Introduction
As one of the most fatal types of human malignant
cancer, pancreatic ductal adenocarcinoma (PDAC) exhibits a poor
prognosis, despite significant advances in diagnostic and
therapeutic options, and has the lowest 5 year relative survival
rate of 6% reported in 2016 in North America (1,2).
Difficulties in detecting the disease at an early stage partially
contribute to the poor prognosis (3). The search for novel biomarkers to
detect and diagnose PDAC has been of interest to clinicians and
researchers. Carbohydrate antigen 19-9 (CA19-9), the only
authenticated marker for clinical application, lacks the
specificity required for a differential diagnosis (4,5). The
majority of other markers are expensive or experimental, and are
not widely used in routine clinical practice (6,7).
Therefore, there is an urgent need to identify convenient and
easily applicable biomarkers for PDAC.
Peripheral blood examination is one of the most
frequently used measures in tumor management. However, it is
relatively rare to regard variables excluded from routine blood
count parameters as prognostic factors (8). The neutrophil-to-lymphocyte ratio
(NLR), platelet-to-lymphocyte ratio (PLR) and
lymphocyte-to-monocyte ratio (LMR) at the initial diagnosis may
serve as simple indexes of immune function, and each one is
reported to be a prognostic factor in a number of different types
of malignant tumor, such as Hodgkin's lymphoma, and bladder and
hepatocellular cancer (9–12). However, their prognostic significance
is controversial for patients with pancreatic cancer. Martin et
al (13) investigated the
effects of systemic inflammation-based factors, including NLR and
PLR, on the outcome of patients with tumors, and concluded that
both NLR and PLR were independent prognostic markers. However,
Aliustaoglu et al (14)
proposed that NLR was a superior marker for patients with
pancreatic cancer. Furthermore, a previous study investigated the
association between LMR and PDAC; one study indicated that low LMR
predicted a poor prognosis in patients with resectable PDAC, but no
further studies were pursued (15).
Therefore, the prognostic value of the peripheral blood NLR, PLR
and LMR in patients with PDAC was examined in the present
study.
S100 calcium-binding protein A4 (S100A4), a member
of the S100 family of calcium-binding proteins, promotes tumor
metastasis, proliferation and immune evasion (16–19). A
previous study has verified the hypothesis that S100A4-mediated
metastasis is associated with extensive T-cell infiltration or
tumor-associated neutrophils (20).
S100A4 tissue expression was associated with a poor prognostic
outcome in a variety of cancer types, including PDAC, and lung and
breast cancer (21,22). In the present study, the association
between the pre-treatment peripheral blood NLR, PLR or LMR and
S100A4 tissue expression was analyzed in 258 patients diagnosed
with PDAC.
Materials and methods
Patient eligibility
The present study was conducted at the Tianjin
Medical University Cancer Institute and Hospital (Tianjin, China).
The study was approved by the Ethics Committee and all patients
provided written informed consent. Patients with PDAC hospitalized
between December 2008 and December 2014 were enrolled in the study.
The clinical records of these patients were reviewed
retrospectively. The inclusion criteria were: i) Patients were
hospitalized for primary diagnosis and had received no treatment
prior to diagnosis (therapy naïve); ii) patients were
histologically diagnosed with primary PDAC and staged according to
the Tumor-Node-Metastasis (TNM) criteria of the American Joint
Committee on Cancer, 2017 (23); and
iii) all clinical data for the patients were available. The
exclusion criteria were: i) Patients did not have primary PDAC; ii)
the detailed and required clinical data were unavailable; iii)
patients had prior clinical evidence of infection, other
inflammation, pulmonary embolism, acute myocardial infarction,
cerebrovascular accident or hematological disease, or were taking
drugs for hematological disorders; iv) patients had received prior
radiation therapy, chemotherapy or surgery; and v) contact with the
patients was lost during the follow-up time.
Clinical and laboratory data
collection
Data regarding the patients, the tumor
characteristics, the diagnosis and the treatment modalities were
collected and retrospectively reviewed. In the current study, the
majority of the complications were anastomotic leakage and
ischemia-reperfusion; drug-controlled complications such as fever
or abdominal infection were not included. For all study subjects,
blood samples were collected at the first consultation in
edathamil-2K preservative tubes, stored at room temperature and
analyzed using the same hematology analyzer within 48 h;
differential leukocyte counts were recorded. The NLR, PLR and LMR
were defined as the absolute neutrophil count divided by the
absolute lymphocyte count, the absolute platelet count divided by
the absolute lymphocyte count and the absolute lymphocyte count
divided by the absolute monocyte count, respectively, in the
laboratory tests prior to treatment. Tumor tissues and adjacent
healthy tissues were collected during surgery or by 18-G needle
biopsy.
Histopathological analysis
Tissues were fixed with 4% formalin at room
temperature within 48 h, embedded in paraffin, and diagnosed
clinically and histopathologically at the Departments of Pancreatic
Cancer and Pathology. All pathological data were analyzed by two
pathologists independently.
Immunohistochemical (IHC) analysis was performed to
evaluate the expression levels of S100A4 as previously described
(24). Briefly, tissue sections with
thickness of 3 µm were incubated at 60°C for 2 h followed by
deparaffinization with xylene and rehydration in concentrations of
100, 95, 85 and 75% alcohol, respectively. The sections were
submerged in ethylenediamine tetraacetic acid antigen retrieval
buffer (Beijing Solarbio Science & Technology Co., Ltd.) and
heated in a microwave for 2 min for antigen retrieval, treated with
3% hydrogen peroxide at room temperature for 10 min in methanol to
quench endogenous peroxidase activity and incubated with 1% bovine
serum albumin (Amresco LLC) to block non-specific binding. The
sections were incubated with mouse anti-S100A4 (1:2,000; cat. no.
ab197896; Abcam,) and diluent (cat. no. ZLI-9030; Origene
Technologies, Inc.) overnight at 4°C. Normal goat serum (Origene
Technologies, Inc.) was used as a negative control. Tissues were
subsequently incubated with the secondary antibody (cat. no.
K183316C; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.)
at 37°C for 1 h. Following 3 PBS washes, the tissue sections were
counterstained with hematoxylin at room temperature for 3 min,
dehydrated and mounted.
The degree of IHC staining was reviewed and scored
independently by two pathologists. IHC was scored by multiplying
the scores of the percentage of positive tumor cells and staining
intensity. The percentage of positive tumor cells was scored as 0
(0%), 1 (1–25%), 2 (26–50%), 3 (51–75%) or 4 (76–100%). Staining
intensity was scored as 0 (negative), 1 (weakly positive), 2
(moderately positive) or 3 (strongly positive). According to the
staining results, the S100A4 tissue expression level was classified
into two groups: Negative (score <3) and positive (score
≥3).
Statistical analysis
Follow-up time was defined as the time between
admission and August 2015. Overall survival (OS) time was defined
as the interval between the time of diagnosis and final follow-up
or death. Statistical analyses were performed using SPSS software
version 21.0 (IBM Corp.). A χ2 test was performed to
compare baseline clinical characteristics between patients of
different subgroups. The survival curves were produced using
Kaplan-Meier analysis. The log-rank and multivariate Cox
proportional hazards regression model analyses were performed to
determine the independent prognostic factors and survival function.
