Introduction
Specificity protein 1 (Sp1) was identified and
cloned by Kadonaga et al (1)
in 1987, and was one of the earliest transcription factors to be
identified. Sp1 belongs to the Sp1/Krüppel-like factor
transcription factor family of sequence-specific DNA binding
proteins (2). Sp1 consists of four
activated functional areas (A, B, C and D). The functional domains
A and B are rich in glutumamide. Domain C is a highly-charged amino
acid enriched area with three zinc fingers at the end of the
hydroxyl group. At the same time, the formation of Sp1 tetramers
can attract more polymers that bind to DNA, and produces positive
feedback regulation of the transcription process (3). Sp1 performs an important regulatory
role for a variety of housekeeping genes, including nucleic acid
metabolism related genes and oxidative phosphorylation related
genes, including mitogen-activated protein kinase 8 and EPH
receptor B2 (4,5). Meanwhile, if the promoter of a gene
lacks the expression of the TATA box, Sp1 can prevent DNA
methylation and maintain gene transcription at the activation state
(3).
It has been proven that Sp1 can upregulate the
expression of Bcl-2 (6), survivin
(7), and TGF-β (8). Studies have shown that Sp1 can form a
compound with the Smad protein to induce the transcription of and
overexpression of Smad7, and negatively regulates the TGF-β
pathway, thus affecting cell growth, differentiation and apoptosis
(9). The abnormal activation of Sp1
can also upregulate the expression of tumor-related factors and
angiogenic factors that provide a good microenvironment for tumor
growth, and promote tumor proliferation, metastasis and
angiogenesis in gastric and pancreatic (10). Sp1 is recruited by the promoter of
vascular endothelial growth factor for its upregulated expression,
promoting vascular endothelial proliferation, angiogenesis and
increasing vascular permeability for tumor growth and metastasis
(11). Increased Sp1 expression has
been found to be positively associated with a worse prognosis for
patients with gastric carcinoma (12).
Reportedly, the expression of Sp1 is significantly
increased in esophageal carcinoma, colorectal cancer, pancreatic
cancer and thyroid cancer (13–16).
Hosoi et al (17) found that
the upregulation of DNA dependent protein kinase Ku70 and Ku80 is
significantly affected by increased expression of Sp1 in small
bowel cancer. It was also found that Sp1 could upregulate the
expression of insulin-like growth factor binding protein and
promote the proliferation of MCF-7 cells (18,19). In
prostate cancer DU145 and PC3cell lines, Sp1 knockdown results in a
high residual glucose level and a low lactic acid level, suggesting
that Sp1 could promote cell metabolism in prostate cancer (20). Liu et al (21) found that decreased expression of Sp1
in prostate cell carcinoma reduces cell proliferation, which
indicates that Sp1 plays an important role in the development of
prostate cancer. Beaver et al (22) found that the knockdown of Sp1 in
mouse embryos, delays the development, causes mutations and may
even result in the death of the embryo. Subsequent studies have
found that Sp1 plays a key role in the development of the mouse
nervous system and male germ cells (23,24). In
the present study, a meta-analysis and a bioinformatics analysis
was performed to provide evidence for clarifying the relationship
between Sp1 expression and clinicopathological factors in gastric
cancer.
Materials and methods
Published study search and selection
criteria
Articles included in the analysis were searched for
on PubMed and China Academic Journal on 8th June 2018 using the key
words: Sp1 AND (gastric OR stomach) AND (cancer OR carcinoma OR
tumor OR adenocarcinoma). The inclusion criteria for studies
included: i) Published Chinese and English literature limited to
patients with gastric cancer; ii) the expression of Sp1 detected
through immunohistochemistry in patients with gastric cancer and
iii) all patients with gastric cancer did not receive radiotherapy
or chemotherapy before surgery. The exclusion criteria included: i)
Abstracts, case reports, reviews and meeting notes; ii) studies
with a small sample size (n<50); iii) repeat publications or
repeat data.
Data extraction and quality
assessment
As shown in Table I,
the information from all eligible publications was extracted by two
reviewers, and included the authors, year of publication, patient
country, antibody company, number of cases and controls, risks for
cancer, and follow-up outcome. The qualities of the studies were
independently assessed by the reviewers according to the
Newcastle-Ottawa Scale (NOS; http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm).
The method consists of sample selection, comparability and
ascertainment of outcome as the number of samples, comparability
and results will affect the accuracy of the statistical results.
Data was extracted from the Kaplan-Meier survival curves using
Engauge Digitizer software (version 4.1;
markummitchell.github.io/engauge-digitizer) and then their hazard
ratios (HR) and corresponding 95% CI were calculated. No
disagreements on the studies to be included were found to exist
between the two reviewers. Publication bias was evaluated using a
funnel plot. Begg's and Egger's tests were used to assess funnel
plot asymmetry.
 | Table I.Characteristics and quality score of
the studies. |
Table I.
Characteristics and quality score of
the studies.
