Introduction
Esophageal adenocarcinoma (EAC) is a highly lethal
malignancy that occurs mainly in the distal esophagus and
gastroesophageal junction (1). EAC
is rare in China; however, it represents the predominant type of
esophageal cancer in North America and Europe. In these continents,
the overall incidence of EAC has rapidly increased over the past
three decades at a rate (5–10%) greater than that of any other
major cancer and the incidence rate is higher in white males
compared with that in white females (2–4). The
reason for this increase is not entirely understood. Previous
studies have reported that EAC differs from esophageal squamous
cell carcinoma (ESCC) in terms of genetic and environmental risk
factors such as tobacco use, alcohol, obesity and germline
mutations (5,6). Systematic therapy for EAC typically
includes endoscopic mucosal resection, surgical resection,
chemoradiotherapy and neoadjuvant chemotherapy; however, the
mortality rate remains high and the overall 5-year survival rate is
17% in the United States (7).
Although endoscopy can accurately diagnose early-stage EAC, most
patients are diagnosed with regional metastasis or distant
metastasis, which are positively correlated with a considerable
decline in the 5-year survival rate (8). There is therefore an urgency to
identify novel potential diagnostic and prognostic biomarkers for
EAC.
Long non-coding RNAs (lncRNAs) represent a new class
of non-coding RNAs (ncRNAs) and are defined as transcripts >200
nucleotides in length (9). Unlike
their shorter counterparts, including microRNAs (miRNAs), the roles
and underlying mechanisms of lncRNAs in human disease remain
largely unknown. Due to improvements in DNA sequencing techniques,
numerous lncRNAs have been discovered. In addition, an increasing
number of lncRNAs have been identified in human cancer, such as
HAGLR opposite strand lncRNA overexpression in gastric cancer
(10). Previous studies have focused
on the biological function and underlying molecular mechanism of
lncRNAs in various types of cancer, including colorectal cancer,
gastric cancer, hepatocellular carcinoma, renal cell carcinoma,
prostate carcinoma and EAC (11–17).
Although studies reported that lncRNAs can be involved in the
development and progression of ESCC (6,14), only
a few studies have determined the function of lncRNAs in EAC
(5,18).
Emerging technologies have increased our ability to
determine the functions of cancer-associated lncRNAs. Significant
progress towards understanding the underlying molecular mechanism
by which lncRNAs can regulate miRNA function has therefore been
made. Salmena et al (19)
proposed a competing endogenous RNA (ceRNA) language where protein
coding genes, microRNAs and lncRNAs communicate with each other by
competitively binding to shared miRNA response elements (MREs).
Competing endogenous RNA networks comprise a new regulatory network
of mRNAs and non-coding RNAs, which reveals a greatly expanded role
for lncRNAs in human disease (20).
This hypothesis has been experimentally validated. For example,
Cesana et al (21) identified
a muscle-specific lncRNA named linc-MD1, which regulates the
expression of mastermind-like 1 and myocyte-specific enhancer
factor 2C by serving as a ‘sponge’ for miR-133. Furthermore, Qu
et al (22) demonstrated that
lncARSR mediates sunitinib resistance in renal cell carcinoma by
competitively binding to miR-34/miR-449 to promote AXL receptor
tyrosine kinase and c-MET expression. Exploration of RNA cross-talk
offers therefore insights into cancer diagnosis and therapy. An
lncRNA-miRNA-mRNA ceRNA network has therefore been constructed for
various types of human cancer, in particular for ESCC (23–26);
however, such a network has not yet been described for EAC.
In order to systematically describe EAC-associated
pseudogenes and to construct a ceRNA network, the present study
comprehensively analyzed RNA sequencing (RNA-Seq) transcript data
that were obtained from The Cancer Genome Atlas (TCGA) esophageal
cancer project (https://www.cancer.gov/types/esophageal). The database
includes lncRNA, microRNA and mRNA data and clinical information
from patients with EAC. The present study included 79 EAC tumor and
11 adjacent non-tumor esophagus tissue samples. By using publicly
available RNA-Seq data from TCGA, some EAC-associated lncRNAs,
mRNAs and miRNAs were identified based on the ceRNA hypothesis.
Furthermore, 561 differentially expressed lncRNAs (DElncRNAs),
1,289 differentially-expressed mRNAs (DEmRNAs) and 44
differentially-expressed miRNAs (DEmiRNAs) were identified.
Subsequently, five dysregulated lncRNAs, 13 mRNAs and 32 miRNAs
were identified and included in a constructed ceRNA network based
on lncRNA-miRNA interactions predicted by miRcode v11 (www.mircode.org/). Potential prognostic biomarkers
were then identified by exploring the influence of dysregulated
RNAs on overall survival using the univariate Cox proportional
hazards regression model and Kaplan-Meier curve analysis. The
results from this comprehensive analysis provided the foundation
for deeper understanding of the cancer-associated lncRNA functions
in EAC and revealed potential prognostic biomarkers.
Materials and methods
Patients and samples
Data for 187 patients with esophageal cancer were
obtained from the TCGA data portal (https://portal.gdc.cancer.gov/). The exclusion
criteria were as follows: i) Patients with ESCC or undetermined
pathological classification; and ii) samples without corresponding
RNA-Seq and miRNA-Seq data. Overall, data from 79 patients with EAC
were enrolled in the present study. This study followed the
publication guidelines provided by TCGA (http://cancergenome.nih.gov/publications/publicationguidelines).
RNA-Seq data
RNA-Seq and miRNA-Seq data (level 3) were downloaded
from 90 tissue samples of the TCGA database, including 79 EAC
samples and 11 adjacent normal samples. The gene expression
profiles generated from Illumina Hiseq platforms (Illumina, Inc.)
were all publicly available data.
Analysis of DEmRNAs, DElcnRNAs and
DEmiRNAs
The raw count data were processed with edgeR v3.25.0
(Bioconductor), which is a package based on the R language (v3.5.0)
(27) for differential gene
expression analysis. For all P-values, a false discovery rate (FDR)
was applied to correct the statistical significance of multiple
testing. Genes with >2.0 fold change (FC) and FDR-adjusted
P<0.01 were considered significant. The volcano plot and heat
map were designed to visualize the results using ggplots v3.0,
which is a package based on the R language.
Association between DElncRNAs and
patient prognosis
All patients were classified into high or low
lncRNA-expression groups according to the median. Kaplan-Meier and
log-rank methods were used to test differences between the two
groups. P<0.05 was considered to indicate a statistically
significant difference.
