Introduction
Gastric cancer (GC) is one of the most common
malignant tumor types according to previous statistics (1). The morbidity of GC ranks fourth and the
associated mortality ranks second worldwide. Eastern Asia has the
highest incidence of GC in the world. Despite comprehensive
post-operative anti-tumor therapy resulting in prolonged survival
following GC resection, long-term survival following surgery
remains poor (2). Precise predictive
tools are critical for individualizing treatment protocols. At
present, the tumor-nodes-metastasis (TNM) stage is the most
frequently used prognostic factor. However, clinical experience has
indicated that even within the same TNM stage, the survival of
patients may differ (3). Therefore,
the development of novel evaluation systems that may include more
prognostic factors is urgently required.
The predictive roles of the preoperative systemic
inflammatory/immune cells in GC have been highlighted by previous
studies, including the neutrophil-to-lymphocyte ratio (NLR), the
monocyte-to-lymphocyte ratio (MLR) and the platelet-to-lymphocyte
ratio (PLR) (4–6). Recently, the
neutrophil-monocyte-lymphocyte ratio (NMLR) has been suggested to
have an improved predictive ability compared with other
inflammatory/immune cell counts or ratios in patients with
hepatocellular carcinoma after curative hepatectomy (7). The most suitable parameter of the
inflammatory/immune system for predicting the outcome for patients
with GC remains to be determined.
To improve and refine the predictive models of
traditional staging systems for GC, several novel nomograms have
been reported (8,9). These included prognostic nomograms for
GC following gastrectomy incorporating systemic inflammatory/immune
parameters. The present study performed a screening to identify the
optimum systemic inflammatory/immune parameter and to develop
reliable nomograms, in order to provide accurate estimations of the
prognosis of patients with GC undergoing R0 resection.
Patients and methods
Ethics statement
The present retrospective cohort study was approved
by the Ethics Review Committee of Wujin Hospital affiliated to
Jiangsu University and was performed in accordance with the ethical
guidelines of the Declaration of Helsinki from 1975. Due to the
retrospective nature of this study, the need for written informed
consent was waived.
Study population
Data were collected from Wujin Hospital and the
Southern Branch of Wujin Hospital Affiliated to Jiangsu University.
A total of 1,023 consecutive patients with GC confirmed by
pathology undergoing radical gastrectomy were considered for the
retrospective analysis The inclusion criteria were as follows: i)
Detailed laboratory test data; ii) no pre-operative metastases
confirmed by computed tomography (CT); iii) no pre-operative
anti-tumor treatments; iv) complete lymph node dissection; v)
complete records and follow-up data, and continuous regular
follow-up. Finally, 679 patients were included into the present
study and further divided into a primary cohort (January 2013 to
December 2013; n=300), an internal validation cohort (January 2014
to October 2014; n=278), and an external validation cohort (May
2012 to May 2015; n=101). The patients in the primary cohort and
internal validation cohort were from Wujin Hospital and the
patients in the external validation cohort were from the Southern
Branch of Wujin Hospital. Wujin Hospital and the Southern Branch of
Wujin Hospital are 2 different centers, independent of each other,
serving different populations. Wujin Hospital serves the people
(~1,300,000) from the Tianning, Zhonglou and Xinbei districts, and
Changzhou city. The Southern Branch of Wujin Hospital serves the
people (~1,400,000) from the Wujin district and Changzhou city. The
grouping method was consistent with a previous study (7).
Data collection
Clinical characteristics, including the status of
the patients, operative features, results of laboratory tests,
histologic and pathologic features of tumors, and prognostic data
were collected. The TNM staging system (American Joint Committee on
Cancer, 8th ed., 2018) was used to stage the tumors (10). Laboratory examinations included
neutrophil, lymphocyte, monocyte and platelet count, and D-dimer
and carcinoembryonic antigen (CEA). The NLR was defined as the
absolute neutrophil count divided by the absolute lymphocyte count.
The MLR was defined as the absolute monocyte count divided by the
absolute lymphocyte count. The PLR was defined as the absolute
platelet count divided by the absolute lymphocyte count. The NMLR
was defined as the product of the neutrophil count and monocyte
count divided by the absolute lymphocyte count. The
platelet-neutrophil-lymphocyte ratio (PNLR) was defined as the
product of the platelet count and neutrophil count divided by the
absolute lymphocyte count. The platelet-monocyte-lymphocyte ratio
(PMLR) was defined as the product of platelet count and monocyte
count divided by the absolute lymphocyte count.
Follow-up
During the first year following surgery, patients
were examined once a month. During the second year, follow-up was
performed every 3 months. For the third year, patients were
followed up twice a year, and then once annually thereafter. The
parameters determined at each visit included thoracic and abdominal
CT scan, blood routine, hepatic and renal function, D-dimer and
CEA.
Statistical analysis
The receiver operating characteristics (ROC) curve
was used to calculate the optimal cutoff values (by Youden index)
of systemic inflammatory/immune cell counts or ratios and the areas
under the ROC curve (AUC). For continuous variables, differences
between groups were analyzed using one-way analysis of variance
with least significant difference test. Categorical variables were
analyzed using the χ2 test. Survival curves were drawn
using the Kaplan-Meier method and compared using log-rank tests.
Parameters in nomograms were selected by univariate and
multivariate analyses, using a Cox proportional-hazards model.
Statistical analyses were performed with SPSS 20.0 for Windows (IBM
Corp.).
Two nomograms were established using the rms package
in R v.3.5.1 (http://www.r-project.org/). Differences between the
predictive model and experimental data were quantified according to
the concordance index (C-index). Bootstraps with 1,000 resamples
were used to estimate bias and present calibration plots. For all
statistical tests, a two-sided P<0.05 was considered to indicate
a statistically significant difference.
Results
Clinicopathological
characteristics
The clinicopathological characteristics of the 679
cases in the primary and validation cohorts are summarized in
Table I. The median follow-up time
was 61, 51 and 49 months, the median age was 67, 66 and 67 years,
and the median tumor size was 4.0, 4.0 and 3.5 cm in the primary,
internal validation and external validation cohorts, respectively.
Among all cases, the neutrophil, monocyte, lymphocyte and platelet
counts ranged from 1.24–13.75×109/l,
0.03–1.61×109/l, 0.33–5.49×109/l and
68–768×109/l, respectively. The laboratory test results
were comparable among the three cohorts, with the exception of the
monocyte (P=0.001), albumin (P<0.001) and globulin levels
(P=0.001), as presented in Table
I.
 | Table I.Characteristics of patients in the
primary and validation cohorts. |
Table I.