| Author, year | Country | Ethnicity | Antibody
supplier | Cases | Ctr | Risk of cancer | Follow-up
outcome | Quality | (Refs.) |
|---|
| Jiang et al,
2015 | China | Asian | Santa Cruz
Biotechnology, Inc. | 227 | – | – | Negative | 8 | (34) |
| Jiang et al,
2009 | China | Asian | Santa Cruz
Biotechnology, Inc. | 78 | 20 | Increased | – | 8 | (33) |
| Zhu et al,
2015 | China | Asian | Santa Cruz
Biotechnology, Inc. | 95 | 20 | Increased | – | 7 | (32) |
| A et al,
2014 | China | Asian | Cusabio Technology
LLC | 66 | 66 | Increased | – | 8 | (31) |
| Cui et al,
2014 | China | Asian | Santa Cruz
Biotechnology, Inc. | 105 | – | – | – | 8 | (35) |
| Zhang et al,
2011 | China | Asian | Bioss, Inc. | 76 | 30 | Increased | – | 8 | (30) |
| Zhang et al,
2014 | China | Asian | Shanghai Long
Island Biotec. Co., Ltd. | 39 | 39 | Increased | – | 8 | (29) |
| Zhang et al,
2015 | China | Asian | Santa Cruz
Biotechnology, Inc. | 64 | 40 | Increased | Negative | 8 | (28) |
| Hua et al,
2013 | China | Asian | Santa Cruz
Biotechnology, Inc. | 75 | 14 | Increased | – | 8 | (27) |
| Wang et al,
2003 | China | Asian | Santa Cruz
Biotechnology, Inc. | 86 | 57 | Increased | Negative | 8 | (26) |
| Yao et al,
2004 | USA | America | Santa Cruz
Biotechnology, Inc. | 86 | – | – | – | 8 | (12) |
| Wei et al,
2009 | China | Asian | Santa Cruz
Biotechnology, Inc. | 68 | – | – | Negative | 8 | (25) |
| Hun et al,
2013 | Japan | Asian | Santa Cruz
Biotechnology, Inc. | 268 | – | – | – | 8 | (36) |
Bioinformatics analysis
Sp1 gene expression level was analyzed using
Oncomine (www.oncomine.org), the largest oncogene
chip database and integrated data mining platform. There are two
analysis methods. Multiple analysis (fold change), where the
expression ratio of each gene under two conditions was calculated,
generally in the range of 0.5–2.0, and there was no significant
differential expression of the gene. T test (P-value), where the
gene whose T statistic exceeds a specific value is detected as an
abnormality. Whether the analysis is statistically significant by
calculating the confidence of the difference. The differences in
Sp1 mRNA level were compared between 80 gastric cancer tissues and
80 normal tissues. All data were log-transformed, median centered
for each array, and standard deviation was normalized to a single
value for each array. Finally, the Kaplan-Meier plots were used to
analyze the prognostic significance of Sp1 mRNA expression. The
expression level of Sp1 was divided into high expression group and
low expression group according to the best cut off value provided
on the Kaplan-Meier plotter.
Statistics analysis
Revman (version 5.3; www.cochrane.es/Download/Files/revman.htm) was
used for data analysis. Odds ratios and 95% CI were used to
estimate the expression of Sp1 based on the clinicopathological
parameters of patients with gastric cancer. First, the
heterogeneity of the original documents obtained from PubMed and
CNKI was determined. If heterogeneity was not significant, the
fixed effect model (Mantel-Haenszel method) was applied. If not,
the random effect model (Der Simonian and Laird method) was
applied. Heterogeneity effect was quantified using the
I2 test. According to the cutoff values, heterogeneity
was subdivided into low, moderate and high degrees of heterogeneity
according to the cut-off values of 25, 50 and 75%, respectively.
Publication bias was evaluated using funnel plot and quantified
using Begg's test and Egger's test to assess funnel plot asymmetry.
Meta-analyses were performed with Revman software 5.3 was analyzed
using SPSS software (version 10.0; SPSS, Inc.) and the Student's
t-test. Two-sided P<0.05 was considered to indicate a
statistically significant difference.
Results
Literature search results
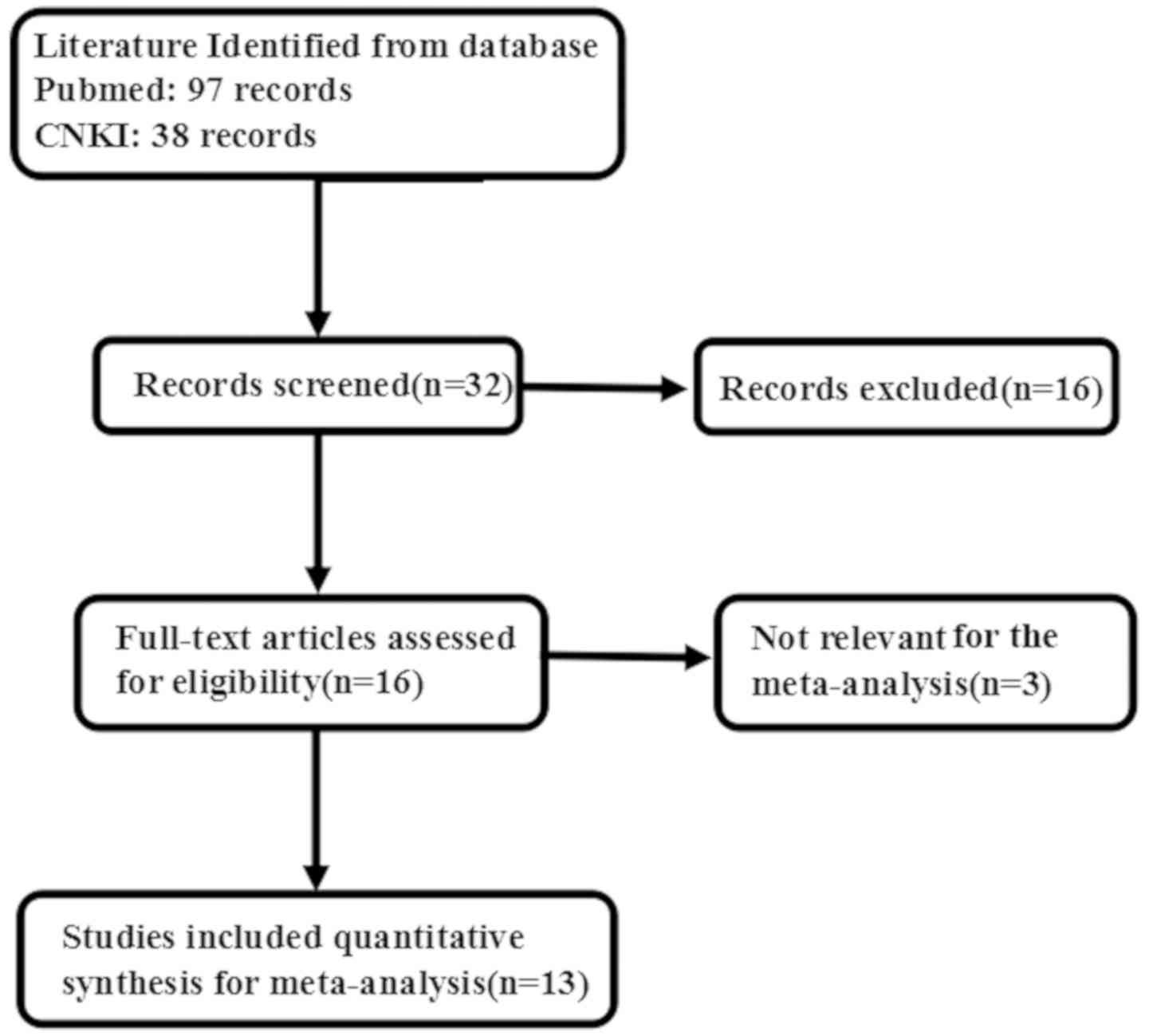
As shown in Fig. 1,
duplicate studies, those that had included animal experiments and
reviews were excluded by reading the abstracts. A total of 135
articles were initially retrieved, but only 13 articles were found
to investigate the relationship between Sp1 expression and
clinicopathological or prognostic indicators of gastric cancer. The
exclusion criteria were as follows: i) Studies for which only the
abstract available and review and conference proceedings; ii)
duplicated studies; iii) studies containing western blot, RT-qPCR,
cDNA microarray, or transcriptomic sequencing for maspin
expression; and iv) insufficient data (Fig. 1).
