Introduction
The incidence rate for multiple primary malignant
neoplasms (MPMNs) is estimated to be between 0.73 and 11.7%
(1). MPMNs are defined as two or
more unassociated primary malignant tumors that occur in the body
synchronously or metachronously (2).
Each tumor originates from different tissues and organs, presents
as a distinct pathological type and excludes lesions that are
secondary or metastatic to other tumors (3). MPMNs can be divided into two categories
(3): i) Synchronous, defined as
malignancies that occur within 6 months of the diagnosis of a
previous malignant neoplasm; and ii) metachronous, defined as
malignancies that occur >6 months apart. In a recent study, the
risks of developing second primary cancers were higher in cancer
survivors compared with the general population with a 3.8% higher
incidence of metachronous second primary cancers within a median
follow-up time of 2.5 years; furthermore, the estimated 10-year
cumulative risk of second primary cancers for patients who were
firstly diagnosed with cancer aged between 60 and 69 was as high as
13% (4). Compared with a single
primary tumor, MPMNs have increased malignant behavior and a worse
prognosis (5).
Case report
A 57-year-old male was admitted to the Chinese
People's Liberation Army 401 Hospital (Qingdao, China) with pain in
the left upper abdomen in August 1996. Enhanced computed tomography
(CT) scans revealed a 10×9.5×12-cm soft-tissue mass, with
heterogeneous density on the greater curvature. A gastric
leiomyosarcoma was suspected and thus, the patient underwent
resection of the gastric lesion and partial transverse colon on
August 21, 1996. Postoperative pathological diagnosis confirmed
gastric leiomyosarcoma with low-grade malignancy. After 11 years,
in April 2007, an abdominal CT scan was performed due to abdominal
pain, which demonstrated a low-density, round, soft-tissue mass
located between the liver and stomach. Considering it a recurrent
tumor, surgeons performed a resection of the gastric lesion and a
left hepatic lobectomy on April 29, 2007. Postoperative
pathological diagnosis confirmed it as a low-grade malignant
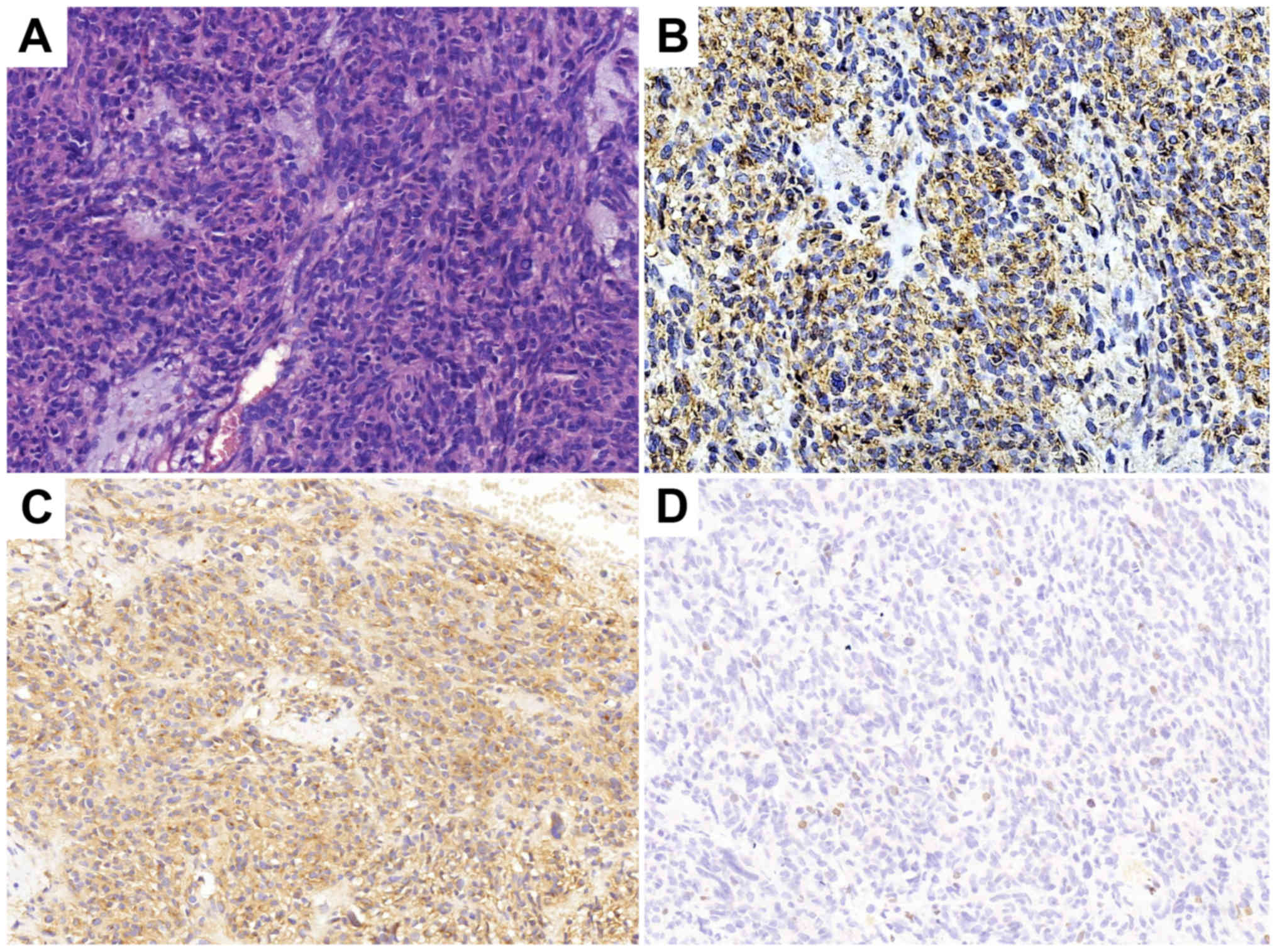
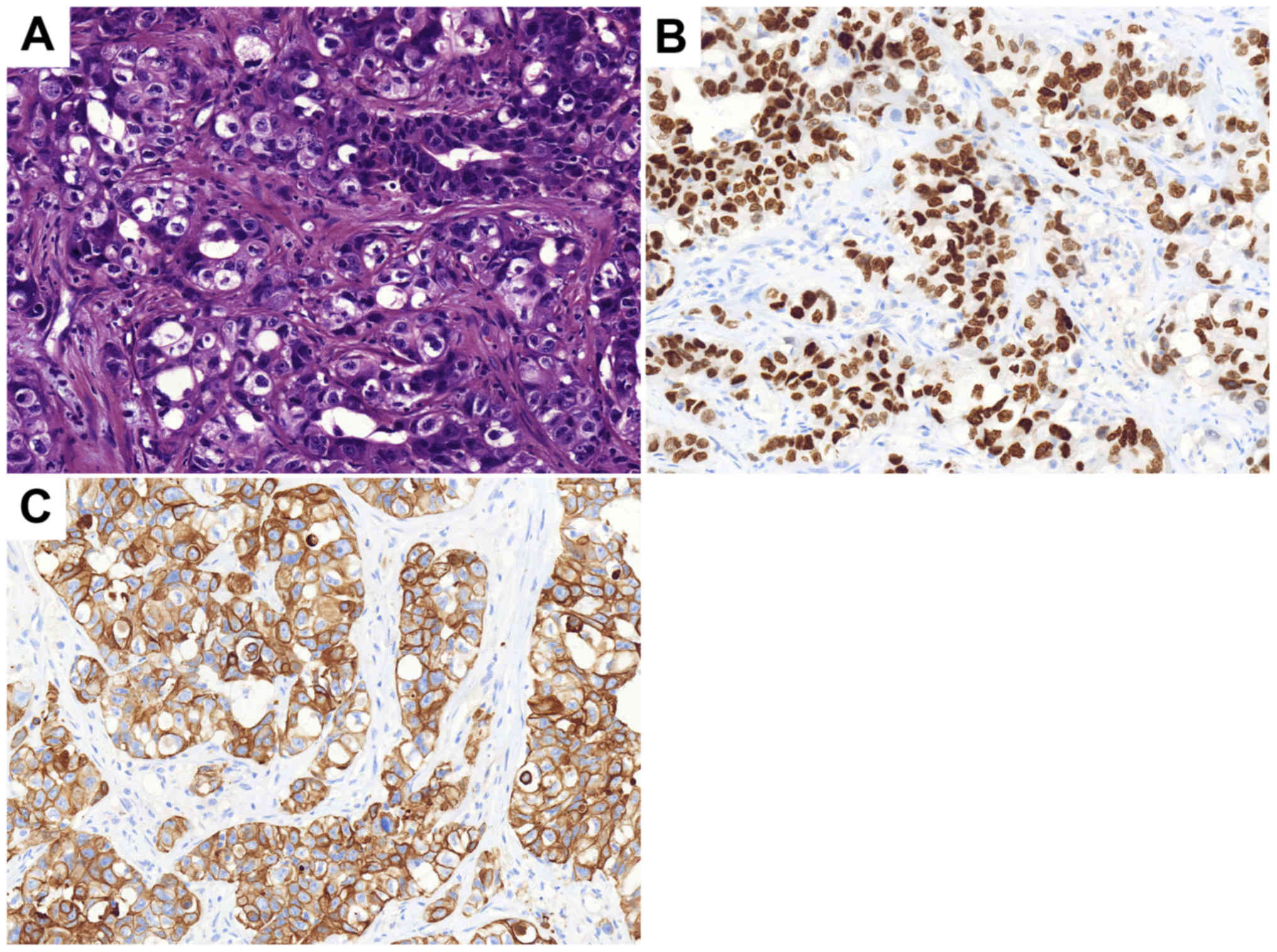
gastrointestinal stromal tumor (GIST). The immunohistochemical
results are presented in Fig. 1. In
April 2013, the annual routine re-examination revealed disease
recurrence again; two large cystic lesions in the abdomen were
discovered by a CT scan, one of which was found between the caudate
lobe of the liver and the right side of the fundus of the stomach,
and the other of which was found on the retroperitoneum.
