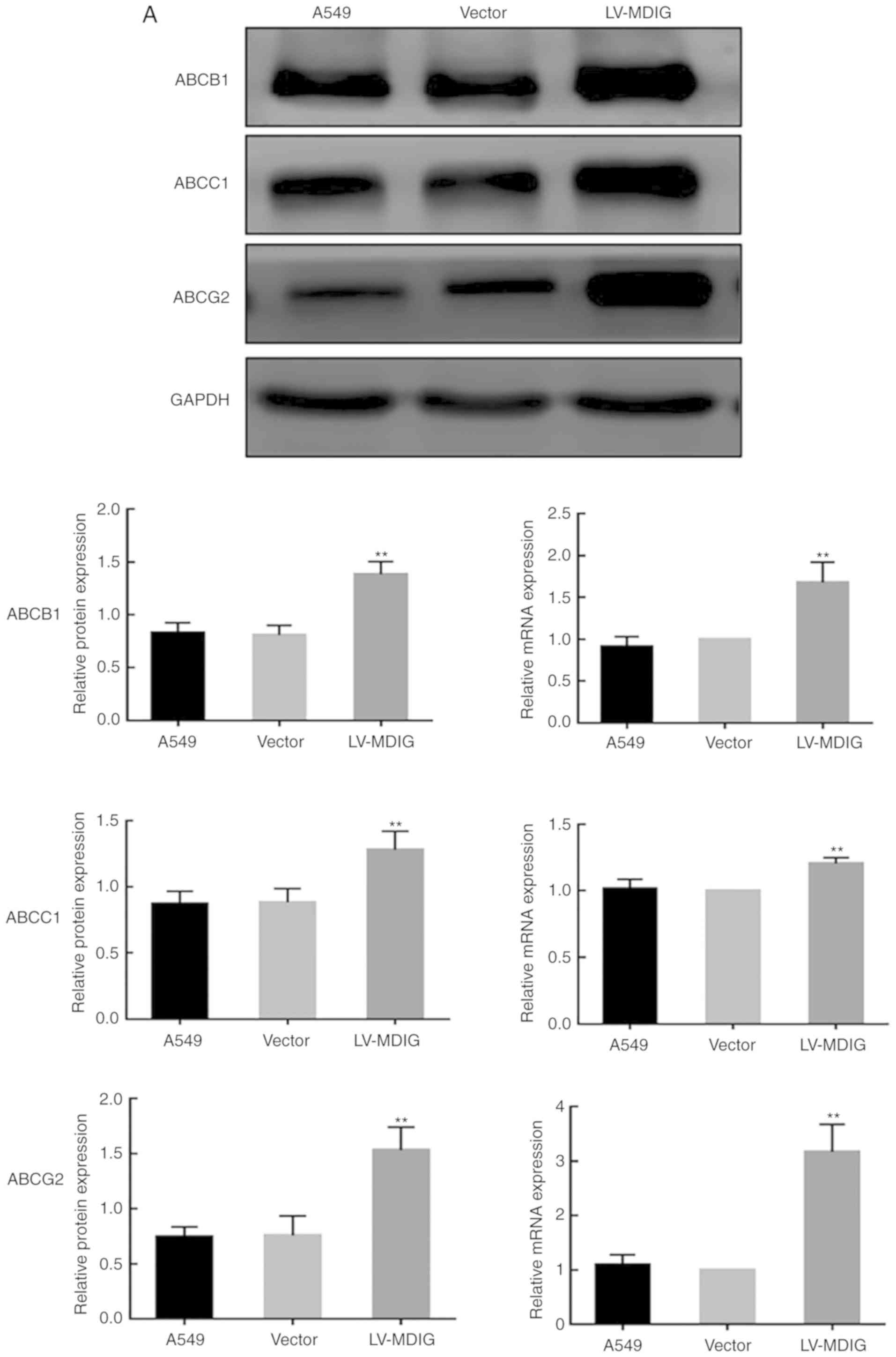 | Figure 7.Alterations in the mRNA and protein
expression levels of ABC transporters in (A) A549 cells following
MDIG overexpression. Alterations in the mRNA and protein expression
levels of ABC transporters in (B) A549/DDP cells following MDIG
overexpression. The LV-MDIG group was compared with the vector and
normal groups. Actin was the reference control for reverse
transcription-quantitative PCR analysis, whereas GAPDH was the
reference control for the western blotting experiments. The naming
of the groups (LV-MDIG-RNAi 1, LV-MDIG, etc.) is detailed in the