Basic characteristics of included
articles
There were 13 articles on the relationship between
Sp1 expression and clinicopathological characteristics of gastric
cancer (12,25–36).
There were 8 articles that included results of normal gastric
tissue (26–33). Finally, there were 4 articles that
discussed the prognostic significance of Sp1 expression and its
relationship with gastric cancer (25–27,34).
Forest plot of odds ratio (OR) for the
association between Sp1 expression and clinicopathological
parameters of gastric cancer
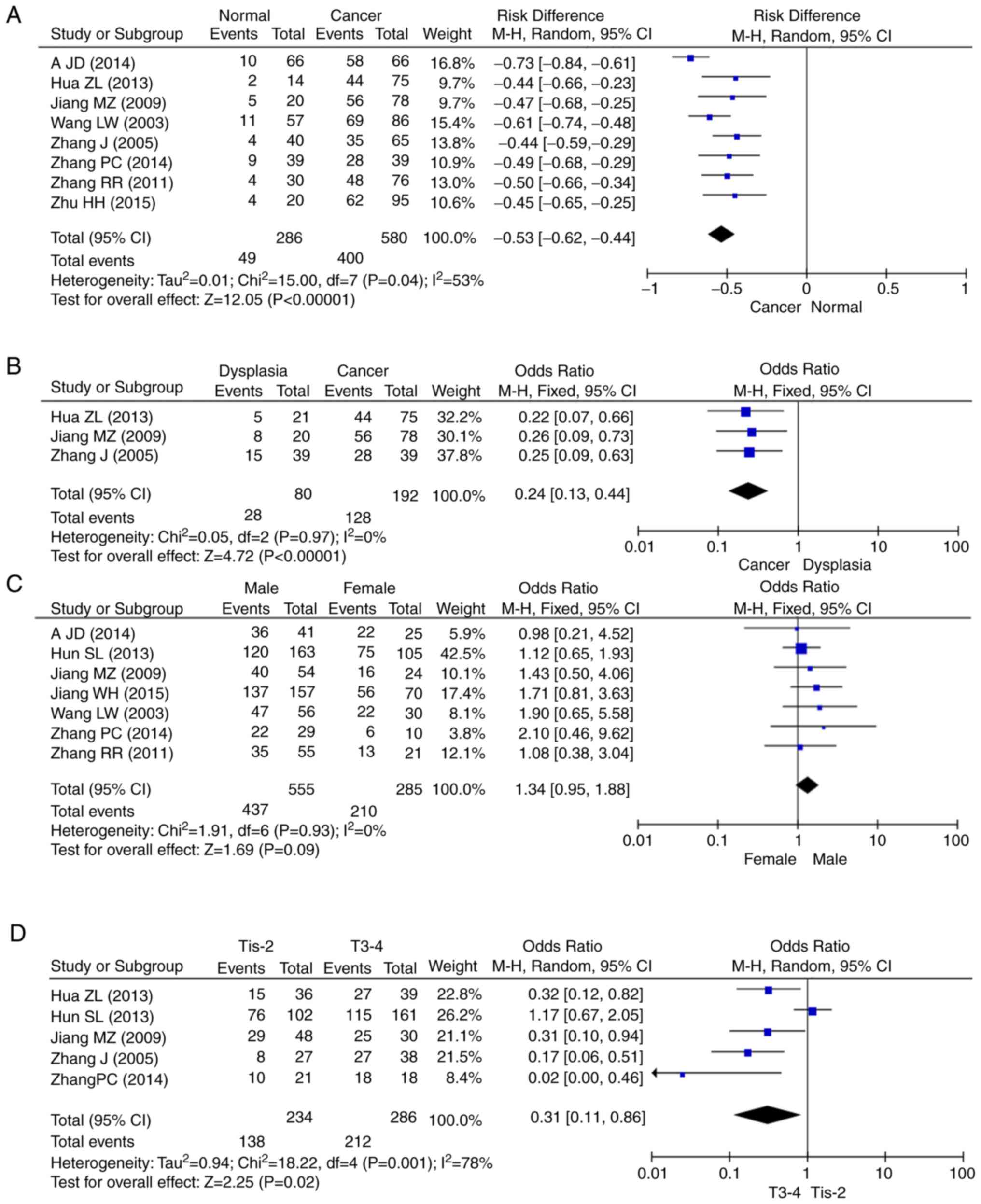
A total of 8 articles included data on 580 patients
with gastric cancer and 286 healthy controls. The overall results
showed that the expression of Sp1 was upregulated in gastric cancer
and dysplasia (Fig. 2A and B),
compared with that of normal mucosa tissue. Forest plots of OR for
the association of Sp1 expression were divided based on sex
(Fig. 2C), depth of invasion
(Fig. 2D), lymph node metastasis
(Fig. 2E), TNM staging (Fig. 2F) and Lauren's classification
(Fig. 2G). The pathological data
available in each article varied. Articles were excluded if a
particular clinicopathological characteristic was missing.
