Introduction
Colorectal cancer (CRC) is one of the most commonly
diagnosed cancers worldwide; according to GLOBOCAN estimates, there
were 1,801,000 new cases of CRC in 2018 worldwide (1). The risk of CRC is increased up to three
times by diabetes mellitus, predominately type 2 diabetes mellitus
(T2DM) (2–4). T2DM is a chronic metabolic disorder
associated with high mortality and morbidity. Patients with
diabetes have an increased risk of various malignancies due to
numerous putative mechanisms, including hyperinsulinemia,
hyperglycemia and inflammation (5).
T2DM has been demonstrated to increase CRC aggressiveness and
mortality (6,7). The key molecular mechanisms by which
T2DM increases the incidence or worsens the prognosis of patients
with CRC are associated with nutrient-sensing pathways coupling
energy metabolism to signals of cell growth and survival, which are
often dysregulated in diabetes, and may be important contributors
to cancer development in patients with diabetes (8). The pathophysiology of T2DM involves
enhanced oxidative stress induced by high plasma glucose, lipid and
cytokine levels, which can trigger endoplasmic reticulum (ER)
stress (9).
Anterior gradient 2 (AGR2) protein, coded by the
AGR2 gene, has recently been described as an important
regulator of ER stress. AGR2 is inducible by ER stress, which is
associated with the acquisition of a pro-inflammatory phenotype
(10). The presence of AGR2 has been
detected in a wide range of human malignancies, including CRC
(11,12). Functionally, AGR2 belongs to the
protein disulfide isomerase family with all the key features of an
ER-resident protein responsible for maintaining ER homeostasis
(13,14). Metformin, which is a biguanide
derivative, is a first-line drug used in T2DM treatment worldwide
(15). Considering the
epidemiological evidence between T2DM and an increased risk of CRC,
the impact of metformin therapy on the incidence and outcome of CRC
has been intensively studied (16).
The beneficial effects of metformin for patients with CRC and
diabetes are already supported by recent clinical trials, which
reported prolonged overall survival for metformin users compared
with nonusers (17). Studies on CRC
cell lines have revealed that metformin inhibits cell proliferation
and migration by arresting the cell cycle in the
G0/G1 phase, and by transient downregulation
of c-Myc and insulin-like growth factor receptor 1 (18). A recent study on the effects of
metformin on ER stress demonstrated that metformin acts as a
modulator of ER stress in patients with T2DM by promoting an
adaptive unfolded protein response (19). Studies on metformin in combination
with 5-fluorouracil (5-FU) and/or oxaliplatin, which are drugs
routinely used during standard chemotherapy of patients with CRC,
confirmed its synergistic anti-cancer effects (20). The potential association between
metformin and AGR2 expression has been demonstrated by
transcriptomic analysis identifying AGR2 as one of the most
downregulated genes in pancreatic cancer cells exposed to combined
treatment with metformin and aspirin (21). Since AGR2 has been identified as a
putative marker of chemoresistance (22–24), and
AGR2 silencing may sensitize tumor cells to ER stress-induced
autophagy (13), the aim of the
present study was to elucidate the role of AGR2 in CRC cells
exposed to metformin in combination with 5-FU and oxaliplatin.
Materials and methods
Cell lines and culture
Human epithelial colorectal adenocarcinoma cell
lines DLD-1 and SW480 (American Type Culture Collection) were
maintained in high-glucose Dulbecco's Modified Eagle's Medium
(Sigma-Aldrich; Merck KGaA) supplemented with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.), 1% pyruvate and 1%
penicillin/streptomycin at 37°C in a humidified atmosphere with 5%
CO2. Unless otherwise stated, cells were grown to 70–80%
confluence prior to treatment. In addition, unless otherwise
stated, the cells were treated with the following concentrations of
drugs: 1 µM bafilomycin A1, (Cell Signaling Technology, Inc.), 5 µM
5-FU (Sigma Aldrich; Merck KGaA), 2 µM oxaliplatin (PLIVA Lachema)
and 5 mM metformin (Sigma Aldrich; Merck KGaA).
Transfection
AGR2-knockout (KOAGR2) DLD-1 cells were
prepared using CRISPR/Cas9 technology. Briefly, the guide RNA
oligonucleotide (5′-AGAGATACCACAGTCAAACC-3′) that targets exon 2 of
the human AGR2 gene (ENSE00003623642) was designed using
Tools for Guide Design (zlab.bio/guide-design-resources). The guide
RNA specifically targeted mRNA coding 21–27 aa of the AGR2
N-terminal region, which is important for AGR2 protein-mediated
cell adhesion. The GFP-scrambled sequence
(5′-AACAGTCGCGTTTGCGACTGG-3′) served as a control (25). Both sequences were cloned into a
LentiCRISPR-v2 vector (cat. no. 52961; Addgene) using Esp3I
restriction cloning. DLD-1 cells (1×106 cells) were
transfected with LentiCRISPR-v2_AGR2 or LentiCRISPR-v2_scrambled
(scr). After 2 days, the cells were exposed to puromycin (2 µg/ml)
and the pool of resistant cells was sorted and seeded as single
colonies in 96-well plates. KOAGR2 cells were tested for AGR2
expression using western blotting. Two clones with an undetectable
expression of AGR2, DLD1 KOAGR2 A9 and F5, were selected for
further experiments. SW480 cells (1×106) were
transiently transfected using an Amaxa Nucleofector II (Lonza Group
Ltd.) with 2 µg pcDNA3-AGR2 or empty pcDNA3 plasmid
(Invitrogen; Thermo Fisher Scientific, Inc.), which served as a
control, and were subsequently selected with G-418 (400 µg/ml;
Sigma-Aldrich; Merck KGaA). A pool of resistant cells was tested
for positive AGR2 expression with western blot analysis.
To determine the doubling time of the transfected
cancer cell lines with manipulated AGR2 gene expression
compared with untransfected cells, equal numbers of cells
(5×105) were seeded into the complete media and
maintained under the standard conditions for 48 h. The culture
medium was removed, and adherent cells were detached by 0.5%
trypsin (Gold Biotechnology, Inc.) and counted using CASY Model TT
cell counter (Roche Diagnostics).
Western blot analysis
Cells were washed twice with cold phosphate-buffered
saline (PBS). The cells were then scraped into NET lysis buffer
[150 mM NaCl, 1% NP-40, 50 mM Tris (pH 8.0), 50 mM NaF, 5 mM EDTA
(pH 8.0)] supplemented with Protease and Phosphatase Inhibitor
Cocktail (Sigma-Aldrich; Merck KGaA) according to the
manufacturer's instructions. Protein concentration was determined
using the Bradford method and 15 µg proteins were loaded onto 10%
acrylamide gels. Following SDS-PAGE, the samples were transferred
to nitrocellulose membranes, blocked with 5% milk diluted in PBS
supplemented with 1% Tween for 1 h at room temperature and
incubated overnight at 4°C with primary antibodies against
phosphorylated-AMPKα at Thr172 (p-AMPK; 1:1,000; cat. no. 2535;
Cell Signaling Technology, Inc.), β-actin and p62 (both 1:1,000;
cat. nos. sc-47778 and sc-28359; Santa Cruz Biotechnology, Inc.),
microtubule-associated proteins 1A/1B light chain 3 (LC3)-II
(1:1,000; cat. no. NB100-2220; Novus Biologicals, LLC) and AGR2 (in
house) (26). The membranes were
washed and probed with horseradish peroxidase-conjugated
anti-rabbit and anti-mouse secondary antibodies (1:1,000; cat. nos.