Characteristics of patients in the
primary and validation cohorts.
|
Characteristics | Primary cohort
(n=300) | Internal validation
cohort (n=278) | External validation
cohort (n=101) | P-value |
|---|
| Age, year, median
(range) | 67 (39–91) | 66 (38–85) | 67 (29–92) | 0.363 |
| Sex
(male/female) | 214/86 | 198/80 | 78/23 | 0.469 |
| Neutrophil, 10×9/l,
median (range) | 4.00
(1.30–13.48) | 3.81
(1.24–13.72) | 3.83
(1.40–13.75) | 0.517 |
| Monocyte, 10×9/l,
median (range) | 0.35
(0.03–1.23) | 0.39
(0.11–1.27) | 0.41
(0.07–1.61) | <0.001 |
| Lymphocyte, 10×9/l,
median (range) | 1.48
(0.33–4.05) | 1.50
(0.51–3.87) | 1.45
(0.49–5.49) | 0.411 |
| Platelet, 10×9/l,
median (range) | 210 (82–585) | 205 (68–768) | 211 (83–492) | 0.372 |
| Albumin, median
(range) | 40 (22.3–50.3) | 39.7
(24.39–59.3) | 42.8
(26.6–55.6) | <0.001 |
| Globulin, median
(range) | 26.6
(16.6–39.4) | 24.9
(14.0–39.0) | 24.5
(13.9–34.4) | <0.001 |
| D-dimer, median
(range) | 0.33
(0.03–40.00) | 0.30
(0.06–6.65) | 0.44
(0.05–22.7) | 0.071 |
| CEA, median
(range) | 2.01
(0.11–527.53) | 2.35
(0.15–391.10) | 2.21
(0.42–193.8) | 0.591 |
| Tumor size, cm
(range) | 4.0 (0.3–14.0) | 4.0 (0.3–14.0) | 3.5 (0.6–11.0) | 0.172 |
| Tumor
differentiation (I–II/III–IV) | 52/248 | 75/203 | 15/86 | 0.005 |
| TNM stage
(I–II/III) | 164/134 | 153/125 | 50/51 | 0.589 |
Overall survival (OS) and
recurrence-free survival (RFS) in the three cohorts
For the primary cohort, the 1-, 3- and 5-year OS
rates were 92.7, 68.0 and 57.7%, and the 1-, 3- and 5-year RFS
rates were 92.3, 58.3 and 39.7%, respectively. For the internal
validation cohort, the 1- and 3-year OS rates were 95.7 and 66.9%;
the 1- and 3-year RFS rates were 93.9 and 52.2%, respectively. For
the external validation cohort, the 1- and 3-year OS rates were
91.1 and 64.4%, and the 1- and 3-year RFS rates were 90.1 and
48.5%, respectively.
Comparison of predictive accuracy of
the systemic inflammatory/immune parameters in the primary
cohort
The optimal cutoff values of systemic
inflammatory/immune cell counts or ratios were estimated from the
ROC curves when the Youden index was maximal, as presented in
Fig. 1. For OS, the optimal cutoff
levels for the neutrophil, lymphocyte, monocyte and platelet
counts, NLR, MLR, PLR, NMLR, PNLR and PMLR were 4.09, 1.10, 0.38,
216.00, 2.50, 0.29, 140.77, 1.15, 580.23 and 63.61, respectively.
For RFS, the optimal cutoff levels for the neutrophil, lymphocyte,
monocyte and platelet counts, NLR, MLR, PLR, NMLR, PNLR and PMLR
were 4.38, 1.28, 0.29, 216.00, 2.65, 0.29, 144.29, 1.17, 668.00 and
59.22, respectively. The final cut-off levels for the neutrophil,
lymphocyte, monocyte and platelet counts, NLR, MLR, PLR, NMLR, PNLR
and PMLR were calculated as the average of the OS and RFS values,
and were set as 4.23, 1.19, 0.33, 216.00, 2.57, 0.29, 143.00, 1.16,
624.00 and 61.40, respectively.
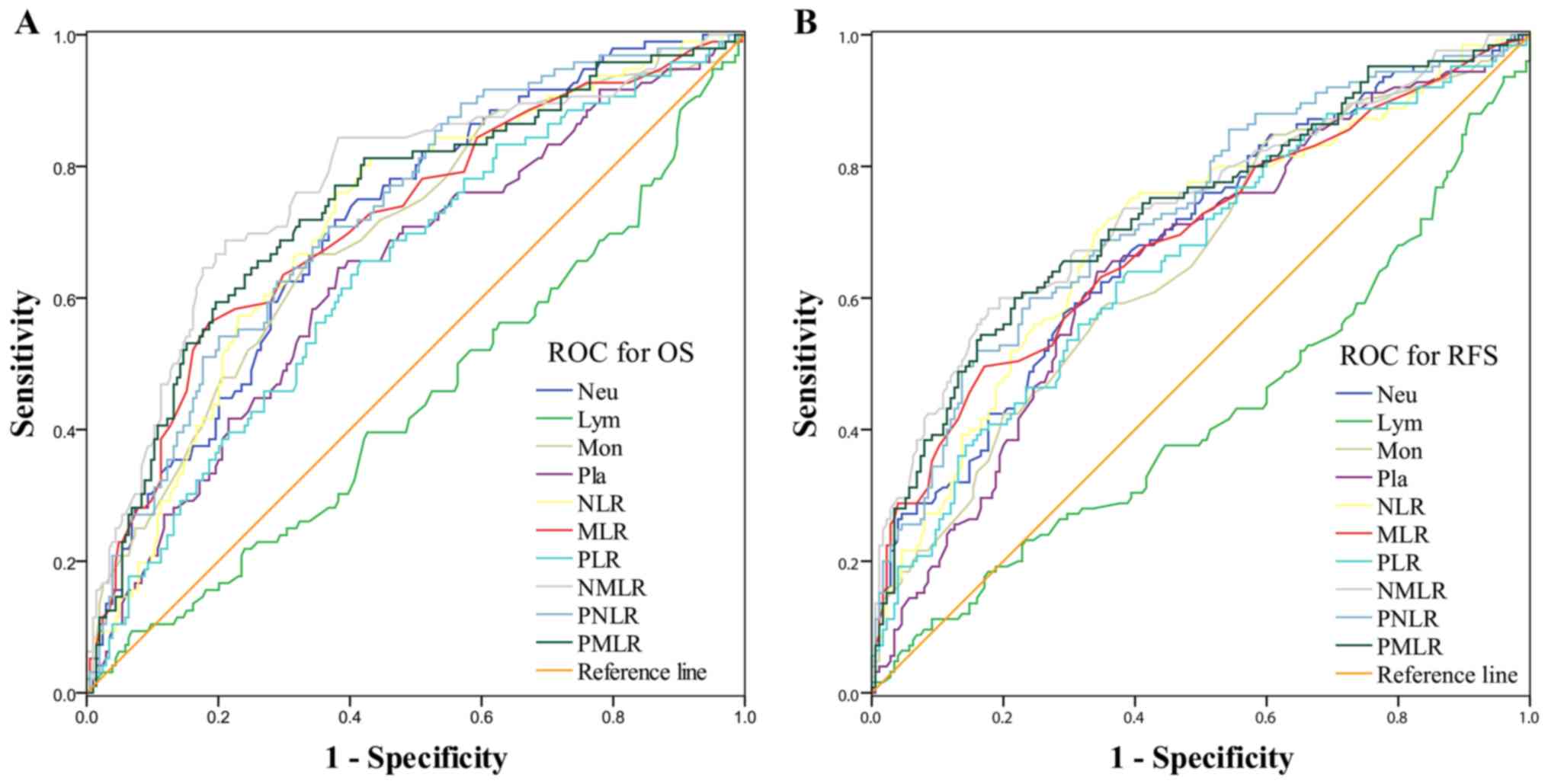 | Figure 1.ROC curves of the prediction index
values in predicting 3-year survival and 3-year overall
recurrence-free survival of patients with gastric cancer. ROC
curves were used to estimate the optimal cutoff values of systemic
inflammatory/immune cell counts or ratios in (A) OS and (B) RFS.