Therefore, the number of articles in the forest map differed to the
initial number of articles. The survival data are showed in
Fig. 2H, and based on the 4
aforementioned datasets. The relationship between Sp1 expression
and decreased survival rate in gastric cancer patients was
investigated and found to be significant (HR, 0.32; 95% CI,
0.22–0.46; P<0.0001).
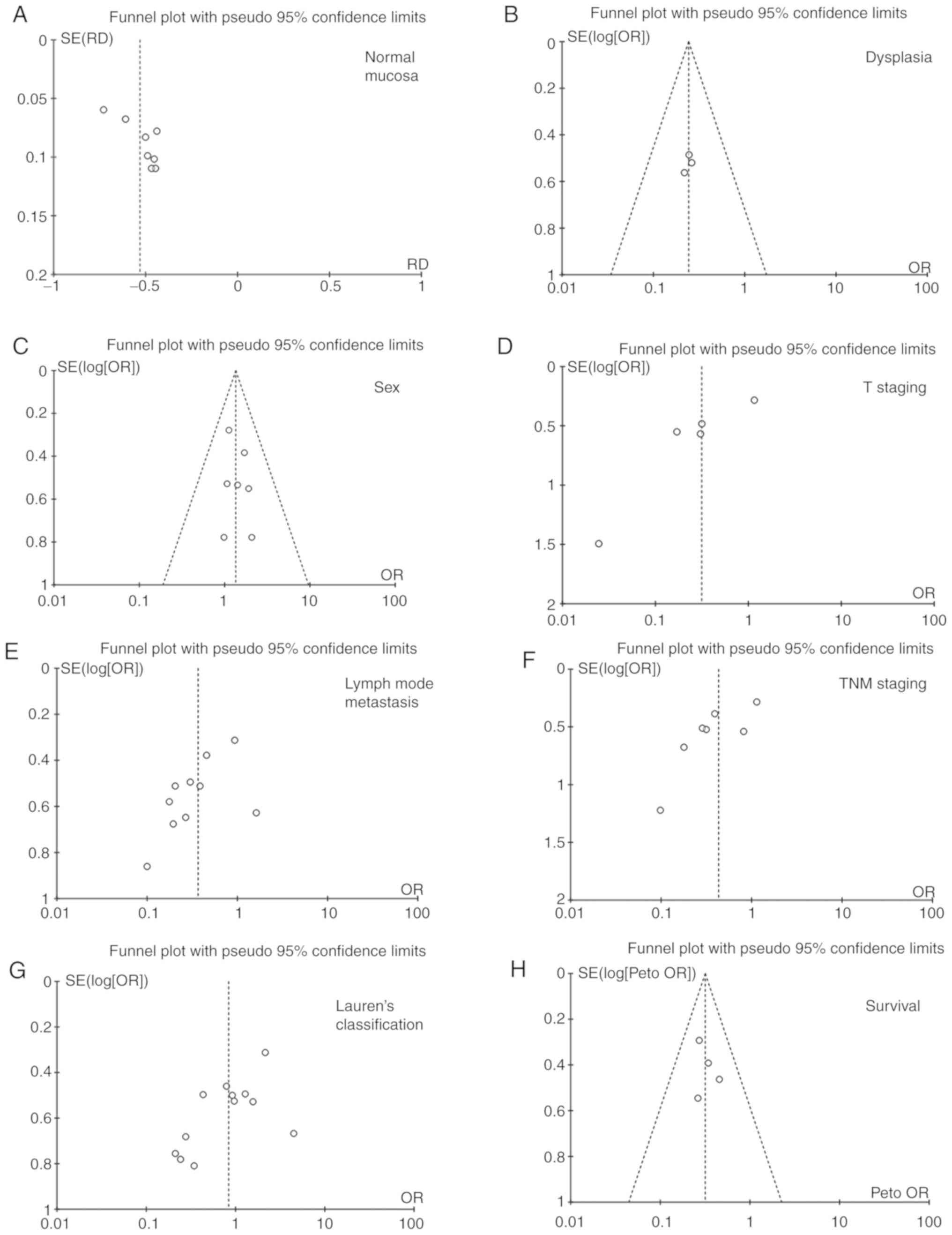
Publication bias
Publication bias can be quantitatively determined
using funnel diagrams, as shown in Fig.
3. Individual studies were removed from the pooled analysis,
and then used sensitivity analysis to assess the impact of the
individual study on aggregated results. According to Egger's test,
this meta-analysis had no apparent publication bias.
The relationship between Sp1
expression and bioinformatics features of gastric cancer
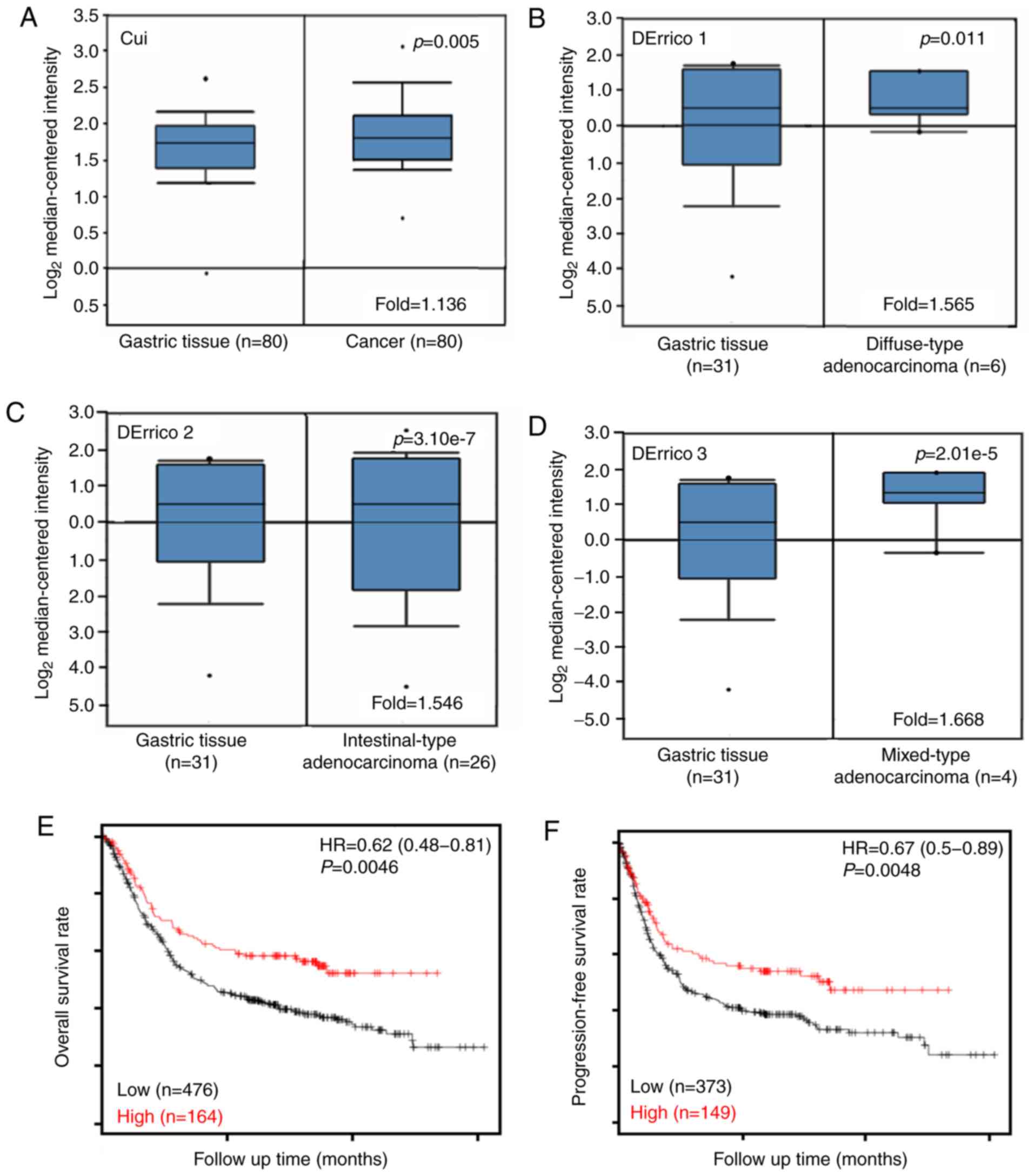
Cui's and D'Errico's datasets showed that Sp1 mRNA
expression was higher in gastric cancer tissue compared with that
in normal tissues based on bioinformatics features (Fig. 4A, P<0.05), even when stratified as
diffuse, intestinal and mixed-type carcinoma (Fig. 4B-D, P<0.05). According to
Kaplan-Meier plots (Fig. 4E and F;
Table II), higher Sp1 mRNA
expression was negatively associated with the overall and
progression-free survival rates of all patients with gastric
cancer. In addition, in patients who received surgery alone or
5-FU-based chemotherapy, those with T2, N0, N1-3, N1 and N2, M0,
intestinal-type moderately-differentiated, or Her2-carcinoma were
also significantly associated with overall and progression-free
survival (P<0.05). Males and T3 patients with gastric cancer
with high Sp1 expression showed shorter overall survival times
compared with those with low expression (P<0.05), while only it
is significantly associated with overall survival (P<0.05). A
similar result was obtained for the progression-free survival rates
in patients with T4 cancer (P<0.05).
 | Table II.Prognostic significance of Sp1 mRNA
in gastric cancer. |
Table II.
Prognostic significance of Sp1 mRNA
in gastric cancer.
|
| Overall
survival | Progression-free
survival |
|---|
|
|
|
|
|---|
| Clinicopathological
features | Hazard ratio | P-value | Hazard ratio | P-value |
|---|
| Sex |
|
|
|
|
|
Female | 0.66
(0.39–1.11) | 0.11 | 0.66
(0.40–1.09) | 0.10 |
|
Male | 0.63
(0.44–0.89) | 0.01 | 0.57
(0.21–1.51) | 0.25 |
| TNM staging |
|
|
|
|
| 1 | 0.19
(0.06–0.57) | <0.01 | 0.17
(0.06–0.53) | <0.01 |
| 2 | 0.57
(0.26–1.24) | 0.15 | 0.47
(0.21–1.06) | 0.06 |
| 3 | 0.60
(0.37–0.97) | 0.04 | 0.66
(0.39–1.12) | 0.12 |
| 4 | 0.73
(0.43–1.25) | 0.25 | 0.74
(0.50–1.08) | 0.12 |
| T |
|
|
|
|
| 2 | 0.52
(0.37–0.87) | 0.01 | 0.61
(0.38–1.00) | 0.05 |
| 3 | 1.29
(0.87–1.89) | 0.20 | 1.31
(0.90–1.91) | 0.16 |
| 4 | 0.49
(0.20–1.20) | 0.11 | 0.44
(0.19–1.01) | 0.05 |
| N |
|
|
|
|
| 0 | 0.40
(0.16–0.97) | 0.04 | 0.39
(0.16–0.94) | 0.03 |
|
1–3 | 0.62
(0.45–0.85) | <0.01 | 0.65
(0.48–0.88) | <0.01 |
| 1 | 0.57
(0.35–0.94) | 0.02 | 0.56
(0.34–0.92) | 0.02 |
| 2 | 0.54
(0.30–0.97) | 0.04 | 0.56
(0.32–1.00) | 0.05 |
| 3 | 1.60
(0.84–3.04) | 0.15 | 0.70
(0.40–1.22) | 0.21 |
| M |
|
|
|
|
| 0 | 0.61
(0.43–0.85) | <0.01 | 0.64
(0.46–0.88) | <0.01 |
| 1 | 0.61
(0.32–1.20) | 0.15 | 0.62
(0.34–1.11) | 0.11 |
| Perforation
complications |
|
|
|
|
| − | 1.27
(0.85–1.90) | 0.25 | 0.79
(0.53–1.18) | 0.25 |
| Treatment |
|
|
|
|
| Surgery
alone | 0.67
(0.48–0.94) | 0.02 | 0.69
(0.49–0.97) | 0.033 |
|
5-fluorouracil-based
adjuvant | 4.36
(1.64–11.6) | <0.01 | 3.22
(1.25–8.24) | 0.01 |
| Other