The mean NLR, PLR and LMR data was compared for the subgroups with
positive and negative S100A4 expression using an unpaired Student's
t-test. X-tile analysis was used to identify the best cut-off value
for low and high NLR, PLR and LMR. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
Among the 258 patients with PDAC, the mean age was
59 years (median, 58.64 years; range, 21–75 years). The median OS
time was 9 months (range, 1–32 months). A total of 105 patients
were diagnosed based on the results of a biopsy, 70 patients
received palliative surgery and 83 received radical surgery.
Adjuvant chemotherapy and radiation therapy were performed
following the biopsy or surgery. The median CA19-9 level was 260.45
U/ml (range, 0–100,254 U/ml). The clinicopathological
characteristics of the patients and treatment modalities are
presented in Table I.
 | Table I.Clinicopathological characteristics
and treatment modalities in patients with pancreatic ductal
adenocarcinoma. |
Table I.
Clinicopathological characteristics
and treatment modalities in patients with pancreatic ductal
adenocarcinoma.
| Characteristic | Number of
patients | Percentage |
|---|
| Sex |
|
|
|
Male | 146 | 56.6 |
|
Female | 112 | 43.4 |
| T stage at
diagnosis |
|
|
| T1 | 1 | 0.4 |
| T2 | 42 | 16.3 |
| T3 | 118 | 45.7 |
| T4 | 97 | 37.6 |
| N stage at
diagnosis |
|
|
| N0 | 111 | 43.0 |
| N1 | 119 | 46.1 |
| N2 | 28 | 10.9 |
| M stage at
diagnosis |
|
|
| M0 | 165 | 64.0 |
| M1 | 93 | 36.0 |
| Stage at
diagnosis |
|
|
| I | 16 | 6.2 |
| II | 24 | 9.3 |
|
III | 125 | 48.5 |
| IV | 93 | 36.0 |
| Tumor
differentiation |
|
|
|
High | 26 | 10.1 |
|
Moderate | 109 | 42.2 |
|
Poor | 123 | 47.7 |
| Tumor location |
|
|
| Head
and neck | 165 | 64.0 |
| Body
and tail | 93 | 36.0 |
| Adjuvant radiation
therapy |
|
|
|
Yes | 17 | 6.6 |
| No | 241 | 93.4 |
| Adjuvant
chemotherapy |
|
|
|
Yes | 219 | 84.9 |
| No | 39 | 15.1 |
| Complications |
|
|
|
Yes | 20 | 7.8 |
| No | 238 | 92.2 |
Patient peripheral blood
characteristics
At diagnosis, the median NLR, PLR and LMR were 2.55
(range, 0.63–19.00), 142.09 (range, 16.49–1,316.67) and 3.13
(range, 0.30–42.33), respectively. The baseline characteristics of
the patients grouped by NLR, PLR or LMR quartiles are presented in
Tables II–IV. The skewed frequency distribution of
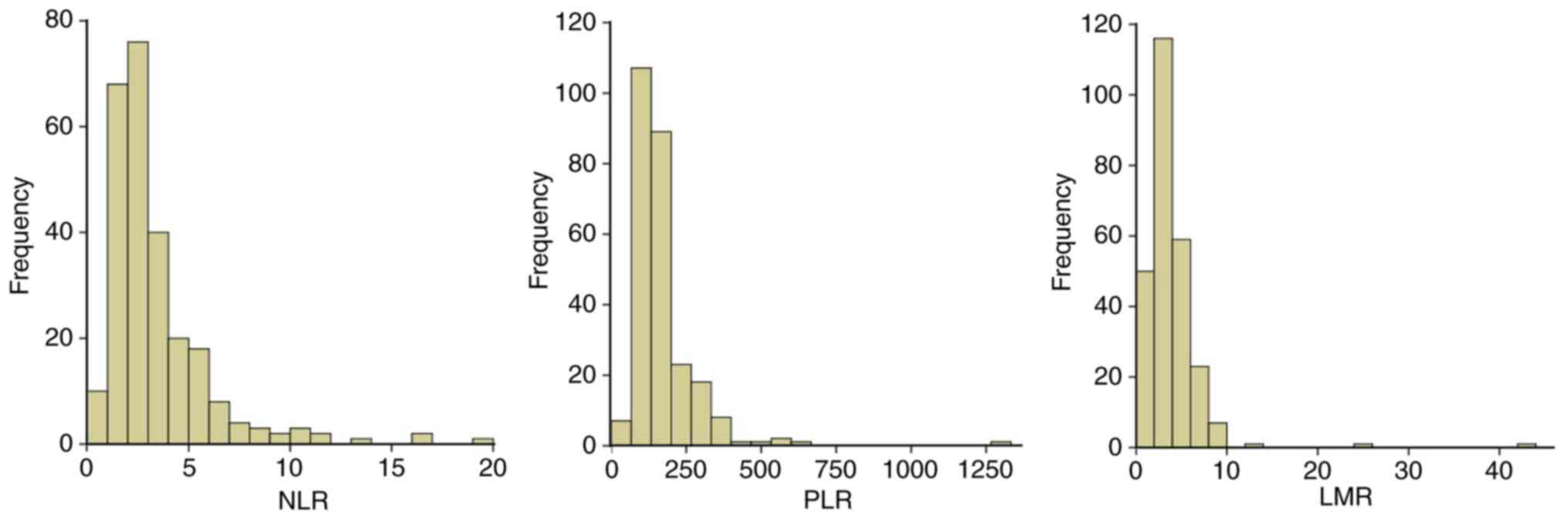
NLR, PLR and LMR is presented in Fig.
1, the majority of the patients exhibited NLR<5, PLR<250
and LMR<10.
 | Table II.Baseline characteristics of patients
with pancreatic ductal adenocarcinoma by neutrophil-to-lymphocyte
ratio quartiles. |
Table II.
Baseline characteristics of patients
with pancreatic ductal adenocarcinoma by neutrophil-to-lymphocyte
ratio quartiles.
