Introduction
Hepatocellular carcinoma (HCC) is one of the leading
causes of morbidity and mortality worldwide. Despite substantial
efforts and advances in drug discovery and clinical trials for
advanced-stage HCC, there is a limited number of available
effective treatments. Sorafenib (Sora), a multikinase inhibitor,
exhibits significant effects on improvement of overall survival
(OS), but it only delays tumor progression (1). To the best of our knowledge, first-line
therapy beyond Sora or attempts to combine Sora with another
targeted agent have not been successful, thus far (2).
Artesunate (Art) is a semisynthetic derivative of
artemisinins and has been used worldwide over the past decade to
combat severe malaria (3). Previous
studies have demonstrated the anticancer effects of Art in various
types of cancer, including leukemia, colorectal cancer, renal cell
carcinoma, lung cancer and ovarian cancer (4–11). The
primary mechanism of antitumor activity of Art is via reactive
oxygen species generated by reactions between Art and iron that has
accumulated in tumor cells (6,8,11). Given its therapeutic significance,
the pharmacokinetics of Art and its active metabolite
dihydroartemisinin (DHA) have been intensively studied in clinical
settings (3,12). Art is rapidly absorbed and converted
to its main active metabolite DHA, and generally cleared within an
hour (12). This rapid clearing may
contribute to its excellent tolerability and lack of adverse
effects, even at high dose or rapid infusion. Accordingly,
strategies combining Art with other drugs are being tested in
vitro and in vivo to combat high systemic toxicity and
chemoresistance, which limit the outcomes of cancer treatment
(13–16).
Furthermore, Art has been demonstrated to decrease
cell viability in a dose-dependent manner and increase caspase-3
activity in human and mouse liver cancer cell lines, HepG2 and
BWTG3 (17). Additionally, it has
been reported that Art may function as a potential inhibitor of
STAT3 in HCC, and that Art modulates STAT3 targets (procaspase-3, B
cell lymphoma 2 like 1 and survivin), leading to apoptosis in
vitro (18). Notably, Art
inhibits angiogenesis by directly downregulating the expression
levels of vascular endothelial growth factor (VEGF) and its
receptor (VEGFR) (17,19). Administration of Art reduces
vascularization/tumor burden in xenograft mice, and when combined
with Sora, these effects are further enhanced (17). This suggests that the combination of
Sora and Art could be an effective treatment strategy for HCC.
However, more reliable and standardized methods need to be employed
in order to evaluate the potency of this drug combination prior to
clinical trials.
The present study attempted to quantitatively
evaluate the type of drug interaction between Sora and Art by
median-drug effect analysis using Calcusyn software (Chou-Talalay
method). Drug combination and reduction indices and isobologram
plots were applied to define drug interactivity in in
vitro-cultured liver cancer cell lines, HepG2 and Huh7.
Furthermore, the combinatorial effect of these two drugs on
apoptosis induction and cell migration suppression was investigated
for clinically achievable concentrations.
Materials and methods
Cell lines and reagents
The liver cancer cell lines, HepG2 and Huh7, were
obtained from American Type Culture Collection, and were cultured
in DMEM and RPMI medium (Thermo Fisher Scientific, Inc.),
respectively. Both cell lines were supplemented with 10% FBS
(HyClone; GE Healthcare Life Sciences) and maintained in a
humidified atmosphere containing 5% CO2 at 37°C. Cell
line authentication was performed by short tandem repeat profiling
and interspecies contamination test (Applied Biological Materials
Inc.). Cryopreserved normal primary human hepatocytes (PHH) derived
from a pool of 5 donors were purchased from Sekisui XenoTech, LLC.
and thawed with OptiThaw Hepatocyte Media (Sekisui XenoTech, LLC.)
according to the manufacturer's protocol. Cells were seeded in
96-well BioCoat™ collagen I cellware (BD Biosciences) in OptiPlate
hepatocyte media (Sekisui XenoTech, LLC.) for 4 h until sufficient
confluency was reached. The media was then replaced with
OptiCulture hepatocyte media containing Penicillin/Streptomycin
(Sekisui XenoTech, LLC.) and the cells were further incubated for
48 h for the hepatotoxicity assays. The multi-kinase inhibitor,
Sora, was ordered from LC Laboratories and dissolved in dimethyl
sulfoxide (DMSO). Art was purchased from TGI Chemicals, Ltd. and
dissolved in 100% ethanol.
Antibodies
Rabbit anti-VEGF receptor 2 (D5B1; cat. no. 9698;
1:1,000), rabbit anti-cleaved PARP (D64E10; cat. no. 5625,
1:1,000), rabbit anti-cleaved caspase 9 (D8I9E; cat. no. 9505;
1:1,000) and rabbit anti-GAPDH (14C10; cat. no. 2118; 1:2,000) were
all purchased from Cell Signaling Technology Inc.. HRP conjugated
goat anti-rabbit secondary antibody (cat. no. sc-2004; 1:1,000) was
purchased from Santa Cruz Biotechnology Inc.
Cell viability and drug combination
assay
At 72 h prior to drug treatments, HepG2 and Huh7
cells (5×103 cells/well) were seeded in 96-well plates.
Cells were treated with Sora, starting at 40 and 20 µM for HepG2
and Huh7, respectively, and Art starting at 400 and 1,000 µM for
HepG2 and Huh7, respectively, or the constant combination ratio of
Sora to Art (1:10 in HepG2 and 1:50 in Huh7). To examine the
hepatotoxicity of the combination of treatments, normal primary
hepatocytes were treated in two constant combination ratios, 1:10
starting at 10 and 100 µM for Sora and Art, respectively and at
ratio of 1:50 starting at 20 and 1,000 µM for Sora and Art,
respectively. Cell viability was monitored using the CyQUANT Cell
Proliferation Assay kit (Thermo Fisher Scientific, Inc.), according
to the manufacturer's protocol. Cell morphology was observed under
an inverted Nikon microscope and images were captured using a
digital camera. Results from cells treated with individual drugs or
the drug combination at a constant ratio (HepG2, Sora:Art, 1:10;
Huh7, Sora:Art, 1:50) were processed, and drug combination and
reduction indices and isobologram plots were calculated using the
Calcusyn software v2.11 (Premier Biosoft International).