adjuvant | 1.58
(0.63–3.96) | 0.3 | 0.66
(0.28–1.54) | 0.34 |
|
Differentiation |
|
|
|
|
|
Moderately-differentiated | 0.48
(0.24–0.94) | 0.03 | 0.45
(0.24–0.87) | 0.02 |
|
Poorly-differentiated | 1.63
(0.98–2.70) | 0.06 | 1.54
(0.95–2.50) | 0.08 |
| Lauren's
classification |
|
|
|
|
|
Intestinal-type | 0.50
(0.33–0.77) | <0.01 | 0.52
(0.36–0.74) | <0.01 |
|
Diffuse-type | 1.43
(0.99–2.06) | 0.05 | 1.36
(0.94–1.96) | 0.10 |
|
Mixed-type | 2.62
(0.57–12.02) | 0.20 | 2.14
(0.59–7.83) | 0.24 |
| Her2
positivity |
|
|
|
|
| − | 0.62
(0.47–0.81) | <0.01 | 0.69
(0.49–0.98) | 0.04 |
| + | 0.65
(0.41–1.02) | 0.06 | 0.60
(0.37–0.98) | 0.04 |
Discussion
Sp1 has a group of zinc-finger proteins that are
important transcriptional components in eukaryotic cells, ranging
from yeast cells to vertebrate cells (37). It was found to be overexpressed in
gastric cancer and associated with a poor outcome (38). Peng et al (39) found that there is a Sp1 binding site
in the promoter region of the dickkopf WNT signaling pathway
inhibitor 1 (DKK1) gene and that Sp1 overexpression could increase
the activity of the DKK1 promoter. Transcriptional enhancer
activator domain 1 was able to increase the expression of Sp1 by
binding to its promoter in colorectal cancer cells (40). Shi et al (41) found that hepatitis B X-interacting
protein may activate the fibroblast growth factor 4 promoter via
Sp1, which then promotes the migration of breast cancer cells. The
transcription activity of Sp1 is enhanced through direct
phosphorylation of threonine by p42/p44 mitogen-activated protein
kinase (42,43). Jiang et al (34) reported that the co-expression of
erb-b2 receptor tyrosine kinase 2 and Sp1 are independent
prognostic factors of patients with gastric cancer. In order to
demonstrate the association with Sp1 expression and its
clinicopathological significance, 13 studies were analyzed that met
specific inclusion criteria and were moderated to ensure high
quality according to NOS scores.
Previous studies show that abnormal Sp1 activation
may improve the growth, metastasis and dedifferentiation of
pancreatic and breast cancers (44–48). In
the present study, the expression of Sp1 at mRNA and protein level
to be upregulated in gastric cancer tissue, compared with that of
normal gastric mucosa, suggesting that Sp1 expression contributes
to gastric carcinogenesis. Sp1 expression was also found to be
positively associated with depth of invasion, lymph node metastasis
and TNM stage of gastric cancer, and the same was true for Sp1 mRNA
expression, which indicates that aberrant Sp1 expression can be
employed to indicate the pathological behavior of gastric cancers.
This result shows that Sp1 mRNA gene expression levels may be used
to predict corresponding protein levels.
Reportedly, Sp1 expression is positively related to
the poor prognosis of patients with ovarian serous adenocarcinoma
and colorectal cancer (49,50). Sp1 upreguation has also been shown to
indicate a worse prognosis of breast cancer and hepatocellular
carcinoma, as an independent factor (51,52).
Chen et al (53) reported
that the overall prognosis of patients with gastric cancer with
high Sp1 levels is significantly poorer compared with that of those
with low Sp1 levels. Our meta-analysis shows that Sp1
overexpression is associated with poor prognosis of human gastric
carcinoma. Additionally, the results show that Sp1 mRNA expression
is also positively associated with overall and progress-free
survival rates of patients with gastric cancer.
Some limitations that exist in our meta-analysis.