Construction of the ceRNA network
Three calculations were performed to construct the
ceRNA network as follows: i) Cancer-associated lncRNA filtration,
where lncRNAs with FC>2.0 (either up- or downregulated) and
FDR-adjusted P<0.01 were considered as cancer-associated lncRNAs
[to improve data reliability, cancer-associated lncRNAs that were
not annotated by GENCODE (http://www.gencodegenes.org/) were excluded]; ii)
lncRNA-miRNA interactions were predicted by miRcode (http://www.mircode.org/) and starBase (http://starbase.sysu.edu.cn/); and iii) target mRNAs
of DEmiRNAs were predicted using the three bioinformatics databases
miRDB (http://mirdb.org/), miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/php/index.php) and
TargetScan (http://www.targetscan.org). Gene
Oncology (GO) was analyzed using Database for Annotation,
Visualization, and Integrated Discovery bioinformatics tools
(DAVID; v6.8; http://david.ncifcrf.gov). In order to improve the
consistency of the bioinformatics analysis, the target genes were
retained. A network graph was constructed and visualized using
Cytoscape v3.5.1 (https://cytoscape.org/).
Results
Patient characteristics
The detailed clinical information and pathological
characteristics of the patients included in the present study,
including sex, age at diagnosis, metastasis status, lymph node
status and tumor-node-metastasis stage, are presented in Table I. The median age for all patients was
69 years (range, 27–86 years). The median overall survival was
14.29 months (range 0.36–83.18 months).
 | Table I.Clinical characteristics of patients
with esophageal adenocarcinoma. |
Table I.
Clinical characteristics of patients
with esophageal adenocarcinoma.
|
Characteristics | No. cases | % |
|---|
| Age at diagnosis,
years |
|
|
|
<60 | 26 | 32.9 |
|
≥60 | 53 | 67.1 |
| Sex |
|
|
|
Male | 11 | 13.9 |
|
Female | 68 | 86.1 |
| Metastasis |
|
|
| M0 | 57 | 72.2 |
| M1 | 10 | 12.7 |
| MX | 10 | 12.7 |
| Lymph node
status |
|
|
| N0 | 22 | 27.8 |
| N1 | 44 | 55.7 |
| N2 | 5 | 6.3 |
| N3 | 5 | 6.3 |
| NX | 3 | 3.8 |
| Stage |
|
|
| I | 9 | 11.4 |
| II | 22 | 27.8 |
|
III | 26 | 32.9 |
| IV | 5 | 6.3 |
| T stage |
|
|
| T0 | 1 | 1.3 |
| T1 | 19 | 24.1 |
| T2 | 10 | 12.7 |
| T3 | 46 | 58.2 |
| T4 | 1 | 1.3 |
| TX | 2 | 2.6 |
| Histological
grade |
|
|
| G1 | 1 | 1.3 |
| G2 | 28 | 35.4 |
| G3 | 24 | 30.4 |
| GX | 26 | 32.9 |
Identification of DElncRNAs
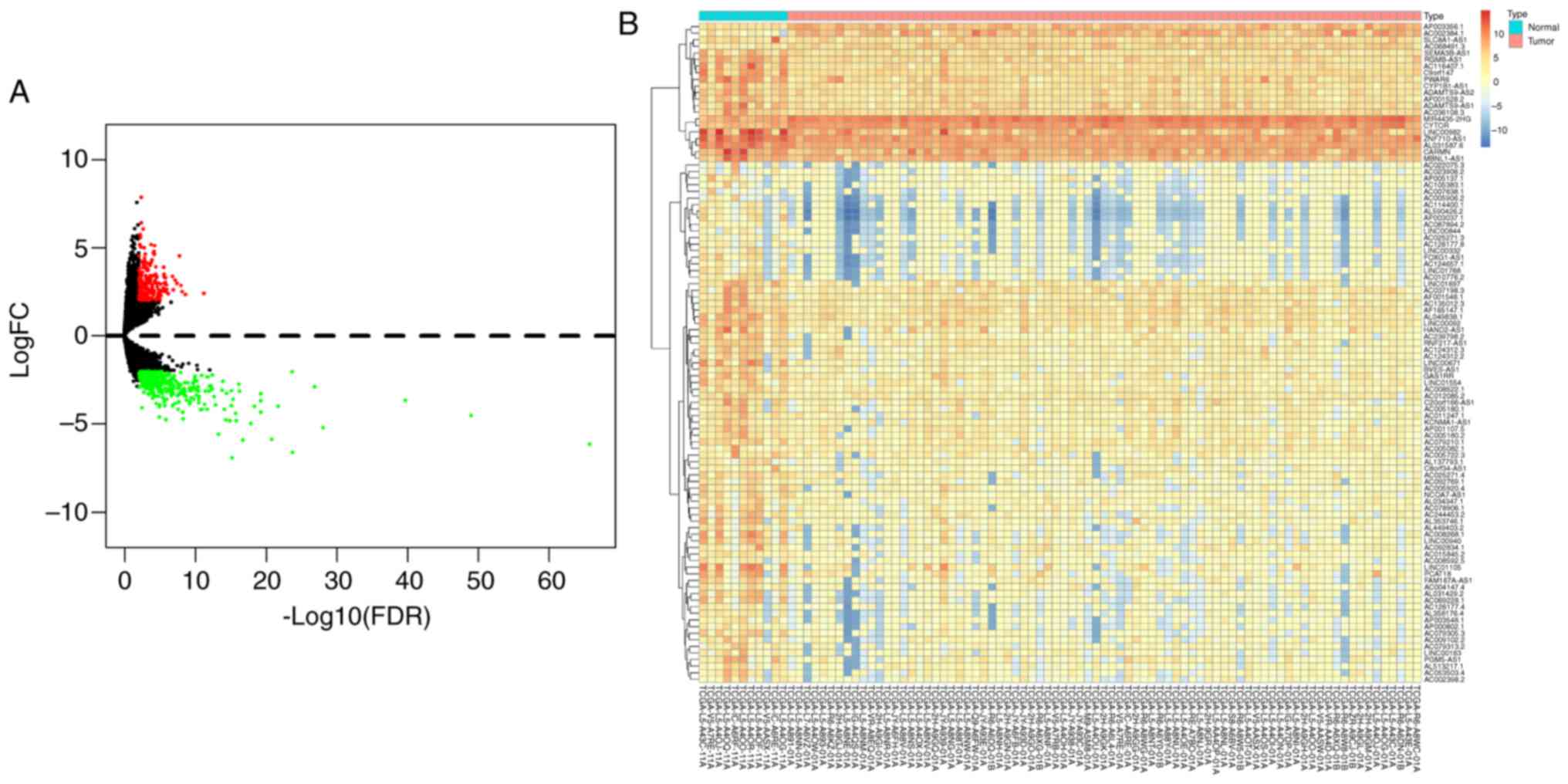
lncRNAs with FDR-adjusted P<0.01 and FC >2.0
were considered to be differentially expressed. A total of 561
DElncRNAs were identified, of which 217 were upregulated and 344
were downregulated (Table SI). A
volcano plot was therefore constructed (Fig. 1A) to visually describe the FDRs and
FCs. In addition, a heat map was designed (Fig. 1B) to highlight the top 100
significant DElncRNAs according to the FDR-adjusted P-values.