Materials and methods section. *P<0.05, **P<0.01 vs. vector
and A549 groups or vs. vector and A549/DDP groups. ABC, ATP binding
cassette; ABCB1, ATP-binding cassette transporter B1; ABCC1,
ATP-binding cassette transporter C1; ABCG2, ATP-binding cassette
transporter G2; DDP, cisplatin; LV, lentiviral vector; MDIG,
mineral dust-induced gene. |
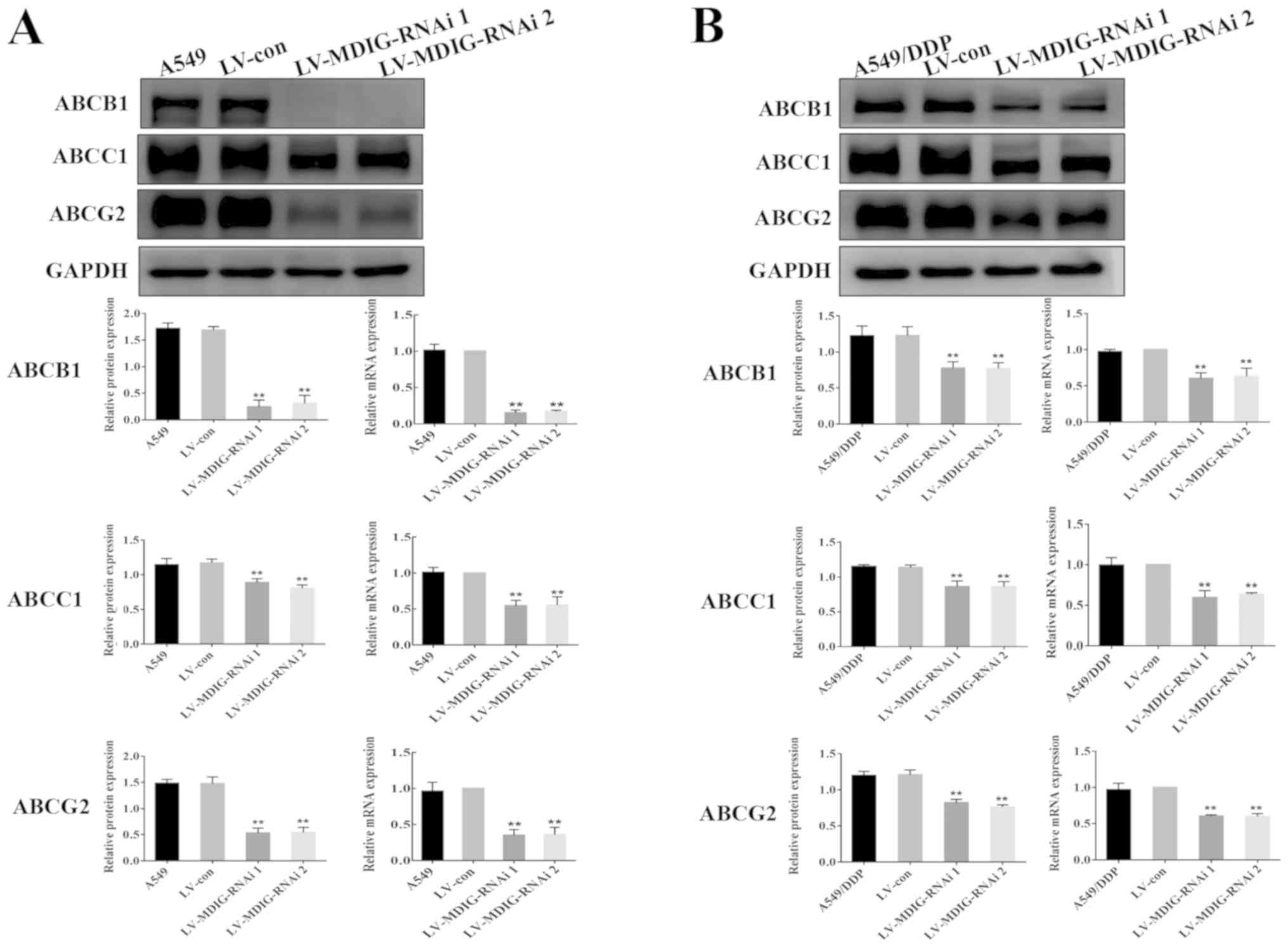 | Figure 8.Alterations in the mRNA and protein
expression levels of the ABC transporters. Expression levels of ABV
transporter proteins in (A) A549 cells following MDIG knockdown.