ROC, receiver operating characteristic; OS, overall survival; RFS,
recurrence-free survival; Neu, neutrophils; Lym, lymphocytes; Mon,
monocytes; Pla, platelets; NLR, neutrophil-to-lymphocyte ratio;
MLR, monocyte-to-lymphocyte ratio; PLR, platelet-to-lymphocyte
ratio; NMLR, neutrophil-monocyte-lymphocyte ratio; PNLR,
platelet-neutrophil-lymphocyte ratio; PMLR,
platelet-monocyte-lymphocyte ratio. |
The quality of the association between each systemic
inflammatory/immune parameter and the OS or RFS rates were compared
using log-rank tests. As presented in Fig. 2, the neutrophil count (both
P<0.001), monocyte count (both P<0.001), platelet count (both
P<0.001), NLR (both P<0.001), MLR (both P<0.001), PLR
(P=0.004 and P=0.003), NMLR (both P<0.001), PNLR (both
P<0.001) and PMLR (both P<0.001) were associated with OS and
RFS. The sensitivities and specificities of each parameter was
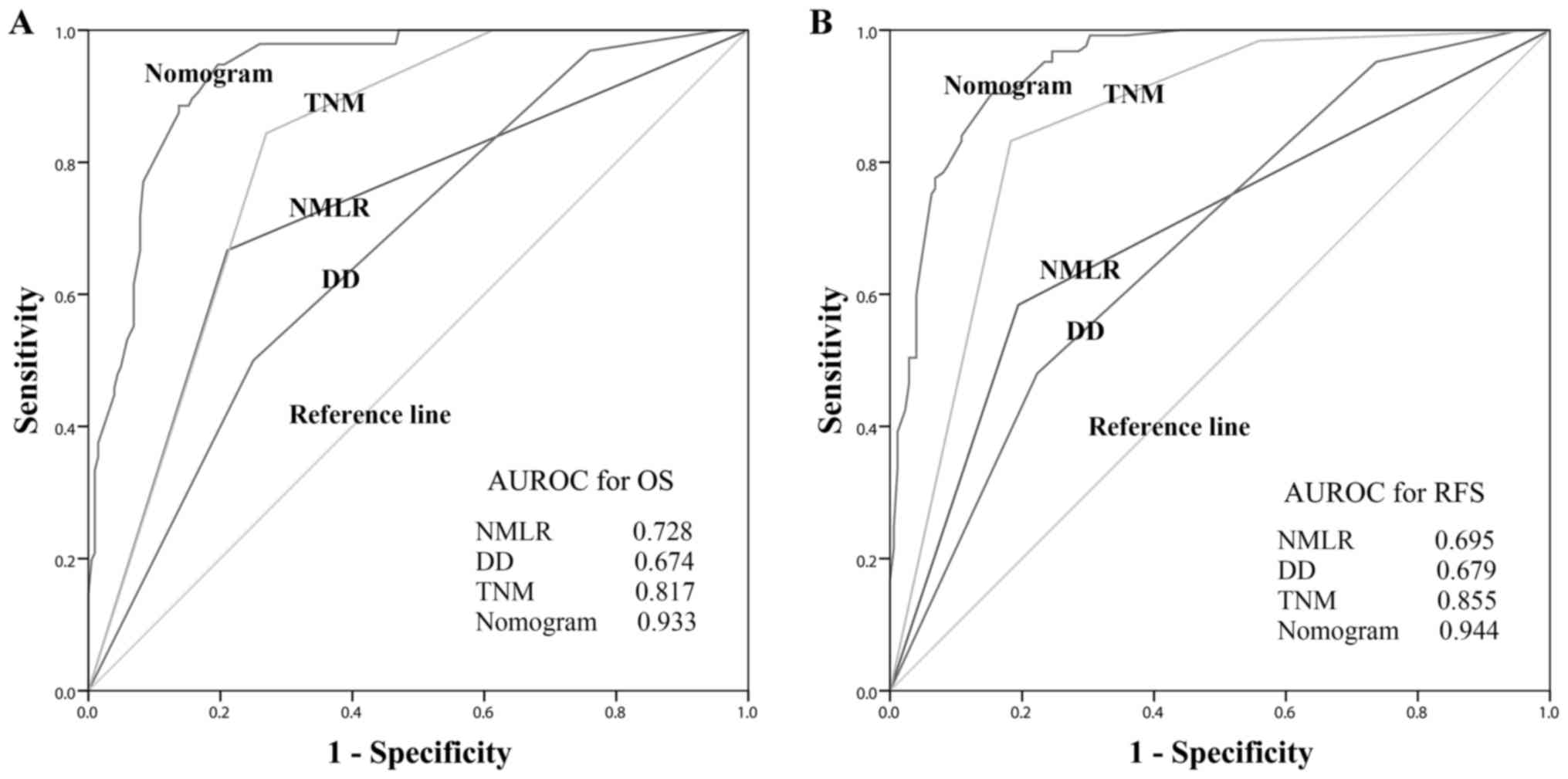
compared using ROC curves, as presented in Fig. 3. Among all inflammatory/immune
parameters, the NMLR consistently exhibited the highest AUC value
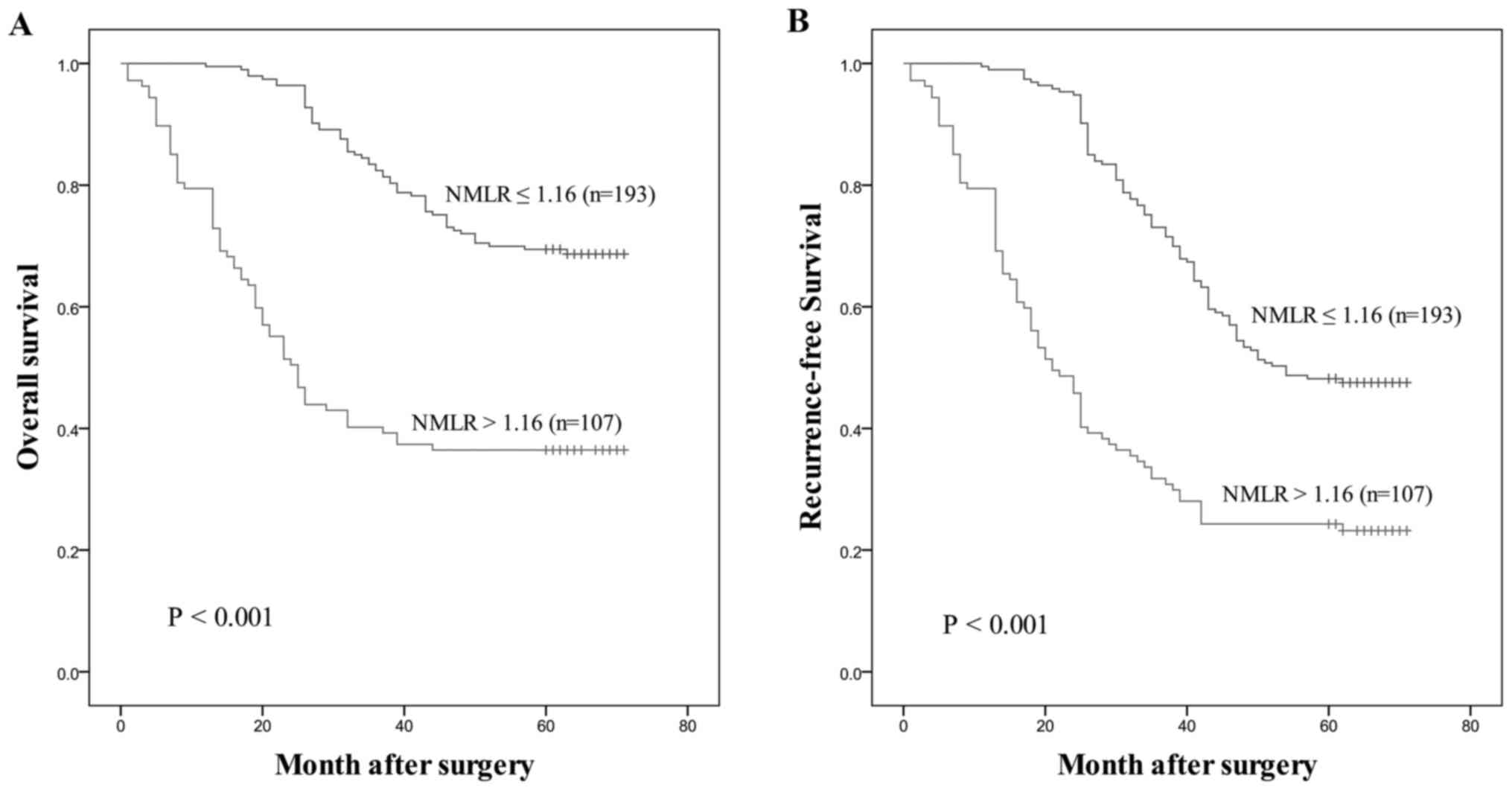
for OS (AUC=0.728) and RFS (AUC=0.695). Patients with a low NMLR
demonstrated significantly improved OS and RFS compared with those
with a high NMLR, as presented in Fig.