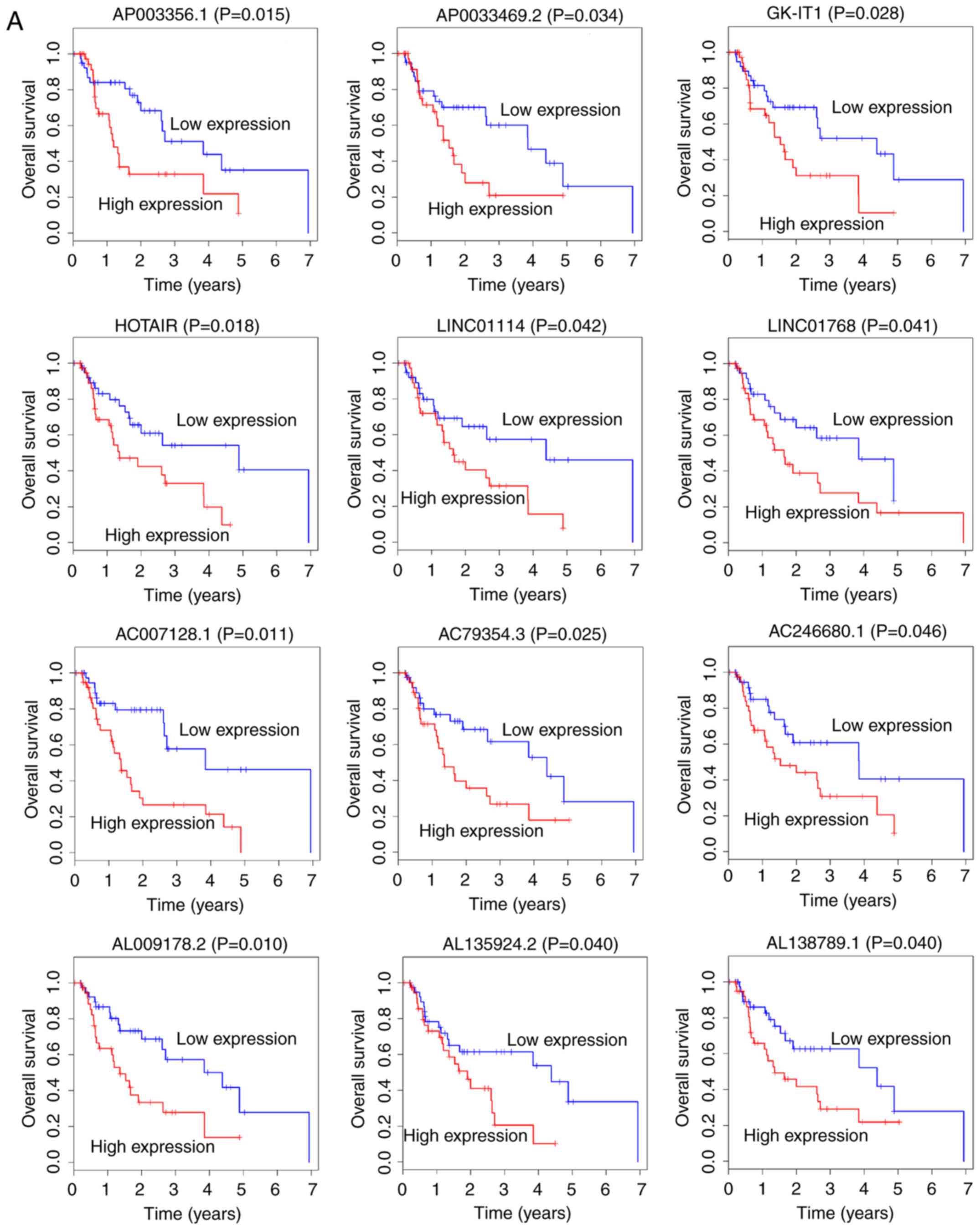
To explore the potential lncRNAs that could possess
prognostic abilities, the expression profiles of 561 lncRNAs and
corresponding clinical data were analyzed using Kaplan-Meier Curve.
The results demonstrated that 26 lncRNAs were positively correlated
with overall survival (OS; P<0.05; Fig. 2). Among these 26, 12 lncRNAs,
AC007128.1, AC079354.3, AC246680.1, AL009178.2, AL135924.2,
AL138789.1, AP003356.1, AP0033469.2, GK-IT1, HOTAIR, LINC01114 and
LINC01768, were negatively correlated with OS (Fig. 2A). Conversely, the remaining 14
lncRNAs, AC004585.1, AC016395.1, AC024337.2, AC087491.1,
AC093583.1, AC104211.1, AL022316.1, AL031429.1, CYP1B1-AS1,
LINC00163, LINC00906, LINC01695, SLCO4A1-AS1 and UG0898H09, were
positively correlated with OS (Fig.
2B).