Expression levels of ABV transporter proteins in (B) A549/DDP cells
following MDIG knockdown. **P<0.01 vs. LV-con and A549 groups or
vs. LV-con and A549/DDP groups. Actin was the reference control for
reverse transcription-quantitative PCR analysis, whereas GAPDH was
the reference control for the western blotting experiments. The
naming of the groups (LV-MDIG-RNAi 1, LV-MDIG, etc.) is detailed in
the Materials and methods section. ABC, ATP-binding cassette;
ABCB1, ATP-binding cassette transporter B1; ABCC1, ATP-binding
cassette transporter C1; ABCG2, ATP-binding cassette transporter
G2; Con, control; DDP, cisplatin; LV, lentiviral vector; MDIG,
mineral dust-induced gene; RNAi, RNA interference. |
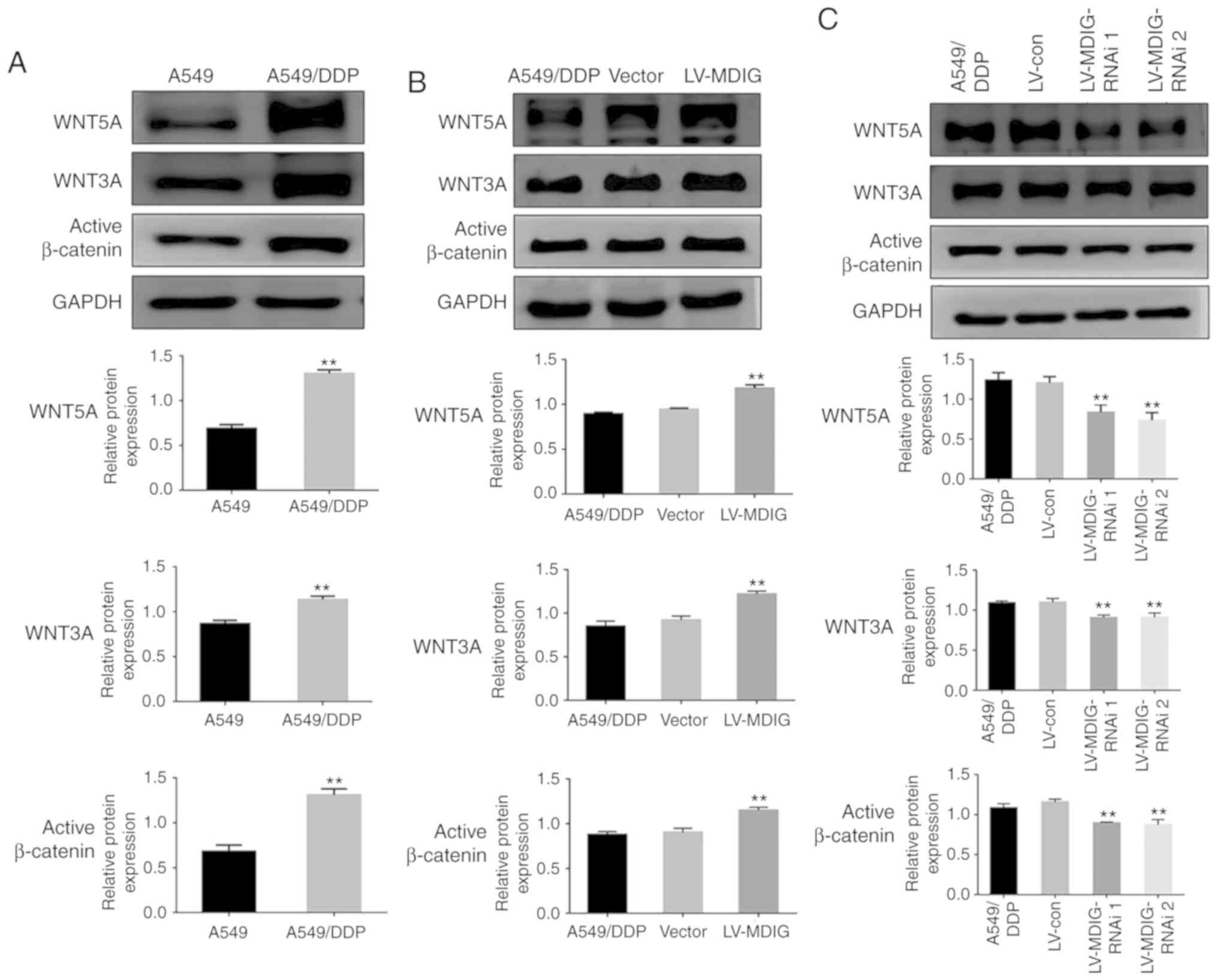
MDIG regulates the expression levels
of ABC transporters by activating the WNT/β-catenin signaling
pathway
A previous study demonstrated that WNT signaling is
activated in A549/DDP cells, and that this is associated with DDP
resistance (19). In addition,
further studies have demonstrated that WN T leads to DDP resistance
by regulating the expression levels of downstream signaling
molecules; the ABC transporters (9,20).
Therefore, in order to investigate whether MDIG also affected the
expression levels of ABC transporters via the WNT signaling
pathway, thereby promoting DDP resistance, the basal expression
levels of WNT signaling pathway-associated proteins were examined
in A549 and A549/DDP cells by western blot analysis. The results
demonstrated that the expression levels of WNT5A, WNT3A and active
β-catenin in the A549/DDP cells were significantly higher compared
with those in the A549 cells (P<0.01; Fig. 9A). Based on this, the expression
levels of WNT signaling pathway-associated proteins following
overexpression or silencing of MDIG in the A549/DDP cells was
examined. The results revealed that, the expression levels of
WNT5A, WNT3A and active β-catenin in the MDIG-overexpression group
were significantly higher compared with those in the normal
A549/DDP and vector groups (P<0.01; Fig. 9B). Conversely, in the MDIG-silencing
experiment, compared with the normal A549/DDP and the LV-con
