Introduction
Pancreatic cancer is one of the deadliest malignant
tumors in humans with ~4% global mortality rate (1,2). In the
past decade, the rate of increase in the incidence of pancreatic
cancer in rural areas has exceeded that in the urban areas
(3). The age standardized rate of
pancreatic cancer in China (8.37 per 100,000), and the associated
mortality rate (7.78 per 100,000), is higher compared with that
recorded in the United States (7.5 and 7.0, respectively), inducing
the increased interests in pancreatic cancer treatment in China
(3). The symptoms of pancreatic
cancer are typically vague and non-specific in the early stages,
which tends to delay the diagnosis (4,5).
Therefore, the majority of patients have metastatic cancer at the
time of diagnosis (5). According to
the American Cancer Society, the 1-and 5-year overall survival
rates of patients with pancreatic cancer (all stages) are 20 and
7%, respectively (6,7). At present, very few effective therapies
are available for pancreatic cancer. Surgery followed by the
administration of compatible drugs is still considered the best
therapeutic approach (8). However,
the surgical mortality and postoperative recurrence rates remain
high (8). Therefore, the development
of new strategies for the treatment of pancreatic cancer is
crucial.
The Hedgehog (Hh) signaling pathway is a highly
conserved pathway involved in the regulation of cell
differentiation and organ development at the embryonic stage
(9,10). This pathway obtains its name from its
ligand, a polypeptide intercellular molecule called Hedgehog, which
has three homologues in mammals: Desert (Dhh), Indian (Ihh) and
Sonic (Shh) (9). Among these, Shh is
the most extensively studied homologue. Hh ligand interacts with
membrane receptors patched 1 (Ptch1) and smoothened frizzled class
receptor (Smo), and activates their downstream molecules and the
nuclear transcription factor of the glioma-associated oncogene
(Gli) family (10,11). The expression of the molecules
involved in the Hh signaling pathway is rarely detected in normal
individuals; however, it is activated in malignant tumors (12), such as pancreatic (13), prostate (14), liver (15), ovarian (16), and breast (17) cancer. A global genomic analysis in 24
pancreatic cancers indicated twelve cellular signaling pathways
that were genetically altered in 67 to 100% of patients, and
genetic alterations in the Hedgehog signaling pathway were observed
in all pancreatic cancers (18).
High expression levels of Shh, Ptch1, Smo and GLI family zinc
finger 1 (Gli1) in early-stage pancreatic cancer indicate an
important role for the Hh signaling pathway in the development of
pancreatic cancer (19). The
abnormal activation of the Hh signaling pathway along with
overexpression of Gli1 in pancreatic cancer (20) forms a positive feedback regulatory
loop that induces the transcription of Ptch1, which further
activates its target genes and results in uncontrolled activation
of the Hh pathway. Gli target genes are involved in tumor cell
growth, apoptosis, metastasis, neovascularization and
epithelial-mesenchymal transition (21). Inhibition of the Hh signaling pathway
reduces the growth, invasion and metastasis of pancreatic cancer
cells (22), which suggests that it
may be a potential therapeutic target in pancreatic cancer.
Dauricine is a bis-benzyltetrahydroisoquinoline
alkaloid isolated from the Asian and Canadian moonseed
(Menispermum dauricum and Menispermum canadense)
(23). This natural compound
exhibits various biological effects, including suppression of
cancer cell growth and inhibition of transmembrane ion channels
(23,24). Dauricine has been demonstrated to
prevent urothelial tumor cell proliferation (23) and to inhibit colon cancer growth by
blocking the nuclear factor κB (NF-κB) pathway (24). Dauricine also attenuates inflammatory
reactions (25), exerts
neuroprotective effects (26,27), and
blocks potassium and calcium channels (28,29). A
previous study by our group examined the effects of a mixture of
phenolic alkaloids containing daurisolin, dauricine, daurinoline
and dauricicoline in pancreatic cancer BxPC-3 cells and BxPC-3
×enograft mice (30). The mixture
exhibited a time-and dose-dependent inhibitory effect on the
proliferation of the BxPC-3 cells, and induced
G0/G1 phase arrest and cell apoptosis; it
also inhibited tumor growth in the BxPC-3 ×enograft in vivo.
However, the therapeutic effects of dauricine in the context of
pancreatic cancer have not been investigated, although the
antitumor function of dauricine has been demonstrated in colon
(24), renal (31) and urothelial cancer cells (23). The present study was designed to
investigate the effects of dauricine on BxPC-3 transplanted
pancreatic cancer in nude mice. Additionally, the underlying
molecular mechanisms of the inhibitory effect of dauricine on
pancreatic cancer growth were investigated.
Materials and methods
Drugs, reagents and kits
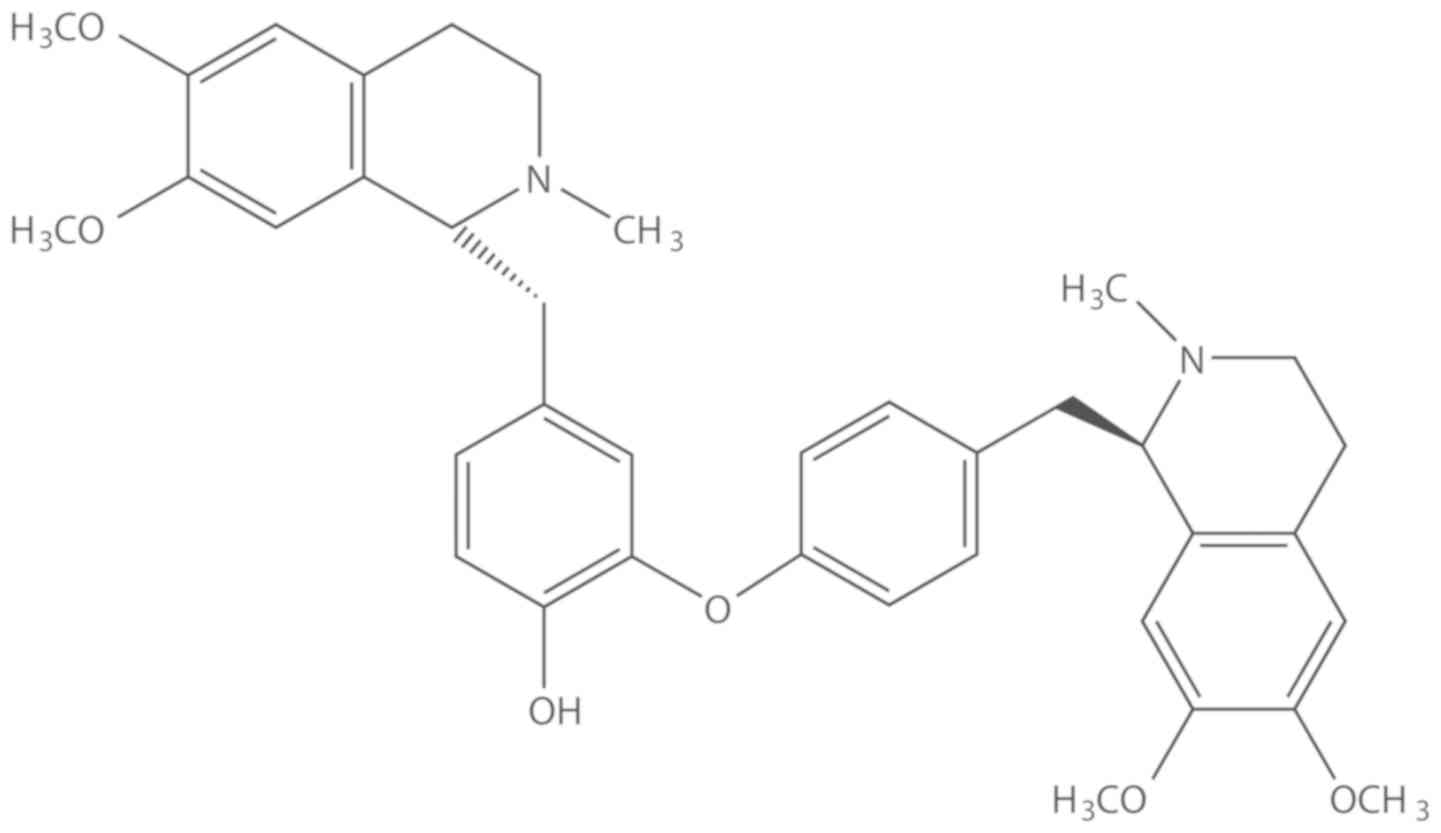
Dauricine (cat. no. MUST-17022102) (Fig. 1) was purchased from Guangzhou Poetry
Man Dan Biotechnology Co., Ltd.. RNase A, TRIzol® and
antibodies against Shh (cat. no. 2207) and Gli1 (cat. no. 3538)
were purchased from Invitrogen; Thermo Fisher Scientific, Inc.
Antibodies against Ptch1 (cat. no. ab39266) and Smo (cat. no.
ab72130) were purchased from Abcam. 5-Fluorouracil (5-FU),
propidium iodide (PI), sodium dodecyl sulfate (SDS; cat. no. L5750)
and Tween-20 (cat. no. P1379) were purchased from Sigma-Aldrich;
Merck KGaA. Anti-GAPDH antibody (cat. no. CW0100A) and a high
sensitivity chemiluminescence detection kit (cat. no. CW0049A) were
purchased from Kangwei Century Biotechnology Co. Ltd. (http://www.cwbiotech.com/). Protein extraction reagent
(cat. no. P0028-1) was purchased from Beyotime Institute of
Biotechnology. PrimeScript Reverse Transcription (RT) Reagent kit
and SYBR Premix Ex Taq were obtained from Takara Bio, Inc..