Identification of DEmRNAs
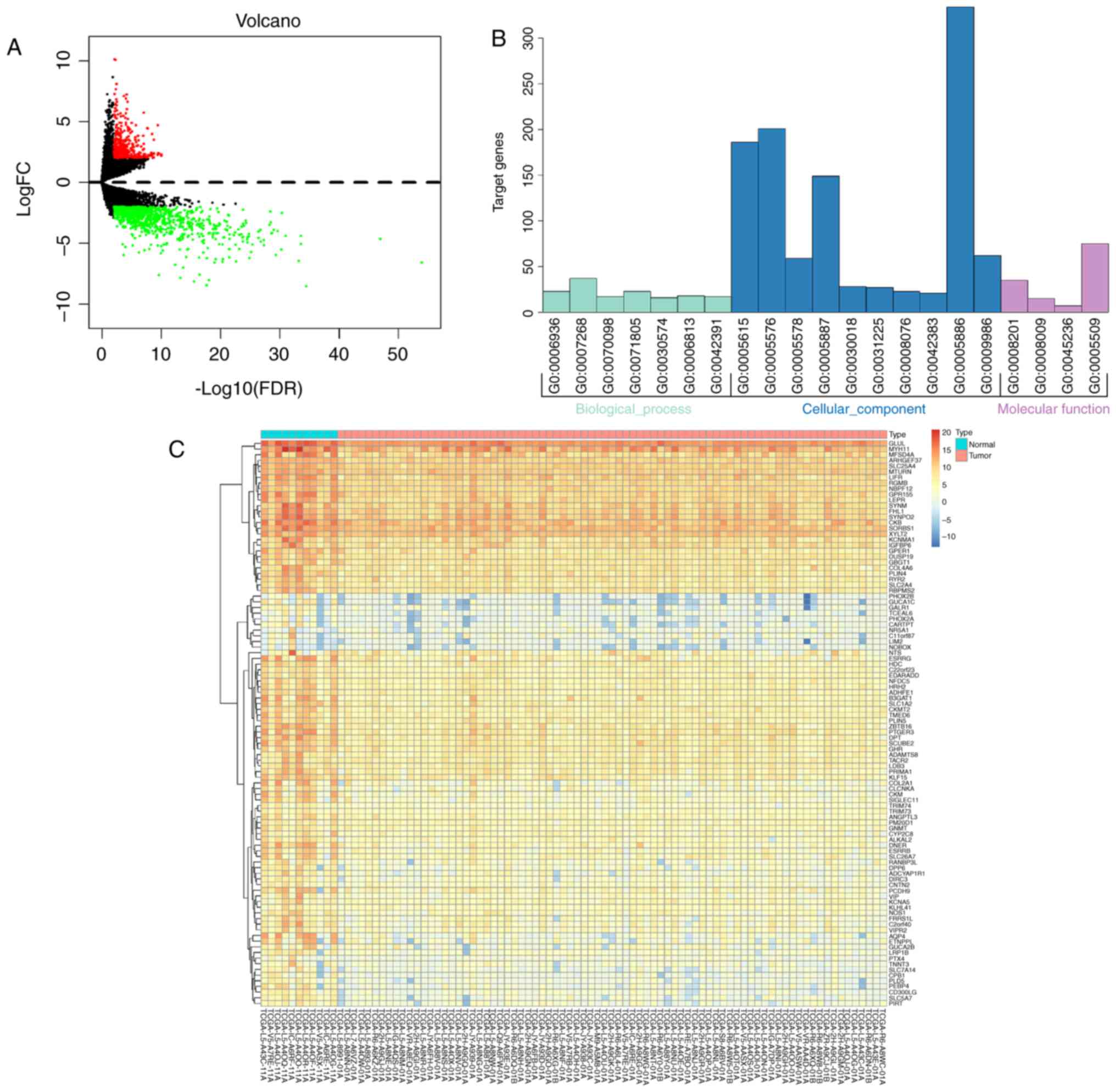
The RNA expression levels in 79 EAC tumor samples
and 11 normal samples were analyzed. With a cut-off value of
FDR-adjusted P<0.01 and FC >2.0, 367 upregulated and 922
downregulated mRNAs were identified (Table SII). A volcano plot was therefore
constructed to visualize the results (Fig. 3A). The top 100 significant DEmRNAs
were then highlighted by plotting FDR-adjusted P-values in a heat
map (Fig. 3C).
To analyze the DEmRNA functions, enrichment analysis
based on enriched functional GO modules was performed. The results
demonstrated that the DEmRNAs were significantly enriched in the
‘chemokine-mediated signaling pathway’ (GO: 0070098), ‘plasma
membrane’ (GO: 0005886) and ‘calcium ion binding’ (GO: 0005509) GO
terms under ‘biological process’, ‘cellular component’ and
‘molecular function’, respectively (Fig.
3B).
Identification of DEmiRNAs
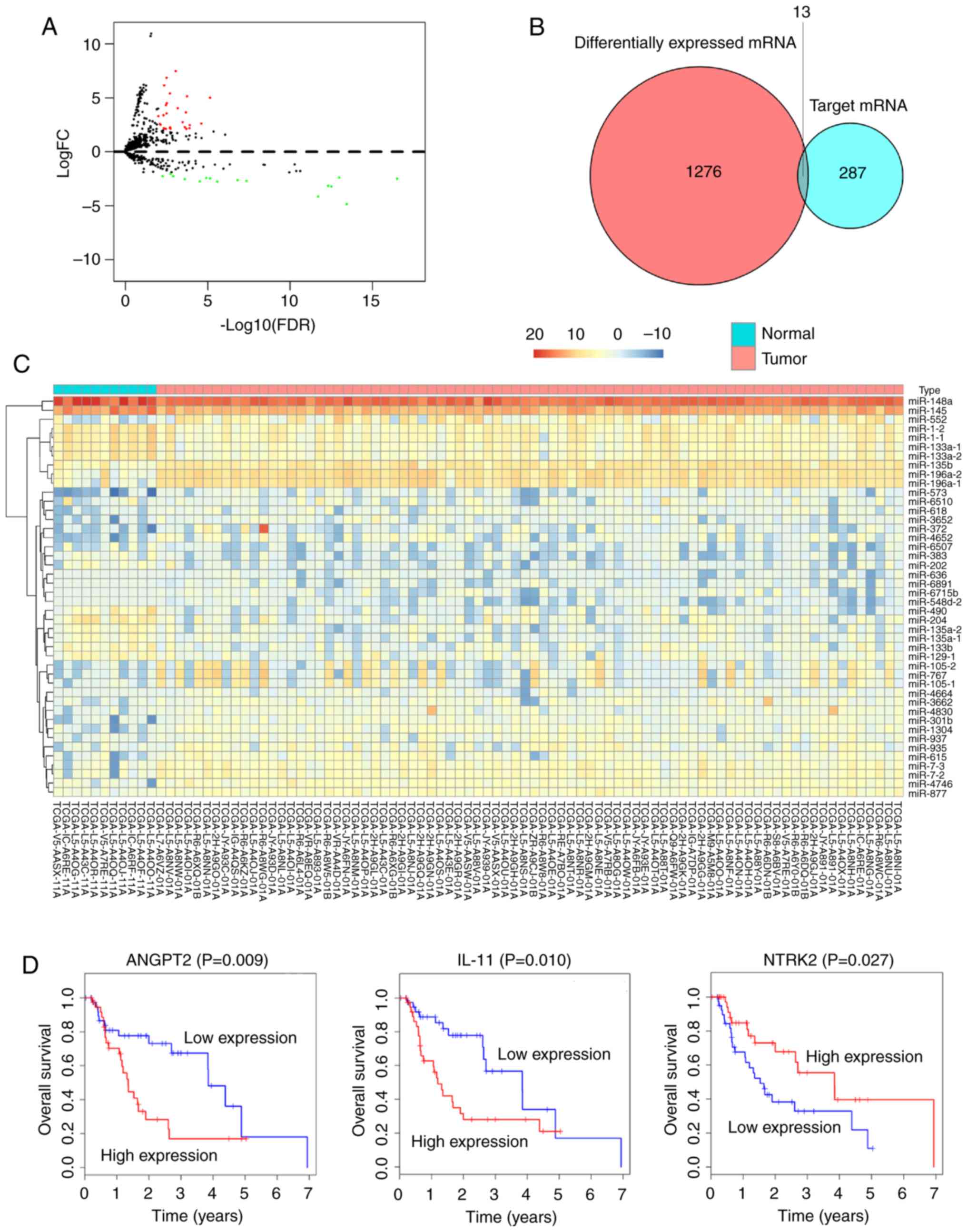
In order to design a ceRNA network for EAC, the
miRNA expression profiles between tumor samples and normal samples
were compared. Subsequently, 44 DEmiRNAs, including 28 upregulated
and 16 downregulated were identified (Fig. 4A and C). The mRNAs that were targeted
by the 44 DEmiRNAs from miRDB, miRTarBase and TargetScan were then
screened. To improve data reliability, mRNAs that were not included
in the set of 1,289 DEmRNAs were excluded. Eventually, 13 DEmiRNAs
remained in the ceRNA network (Fig.