groups, the expression levels of WNT5A, WNT3A and active β-catenin
in the MDIG-silencing groups were significantly lower (P<0.01;
Fig. 9C).
Discussion
Lung cancer is the leading cause of
cancer-associated mortality worldwide (1). NSCLC accounts for 80–85% of all cases
of lung cancer (1,2). Out of all patients with NSCLC, ~70% are
diagnosed with advanced metastasis. Chemotherapy is able to prolong
the life of patients with advanced NSCLC, and to improve their
quality of life. DDP is one of the most important first-line
chemotherapy drugs for patients with advanced lung cancer. However,
the development of lung cancer resistance to DDP leads to failure
of chemotherapy, rendering the treatment of patients with advanced
cancer less effective. According to previous studies, the overall
5-year survival rate of patients with stage IIIB/IV NSCLC is only
1–5% (21–23).
MDIG is a proto-oncogene associated with lung
cancer, and is highly expressed in lung cancer tissues and the
majority of lung cancer cell lines, but not in normal lung tissues
(11). Previous studies have
demonstrated that MDIG is highly expressed in a variety of tumor
types in addition to lung cancer, including breast cancer, colon
cancer, liver cancer, renal cell carcinoma and neuroblastoma
(24–28). In addition, it exerts different
biological effects in tumor progression, including regulation of
tumor cell proliferation, invasion and migration, differentiation,
and genomic stability (11–16). However, limited research has been
conducted on whether MDIG is associated with tumor resistance. One
study in this area was conducted by Huo et al (29), who reported in 2017 that MDIG is
associated with the resistance of sorafenib in hepatoma cells in
vitro. They demonstrated that MDIG knockdown increased the
sensitivity of Huh7 (human hepatoma) cells and MHCC97-H (human
high-metastatic-potential hepatoma) cells to sorafenib; however,
the specific mechanism of drug resistance was not further explored,
and the association between MDIG and DDP sensitivity in lung cancer
and other tumor cells, to the best of our knowledge, has not yet
been reported on.