| Variable | N | 1st quartile | 2nd quartile | 3rd quartile | 4th quartile | P-value |
|---|
| Sex |
|
|
|
|
| 0.008a |
|
Male | 146 | 25 | 39 | 44 | 38 |
|
|
Female | 112 | 39 | 26 | 21 | 26 |
|
| T stage at
diagnosis |
|
|
|
|
| 0.816 |
|
T1+T2 | 43 | 10 | 10 | 8 | 15 |
|
| T3 | 118 | 25 | 33 | 32 | 28 |
|
| T4 | 97 | 26 | 27 | 24 | 20 |
|
| N stage at
diagnosis |
|
|
|
|
| 0.450 |
| N0 | 111 | 30 | 30 | 30 | 21 |
|
| N1 | 119 | 31 | 28 | 27 | 33 |
|
| N2 | 28 | 8 | 7 | 6 | 7 |
|
| M stage at
diagnosis |
|
|
|
|
| 0.116 |
| M0 | 165 | 45 | 44 | 43 | 33 |
|
| M1 | 93 | 19 | 21 | 22 | 31 |
|
| Stage at
diagnosis |
|
|
|
|
| 0.617 |
| I | 16 | 4 | 3 | 4 | 5 |
|
| II | 24 | 7 | 6 | 6 | 5 |
|
|
III | 125 | 34 | 28 | 36 | 27 |
|
| IV | 93 | 19 | 19 | 23 | 23 |
|
| Tumor
differentiation |
|
|
|
|
| 0.212 |
|
High | 26 | 12 | 6 | 4 | 4 |
|
|
Moderate | 109 | 25 | 30 | 26 | 28 |
|
|
Poor | 123 | 27 | 29 | 35 | 32 |
|
| Tumor location |
|
|
|
|
| 0.007a |
| Head
and neck | 165 | 32 | 39 | 51 | 43 |
|
| Body
and tail | 93 | 32 | 26 | 14 | 21 |
|
| Adjuvant radiation
therapy |
|
|
|
|
| 0.741 |
| No | 241 | 60 | 61 | 62 | 58 |
|
|
Yes | 17 | 4 | 4 | 3 | 6 |
|
| Surgery |
|
|
|
|
| 0.833 |
|
None | 105 | 25 | 24 | 27 | 29 |
|
|
Radical | 83 | 24 | 20 | 19 | 20 |
|
|
Palliative | 70 | 15 | 21 | 19 | 15 |
|
| Adjuvant
chemotherapy |
|
|
|
|
| 0.906 |
|
None | 39 | 9 | 8 | 12 | 10 |
|
|
GEM | 144 | 34 | 36 | 37 | 37 |
|
| GEM +
others | 75 | 21 | 21 | 16 | 17 |
|
| Complications |
|
|
|
|
| 0.735 |
| No | 238 | 61 | 59 | 60 | 58 |
|
|
Yes | 20 | 3 | 6 | 5 | 6 |
|
| CA19-9, U/ml |
|
|
|
|
| 0.037a |
|
<73.68 | 64 | 16 | 20 | 12 | 16 |
|
|
73.68–260.45 | 65 | 24 | 18 | 16 | 7 |
|
|
>260.45 to ≤1,357 | 65 | 12 | 15 | 19 | 19 |
|
|
>1,357 | 64 | 12 | 12 | 18 | 22 |
|
 | Table IV.Baseline characteristics of patients
with pancreatic ductal adenocarcinoma by lymphocyte-to-monocyte
ratio quartiles. |
Table IV.
Baseline characteristics of patients
with pancreatic ductal adenocarcinoma by lymphocyte-to-monocyte
ratio quartiles.
| Variable | N | 1st quartile | 2nd quartile | 3rd quartile | 4th quartile | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
Male | 146 | 34 | 47 | 40 | 25 | 0.001a |
|
Female | 112 | 30 | 18 | 25 | 39 |
|
| T stage at
diagnosis |
|
|
|
|
|
|
|
T1+T2 | 43 | 11 | 8 | 14 | 10 | 0.469 |
| T3 | 118 | 33 | 28 | 29 | 28 |
|
| T4 | 97 | 20 | 29 | 26 | 22 |
|
| N stage at
diagnosis |
|
|
|
|
|
|
| N0 | 111 | 25 | 31 | 28 | 27 | 0.672 |
| N1 | 119 | 26 | 34 | 32 | 27 |
|
| N2 | 28 | 6 | 7 | 5 | 10 |
|
| M stage at
diagnosis |
|
|
|
|
|
|
| M0 | 165 | 32 | 44 | 44 | 45 | 0.062 |
| M1 | 93 | 32 | 21 | 21 | 19 |
|
| Stage at
diagnosis |
|
|
|
|
|
|
| I | 16 | 4 | 3 | 6 | 3 | 0.347 |
| II | 24 | 6 | 5 | 7 | 6 |
|
|
III | 125 | 27 | 32 | 29 | 27 |
|
| IV | 93 | 24 | 16 | 29 | 24 |
|
| Tumor
differentiation |
|
|
|
|
|
|
|
High | 26 | 3 | 6 | 6 | 11 | 0.225 |
|
Moderate | 109 | 26 | 29 | 32 | 22 |
|
|
Poor | 123 | 35 | 30 | 27 | 31 |
|
| Tumor location |
|
|
|
|
|
|
| Head
and neck | 165 | 41 | 45 | 37 | 42 | 0.521 |
| Body
and tail | 93 | 23 | 20 | 28 | 22 |
|
| Adjuvant radiation
therapy |
|
|
|
|
|
|
| No | 241 | 60 | 62 | 61 | 58 | 0.741 |
|
Yes | 17 | 4 | 3 | 4 | 6 |
|
| Surgery |
|
|
|
|
|
|
|
None | 105 | 28 | 25 | 24 | 28 | 0.906 |
|
Radical | 83 | 20 | 19 | 24 | 20 |
|
|
Palliative | 70 | 16 | 21 | 17 | 16 |
|
| Adjuvant
chemotherapy |
|
|
|
|
|
|
|
None | 39 | 12 | 10 | 10 | 7 | 0.852 |
|
GEM | 144 | 35 | 33 | 37 | 39 |
|
| GEM +
others | 75 | 17 | 22 | 18 | 18 |
|
| Complications |
|
|
|
|
|
|
| No | 238 | 57 | 61 | 60 | 60 | 0.719 |
|
Yes | 20 | 7 | 4 | 5 | 4 |
|
| CA19-9, U/ml |
|
|
|
|
|
|
|
<73.68 | 64 | 17 | 15 | 18 | 14 | 0.228 |
|
73.68–260.45 | 65 | 7 | 18 | 20 | 20 |
|
|
>260.45 to ≤1,357 | 65 | 19 | 15 | 16 | 15 |
|
|
>1,357 | 64 | 21 | 17 | 11 | 15 |
|
Patients in the highest NLR quartile were primarily
male and had more pancreatic tumors of head and neck origin
compared with the lowest quartile. In addition, the CA19-9 value
was higher compared with that in the lowest NLR quartile (Table II). The patients in the highest PLR
quartile had more pancreatic tumors of head and neck origin
compared with those in the lowest PLR quartile. In addition,
patients in the highest quartile were less likely to receive
radiotherapy (Table III). The
patients in the highest LMR quartile were primarily female compared
with those in the lowest LMR quartile and no significant
differences existed in the characteristics of the patients between
the two groups (Table IV).
 | Table III.Baseline characteristics of patients
with pancreatic ductal adenocarcinoma by platelet-to-lymphocyte
ratio quartiles. |
Table III.
Baseline characteristics of patients
with pancreatic ductal adenocarcinoma by platelet-to-lymphocyte
ratio quartiles.