P0217 and P0161; Dako) for 1 h at room temperature.
Chemiluminescent signals were developed using a mixture of 1:1
ECL-A and ECL-B solutions [ECL-A: 0.5 M EDTA (pH 8), 90 mM coumaric
acid, 1 M luminol, 200 mM Tris (pH 9.4) and ECL-B: 0.5 M EDTA (pH
8), 0.123 g NaBO3 × 4H2O, 50 mM
CH3COONa (pH 5)] and visualized with the SYNGENE G:BOX
Chem XX6 gel doc system (Syngene; Synoptics Ltd.).
Clonogenic assay
Cells were plated at a density of 250 cells/well in
a 6-well plate. Following 24-h incubation at 37°C with 5%
CO2, 500 µM metformin, 5 µM 5-FU and 2 µM oxaliplatin
were applied and further incubation for ~10 days was performed. The
medium was removed, and the colonies were stained with 1% crystal
violet at room temperature for 20 min and counted. Recombinant
extracellular AGR2 (eAGR2) was prepared as described previously
(27) and was used in the clonogenic
assay as an extracellular protein supplied in the medium (1 ng/ml)
throughout the experiment.
Determination of mitochondrial
depolarization
To analyze mitochondrial depolarization, the JC-1
probe was used. At 24 h post-treatment, ~2×105 cells
were harvested and washed with PBS, stained with 5 µg/ml JC-1 dye
(Invitrogen; Thermo Fisher Scientific, Inc.) for 30 min at 37°C and
analyzed using a BD FACSAria III flow cytometer (BD Biosciences).
Viable population of cells was gated according to forward scatter
(FSC) vs. side scatter (SSC). Individual cells were gated by FSC-A
vs. FSC-H parameters. The ratio of green (FITC) to red
(phycoerythrin) fluorescence was analyzed by FCS Express version 4
software (De Novo Software) as a loss of mitochondrial potential
(Δψm). Treatment with valinomycin (25 µM; Thermo Fisher Scientific,
Inc.) for 2 h at 37°C was used as a positive control for
mitochondrial depolarization.
Detection of reactive oxygen species
(ROS) production
CM-H2DCFDA was used as an indicator for ROS in CRC
cells. Briefly, the cells were seeded in 96-well plates
(4×103 cells/well) and incubated at 37°C overnight. The
next day, the cells were treated with respective drugs diluted in
Hank's Balanced Salt Solution (HBSS; Sigma-Aldrich; Merck KGaA) for
6 h at 37°C. Hydrogen peroxide (50 µM; Penta s.r.o.) served as a
positive control, whereas N-acetyl cysteine (10 mM; Sigma-Aldrich;
Merck KGaA) was used to block ROS production. Subsequently, the
drugs were removed, and 5 µM CM-H2DCFDA (Thermo Fisher
Scientific, Inc.) in HBSS was added for 30 min at 37°C. The cells
were washed twice with HBSS, and the fluorescence was examined
using an Infinite 100 plate reader (Tecan Group, Ltd.).
Statistical analysis
Statistical analyses were performed using the Online
Web Statistical Calculators for Categorical Data Analysis
(https://astatsa.com). One-way ANOVA with post-hoc
Tukey HSD calculator was used to determine statistically
significant differences between the groups. For western blot
analyses, the protein level changes were first normalized to
β-actin and were then statistically analyzed. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of metformin in combination
with 5-FU and oxaliplatin on CRC cells with manipulated AGR2
expression
Two clones with AGR2 gene knockout, KOAGR2 A9
and KOAGR2 F5, were prepared by CRISPR/Cas9 using DLD-1 cells
(Fig. 1A). SW480 cells with no
detectable AGR2 expression were used as the second model; an
AGR2-knock-in SW480 cell line was prepared by stable
transfection (Fig. 1A). The effects
of AGR2 expression on cell proliferation were tested in DLD-1 and
SW480 cells. Consistently with previous studies (28,29), the
results demonstrated a positive effect of AGR2 expression on cell
proliferation rate in the two models (Fig. 1B). The cells were then exposed to
selected drugs to determine their effects on the phosphorylation of
AMPK. It has previously been reported that the actions of metformin
are attributable to AMPK (30).
Metformin treatment exhibited a negligible effect on AMPK
phosphorylation in DLD-1 scr cells. Treatment with metformin in
combination with 5-FU or oxaliplatin induced a moderate increase in
AMPK phosphorylation (Fig. 1C).
Notably, a significant increase in the expression of p-AMPK was
observed in both KOAGR2 clones treated with 5-FU and oxaliplatin in
combination with metformin (Fig.
1C). To confirm the involvement of AGR2 in the regulation of
AMPK activation, the same experiment was performed using SW480
cells. A non-significant increase in p-AMPK expression was observed
in SW480 cells without AGR2 (SW480-pcDNA3) treated with the
combination of metformin and 5-FU (Fig.
1D). Since both A9 and F5 KOAGR2 clones showed very similar
p-AMPK/AMPK expression patterns, subsequent experiments were
performed with only KOAGR2 clone A9.