Pancreatic cancer animal model
The human pancreatic cancer BxPC-3 cell line was
obtained from the Cell Bank/Stem Cell Bank, Shanghai Institute of
Life Sciences, Chinese Academy of Sciences. The cells were cultured
in RPMI 1640 complete medium (HyClone; GE Healthcare Life Sciences)
and incubated at 37°C in 5% CO2. The cells were digested
with 0.25% trypsin-EDTA, collected and adjusted to a concentration
of 5×106 cells/ml of medium.
A total of 50 BALB/c nude mice (age, 4–6 weeks; 25
male and 25 female; weight, 18±2 g) were obtained from the Shanghai
SLAC Laboratory Animal Co. Ltd. Mice were housed at 23°C and 55%
humidity with 12 light/12 dark cycle at the Comparative Medicine
Facility, Heilongjiang University of Chinese Medicine (Harbin,
China) and provided with ad libitum access to water and
food. The experimental protocols were approved by the Animal Care
and Use Committee of the Heilongjiang University of Chinese
Medicine.
The pancreatic cancer BxPC-3 ×enograft animal model
was established according to previously described methods (30). Cell suspension (0.2 ml;
5×106 cells/ml) was inoculated subcutaneously into the
right armpit of the nude mice. Flattened or round lumps were
observed on days 7 to 10 post-injection, which indicated successful
inoculation. The tumor size was monitored by visual observation
daily. Only one tumor was observed in each animal; no animals
exhibited multiple subcutaneous tumors. Specific criteria for
humane endpoints included: i) A tumor size that >1.5 cm; ii)
tumors becoming ulcerated, infected or necrotic with breaks in the
overlying skin; and iii) body weight loss of >20%. Other general
signs of illness, such as inactivity, hunched posture or ruffled
appearance, were also included. The use of humane endpoints was
monitored and reviewed throughout the experiment. None of the
animals exhibited the signs of these endpoints until the end of the
experiment.
Experimental design
A total of 40 pancreatic cancer BxPC-3
×enograft-bearing animals were randomly divided into four groups
(n=10 mice/group) as follows: i) The saline group; ii) the 5-FU
group; iii) the low-dose dauricine group; and iv) the high-dose
dauricine group. Healthy BALB/c nude mice without BxPC-3 ×enografts
were used as the control group (n=10). Mice in the saline group
received intraperitoneal injections of saline, whereas mice in the
low-and high-dose dauricine groups received intraperitoneal
injections of dauricine at 6 and 12 mg/kg body weight,
respectively. Mice in the 5-FU group were inoculated with 5-FU (20
mg/kg). 5-FU was selected as the positive control for the
evaluation of the effects of dauricine. Control mice received
injections of an equal volume of saline. The treatments were
administered at 10 a.m. daily for 21 days. Following the completion
of treatment, the animals were weighed and euthanized the next day
by cervical dislocation for sample collection. Death was verified
by the absence of a heart beat and the onset of rigor mortis.
Tumor inhibition rate and spleen
index
The tumor and spleen of each mouse were dissected,
washed with sterile saline and dried using filter paper. The tumor
volume was calculated using the following formula: Volume=(4π/3) ×
(L/2) 3, where L is the mean tumor length measured in 3
dimensions. It was observed that maximum tumor diameter was ~1.2
cm, and the tumor volume in the saline control group reached ~1,000
mm3 at 21 days, which was in accordance with the results
from our previous study (30). The
weights of the tumor and spleen were measured. The spleen index was
calculated as spleen weight (g) × 1,000/body weight (g).
Transmission electron microscopy
(TEM)
Fresh tumor tissues from each group were cut into ~1
mm3 cubes, immersed in 2.5% glutaraldehyde for 2 h,
washed 3 times with 1X PBS, and fixed in 1% citric acid for 1.5 h.
Following washing 3 times, dehydration was performed by a graded
ethanol series with 50, 70 and 90% ethanol, 90% ethanol and 90%
acetone (1:1), and 90% acetone at 4°C followed by 100% acetone at
room temperature. The duration of each step was 15–20 min. The
samples were soaked in propylene oxide and epoxy resin Epon 812
mixture (1:1), and embedded at 37°C in epoxy resin Epon 812. The
cubes were sliced (4 µm) and stained with 2% toluidine blue at room
temperature for 3 min. Ultra-thin sections were placed in 200-µm
copper mesh, stained with uranyl acetate and lead citrate at room
temperature for 3 min, and examined at ×10,000 magnification, under
a transmission electron microscope HT7700 (Hitachi
High-Technologies Corporation) equipped with Hitachi's EMIP-SP
database software (Hitachi High-Technologies Corporation).
Apoptotic assay
The surface fiber membrane of tumor tissues was
removed and tissues were placed on ice, smashed and ground to
obtain a single cell suspension. The late apoptotic rate of tumor
cells was evaluated using Annexin V-FITC Apoptosis Detection Kit I
(BD Biosciences) according to the manufacturer's protocol. Cells
were incubated with 5 µl/ml Annexin V-FITC for 30 min in the dark
and florescence was measured using a BD FACSCanto™ II flow
cytometer (BD Biosciences).
Cell cycle analysis
Single cell suspension was obtained as described
above. Cell concentration was adjusted to 2×106/ml.
Cells from different groups were seeded in a 6-well plate and
treated with saline, 5-FU, low-dose dauricine or high-dose
dauricine at 37°C for 24 h. Cells were fixed with 70% ethanol
overnight at −20°C, washed and resuspended. A total of 1 ml of the
cell suspension was incubated with 1.6 ml RNase A (1 mg/ml) at 37°C
for 30 min and centrifuged at 12,000 × g for 5 min. The cell pellet
was suspended in 0.4 ml PBS, mixed with PI at 100 µg/ml for 10 min
at room temperature and subjected to cell cycle analysis by flow
cytometry using a BD FACSCanto™ II flow cytometer (BD
Biosciences).
Immunohistochemical analysis
Tumor tissues were harvested, fixed in 10% formalin
for 4 h at room temperature, dehydrated by a graded ethanol series,
and placed in xylene for 30 min at room temperature. Tissues were
embedded in paraffin, sectioned into 4-µm thick slices using a
microtome and mounted on Poly-L-lysine slides. Following
deparaffination by immersing the slides in xylene for 3 min twice,
1:1 ×ylene, 100% ethanol for 3 min, and a graded descending ethanol
series (100–50%), slides were washed by running tap water and
incubated with 3% H2O2 for 10 min at room
temperature, rinsed with distilled water and heated in a microwave
oven for 5 min for antigen retrieval. Slides were blocked with 0.5%
BSA at room temperature for 20 min, incubated with respective
primary antibodies (1:1,000 dilution) for 2 h at 37°C and rinsed
with PBS for 5 min, followed by the addition of the secondary
antibody (1:10,000 dilution) at 37°C for 30 min. Following rinsing
with PBS for 3 min, the slides were incubated with
3,3′-diaminobenzidine for 10 min at room temperature, washed with
water and counterstained with hematoxylin for 5 min. The slides
were covered, sealed and examined under a light microscope (Olympus
Corporation). Seven fields of view (×100 magnification) were
randomly selected and photographed. The integrated optical density
(OD) of positive cells was analyzed and compared using the Motic
Med 6.0 digital medical image analysis system (Motic Instruments
Inc.).
Western blot analysis
Tumor tissues were homogenized in protein extraction
reagent (cat. no. P0028-1; Beyotime Institute of Biotechnology) and
centrifuged at 15,000 × g for 10 min at 4°C. Total protein was
quantified using a bicinchoninic acid assay, denatured at 100°C for
5 min, and stored at −80°C. Protein samples (20 µg) were separated
by electrophoresis for 1 h at 75 V on a 10% SDS-PAGE gel. The
proteins were transferred onto a PVDF membrane, blocked with 5% BSA
overnight at 4°C, and incubated with primary antibodies against
GAPDH (1:3,000), Shh (1:800), Ptch (1:1,000), Smo (1:2,000) and
Gli1 (1:2,000) at 37°C for 2 h. The membrane was washed, incubated
with horseradish peroxidase-conjugated secondary antibodies at 37°C
for 1 h, and examined using a chemiluminescence detection kit. The
images were captured and analyzed with an HMIAS-2000
High-Definition Color Medical Analysis system (Wuhan Qianping
Imaging Technology Co., Ltd.). GAPDH was used as an internal
control. The results are expressed as the ratio of density of
individual target proteins to the expression of respective
GAPDH.
RT-quantitative polymerase chain
reaction (RT-qPCR)
mRNA from tumor tissues was extracted using TRIzol
according to the manufacturer's protocol. A total of 500 ng RNA was
mixed in a 10 µl reaction containing 2 µl 5X PrimeScript Buffer,
0.5 µl PrimeScript RT Enzyme Mix, 0.5 µl Oligo dT Primer (50 µM),
and 0.5 µl Random 6 mers (100 µM). The reaction mixture was
incubated under the following condition: 37°C for 15 min, and 85°C
for 5 sec. SYBR Premix Ex Taq was used for PCR reaction. The qPCR
system was prepared according to the manufacturer's instructions.