| Variable | N | 1st quartile | 2nd quartile | 3rd quartile | 4th quartile | P-value |
|---|
| Sex |
|
|
|
|
| 0.318 |
|
Male | 146 | 38 | 42 | 34 | 32 |
|
|
Female | 112 | 26 | 23 | 31 | 32 |
|
| T stage at
diagnosis |
|
|
|
|
| 0.764 |
|
T1+T2 | 43 | 10 | 10 | 10 | 13 |
|
| T3 | 118 | 27 | 28 | 33 | 30 |
|
| T4 | 97 | 26 | 30 | 21 | 20 |
|
| N stage at
diagnosis |
|
|
|
|
| 0.803 |
| N0 | 111 | 27 | 28 | 27 | 29 |
|
| N1 | 119 | 30 | 29 | 35 | 25 |
|
| N2 | 28 | 7 | 6 | 9 | 8 |
|
| M stage at
diagnosis |
|
|
|
|
| 0.381 |
| M0 | 165 | 41 | 38 | 47 | 39 |
|
| M1 | 93 | 23 | 27 | 18 | 25 |
|
| Stage at
diagnosis |
|
|
|
|
| 0.534 |
| I | 16 | 5 | 4 | 4 | 3 |
|
| II | 24 | 7 | 7 | 6 | 4 |
|
|
III | 125 | 32 | 30 | 29 | 34 |
|
| IV | 93 | 23 | 26 | 18 | 26 |
|
| Tumor
differentiation |
|
|
|
|
| 0.456 |
|
High | 26 | 7 | 3 | 9 | 7 |
|
|
Moderate | 109 | 26 | 33 | 22 | 28 |
|
|
Poor | 123 | 31 | 29 | 34 | 29 |
|
| Tumor location |
|
|
|
|
| 0.007a |
| Head
and neck | 165 | 32 | 39 | 51 | 43 |
|
| Body
and tail | 93 | 32 | 26 | 14 | 21 |
|
| Adjuvant radiation
therapy |
|
|
|
|
| 0.048a |
| No | 241 | 57 | 64 | 63 | 57 |
|
|
Yes | 17 | 7 | 1 | 2 | 7 |
|
| Surgery |
|
|
|
|
| 0.457 |
|
None | 105 | 32 | 25 | 23 | 25 |
|
|
Radical | 83 | 17 | 21 | 20 | 25 |
|
|
Palliative | 70 | 15 | 19 | 22 | 14 |
|
| Adjuvant
chemotherapy |
|
|
|
|
| 0.173 |
|
None | 39 | 12 | 8 | 9 | 10 |
|
|
GEM | 144 | 31 | 33 | 45 | 35 |
|
| GEM +
others | 75 | 21 | 24 | 11 | 19 |
|
| Complications |
|
|
|
|
| 0.940 |
| No | 238 | 60 | 59 | 60 | 59 |
|
|
Yes | 20 | 4 | 6 | 5 | 5 |
|
| CA19-9, U/ml |
|
|
|
|
| 0.118 |
|
<73.68 | 64 | 14 | 18 | 14 | 18 |
|
|
73.68–260.45 | 65 | 15 | 16 | 23 | 11 |
|
|
>260.45 to ≤1,357 | 65 | 19 | 9 | 16 | 21 |
|
|
>1,357 | 64 | 16 | 22 | 12 | 14 |
|
A significant increase was observed in OS among the
patients in the lowest NLR quartile compared with those in the
highest NLR quartile (median survival rate, 50.7 vs. 31.3%,
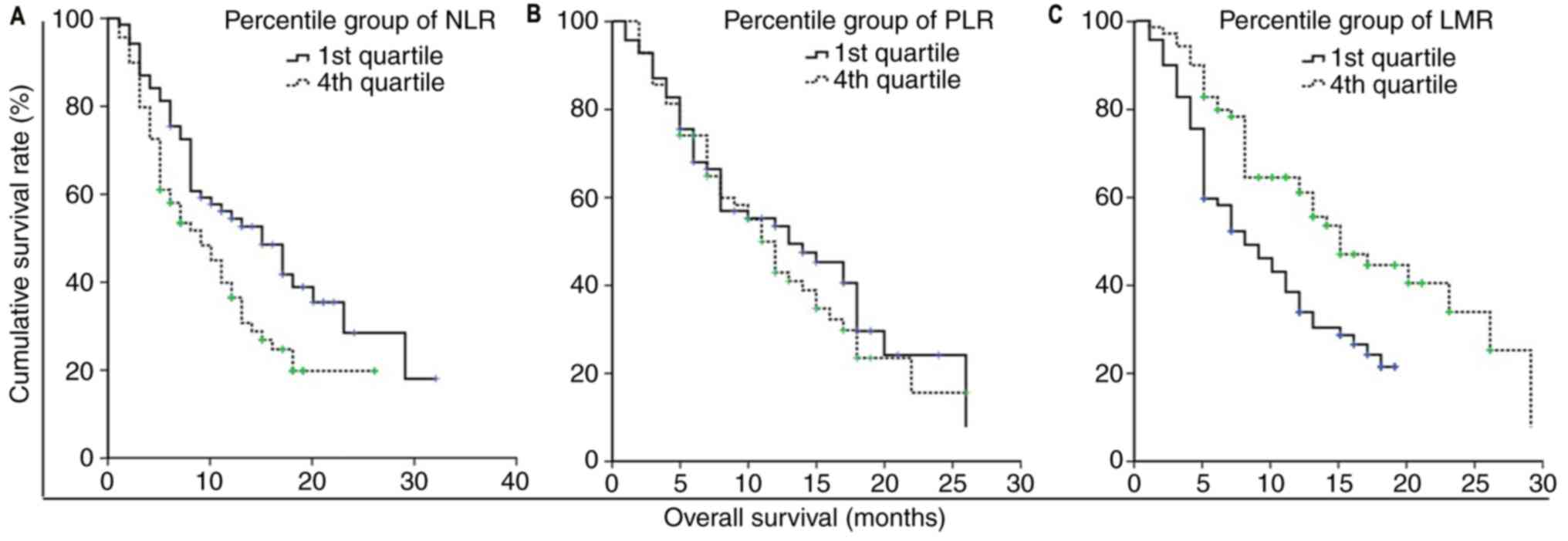
respectively; P<0.05; Fig. 2A).
No statistically significant difference was observed in the OS
between patients in the lowest PRL quartile and those in the
highest quartile (49.5 vs. 38.1%, respectively; P>0.05; Fig. 2B). By contrast, there was a
significant decrease in OS between patients in the lowest LMR
quartile compared with those with in the highest LMR quartile (28.3
vs. 57.8%; P<0.05; Fig. 2C).
Risk factors of mortality
According to the univariate analysis, higher NLR,
age, CA19-9 level, stage and histological grade were associated
with a higher risk of mortality, whereas surgery, chemotherapy and
higher LMR were associated with lower mortality (Table V). The univariate analysis revealed
that PLR had no significant effect on OS. The hazard ratio of
mortality of patients with PDAC in the highest NLR quartile
increased 1.765-fold (P=0.007) compared with those in the lowest.
However, the hazard ratio of mortality of patients with PDAC in the
highest LMR quartile decreased 0.501-fold (P=0.001) compared with
those in the lowest LMR quartile (Table
V).
 | Table V.Hazard ratios of baseline
characteristics for mortality in patients with pancreatic ductal
adenocarcinoma. |
Table V.