Considering the previous diagnosis of a GIST, the surgeon did not
preform a biopsy again before surgery. The patient underwent a
total gastrectomy, as well as stern resection of the pancreas,
splenectomy, left adrenalectomy and Roux-en-Y esophagojejunostomy.
Following this major surgery, the postoperative reactions of the
patient included weight loss and impaired digestive function. The
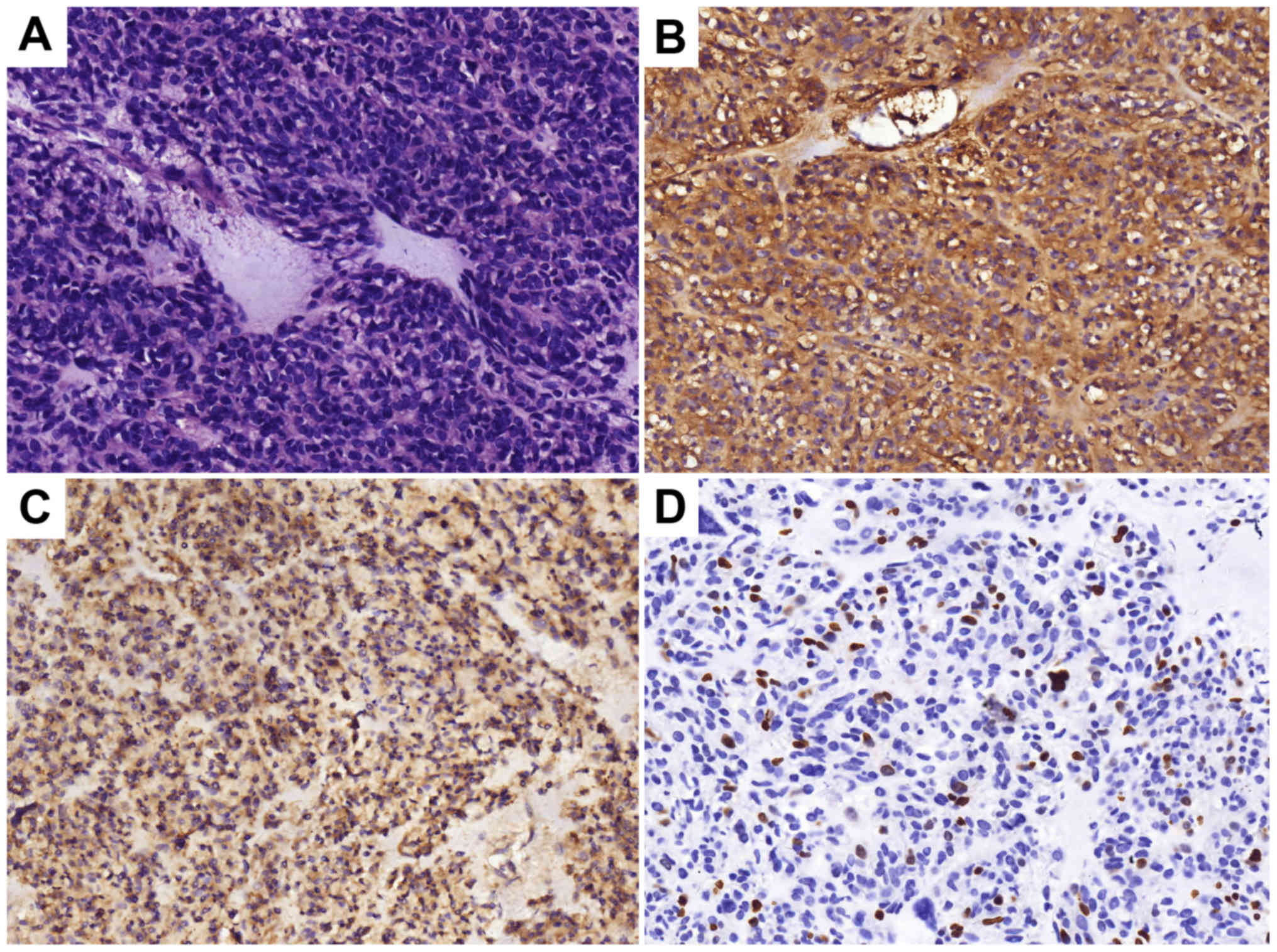
postoperative pathology confirmed GIST and the immunohistochemistry
results of the tumor are presented in Fig. 2. The patient was prescribed daily
oral imatinib (400 mg) from May 2013. During this period, diarrhea
and edema occurred, which spontaneously resolved after 2 months and
thus, the dosage of imatinib was not decreased.
The patient was referred to the Qilu Hospital of
Shandong University in November 2015 due to pain on the left side
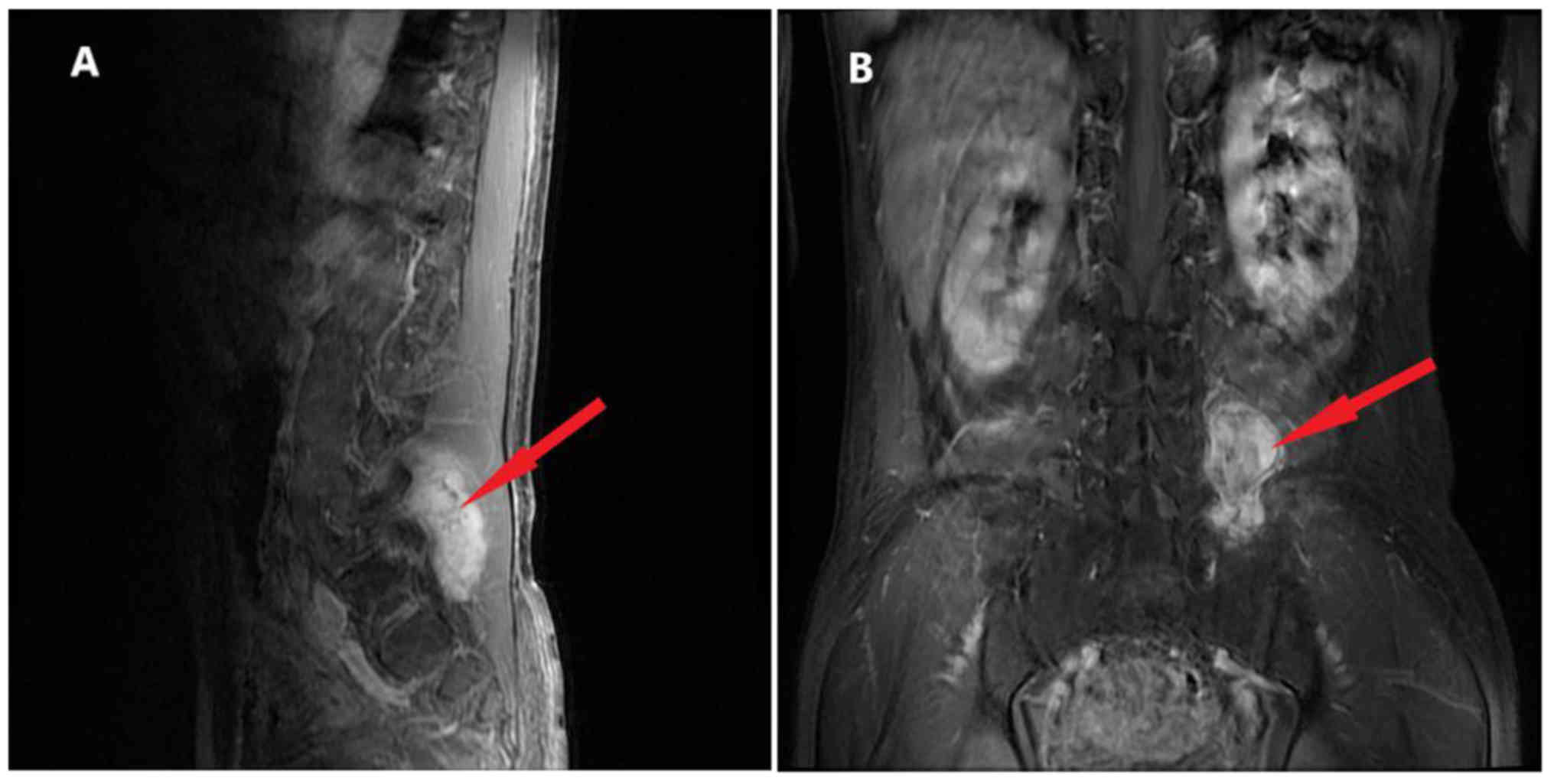
of the back. As shown in Fig. 3,
there was an irregular lesion measuring 4.2×5.7×6.7 cm between the
left transverse process of the 5th lumbar vertebrae (L5) and the
sacroiliac joint, partly involving the sacrum and left iliac bone.