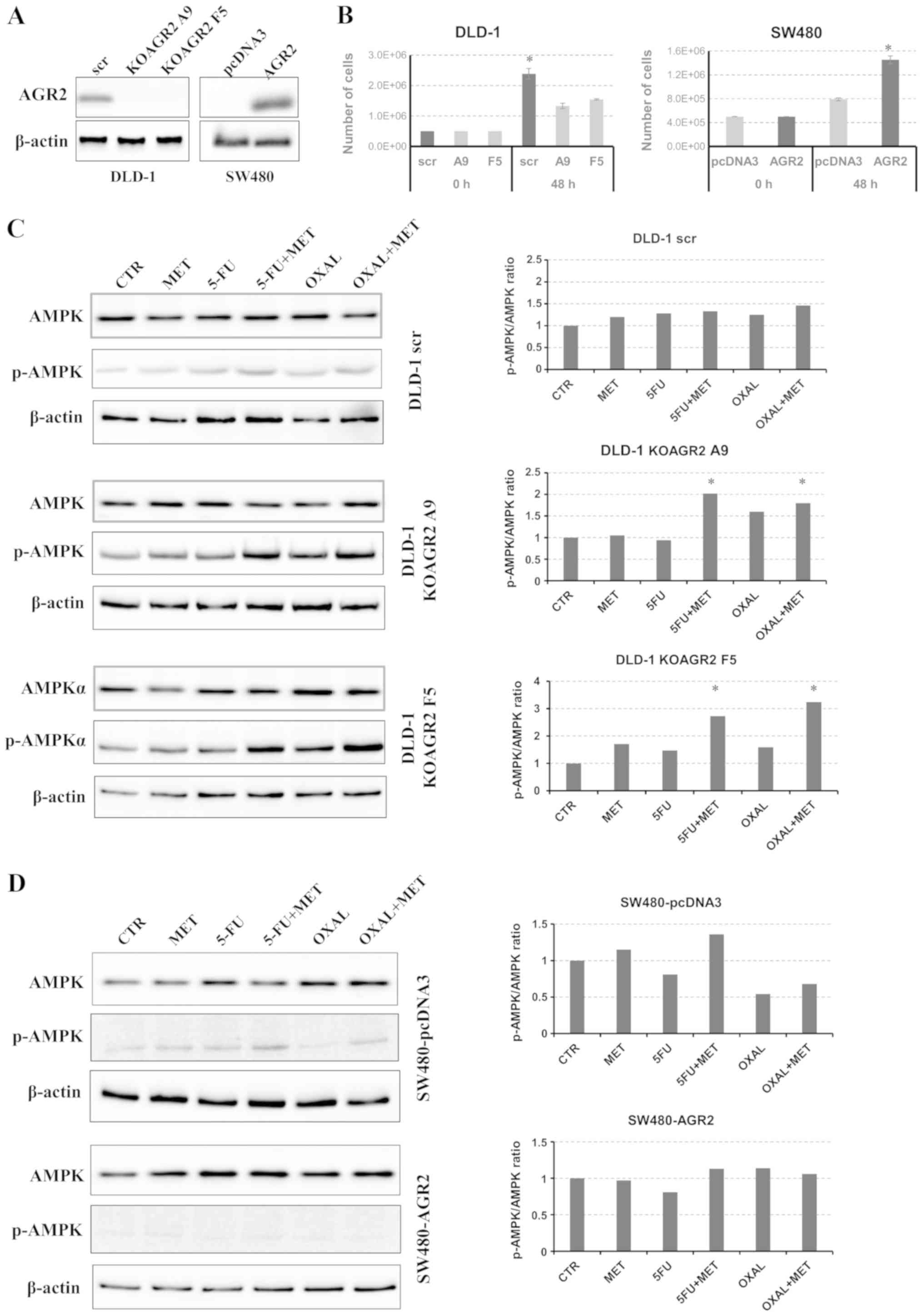 | Figure 1.Determination of AMPK expression. (A)
Evaluation of AGR2 protein levels in DLD-1 cells with AGR2 gene
knockout and in SW480 cells with established production of AGR2.
(B) Proliferation rate of DLD-1 and SW480 cells with different AGR2
expression levels. Cells producing AGR2 showed enhanced
proliferation. *P<0.05 vs. A9 and F5 or pcDNA3. (C and D)
Western blot analysis of total and p-AMPK levels in (C) DLD-1 cells
and (D) SW480 cells treated with MET, 5-FU, OXAL and their
combination in relation to CTR cells. β-actin was used as a loading
control and for normalization. *P<0.05 vs. CTR. 5-FU,
5-fluorouracil; AMPK, AMP-activated protein kinase; AGR2, anterior
gradient 2; CTR, control; KOAGR2, AGR2-knockout; MET, metformin;
OXAL, oxaliplatin; scr, scrambled guide RNA. |
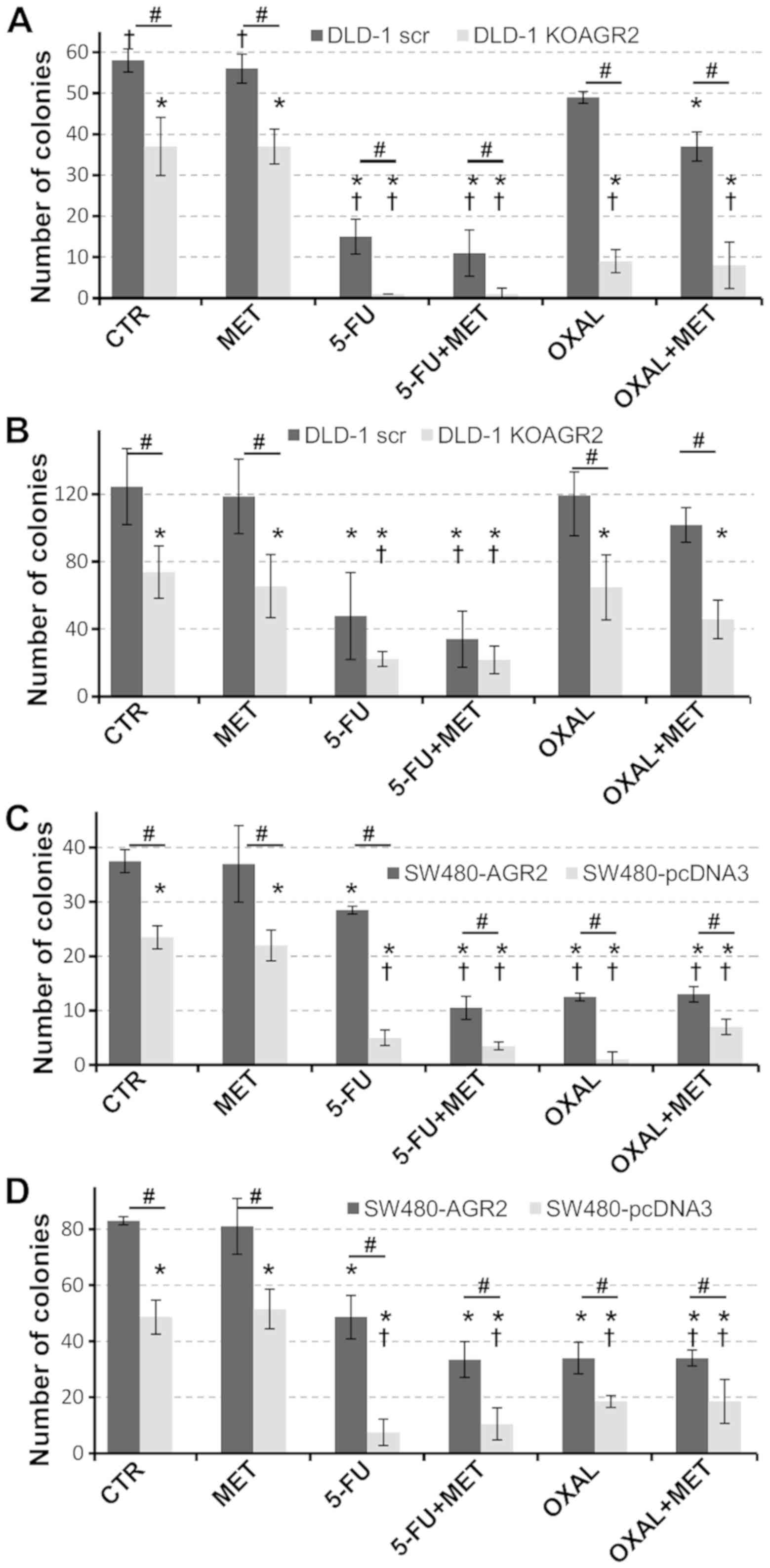
Effects of metformin on colony
formation
The effects of metformin on the ability of cells to
sufficiently form colonies, based on AGR2 expression and in
response to standard chemotherapy drugs, were tested using a
clonogenic assay. A significant loss in the ability of cells to
develop colonies was associated with the absence of AGR2 (Fig. 2). Metformin alone exhibited no effect
on the number of colonies developed by DLD-1 and SW480 cells
irrespective of AGR2 expression. However, metformin sensitized
DLD-1 cells producing AGR2 to 5-FU and oxaliplatin, which was
reflected by significantly reduced colony formation compared with
untreated ARG2-negative cells (Fig.