In order to investigate the association between MDIG
and platinum-like resistance in lung adenocarcinoma cells, in the
present study, the mRNA and protein expression levels of MDIG in
DDP-resistant lung adenocarcinoma cells (A549/DDP) were revealed to
be significantly higher compared with those in normal lung
adenocarcinoma cells (A549) according to RT-qPCR and western
blotting analyses. Additionally, the expression levels of MDIG in
the two cell lines increased in a dose-dependent manner with
increasing DDP concentration, which suggested that DDP resistance
in lung adenocarcinoma may be associated with the upregulation of
MDIG. The expression levels of MDIG were positively associated with
the degree of DDP resistance.
To further investigate the association between MDIG
and DDP resistance in lung cancer cells, the present study
initially employed a CCK-8 assay to detect the IC50
value for DDP in A549 and A549/DDP cells. The results demonstrated
that the IC50 value for DDP of the A549/DDP cells was
significantly higher compared with that of the A549 cells, and the
resistance coefficient was 3.14. Based on this, following MDIG
overexpression (LV-MDIG group) or MDIG knockdown (LV-MDIG-RNAi
groups) in A549 and A549/DDP cells, the IC50 values for
DDP of each group were determined, revealing that, compared with
the normal A549 and the vector groups, the IC50 values
were significantly increased in the MDIG-overexpressing A549
groups, whereas the IC50 values were significantly
decreased in the MDIG-silencing A549 groups, when compared with the
normal A549 and the LV-con groups. Following overexpression and
knockdown of MDIG in the A549/DDP cells, similar results to those
described for the A549 cells were obtained. These results suggested
that MDIG expression may promote the resistance of lung
adenocarcinoma cells to DDP, and that the sensitivity to DDP can be
restored following MDIG silencing.
The emergence of MDR is the most important cause of
the failure of tumors to respond to chemotherapy drugs, and the ABC
transporters in the efflux pump on the tumor cell membrane serve
the most important role in this regard (6,7). The ABC
transporter family is a group of transmembrane proteins, including
seven subfamilies: ABCA-ABCG, of which the three subfamilies of
ABCB, ABCC and ABCG are the ones that are mainly associated with
tumor MDR. The most studied proteins are ABCB1 in the ABCB
subfamily (also known as P-gp), ABCC1 in the ABCC subfamily (also
known as MRP1) and ABCG2 in the ABCG subfamily (also known as
BCRP). These proteins function predominantly by using the energy
released by ATP hydrolysis to pump the intracellular drugs out of
the cell, leading to a reduction in the intracellular drug
concentration and consequent drug resistance (8). Robey et al (30) demonstrated that the use of specific
targeting inhibitors of ABCB1 and ABCC1 is able to overcome the
resistance of osteosarcoma to DDP. Similarly, Shi et al
(31) reported that the epidermal
growth factor tyrosine kinase inhibitor, AG1478, and erlotinib
effectively reverse ABCG2-mediated MDR by directly inhibiting the
drug efflux function of ABCG2 in ABCG2-overexpressing cells.
In order to investigate the molecular mechanism of
MDIG-promoted DDP resistance in lung adenocarcinoma cells, the
present study examined the basal expression levels of efflux pump
ABC transporters in A549 and A549/DDP cells, and it was revealed
that the mRNA and protein expression levels of ABCB1, ABCC1 and
ABCG2 in A549/DDP cells were significantly higher compared with
those of A549 cells. This was consistent with previous studies
(32–34), indicating that efflux pump ABC
transporters are involved in DDP resistance in lung adenocarcinoma
cells. To further investigate whether MDIG affected the resistance
of lung adenocarcinoma to DDP by regulating the expression levels
of the ABC transporter family, MDIG was overexpressed and silenced
in A549 and A549/DDP cells, and the mRNA and protein expression
levels of the ABC transporters were subsequently determined by
RT-qPCR and western blot analysis, respectively. The results
revealed that the mRNA and protein expression levels of the
extracellular pumping transporters, ABCB1, ABCC1 and ABCG2, were
upregulated in the two cell lines compared with the normal and the
control vector groups. On the other hand, following MDIG knockdown,
the expression levels were significantly lower compared with the
normal and LV-con groups. These findings indicated that MDIG
regulated the expression levels of ABC transporters, which may
cause DDP resistance in lung adenocarcinoma cells by promoting the
expression levels of the efflux pump ABC transporters.