The CT scan showed heterogeneous enhancement. The patient underwent
surgery to remove the lumbar soft tissue tumors on January 11,
2016, and the postoperative pathology indicated malignant ossifying
myxofibrosarcoma (MFS) with infiltrating growth and satellite
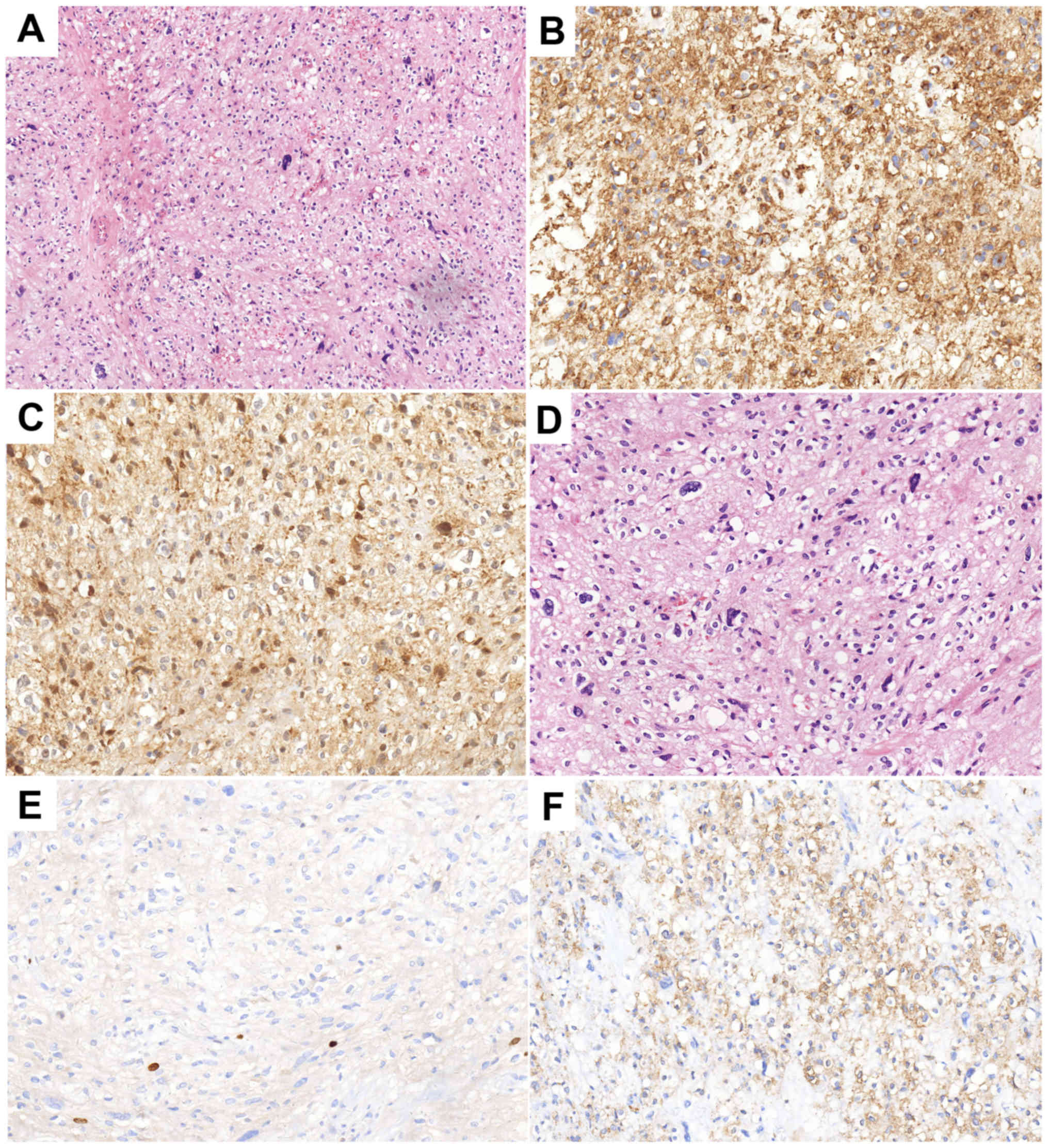
nodules besides the main body of the tumor. The
immunohistochemistry results were as follows: Smooth muscle actin
positivity, CD34 positivity, cyclin-dependent kinase 4 (CDK-4)
positivity, p63 negativity, S-100 negativity, glial fibrillary
acidic protein negativity, epithelial membrane antigen (EMA)
negativity, pan-cytokeratin (CKpan) negativity, vimentin (foci)
positivity and Ki-67 positivity (1–2%) (Fig. 4). Annual re-examination of CT and
magnetic resonance imaging scans after the surgery showed no
obvious changes in L5.
On March 6, 2018, a routine CT scan revealed two
hypoechoic nodules in the right liver with a bull's-eye
configuration. Multiple enlarged lymph nodes were found on the
right anterior cervical region through physical examination. A
cervical lymph node dissection biopsy was performed, and
pathological examination revealed metastatic low-differentiated
adenocarcinoma of the right cervical lymph nodes.