4.
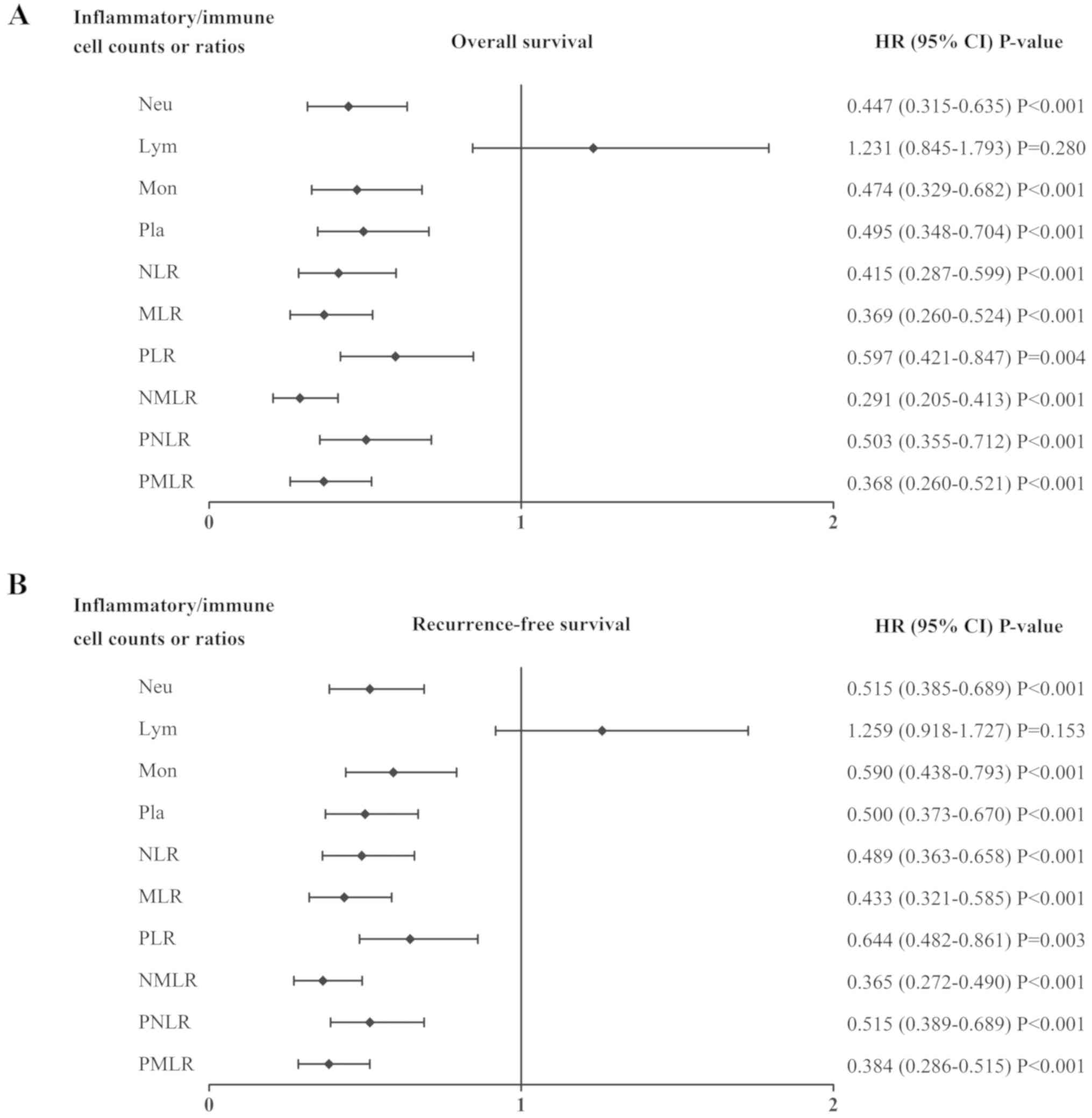 | Figure 2.Association between the systemic
inflammatory/immune parameters and prognosis. HR and CI of (A)
overall survival and (B) recurrence-free survival rates were
analyzed using the log-rank method for the systemic
inflammatory/immune cells counts and ratios. HR, hazard ratio; CI,
confidence interval; Neu, neutrophils; Lym, lymphocytes; Mon,
monocytes; Pla, platelets; NLR, neutrophil-to-lymphocyte ratio;
MLR, monocyte-to-lymphocyte ratio; PLR, platelet-to-lymphocyte
ratio; NMLR, neutrophil-monocyte-lymphocyte ratio; PNLR,
platelet-neutrophil-lymphocyte ratio; PMLR,
platelet-monocyte-lymphocyte ratio. |
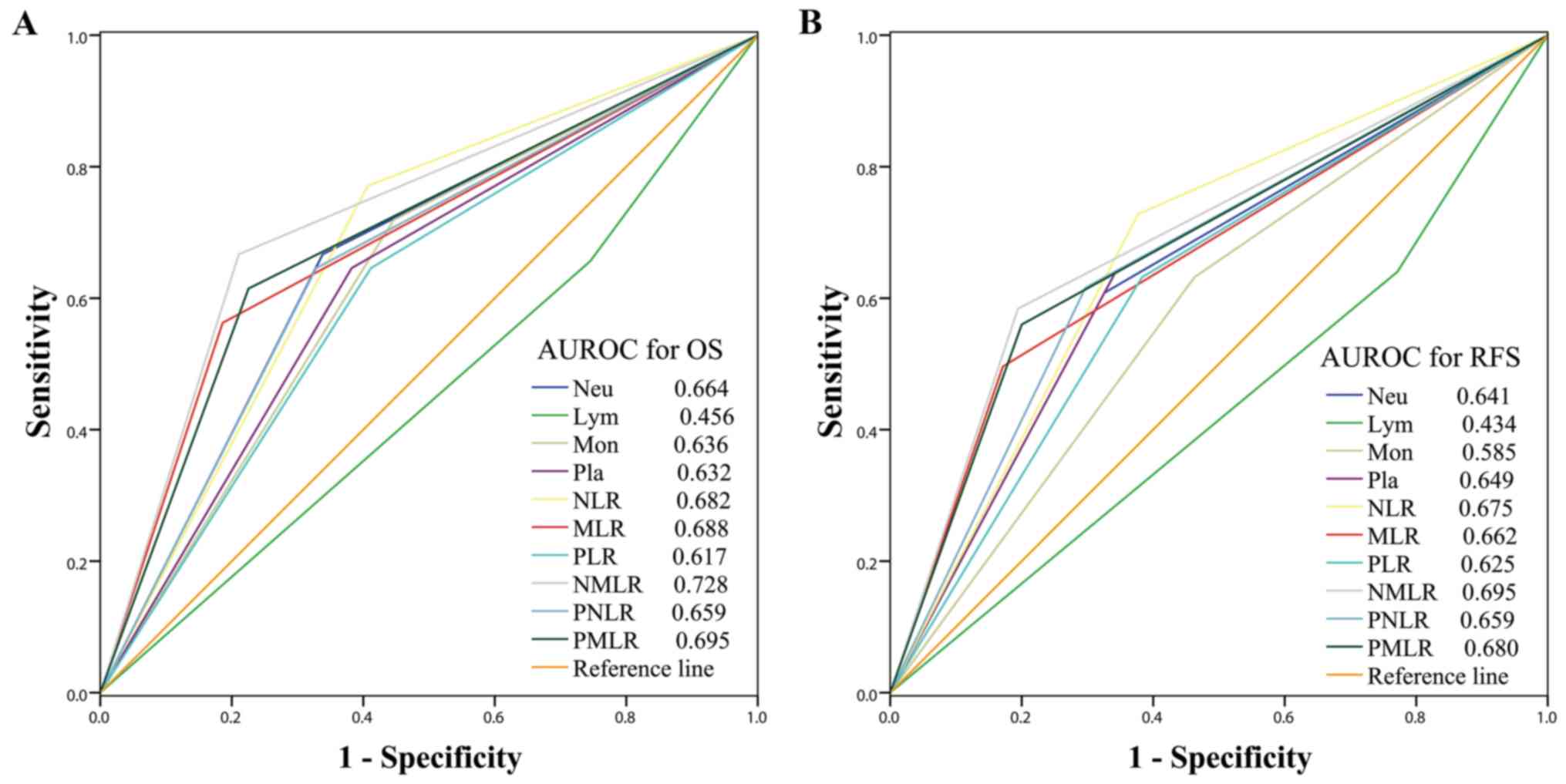 | Figure 3.Predictive accuracy comparison of the
systemic inflammatory/immune parameters for prognosis. AUROC was
used to compare the sensitivities and specificities of the systemic
inflammatory/immune cells counts and ratios to (A) OS or (B) RFS.
AUROC, area under the receiver operating characteristic curve; OS,
overall survival; RFS, recurrence-free survival; Neu, neutrophils;
Lym, lymphocytes; Mon, monocytes; Pla, platelets; NLR,
neutrophil-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio;
PLR, platelet-to-lymphocyte ratio; NMLR,
neutrophil-monocyte-lymphocyte ratio; PNLR,
platelet-neutrophil-lymphocyte ratio; PMLR,
platelet-monocyte-lymphocyte ratio. |
Prognostic factors according to
univariate and multivariate analyses in the primary cohort
In the primary cohort, univariate analyses were used
to identify the potential predictive factors. Subsequently, the