Primers were designed and synthesized by Shanghai Shenggong Biology
Engineering Technology Service, Ltd. The primer sequences were as
follows: Shh forward, 5′-GCTCGGTGAAAGCAGAGAACT-3′ and reverse
5′-CCAGGAAAGTGAGGAAGTCG-3′; Smo forward, 5′-CTGGTGTGGTTTGGTTTGTG-3′
and reverse, 5′-CAGGTGGAAGTAGGAGGTCTTG-3′; Ptch1 forward,
5′-CTCCTTTGCGGTGGACAA-3′ and reverse, 5′-CCTCAGCCTTATTCAGCATTTC-3′;
Gli1 forward, 5′-ATCCTTACCTCCCAACCTCTGT-3′ and reverse,
5′-CCAACTTCTGGCTCTTCCTGTA-3′; and GAPDH forward,
5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse, 5′-AGGGGCCATCCACAGTCTTC-3′.
GAPDH was used as an internal control. The qPCR thermocycling
conditions were as follows: Initial denaturation at 95°C for 10
sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 34 sec
for amplification. The reaction was performed using an Applied
Biosystems 7300 Real-Time PCR System (Thermo Fisher Scientific,
Inc.). The data were processed using the 2−ΔΔCq method
(32) and gene expression was
expressed as a fold-change relative to the saline group.
Statistical analysis
The statistical software SPSS v21.0 (IBM Corp.) was
used for data analysis. Normally distributed data are expressed as
the mean ± standard deviation. One-way analysis of variance
followed by Tukey's post hoc test was used for multiple group
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Dauricine inhibits the proliferation
of BxPC-3 pancreatic cancer xenografts in mice
Following the injection of BxPC-3 cells, the mice
developed pancreatic cancer xenografts (mean tumor weight in
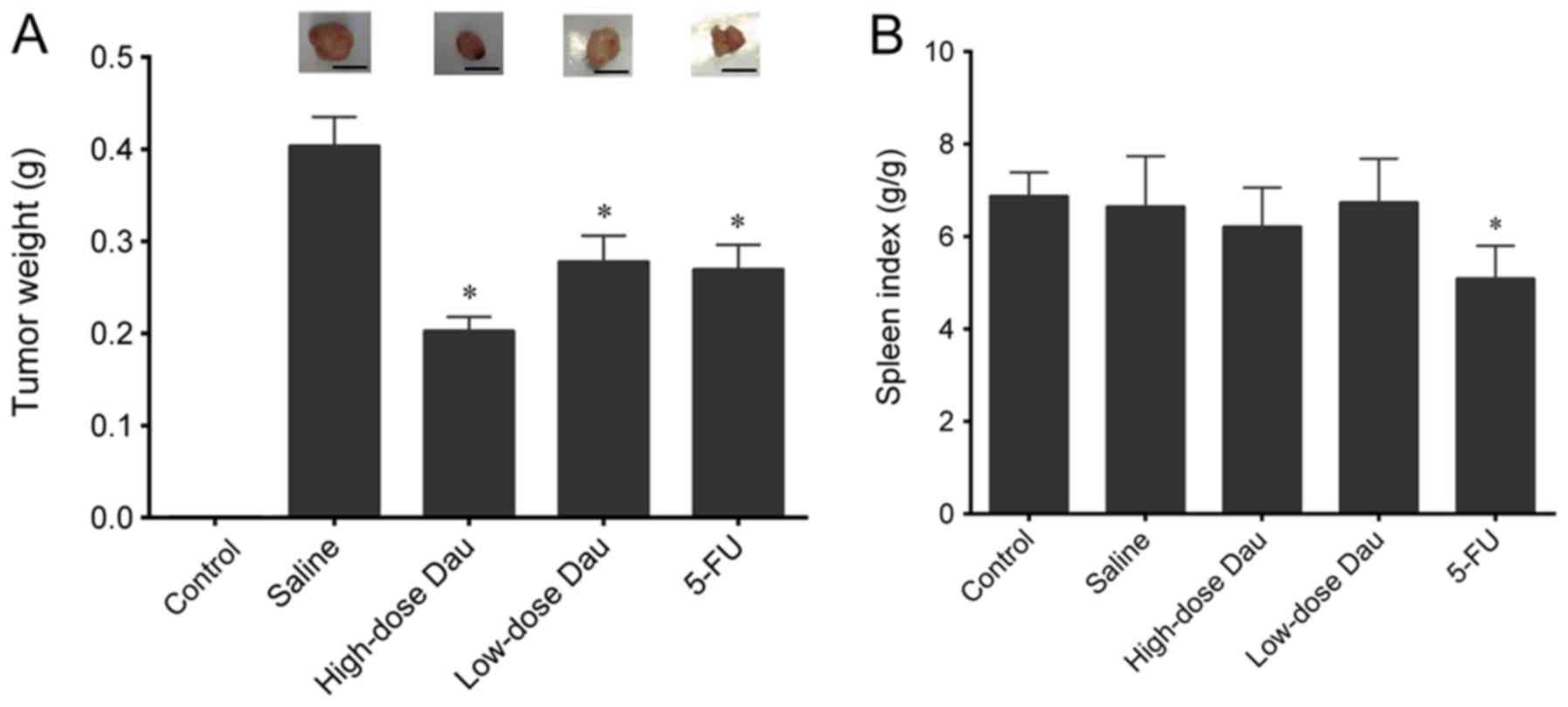
saline-treated mice, 0.4052±0.0309 g) (Fig. 2A). Treatment with 5-FU, low-dose
dauricine or high-dose dauricine significantly inhibited the tumor
growth (0.2739±0.0249, 0.2801±0.0262 and 0.2058±0.0161 g,
respectively; P<0.05) compared with the control saline group.
High-dose dauricine exhibited the most potent antitumor effect, as
indicated by the lowest tumor weight (Fig. 2A). Mice in the control group did not
develop xenografts of pancreatic cancer.
Effects of dauricine on the spleen
index of mice with pancreatic cancer BxPC-3 ×enografts
No significant difference in the spleen index was
observed between mice in the control and saline groups. However,
treatment with 5-FU significantly decreased the spleen index as
compared with the saline group (P<0.05) (Fig. 2B), which suggested that 5-FU may have
an effect on spleen function. No significant differences were
observed between the control and the low-or high-dose dauricine
treatment groups (Fig. 2B), which
indicated that dauricine had no influence on spleen weight and
function.
TEM
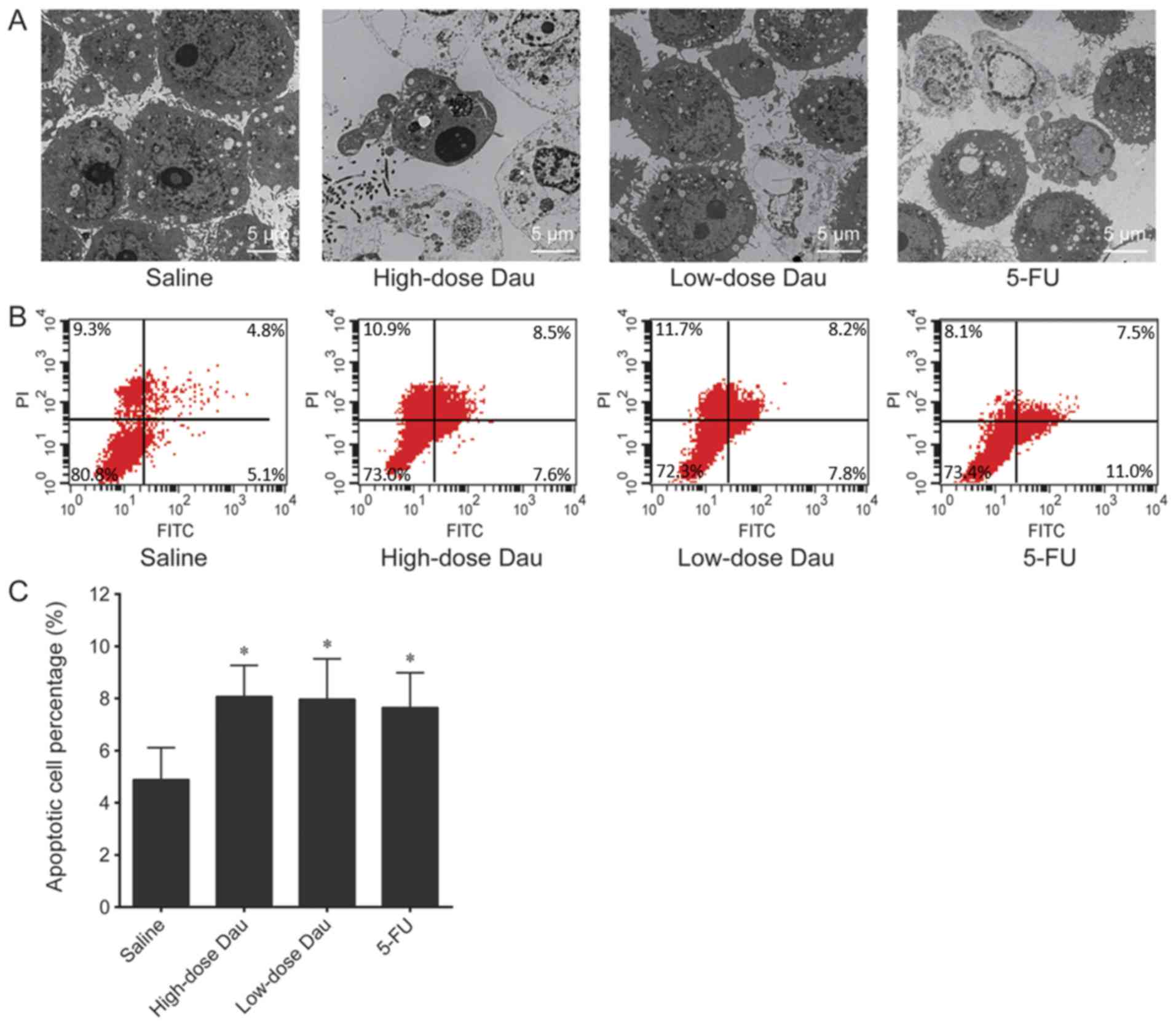
TEM examination demonstrated that tumor cells in the
saline group were elliptical with a large nucleus, high
heterogeneity and a clear double-layered nuclear membrane (Fig. 3A). A high quantity of organelles and
abundant free ribosomes was observed in the cytoplasm.