2A).
To investigate the impact of eAGR2 on cell
chemosensitivity, recombinant eAGR2 protein was added into the
culture medium (Fig. 2B). Similar
trends were observed as in Fig. 2A;
however, with eAGR2, twice the number of colonies was formed
compared with cells cultured in medium without eAGR2. In addition,
the effect of eAGR2 was stronger in AGR2-negative cells treated
with chemotherapy drugs, which exhibited approximately twofold
increase in the number of colonies compared with cells maintained
in media without eAGR2 (Fig. 2A and
B).
SW480-AGR2 cells exhibited a significant decrease in
the number of colonies in response to the combination of metformin
and 5-FU (Fig. 2C). The presence of
eAGR2 protein approximately doubled the number of colonies
(Fig. 2D). Taken together, these
data support the antiproliferative activity of metformin
administered in combination with standard chemotherapy and suggest
a significant contribution of eAGR2 to the resistance of tumor
cells to chemotherapy.
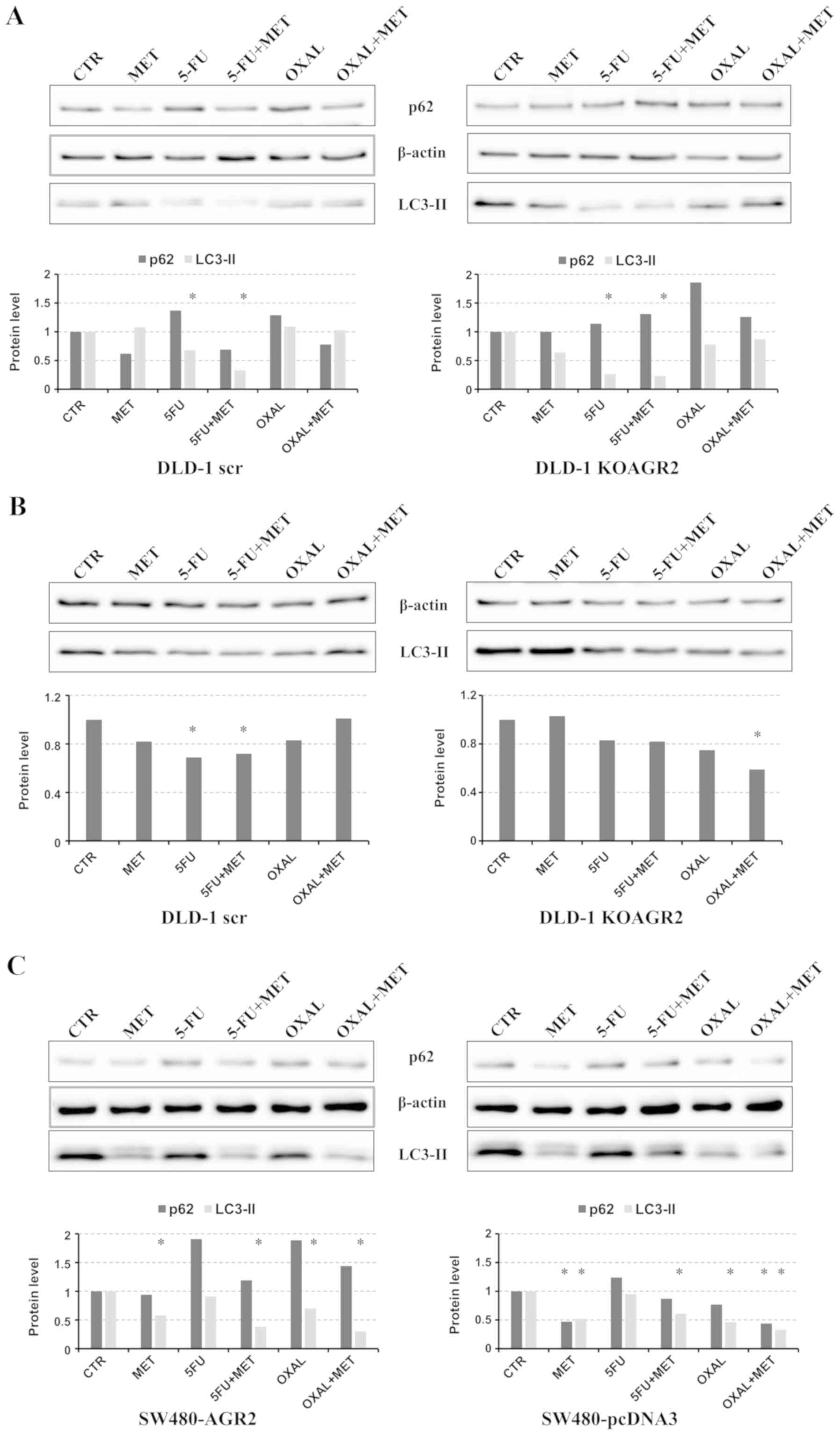
Effect of metformin combined with
standard chemotherapy on autophagy and oxidative stress
Since AMPK has been demonstrated to regulate
autophagy, the effects of metformin on LC3-II cleavage were
determined (Fig. 3). In agreement
with the elevated p-AMPK levels in AGR2-negative cells (Fig. 1), markedly higher signals of LC3-II
were detected in DLD-1 KOAGR2 cells in comparison with DLD-1 scr
cells (Fig. 3), which indicated that
AGR2 may attenuate autophagy. No significant induction of LC3-II
was observed in cells exposed to all drugs compared to untreated
cells (Fig. 3A). 5-FU treatment was
even associated with a sharp decrease in LC3-II, indicating that
autophagy may serve an important role in survival of these tumor
cells. The role of autophagy may correspond with the high
sensitivity of DLD-1 cells to 5-FU, as demonstrated in Fig. 2. Although the amount of LC3-II is
clearly correlated with the number of autophagosomes, LC3-II itself
is also degraded by autophagy (31).
Therefore, it is important to measure the amount of LC3-II
delivered to lysosomes by comparing LC3-II levels in the presence
and absence of lysosomal protease inhibitors e.g. bafilomycin A1.
However, the addition of bafilomycin A1 was not associated with
increased LC3-II cleavage in cells treated with metformin in
combination with 5-FU or oxaliplatin (Fig. 3B), which indicates that autophagy was
inhibited. An alternative method for detecting the autophagic flux
is the determination of p62/sequestosome-1 degradation, since p62
can bind LC3, serving as a selective substrate of autophagy
(32). However, no significant
decrease in p62 levels was observed in response to metformin
combined with 5-FU or oxaliplatin treatments; by contrast, an
increase in p62 levels was observed in response to 5-FU and
oxaliplatin administered alone. Western blot analysis of SW480
cells revealed a reduction in LC3-II isoform expression in response
to metformin and its combinations with 5-FU and oxaliplatin
(Fig. 3C). These findings support
the hypothesis that autophagy-dependent clearance of misfolded
proteins in non-metformin-treated patients with T2DM may be
suppressed by metformin treatment (19).