significant predictive factors were subjected to multivariate
analyses. It was demonstrated that the degree of differentiation
(DD; P=0.003 and P=0.010), T stage (both P<0.001), N stage (both
P<0.001), and NMLR (both P<0.001) were independent prognostic
factors for OS and RFS, respectively (Table II).
 | Table II.Univariate and multivariate analyses,
using a Cox proportional-hazards model of OS and recurrence-free
survival of gastric cancer in primary cohort. |
Table II.
Univariate and multivariate analyses,
using a Cox proportional-hazards model of OS and recurrence-free
survival of gastric cancer in primary cohort.
|
| OS | RFS |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Prognostic
variables | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 0.998 | 0.833 | – | – | 1.004 | 0.620 | – | – |
|
| (0.979–1.017) |
|
|
| (0.988–1.020) |
|
|
|
| Sex | 1.532 | 0.046 | – | – | 1.082 | 0.345 | – | – |
|
| (1.007–2.330) |
|
|
| (0.919–1.274) |
|
|
|
| Tumor size | 1.161 | <0.001 | – | – | 1.181 | <0.001 | – | – |
|
| (1.089–1.239) |
|
|
| (1.120–1.245) |
|
|
|
| DD | – | <0.001 | – | 0.003 | – | <0.001 | – |
0.010 |
| T-stage | – | <0.001 | – | <0.001 | – | <0.001 | – | <0.001 |
| N-stage | – | <0.001 | – | <0.001 | – | <0.001 | – | <0.001 |
| NMLR | 0.291 | <0.001 | 0.162 | <0.001 | 0.365 | <0.001 | 0.229 | <0.001 |
|
| (0.205–0.413) |
| (0.107–0.244) |
| (0.272–0.490) |
| (0.165–0.318) |
|
| D-dimer | 0.984 | 0.639 | – | – | 1.021 | 0.356 | – | – |
|
| (0.918–1.054) |
|
|
| (0.977–1.066) |
|
|
|
| CEA | 1.003 | 0.025 | – | – | 1.003 | 0.033 | – | – |
|
| (1.000–1.005) |
|
|