Mitochondrial cristae were clearly visible. In the high-dose
dauricine group, the tumor cells were small in size and exhibited
nuclear condensation; chromatin clumps were distributed below the
nuclear membrane. Heterochromatin was clustered on one side of the
nucleus in a half-moon shape. The nuclear membrane was clear and
the plasma membrane was intact. Parts of the cytoplasm and
nucleoplasm were detached from the cell body, forming apoptotic
bodies, accompanied by tumor cell necrosis and granulocyte
infiltration. A decrease in the number of free ribosomes was also
observed. Mitochondrial cristae were dissolved and there was an
increase in the number of apoptotic cells (Fig. 3A). In the low-dose dauricine group,
tumor cells were irregular in shape with a clear cell membrane and
an intact nuclear membrane. Nuclear material was condensed and
heterochromatin was clustered on one side of the nucleus.
Mitochondrial vacuoles were degenerated and free ribosomes were
abundant, which was consistent with the early apoptotic state
(Fig. 3A). In the 5-FU group, tumor
cells exhibited a regular shape with a clear nuclear membrane and
obvious nucleoli. Free ribosomes were abundant and the rough
endoplasmic reticulum was slightly expanded. Mitochondrial vacuoles
were generated, and some tumor cells exhibited early apoptosis and
lymphocytic infiltration (Fig.
3A).
Effects of dauricine on apoptosis and
the cell cycle of pancreatic cancer BxPC-3 ×enografts
The apoptotic cell percentages in the saline,
high-low-dose dauricine, and 5-FU groups were 5.0017±1.2329,
8.1800±1.1675, 8.0717±1.5027 and 7.6933±1.3402%, respectively
(Fig. 3B and C). The percentages of
apoptotic cells in the high-and low-dose dauricine, and 5-FU groups
were significantly increased compared with that in the control
group (P<0.05; Fig. 3B and C). No
significant differences were observed between the high-dose
dauricine, low-dose dauricine and 5-FU groups.
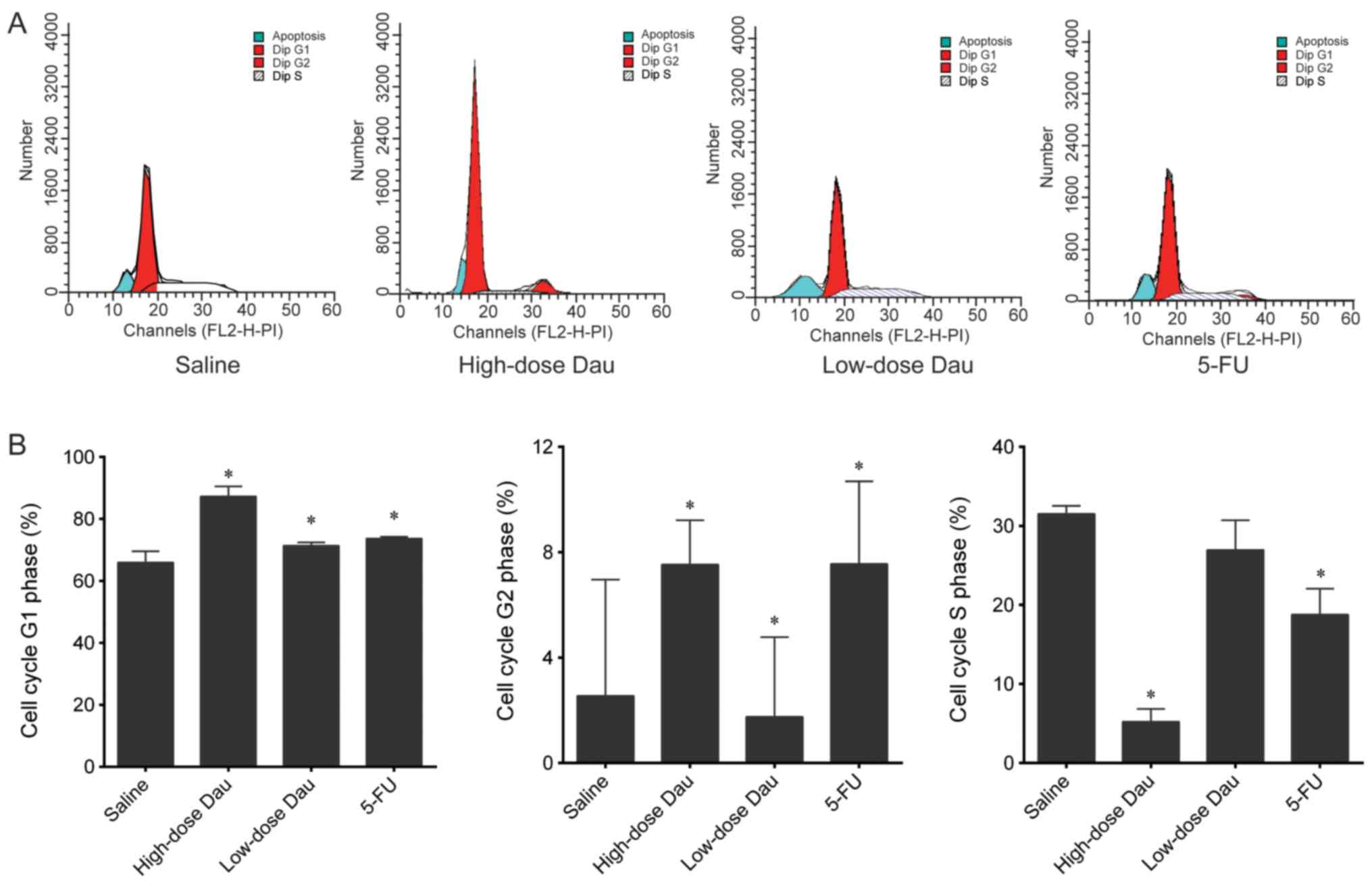
In the high- and low-dose dauricine, and 5-FU
groups, the number of cells in the G1 phase was
significantly higher, whereas the number of cells in the G2 phase
was significantly lower compared with that in the saline group (all
P<0.05; Fig. 4). These results
indicated cell cycle arrest in the G1 phase following
drug treatment. The number of cells in the S phase in the high-dose
dauricine and 5-FU groups was significantly lower compared with
that in the saline group; however, no significant difference was
observed between the low-dose dauricine group and the saline group
(Fig. 4).