Previous studies have reported that DDP resistance
of tumor cells is associated with activation of the WNT/β-catenin
signaling pathway (9,10). For example, Luo et al
(19) reported that the sensitivity
of DDP-resistant lung cancer cells was enhanced upon downregulating
WNT/β-catenin signaling and inhibiting BCRP and MRP4 expression,
and Huang et al (35)
demonstrated that abnormal activation of the WNT/β-catenin
signaling pathway in ovarian cancer cells promoted DDP resistance,
and inhibition of WNT signaling effectively reversed DDP
chemoresistance in SKOV3/DDP cells. Furthermore, Li et al
(36) reported that the long
non-coding RNA HOXA distal transcript antisense RNA induced
resistance to DDP in osteosarcoma cells by activating the
WNT/β-catenin pathway, a process that could be reversed by the
addition of WNT signaling pathway inhibitors. Previous studies have
also demonstrated that ABC transporters are the downstream
signaling molecules of the WNT/β-catenin signaling pathway
(9,20). In addition, WNT leads to DDP
resistance by regulating the expression levels of the downstream
signaling molecule ABC transporter (9,20). In
order to investigate whether MDIG also affected ABC transporter
expression via the WNT signaling pathway and promoted DDP
resistance in lung adenocarcinoma cells, the present study examined
the basal expression levels of WNT signaling pathway-associated
proteins in A549 and A549/DDP cells by western blot analysis. The
results demonstrated that the expression levels of WNT5A, WNT3A and
active β-catenin in A549/DDP cells were significantly higher
compared with those in A549 cells, indicating that the
WNT/β-catenin signaling pathway was activated in DDP-resistant lung
adenocarcinoma cells, a finding consistent with a previous study
(19). On this basis, in the present
study, MDIG in A549/DDP cells was respectively overexpressed or
silenced, revealing that the MDIG-overexpression group was
associated with significantly increased expression levels of WNT5A,
WNT3A and active β-catenin. Conversely, the MDIG-silenced group was
associated with significantly reduced expression levels of WNT5A,
WNT3A and active β-catenin. These results suggested that MDIG
promoted the expression of the downstream signaling molecules, the
ABC transporters (ABCB1, ABCC1 and ABCG2), by activating the
WNT/β-catenin signaling pathway, which may in turn lead to DDP
resistance in lung adenocarcinoma cells.
In conclusion, in the present study, A549 cells and
DDP-resistant A549 cells (A549/DDP cells) were used as target
cells. Through silencing and overexpression of MDIG, it was
demonstrated that: i) DDP resistance in lung adenocarcinoma cells
may be associated with the upregulation of MDIG; ii) the expression
level of MDIG was positively associated with the degree of DDP
resistance; and iii) MDIG promoted the expression of downstream
signaling efflux ABC transporters (ABCB1, ABCC1 and ABCG2) by
activating the WNT/β-catenin signaling pathway, which may lead to
DDP resistance in lung adenocarcinoma cells. Although a lack of
experiments investigating ABC transporter inhibitors was a
limitation of the present study, the present study has provided a
putative novel target for effective DDP-based targeted therapy in
the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81472194) and by the
Project of Liaoning Distinguished Professor [grant no. (2013) 204]
to HZ.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
QW, FG, HZ, YC, JD, XZ, DS and HZ contributed to the
experimental design. QW participated in the writing of manuscript
and data interpretation. QW and FG conducted the transfection
experiment. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de Groot P and Munden RF: Lung cancer
epidemiology, risk factors, and prevention. Radiol Clin North Am.
50:863–876. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma D, Guo D, Li W and Zhao H: MDIG, a lung
cancer-associated gene, regulates cell cycle progression through
p27(KIP1). Tumour Biol. 36:6909–6917. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu HY, Wang XJ and Mao WM: Targeted
therapies in small cell lung cancer. Oncol Lett. 5:3–11.