4B).
In order to identify the DEmRNAs that may have
potential prognostic ability, the expression profiles of the 13
DEmRNAs included in the ceRNA network were analyzed using
Kaplan-Meier curve. The results demonstrated that the expression
profiles of three DEmRNAs were positively correlated with OS
(P<0.05). Two of these DEmRNAs, angiopoietin 2 and interleukin
11 (IL11) were negatively correlated with OS, whereas neurotrophic
receptor tyrosine kinase 2 (NTRK2) was positively correlated with
OS (Fig. 4D).
To identify the lncRNA-miRNA interactions in EAC,
the potential MREs in DElncRNAs were screened using miRcode. For
miRNA-mRNA interactions, miRDB, miRTarBase, and TargetScan were
used to identify the DEmRNAs targeted by DEmiRNAs. The results are
listed in Tables II and III.
 | Table II.Potential miRNAs that may target
cancer-associated lncRNAs by miRNA response elements. |
Table II.
Potential miRNAs that may target
cancer-associated lncRNAs by miRNA response elements.
| lncRNAs | miRNAs |
|---|
| LINC00525 | hsa-mir-301b,
hsa-mir-383 |
| PART1 | hsa-mir-301b,
hsa-mir-145, hsa-mir-204 |
| C2orf48 | hsa-mir-372,
hsa-mir-372 |
| LINC00483 | hsa-mir-372 |
| C20orf166-AS1 | hsa-mir-301b,
hsa-mir-372 |
| C15orf54 | hsa-mir-301b,
hsa-mir-372 |
| C8orf31 | hsa-mir-372,
hsa-mir-204 |
| AP002478.1 | hsa-mir-372 |
| LINC00314 | hsa-mir-372,
hsa-mir-204 |
| LINC00501 | hsa-mir-301b,
hsa-mir-204 |
| C1orf137 | hsa-mir-204,
hsa-mir-383 |
| MUC19 | hsa-mir-301b,
hsa-mir-372, hsa-mir-145, hsa-mir-204 |
| KRBOX1-AS1 | hsa-mir-301b,
hsa-mir-372, hsa-mir-204 |
| LINC00114 | hsa-mir-204 |
| SNHG14 | hsa-mir-301b,
hsa-mir-372, hsa-mir-145, hsa-mir-204 |
| LINC00337 | hsa-mir-372,
hsa-mir-145, hsa-mir-383 |
| HOTAIR | hsa-mir-301b,
hsa-mir-204 |
| LINC00332 | hsa-mir-204 |
| CYP1B1-AS1 | hsa-mir-301b,
hsa-mir-145, hsa-mir-204 |
| AC110491.1 | hsa-mir-204 |
| LINC00494 | hsa-mir-372,
hsa-mir-145, hsa-mir-383 |
| LINC00299 | hsa-mir-145,
hsa-mir-204, hsa-mir-383 |
| CADM2-AS1 | hsa-mir-301b,
hsa-mir-145, hsa-mir-383 |
| AC011374.1 | hsa-mir-372 |
| ADAMTS9-AS1 | hsa-mir-301b,
hsa-mir-145 |
| AC002464.1 | hsa-mir-383 |
| AC007834.1 | hsa-mir-301b |
| ADAMTS9-AS2 | hsa-mir-301b,
hsa-mir-372, hsa-mir-145, hsa-mir-204 |
| GK-AS1 | hsa-mir-145 |
| LIFR-AS1 | hsa-mir-372,
hsa-mir-204 |
| ALDH1L1-AS2 | hsa-mir-301b,
hsa-mir-372, hsa-mir-145 |
| PVT1 | hsa-mir-372,
hsa-mir-145, hsa-mir-383 |
| LINC00536 | hsa-mir-204 |
| AC006487.1 | hsa-mir-204 |
| AC090206.1 | hsa-mir-204 |
| FAM181A-AS1 | hsa-mir-204 |
| LINC00524 | hsa-mir-204 |
 | Table III.Differentially expressed mRNAs
targeted by differentially expressed miRNAs. |
Table III.
Differentially expressed mRNAs
targeted by differentially expressed miRNAs.