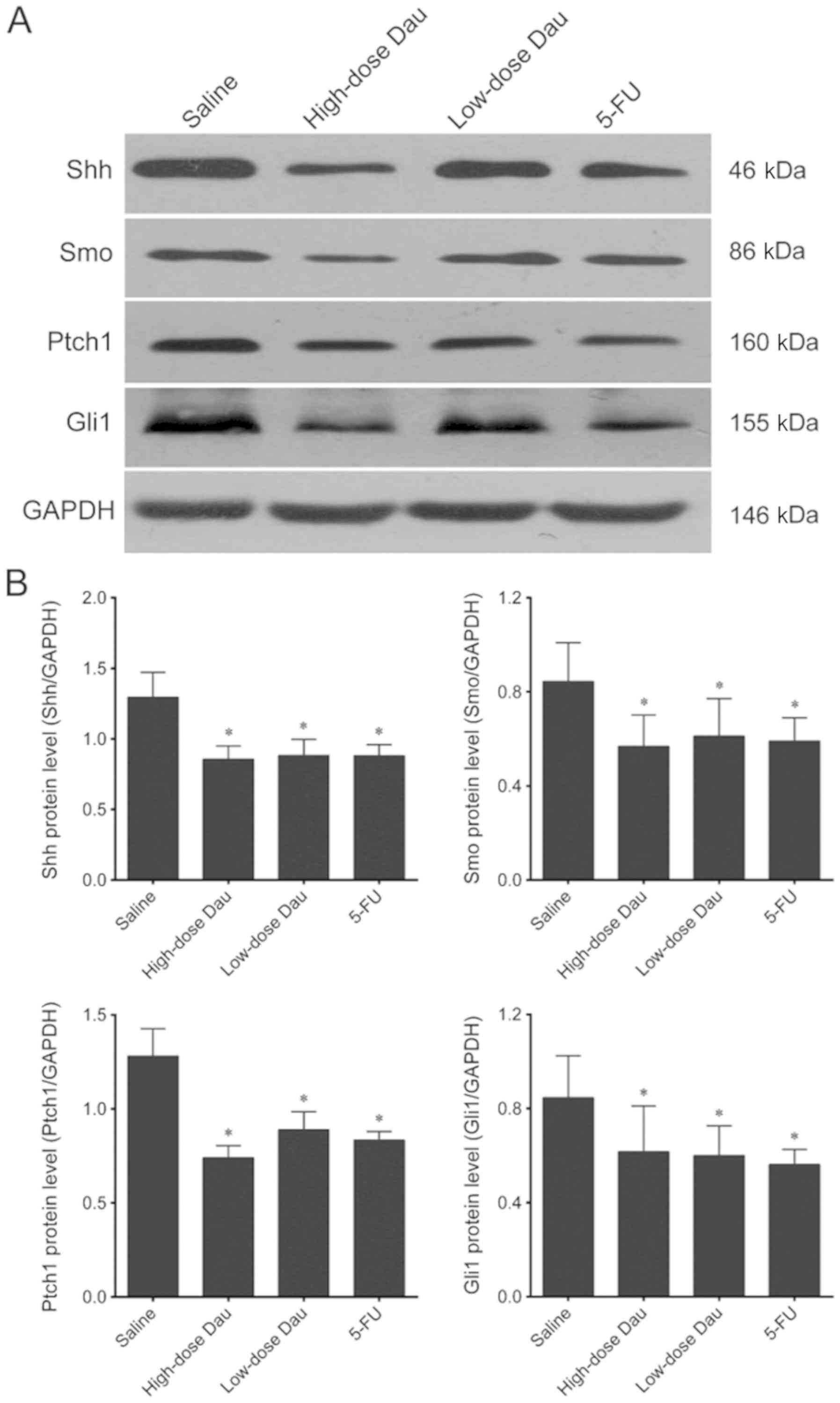
Effects of dauricine on Hh pathway
molecule protein expression levels in pancreatic cancer BxPC-3
×enografts
Immunohistochemistry results demonstrated that the
protein expression levels of Shh, Smo, Ptch1 and Gli1 in the
high-dose dauricine and 5-FU groups were significantly lower
compared with those in the saline group (Fig. 5). This indicated that dauricine may
inhibit the Hh signaling pathway and suppress the growth of cancer
cells. This result was validated by western blotting, which
revealed significantly lower protein expression levels of Shh, Smo,
Ptch1 and Gli1 in the high-dose dauricine and 5-FU groups (Fig. 6).
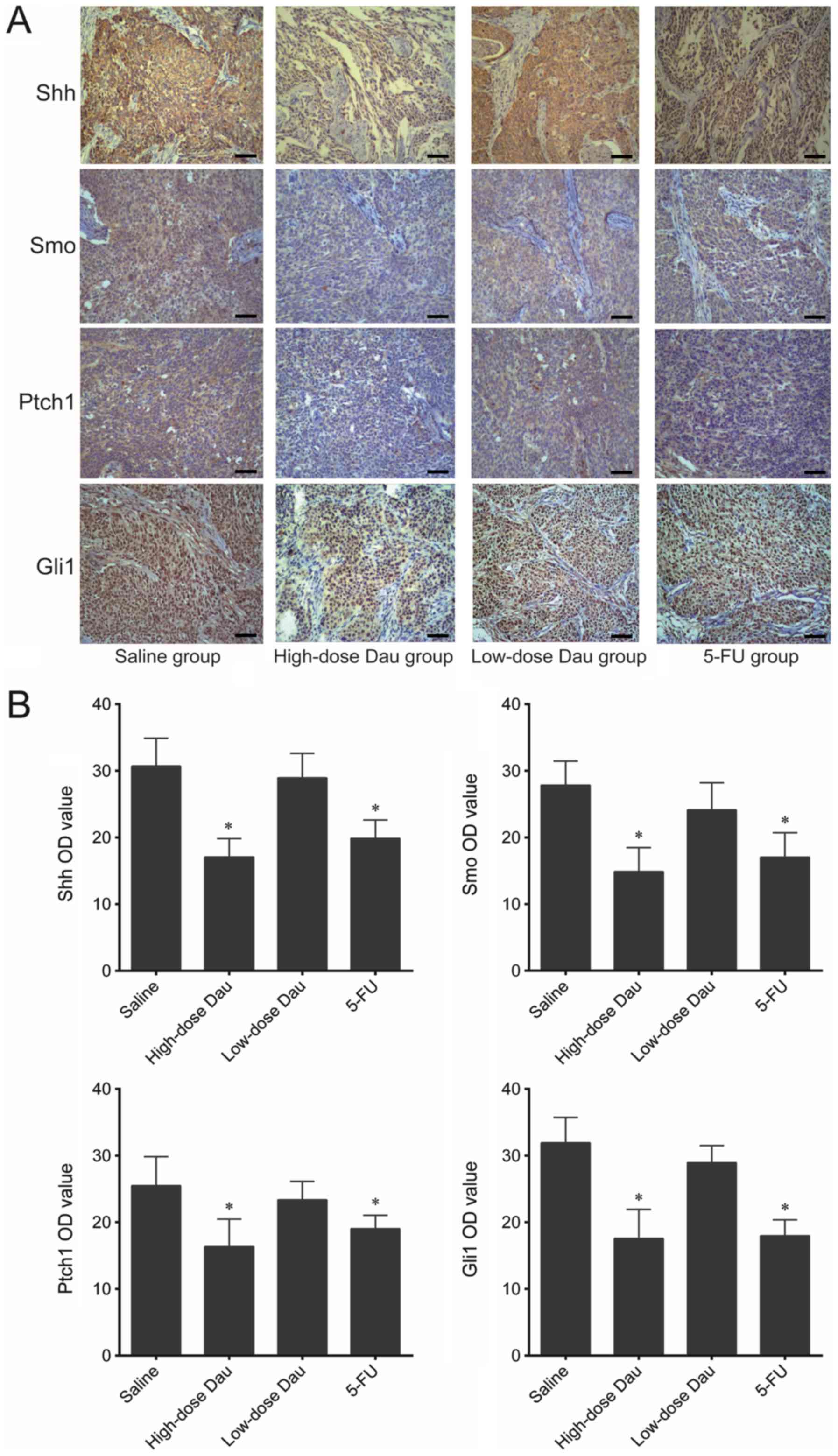 | Figure 5.Immunohistochemistry analysis of Shh,
Smo, Ptch1 and Gli1 in BxPC-3 pancreatic cancer xenografts. (A)
Representative images of immunohistochemistry (×100 magnification).
Scale bar, 200 µm. (B) OD analysis of the immunohistochemistry
results. *P<0.05 vs. saline. Dau, dauricine; 5-FU,
5-fluorouracil; Gli1, glioma-associated oncogene family zinc finger
1; OD, optical density; Ptch1, patched 1; Shh, sonic hedgehog
signaling molecule; Smo, smoothened, frizzled class receptor. |
Western blotting results also demonstrated that Shh,
Smo, Ptch1 and Gli1 protein expression levels in the low-dose
dauricine group were significantly lower compared with those in the
saline group (Fig. 6); however, this
difference was not observed in the immunohistochemistry assay
(Fig. 5).