Metformin inhibits mitochondrial respiratory complex
I at a cellular level, possibly leading to mitochondrial membrane
depolarization and the release of ROS (33,34).
Therefore, alterations in mitochondrial membrane potential were
investigated in cells exposed to metformin in combination with 5-FU
or oxaliplatin. JC-1 staining followed by flow cytometric analysis
revealed a significant mitochondrial depolarization in response to
metformin alone and in combination with oxaliplatin in
AGR2-negative DLD-1 cells compared with in untreated cells
(Fig. 4A-C).
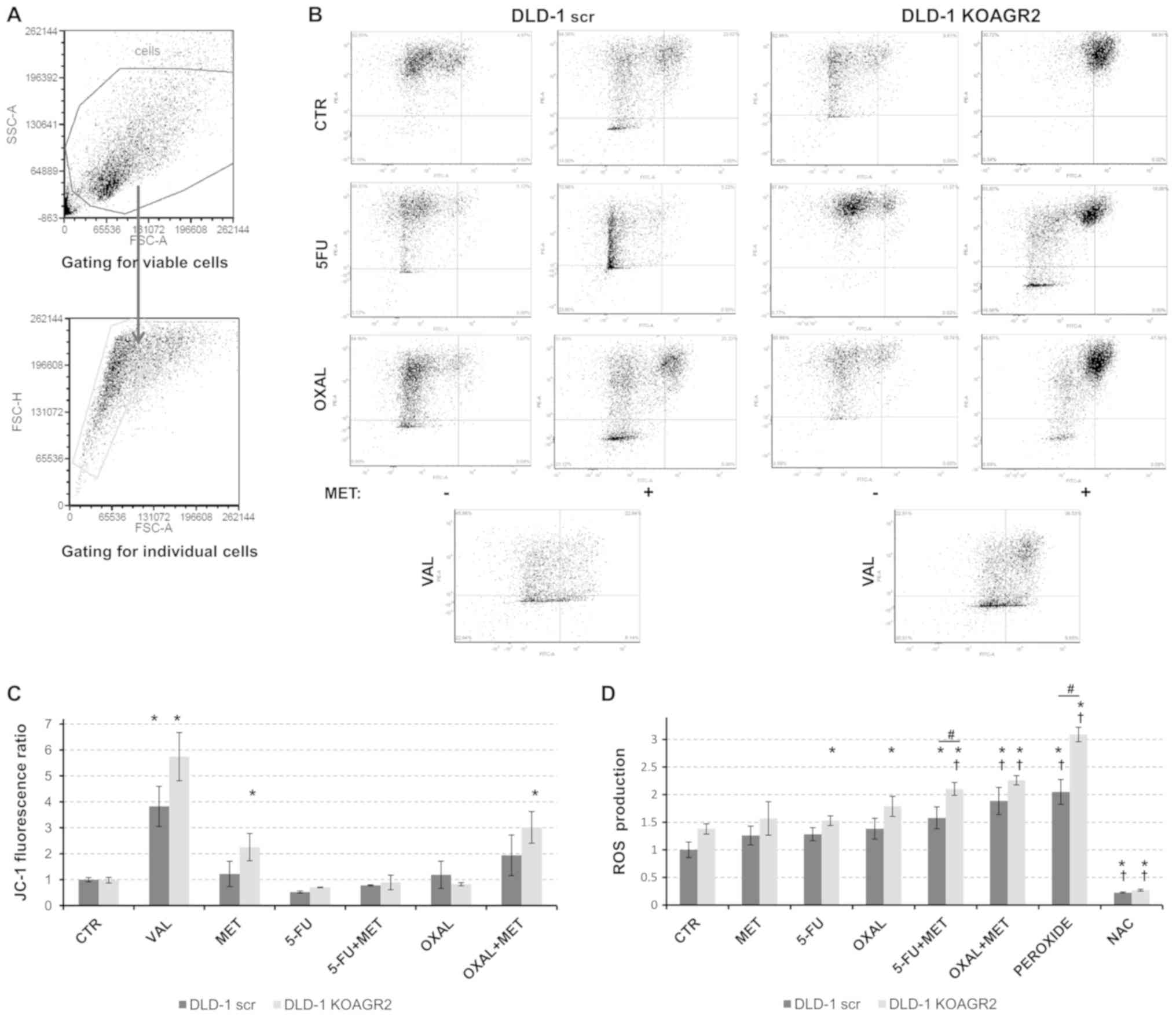 | Figure 4.Analysis of mitochondrial
depolarization and oxidative stress. (A) Flow cytometry plots
showing the gating strategy to determine viable and individual
cellular population. (B) Representative plots demonstrating
mitochondrial depolarization. (C) At 24 h post-treatment with 5-FU
(5 µM), OXAL (2 µM), MET (0.5 mM) and their combination, the JC-1
probe was used to measure the FITC/PE ratio to identify alterations
in mitochondrial membrane potential compared with in control cells.
25 µM VAL was used as a positive control. (D) ROS production was
determined relative to untreated cells. Hydrogen peroxide was used
as a positive control, whereas NAC was used to inhibit ROS
development. *P<0.05 vs. untreated DLD-1 scr; †P<0.05 vs.
untreated DLD-1 KOAGR2; #P<0.05 as indicated. 5-FU,
5-fluorouracil; CTR, control; MET, metformin; NAC, N-acetyl
cysteine; OXAL, oxaliplatin; VAL, valinomycin. At least three
independent biological experiments were performed to construct the
graphs. |
The impact of metformin on ROS production was
monitored using an indicator for reactive oxygen species in cells,
CM-H2DCFDA. All tested drugs administered alone resulted in a low
or moderate induction of ROS. However, the combined administration
of metformin with 5-FU or oxaliplatin significantly increased ROS
production in both DLD-1 scr and DLD-1 KOAGR2 cells. The effect was
significantly enhanced in AGR2-negative cells exposed to a
combination of 5-FU and metformin, whereas combined treatment with
oxaliplatin and metformin reached only a marginal effect (Fig. 4D).
Discussion
CRC is a type of cancer, the relative risk of which
is increased by diabetes (35); in
addition, the outcomes of CRC are significantly worse in patients
with diabetes compared with in non-diabetic subjects (36). Metformin is used for treating
patients with T2DM, including those with CRC, as demonstrated in
the clinical setting (17).
Beneficial effects of metformin are not limited to primary CRC.
Metformin also significantly increases the therapeutic
effectiveness of standard chemotherapeutics, such as 5-FU or
oxaliplatin, on recurring CRC by targeting chemoresistant CRC cells
enriched in stem or stem-like cells (37,38). The
effects of metformin on CRC cells depend predominately on
regulation of the AMPK/mammalian target of rapamycin (mTOR)
pathway. Under low energy conditions, AMPK phosphorylates specific
enzymes and growth control nodes to increase ATP generation and
decrease ATP consumption (39). A
decreased level of AMPK in T2DM attenuates the inhibition of
protein, fatty acid and cholesterol synthesis to favor cancer cell
growth. AMPK regulates these processes by interfering with mTOR,
which is a regulator of growth. Thus, under nutrient-rich
conditions, AMPK is inactive and mTOR is active, whereas under
energy deficient conditions, increased AMPK activity leads to a
decrease in mTOR activity, resulting in reduced protein synthesis
and cell growth (40). The
PI3K/AKT/mTOR axis has also been shown to be involved in the
positive regulation of AGR2 expression (41–43).