| miRNAs | mRNAs |
|---|
| hsa-mir-145 | ANGPT2, PDGFD, DDC,
MEST |
| hsa-mir-148a | HOXC8, NPTX1 |
| hsa-mir-196b | HOXB7, IGF2BP3,
HOXC8 |
| hsa-mir-204 | HOXC8, NTRK2,
SLC22A6, CHRDL1, IL11, NPTX1, CDH2 |
| hsa-mir-372 | TMEM100, CADM2 |
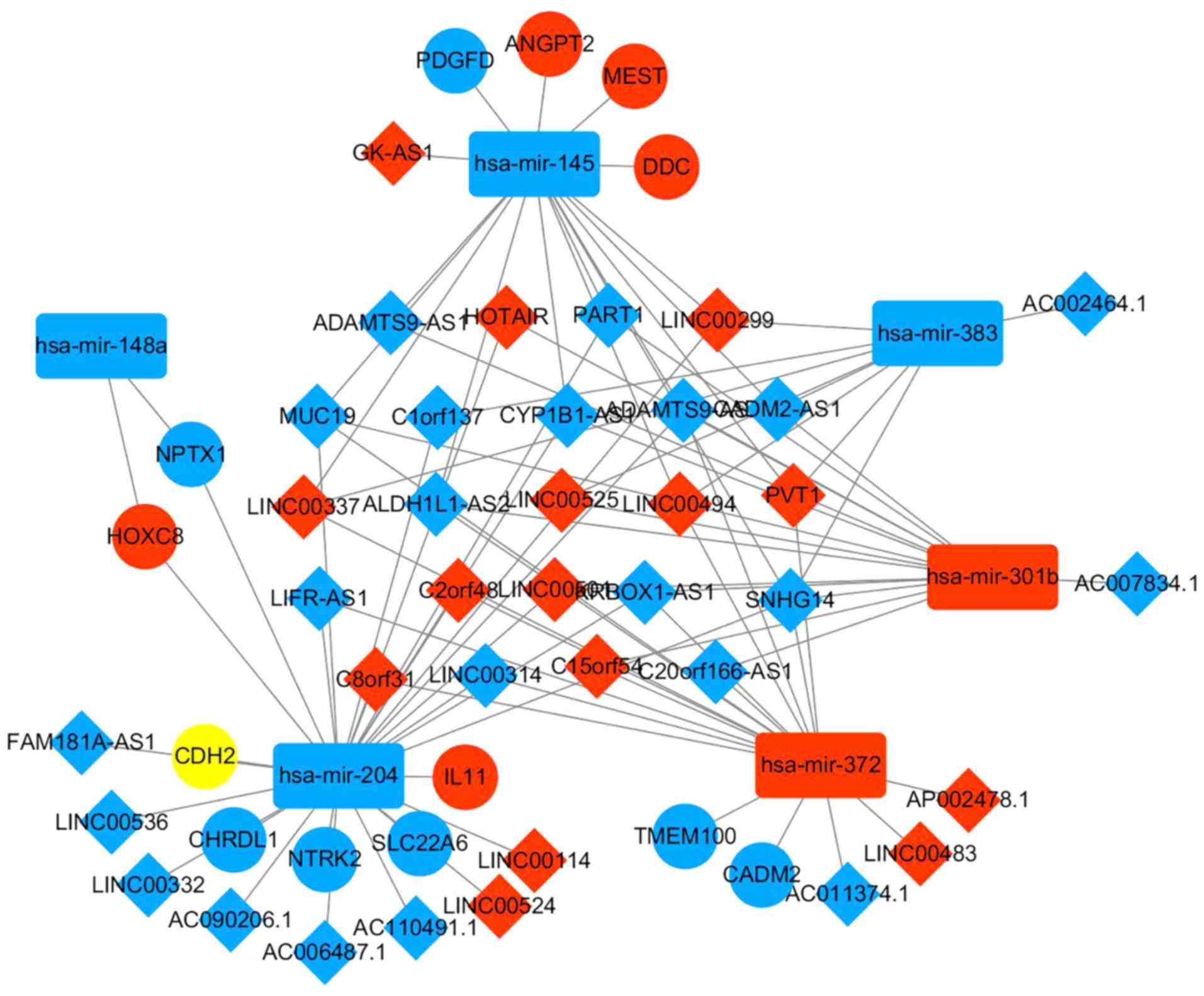
ceRNA network construction
In order to improve knowledge on DElncRNA function
in EAC, a dysregulated lncRNA-miRNA-mRNA ceRNA network based on the
aforementioned data (presented in Tables II and III) was constructed. The results
demonstrated that in the ceRNA network, interaction of five
DEmiRNAs with 37 DElncRNAs was predicted, according to the results
retrieved from miRcode. The ceRNA network is presented in Fig 5.
Discussion
lncRNAs represent a crucial category of non-coding
genes in the transcriptome that act as pivotal regulators of cell
physiology and pathology in human cancer by mediating gene
expression through multiple mechanisms (28). The dysregulation of lncRNA expression
is involved in the pathogenesis of various types of solid tumor
(29,30). Numerous novel biological functions
have been attributed to lncRNAs, which have become the focal point
of many studies (10,17). The ceRNA hypothesis describes
regulatory networks among protein-coding mRNAs and non-coding RNAs,
including miRNAs and lncRNAs at the post-transcription level.
According to this hypothesis, changes in the expression of one or
multiple miRNA targets can alter the number of unbound miRNAs and
lead to observable changes in miRNA activity. The various
transcripts from the transcriptome communicate with one another by
competitively binding to shared MREs (20). ceRNA networks in human cancer include
cancer-associated lncRNAs, microRNAs and mRNAs. A previous study
demonstrated a miRNA-lncRNA-mRNA interaction in ESCC (31). However, the ceRNA network in EAC
remains poorly understood.
At present, since lncRNAs are able to regulate miRNA
functions by competitively binding to shared MREs in mRNA, they are
considered as diagnostic and prognostic biomarkers. Numerous
well-studied lncRNAs have been identified as potential targets or
powerful predictors in various types of cancer, including
LINC00668, H19 and UCA1 (32–34).
However, studies on EAC remain rare. Based on the RNA-Seq data and
clinical data from 79 patients with EAC, the present study
demonstrated that 26 cancer-associated lncRNAs may affect the OS of
patients with EAC. In particular, the results from this study
reported that two DElncRNAs, CYP1B1-AS1 and HOTAIR, were not only
identified as part of the ceRNA network, but were also positively
and negatively correlated with OS, respectively, which suggested
that these two lncRNAs may serve as essential oncogenes and as
prognostic markers in EAC.
HOTAIR is a highly studied lncRNA. Previous studies
demonstrated that it serves a role in the development and
progression of various types of solid tumor, including renal cell
carcinoma, colorectal cancer, breast cancer, gastric cancer,
non-small cell lung cancer, cervical cancer and ovarian epithelial
carcinoma (35–45). In addition, Ren et al
(46) demonstrated that HOTAIR can
control the cell cycle by acting as a competing endogenous ‘sponge’
to downregulate miR-1 and upregulate cyclin D1 in ESCC. The present
study reported that HOTAIR expression was upregulated in EAC tumor
tissues. In addition, patients with highly expressed HOTAIR had
worse survival outcomes. HOTAIR may therefore compete with miR-301b
and miR-204 to regulate chordin like 1, NTRK2, IL11, neuronal
pentraxin 1, homeobox C8 and solute carrier family 22 member 6
expression. Although these mRNAs have been identified as aberrantly
expressed, their roles have not been fully investigated in EAC.
In addition to HOTAIR, LINC00163 and SLCO4A1-AS1
have also been reported to be associated with cancer prognosis. Guo
et al (47) demonstrated that
the LINC00163 level is significantly decreased in lung cancer
tissues and cell lines following bioinformatics and reverse
transcription-quantitative PCR analyses. LINC00163 expression was
lower in metastatic tissues compared with non-metastatic tissues,
and a higher LINC00163 expression in patients with lung cancer
could predict a better prognosis. Yang et al (48) reported that SLCO4A1-AS1 expression
was more upregulated in bladder cancer tissues compared with that
in adjacent normal tissues, and that SLCO4A1-AS1 overexpression is
associated with poor prognosis and tumor metastasis. Yu et
al (49) demonstrated that a
high SLCO4A1-AS1 expression level is associated with bladder cancer
progression and that SLCO4A1-AS1 promotes malignant phenotypes of
bladder cancer cells via the miRNA-335-5p/OCT4 axis.