First, potential publication bias stems from the fact that
published results were predominantly positive. Second, the patients
included in the studies were only from Asia and America. Different
levels of medical development in different areas may also influence
the results as different experimental methods may have been used to
detect Sp1 expression. Third, survival data were extracted from
survival curves, which may affect the results. Fourth, small sample
size may influence associations in some articles.
In conclusion, Sp1 protein expression is upregulated
in gastric carcinogenesis. Sp1 is positively associated with the
depth of invasion and TNM stage of gastric cancer. Sp1 protein
expression can be employed as a good potential marker for the
prognosis of patients with gastric cancer.
Acknowledgements
No applicable.
Funding
No funding was received.
Availability data and materials
The datasets generated and/or analyzed during the
current study are available in the Oncomine database (www.oncomine.org) and Kaplan-Meier plotter (kmplot.com).
Authors' contributions
SS and ZGZ extracted all the relevant information
from the eligible publications, independently assessed the quality
of the studies and wrote the manuscript. Both authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kadonaga JT, Carner KR, Masiarz FR and
Tjian R: Isolation of cDNA encoding transcription factor Sp1 and
functional analysis of the DNA binding domain. Cell. 51:1079–1090.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roder K, Kim KH and Sul HS: Induction of
murine H-rev107 gene expression by growth arrest and histone
acetylation: Involvement of an Sp1/Sp3-binding GC-box. Biochem
Biophys Res Commun. 294:63–70. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Samson SL and Wong NC: Role of Sp1 in
insulin regulation of gene expression. J Mol Endocrinol.
29:265–279. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Safe S and Abdelrahim M: Sp transcription
factor family and its role in cancer. Eur J Cancer. 41:2438–2448.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zaid A, Li R, Luciakova K, Barath P, Nery
S and Nelson BD: On the role of the general transcription factor
Sp1 in the activation and repression of diverse mammalian oxidative
phosphorylation genes. J Bioenerg Biomembr. 31:129–135. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duan H, Heckman CA and Linda MB: Histone
deacetylase inhibitors down-regulate bcl-2 expression and induce
apoptosis in t(14;18) lymphomas. Mol Cell Biol. 25:1608–1619. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu J, Ling X, Pan D, Apontes P, Song L,
Liang P, Altieri DC, Beerman T and Li F: Molecular mechanism of
inhibition of survivin transcription by the GC-rich
sequence-selective DNA binding antitumor agent, hedamycin: Evidence
of survivin down-regulation associated with drug sensitivity. J
Biol Chem. 280:9745–9751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martin M, Le J and Delanlan S: TGF-beta1
and radiation fibrosis: A master switch and a specific therapeutic
target? Int J Rad Oncol Biol Phys. 47:277–290. 2000. View Article : Google Scholar
|
|
9
|
Jungert K, Buck A, Buchholz M, Wagner M,
Adler G, Gress TM and Ellenrieder V: Smad-Sp1 complexes mediate
TGFbeta-induced early transcription of oncogenic Smad7 in
pancreatic cancer cells. Carcinogenesis. 27:2392–2401. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S, Wang W, Wesley RA and Danner RL: A
Sp1 binding site of the tumor necrosis factor alpha promoter
functions as a nitric oxide response element. J Biol Chem.