Western blot analysis
Protein extracts were prepared in 1% NP-40 lysis
buffer (50 mM sodium fluoride, 1 mM orthovanadate, 10 mM
iodoacetamide, 1 mM ethylenediaminetetraacetic acid, 0.25% sodium
deoxycholate, 1 mM phenylmethylsulfonyl fluoride and protease
inhibitor cocktail). Protein concentrations were determined by
Pierce BCA protein assay kit (Thermo Fisher Scientific, Inc.). Cell
protein lysates (50 µg) were separated by 10% SDS-PAGE gels and
then transferred onto PVDF membranes. The membranes were blocked
with Tris-buffered saline containing 3% BSA and 0.05% Tween-20
(TBST) for 30 min, followed by incubation with aforementioned
primary antibodies overnight at 4°C and aforementioned secondary
antibody for 1 h at room temperature. A total of three 5-min washes
with TBST were performed after incubation with the primary or
secondary antibodies. The protein expression was detected using
RapidStep™ Enhanced Chemiluminescence (Merck KGaA). GAPDH was used
as a reference protein.
Wound healing assay
HepG2 and Huh7 cells (20,000 cells/well) were seeded
in triplicate in ImageLock 96-well plates (Essen Bioscience). Cells
were treated with 1 mg/ml mytomycin C for 2 h in order to inhibit
proliferation prior to wound scratching. Confluent cell layers were
scratched using the Essen Bioscience Wound Maker in order to
generate wounds that were 700–800 µm wide. Cells were washed twice
with PBS and allowed to grow in 10% FBS growth medium with vehicle
(DMSO or 100% ethanol), 2.5 µM Sora, 25 µM (HepG2)/125 µM (Huh7)
Art or Sora + Art. Images were captured at 6 h intervals for 36 h
using the IncuCyte ZOOM imaging system with time-lapse bright field
microscopy (Essen Bioscience). Relative wound density was
calculated based on the ratio of cell density in the wound/cell
density outside the wound.
Flow cytometry
Liver cancer cell lines were plated into 6-well
plates at a density of 300,000 cells/well and treated with vehicle,
2.5 µM Sora, 25 µM (HepG2)/125 µM (Huh7) Art or Sora + Art for 72
h. Cell cycle analysis with propidium iodide (PI) staining was
performed according to a standard protocol. Briefly, drug-treated
cells were harvested in cell suspensions in PBS and fixed in a
final concentration of 70% ethanol on ice for 30 min, followed by
incubation with the PI/Triton X-100 staining solution (50 mg/ml PI,
0.05% Triton X-100 and 100 mg/ml RNase A) at 37°C for 1 h. Data was
acquired by collecting the area and width on a linear scale for the
DNA channel in addition to forward scatter and side scatter.
Relative DNA contents were analyzed using the FACSCanto II flow
cytometer and BD FACSDiva software v5.0.3 (Becton, Dickinson and
Company). Apoptotic cells were indicated by the percentage of
subG0/G1 phase.
Statistical analysis
All values are presented as the means ± standard
deviation of at least three independent experiments. Statistical
analyses were performed to analyse differences in growth inhibition
and apoptosis using one-way ANOVA followed by Tukey's post hoc
analysis. The wound scratch assays were analysed using Friedman's
test followed by the Dunn's post hoc test using GraphPad Prism
v6.0.1 software (GraphPad Software, Inc.). P<0.05 (two-tailed)
was considered to indicate a statistically significant
difference.
Results
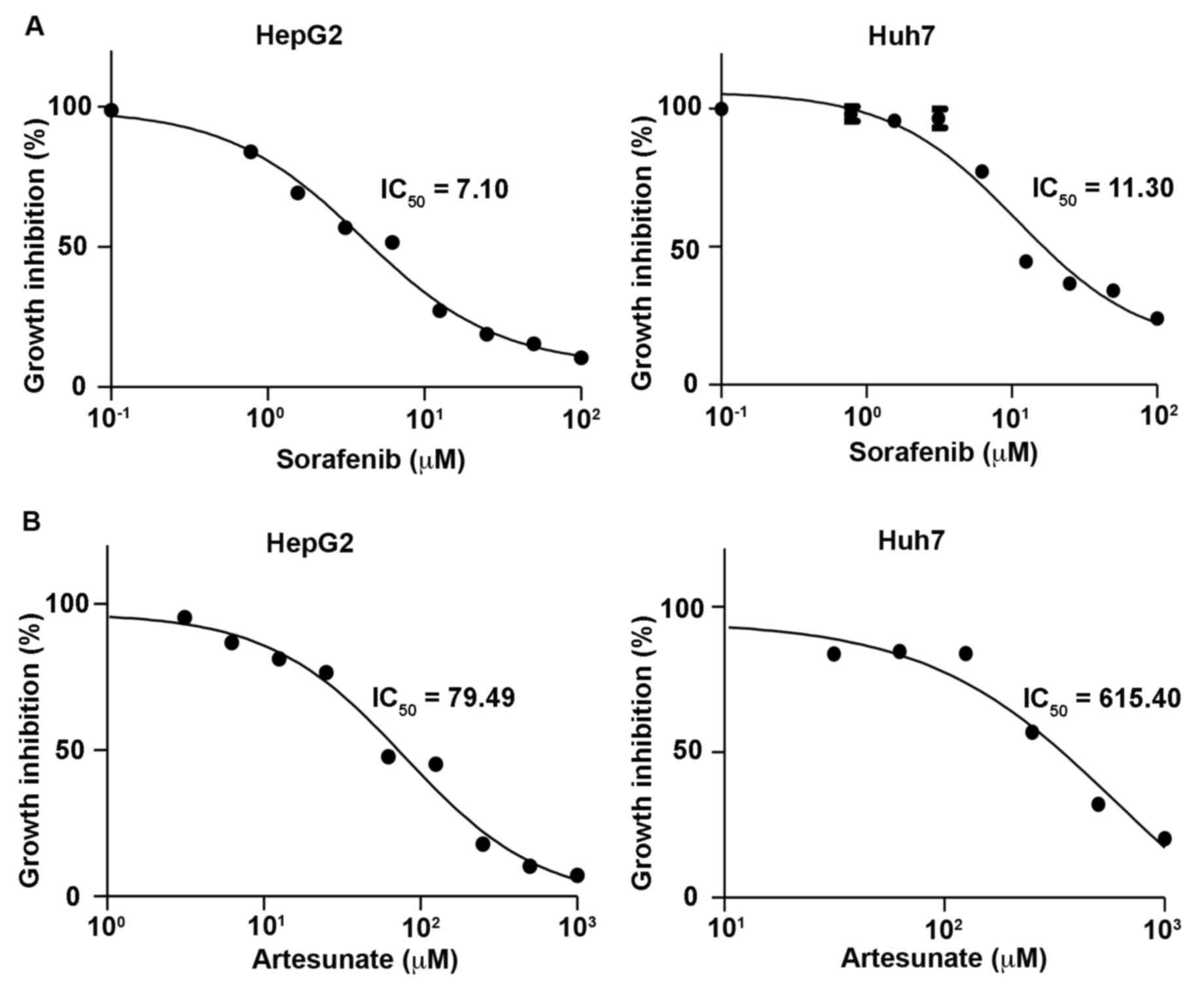
Determination of dose-response curves
and half-maximal inhibitory concentration (IC50) values
in liver cancer cell lines
To determine accurate IC50 values of Sora
and Art used in the present study, dose-response curves were
initially generated for these drugs using the CyQUANT cell
proliferation assay. This assay is a cell metabolic
activity-independent and sensitive method, which is used to
directly measure DNA content to quantify cells. The dose response
curves of Sora in HepG2 and Huh7 cells were similar to those
reported previously, with IC50 values of 5.93–8.51 (mean
7.10) µM in HepG2 and 7.11–17.11 (mean 11.03) µM in Huh7 cells
(Fig. 1; Table I) (20,21). It
has been reported that the maximum drug concentration of Art in
human plasma is 3,260 (1,020–164,000) ng/ml [8.48 (2.65–427.08) µM]
and the terminal elimination half-life is 0.25 (0.1–1.8) h
(22). To obtain clinically relevant
concentrations, the inhibitory dose response of Art was tested for
72 h in the two cell lines at a starting concentration of 1,000 µM.