Activation of AKT signaling and impaired expression of its negative
regulator phosphatase and tensin homolog has been reported in
60–70% of human colon cancer cases (44). Recent meta-analysis has reported that
AGR2 overexpression has an unfavorable impact on overall survival
and time to tumor progression in patients with solid tumors
(45). Although elevated expression
of AGR2 is generally perceived as undesirable due to the prediction
of poor outcome, in several tumor types, such as lung, ovarian and
colorectal cancer, contradictory findings have been reported;
therefore, further analyses and clinical trials on certain types of
cancer are required (46,47).
Autophagy may exhibit dual functions in tumors,
including CRC; it may contribute to cancer development; however, it
may also act as a tumor suppressor by inducing cell death. The
decision to trigger either induction of processes leading to cell
death or activation of pro-survival functions depends on the stage
of the neoplastic process (48).
Early stages of CRC carcinogenesis are usually associated with the
tumor suppressive role of autophagy, whereas late stages are
associated with pro-survival functions (49). Development of chemoresistance
represents an event that is frequently observed in later stages of
CRC due to activation of autophagy. For instance, Yang et al
(50) demonstrated that autophagy
was induced in response to oxaliplatin treatment, along with the
enrichment of the CRC stem cell population, which increased cancer
cell resistance to chemotherapy and prevented apoptosis. Li et
al (51) reported that the
combination of fluorouracil treatment with autophagic inhibitors,
such as bafilomycin A1 or 3-methyladenine, enhanced the
chemotherapeutic effect of fluorouracil by stimulating cell
death.
The results of the present study demonstrated that,
compared with in AGR2-positive cells, CRC cells with silenced AGR2
expression exhibited increased levels of autophagy. However, the
activation of AMPK in response to chemotherapy combined with
metformin did not induce autophagy. A clear decrease in LC3-II was
observed in SW480 cells irrespective of AGR2 status. Although the
mechanism of metformin as an antineoplastic agent is not yet fully
understood (52), its activity is at
least partially attributable to AMPK activation through the
inhibition of complex I of the mitochondrial respiratory chain,
leading to an increased AMP:ATP ratio (30,34),
membrane depolarization (33) and
the release of mitochondrial ROS (53). Notably, the present study revealed
that metformin in combination with 5-FU or oxaliplatin attenuated
autophagy in CRC cells, but increased ROS production responsible
for decreased cell viability, as demonstrated by the clonogenic
assay. This effect was also significantly enhanced by knocking out
AGR2, supporting its potential catalytic redox activity in
the regulation of redox balance in cells, which is a typical
feature of protein disulfide isomerases (54). The results of the present study are
also in agreement with a recent study that demonstrated increased
ROS production in CRC cells induced by metformin (18). By contrast, a study on breast cancer
MDA-MB-231 cells revealed that low doses of metformin inhibited ROS
production and inflammatory signaling (55). This discrepancy suggests that
metformin may function through distinct mechanisms at lower versus
higher concentrations (56).
Another important aspect is the cellular
localization of AGR2. Although AGR2 is overexpressed in various
types of human cancer and has been reported to promote aggressive
tumor features, including the resistance to anticancer treatment
(23,24,26,57),
little is known regarding the extracellular functions of AGR2 in
tumorigenesis. Secreted eAGR2 has been demonstrated to promote cell
migration and metastasis of CRC in vitro and in vivo
(12). A comprehensive
protein-protein interaction screen identified AGR2 as an
interacting partner of the mTOR complex 2 pathway; eAGR2 promoted
increased phosphorylation of rapamycin-insensitive companion of
mTOR (T1135), whereas intracellular AGR2 antagonized its levels and
phosphorylation (58). A subsequent
study aiming to distinguish between the roles of intracellular AGR2
and eAGR2 in response to chemotherapy using an in vitro
prostate cancer model revealed that eAGR2 promoted significant
resistance to docetaxel (58).
Recently, the interaction between AGR2 and transmembrane p24
trafficking protein 2 has been described to serve a key role in
AGR2 dimerization and following autophagy-dependent release of AGR2
in the extracellular milieu (59).
These findings support the present results, which indicated that
autophagy may contribute to survival of CRC cells exposed to 5-FU
and oxaliplatin, and the presence of eAGR2 may significantly
enhance cell survival and colony formation.
Notably, 5-FU and oxaliplatin represent the standard
therapy option for patients with CRC, although with a limited
therapeutic success rate (60).
Therefore, compounds sensitizing CRC cells to these routinely used
drugs are urgently required to improve therapeutic outcome.
Metformin is a promising candidate, as documented by several
clinical trials (61,62). The results of the present study
demonstrated that metformin augmented the anticancer activity of
5-FU and oxaliplatin, and that the effect was enhanced in CRC cells
with disrupted AGR2 expression. However, the non-canonical
mechanisms by which metformin activates AMPK and induces oxidative
stress are areas that require further investigation.
Acknowledgements
The authors would like to thank Ms. Tamara Kolarova
(Regional Centre for Applied Molecular Oncology, Masaryk Memorial
Cancer Institute) for technical assistance.
Funding
The present study was supported by the Ministry of
Health, Czech Republic-Conceptual Development of Research
Organization (Masaryk Memorial Cancer Institute; grant no.
00209805), by the project MEYS-NPS I-LO1413 and GACR 16-14829S and
19-02014S.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AM conducted the majority of western blotting
experiments and prepared the manuscript. LS prepared stable
AGR2 gene knockout cell lines and prepared the manuscript.
KKu was responsible for functional analyses. JP participated in
autophagy experiments and reactive oxygen species determination. BV
provided materials and tools, designed the preparation of stable
AGR2 knockout cell lines and drafted the manuscript. KKa
participated in the design of the study and revised the manuscript.
RH conceived and approved all experiments, performed functional
biological assays and finalized the manuscript. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AGR2
|
anterior gradient 2
|
|
T2DM
|
type 2 diabetes mellitus
|
|
CRC
|
colorectal cancer
|
|
AMPK
|
AMP-activated protein kinase
|
|
mTOR
|
mammalian target of rapamycin
|
|
5-FU
|
5-fluorouracil
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019.PubMed/NCBI
|
|
2
|
de Kort S, Masclee AAM, Sanduleanu S,
Weijenberg MP, van Herk-Sukel MPP, Oldenhof NJJ, van den Bergh JPW,
Haak HR and Janssen-Heijnen ML: Higher risk of colorectal cancer in
patients with newly diagnosed diabetes mellitus before the age of
colorectal cancer screening initiation. Sci Rep. 7:465272017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun L and Yu S: Diabetes mellitus is an
independent risk factor for colorectal cancer. Dig Dis Sci.