274:33190–33193. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chae YS, Kim JG, Sohn SK, Cho YY, Moon JH,
Bae HI, Park JY, Lee MH, Lee HC, Chung HY and Yu W: Investigation
of vascular endothelial growth factor gene polymorphisms and its
association with clinicopathologic characteristics in gastric
cancer. Oncology. 71:266–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao JC, Wang L, Wei D, Gong W, Hassan M,
Wu TT, Mansfield P, Ajani J and Xie K: Association between
expression of transcription factor Sp1 and increased vascular
endothelial growth factor expression, advanced stage, and poor
survival in patients with resected gastric cancer. Clin Cancer Res.
15:4109–4117. 2004. View Article : Google Scholar
|
|
13
|
Maurer GD, Leupold JH, Schewe DM, Biller
T, Kates RE, Hornung HM, Lau-Werner U, Post S and Allgayer H:
Analysis of specific transcriptional regulators as early predictors
of independent prognostic relevance in resected colorectal cancer.
Clinic Cancer Res. 13:1123–1132. 2007. View Article : Google Scholar
|
|
14
|
Chen QN, Yuan P and Fan YZ: Expression of
transcription factor Sp1 in pancreatic cancer and its relationship
with prognosis. J Tongji Univ. 30:5–8. 2009.
|
|
15
|
Zhang W, Kadam S, Emerson BM and Bieker
JJ: Site-specific acetylation by p300 or CREB binding protein
regulates erythroid Krüppel-like factor transcriptional activity
via its interaction with the SWI-SNF complex. Mol Cell Biol.
21:2413–2422. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiefari E, Brunetti A, Arturi F, Bidart
JM, Russo D, Schlumberger M and Filetti S: Increased expression of
AP2 and Sp1 transcription factors in human thyroid tumors: A role
in NIS expression regulation? BMC Cancer. 2:352002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hosoi Y, Watanabe T, Nakagawa K, Matsumoto
Y, Enomoto A, Morita A, Nagawa H and Suzuki N: Up-regulation of
DNA-dependent protein kinase activity and Sp1 in colorectal cancer.
Int J Oncol. 25:461–468. 2004.PubMed/NCBI
|
|
18
|
Maor S, Mayer D, Yarden RI, Lee AV,
Sarfstein R, Werner H and Papa MZ: Estrogen receptor regulates
insulin-like growth factor-I receptor gene expression in breast
tumor cells: Involvement of transcription factor Sp1. J Endocrinol.
191:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han WD, Mu YM, Lu XC, Xu ZM, Li XJ, Yu L,
Song HJ, Li M, Lu JM, Zhao YL and Pan CY: Up-regulation of LRP16
mRNA by 17beta-estradiol through activation of estrogen receptor
alpha (ERalpha), but not ERbeta, and promotion of human breast
cancer MCF-7 cell proliferation: A preliminary report. Endocr Relat
Cancer. 10:217–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marin M, Karis A, Visser P, Grosveld F and
Philipsen S: Transcription factor Sp1 is essential for early
embryonic development but dispensable for cell growth and
differentiation. Cell. 89:619–628. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu DC, Liang Q, Tao T, Huang YQ, Xu B,
Chen NG, Zhang ZG, Dong Y, Han CH and Chen M: Sp1 promotes cell
metabolism and proliferation through PKM2 pathway in prostate
cancer. J Southeast Univ (Med Sci Edi). 10:337–341. 2015.
|
|
22
|
Beaver LM, Buchanan A, Sokolowski EI,
Riscoe AN, Wong CP, Chang JH, Löhr CV, Williams DE, Dashwood RH and
Ho E: Transcriptome analysis reveals a dynamic and differential
transcriptional response to sulforaphane in normal and prostate
cancer cells and suggests a role for Sp1 in chemoprevention. Mol
Nutr Food Res. 58:2001–2013. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hao C and Meng A: Sp1-like transcription
factors are regulators of embryonic development in vertebrates. Dev
Growth Differ. 47:201–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Homas K, Wu J, Sung DY, Thompson W, Powell
M, McCarrey J, Gibbs R and Walker W: SP1 transcription factors in
male germ cell development and differentiation. Mol Cell
Endocrinol. 270:1–7. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei YZ, Li CF and Xue YW: Expression of
transcription factor SP1, vascular endothelial growth factor and
CD34 in serosa-infiltrating gastric cancer and their relationship
with biological behavior and prognosis. Zhonghua Wei Chang Wai Ke
Za Zhi. 12:145–149. 2009.(In Chinese). PubMed/NCBI
|
|
26
|
Wang LW, Wei D, Huang S, Peng Z, Le X, Wu
TT, Yao J, Ajani J and Xie K: Transcription factor Sp1 expression
is a significant predictor of survival in human gastric cancer.
Clin Cancer Res. 9:6371–6380. 2003.PubMed/NCBI
|
|
27
|
Hua ZL, Zhu YC, Guo GP, Ling RB, Zhou Q
and Shi AW: Expression of transcription factors in gastric cancer
and its relationship with clinical pathology. Med Imaging.
9:162–163. 2013.
|
|
28
|
Zhang J, Zhu ZG, Ji J, Yuan F, Yu YY, Liu
BY and Lin YZ: Transcription factor Sp1 expression in gastric
cancer and its relationship to long-term prognosis. World J
Gastroenterol. 11:2213–2217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang PC, Liang L, Zhang DK, Yao BH, Song
L, Li Y and Zhao JS: Expression and significance of transcription
factor Sp1 and Stat-3 in gastric carcinoma. Chin J Gen Surg.