This revealed that the IC50 values of Art ranged between
63.28 and 99.85 (mean 79.49) µM in HepG2, and 344.70–1,099 (mean
615.40) µM in Huh7 cells (Fig. 1;
Table I). The results indicated that
HepG2 and Huh7 cells exhibited low sensitivity to Art treatment,
compared with other types of cancer cell lines, including leukemia
or colon cancer cell lines, which have been reported to be
responsive in vitro to Art at 1.11±0.56 and 2.13±0.74 µM,
respectively (4). However, the
benefits of the drug combination include mutual enhancement of
therapeutic effects, prevention of drug resistance and reduction of
dose used per single treatment.
 | Table I.Cytotoxic effects of sorafenib and
artesunate in liver cancer cell lines. |
Table I.
Cytotoxic effects of sorafenib and
artesunate in liver cancer cell lines.
|
| Half-maximal
inhibitory concentration, µM (95% confidence interval) |
|---|
|
|
|
|---|
| Drug | HepG2 cells | Huh7 cells |
|---|
| Sorafenib | 7.10
(5.93–8.51) | 11.03
(7.11–17.11) |
| Artesunate | 79.49
(63.28–99.85) | 615.40
(344.70–1099.00) |
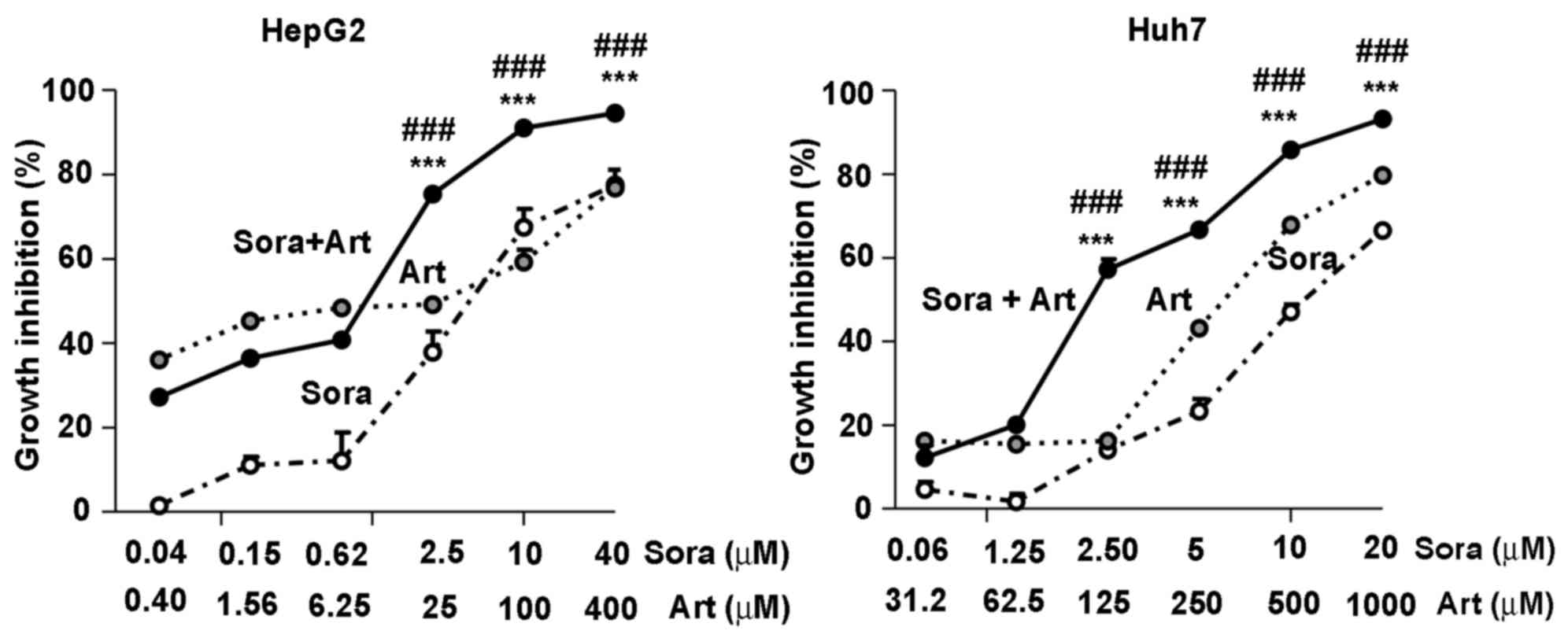
Combinatorial inhibition of Sora and
Art of liver cancer cell growth
To leverage the synergistic inhibitory effects of
Sora and Art, a constant combination ratio of Sora to Art (1:10 in
HepG2 and 1:50 in Huh7) was used, based on IC50 values
of the individual drugs in liver cancer cell lines. The results
demonstrated that the combination of Sora and Art significantly
augmented cell growth inhibition compared with single drug
treatments, the starting concentrations at which this effect became
significant were 2.5 µM for Sora combined with Art at 25 µM for
HepG2 and 2.5 µM Sora with 125 µM Art for Huh7 (Fig. 2). In addition, PHH were tested for
the potential hepatotoxic effects associated with this drug
combination. Due to the limitations of the utility of this model,
the hepatotoxicity evaluation here is focused on cell viability.