Immunohistochemistry results showed positivity for EMA, CKpan,
cytokeratin (CK) 7, CK19, carcinoembryonic antigen, transcription
termination factor 1 (TTF-1) and S-100 (Fig. 5), supporting the hypothesis that the
tumor was derived from the lung, and it was classified as
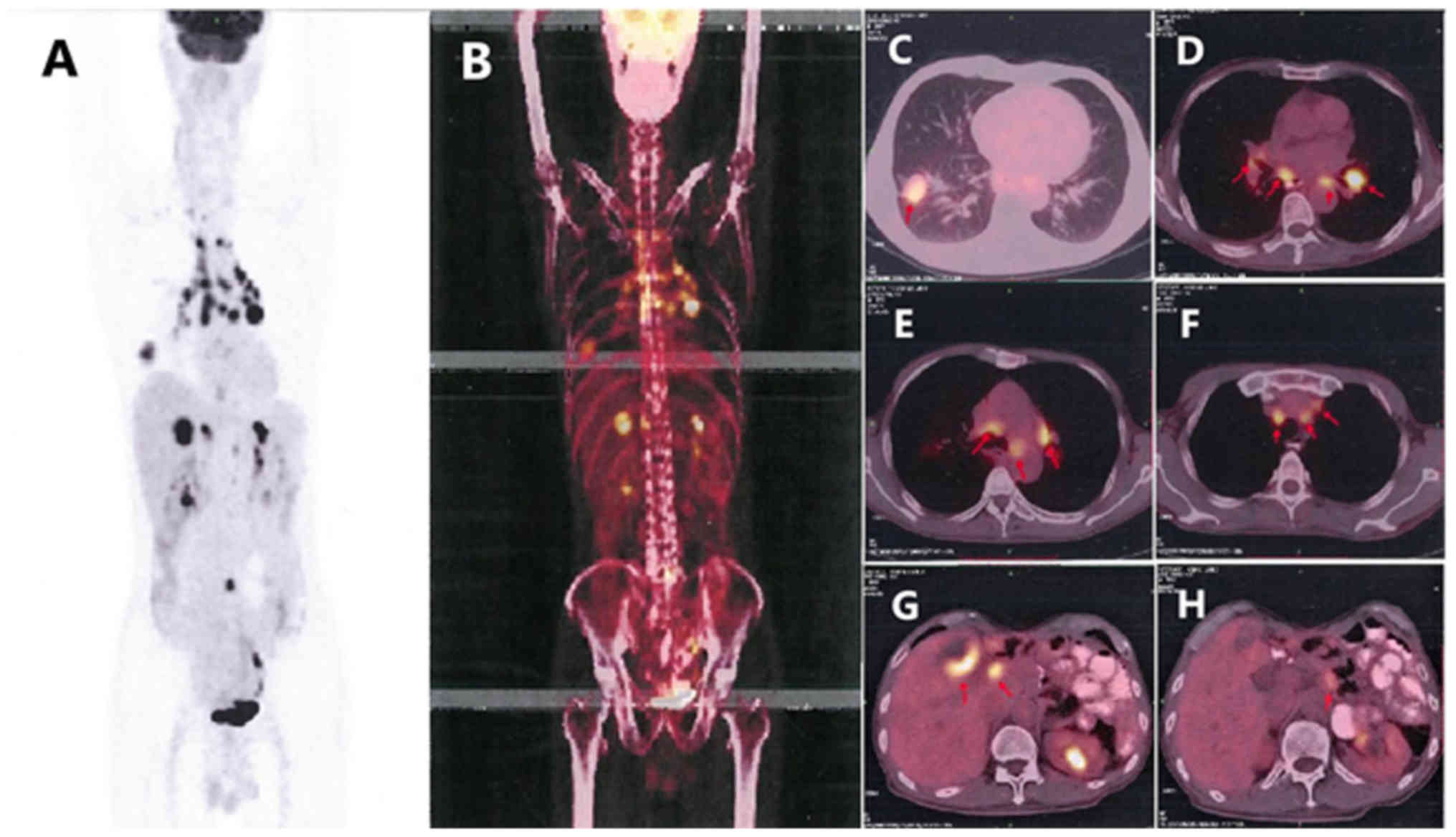
adenocarcinoma. When these findings were combined with the results
from a positron emission tomography (PET)-CT scan (Fig. 6), pulmonary adenocarcinoma located on
the inferior lobe of the right lung and liver metastases were
diagnosed. Clinical Tumor-Node-Metastasis stage was shown to be
T1bN3Mx. Next-generation sequencing technology (NGS) was used on
tissues and revealed positivity for the B-Raf proto-oncogene
serine/threonine kinase (BRAF) V600E mutation (Table I). NGS analyses were performed using
a long-range PCR-based target enrichment method (LR-PCR-based NGS)
(6). Considering the poor physique
and intolerance of the patient to chemotherapy, targeted therapy
was recommended. The patient was recommended dabrafenib (300
mg/day) and trametinib (2 mg/day) as therapy from April 2018, and
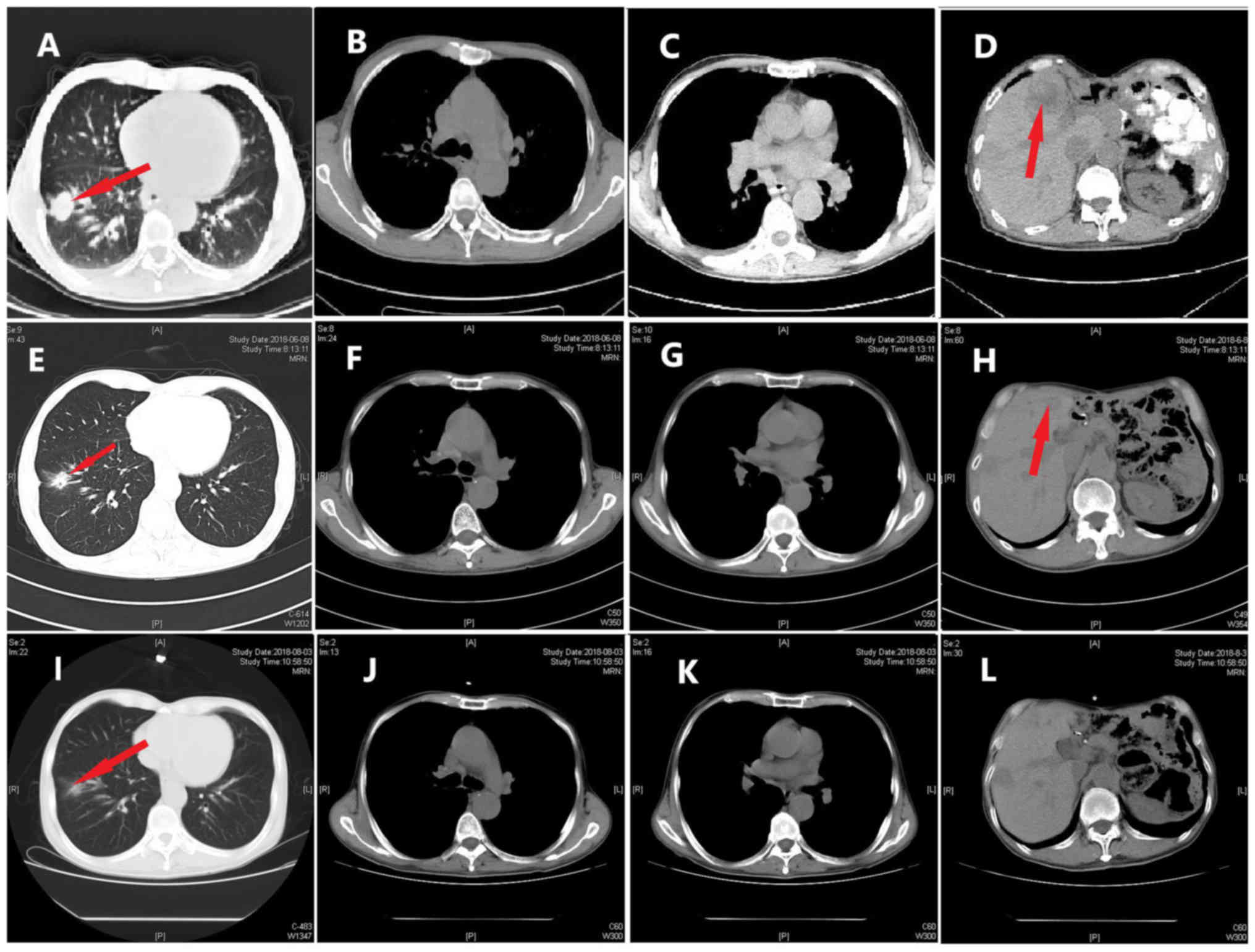
no obvious adverse reactions were noted. On June 8, 2018, and
August 3, 2018, re-examination of the chest by a CT scan (Fig. 7) revealed significant reduction in
the size and number of the nodule in the inferior lobe of the right
lung, the multiple pulmonary nodules and the lymph nodes in the
mediastinum. According to the Response Evaluation Criteria In Solid
Tumors (RECIST version 1.1) (7), the
evaluation result indicated stable disease (SD). On October 24
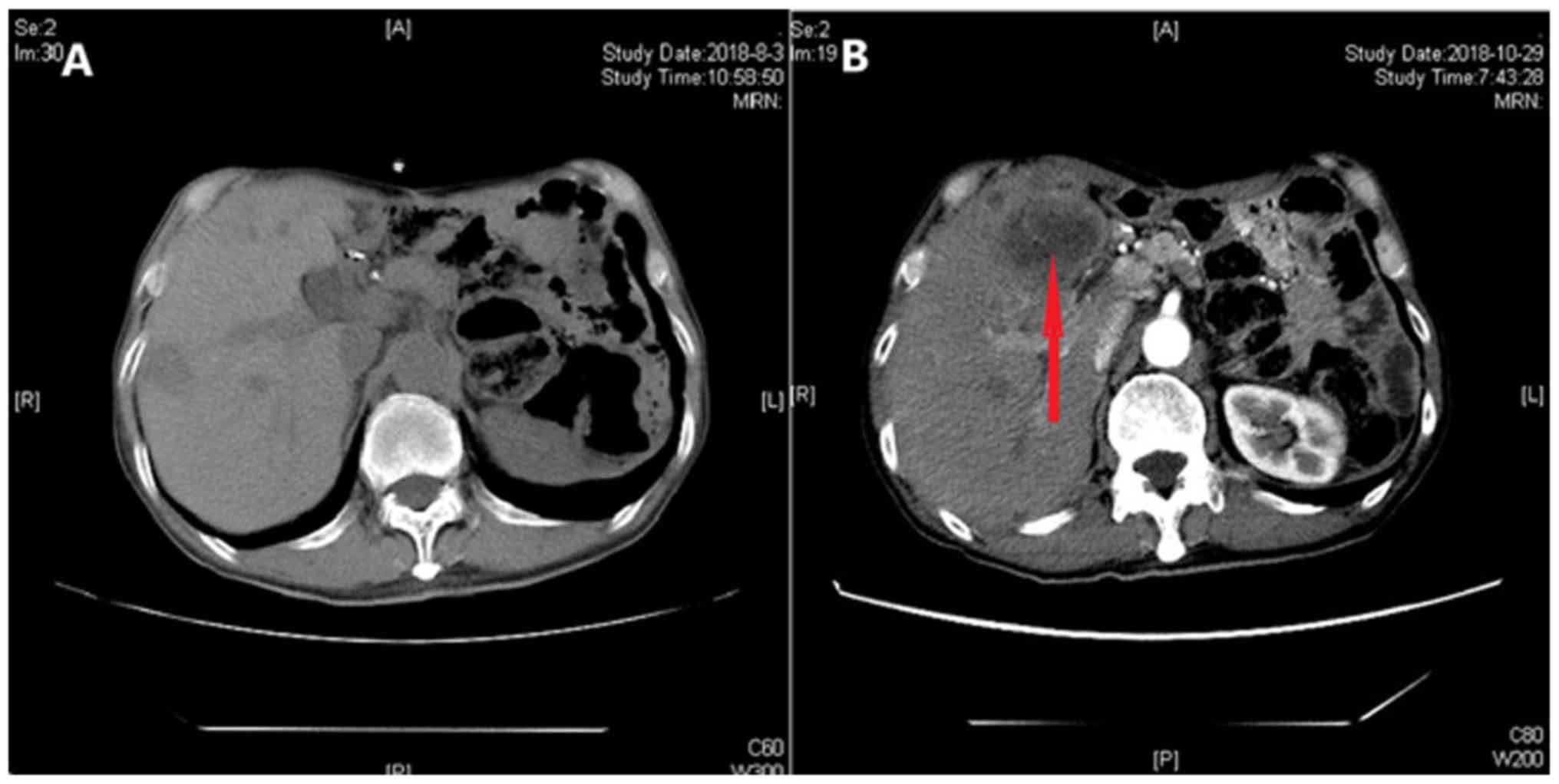
2018, re-examination of the upper abdomen by a CT scan (Fig. 8) revealed larger hypoechoic nodules
in the right liver compared with those observed on the previous CT
scan. This demonstrated disease progression. To evaluate the
properties of the intrahepatic lesions, a CT-guided percutaneous
liver biopsy was performed on October 29, 2018. Pathological
examination revealed invasion of malignant tumor cells in liver
tissue. Furthermore, immunohistochemistry results showed positivity
for CKpan, CK7, p40, Napsin A part, TTF-1, and negativity for
CD117, Dog-1, CD34, hepatocyte antigen, arginase-1 and programmed
death ligand 1 (PD-L1; 70%), supporting the hypothesis that the
intrahepatic lesions were metastases from lung cancer. Following
this, two cycles of chemotherapy, pemetrexed (700 mg, on day 2
every 3 weeks) combined with bevacizumab (400 mg, on day 1 every 3
weeks), were performed. The evaluation of the effect of these
cycles of chemotherapy indicated SD according to RECIST version
1.1.
 | Table I.Mutation frequency detected by
next-generation sequencing technology. |
Table I.
Mutation frequency detected by
next-generation sequencing technology.
| Gene | Base mutation | Amino acid
mutation | Gene subregion | Mutation frequency,
% |
|---|
| BRAF | c.1799T>A | p.V600E | EX15 | 27.69 |
| TP53 | c.811G>A | p.R237H | EX8 | 24.57 |
| EGFR | c.728C>G | p.P234R | EX6 | 25.30 |
| NTRK3 | c.1685C>T | p.P562L | EX15 | 21.64 |
| BTK | c.286G>A | p.E96K | EX4 | 20.83 |
| PDGFRA | c.509C>T | p.A170V | EX3 | 9.12 |
Discussion
MPMNs are defined as the occurrence of two or more
primary malignant tumors, each occurring at a different site and
not representing extension, recurrence or metastasis (8). According to Warren and Gates (9), if the interval between occurrences is
<6 months, the diagnosis is considered to be of synchronous
MPMN, and if >6 months, the diagnosis is considered to be a
metachronous MPMN (10). The patient
in the present case report conformed to the diagnosis of
metachronous MPMN for the following reasons: Firstly, the patient