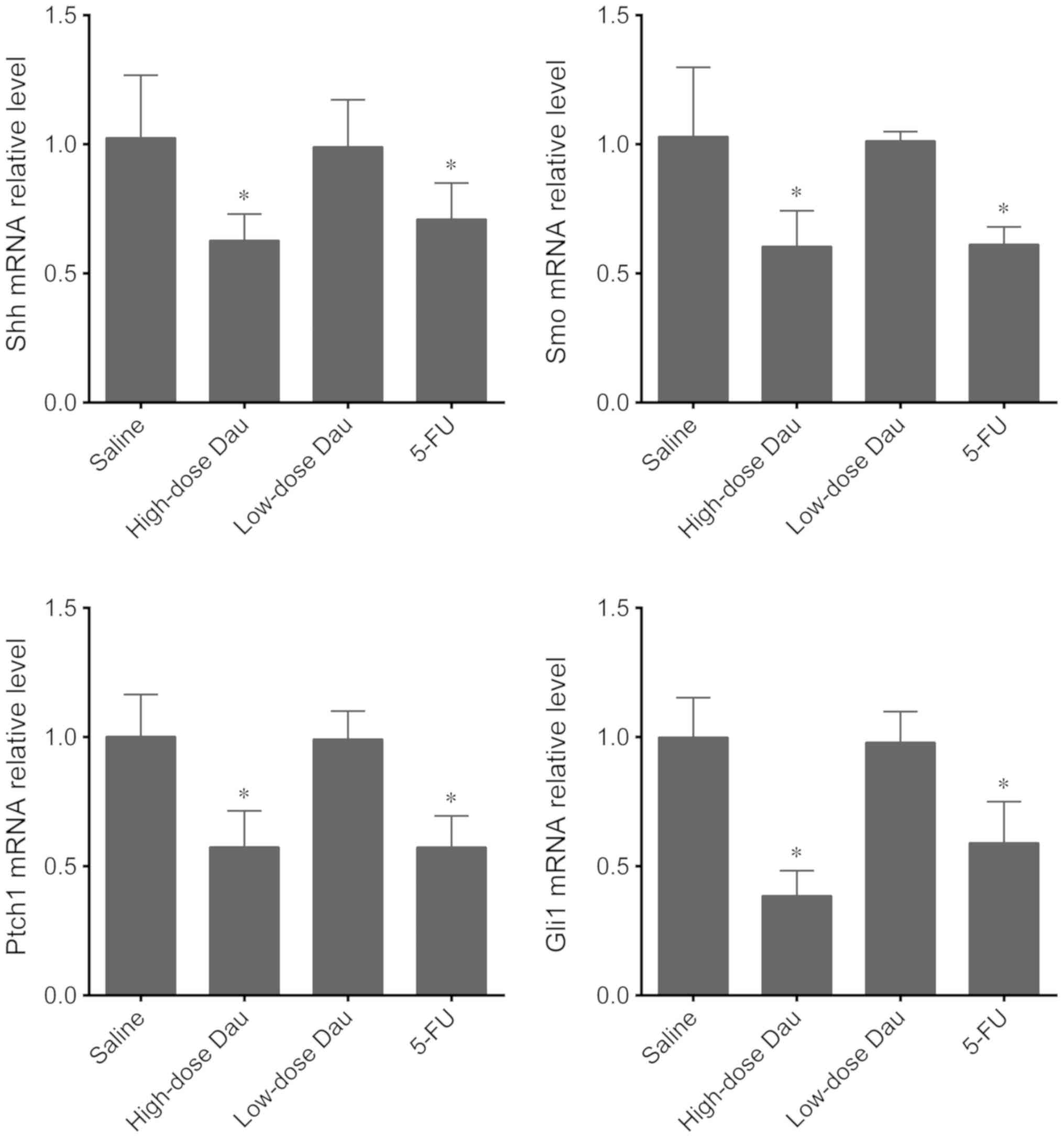
Effects of dauricine on the expression
of genes in the Hh pathway in pancreatic cancer BxPC-3
×enografts
The mRNA expression levels of Shh, Smo, Ptch1 and
Gli1 were further tested in BxPC-3 pancreatic cancer xenografts
following different treatments. The high-dose dauricine and 5-FU
groups exhibited a significant decrease in the expression levels of
these genes compared with the saline group (Fig. 7). However, in the low-dose dauricine
group, the mRNA expression levels of Shh, Ptch1, Smo and Gli1 were
not significantly different compared with those of the saline group
(Fig. 7).
Discussion
Pancreatic cancer is an aggressive and highly
lethal malignancy with limited effective treatment options.
In the present study, the effect of dauricine on BxPC-3 pancreatic
cancer xenografts and the role of Hh signaling pathway as an
underlying molecular mechanism were investigated.
Dauricine, a bioactive component extracted from the
moonseed rhizome, has demonstrated promising pharmacological
attributes in clinical settings (24,30). It
has long been used as a traditional Chinese medicine remedy for
inflammatory disorders. Several studies have investigated the
inhibitory effect of dauricine on tumor growth. In a study by Zhang
et al (31), dauricine
effectively inhibited the viability of four renal cell carcinoma
cell lines through the induction of cell cycle arrest at the
G0/G1 phase and the promotion of apoptosis.
Dauricine has been demonstrated to exhibit an inhibitory effect on
urinary tract tumor cells at minimum drug concentrations of
3.81–5.15 µg/ml (23). In a previous
study, dauricine suppressed cell proliferation and invasion, and
induced the apoptosis of colon cancer cells by inhibiting the NF-κB
pathway and its downstream gene expression (24). Dauricine has been demonstrated to
inhibit angiogenesis in human breast cancer by blocking vascular
endothelial growth factor expression and inducing accumulation of
hypoxia-inducible factor 1 subunit α protein (33). These findings suggest that dauricine
may be a potential therapeutic agent for the treatment of cancer.
However, the antitumor effects of dauricine in the context of
pancreatic cancer have not been reported. A previous study on colon
cancer in mice used 40 mg/kg dauricine injected every 2 days until
day 9 (24), which showed that
dauricine significantly suppressed colonic tumor growth. Our
previous study demonstrated the significant in vivo effects
of a 21-day treatment with 10 and 20 mg/kg phenolic alkaloid
mixture on pancreatic cancer (30).
Therefore, a 21-day treatment with two doses of dauricine (6 and 12
mg/kg body weight) was tested in the present study. H&E
staining was conducted in our previous study (30), which demonstrated that the model was
well established; therefore, it was not performed in the present
study. The absence of H&E staining is a limitation of the
present study, as the morphological changes of pancreatic cancer
tissues in the animal models were not demonstrated. However, 12
mg/kg dauricine treatment exhibited stronger effects in decreasing
the tumor size compared with 6 mg/kg, although 6 mg/kg dauricine
also had a minor inhibitory effect. The effects of dauricine were
comparable to those of 5-FU. 5-FU is a chemotherapeutic agent
widely used for the treatment of a number of cancer types,
including breast (34), skin
(35), stomach (36), gullet (37), bowel (38) and pancreatic cancer (39). 5-FU works as an antimetabolite and
prevents cell proliferation through inhibition of thymidylate
synthase, which in turn prevents nucleotide synthesis and arrests
cell division (40). However, it is
associated with several side effects, including mouth sores,
anorexia, nausea, vomiting, diarrhea, photophobia, leukocytopenia,
erythrocytopenia and thrombocytopenia (41). These side effects increase the risk
of infection, bleeding and anemia (42). In the present study, 5-FU was
selected as the positive control for the evaluation of the effects
of dauricine. Animals treated with 5-FU exhibited abnormal spleen
function, as reflected in the significantly decreased spleen index
compared with that of the saline and control groups. The spleen
index results demonstrated that the subcutaneous injection of
BxPC-3 pancreatic cancer cells and the subsequent growth of cancer
did not affect the spleen weight when compared with the control
mice. Animals in the high-and low-dose dauricine groups also
exhibited no significant differences in spleen index compared with
the saline and control groups. Thus, dauricine may be a safer agent
with fewer side effects compared with 5-FU. The metabolic effects
and pulmonary toxicity of dauricine have been investigated in CD-1
mice in a previous study; mice that received intraperitoneal
injections of dauricine at 50, 100 or 150 mg/kg did not exhibit any
changes in blood urea nitrogen, serum aspartate aminotransferase or
serum alanine aminotransferase, whereas a dose-dependent increase
in lactate dehydrogenase activity was observed in lung lavage
fluids (43). The results of the
present study demonstrated that intraperitoneal injection of
dauricine at 6 and 12 mg/kg significantly inhibited tumor growth in
nude mice with no concomitant changes in the spleen index. However,
the molecular mechanisms that mediate this effect are not
understood.
A potential mechanism of the effects of dauricine in
pancreatic cancer is through promotion of apoptosis and
G1 cell cycle arrest. The TEM results of the present
study revealed that treatment with dauricine and 5-FU induced
apoptosis. The TEM results were further verified by flow cytometry,
which demonstrated an increased number of apoptotic cells following
treatment with dauricine or 5-FU. The effects of dauricine on the
apoptosis of BxPC-3 cells were also tested in vitro (data
not shown) and were similar to the in vivo results. Cell
cycle analysis also demonstrated G1 phase arrest
following drug treatment. The results of the present study were
consistent with a previous study in which dauricine inhibited the
viability and proliferation of renal carcinoma cell lines by
enhancing apoptosis and inducing cell cycle arrest in the
G0/G1 phase, and by activating caspase-9 and
caspase-3 (31). Dauricine has also
been demonstrated to downregulate the expression levels of
anti-apoptotic genes (survivin, BCL2 apoptosis regulator, X-linked
inhibitor of apoptosis and inhibitor of apoptosis protein 1) in
colon cancer cells (24). The
effects of dauricine on pro-and anti-apoptotic gene expression need
to be further investigated in pancreatic cancer cells.
The induction of apoptosis and cell cycle arrest by
dauricine and 5-FU in pancreatic cancer may be mediated by the
inhibition of the Hh signaling pathway. The Hh signaling pathway is
activated in several malignant tumors, including pancreatic,
prostate, liver, ovarian and breast cancer. Genetic alterations in
the Hedgehog signaling pathway were observed in 24 pancreatic
cancer patients studied by a global genomic analysis (18). Additionally, our previous study using
a phenolic alkaloid mixture demonstrated the involvement of the Hh
signaling pathway (30). To the best
of our knowledge, no previous data has been published regarding the
effects of pure dauricine on pancreatic cancer. However, dauricine
has been demonstrated to inhibit cell viability and proliferation
and, to induce apoptosis of renal and colon cancer cells via the
phosphoinositide 3-kinase/protein kinase B and NF-κB signaling
pathways (26,31).
The Hh signaling pathway was first identified in the
common fruit fly (44). The
biological effects of the Hh pathway are exerted through signal
transmission from the cell membrane to the nucleus, which regulates
the balance of activators and repressors of Gli transcription
factors (10,11). The components of the Hh signaling
pathway include Hedgehog ligands, Ptch1, Ptch2 and Smo. Shh
activates Smo by relieving the inhibitory effect of Ptch on Smo
(45). Ptch is a membrane receptor
and a tumor suppressor (26,46–48).