The cells were treated with the drug combination ratios of Sora to
Art, 1:10 and 1:50, with Art starting at 100 µM and 1,000 µM,
respectively. The former ration did not present hepatotoxicity in
human normal primary hepatocytes, whereas with the latter ratio,
Art treatment alone at higher concentrations (>250 µM),
significantly reduced the cell viability by ~40% (the combination
ratio at 1:50). Notably, no significant difference was observed
between Art alone and the combination treatments, suggesting that
the drug combination in PHH does not show augmented hepatotoxicity
beyond the single drug treatments.
Synergistic effects of Sora and Art on
suppression of liver cancer cell growth
To further determine the types of drug interactions,
the data were analyzed by median-drug effect analysis (Calcusyn) to
determine antagonism [combination index (CI)>1], additivity
(CI=1) and synergism (CI<1). The combinatorial effects of Sora
and Art in HepG2 and Huh7 cells as identified by the CI,
isobologram and dose reduction index (DRI) at three dose-effect
levels of cell growth inhibition (IC50, IC75
or IC90) are summarized in Table II. The combinations exhibited strong
synergy in HepG2 cells (CI range, 0.11–0.22) or moderate to strong
synergy in Huh7 cells (CI range, 0.75–0.43), which was also
indicated by the isobolograms (Fig.
3; Table II). The isobolograms
indicate the nature of the drug interaction at constant ratios in
each liver cancer cell line. They present drug combination effects
at the ED50 (50% effective dose), ED75 (75%
effective dose), and ED90 (90% effective dose) and the
data points below the line, on the line or above the line suggest
synergistic, additive or antagonistic effects, respectively
(Fig. 3). Although there are
differences between HepG2 and Huh7 cells in response to this drug
combination which showed opposing trends in the CIs and DRIs
(Table II), the CIs clearly
indicated strong synergism in HepG2 and moderate synergism in Huh7
cells. Furthermore, the DRIs exhibited considerable dose reduction
for Sora and Art as a result of synergism. When applied at the
indicated constant ratio (Table
II), the IC50 of Sora was decreased 14.69-fold
(HepG2) or 3.04-fold (Huh7). For Art, it was reduced 21.86-fold
(HepG2) or 2.34-fold (Huh7). The dose reduction levels were
specific to each cell line.
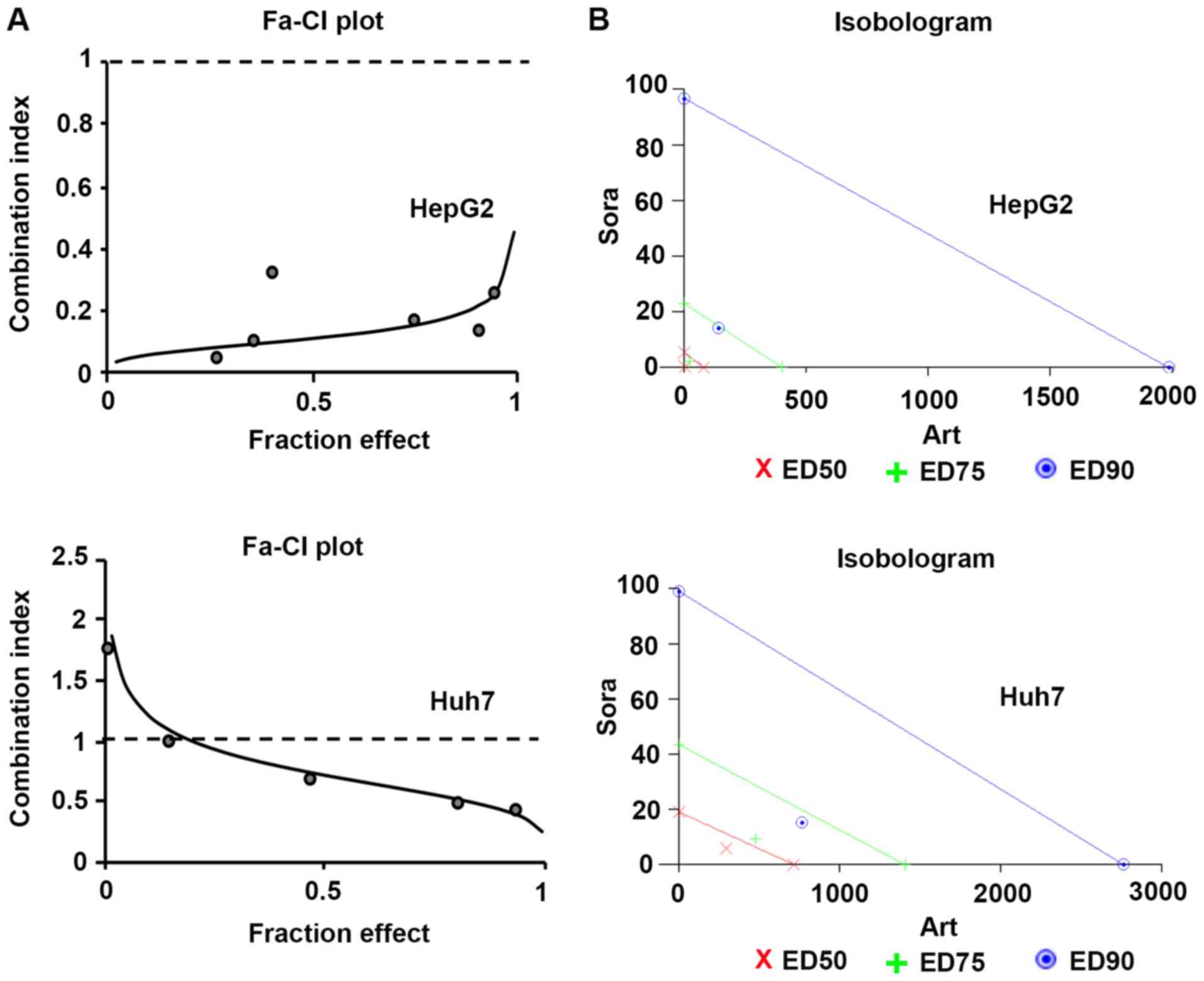 | Figure 3.Dose-effect relationship of Sora and
Art combination in liver cancer cell lines. (A) Fa-CI plots were
obtained from the median-effect analysis (Calcusyn). Solid lines
show computer-simulated Fa-CI plots. Circles represent experimental
data points. CI<1, CI=1 and CI>1 indicate synergism, additive
effects and antagonism, respectively. (B) Isobolograms indicate the
nature of the drug interaction at constant ratios in each liver
cancer cell line. Respective drug combination at the
ED50, ED75, and ED90 effect
levels, data points below the line = synergistic, on the line =
additive and above the line = antagonistic effects. The degree of
synergism in this drug combination is reflected by the distance of
the data point from its respective line (same color). Fa, fraction
affected; CI, combination index; Sora, sorafenib; Art, artesunate;
ED50, median effective dose to inhibit 50% of cells;
ED75, median effective dose to inhibit 75% of cells;
ED90, median effective dose to inhibit 90% of cells. |
 | Table II.Calcusyn output of median-effect
analysis of Sora and Art combination in liver cancer cell
lines. |
Table II.