suffered from three tumors, namely a GIST, malignant fibrous
mucinous sarcoma of the lumbar spine and lung adenocarcinoma.
Secondly, the onset time of these three malignant tumors exceeded
the cut-off time of 6 months. Finally, the pathological types of
these three malignant tumors were different, and showed no
recurrence or metastases. The pathogenic factors of MPMNs are
unclear, but may be associated with the following factors: i)
Endogenous factors such as abnormal development of the embryo,
immunity-associated diseases and endocrine diseases affecting
carcinogen sensitivity; ii) environmental exposure, including
long-term effects of radiation and industrial pollution, and
lifestyle; iii) genetic determinants; and iv) iatrogenic effects,
particularly radiation therapy and drug therapy (11). There are no currently available
standard guidelines for MPMN treatment. Malignancy type, disease
stage and the overall health of the patient are each considered
(12), and each patient has
multidisciplinary individualized treatment.
The patient evaluated in the present case report
successively suffered from GIST, MFS and NSCLC. The patient had no
immune-associated diseases, no history of exposure to toxic and
harmful substances, and the family history revealed that the
patient's brother died of lung cancer several years ago.
Similarities in gene abnormalities, lifestyle and living
environment to the brother may have been associated with the
disease status of the patient. Following the diagnosis of NSCLC in
2018, NGS was performed on the tumor tissue, which revealed
mutations in tumor protein p53, epidermal growth factor receptor
(EGFR), NT-3 growth factor receptor (NTRK3), Bruton tyrosine kinase
(BTK), and platelet-derived growth factor receptor α (PDGFRA)
(Table I). BRAF V600E mutation has
clinical significance, and is associated with the occurrence of
multiple tumors, including NSCLC and a few non-KIT proto-oncogene
receptor tyrosine kinase KIT/PDGFRA-mutated GISTs (13). The mutations in EGFR, NTRK3, BTK and
PDGFRA genes are uncommon missense mutations that have ambiguous
significance, nevertheless, they are not considered irrelevant to
the occurrence of multiple tumors in the patient mentioned in the
present case report. Since 2013, the patient was taking oral
imatinib for the GIST. Thus, long-term application of targeted drug
therapy may also increase the incidence of secondary malignant
tumors. In 2002, Surveillance Epidemiology and End Results data
indicated that ~16% of all new cancer cases were secondary or
higher-order primary cancer in the United States (12).
The BRAF gene is mutated in 1–5% of NSCLC cases and
most of these mutations occur in adenocarcinoma (14). BRAF is a member of the RAF family of
serine/threonine kinases that mediate signal transduction between
RAS and mitogen-activated protein kinase (MAPK) signaling pathways
(15). Once the mutation is
activated, the downstream mitogen-actiavted protein kinase
(MEK)-extracellular regulated protein kinase signaling pathway is
activated continuously, resulting in the excessive proliferation
and survival of tumor cells (Fig. 9)
(16). The V600E mutation accounts
for ~50% of BRAF mutations (14). In
a multicenter, single arm, non-randomized phase II study
(BRF113928; ClinicalTrials.gov identifier,
NCT01336634) (17), the efficacy of
dabrafenib as a monotherapy was compared with that of dabrafenib
administered in combination with trametinib. The primary endpoint
included the objective response rate (ORR), disease control rate
(DCR), median progression-free survival (PFS) time and overall
survival (OS) time of 84 patients treated with oral dabrafenib.