2013.PubMed/NCBI
|
|
4
|
Guo Y, Chu M, Tan S, Zhao S, Liu H, Otieno
BO, Yang X, Xu C and Zhang Z: Chitosan-g-TPGS nanoparticles for
anticancer drug delivery and overcoming multidrug resistance. Mol
Pharm. 11:59–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Q, Yang Z, Nie Y, Shi Y and Fan D:
Multi-drug resistance in cancer chemotherapeutics: Mechanisms and
lab approaches. Cancer Lett. 347:159–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calatozzolo C, Gelati M, Ciusani E,
Sciacca FL, Pollo B, Cajola L, Marras C, Silvani A,
Vitellaro-Zuccarello L, Croci D, et al: Expression of drug
resistance proteins Pgp, MRP1, MRP3, MRP5 and GST-pi in human
glioma. J Neurooncol. 74:113–121. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui H, Zhang AJ, Chen M and Liu JJ: ABC
transporter inhibitors in reversing multidrug resistance to
chemotherapy. Curr Drug Targets. 16:1356–1371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mohammad IS, He W and Yin L: Understanding
of human ATP binding cassette superfamily and novel multidrug
resistance modulators to overcome MDR. Biomed Pharmacother.
100:335–348. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hung TH, Hsu SC, Cheng CY, Choo KB, Tseng
CP, Chen TC, Lan YW, Huang TT, Lai HC, Chen CM and Chong KY: WNT5A
regulates ABCB1 expression in multidrug-resistant cancer cells
through activation of the non-canonical PKA/β-catenin pathway.
Oncotarget. 5:12273–12290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen B, Zhang D, Kuai J, Cheng M, Fang X
and Li G: Upregulation of miR-199a/b contributes to cisplatin
resistance via WNT/β-catenin-ABCG2 signaling pathway in
ALDHA1+ colorectal cancer stem cells. Tumour Biol.
39:10104283177151552017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Lu Y, Yuan BZ, Castranova V, Shi
X, Stauffer JL, Demers LM and Chen F: The Human mineral
dust-induced gene, MDIG, is a cell growth regulating gene
associated with lung cancer. Oncogene. 24:4873–4882. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsuneoka M, Koda Y, Soejima M, Teye K and
Kimura H: A novel myc target gene, mina53, that is involved in cell
proliferation. J Biol Chem. 277:35450–35459. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eilbracht J, Kneissel S, Hofmann A and
Schmidt-Zachmann MS: Protein NO52-a constitutive nucleolar
component sharing high sequence homologies to protein NO66. Eur J
Cell Biol. 84:279–294. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu M, Sun J, Thakur C, Chen B, Lu Y, Zhao
H and Chen F: Paradoxical roles of mineral dust induced gene on
cell proliferation and migration/invasion. PLoS One. 9:e879982014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thakur C, Wolfarth M, Sun J, Zhang Y, Lu
Y, Battelli L, Porter DW and Chen F: Oncoprotein MDIG contributes
to silica-induced pulmonary fibrosis by altering balance between
Th17 and Treg T cells. Oncotarget. 6:3722–3736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen B, Yu M, Chang Q, Lu Y, Thakur C, Ma
D, Yi Z and Chen F: MDIG de-represses H19 large intergenic
non-coding RNA (lincRNA) by down-regulating H3K9me3 and
heterochromatin. Oncotarget. 4:1427–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu K, Tan MY, Jiang JT, Mu XY, Wang JR,
Zhou WJ, Wang X, Li MQ, He YY and Liu ZH: Cisplatin inhibits the
progression of bladder cancer by selectively depleting G-MDSCs: A
novel chemoimmunomodulating strategy. Clin Immunol. 193:60–69.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo K, Gu X, Liu J, Zeng G, Peng L, Huang
H, Jiang M, Yang P, Li M, Yang Y, et al: Inhibition of disheveled-2
resensitizes cisplatin-resistant lung cancer cells through
down-regulating WNT/β-catenin signaling. Exp Cell Res. 347:105–113.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Corrêa S, Binato R, Du Rocher B,
Castelo-Branco MT, Pizzatti L and Abdelhay E: WNT/β-catenin pathway
regulates ABCB1 transcription in chronic myeloid leukemia. BMC
Cancer. 12:3032012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou C: Lung cancer molecular epidemiology
in China: Recent trends. Transl Lung Cancer Res. 3:270–279.