57:1586–1597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van de Poll-Franse LV, Haak HR, Coebergh
JW, Janssen-Heijnen ML and Lemmens VE: Disease-specific mortality
among stage I–III colorectal cancer patients with diabetes: A large
population-based analysis. Diabetologia. 55:2163–2172. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giovannucci E, Harlan DM, Archer MC,
Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG and
Yee D: Diabetes and cancer: A consensus report. Diabetes Care.
33:1674–1685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mills KT, Bellows CF, Hoffman AE, Kelly TN
and Gagliardi G: Diabetes mellitus and colorectal cancer prognosis:
A meta-analysis. Dis Colon Rectum. 56:1304–1319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharma A, Ng H, Kumar A, Teli K, Randhawa
J, Record J and Maroules M: Colorectal cancer: Histopathologic
differences in tumor characteristics between patients with and
without diabetes. Clin Colorectal Cancer. 13:54–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang J, Nishihara R, Zhang X, Ogino S and
Qian ZR: Energy sensing pathways: Bridging type 2 diabetes and
colorectal cancer? J Diabetes Complications. 31:1228–1236. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ariyasu D, Yoshida H and Hasegawa Y:
Endoplasmic Reticulum (ER) stress and endocrine disorders. Int J
Mol Sci. 18:2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dumartin L, Alrawashdeh W, Trabulo SM,
Radon TP, Steiger K, Feakins RM, di Magliano MP, Heeschen C,
Esposito I, Lemoine NR and Crnogorac-Jurcevic T: ER stress protein
AGR2 precedes and is involved in the regulation of pancreatic
cancer initiation. Oncogene. 36:3094–3103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brychtova V, Vojtesek B and Hrstka R:
Anterior gradient 2: A novel player in tumor cell biology. Cancer
Lett. 304:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian S, Hu J, Tao K, Wang J, Chu Y, Li J,
Liu Z, Ding X, Xu L, Li Q, et al: Secreted AGR2 promotes invasion
of colorectal cancer cells via Wnt11-mediated non-canonical Wnt
signaling. Exp Cell Res. 364:198–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Higa A, Mulot A, Delom F, Bouchecareilh M,
Nguyên DT, Boismenu D, Wise MJ and Chevet E: Role of pro-oncogenic
protein disulfide isomerase (PDI) family member anterior gradient 2
(AGR2) in the control of endoplasmic reticulum homeostasis. J Biol
Chem. 286:44855–44868. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chevet E, Fessart D, Delom F, Mulot A,
Vojtesek B, Hrstka R, Murray E, Gray T and Hupp T: Emerging roles
for the pro-oncogenic anterior gradient-2 in cancer development.
Oncogene. 32:2499–2509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inzucchi SE, Bergenstal RM, Buse JB,
Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R and
Matthews DR; American Diabetes Association (ADA); European
Association for the Study of Diabetes (EASD), : Management of
hyperglycemia in type 2 diabetes: A patient-centered approach:
Position statement of the American Diabetes Association (ADA) and
the European Association for the Study of Diabetes (EASD). Diabetes
Care. 35:1364–1379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Higurashi T and Nakajima A: Metformin and
colorectal cancer. Front Endocrinol. 9:6222018. View Article : Google Scholar
|
|
17
|
Meng F, Song L and Wang W: Metformin
improves overall survival of colorectal cancer patients with
diabetes: A meta-analysis. J Diabetes Res. 2017:50632392017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mogavero A, Maiorana MV, Zanutto S,
Varinelli L, Bozzi F, Belfiore A, Volpi CC, Gloghini A, Pierotti MA
and Gariboldi M: Metformin transiently inhibits colorectal cancer
cell proliferation as a result of either AMPK activation or
increased ROS production. Sci Rep. 7:159922017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Diaz-Morales N, Iannantuoni F,
Escribano-Lopez I, Bañuls C, Rovira-Llopis S, Sola E, Rocha M,
Hernandez-Mijares A and Victor VM: Does metformin modulate
endoplasmic reticulum stress and autophagy in type 2 diabetic
peripheral blood mononuclear cells? Antioxid Redox Signal.
28:1562–1569. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobiela J, Dobrzycka M, Jędrusik P,
Kobiela P, Spychalski P, Śledziński Z and Zdrojewski T: Metformin
and colorectal cancer-a systematic review. Exp Clin Endocrinol
Diabetes. 127:445–454. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yue W, Wang T, Zachariah E, Lin Y, Yang
CS, Xu Q, DiPaola RS and Tan XL: Transcriptomic analysis of
pancreatic cancer cells in response to metformin and aspirin: An
implication of synergy. Sci Rep. 5:133902015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arumugam T, Deng D, Bover L, Wang H,
Logsdon CD and Ramachandran V: New blocking antibodies against
novel AGR2-C4.4A pathway reduce growth and metastasis of pancreatic
tumors and increase survival in mice. Mol Cancer Ther. 14:941–951.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hrstka R, Bouchalova P, Michalova E,
Matoulkova E, Muller P, Coates PJ and Vojtesek B: AGR2 oncoprotein
inhibits p38 MAPK and p53 activation through a DUSP10-mediated
regulatory pathway. Mol Oncol. 10:652–662. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hrstka R, Brychtova V, Fabian P, Vojtesek
B and Svoboda M: AGR2 predicts tamoxifen resistance in
postmenopausal breast cancer patients. Dis Markers. 35:207–212.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Q, Gu J, Li L, Liu J, Luo B, Cheung
HW, Boehm JS, Ni M, Geisen C, Root DE, et al: Control of cyclin D1
and breast tumorigenesis by the EglN2 prolyl hydroxylase. Cancer
Cell. 16:413–424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hrstka R, Nenutil R, Fourtouna A, Maslon
MM, Naughton C, Langdon S, Murray E, Larionov A, Petrakova K,
Muller P, et al: The pro-metastatic protein anterior gradient-2
predicts poor prognosis in tamoxifen-treated breast cancers.
Oncogene. 29:4838–4847. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ostatná V, Vargová V, Hrstka R, Ďurech M,
Vojtěšek B and Paleček E: Effect of His6-tagging of
anterior gradient 2 protein on its electro-oxidation. Electrochim
Acta. 150:218–222. 2014. View Article : Google Scholar
|
|
28
|
Ma SR, Mao L, Deng WW, Li YC, Bu LL, Yu
GT, Zhang WF and Sun ZJ: AGR2 promotes the proliferation, migration
and regulates epithelial-mesenchymal transition in salivary adenoid
cystic carcinoma. Am J Transl Res. 9:507–519. 2017.PubMed/NCBI
|
|
29
|
Park K, Chung YJ, So H, Kim K, Park J, Oh
M, Jo M, Choi K, Lee EJ, Choi YL, et al: AGR2, a mucinous ovarian
cancer marker, promotes cell proliferation and migration. Exp Mol
Med. 43:91–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim J, Yang G, Kim Y, Kim J and Ha J: AMPK
activators: Mechanisms of action and physiological activities. Exp
Mol Med. 48:e2242016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mizushima N and Yoshimori T: How to
interpret LC3 immunoblotting. Autophagy. 3:542–545. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Andrzejewski S, Gravel SP, Pollak M and
St-Pierre J: Metformin directly acts on mitochondria to alter
cellular bioenergetics. Cancer Metab. 2:122014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Owen MR, Doran E and Halestrap AP:
Evidence that metformin exerts its anti-diabetic effects through
inhibition of complex 1 of the mitochondrial respiratory chain.