Hazard ratios of baseline
characteristics for mortality in patients with pancreatic ductal
adenocarcinoma.
| Variable | Hazard ratio (95%
CI) | P-value |
|---|
| Sex | 0.877
(0.650–1.182) | 0.388 |
| Age | 1.108
(1.002–1.035) | 0.026a |
| Stage at diagnosis
(ref: I) |
|
<0.00a |
| II | 0.431
(0.219–0.969) | 1.512 |
|
III | 4.652
(1.773–8.437) |
<0.001a |
| IV | 3.273
(1.728–6.202) |
<0.001a |
| Tumor
differentiation (ref: High) |
|
<0.001a |
|
Moderate | 2.898
(1.538–5.459) | 0.001a |
|
Poor | 5.524
(2.900–10.522) |
<0.001a |
| Tumor location | 1.140
(0.839–1.551) | 0.402 |
| Radiation
therapy | 0.934
(0.531–1.645) | 0.814 |
| Complications | 0.737
(0.426–1.275) | 0.275 |
| Surgery (ref:
None) |
|
<0.001a |
|
Radical | 0.372
(0.257–0.539) |
<0.001a |
|
Palliative | 0.688
(0.483–0.979) | 0.038a |
| Chemotherapy (ref:
None) |
| 0.037a |
|
GEM | 0.628
(0.421–0.939) | 0.023 a |
| GEM +
others | 0.579
(0.371–0.901) | 0.016a |
| CA19-9 (ref:
≤73.68) |
| 0.004a |
|
>73.68 to 260.45 | 1.367
(0.881–2.121) | 0.164 |
|
>260.45 to ≤1357 | 1.494
(0.970–2.300) | 0.068 |
|
>1357 | 2.163
(1.419–3.297) |
<0.001a |
| NLR quartile (ref:
1st) |
| 0.009a |
|
2nd | 0.945
(0.612–1.459) | 0.797 |
|
3rd | 1.414
(0.925–2.161) | 0.109 |
|
4th | 1.765
(1.164–2.676) | 0.007a |
| PLR quartile (ref:
1st) |
| 0.767 |
|
2nd | 0.927
(0.608–1.414) | 0.725 |
|
3rd | 1.045
(0.687–1.588) | 0.838 |
|
4th | 1.156
(0.762–1.753) | 0.495 |
| LMR quartile (ref:
1st) |
| 0.007a |
|
2nd | 0.763
(0.513–1.133) | 0.179 |
|
3rd | 0.586
(0.387–0.886) | 0.011a |
|
4th | 0.501
(0.328–0.765) | 0.001a |
Role of NLR, PLR and LMR as an
independent predictor of mortality in PDAC
The variables associated with NLR quartile and
survival status in the Cox regression analyses were included in the
Cox proportional hazard multivariate model, and all variables
included in Table VI were
associated with NLR quartile in previous analyses. The Cox
proportional hazard multivariate analysis was performed separately
to avoid combining NLR, PLR and LMR into one model, as they were
highly associated with absolute lymphocyte counts. The results
revealed that NLR was an independent predictor of mortality with a
hazard ratio of 1.198 (P=0.017) as a continuous variable, whereas
1.543 as a categorical variable (P=0.058), therefore NLR cannot be
used as an independent predictor of mortality as a categorical
variable. As a continuous variable, LMR was an independent
predictor of mortality with a hazard ratio of 0.846 (P=0.021), but
it was not a predictor as a categorical variable (hazard ratio,
0.663; P=0.074). PLR was not an independent predictor as a
continuous or categorical variable (Table VI).
 | Table VI.Cox proportional multivariate hazard
models in patients with pancreatic ductal adenocarcinoma. |
Table VI.
Cox proportional multivariate hazard
models in patients with pancreatic ductal adenocarcinoma.
| A, Model A1 (NLR as
a continuous variable) |
|---|
|
|---|
| Variable | Hazard ratio (95%
CI) | P-value |
|---|
| NLR | 1.198