To confirm the accuracy of the ceRNA network
prediction presented in this study, interactions among lncRNAs,
miRNAs and mRNAs in EAC were measured. Only cancer-associated
lncRNAs and miRNAs with >2.0 FC and FDR <0.01 were selected.
These non-coding genes were then annotated by GENCODE. Interactions
among lncRNAs, miRNAs and mRNAs were predicted by experimentally
conformed algorithms or by using miRDB, miRcode, miRTarBase and
TargetScan databases. In the present study, cancer-associated
lncRNAs in EAC were identified based on the RNA-Seq data of 79 EAC
tissues and 11 normal tissues. Subsequently, cancer-associated
miRNAs and mRNAs were identified. Eventually, interactions between
lncRNAs, miRNAs and mRNAs was identified by constructing an
lncRNA-miRNA-mRNA ceRNA network. A total of 37 DElncRNAs, five
miRNAs and 13 mRNAs were selected to construct this
newly-identified ceRNA-mediated gene regulatory network. This
network included numerous active lncRNA-miRNA-mRNA interactions
that may be used as prognostic biomarkers in EAC.
In conclusion, the present study identified some
cancer-associated lncRNAs and revealed their potential use in
prognosis prediction. In particular, some cancer-associated lncRNAs
may serve as ceRNAs. The ceRNA network that was built in the
present study may help understanding the mechanisms involved in the
development and progression of EAC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SC and YY conceived and designed the study. XC
acquired the data. YY analyzed and interpreted the data. YY and XC
wrote and revised the manuscript. SC supervised the study. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mannath J and Ragunath K: Role of
endoscopy in early oesophageal cancer. Nat Rev Gastroenterol
Hepatol. 13:720–730. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pera M, Manterola C, Vidal O and Grande L:
Epidemiology of esophageal adenocarcinoma. J Surg Oncol.
92:151–159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coleman HG, Xie SH and Lagergren J: The
epidemiology of esophageal adenocarcinoma. Gastroenterology.
154:390–405. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang X, Zhou X, Hu Q, Sun B, Deng M, Qi X
and Lü M: Advances in esophageal cancer: A new perspective on
pathogenesis associated with long non-coding RNAs. Cancer Lett.
413:94–101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lagergren J and Lagergren P: Recent
developments in esophageal adenocarcinoma. CA Cancer J Clin.
63:232–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rustgi AK and El-Serag HB: Esophageal
carcinoma. N Engl J Med. 371:2499–2509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen JF, Wu P, Xia R, Yang J, Huo XY, Gu
DY, Tang CJ, De W and Yang F: STAT3-induced lncRNA HAGLROS
overexpression contributes to the malignant progression of gastric
cancer cells via mTOR signal-mediated inhibition of autophagy. Mol
Cancer. 17:62018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou DD, Liu XF, Lu CW, Pant OP and Liu
XD: Long non-coding RNA PVT1: Emerging biomarker in digestive
system cancer. Cell Prolif. 50:2017. View Article : Google Scholar
|
|
12
|
Song YX, Sun JX, Zhao JH, Yang YC, Shi JX,
Wu ZH, Chen XW, Gao P, Miao ZF and Wang ZN: Non-coding RNAs
participate in the regulatory network of CLDN4 via ceRNA mediated
miRNA evasion. Nat Commun. 8:2892017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Parasramka MA, Maji S, Matsuda A, Yan IK
and Patel T: Long non-coding RNAs as novel targets for therapy in
hepatocellular carcinoma. Pharmacol Ther. 161:67–78. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li W, Zheng J, Deng J, You Y, Wu H, Li N,
Lu J and Zhou Y: Increased levels of the long intergenic
non-protein coding RNA POU3F3 promote DNA methylation in esophageal
squamous cell carcinoma cells. Gastroenterology. 146:1714–1726.e5.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao ZD, Han L, Lee H, Zhuang L, Zhang Y,
Baddour J, Nagrath D, Wood CG, Gu J, Wu X, et al: Energy
stress-induced lncRNA FILNC1 represses c-Myc-mediated energy
metabolism and inhibits renal tumor development. Nat Commun.
8:7832017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martens-Uzunova ES, Böttcher R, Croce CM,
Jenster G, Visakorpi T and Calin GA: Long noncoding RNA in
prostate, bladder, and kidney cancer. Eur Urol. 65:1140–1151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang P, Xu J, Wang Y and Cao X: An
interferon-independent lncRNA promotes viral replication by
modulating cellular metabolism. Science. 358:1051–1055. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Su W, Guo C, Wang L, Wang Z, Yang X, Niu
F, Tzou D, Yang X, Huang X, Wu J, et al: LncRNA MIR22HG abrogation
inhibits proliferation and induces apoptosis in esophageal
adenocarcinoma cells via activation of the STAT3/c-Myc/FAK
signaling. Aging (Albany NY). 11:4587–4596. 2019.PubMed/NCBI
|
|
19
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The rosetta stone of a hidden RNA
language. Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qi X, Zhang DH, Wu N, Xiao JH, Wang X and
Ma W: ceRNA in cancer: Possible functions and clinical
implications. J Med Genet. 52:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cesana M, Cacchiarelli D, Legnini I,
Santini T, Sthandier O, Chinappi M, Tramontano A and Bozzoni I: A
long noncoding RNA controls muscle differentiation by functioning
as a competing endogenous RNA. Cell. 147:358–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y,
Chen W, Liu F, Sun W, Li XF, et al: Exosome-transmitted lncARSR
promotes Sunitinib resistance in renal cancer by acting as a
competing endogenous RNA. Cancer Cell. 29:653–668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao C, Zhang T, Zhang D, Xie L, Zou X, Lei
L, Wu D and Liu L: The long non-coding RNA, SNHG6-003, functions as
a competing endogenous RNA to promote the progression of
hepatocellular carcinoma. Oncogene. 36:1112–1122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Conte F, Fiscon G, Chiara M, Colombo T,
Farina L and Paci P: Role of the long non-coding RNA PVT1 in the
dysregulation of the ceRNA-ceRNA network in human breast cancer.