Evaluation of these primary endpoints in patients treated with
dabrafenib alone revealed the following: ORR, 33%; DCR, 58%; PFS
time, 5.5 months; and OS time, 12.7 months. The ORR, DCR and median
PFS time of the patients who were treated with dabrafenib and
trametinib were 63, 79% and 9.7 months, respectively, and 65% of
the patients achieved a PFS time of >6 months (17). Thus, the combination of MEK
inhibitors with BRAF inhibitors may demonstrate improved results
compared with BRAF inhibitors alone (18). The patient in the present case report
was diagnosed with NSCLC harboring a BRAF V600E mutation. The Food
and Drug Administration regulations approved dabrafenib and
trametinib in combination for treatment of metastatic NSCLC with a
BRAF V600E mutation on June 22, 2017 (19), therefore combined dabrafenib and
trametinib treatment was administered. At 6 months after the
treatment, the lesions in the liver had progressed, which suggested
drug resistance. Kim et al (20) recently reported that the expression
of PD-L1 was changed in 28% of patients following EGFR-tyrosine
kinase inhibitor (TKI) therapy. Su et al (21) performed a retrospective analysis,
which revealed high PD-L1 expression to be a predictor of a poor
response to EGFR-TKI treatment among patients with EGFR-mutated
NSCLC. Thus, resistance to the BRAF inhibitor may be associated
with the high PD-L1 expression in the patient described in the
present case report. A previous study has demonstrated that, in
melanoma models, cells resistant to BRAF inhibitors exhibited
increased MAPK signaling and PD-L1 expression (22). Moreover, increased expression of
markers of T-cell exhaustion and PD-L1 were detected in patients
with melanoma following treatment with a BRAF inhibitor.
Immunotherapy plays an important role in advanced NSCLC patients
with high expression of PD-L1. In a phase II study, the combination
of pembrolizumab with carboplatin and pemetrexed in
chemotherapy-naïve, advanced non-squamous NSCLCs was evaluated
(23). The pembrolizumab combined
with chemotherapy group achieved an ORR of 55% in comparison to 29%
in the chemotherapy alone group (23). The incidence of grade 3 or worse
treatment-related adverse events was similar between the two
groups. PFS time was also significantly longer in patients treated
with pembrolizumab in combination with chemotherapy compared with
that in patients who received chemotherapy alone (23).
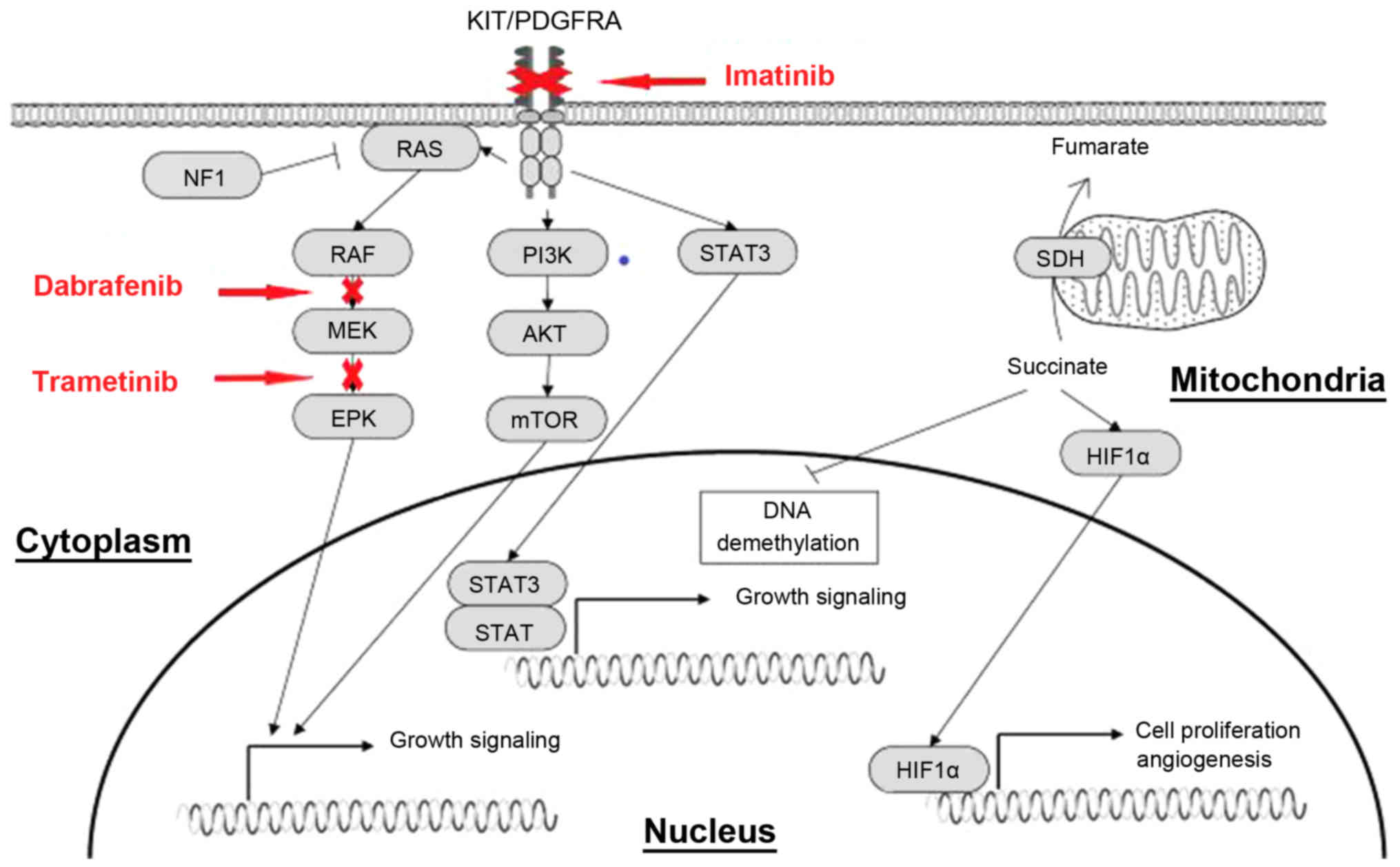 | Figure 9.Effect of imatinib, dabarafenib and
trametinib on downstream signaling pathways. Activating mutations
of KIT and PDGFRA genes permit ligand-independent phosphorylation
of the receptor tyrosine kinases, allowing the receptor-initiated
signal and causing activation of the downstream effectors, such as
PI3K/AKT, Ras/MAPK and JAK/STAT. PDGFRA, platelet-derived growth
factor receptor α; JAK, Janus kinase; MAPK, mitogen-activated
protein kinase; PI3K, phophoinositide-3 kinase; mTOR, mechanistic
target of rapamycin; STAT, signal transducer and activator of
transcription; c-Kit, KIT proto-oncogene receptor tyrosine kinase;
HIF1α, hypoxia inducible factor-1α; SDH, succinate dehydrogenase;
ERK, extracellular regulated protein kinases. |
GISTs are known to be the most common type of
mesenchymal neoplasm of the gastrointestinal tract. From a previous
study, the worldwide incidence and prevalence rates were estimated
to be between 1 and 1.5 per 100,000 per year and 13 per 100,000
individuals, respectively in 2005 (24). Peixoto et al (25) demonstrated that the stomach was the
most frequently involved organ with regard to GISTs, accounting for
64.1% of all cases, and that the lesions were distributed in the
fundus (15.5%), body (61.9%) and antrum (22.6%) (25). Other organs that were involved
included the small bowel (29.8%), duodenum (30.8%), jejunum (30.8%)
and ileum (38.5%). Occasionally, the omentum, mesentery and
peritoneum may also be affected. GIST metastases often involved the
liver and could present as a primary disease or as recurrence
following surgery (26).
A GIST is associated with a mutation in the KIT gene
(~80% of cases) or the PDGFRA gene, coding for type III receptor
tyrosine kinases (27). Mutations in
the PDGFRA gene can be detected in ~10% of GISTs without a KIT gene
mutation. The landscape of mutations in PDGFRA mostly occur in exon
18, accounting for >90% of all PDGFRA mutations, and among the
patients who do not have a KIT or PDGFRA mutation, 7–15% have now
been found to harbor a BRAF V600E mutation (28). The patient evaluated in the present
case report had a rare PDGRFA A107V mutation in exon 3, with a
mutation frequency of ~9.4%, which was considered as an uncommon
pathogenic mutation, according to the review of previous studies.
The association of this mutation with the occurrence of GIST or
imatinib resistance is worthy of further investigation. GISTs
usually differ in size, and this is associated with different
degrees of risk: Ultra-low risk, <2 cm; low-risk, 2–5 cm;
moderate-risk, 5–10 cm; and high-risk, >10 cm (29). Surgical treatment is the first
therapeutic choice and is considered as the only possible cure for
GISTs (26). High-risk patients have
a high rate of postoperative recurrence and metastasis (up to
55–90%); 80% have a 3–4 local recurrence within 1–2 years of
surgery and 50% have liver metastases (30). Imatinib is a KIT/PDGFRA TKI that is
mainly used for KIT-positive patients with non-operable, metastatic
or recurrent tumors, or as an adjuvant therapy for primary
KIT-positive GIST patients (30). In
the Scandinavian/German SSG XVIII/AIO trial (31), patients at high risk or with
metastasis following R0/R1 resection were divided into two groups.