Ptch1 and Ptch2 are homologs of Ptch in humans, which bind with Shh
and activate Smo (49). Smo is a
proto-oncogene and a key factor in the Hh signaling pathway
(50), which activates the
downstream transcription factor of the Gli family. The Gli family
includes three molecules: Gli1, Gli2 and Gli3 (51). Gli1 is a potent transcriptional
activator (10); Gli2 also acts as a
transcriptional activator, whereas Gli3 mainly acts as a
transcriptional repressor (52).
The activation of the Hh signaling pathway is rarely
detected in normal human tissue samples; however, it is necessary
for the survival and proliferation of pancreatic cancer cells
(53). The expression of Hh
molecules is increased in pancreatic cancer cell lines and human
pancreatic cancer tissue samples (54,55). The
proliferation and metastasis of human pancreatic cancer cells have
been demonstrated to be closely associated with abnormal expression
levels of Shh, Ptch1 and Smo (56,57). In
the early stage of pancreatic cancer development, activation of the
Hh signaling pathway induces malignant transformation of the
pancreatic ductal epithelium. Several Hh signaling pathway
inhibitors, including vismodegib and sonidegib, have been developed
for cancer treatment (12,18). Inhibition of the Hh signaling pathway
may be a potential therapeutic target for pancreatic cancer. The
present study identified high mRNA and protein expression levels of
Shh, Ptch1, Smo and Gli1 in pancreatic cancer BxPC-3 ×enografts in
nude mice, which was consistent with the aforementioned
observations. High-dose dauricine and 5-FU significantly decreased
the mRNA and protein expression levels of these molecules in
pancreatic cancer xenografts, which was demonstrated by RT-qPCR,
immunohistochemistry and western blotting. The low-dose dauricine
treatment group exhibited no differences in mRNA expression, but
lower protein expression levels of Shh, Ptch1, Smo and Gli1
compared with the saline group were observed in the western blot,
but not in the immunohistochemistry assay. To date, the inhibitory
effect of dauricine on the Hh signaling pathway has not been
reported. However, some studies have demonstrated that the
inhibitory effects of other natural products on pancreatic cancer
are mediated by the inhibition of the Hh signaling pathway. An
active component from Siegesbeckia glabrescens,
germacranolide sesquiterpene lactone, inhibited Gli-mediated
transcriptional activity in human pancreatic cancer AsPC-1 and
PANC-1 cells, and decreased cancer cell proliferation and the
expression of Gli-targeted genes (51). Deguelin, a natural compound from the
flavonoid family, has been reported to suppress growth, migration
and invasion, and to promote apoptosis in two pancreatic cancer
cell lines, PANC-1 and BxPC-3, through suppression of the Hh
signaling pathway (58). These
findings suggested that suppression of the Hh pathway expression
may represent a new mechanism for the treatment of pancreatic
cancer.
BxPC-3 cells are a wild-type KRAS proto-oncogene,
GTPase (KRAS) pancreatic cancer cell line. KRAS is the most common
mutated oncogene; studies have indicated that 90% of patients with
pancreatic cancer have KRAS mutations (59). However, one recent study demonstrated
that out of 91 patients with pancreatic cancer, 49 had KRAS
mutations, whereas 42 had a wild-type KRAS genotype (60). The study also revealed that mutant
KRAS tumors exhibit higher expression levels of Shh and Ihh
compared with wild-type KRAS tumors, which suggested that
KRAS-mutated tumors may benefit from Hh inhibitors. To date, to the
best of our knowledge, there are no published studies on the
effects of dauricine on mutant KRAS pancreatic cancer cells. Future
work is necessary to compare the antitumor function of dauricine in
wild-type and mutant KRAS pancreatic cancer cells.
In conclusion, high-and low-dose dauricine treatment
suppressed the growth of BxPC-3 pancreatic cancer xenografts in
nude mice with no significant changes in the spleen index.
Dauricine induced apoptosis and cell cycle arrest in tumor cells
from BxPC-3 pancreatic cancer xenografts. The inhibitory effect of
dauricine was likely mediated by suppression of the abnormally
activated Hh signaling pathway, as gene and protein expression
levels of Shh, Ptch1, Smo and Gli1 were decreased following
dauricine treatment. The effects of dauricine were similar to those
of 5-FU. The results of the present study suggest that dauricine
may represent a promising anticancer agent for the treatment of
pancreatic cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by the Heilongjiang
Provincial Department of Education Project (grant nos. 11521323 and
12531788), the Heilongjiang Qiqihar Medical College Doctor
Scientific Research Fund (grant nos. QY2016B-26 and QY2016B-21),
the Heilongjiang Qiqihar Technology Office Fund (grant no.
SFGG-201630), the National Natural Science Foundation of China
(grant nos. 81373777 and 81173599), the Heilongjiang Provincial
Postdoctoral Program (grant no. LBH-Z14196), the China Postdoctoral
Fund Project (grant no. 2015M581496) and the Heilongjiang Province
Natural Science Fund Project (grant no. QC2015101).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YBZ, HXF and JG designed the study. YJZ, HXF, JG,
XJZ, SLW and LLZ collected and analyzed the data. YBZ, HXF and JG
drafted and wrote the manuscript. HXF critically revised the
manuscript. All authors had intellectual input into the study and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All animal protocols were approved by the Animal
Care and Use Committee at the Heilongjiang University of Chinese
Medicine (Harbin, China). All procedures in the animal studies were
performed in accordance with the ethical standards of the
institution or practice.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Veisani Y, Jenabi E, Khazaei S and
Nematollahi S: Global incidence and mortality rates in pancreatic
cancer and the association with the human development index:
Decomposition approach. Public Health. 156:87–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin QJ, Yang F, Jin C and Fu DL: Current
status and progress of pancreatic cancer in China. World J
Gastroenterol. 21:7988–8003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu AA, Jaffee E and Lee V: Current status
of immunotherapies for treating pancreatic cancer. Curr Oncol Rep.
21:602019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moutinho-Ribeiro P, Macedo G and Melo SA:
Pancreatic cancer diagnosis and management: Has the time come to
prick the bubble? Front Endocrinol (Lausanne). 9:7792019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rahib L, Fleshman JM, Matrisian LM and
Berlin JD: Evaluation of pancreatic cancer clinical trials and
benchmarks for clinically meaningful future trials: A systematic
review. JAMA Oncol. 2:1209–1216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ilic M and Ilic I: Epidemiology of
pancreatic cancer. World J Gastroenterol. 22:9694–9705. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeune F, Coriat R, Prat F, Dousset B,
Vaillant JC and Gaujoux S: Pancreatic cancer surgical management.
Presse Med. 48:e147–e158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marigo V, Roberts DJ, Lee SM, Tsukurov O,
Levi T, Gastier JM, Epstein DJ, Gilbert DJ, Copeland NG and Seidman
CE: Cloning, expression, and chromosomal location of SHH and IHH:
Two human homologues of the Drosophila segment polarity gene
hedgehog. Genomics. 28:44–51. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rimkus TK, Carpenter RL, Qasem S, Chan M
and Lo HW: Targeting the sonic hedgehog signaling pathway: Review
of smoothened and GLI inhibitors. Cancers (Basel). 8(pii): E222016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin S, Sun D, Li H, Li X, Pan W, Yan C,
Tang R and Liu X: The effect of SHH-Gli signaling pathway on the
synovial fibroblast proliferation in rheumatoid arthritis.
Inflammation. 39:503–512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Skoda AM, Simovic D, Karin V, Kardum V,
Vranic S and Serman L: The role of the Hedgehog signaling pathway
in cancer: A comprehensive review. Bosn J Basic Med Sci. 18:8–20.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu J, Saiyin H, Fu D and Li J: Stroma-a
double-edged sword in pancreatic cancer: A lesson from targeting
stroma in pancreatic cancer with hedgehog signaling inhibitors.
Pancreas. 47:382–389. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tong W, Qiu L, Qi M, Liu J, Hu K, Lin W,
Huang Y and Fu J: GANT-61 and GDC-0449 induce apoptosis of prostate
cancer stem cells through a GLI-dependent mechanism. J Cell
Biochem. 119:3641–3652. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ding J, Zhou XT, Zou HY and Wu J: Hedgehog
signaling pathway affects the sensitivity of hepatoma cells to drug
therapy through the ABCC1 transporter. Lab Invest. 97:819–832.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song X, Yan L, Lu C, Zhang C, Zhu F, Yang
J, Jing H, Zhang Y, Qiao J and Guo H: Activation of hedgehog
signaling and its association with cisplatin resistance in ovarian
epithelial tumors. Oncol Lett. 15:5569–5576. 2018.PubMed/NCBI
|
|
17
|
Wang X, Wei S, Zhao Y, Shi C, Liu P, Zhang
C, Lei Y, Zhang B, Bai B and Huang Y: Anti-proliferation of breast
cancer cells with itraconazole: Hedgehog pathway inhibition induces
apoptosis and autophagic cell death. Cancer Lett. 385:128–136.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jones S, Zhang X, Parsons DW, Lin JC,
Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et
al: Core signaling pathways in human pancreatic cancers revealed by
global genomic analyses. Science. 321:1801–1806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pasca di Magliano M, Sekine S, Ermilov A,
Ferris J, Dlugosz AA and Hebrok M: Hedgehog/Ras interactions
regulate early stages of pancreatic cancer. Genes Dev.