| (1.000–1.005) |
|
|
|
Establishment and evaluation of
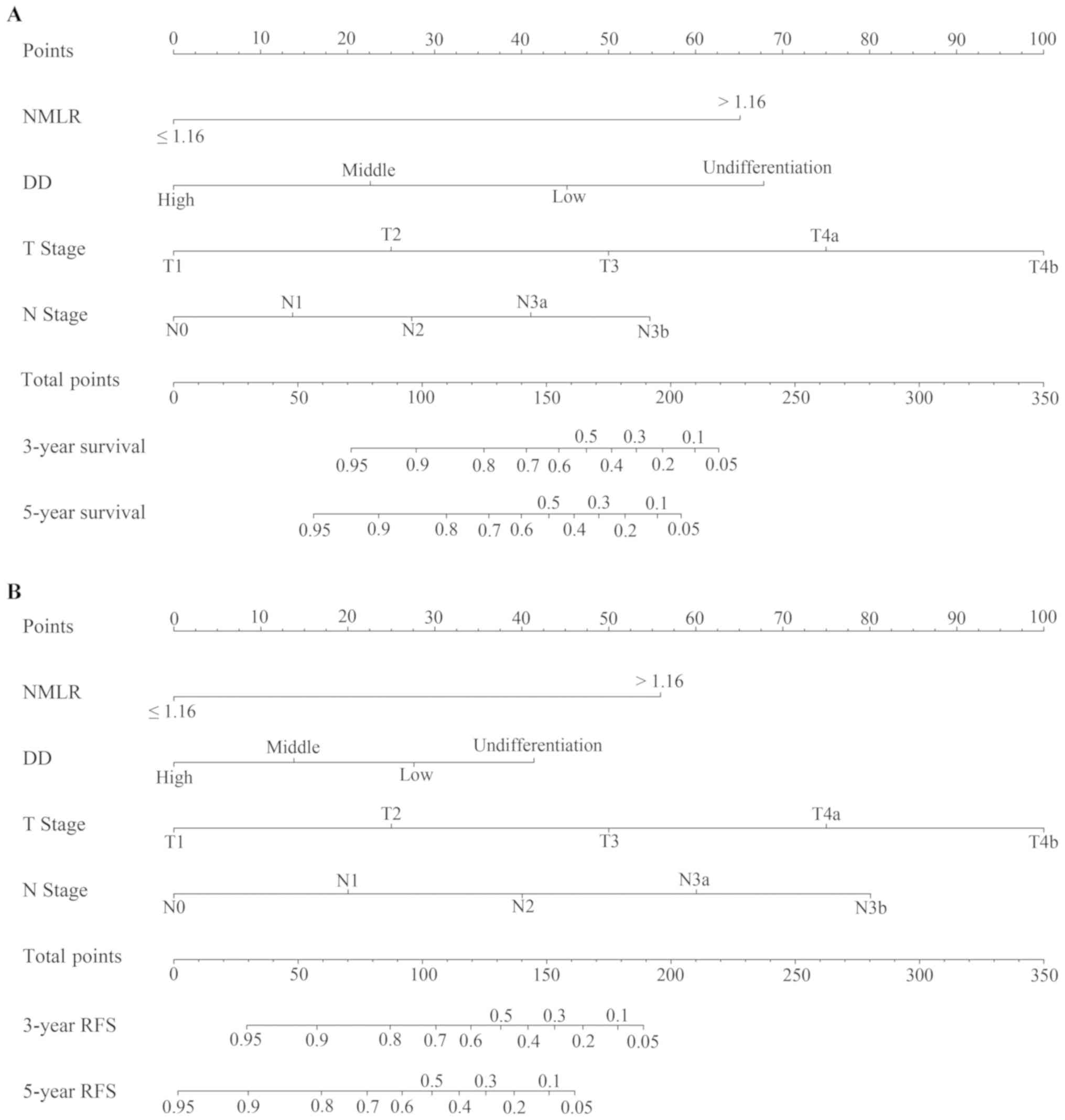
nomograms for OS and RFS
The nomograms for OS (Fig. 5A) and RFS (Fig. 5B) were established using independent
prognostic factors identified in the multivariate analysis
conducted in the primary cohort. The C-index of the nomograms for
OS was 0.851 [95% confidence interval (CI), 0.817–0.883], which was
increased compared with that of DD (0.636; 95% CI, 0.593–0.679),
the NMLR (0.667; 95% CI, 0.628–0.706) and the TNM stage (0.731; 95%
CI, 0.698–0.764). The C-index of the nomograms for RFS was 0.860
(95% CI, 0.831–0.889), which was increased compared with that of DD
(0.629; 95% CI, 0.591–0.667), the NMLR (0.645; 95% CI, 0.612–0.678)
and the TNM stage (0.740; 95% CI, 0.712–0.768). Concomitantly, the
nomograms for OS and RFS exhibited the largest AUC value (0.933 for
OS and 0.944 for RFS) compared with DD (0.674 for OS and 0.679 for
RFS), NMLR (0.728 for OS and 0.695 for RFS) and TNM stage (0.817
for OS and 0.855 for RFS), as presented in Fig. 6. In the internal validation cohort,
the C-indexes of the nomogram for OS and RFS were 0.840 (95% CI,
0.803–0.877) and 0.916 (95% CI, 0.895–0.937), respectively. In the
external validation cohort, the C-indexes of the nomogram for OS
and RFS were 0.827 (95% CI, 0.763–0.891) and 0.891 (95% CI,
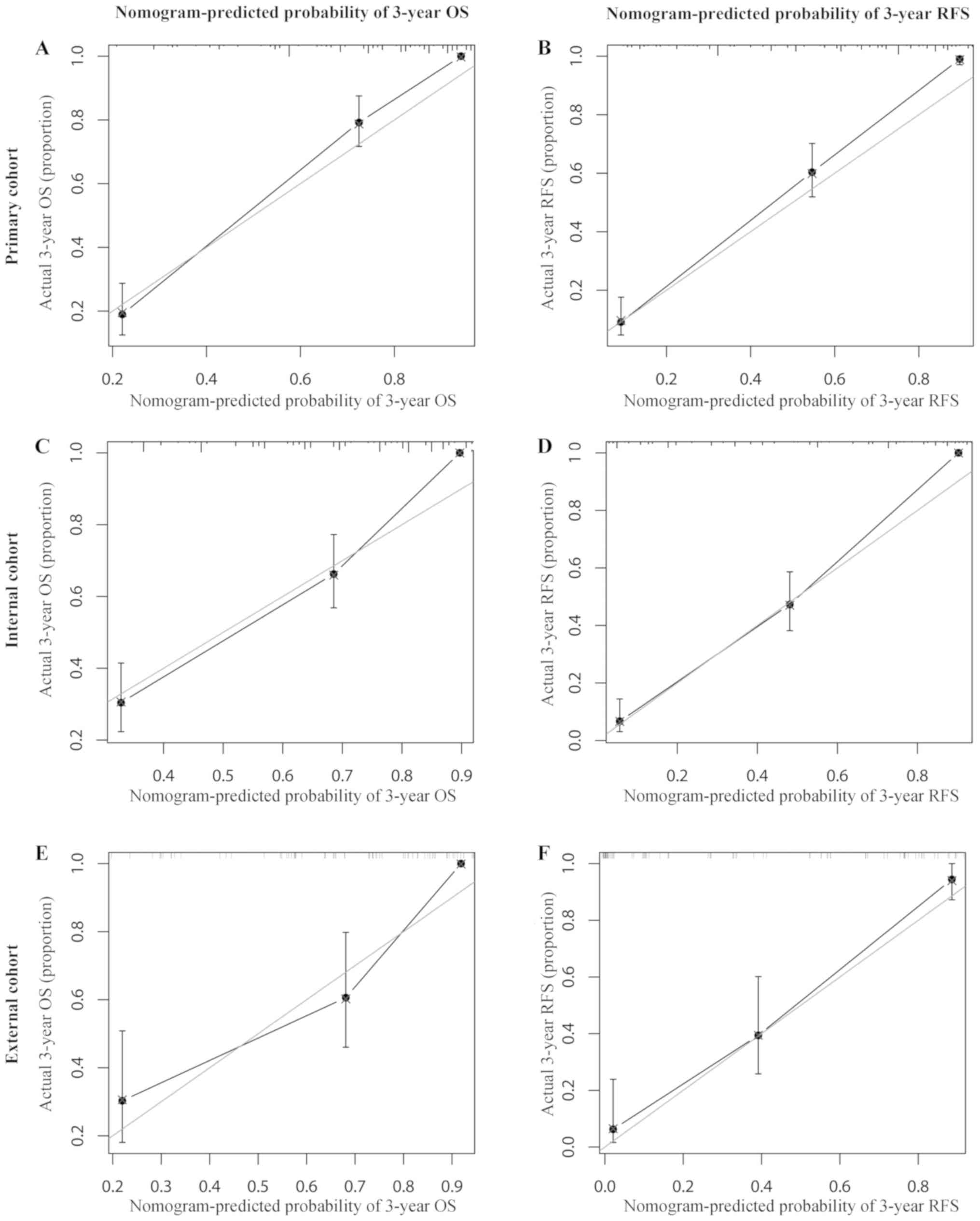
0.852–0.930), respectively. The calibration plots generated in the
present study exhibited a good coherence between the predictions
and observations regarding 3-year survival and recurrence, as
presented in Fig. 7.