Calcusyn output of median-effect
analysis of Sora and Art combination in liver cancer cell
lines.
|
|
| Parameters | CI value | DRI value |
|---|
|
|
|
|
|
|
|---|
| Cell line | Drugs | Dm (µM) | m | r |
IC50 |
IC75 |
IC90 |
IC50 |
IC75 |
IC90 |
|---|
| HepG2 | Sora | 5.48 | 0.76 | 0.98 |
|
|
| 14.69 | 10.01 | 6.81 |
|
| Art | 81.5 | 0.69 | 0.93 |
|
|
| 21.86 | 17.48 | 13.98 |
|
| Sora + Art
(1:10) | 0.37+3.73 | 0.6 | 0.97 | 0.11 | 0.16 | 0.22 |
|
|
|
| Huh7 | Sora | 16.44 | 1.33 | 0.91 |
|
|
| 3.04 | 4.57 | 6.46 |
|
| Art | 631.93 | 1.62 | 0.87 |
|
|
| 2.34 | 2.96 | 3.61 |
|
| Sora + Art
(1:50) | 5.39+270.05 | 2.29 | 0.98 | 0.75 | 0.56 | 0.43 |
|
|
|
Additionally, a fixed constant ratio of drug
combination (Sora:Art, 1:5) was tested in HepG2 and Huh7 cells for
48 h (Fig. S2). Art treatment at
concentrations <100 µM demonstrated moderate effects on growth
inhibition within 48 h (17–40%) for the two cell lines, while it
exhibited synergistic effects with Sora on suppression of cell
growth in HepG2 cells as indicated by CI (Fig. S2A). By contrast, concurrent
treatment of Art and Sora in Huh7 cells for 48 h did not comply
with the Calcusyn mathematical model since the median-effect curves
in this cell line exhibited a negative slope due to its poor
response to Art treatment (<100 µM; Fig. S2B). However, this was overcome by 24
h pre-treatment with Art, followed by the combination treatment
(Fig. S2B), which suggested that
sequential treatment of Art followed by Sora may improve the
overall outcome of this drug combination in liver cancer
treatment.
Combination of Sora and Art
significantly increases cell apoptosis
It has been previously reported that Art exerts
anticancer properties in a variety of tumor types via the
activation of mitochondrial apoptosis (17). Art also has been revealed to reduce
tumor vessel formation via downregulation of VEGF and VEGFR protein
expression levels (12,14). To further assess the biological
effects of the combination of Sora and Art, concentrations
(Sora:Art, 2.5:25 µM for HepG2; 2.5:125 µM for Huh7) at which the
combination started exhibiting noticeable enhancement of growth
inhibition at 72 h compared with the single drug treatments were
selected (Fig. 2). This
significantly reduced cell numbers, which was partially caused by
cell apoptosis induction as indicated by the percentage of cells in
the subG0/G1 phases of the cell cycle
(Fig. 4A and B). Consistent with
these results, western blot analysis revealed that the initiator
and the final substrate of the caspase cascade, cleaved caspase-9
and cleaved poly ADP ribose polymerase expression levels were
significantly increased following combination treatment. Sora and
Art reduced VEGFR2 protein expression, and this effect was enhanced
by drug combination in HepG2 and Huh7 cells (Fig. 4C).