PLoS One. 12:e01716612017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang S, Ning Q, Zhang G, Sun H, Wang Z and
Li Y: Construction of differential mRNA-lncRNA crosstalk networks
based on ceRNA hypothesis uncover key roles of lncRNAs implicated
in esophageal squamous cell carcinoma. Oncotarget. 7:85728–85740.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li CY, Liang GY, Yao WZ, Sui J, Shen X,
Zhang YQ, Peng H, Hong WW, Ye YC, Zhang ZY, et al: Integrated
analysis of long non-coding RNA competing interactions reveals the
potential role in progression of human gastric cancer. Int J Oncol.
48:1965–1976. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Team RC: R:, . A language and environment
for statistical computingR Foundation for Statistical Computing;
Vienna, Austria: 2013
|
|
28
|
Marchese FP, Raimondi I and Huarte M: The
multidimensional mechanisms of long noncoding RNA function. Genome
Biol. 18:2062017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alvarez-Dominguez JR and Lodish HF:
Emerging mechanisms of long noncoding RNA function during normal
and malignant hematopoiesis. Blood. 130:1965–1975. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Klingenberg M, Matsuda A, Diederichs S and
Patel T: Non-coding RNA in hepatocellular carcinoma: Mechanisms,
biomarkers and therapeutic targets. J Hepatol. 67:603–618. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin C, Zhang N, Wang Y, Wang Y, Nice E,
Guo C, Zhang E, Yu L, Li M, Liu C, et al: Functional role of a
novel long noncoding RNA TTN-AS1s in esophageal squamous cell
carcinoma progression and metastasis. Clin Cancer Res. 24:486–498.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang E, Yin D, Han L, He X, Si X, Chen W,
Xia R, Xu T, Gu D, De W, et al: E2F1-induced upregulation of long
noncoding RNA LINC00668 predicts a poor prognosis of gastric cancer
and promotes cell proliferation through epigenetically silencing of
CKIs. Oncotarget. 7:23212–23226. 2016.PubMed/NCBI
|
|
33
|
Liu L, Liu L and Lu S: lncRNA H19 promotes
viability and epithelial-mesenchymal transition of lung
adenocarcinoma cells by targeting miR-29b-3p and modifying STAT3.
Int J Oncol. 54:929–941. 2019.PubMed/NCBI
|
|
34
|
Cui X, Zhao C, Yao X, Qian B, Su C, Ren Y,
Yao Z, Gao X and Yang J: SND1 acts as an anti-apoptotic factor via
regulating the expression of lncRNA UCA1 in hepatocellular
carcinoma. RNA Biol. 15:1364–1375. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Katayama H, Tamai K, Shibuya R, Nakamura
M, Mochizuki M, Yamaguchi K, Kawamura S, Tochigi T, Sato I,
Okanishi T, et al: Long non-coding RNA HOTAIR promotes cell
migration by upregulating insulin growth factor-binding protein 2
in renal cell carcinoma. Sci Rep. 7:120162017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li M, Li X, Zhuang Y, Flemington EK, Lin Z
and Shan B: Induction of a novel isoform of the lncRNA HOTAIR in
Claudin-low breast cancer cells attached to extracellular matrix.
Mol Oncol. 11:1698–1710. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li P, Zhang X, Wang L, Du L, Yang Y, Liu
T, Li C and Wang C: lncRNA HOTAIR contributes to 5FU resistance
through suppressing miR-218 and activating NF-κB/TS signaling in
colorectal cancer. Mol Ther Nucleic Acids. 8:356–369. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma J, Fan Y, Feng T, Chen F, Xu Z, Li S,
Lin Q, He X, Shi W, Liu Y, et al: HOTAIR regulates HK2 expression
by binding endogenous miR-125 and miR-143 in oesophageal squamous
cell carcinoma progression. Oncotarget. 8:86410–86422. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chi S, Shen L, Hua T, Liu S, Zhuang G,
Wang X, Zhou X, Wang G and Wang H: Prognostic and diagnostic
significance of lncRNAs expression in cervical cancer: A systematic
review and meta-analysis. Oncotarget. 8:79061–79072. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fan CN, Ma L and Liu N: Systematic
analysis of lncRNA-miRNA-mRNA competing endogenous RNA network
identifies four-lncRNA signature as a prognostic biomarker for
breast cancer. J Transl Med. 16:2642018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao S, Zhao ZY, Wu R, Zhang Y and Zhang
ZY: Prognostic value of long noncoding RNAs in gastric cancer: A
meta-analysis. OncoTargets Ther. 11:4877–4891. 2018. View Article : Google Scholar
|
|
42
|
Guo F, Cao Z, Guo H and Li S: The action
mechanism of lncRNA-HOTAIR on the drug resistance of non-small cell
lung cancer by regulating Wnt signaling pathway. Exp Ther Med.
15:4885–4889. 2018.PubMed/NCBI
|
|
43
|
Qiu JJ, Lin YY, Ye LC, Ding JX, Feng WW,
Jin HY, Zhang Y, Li Q and Hua KQ: Overexpression of long non-coding
RNA HOTAIR predicts poor patient prognosis and promotes tumor
metastasis in epithelial ovarian cancer. Gynecol Oncol.
134:121–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang WJ, Li HT, Yu JP, Han XP, Xu ZP, Li
YM, Jiao ZY and Liu HB: A competing endogenous RNA network reveals
novel potential lncRNA, miRNA, and mRNA biomarkers in the prognosis
of human colon adenocarcinoma. J Surg Res. 235:22–33. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang H, Wang Z, Wu J, Ma R and Feng J:
Long noncoding RNAs predict the survival of patients with
colorectal cancer as revealed by constructing an endogenous RNA
network using bioinformation analysis. Cancer Med. 8:863–873. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ren K, Li Y, Lu H, Li Z, Li Z, Wu K, Li Z
and Han X: Long noncoding RNA HOTAIR controls cell cycle by
functioning as a competing endogenous RNA in esophageal squamous
cell carcinoma. Transl Oncol. 9:489–497. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guo X, Wei Y, Wang Z, Liu W, Yang Y, Yu X
and He J: LncRNA LINC00163 upregulation suppresses lung cancer
development though transcriptionally increasing TCF21 expression.
Am J Cancer Res. 8:2494–2506. 2018.PubMed/NCBI
|
|
48
|
Yang Y, Wang F, Huang H, Zhang Y, Xie H
and Men T: lncRNA SLCO4A1-AS1 promotes growth and invasion of
bladder cancer through sponging miR-335-5p to upregulate OCT4.
OncoTargets Ther. 12:1351–1358. 2019. View Article : Google Scholar
|
|
49
|
Yu J, Han Z, Sun Z, Wang Y, Zheng M and
Song C: LncRNA SLCO4A1-AS1 facilitates growth and metastasis of
colorectal cancer through β-catenin-dependent Wnt pathway. J Exp
Clin Cancer Res. 37:2222018. View Article : Google Scholar : PubMed/NCBI
|