Discussion
In the development of GC, sustained inflammatory and
immune responses are hypothesized to serve a role, and they are
considered to be the most important risk factors for prognosis
(11,12). GC is associated with Helicobacter
pylori infection, which stimulates Toll-like receptors, induces
infection-associated inflammation and generates an inflammatory
microenvironment by activating innate immunity. Immune cells,
particularly regulatory T cells, have been considered to be
involved in inflammatory and immune response during the development
of GC (13). These responses result
in neutrophilia, lymphopenia and thrombocytosis. A high absolute
neutrophil, monocyte and platelet count, and a low absolute count
of lymphocytes have been demonstrated to be associated with poor
prognosis of patients with GC (14,15). The
tumor immune microenvironment of GC is complex and changeable, and
it involves various inflammatory cells, immune cells and tumor
cells. The majority of single inflammatory or immune cell type
counts are not sufficient to predict the prognosis of patients with
GC after R0 resection. Numerous studies have revealed that systemic
inflammation/immune cell ratios may be recognized as significant
independent risk factors for the prognosis of GC (5,16–18).
However, to date, only a few studies have compared the predictive
value of all inflammatory/immune parameters (7,19). The
present study demonstrated that the NMLR exhibited the highest
accuracy and predictive power; among all inflammatory/immune
parameters assessed, it was the only parameter that was
independently associated with OS and RFS.
The roles of inflammatory and immune cells in
tumorigenesis may explain their predictive capacities regarding
prognosis. Increasing evidence suggests that inflammatory
environments accelerate the progression of metastasis by
neutrophil-mediated mechanisms (20). For example, neutrophils contribute to
the initiation of natural killer cell and monocyte recruitment by
various mechanisms (21).
Neutrophils and monocytes may contribute to tumor progression by
releasing prostaglandin E2 to amplify inflammation and create the
tumor microenvironment (22).
Conversely, lymphocytes may kill tumor cells through cytotoxic
effects from the release of chemokines and cytokines, including
interleukin-16, C-C motif chemokine ligand 21 and vascular
endothelial growth factor A, which attract monocytes, dendritic
cells and endothelial cells to the tumor core and invasive margin
(23,24). Therefore, the NMLR may reflect the
complex interaction between neutrophils, monocytes and lymphocytes
in the tumor microenvironment.
The present study analyzed the predictive ability of
NMLR. To the best of our knowledge, the present study was the first
to demonstrate that the NMLR, which reflects the homeostasis
between host inflammatory and immune status, exhibited a greater
prognostic value in GC compared with any other inflammatory/immune
parameters; it was also the first to demonstrate that 2 specific OS
and RFS nomograms, which included NMLR as one of their factors,
exhibited high predictive values compared with measuring NMLR and
the TNM stage separately. Among all factors involved in the 2
nomograms, the T stage, N stage and DD have been previously
suggested to be associated with the prognosis of GC after
gastrectomy (25,26). Although certain risk factors,
including CEA, sex, age and D-dimer, are associated with the
prognosis of patients with GC (27–30),
these factors were not applicable in the present study.
The nomogram described in the present study has
several specific characteristics that distinguish it from previous
nomograms. Firstly, the clinical and pathological factors included
in the nomograms of the present study are much simpler to determine
by routine clinical analysis. Furthermore, the nomogram did not
just include the severity of GC, but the immune status of the
patient was also considered. Finally, internal and external
validation confirmed this accuracy.
There are several limitations which should be taken
into consideration when interpreting the conclusions of the present
study. Firstly, the present study was limited by its retrospective
nature. Furthermore, as certain cases were followed up for <5
years, the 5-year survival rate and 5-year recurrence-free survival
rate were not sufficiently accurate. In addition, the effects of
adjuvant treatment, including chemotherapy or radiation treatment,
were not evaluated. As an additional limitation, nutritive indexes
were not considered in the present study. Previous studies
demonstrated that certain nutritive indexes, including Controlling
Nutritional Status, prognostic nutritional index and pre-operative
body weight, were closely associated with the prognosis of GC
(31–33). Finally, comorbidities, including
hypertension and diabetes, were not reflected in the nomograms. It
may be assumed that comorbidities may affect the prognosis to a
certain extent.
In conclusion, 2 nomograms were described in the
present study, which demonstrated predictive value for survival and
recurrence in patients with GC after R0 resection with improved
sensitivity and accuracy. This evaluation system may provide
valuable insight into identifying patients with a high risk of poor
prognosis following surgery. Close follow-up and comprehensive
anti-tumor therapy are more suitable for these people. However, a
large-sample prospective study is required to determine whether
these nomograms are sufficiently accurate, and whether any further
risk factors should be considered for inclusion in the
assessment.
Acknowledgments
Not applicable.
Funding
The present study received a grant (grant no.
WS201515) from the Science and Technology Bureau of Changzhou
Municipal Wujin District.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YT, HW, YW and PJ were responsible for data curation
and YQ performed the statistical analysis. XX was responsible for
acquiring the funding. WD, XX and XZ developed the methodology of
the present study and WD, WX and YX performed the software
analysis. WD and XX supervised the study. WD and WX wrote the
original draft of the manuscript, and WD performed the review and
editing of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Wujin Hospital Affiliated to Jiangsu University. Due
to the retrospective nature of this study, the need for written
informed consent was waived.
Patient consent for publication
Due to the retrospective nature of this study,
informed consent was waived.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GC
|
gastric cancer
|
|
OS
|
overall survival
|
|
RFS
|
recurrence-free survival
|
|
TNM
|
tumor-nodes-metastasis
|
|
DD
|
degree of differentiation
|
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
MLR
|
monocyte-to-lymphocyte ratio
|
|
PLR
|
platelet-to-lymphocyte ratio
|
|
CT
|
computed tomography
|
|
CEA
|
carcinoembryonic antigen
|
|
NMLR
|
neutrophil-monocyte-lymphocyte
ratio
|
|
PNLR
|
platelet-neutrophil-lymphocyte
|
|
PMLR
|
platelet-monocyte-lymphocyte
|
|
ROC
|
receiver operating characteristics
|
|
AUC
|
area under the ROC curve
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fornaro L, Vasile E, Aprile G, Goetze TO,
Vivaldi C, Falcone A and Al-Batran SE: Locally advanced
gastro-oesophageal cancer: Recent therapeutic advances and research
directions. Cancer Treat Rev. 69:90–100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah MA and Ajani JA: Gastric cancer-an
enigmatic and heterogeneous disease. JAMA. 303:1753–1754. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arigami T, Uenosono Y, Matsushita D,
Yanagita S, Uchikado Y, Kita Y, Mori S, Kijima Y, Okumura H,
Maemura K, et al: Combined fibrinogen concentration and
neutrophil-lymphocyte ratio as a prognostic marker of gastric
cancer. Oncol Lett. 11:1537–1544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen L, Hao Y, Zhu L, Li S, Zuo Y, Zhang
Y, Song H and Xue Y: Monocyte to lymphocyte ratio predicts survival
in patients with advanced gastric cancer undergoing neoadjuvant
chemotherapy. Onco Targets. 10:4007–4016. 2017. View Article : Google Scholar
|
|
6
|
Zhang Y, Lu JJ, Du YP, Feng CX, Wang LQ
and Chen MB: Prognostic value of neutrophil-to-lymphocyte ratio and
platelet-to-lymphocyte ratio in gastric cancer. Medicine
(Baltimore). 97:e01442018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liao R, Peng C, Li M, Li DW, Jiang N, Li
PZ, Ding X, Wu Q, Du CY and Gong JP: Comparison and validation of
the prognostic value of preoperative systemic immune cells in
hepatocellular carcinoma after curative hepatectomy. Cancer Med.