One group was treated with imatinib (400 mg/day) for 3 years and
the other group was treated with imatinib (400 mg/day) for 1 year.
The median follow-up time was 4.5 years, and the 5-year PFS times
for the two groups were 65 and 48%, respectively. PFS and OS times
were longer in the 3-year group. Furthermore, treatment with
imatinib for 5 years was found to delay recurrence in 90% of
patients, whereas treatment for 3 years only delayed recurrence in
66% of patients. The 5-year OS time did not notably differ in the
3-year arm of the SSG XVIII trial when compared with the 5-year arm
(95 vs. 92%, respectively). Recurrence of GISTs occurred twice in
the patient described in the present case study, following the
initial surgery in 1997. As imatinib was listed and included in the
medical insurance in China, the patient began to take imatinib (400
mg/day) from May 2013. Regular follow-up with abdominal ultrasound
showed no abnormalities. Considering the three recurrences, the
patient did not stop the intake of imatinib, and the treatment
continued for 5 years.
MFS, formerly known as a myxoid variant of malignant
fibrous histiocytoma, is the most frequently occurring sarcoma of
the extremities in adults, with characteristic high local
recurrence rates (32). At present,
the World Health Organization (32)
describes MFS as a spectrum of malignant fibroblastic lesions with
myxoid stroma, pleomorphism and curvilinear vessels. MFS mainly
occurs in the extremities of elderly patients, particularly in the
lower limbs; however, it can also appear on the trunk and in the
head and neck regions (33). An MFS
diagnosis is based on characteristic histopathological features,
including the presence of alternating hypocellular myxoid and
hypercellular fibrous areas, pleomorphic nuclei, curvilinear
thin-walled blood vessels prominent in myxoid areas, the
aggregation of neoplastic cells or inflammatory cells, and spindle
and stellate cells in the myxoid matrix (34). In previous studies, immunological
staining often revealed vimentin and CD34 positivity, which also
indicated that the tumor was of fibroblast origin, and the samples
could also be SMA-positive and S-100 protein-negative (34). Compared with other soft-tissue
sarcoma types, MFS showed better overall prognosis, with an OS rate
of ~70% and an overall risk of metastases between 20 and 25% in
high-grade variants. However, MFS is inclined to have a higher rate
of local recurrence compared with other types, with a rate reported
to be between 20 and 75% (33).
Previous studies reported that the distant metastasis rate of MFS
patients ranged from 9.5–23.6%, and the lung was considered to be
the most common metastatic organ of soft-tissue sarcomas (35). Clinically, once the local recurrence
of MFS occurs, the stages and grades of the tumors also increase,
leading to metastatic disease (36).
Treatment strategies for MFS include extensive local resection,
chemotherapy and radiotherapy. The recurrence rate after extensive
resection ranged from 16–54%, and it was therefore recommended as
the treatment strategy for low-grade MFS (37). In the present study, 2 years after
the patient accepted the first surgery, the PET-CT scan showed bone
destruction in L5, with a maximum standardized uptake value of
~11.2, suggesting regional recurrence of fibrous sarcoma. The
treatment options were radiotherapy or surgery. In view of the fact
that the patient showed no obvious discomfort and possessed a
physique unsuitability for radiotherapy or surgery, close
observation was suggested.
The patient was treated with three targeted drugs,
including imatinib, dabrafenib and trametinib, and to the best of
our knowledge, no study has previously assessed the adverse effects
of this combination. A previous study demonstrated that the most
common adverse reactions to dabrafenib and trametinib were pyrexia,
peripheral edema, increased aspartate aminotransferase and blood
alkaline phosphatase levels, nasopharyngitis, erythema, stomatitis
and headaches (38). Few studies
demonstrated that the adverse effects associated with the eye
should not be ignored. These effects included retinal vein
occlusion, with an incidence of <1.5% with trametinib,
chorioretinopathy, with an incidence of up to 2% with both
trametinib and dabrafenib, and uveitis, with unknown incidence
(39). Toxicities as a result of
imatinib treatment include the retention of fluid, nausea,
diarrhea, fatigue, abdominal pain, muscle cramps and rashes.
Symptoms of congestive heart failure were observed in ~8.2% of the
patients treated with imatinib for GISTs (40). Following the use of imatinib, the
patient in the present case had mild edema and diarrhea, which
continued for ~6 months. However, these adverse reactions gradually
resolved without any intervention. Since April 2018, combined
dabrafenib and trametinib treatment was used, and the patient
showed no signs of other significant adverse reactions.
The potential of genetic mutations of ambiguous
significance as therapeutic indicators was investigated in the
present case report. For example, the patient had a NTRK3 P562L
mutation in exon 15. A previous study demonstrated that
larotrectinib had marked and durable antitumor activity in patients
with tropomyosin receptor kinase fusion-positive cancer, regardless
of the age of the patient or of the type of the tumor, and the
overall response rate was 75% (41).
The patient in the present case also had a BTK E96K mutation in
exon 4, with a mutation frequency of ~20.83%. Ibrutinib was
previously demonstrated to significantly improve the prognosis of
patients with mantle cell lymphoma who had high expression of BTK
(42), and ths drug could be
considered as treatment for the present patient. Additionally,
PD-L1 positivity (70%) enables the use of PD1/PD-L1 inhibitors.
Thus, the decision on the best treatment option may benefit from
the knowledge on the patients genetic mutation profile. Moreover,
the best time and whether various anticancer agents should be
combined require further investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ, LF and JW collaborated in data collection,
literature review and writing the manuscript. HC analyzed the data
from the patient. ZY, HW and YD were responsible for the treatment
and management of the patient. All authors were involved in writing
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Verbal consent for publication was provided by the
patient included in this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DE Luca A, Frusone F, Vergine M, Cocchiara
R, LA Torre G, Ballesio L, Monti M and Amabile MI: Breast cancer
and multiple primary malignant tumors: Case report and review of
the literature. In vivo. 33:1313–1324. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao J, Tan Y, Wu Y, Zhao W, Wu J, Ji M,
Shi L, Jiang J and Wu C: A rare case of eight multiple primary
malignant neoplasms in a female patient: A case report and review
of the literature. Oncol Lett. 9:587–590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mukaiyama Y, Suzuki M, Morikawa T, Mori Y,
Takeshima Y, Fujimura T, Fukuhara H, Nakagawa T, Nishimatsu H, Kume
H and Homma Y: Multiple primary malignant neoplasms of the glottis,
renal pelvis, urinary bladder, oral floor, prostate, and esophagus
in a Japanese male patient: A case report. World J Surg Oncol.
12:2942014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang H, Hou J, Zhang G, Zhang M, Li P, Yan
X and Ma Z: Clinical characteristics and prognostic analysis of
multiple primary malignant neoplasms in patients with lung cancer.
Cancer Gene Ther. Jan 31–2019.(Epub ahead of print). View Article : Google Scholar
|
|
5
|
Lee J, Park S, Kim S, Kim J, Ryu J, Park
HS, Kim SI and Park BW: Characteristics and survival of breast
cancer patients with multiple synchronous or metachronous primary
cancers. Yonsei Med J. 56:1213–1220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mochizuki T, Teraoka A, Akagawa H, Makabe
S, Akihisa T, Sato M, Kataoka H, Mitobe M, Furukawa T, Tsuchiya K
and Nitta K: Mutation analyses by next-generation sequencing and
multiplex ligation-dependent probe amplification in Japanese
autosomal dominant polycystic kidney disease patients. Clin Exp
Nephrol. 23:1022–1030. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kataoka Y, Hirano K, Narabayashi T, Hara
S, Fujimoto D, Tanaka T, Ebi N, Tomii K and Yoshioka H: Concordance
between the response evaluation criteria in solid tumors version
1.1 and the immune-related response criteria in patients with
non-small cell lung cancer treated with nivolumab: A multicenter
retrospective cohort study. Cancer Chemother Pharmacol. 81:333–337.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han Y, Shao N, Xi X and Hao X: Use of
microwave ablation in the treatment of patients with multiple
primary malignant tumors. Thoracic Cancer. 8:365–371. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Warren S and Gates O: Multiple primary
malignant tumors: A survey of the literature and statistical study.
Am J Cancer. 16:1358–1414. 1932.
|
|
10
|
Etiz D, Metcalfe E and Akcay M: Multiple
primary malignant neoplasms: A 10-year experience at a single
institution from Turkey. J Cancer Res Ther. 13:16–20. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Motuzyuk I, Sydorchuk O, Kovtun N, Palian
Z and Kostiuchenko Y: Analysis of trends and factors in breast
multiple primary malignant neoplasms. Breast Cancer (Auckl).