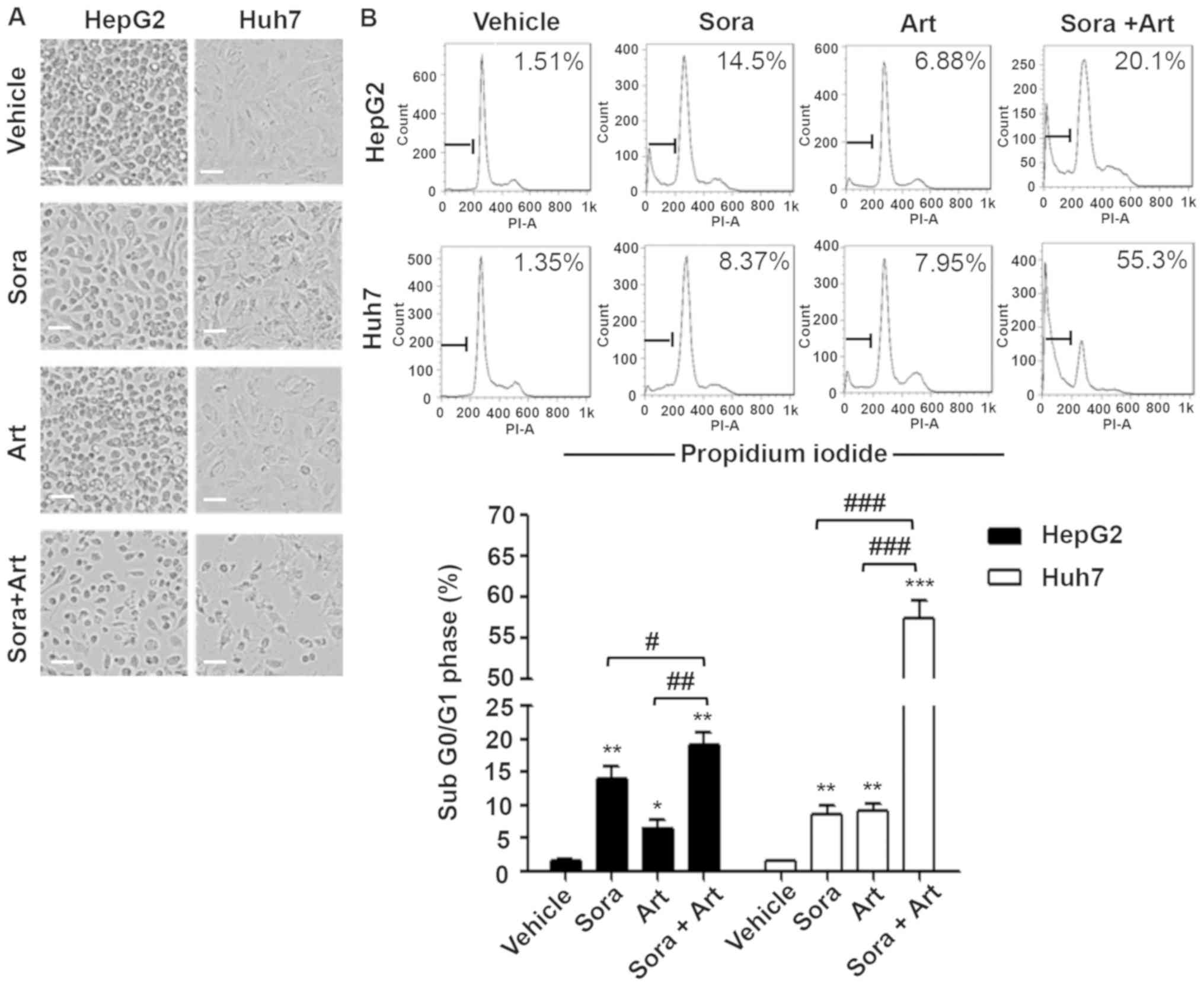 | Figure 4.Combination of Sora and Art markedly
increases apoptosis. (A) Morphological alterations of liver cancer
cells treated with Sora, Art or Sora + Art. HepG2 and Huh7 cells
were treated with 2.5 µM Sora, 25 µM (HepG2) or 125 µM (Huh7) Art,
or Sora + Art for 72 hrs, followed by phase-contrast microscopy.
Scale bar, 100 µm. (B) Effects of Sora and Art on the induction of
apoptosis in HepG2 and Huh7 cells. Cells were stained with PI and
DNA contents were examined by flow cytometry. Apoptotic cells were
gated in the subG0/G1 phase. (C) Western blot
analysis of the VEGFR2 (Sora target) and apoptosis-associated
proteins, cleavage and activation of initiator Casp9, and PARP
proteolysis (effector; caspase-3 substrate). GAPDH was used as a
loading control. Data are presented as the mean + standard
deviation. *P<0.05, **P<0.01, ***P<0.001 vs. vehicle
control; #P<0.05, ##P<0.01,
###P<0.001. Sora, sorafenib; Art, artesunate; PI,
propidium iodide; VEGFR2, vascular endothelial growth factor
receptor 2; PARP, poly ADP ribose polymerase; Casp9, caspase-9. |
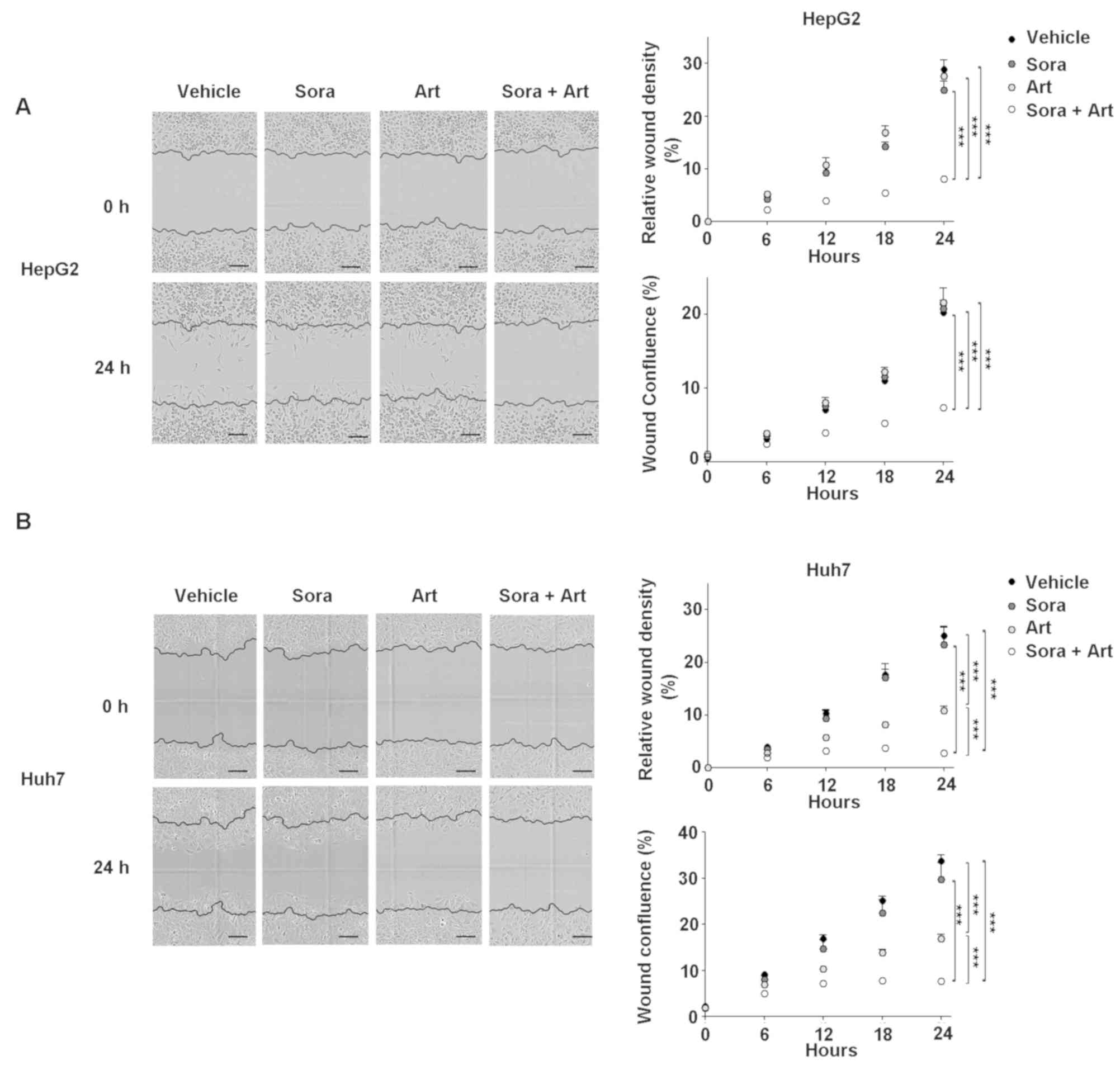
Combination of Sora and Art
significantly reduces cell migration
Subsequently, the combined effect of the drugs on
cell migration was determined using an in vitro wound
healing assay (Fig. 5). Cell
migration was kinetically monitored in Essen ImageLock plates using
the IncuCyte Zoom imaging system. Cell growth was controlled by
mitomycin C treatment. For the selected drug combination ratio
(Sora:Art, 2.5:25 µM for HepG2 and 2.5:125 µM for Huh7), no
significant apoptotic effects were observed at 24 h (data not
shown). However, the results revealed a substantial inhibition of
migration in the combination-treated cells compared with vehicle,
Sora or Art treated cells (Fig. 5A and
B).