23:1714–1716. 2014.
|
|
30
|
Zhang RR: Expression of correlation
relationship and significance of Sp1, Notch1 and KLF4 in gastric
cancer. Msater Thesis, Shanxi Med Univ China. 2011.
|
|
31
|
A JD: The clinical study on expression and
significance of Sp1 protein in gastric carcinoma. Master Thesis,
Qinghai Univ China. 2011.
|
|
32
|
Zhu HH, Wu XM and Ye CJ: Expression of
matrix metalloproteinase-11 and the transcription factor Sp1 in
gastric cancer. J Qinghai Med College. 36:49–53. 2015.
|
|
33
|
Jiang MZ: Study about the expression and
significance of KLF5, Sp1 and CyclinD1 in carcinoma of stomach.
Kunming. Msater Thesis, Med Univ China. 2009.
|
|
34
|
Jiang W, Jin Z, Zhou F, Cui J and Wang L
and Wang L: High co-expression of Sp1 and HER-2 is correlated with
poor prognosis of gastric cancer patients. Surg Oncol. 24:220–225.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cui JF, Zhao CY, Cao LY, Wu WN, Li YH,
Wang Y, Xue LY and Zhang XG: Comparison of KLF4, SP1, and Cyclin D1
expressions between ad-enocarcinanoma of the esophagogastric
junction and distal gastric adenocarcinoma. Chin J Clin Oncol.
41:108–112. 2014.
|
|
36
|
Lee HS, Park CK, Oh E, Erkin ÖC, Jung HS,
Cho MH, Kwon MJ, Chae SW, Kim SH, Wang LH, et al: Low SP1
expression differentially affects intestinal-type compared with
diffuse-type gastric adenocarcinoma. PLoS One. 8:e555222013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumar AP and Butler AP: Serum responsive
gene expression mediated by Sp1. Biochem Biophys Res Commun.
252:517–523. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen F, Zhang F, Rao J and Studzinski GP:
Ectopic expression of truncated Sp1 transcription factor prolongs
the S phase and reduces the growth rate. Anticancer Res.
20:661–667. 2000.PubMed/NCBI
|
|
39
|
Peng H, Li Y, Liu Y, Zhang J, Chen K,
Huang A and Tang H: HBx and SP1 upregulate DKK1 expression. Acta
Biochim Pol. 64:35–39. 2017.PubMed/NCBI
|
|
40
|
Yu MH and Zhang W: TEAD1 enhances
proliferation via activating SP1 in colorectal cancer. Biomed
Pharmacother. 83:496–501. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shi H, Li Y, Feng G, Li L, Fang R, Wang Z,
Qu J, Ding P, Zhang X and Ye L: The oncoprotein HBXIP up-regulates
FGF4 through activating transcriptional factor Sp1 to promote the
migration of breast cancer cells. Biochem Biophys Res Commun.
471:89–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Milanini-Mongiat J, Pouysségur J and Pagès
G: Identification of two Sp1 phosphorylation sites for p42/p44
mitogen-activated protein kinases. Their implication in vascular
endothelial growth factor gene transcription. J Biol Chem.
277:20631–20639. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Merchant JL, Du M and Todisco A: Sp1
phosphorylation by Erk 2 stimulates DNA binding. Biochem Biophys
Res Commun. 254:454–461. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wei D, Wang L, He Y, Xiong HQ, Abbruzzese
JL and Xie K: Celecoxib inhibits vascular endothelial growth factor
expression in and reduces angiogenesis and metastasis of human
pancreatic cancer via suppression of Sp1 transcription factor
activity. Cancer Res. 64:2030–2038. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao S, Venkatasubbarao K, Li S and
Freeman JW: Requirement of a specific Sp1 site for histone
deacetylase-mediated repression of transforming growth factor beta
Type II receptor expression in human pancreatic cancer cells.
Cancer Res. 63:2624–2630. 2003.PubMed/NCBI
|
|
46
|
Suske G: The Sp-family of transcription
factors. Gene. 238:291–300. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wright C, Angus B, Napier J, Wetherall M,
Udagawa Y, Sainsbury JR, Johnston S, Carpenter F and Horne CH:
Prognostic factors in breast cancer: Immunohistochemical staining
for SP1 and NCRC 11 related to survival, tumour epidermal growth
factor receptor and oestrogen receptor status. J Pathol.
153:325–331. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shi Q, Le X, Abbruzzese JL, Peng Z, Qian
CN, Tang H, Xiong Q, Wang B, Li XC and Xie K: Constitutive Sp1
activity is essential for differential constitutive expression of
vascular endothelial growth factor in human pancreatic
adenocarcinoma. Cancer Res. 61:4143–4154. 2001.PubMed/NCBI
|
|
49
|
Zhang J, Jiang RX, Fan XM, Lou L, Liu WN,
Zhao XW and Li YH: Expression and prognostic significance of Sp1.
Klf4 and p21 in the ovary serous adenocarcinoma. J Clin Exp Pathol.
33:22–26. 2017.
|
|
50
|
Liu CF, Ji YC, Sun YY and Li XD:
Expression of SP1 in colorectal cancer and its relation ship with
biological behavior and prognosis. Chin J Gastroenterol Hepatol.
20:51–53. 2011.
|
|
51
|
Wang XB, Peng WQ, Yi ZJ, Zhu SL and Gan
QH: Expression and prognostic value of transcriptional factor sp1
in breast cancer. Ai Zheng. 26:996–1000. 2007.(In Chinese).
PubMed/NCBI
|
|
52
|
Pan Q, Zhu K, Chen WY, Zhang JB, Sun HC,
Wang L and Ren N: Correlation of transcription factor Sp1
expression with clinical and pathological characteristics and
prognosis of hepatocellular carcinoma. Chin J Clin Oncol.
41:1284–1287. 2014.
|
|
53
|
Chen QN, Yuan P, Fan YZ, et al: Expression
of transcription factor Sp1 in human pancreatic ductal carcinoma
and its relationship with prognosis. J Tongji Univ (Med Sci).
30:5–8. 2009.
|