Biochem J 348 Pt. 3:607–614. 2000. View Article : Google Scholar
|
|
35
|
Larsson SC, Orsini N and Wolk A: Diabetes
mellitus and risk of colorectal cancer: A meta-analysis. J Natl
Cancer Inst. 97:1679–1687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zanders MM, Vissers PA, Haak HR and van de
Poll-Franse LV: Colorectal cancer, diabetes and survival:
Epidemiological insights. Diabetes Metab. 40:120–127. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim JH, Lee KJ, Seo Y, Kwon JH, Yoon JP,
Kang JY, Lee HJ, Park SJ, Hong SP, Cheon JH, et al: Effects of
metformin on colorectal cancer stem cells depend on alterations in
glutamine metabolism. Sci Rep. 8:4092018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nangia-Makker P, Yu Y, Vasudevan A,
Farhana L, Rajendra SG, Levi E and Majumdar AP: Metformin: A
potential therapeutic agent for recurrent colon cancer. PLoS One.
9:e843692014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Herzig S and Shaw RJ: AMPK: Guardian of
metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol.
19:121–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Emami Riedmaier A, Fisel P, Nies AT,
Schaeffeler E and Schwab M: Metformin and cancer: From the old
medicine cabinet to pharmacological pitfalls and prospects. Trends
Pharmacol Sci. 34:126–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hrstka R, Murray E, Brychtova V, Fabian P,
Hupp TR and Vojtesek B: Identification of an AKT-dependent
signalling pathway that mediates tamoxifen-dependent induction of
the pro-metastatic protein anterior gradient-2. Cancer Lett.
333:187–193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Z, Wu Z, Chen H, Zhu Q, Gao G, Hu L,
Negi H, Kamle S and Li D: Induction of anterior gradient 2 (AGR2)
plays a key role in insulin-like growth factor-1 (IGF-1)-induced
breast cancer cell proliferation and migration. Med Oncol.
32:5772015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Matoulkova E, Sommerova L, Pastorek M,
Vojtesek B and Hrstka R: Regulation of AGR2 expression via 3′UTR
shortening. Exp Cell Res. 356:40–47. 2017.PubMed/NCBI
|
|
44
|
Pandurangan AK: Potential targets for
prevention of colorectal cancer: A focus on PI3K/Akt/mTOR and Wnt
pathways. Asian Pac J Cancer Prev. 14:2201–2205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tian SB, Tao KX, Hu J, Liu ZB, Ding XL,
Chu YN, Cui JY, Shuai XM, Gao JB, Cai KL, et al: The prognostic
value of AGR2 expression in solid tumours: A systematic review and
meta-analysis. Sci Rep. 7:155002017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Alves MR, E Melo NC, Barros-Filho MC, do
Amaral NS, Silva FIB, Baiocchi Neto G, Soares FA, de Brot Andrade L
and Rocha RM: Downregulation of AGR2, p21, and cyclin D and
alterations in p53 function were associated with tumor progression
and chemotherapy resistance in epithelial ovarian carcinoma. Cancer
Med. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Riener MO, Thiesler T, Hellerbrand C,
Amann T, Cathomas G, Fritzsche FR, Dahl E, Bahra M, Weichert W,
Terracciano L and Kristiansen G: Loss of anterior gradient-2
expression is an independent prognostic factor in colorectal
carcinomas. Eur J Cancer. 50:1722–1730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen N and Karantza-Wadsworth V: Role and
regulation of autophagy in cancer. Biochim Biophys Acta.
1793:1516–1523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Madia F, Grossi V, Peserico A and Simone
C: Updates from the intestinal front line: Autophagic weapons
against inflammation and cancer. Cells. 1:535–557. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang HZ, Ma Y, Zhou Y, Xu LM, Chen XJ,
Ding WB and Zou HB: Autophagy contributes to the enrichment and
survival of colorectal cancer stem cells under oxaliplatin
treatment. Cancer Lett. 361:128–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li J, Hou N, Faried A, Tsutsumi S and
Kuwano H: Inhibition of autophagy augments 5-fluorouracil
chemotherapy in human colon cancer in vitro and in vivo model. Eur
J Cancer. 46:1900–1909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Abdelsatir AA, Husain NE, Hassan AT,
Elmadhoun WM, Almobarak AO and Ahmed MH: Potential benefit of
metformin as treatment for colon cancer: The evidence so far. Asian
Pac J Cancer Prev. 16:8053–8058. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hur KY and Lee MS: New mechanisms of
metformin action: Focusing on mitochondria and the gut. J Diabetes
Investig. 6:600–609. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang L, Wang X and Wang CC: Protein
disulfide-isomerase, a folding catalyst and a redox-regulated
chaperone. Free Radic Biol Med. 83:305–313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schexnayder C, Broussard K, Onuaguluchi D,
Poché A, Ismail M, McAtee L, Llopis S, Keizerweerd A, McFerrin H
and Williams C: Metformin inhibits migration and invasion by
suppressing ROS production and COX2 expression in MDA-MB-231 breast
cancer cells. Int J Mol Sci. 19:2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Queiroz EA, Puukila S, Eichler R, Sampaio
SC, Forsyth HL, Lees SJ, Barbosa AM, Dekker RF, Fortes ZB and
Khaper N: Metformin induces apoptosis and cell cycle arrest
mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast
cancer cells. PLoS One. 9:e982072014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ramachandran V, Arumugam T, Wang H and
Logsdon CD: Anterior gradient 2 is expressed and secreted during
the development of pancreatic cancer and promotes cancer cell
survival. Cancer Res. 68:7811–7818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tiemann K, Garri C, Lee SB, Malihi PD,
Park M, Alvarez RM, Yap LP, Mallick P, Katz JE, Gross ME and Kani
K: Loss of ER retention motif of AGR2 can impact mTORC signaling
and promote cancer metastasis. Oncogene. 38:3003–3018. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Maurel M, Obacz J, Avril T, Ding YP,
Papadodima O, Treton X, Daniel F, Pilalis E, Hörberg J, Hou W, et
al: Control of anterior GRadient 2 (AGR2) dimerization links
endoplasmic reticulum proteostasis to inflammation. EMBO Mol Med.
11:2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Meyerhardt JA and Mayer RJ: Systemic
therapy for colorectal cancer. N Engl J Med. 352:476–487. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Miranda VC, Braghiroli MI, Faria LD,
Bariani G, Alex A, Bezerra Neto JE, Capareli FC, Sabbaga J, Lobo
Dos Santos JF, Hoff PM and Riechelmann RP: Phase 2 trial of
metformin combined with 5-Fluorouracil in patients with refractory
metastatic colorectal cancer. Clin Colorectal Cancer.
15:321.e1–328.e1. 2016. View Article : Google Scholar
|
|
62
|
Richard SM and Martinez Marignac VL:
Sensitization to oxaliplatin in HCT116 and HT29 cell lines by
metformin and ribavirin and differences in response to
mitochondrial glutaminase inhibition. J Cancer Res Ther.
11:336–340. 2015. View Article : Google Scholar : PubMed/NCBI
|