7:1170–1182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng ZF, Lu J and Huang CM: ASO author
reflections: Simplified nomogram predictive of survival after R0
resection for gastric cancer. Ann Surg Oncol. 25 (Suppl
3):S733–S734. 2018. View Article : Google Scholar
|
|
9
|
Liu X, Wu Z, Lin E, Li W, Chen Y, Sun X
and Zhou Z: Systemic prognostic score and nomogram based on
inflammatory, nutritional and tumor markers predict cancer-specific
survival in stage II–III gastric cancer patients with adjuvant
chemotherapy. Clin Nutr. 38:1853–1860. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ajani JA, In H, Sano T, et al: StomachAmin
MB: AJCC cancer staging manual. 8th. New York: Springer-Verlag;
2016
|
|
11
|
Matsueda S and Graham DY: Immunotherapy in
gastric cancer. World J Gastroenterol. 20:1657–1666. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Echizen K, Hirose O, Maeda Y and Oshima M:
Inflammation in gastric cancer: Interplay of the
COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways.
Cancer Sci. 107:391–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kandulski A, Malfertheiner P and Wex T:
Role of regulatory T-cells in H. pylori-induced gastritis and
gastric cancer. Anticancer Res. 30:1093–1103. 2010.PubMed/NCBI
|
|
14
|
Shen Q, Liu W, Quan H, Pan S, Li S, Zhou
T, Ouyang Y and Xiao H: Prealbumin and lymphocyte-based prognostic
score, a new tool for predicting long-term survival after curative
resection of stage II/III gastric cancer. Br J Nutr. 120:1359–1369.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng F, Zheng G and Wang Q: Low lymphocyte
count and high monocyte count predicts poor prognosis of gastric
cancer. BMC Gastroenterol. 18:1482018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim H, Ro SM, Yang JH, Jeong JW, Lee JE,
Roh SY and Kim IH: The neutrophil-to-lymphocyte ratio
prechemotherapy and postchemotherapy as a prognostic marker in
metastatic gastric cancer. Korean J Intern Med. 33:990–999. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma M, Wang J, Hu Y, Weng M, Liu X and Wang
Y: Prognostic value of inflammatory biomarkers in gastric cancer
patients and the construction of a predictive model. Dig Surg.
36:433–442. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wen J, Bedford M, Begum R, Mitchell H,
Hodson J, Whiting J and Griffiths E: The value of inflammation
based prognostic scores in patients undergoing surgical resection
for oesophageal and gastric carcinoma. J Surg Oncol. 117:1697–1707.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou ZQ, Pang S, Yu XC, Xue Q, Jiang HY,
Liang XJ and Liu L: Predictive values of postoperative and dynamic
changes of inflammation indexes in survival of patients with
resected colorectal cancer. Curr Med Sci. 38:798–808. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McDonald B, Spicer J, Giannais B,
Fallavollita L, Brodt P and Ferri LE: Systemic inflammation
increases cancer cell adhesion to hepatic sinusoids by neutrophil
mediated mechanisms. Int J Cancer. 125:1298–1305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nowarski R, Gagliani N, Huber S and
Flavell RA: Innate immune cells in inflammation and cancer. Cancer
Immunol Res. 1:77–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spiegel A, Brooks MW, Houshyar S,
Reinhardt F, Ardolino M, Fessler E, Chen MB, Krall JA, DeCock J,
Zervantonakis IK, et al: Neutrophils suppress intraluminal NK
cell-mediated tumor cell clearance and enhance extravasation of
disseminated carcinoma cells. Cancer Discov. 6:630–649. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He W, Zhang H, Han F, Chen X, Lin R, Wang
W, Qiu H, Zhuang Z, Liao Q, Zhang W, et al: CD155T/TIGIT signaling
regulates CD8+ T-cell metabolism and promotes tumor
progression in human gastric cancer. Cancer Res. 77:6375–6388.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fridman WH, Pagès F, Sautès-Fridman C and
Galon J: The immune contexture in human tumours: Impact on clinical
outcome. Nat Rev Cancer. 12:298–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanesaka T, Nagahama T, Uedo N, Doyama H,
Ueo T, Uchita K, Yoshida N, Takeda Y, Imamura K, Wada K, et al:
Clinical predictors of histologic type of gastric cancer.
Gastrointest Endosc. 87:1014–1022. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng J, Zhang R, Pan Y, Wang B, Wu L, Jiao
X, Bao T, Hao X and Liang H: Comparison of the staging of regional
lymph nodes using the sixth and seventh editions of the
tumor-node-metastasis (TNM) classification system for the
evaluation of overall survival in gastric cancer patients: Findings
of a case-control analysis involving a single institution in China.
Surgery. 156:64–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng F, Tian Y, Xu G, Liu Z, Liu S, Zheng
G, Guo M, Lian X, Fan D and Zhang H: Diagnostic and prognostic
value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC
Cancer. 17:7372017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mori G, Nakajima T, Asada K, Shimazu T,
Yamamichi N, Maekita T, Yokoi C, Fujishiro M, Gotoda T, Ichinose M,
et al: Incidence of and risk factors for metachronous gastric
cancer after endoscopic resection and successful Helicobacter
pylori eradication: Results of a large-scale, multicenter
cohort study in Japan. Gastric Cancer. 19:911–918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kono Y, Kanzaki H, Tsuzuki T, Takatani M,
Nasu J, Kawai D, Takenaka R, Tanaka T, Iwamuro M, Kawano S, et al:
A multicenter observational study on the clinicopathological
features of gastric cancer in young patients. J Gastroenterol.
54:419–426. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu L, Zhang X, Yan B, Gu Q, Zhang X, Jiao
J, Sun D, Wang N and Yue X: Elevated plasma D-dimer levels
correlate with long term survival of gastric cancer patients. PLoS
One. 9:e905472014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuroda D, Sawayama H, Kurashige J,
Iwatsuki M, Eto T, Tokunaga R, Kitano Y, Yamamura K, Ouchi M,
Nakamura K, et al: Controlling nutritional status (CONUT) score is
a prognostic marker for gastric cancer patients after curative
resection. Gastric Cancer. 21:204–212. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun KY, Xu JB, Chen SL, Yuan YJ, Wu H,
Peng JJ, Chen CQ, Guo P, Hao YT and He YL: Novel immunological and
nutritional-based prognostic index for gastric cancer. World J
Gastroenterol. 21:5961–5971. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu X, Qiu H, Kong P, Zhou Z and Sun X:
Gastric cancer, nutritional status, and outcome. Onco Targets Ther.
10:2107–2114. 2017. View Article : Google Scholar : PubMed/NCBI
|