Discussion
In the SHARP trial (2008), Sora treatment was
associated with a modest improvement in survival (2.8 months)
compared with placebo treatment; however, the treatment was
commonly associated with adverse effects, resulting in
discontinuation of the drug in certain cases (1). Since then, there have been a number of
trials investigating the combination therapy of Sora with various
interventions, including VEGF-targeted monoclonal antibody,
bevacizumab (Avastin®; Genentech) (2,23). The
overall assessment of the majority of studies is that they do not
support the combination of two chemotherapeutic agents for the
treatment of unresectable HCC (23–25).
Recently, another multikinase inhibitor, regorafenib, first
appeared to improve survival rates in patients who progressed on
Sora, with an increased median OS over 24 months across the two
lines of therapy with Sora as a first-line and regorafenib as a
second-line treatment (24,25). However, due to common adverse events,
patients intolerant to Sora were excluded from the study (24). Therefore, further investigation is
required to determine the benefits or lack of benefits of
combination therapy. Similarly, identification of a synergistic
partner drug would provide an opportunity for dose-reduction; and
therefore, increase the therapeutic window.
Drug repurposing of Art in cancer therapy has been
proposed in numerous studies (4–11).
Synergistic effects of Art and other chemotherapy drugs have been
reported in a number of types of cancer (15,16,26–28).
Therefore, its combination with Sora has been investigated in HCC
in in vitro and in vivo (17). However, the lack of evidence from
reliable and standardised methods defining the in vitro
synergistic potential of this drug combination remains a major
drawback for its practical use. It is often unclear whether it has
greater or lesser effects in combination with other drugs compared
with the simple additive effect expected from the combination of
the effects of each drug individually. This is a great limitation
in providing valuable insights for developing drug combination in
cancer therapeutics. In the present study, an effort was made to
measure the dose-effect relationship of Sora or Art alone or in
combination, and to quantitatively determine whether this
combination yielded a synergistic effect in liver cancer cells.
Median-drug effect analysis (Calcusyn) was used to
define drug interactivity by generating the CI, isobologram and DRI
in an objective manner. The drug combination ratio reported in the
present study was determined based on the IC50 values of
each drug, and the same ratio was applied to additional functional
studies. The results of the present study indicated that
combination at the fixed ratio was associated with strong to
moderate synergistic growth inhibition in HepG2 and Huh7 cells. The
DRI of Sora ranged between 14.69- and 3.04-fold and that of Art
ranged between 21.86- and 2.34-fold at the IC50 level.
The synergistic effects included apoptosis induction, cell
migration inhibition and anti-angiogenesis activity. Notably, the
combination treatment reduced VEGFR2 protein expression more than
Sora or Art alone, indicating these two drugs cooperatively exert
anti-angiogenesis roles. Additionally, another drug combination
ratio of Sora:Art (1:5) was applied in HepG2 and Huh7 cells, and
sequential combination treatment in Huh7 cells that were not
responding well in the first setting was assessed. It was revealed
that the drug combinatorial effect of Art and Sora was synergistic.
The benefit of this particular drug combination in HCC is not only
due to the characteristics of the drugs, but also dependent on the
dose ratio and scheduling of treatments. Furthermore, it would be
reasonable to consider what dose ratio or scheduling of treatments
may optimize the synergy. Ideally, these two factors should be
extensively optimized in preclinical studies prior to proceeding to
a clinical setting in humans.
In conclusion, the present study provided
methodological evidence to facilitate the development of the drug
combination of Sora and Art in HCC treatment. This could be a
potential treatment option for patients with HCC and may harness
the overall therapeutic efficacy of Sora with Art, an affordable
and well-characterized medication. The combination ratio and
concurrent/sequential dosing schedule requires further
investigation in clinical trials.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Na Li (Vancouver
Prostate Centre, Vancouver, BC, Canada) for their advice regarding
experimental design and data analysis.