(1.033–1.389) | 0.017a |
| Age | 1.014
(0.996–1.032) | 0.133 |
| Stage at diagnosis
(ref: I) |
| 0.012a |
| II | 1.199
(0.583–2.465) | 0.622 |
|
III | 2.968
(1.382–6.056) | 0.004a |
| IV | 2.366
(1.047–5.345) | 0.038a |
| Tumor
differentiation (ref: High) |
|
<0.001a |
|
Moderate | 2.248
(1.175–4.299) | 0.014a |
|
Poor | 3.942
(2.004–7.752) |
<0.001a |
| Surgery (ref:
None) |
|
<0.001a |
|
Radical | 0.578
(0.335–0.997) | 0.049a |
|
Palliative | 0.874
(0.595–1.285) | 0.495 |
| Chemotherapy (ref:
None) |
| 0.037a |
|
GEM | 0.682
(0.433–1.073) | 0.098 |
| GEM +
others | 0.650
(0.402–1.052) | 0.080 |
| CA19-9 (ref:
≤73.68) |
| 0.004a |
|
>73.68 to 260.45 | 1.373
(0.849–2.221) | 0.197 |
|
>260.45 to ≤1357 | 1.249
(0.802–1.944) | 0.326 |
|
>1357 | 1.503
(0.967–2.337) | 0.070 |
|
| B, Model B1 (LMR
as a continuous variable) |
|
|
Variable | Hazard ratio
(95% CI) | P-value |
|
| LMR | 0.846
(0.734–0.975) | 0.021a |
| Age | 1.012
(0.994–1.031) | 0.181 |
| Stage at diagnosis
(ref: I) |
| 0.012a |
| II | 1.169
(0.570–2.399) | 0.669 |
|
III | 2.786
(1.438–5.894) | 0.010a |
| IV | 2.214
(0.985–4.975) | 0.054 |
| Tumor
differentiation |
|
<0.001a |
| (ref: High) |
|
Moderate | 2.215
(1.160–4.232) | 0.016a |
|
Poor | 3.861
(1.964–7.589) |
<0.001a |
| Surgery (ref:
None) |
|
<0.001a |
|
Radical | 0.566
(0.329–0.973) | 0.040a |
|
Palliative | 0.855
(0.581–1.260) | 0.429 |
| Chemotherapy (ref:
None) |
| 0.037a |
|
GEM | 0.693
(0.441–1.090) | 0.113 |
| GEM +
others | 0.638
(0.394–1.034) | 0.068 |
| CA19-9, U/ml (ref:
≤73.68) |
| 0.004a |
|
>73.68 to 260.45 | 1.345
(0.833–2.174) | 0.226 |
|
>260.45 to ≤1,357 | 1.272
(0.815–1.985) | 0.289 |
|
>1,357 | 1.504
(0.965–2.345) | 0.071 |
|
| C, Model A2 (NLR
as a categorical variable) |
|
|
Variable | Hazard ratio
(95% CI) | P-value |
|
| NLR quartile (ref:
1st) |
|
0.019a |
|
2nd | 0.769
(0.487–1.214) | 0.259 |
|
3rd | 1.124
(0.725–1.743) | 0.602 |
|
4th | 1.543
(0.986–2.415) | 0.058 |
|
| D, Model B2 (LMR
as a categorical variable) |
|
|
Variable | Hazard ratio
(95% CI) | P-value |
|
| LMR quartile (ref:
1st) |
| 0.088 |
|
2nd | 0.952
(0.621–1.458) | 0.820 |
|
3rd | 0.642
(0.414–0.996) |
0.048a |
|
4th | 0.663
(0.422–1.040) | 0.074 |
S100A4 expression and its association
with peripheral blood NLR, PLR and LMR
The levels of peripheral blood NLR, PLR and LMR were
analyzed in the subgroups of different expression levels of S100A4
(Fig. 3A). The number of patients
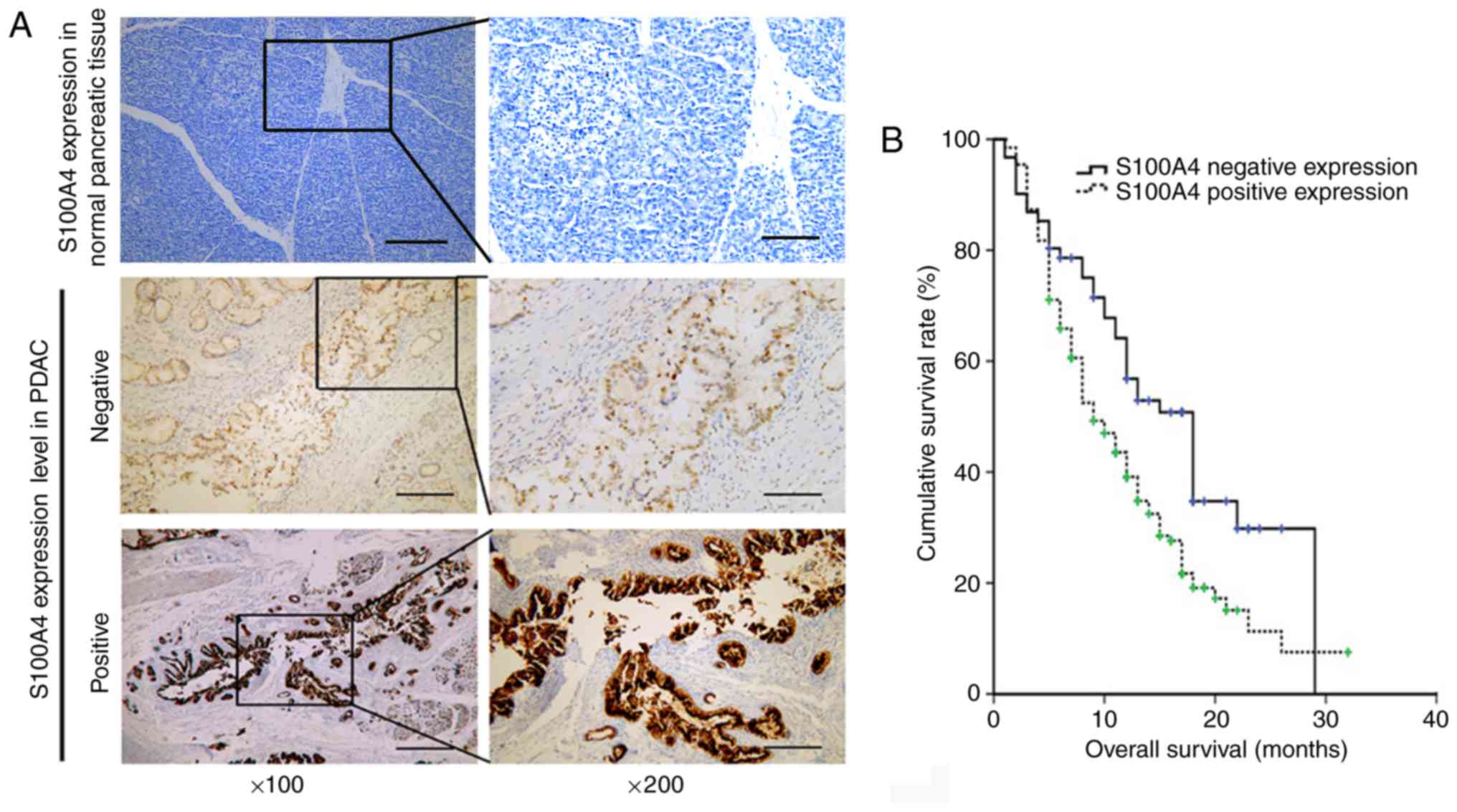
with negative and positive tissue expression levels of S100A4 was
60 and 198, respectively. High expression levels of S100A4 were
demonstrated to be associated with worse OS (P=0.003; Fig. 3B). The median value of NLR, PLR and
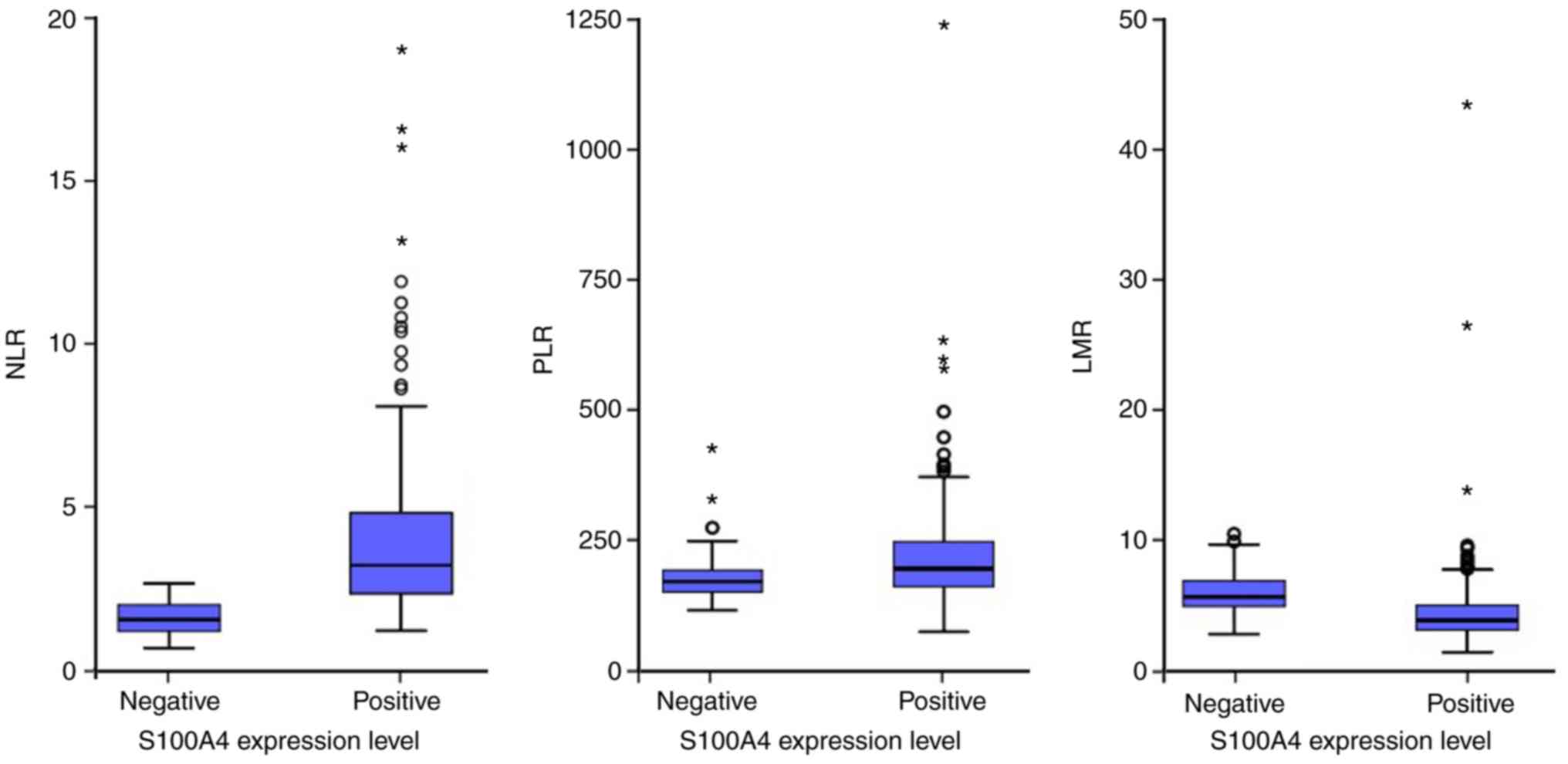
LMR in the subgroup of negative S100A4 expression was 1.50, 114.19
and 4.70, respectively (Fig. 4). The
mean value of NLR, PLR and LMR in the subgroup of positive S100A4
expression was 3.93 (median, 3.16), 181.10 (median, 152.45) and
3.46 (median, 2.73), respectively (Fig.