2014.PubMed/NCBI
|
|
23
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: Recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thakur C, Lu Y, Sun J, Yu M, Chen B and
Chen F: Increased expression of MDIG predicts poorer survival of
the breast cancer patients. Gene. 535:218–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Teye K, Tsuneoka M, Arima N, Koda Y,
Nakamura Y, Ueta Y, Shirouzu K and Kimura H: Increased expression
of a Myc target gene Mina53 in human colon cancer. Am J Pathol.
164:205–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ogasawara S, Komuta M, Nakashima O, Akiba
J, Tsuneoka M and Yano H: Accelerated expression of a Myc target
gene Mina53 in aggressive hepatocellular carcinoma. Hepatol Res.
40:330–336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ishizaki H, Yano H, Tsuneoka M, Ogasawara
S, Akiba J, Nishida N, Kojiro S, Fukahori S, Moriya F, Matsuoka K
and Kojiro M: Overexpression of the myc target gene Mina53 in
advanced renal cell carcinoma. Pathol Int. 57:672–680. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fukahori S, Yano H, Tsuneoka M, Tanaka Y,
Yagi M, Kuwano M, Tajiri T, Taguchi T, Tsuneyoshi M and Kojiro M:
Immunohistochemical expressions of Cap43 and Mina53 proteins in
neuroblastoma. J Pediatr Surg. 42:1831–1840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huo Q, Ge C, Tian H, Sun J, Cui M, Li H,
Zhao F, Chen T, Xie H, Cui Y, et al: Dysfunction of IKZF1/MYC/MDIG
axis contributes to liver cancer progression through regulating
H3K9me3/p21 activity. Cell Death Dis. 8:e27662017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Robey RW, Shukla S, Finley EM, Oldham RK,
Barnett D, Ambudkar SV, Fojo T and Bates SE: Inhibition of
P-glycoprotein (ABCB1)- and multidrug resistance-associated protein
1 (ABCC1)-mediated transport by the orally administered inhibitor,
CBT-1((R)). Biochem Pharmacol. 75:1302–1312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi Z, Parmar S, Peng XX, Shen T, Robey
RW, Bates SE, Fu LW, Shao Y, Chen YM, Zang F and Chen ZS: The
epidermal growth factor tyrosine kinase inhibitor AG1478 and
erlotinib reverse ABCG2-mediated drug resistance. Oncol Rep.
21:483–489. 2009.PubMed/NCBI
|
|
32
|
Yoh K, Ishii G, Yokose T, Minegishi Y,
Tsuta K, Goto K, Nishiwaki Y, Kodama T, Suga M and Ochiai A: Breast
cancer resistance protein impacts clinical outcome in
platinum-based chemotherapy for advanced non-small cell lung
cancer. Clin Cancer Res. 10:1691–1697. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li A, Song J, Lai Q, Liu B, Wang H, Xu Y,
Feng X, Sun X and Du Z: Hypermethylation of ATP-binding cassette B1
(ABCB1) multidrug resistance 1 (MDR1) is associated with cisplatin
resistance in the A549 lung adenocarcinoma cell line. Int J Exp
Pathol. 97:412–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pei K, Zhu JJ, Wang CE, Xie QL and Guo JY:
MicroRNA-185-5p modulates chemosensitivity of human non-small cell
lung cancer to cisplatin via targeting ABCC1. Eur Rev Med Pharmacol
Sci. 20:4697–4704. 2016.PubMed/NCBI
|
|
35
|
Huang L, Jin Y, Feng S, Zou Y, Xu S, Qiu
S, Li L and Zheng J: Role of WNT/β-catenin, WNT/c-Jun N-terminal
kinase and WNT/Ca2+ pathways in cisplatin-induced
chemoresistance in ovarian cancer. Exp Ther Med. 12:3851–3858.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Z, Zhao L and Wang Q: Overexpression of
long non-coding RNA HOTTIP increases chemoresistance of
osteosarcoma cell by activating the WNT/β-catenin pathway. Am J
Transl Res. 8:2385–2393. 2016.PubMed/NCBI
|