20:3161–3173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gan H, Liu H, Zhang H, Li Y, Xu X, Xu X
and Xu J: SHh-Gli1 signaling pathway promotes cell survival by
mediating baculoviral IAP repeat-containing 3 (BIRC3) gene in
pancreatic cancer cells. Tumour Biol. 37:9943–9950. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Katoh Y and Katoh M: Hedgehog target
genes: Mechanisms of carcinogenesis induced by aberrant hedgehog
signaling activation. Curr Mol Med. 9:873–886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feldmann G, Dhara S, Fendrich V, Bedja D,
Beaty R, Mullendore M, Karikari C, Alvarez H, Iacobuzio-Donahue C,
Jimeno A, et al: Blockade of hedgehog signaling inhibits pancreatic
cancer invasion and metastases: A new paradigm for combination
therapy in solid cancers. Cancer Res. 67:2187–2196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Li Y, Zu XB, Chen MF and Qi L:
Dauricine can inhibit the activity of proliferation of urinary
tract tumor cells. Asian Pac J Trop Med. 5:973–976. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Z, Li C, Wang X, Zhai C, Yi Z, Wang
L, Liu B, Du B, Wu H, Guo X, et al: Dauricine induces apoptosis,
inhibits proliferation and invasion through inhibiting NF-kappaB
signaling pathway in colon cancer cells. J Cell Physiol.
225:266–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen S, Liu L, Yang Y, Dai Z and Zeng F:
Metabolism of dauricine and identification of its main metabolites.
J Tongji Med Univ. 20:253–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Y, Tian X, Xie X, Zhuang Y, Wu W and
Wang W: Expression and regulation of hedgehog signaling pathway in
pancreatic cancer. Langenbecks Arch Surg. 395:515–525. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li YH and Gong PL: Neuroprotective effect
of dauricine in cortical neuron culture exposed to hypoxia and
hypoglycemia: Involvement of correcting perturbed calcium
homeostasis. Can J Physiol Pharmacol. 85:621–627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu QN, Zhang L, Gong PL, Yang XY and Zeng
FD: Inhibitory effects of dauricine on early afterdepolarizations
and L-type calcium current. Can J Physiol Pharmacol. 87:954–962.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao J, Lian Y, Lu C, Jing L, Yuan H and
Peng S: Inhibitory effects of a bisbenzylisoquinline alkaloid
dauricine on HERG potassium channels. J Ethnopharmacol.
141:685–691. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou ZG, Zhang CY, Fei HX, Zhong LL and
Bai Y: Phenolic alkaloids from Menispermum dauricum inhibits
BxPC-3 pancreatic cancer cells by blocking of Hedgehog signaling
pathway. Pharmacogn Mag. 11:690–697. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang S, Ren Y and Qiu J: Dauricine
inhibits viability and induces cell cycle arrest and apoptosis via
inhibiting the PI3K/Akt signaling pathway in renal cell carcinoma
cells. Mol Med Rep. 17:7403–7408. 2018.PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang XD, Zhou X and Zhou KY: Dauricine
inhibits insulin-like growth factor-I-induced hypoxia inducible
factor 1alpha protein accumulation and vascular endothelial growth
factor expression in human breast cancer cells. Acta Pharmacol Sin.
30:605–616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deveci HA, Nazıroğlu M and Nur G:
5-Fluorouracil-induced mitochondrial oxidative cytotoxicity and
apoptosis are increased in MCF-7 human breast cancer cells by TRPV1
channel activation but not Hypericum perforatum treatment. Mol Cell
Biochem. 439:189–198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cunningham TJ, Tabacchi M, Eliane JP,
Tuchayi SM, Manivasagam S, Mirzaalian H, Turkoz A, Kopan R,
Schaffer A, Saavedra AP, et al: Randomized trial of calcipotriol
combined with 5-fluorouracil for skin cancer precursor
immunotherapy. J Clin Invest. 127:106–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mahlberg R, Lorenzen S, Thuss-Patience P,
Heinemann V, Pfeiffer P and Möhler M: New perspectives in the
treatment of advanced gastric cancer: S-1 as a novel oral 5-fu
therapy in combination with cisplatin. Chemotherapy. 62:62–70.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu J, Wang Z, Wu K, Li J, Chen W, Shen Y
and Guo S: Paclitaxel or 5-fluorouracil/esophageal stent
combinations as a novel approach for the treatment of esophageal
cancer. Biomaterials. 53:592–599. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bash-Imam Z, Thérizols G, Vincent A,
Lafôrets F, Polay Espinoza M, Pion N, Macari F, Pannequin J, David
A, Saurin JC, et al: Translational reprogramming of colorectal
cancer cells induced by 5-fluorouracil through a miRNA-dependent
mechanism. Oncotarget. 8:46219–46233. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shim IK, Yi HJ, Yi HG, Lee CM, Lee YN,
Choi YJ, Jeong SY, Jun E, Hoffman RM, Cho DW and Kim SC:
Locally-applied 5-fluorouracil-loaded slow-release patch prevents
pancreatic cancer growth in an orthotopic mouse model. Oncotarget.
8:40140–40151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, Han Q and Zhang H: Evaluation of
the toxicity of 5-fluorouracil on three digestive enzymes from the
view of side effects. Spectrochim Acta A Mol Biomol Spectrosc.
220:1171052019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Esin E, Telli TA, Yuce D and Yalcin S: A
correlation study of fluorouracil pharmacodynamics with clinical
efficacy and toxicity. Tumori. 104:157–164. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jin H, Dai J, Chen X, Liu J, Zhong D, Gu Y
and Zheng J: Pulmonary toxicity and metabolic activation of
dauricine in CD-1 mice. J Pharmacol Exp Ther. 332:738–746. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mohler J: Requirements for hedgehog, a
segmental polarity gene, in patterning larval and adult cuticle of
Drosophila. Genetics. 120:1061–1072. 1988.PubMed/NCBI
|
|
45
|
Du Z, Zhou F, Jia Z, Zheng B, Han S, Cheng
J, Zhu G and Huang P: The hedgehog/Gli-1 signaling pathways is
involved in the inhibitory effect of resveratrol on human
colorectal cancer HCT116 cells. Iran J Basic Med Sci. 19:1171–1176.
2016.PubMed/NCBI
|
|
46
|
Mizukoshi K, Koyama N, Hayashi T, Zheng L,
Matsuura S and Kashimata M: Shh/Ptch and EGF/ErbB cooperatively
regulate branching morphogenesis of fetal mouse submandibular
glands. Dev Biol. 412:278–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang LW, Lin H, Lu Y, Xia W, Gao J and Li
ZS: Sonic hedgehog expression in a rat model of chronic
pancreatitis. World J Gastroenterol. 20:4712–4717. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kayed H, Kleeff J, Keleg S, Guo J,
Ketterer K, Berberat PO, Giese N, Esposito I, Giese T, Buchler MW
and Friess H: Indian hedgehog signaling pathway: Expression and
regulation in pancreatic cancer. Int J Cancer. 110:668–676. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gao J, Li Z, Chen Z, Shao J, Zhang L, Xu
G, Tu Z and Gong Y: Antisense Smo under the control of the PTCH1
promoter delivered by an adenoviral vector inhibits the growth of
human pancreatic cancer. Gene Ther. 13:1587–1594. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Han JB, Hua YQ, Chen LY and Liu LM:
Advances in Smoothened-targeting therapies for pancreatic cancer:
Implication for drug discovery from herbal medicines. Zhong Xi Yi
Jie He Xue Bao. 10:256–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee HJ, Wu Q, Li H, Bae GU, Kim AK and Ryu
JH: A sesquiterpene lactone from Siegesbeckia glabrescens
suppresses Hedgehog/Gli-mediated transcription in pancreatic cancer
cells. Oncol Lett. 12:2912–2917. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bai CB, Stephen D and Joyner AL: All mouse
ventral spinal cord patterning by hedgehog is Gli dependent and
involves an activator function of Gli3. Dev Cell. 6:103–115. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gupta S, Takebe N and Lorusso P: Targeting
the Hedgehog pathway in cancer. Ther Adv Med Oncol. 2:237–250.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hao K, Tian XD, Qin CF, Xie XH and Yang
YM: Hedgehog signaling pathway regulates human pancreatic cancer
cell proliferation and metastasis. Oncol Rep. 29:1124–1132. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hidalgo M and Maitra A: The hedgehog
pathway and pancreatic cancer. N Engl J Med. 361:2094–2096. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kelleher FC: Hedgehog signaling and
therapeutics in pancreatic cancer. Carcinogenesis. 32:445–451.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dosch JS, Pasca di Magliano M and Simeone
DM: Pancreatic cancer and hedgehog pathway signaling: New insights.
Pancreatology. 10:151–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zheng W, Lu S, Cai H, Kang M, Qin W, Li C
and Wu Y: Deguelin inhibits proliferation and migration of human
pancreatic cancer cells in vitro targeting hedgehog pathway. Oncol
Lett. 12:2761–2765. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lennerz JK and Stenzinger A: Allelic ratio
of KRAS mutations in pancreatic cancer. Oncologist. 20:e8–e9. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Agarwal A and Saif MW: KRAS in pancreatic
cancer. Jop. 15:303–305. 2014.PubMed/NCBI
|