4). The levels of NLR and PLR in patients with positive S100A4
expression were higher compared with those in the negative S100A4
expression group (P<0.001 and P=0.001, respectively), whereas
the level of LMR in patients with positive S100A4 expression was
lower compared with that in the negative S100A4 expression group
(P=0.001; Fig. 4).
Kaplan Meier survival analysis demonstrated better
prognosis when NLR was lower than the cut-off value (P<0.001;
Fig. S1A). Furthermore, PLR had no
significant effects on the prognosis (P>0.05; Fig. S1B). In contrast, LMR higher than the
cut-off value predicted poor prognosis (P<0.001; Fig. S1C).
Discussion
The aim of the present study was to identify simply
obtained and inexpensive prognostic factors for PDAC. The
prognostic significance of the peripheral blood NLR, PLR and LMR at
diagnosis and their association with S100A4 expression in patients
with PDAC were investigated. A number of studies have assessed the
role of NLR in the outcome of PDAC and suggested that NLR may offer
important prognostic information for the survival rate in patients
with resectable PDAC (6,25). In the present study, the prognostic
role that NLR and LMR serve in PDAC was elucidated. Similarly to
previous studies, a high NLR was an independent prognostic marker
for the OS of patients with PDAC (6,14,26). LMR
has also been suggested to serve as a simple index of the immune
function, and low LMR has been regarded as an independent predictor
of poor prognosis in PDAC in a previous study (15). Consistent with the results of the
previous study, the present study demonstrated that LMR possessed
important prognostic information for PDAC and was associated with
poor OS. According to studies by Kakkat et al (27) and Asari et al (28), high pre-treatment PLR is an
independent predictive risk factor for patients with PDAC, which
was not demonstrated in the present study. In addition, the
majority of the patients received chemotherapy, but the effect of
chemotherapy on overall survival was not investigated; the effect
of chemotherapy on the statistical importance of NLR and PLR in OS
needs to be further explored in the future.
S100A4 is involved in the proliferation,
angiogenesis and invasion of tumor cells (29,30). In
the present study, it was revealed that patients with high S100A4
tissue expression exhibited unfavorable OS outcomes, which was
similar to the results from previous studies (29–31).
However, to the best of our knowledge, there are currently no
studies that have been performed with the aim of evaluating the
association between peripheral blood NLR, PLR and LMR and S100A4
expression. In the present study, NLR and PLR were positively
associated with S100A4 expression, whereas LMR was negatively
associated with S100A4 expression. The tumor microenvironment,
comprising multiple cellular and molecular factors, serves a
pivotal role in the biological behavior of numerous different types
of cancer, including PDAC (1,32). The
microenvironment surrounding the tumor cells, containing cells of
the immune system, is a prerequisite for regulating the initiation
of metastasis and affects the prognosis of the malignancy (32,33). The
mechanism by which the microenvironment influences tumor metastasis
is currently unknown, although it has been suggested to be caused
by S100A4 promoting tumor progression, metastasis and inflammation,
either systemically or in the tumor microenvironment (34).
Certain studies focused on the genetic
characteristics of the tumor (35,36).
However, a limited number of these prognostic models consider the
role of host immunity (i.e., lymphocytes) and the microenvironment
produced by the tumor (i.e., monocytes, neutrophils and S100A4)
(15). In the present study, as well
as NLR, peripheral blood LMR was revealed to serve a prognostic
role in patients with PDAC. In addition, the association between
peripheral blood NLR, PLR and LMR and the tissue expression of
S100A4 was thoroughly analyzed in sufficient sample size. However,
there were limitations to the present study, including the
retrospective design, short follow-up periods and a relatively
small sample size.
The present study provided evidence to support the
prognostic use of NLR and LMR in patients with PDAC and
demonstrated the prognostic relevance of host immunity and
tumor-associated microenvironment when determining the clinical
outcome. Further studies, including prospective clinical trials and
mechanistic studies, are required in order to confirm the
conclusions of the present study and reveal the underlying
molecular mechanisms.
In conclusion, the present study demonstrated that
in the peripheral blood from patients with PDAC, the highest NLR
quartile and the lowest LMR quartile were associated with an
unfavorable prognosis. The results of the present study also
support the prognostic relevance of host immunity and the
tumor-associated microenvironment when determining the clinical
outcomes of patients with PDAC. As a simply obtained and widely
available index at diagnosis, NLR and LMR may be a valid novel
predictive and stratification marker for PDAC in clinical
practice.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was funded by the Science and Technology
Project of Tianjin colleges and Universities (grant no. 20130122),
the Science Foundation of Tianjin Health Bureau (grant no.
2015KZ088), the Joint Funding Project of Tianjin Science Committee
(grant no. 15JCYBJC49600) and the Scientific Research Foundation of
Tianjin Medical University Cancer Institute and Hospital (grant no.
1706).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZS and HL conceived and designed the study. HL and
DZ designed the experiments. HL and XT performed the experiments
and analyzed the data. YH obtained the epidemiological data. YX and
YP performed the pathological analysis. HL and XT wrote, edited and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Tianjin Medical University Cancer Institute and
Hospital (Tianjin, China), and all patients provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Foucher ED, Ghigo C, Chouaib S, Galon J,
Iovanna J and Olive D: Pancreatic ductal adenocarcinoma: A strong
imbalance of good and bad immunological cops in the tumor
microenvironment. Front Immunol. 9:10442018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng L, Xue J, Jaffee EM and Habtezion A:
Role of immune cells and immune-based therapies in pancreatitis and
pancreatic ductal adenocarcinoma. Gastroenterology. 144:1230–1240.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi C, Merchant N, Newsome G, Goldenberg
DM and Gold DV: Differentiation of pancreatic ductal adenocarcinoma
from chronic pancreatitis by PAM4 immunohistochemistry. Archives
Pathol Lab Med. 138:220–228. 2014. View Article : Google Scholar
|
|
4
|
Hernandez JM, Cowgill SM, Al-Saadi S,
Collins A, Ross SB, Cooper J, Villadolid D, Zervos E and Rosemurgy
A: CA 19-9 velocity predicts disease-free survival and overall
survival after pancreatectomy of curative intent. J Gastrointest
Surg. 13:349–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winter JM, Yeo CJ and Brody JR:
Diagnostic, prognostic, and predictive biomarkers in pancreatic
cancer. J Surg Oncol. 107:15–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marengo E and Robotti E: Biomarkers for
pancreatic cancer: Recent achievements in proteomics and genomics
through classical and multivariate statistical methods. World J
Gastroenterol. 20:13325–13342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roxburgh CS and McMillan DC: Role of
systemic inflammatory response in predicting survival in patients
with primary operable cancer. Future Oncol. 6:149–163. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koh YW, Kang HJ, Park C, Yoon DH, Kim S,
Suh C, Go H, Kim JE, Kim CW and Huh J: The ratio of the absolute
lymphocyte count to the absolute monocyte count is associated with
prognosis in hodgkin's lymphoma: Correlation with tumor-associated
macrophages. Oncologist. 17:871–880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaynar M, Yildirim ME, Badem H, Cavis M,
Tekinarslan E, Istanbulluoglu MO, Karatas OF and Cimentepe E:
Bladder cancer invasion predictability based on preoperative
neutrophil-lymphocyte ratio. Tumour Biol. 35:6601–6605. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanoglu A and Karagoz E: Neutrophil and
platelet-to-lymphocyte ratio: New predictors of dropout and
recurrence after liver transplantation for hepatocellular cancer?