12:1178223418759952018. View Article : Google Scholar
|
|
12
|
Parekh JD, Kukrety S, Thandra A and
Valenta C: Multiple primary malignant neoplasms in an elderly
patient. Cureus. 10:e23842018.PubMed/NCBI
|
|
13
|
Huss S, Pasternack H, Ihle MA,
Merkelbach-Bruse S, Heitkötter B, Hartmann W, Trautmann M,
Gevensleben H, Büttner R, Schildhaus HU and Wardelmann E:
Clinicopathological and molecular features of a large cohort of
gastrointestinal stromal tumors (GISTs) and review of the
literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rere
events. Hum Pathol. 62:206–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Y, Meng Y, Zhang H, Shen X, Li R, Yu
L, Liu B and Wang L: Detection of EGFR and BRAF mutations by
competitive allele-specific TaqMan polymerase chain reaction in
lung adenocarcinoma. Oncol Lett. 15:3295–3304. 2018.PubMed/NCBI
|
|
15
|
Mitsogianni M, Mitsimponas N, Crespo F,
Hartmann KA, Klosterhalfen B, Haase S and Giagounidis A:
Concomitant non-small cell lung cancer and hairy cell leukemia in a
patient harboring BRAF-V600E mutation in both tissues: A case
report. Case Rep Oncol. 11:109–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding X, Zhang Z, Jiang T, Li X, Zhao C, Su
B and Zhou C: Clinicopathologic characteristics and outcomes of
Chinese patients with non-small-cell lung cancer and BRAF mutation.
Cancer Med. 6:555–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khunger A, Khunger M and Velcheti V:
Dabrafenib in combination with trametinib in the treatment of
patients with BRAF V600-positive advanced or metastatic non-small
cell lung cancer: Clinical evidence and experience. Ther Adv Respir
Dis. 12:17534666187676112018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mufti M, Ching S, Farjami S, Shahangian S
and Sobnosky S: A case series of two patients presenting with
pericardial effusion as first manifestation of non-small cell lung
cancer with BRAF mutation and expression of PD-L1. World J Oncol.
9:56–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weart TC, Miller KD and Simone CB 2nd:
Spotlight on dabrafenib/trametinib in the treatment of
non-small-cell lung cancer: Place in therapy. Cancer Manag Res.
10:647–652. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim TJ, Hong SA, Kim O, Kim SJ, Yang JH,
Joung EK, Kang JH and Hong SH: Changes in PD-L1 expression
according to tumor infiltrating lymphocytes of acquired EGFR-TKI
resistant EGFR-mutant non-small-cell lung cancer. Oncotarget.
8:107630–107639. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su S, Dong ZY, Xie Z, Yan LX, Li YF, Su J,
Liu SY, Yin K, Chen RL, Huang SM, et al: Strong programmed death
ligand 1 expression predicts poor response and de novo resistance
to EGFR TKIs among non-small cell lung cancer patients with EGFR
mutation. J Thorac Oncol. 13:1668–1675. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang X, Zhou J, Giobbie-Hurder A, Wargo J
and Hodi FS: The activation of MAPK in melanoma cells resistant to
BRAF inhibition promotes PD-L1 expression that is reversible by MEK
and PI3K inhibition. Clin Cancer Res. 19:598–609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li SD, Martial A, Schrock AB and Liu JJ:
Extraordinary clinical benefit to sequential treatment with
targeted therapy and immunotherapy of a BRAF V600E and PD-L1
positive metastatic lung adenocarcinoma. Exp Hematol Oncol.
6:292017.PubMed/NCBI
|
|
24
|
Liu J, Chen Z, Chen H, Hou Y, Lu W, He J,
Tong H, Zhou Y and Cai W: Genetic polymorphisms contribute to the
individual variations of imatinib mesylate plasma levels and
adverse reactions in chinese GIST patients. Int J Mol Sci. 18(pii):
E6032017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peixoto A, Costa-Moreira P, Silva M,
Santos AL, Lopes S, Vilas-Boas F, Moutinho-Ribeiro P and Macedo G:
Gastrointestinal stromal tumors in the imatinib era: 15 years'
experience of a tertiary center. J Gastrointest Oncol. 9:358–362.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sanchez-Hidalgo JM, Duran-Martinez M,
Molero-Payan R, Rufian-Peña S, Arjona-Sanchez A, Casado-Adam A,
Cosano-Alvarez A and Briceño-Delgado J: Gastrointestinal stromal
tumors: A multidisciplinary challenge. World J Gastroenterol.
24:1925–1941. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rubin BP: Gastrointestinal stromal
tumours: An update. Histopathology. 48:83–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Balachandran VP and Dematteo RP:
Gastrointestinal stromal tumors: Who should get imatinib and for
how long. Adv Surg. 48:165–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alecu L, Tulin A, Enciu O, Bărbulescu M,
Ursuţ B and Obrocea F: Gastrointestinal stromal tumors-Diagnosis
and surgical treatment. Chirurgia (Bucur). 110:525–529.
2015.PubMed/NCBI
|
|
30
|
Mei L, Du W, Idowu M, von Mehren M and
Boikos S: Advances and challenges on management of gastrointestinal
stromal tumors. Front Oncol. 8:1352018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Joensuu H, Eriksson M, Sundby Hall K,
Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster
J, Al-Batran SE, et al: One vs three years of adjuvant imatinib for
operable gastrointestinal stromal tumor: A randomized trial. JAMA.
307:1265–1272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
De Vita A, Recine F, Mercatali L,
Miserocchi G, Liverani C, Spadazzi C, Casadei R, Bongiovanni A,
Pieri F, Riva N, et al: Myxofibrosarcoma primary cultures:
Molecular and pharmacological profile. Ther Adv Med Oncol.
9:755–767. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Colia V, Fiore M, Provenzano S, Fumagalli
E, Bertulli R, Morosi C, Dei Tos AP, Barisella M, Gronchi A, Casali
PG and Sanfilippo R: Activity of anthracycline- and
ifosfamide-based chemotherapy in a series of patients affected by
advanced myxofibrosarcoma. Clin Sarcoma Res. 7:162017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wong A, Chan Woo Park R, Mirani NM and
Eloy JA: Myxofibrosarcoma of the maxillary sinus. Allergy Rhinol
(Providence). 8:e95–e99. 2017. View Article : Google Scholar
|
|
35
|
Tsuchie H, Kaya M, Nagasawa H, Emori M,
Murahashi Y, Mizushima E, Miyakoshi N, Yamashita T and Shimada Y:
Distant metastasis in patients with myxofibrosarcoma. Ups J Med
Sci. 122:190–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lohberger B, Stuendl N, Leithner A, Rinner
B, Sauer S, Kashofer K and Liegl-Atzwanger B: Establishment of a
novel cellular model for myxofibrosarcoma heterogeneity. Sci Rep.
7:447002017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nomura T, Sakakibara S, Moriwaki A,
Kawamoto T, Suzuki S, Ishimura T, Hashikawa K and Terashi H:
Terashi, low-grade myxofibrosarcoma of the rectus abdominus muscle
infiltrating into abdominal cavity: A case report. Eplasty.
17:e62017.PubMed/NCBI
|
|
38
|
Yamazaki N, Tsutsumida A, Takahashi A,
Namikawa K, Yoshikawa S, Fujiwara Y, Kondo S, Mukaiyama A, Zhang F
and Kiyohara Y: Phase 1/2 study assessing the safety and efficacy
of dabrafenib and trametinib combination therapy in Japanese
patients with BRAF V600 mutation-positive advanced cutaneous
melanoma. J Dermatol. 45:397–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dy GK and Adjei AA: Understanding,
recognizing, and managing toxicities of targeted anticancer
therapies. CA Cancer J Clin. 63:249–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ghias AAP, Bhayani S, Gemmel DJ and Garg
SK: Rapidly progressive dyspnea in gastrointestinal stromal tumor
(GIST) with imatinib cardiac toxicity. J Community Hosp Intern Med
Perspect. 8:87–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Drilon A, Laetsch TW, Kummar S, DuBois SG,
Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo
AS, et al: Efficacy of larotrectinib in TRK fusion-positive cancers
in adults and children. N Engl J Med. 378:731–739. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Noy A, de Vos S, Thieblemont C, Martin P,
Flowers CR, Morschhauser F, Collins GP, Ma S, Coleman M, Peles S,
et al: Targeting Bruton tyrosine kinase with ibrutinib in
relapsed/refractory marginal zone lymphoma. Blood. 129:2224–2232.
2017. View Article : Google Scholar : PubMed/NCBI
|