Funding
The present study was supported by research funding
from Yanbian University (Jilin, China) for junior investigators
(grant no. 20120010) and the Natural Science Foundation of Jilin
Province, China (grant no. 201115239).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL contributed to the overall experimental design,
performed the experiments, and analyzed the data for the cell
growth assay and cell migration assay. KX and GP contributed to
western blot analysis and flow cytometry. SS contributed to the
experimental design, data interpretation and preparation of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun W and Cabrera R: Systemic treatment of
patients with advanced, unresectable hepatocellular carcinoma:
Emergence of therapies. J Gastrointest Cancer. 49:107–115. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dondorp A, Nosten F, Stepniewska K, Day N
and White N; South East Asian Quinine Artesunate Malaria
(SEAQUAMAT) Group, : Artesunate versus quinine for treatment of
severe falciparum malaria: A randomised trial. Lancet. 366:717–725.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Efferth T, Dunstan H, Sauerbrey A, Miyachi
H and Chitambar CR: The anti-malarial artesunate is also active
against cancer. Int J Oncol. 18:767–773. 2001.PubMed/NCBI
|
|
5
|
Crespo-Ortiz MP and Wei MQ: Antitumor
activity of artemisinin and its derivatives: From a well-known
antimalarial agent to a potential anticancer drug. J Biomed
Biotechnol. 2012:2475972012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar B, Kalvala A, Chu S, Rosen S, Forman
SJ, Marcucci G, Chen CC and Pullarkat V: Antileukemic activity and
cellular effects of the antimalarial agent artesunate in acute
myeloid leukemia. Leuk Res. 59:124–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krishna S, Ganapathi S, Ster IC, Saeed ME,
Cowan M, Finlayson C, Kovacsevics H, Jansen H, Kremsner PG, Efferth
T and Kumar D: A randomised, double blind, placebo-controlled pilot
study of oral artesunate therapy for colorectal cancer.
EBioMedicine. 2:82–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chauhan AK, Min KJ and Kwon TK:
RIP1-dependent reactive oxygen species production executes
artesunate-induced cell death in renal carcinoma Caki cells. Mol
Cell Biochem. 435:15–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tong Y, Liu Y, Zheng H, Zheng L, Liu W, Wu
J, Ou R, Zhang G, Li F, Hu M, et al: Artemisinin and its
derivatives can significantly inhibit lung tumorigenesis and tumor
metastasis through Wnt/beta-catenin signaling. Oncotarget.
7:31413–31428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang CZ, Zhang H, Yun J, Chen GG and Lai
PB: Dihydroartemisinin exhibits antitumor activity toward
hepatocellular carcinoma in vitro and in vivo. Biochem Pharmacol.
83:1278–1289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greenshields AL, Shepherd TG and Hoskin
DW: Contribution of reactive oxygen species to ovarian cancer cell
growth arrest and killing by the anti-malarial drug artesunate. Mol
Carcinog. 56:75–93. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morris CA, Duparc S, Borghini-Fuhrer I,
Jung D, Shin CS and Fleckenstein L: Review of the clinical
pharmacokinetics of artesunate and its active metabolite
dihydroartemisinin following intravenous, intramuscular, oral or
rectal administration. Malar J. 10:2632011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Efferth T, Giaisi M, Merling A, Krammer PH
and Li-Weber M: Artesunate induces ROS-mediated apoptosis in
doxorubicin-resistant T leukemia cells. PLoS One. 2:e6932007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang B, Hou D, Liu Q, Wu T, Guo H, Zhang
X, Zou Y, Liu Z, Liu J, Wei J, et al: Artesunate sensitizes ovarian
cancer cells to cisplatin by downregulating RAD51. Cancer Biol
Ther. 16:1548–1556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Efferth T: Cancer combination therapy of
the sesquiterpenoid artesunate and the selective EGFR-tyrosine
kinase inhibitor erlotinib. Phytomedicine. 37:58–61. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nunes JJ, Pandey SK, Yadav A, Goel S and
Ateeq B: Targeting NF-kappa B signaling by artesunate restores
sensitivity of castrate-resistant prostate cancer cells to
antiandrogens. Neoplasia. 19:333–345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vandewynckel YP, Laukens D, Geerts A,
Vanhove C, Descamps B, Colle I, Devisscher L, Bogaerts E, Paridaens
A, Verhelst X, et al: Therapeutic effects of artesunate in
hepatocellular carcinoma: Repurposing an ancient antimalarial
agent. Eur J Gastroenterol Hepatol. 26:861–870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ilamathi M, Santhosh S and
Sivaramakrishnan V: Artesunate as an anti-cancer agent targets
stat-3 and favorably suppresses hepatocellular carcinoma. Curr Top
Med Chem. 16:2453–2463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen HH, Zhou HJ, Wu GD and Lou XE:
Inhibitory effects of artesunate on angiogenesis and on expressions
of vascular endothelial growth factor and VEGF receptor KDR/flk-1.
Pharmacology. 71:1–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cervello M, Bachvarov D, Lampiasi N,
Cusimano A, Azzolina A, McCubrey JA and Montalto G: Molecular
mechanisms of sorafenib action in liver cancer cells. Cell Cycle.
11:2843–2855. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Liu Y, Meng L, Ji B and Yang D:
Synergistic antitumor effect of sorafenib in combination with ATM
inhibitor in hepatocellular carcinoma cells. Int J Med Sci.
14:523–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Byakika-Kibwika P, Lamorde M, Mayito J,
Nabukeera L, Mayanja-Kizza H, Katabira E, Hanpithakpong W, Obua C,
Pakker N, Lindegardh N, et al: Pharmacokinetics and
pharmacodynamics of intravenous artesunate during severe malaria
treatment in Ugandan adults. Malar J. 11:1322012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hubbard JM, Mahoney MR, Loui WS, Roberts
LR, Smyrk TC, Gatalica Z, Borad M, Kumar S and Alberts SR: Phase
I/II randomized trial of sorafenib and bevacizumab as first-line
therapy in patients with locally advanced or metastatic
hepatocellular carcinoma: North central cancer treatment group
trial N0745 (Alliance). Target Oncol. 12:201–209. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Finn RS, Merle P, Granito A, Huang YH,
Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Gerolami R, Caparello
C, et al: Outcomes of sequential treatment with sorafenib followed
by regorafenib for HCC: Additional analyses from the phase III
RESORCE trial. J Hepatol. 69:353–358. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tran BN, Nguyen HT, Kim JO, Yong CS and
Nguyen CN: Developing combination of artesunate with paclitaxel
loaded into poly-d,l-lactic-co-glycolic acid nanoparticle for
systemic delivery to exhibit synergic chemotherapeutic response.
Drug Dev Ind Pharm. 43:1952–1962. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goswami U, Kandimalla R, Kalita S,
Chattopadhyay A and Ghosh SS: Polyethylene glycol-encapsulated
histone deacetylase inhibitor drug-composite nanoparticles for
combination therapy with artesunate. ACS Omega. 3:11504–11516.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Efferth T: Cancer combination therapies
with artemisinin-type drugs. Biochem Pharmacol. 139:56–70. 2017.
View Article : Google Scholar : PubMed/NCBI
|