Transplant Int. 27:e80–e81. 2014. View Article : Google Scholar
|
|
12
|
McMillan DC: Systemic inflammation,
nutritional status and survival in patients with cancer. Curr Opin
Clin Nutr Metab Care. 12:223–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martin HL, Ohara K, Kiberu A, Van Hagen T,
Davidson A and Khattak MA: Prognostic value of systemic
inflammation-based markers in advanced pancreatic cancer. Intern
Med J. 44:676–682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aliustaoglu M, Bilici A, Seker M, Dane F,
Gocun M, Konya V, Ustaalioglu BB and Gumus M: The association of
pre-treatment peripheral blood markers with survival in patients
with pancreatic cancer. Hepatogastroenterology. 57:640–645.
2010.PubMed/NCBI
|
|
15
|
Sierzega M, Lenart M, Rutkowska M, Surman
M, Mytar B, Matyja A, Siedlar M and Kulig J: Preoperative
neutrophil-lymphocyte and lymphocyte-monocyte ratios reflect immune
cell population rearrangement in resectable pancreatic cancer. Ann
Surg Oncol. 24:808–815. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garrett SC, Varney KM, Weber DJ and
Bresnick AR: S100A4, a mediator of metastasis. J Biol Chem.
281:677–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang J, Piao Y, Holmes L, Fuller GN,
Henry V, Tiao N and de Groot JF: Neutrophils promote the malignant
glioma phenotype through S100A4. Clin Cancer Res. 20:187–198. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hansen MT, Forst B, Cremers N, Quagliata
L, Ambartsumian N, Grum-Schwensen B, Klingelhofer J, Abdul-Al A,
Herrmann P, Osterland M, et al: A link between inflammation and
metastasis: Serum amyloid A1 and A3 induce metastasis, and are
targets of metastasis-inducing S100A4. Oncogene. 34:424–435. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dahlmann M, Kobelt D, Walther W, Mudduluru
G and Stein U: S100A4 in cancer metastasis: Wnt signaling-driven
interventions for metastasis restriction. Cancers (Basel).
8:E592016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grum-Schwensen B, Klingelhofer J,
Grigorian M, Almholt K, Nielsen BS, Lukanidin E and Ambartsumian N:
Lung metastasis fails in MMTV-PyMT oncomice lacking S100A4 due to a
T-cell deficiency in primary tumors. Cancer Res. 70:936–947. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao R, Davidson DD, Lopez-Beltran A,
MacLennan GT, Montironi R and Cheng L: The S100 proteins for
screening and prognostic grading of bladder cancer. Histol
Histopathol. 22:1025–1032. 2007.PubMed/NCBI
|
|
22
|
Ai KX, Lu LY, Huang XY, Chen W and Zhang
HZ: Prognostic significance of S100A4 and vascular endothelial
growth factor expression in pancreatic cancer. World J
Gastroenterol. 14:1931–1935. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kamarajah SK, Burns WR, Frankel TL, Cho CS
and Nathan H: Validation of the american joint commission on cancer
(AJCC) 8th edition staging system for patients with pancreatic
adenocarcinoma: A surveillance, epidemiology and end results (SEER)
analysis. Ann Surg Oncol. 24:2023–2030. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tian X, Zhou D, Chen L, Tian Y, Zhong B,
Cao Y, Dong Q, Zhou M, Yan J, Wang Y, et al: Polo-like kinase 4
mediates epithelial-mesenchymal transition in neuroblastoma via
PI3K/Akt signaling pathway. Cell Death Dis. 9:542018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garcea G, Ladwa N, Neal CP, Metcalfe MS,
Dennison AR and Berry DP: Preoperative neutrophil-to-lymphocyte
ratio (NLR) is associated with reduced disease-free survival
following curative resection of pancreatic adenocarcinoma. World J
Surg. 35:868–872. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smith RA, Bosonnet L, Raraty M, Sutton R,
Neoptolemos JP, Campbell F and Ghaneh P: Preoperative
platelet-lymphocyte ratio is an independent significant prognostic
marker in resected pancreatic ductal adenocarcinoma. Am J Surg.
197:466–472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kakkat S, Rajan R, Sindhu RS, Natesh B and
Raviram S: Comparison of platelet-lymphocyte ratio and CA 19-9 in
differentiating benign from malignant head masses in patients with
chronic pancreatitis. Indian J Gastroenterol. 36:263–267. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Asari S, Matsumoto I, Toyama H, Shinzeki
M, Goto T, Ishida J, Ajiki T, Fukumoto T and Ku Y: Preoperative
independent prognostic factors in patients with borderline
resectable pancreatic ductal adenocarcinoma following curative
resection: The neutrophil-lymphocyte and platelet-lymphocyte
ratios. Surg Today. 46:583–592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jia W, Gao XJ, Zhang ZD, Yang ZX and Zhang
G: S100A4 silencing suppresses proliferation, angiogenesis and
invasion of thyroid cancer cells through downregulation of MMP-9
and VEGF. Eur Rev Med Pharmacol Sci. 17:1495–1508. 2013.PubMed/NCBI
|
|
30
|
Tsukamoto N, Egawa S, Akada M, Abe K,
Saiki Y, Kaneko N, Yokoyama S, Shima K, Yamamura A, Motoi F, et al:
The expression of S100A4 in human pancreatic cancer is associated
with invasion. Pancreas. 42:1027–1033. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mahon PC, Baril P, Bhakta V, Chelala C,
Caulee K, Harada T and Lemoine NR: S100A4 contributes to the
suppression of BNIP3 expression, chemoresistance, and inhibition of
apoptosis in pancreatic cancer. Cancer Res. 67:6786–6795. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee SH, Kim H, Hwang JH, Shin E, Lee HS,
Hwang DW, Cho JY, Yoon YS, Han HS and Cha BH: CD24 and S100A4
expression in resectable pancreatic cancers with earlier disease
recurrence and poor survival. Pancreas. 43:380–388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arumugam T, Ramachandran V, Gomez SB,
Schmidt AM and Logsdon CD: S100P-derived RAGE antagonistic peptide
reduces tumor growth and metastasis. Clin Cancer Res. 18:4356–4364.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Langley RR and Fidler IJ: The seed and
soil hypothesis revisited-the role of tumor-stroma interactions in
metastasis to different organs. Int J Cancer. 128:2527–2535. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Knudsen ES, Vail P, Balaji U, Ngo H,
Botros IW, Makarov V, Riaz N, Balachandran V, Leach S, Thompson DM,
et al: Stratification of pancreatic ductal adenocarcinoma:
Combinatorial genetic, stromal, and immunological markers. Clin
Cancer Res. 23:4429–4440. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Raphael BJ, Hruban RH, Aguirre AJ, Moffitt
RA, Yeh JJ, Stewart C, Robertson AG, Cherniack AD, Gupta M, Getz G,
et al: Integrated genomic characterization of pancreatic ductal
adenocarcinoma. Cancer Cell. 14:185–203. 2017. View Article : Google Scholar
|