Introduction
Previous epidemiological studies have illustrated
increased morbidity and mortality in patients with pancreatic
cancer globally; in the past two decades, the associated morbidity
has reached 5.1/100,000, accounting for 7% of malignant cancers
(1,2). Existing clinical treatments for
pancreatic cancer rely heavily on early diagnosis and treatment,
and diagnosis at a late stage is typically associated with a high
rate of treatment failure. Thus, it is necessary to investigate the
metastatic mechanisms of pancreatic cancer, and to identify
molecular targets that are able to block the invasion and
metastasis of pancreatic cancer cells for successful clinical
diagnosis and treatment (3,4).
Deleted in liver cancer (DLC) is a tumor suppressor
gene that encodes a GTPase-activating protein (GAP), which
regulates small GTP-binding proteins and cell processes associated
with cytoskeletal alterations. The DLC-1 gene is 6 kb in length,
encoding a 1,092 amino acid protein with a molecular weight of 122
KDa. DLC exits in 3 subtypes; DLC-1, DLC-2 and DLC-3, all of which
are able to regulate Rho-GTP enzymes through the activation of
GTPases (5). DLC-1 and 2 activate
Rho GTPase proteins and are downregulated in cancer [where DLC-2
impacts Rho-GTPase-activating proteins (RhoGAP)], and DLC-3 is an
essential component for junction integrity. DLC-1 acts as a tumor
suppressor gene in multiple types of cancer, including breast and
colorectal tumors. Previous studies have associated DLC-1 with
tumor growth, differentiation and metastasis (6). DLC-1 exists widely in normal human
tissues, but existing studies have reported frequently lowered
expression levels in various cancer cell lines (7,8).
Additionally, the overexpression of the DLC-1 gene inhibits tumor
cell proliferation and has potential therapeutic effects in
prostate, gastric, liver, nasopharyngeal, breast, colon and ovarian
cancer, as well as lymphoma (9–11).
Despite partial illustration of the mechanism of
DLC-1 in specific cancers (such as hepatocellular cancer), existing
studies have only attempted to review its influence on the
pathogenesis of pancreatic cancer (12,13). A
previous study revealed that DLC-1 was able to suppress the
progression of hepatocellular cancer, retarding its invasioness and
metastasis; DLC-1 was also indicated to regulate the expression of
Rho A, Rho-associated protein kinase 2 and moesin (14). Using clinical samples, the authors
illustrated the reduced expression of DLC-1 in hepatocellular
cancer tissues, thus suggesting its role as a therapeutic target
for the treatment of the disease. However, the exact influence of
this variation in DLC-1 expression on pancreatic cancer progression
is yet to be elucidated. Additional investigations have also
associated DLC-1 with pancreatic cancers (9,15); Xue
et al (16) found that the
expression level of DLC-1 in patients with stage 3–4 pancreatic
cancer was lower than those at stages 1–2. Also, prognostic
analysis revealed that patients with a hypermethylated DLC-1 gene
exhibited a reduced 5 year survival rate compared with patients
without hypermethylation (14). This
result was also confirmed by the promotion of tumor progression in
human cancer cells following deletion of the DLC-1 gene (17). Furthermore, DLC-1 inactivation in
mouse embryonic fibroblasts promoted neoplastic transformation,
which resulted in increased Rho and cell division control protein
42 homolog (Cdc42) activity (18,19).
Further studies also showed that the Rho-GAP activity and tumor
suppressive capacity of DLC-1 were associated with protein kinase A
(PKA) (20).
Despite the indicated association between DLC-1 and
pancreatic cancer, further studies are required to support this
discovery, including experimental in vitro and in
vivo analysis. Therefore, the present study aimed to
investigate the inhibition of DLC-1 in clinical tissues and its
subsequent effects in vitro and in vivo. It was
revealed that the level of DLC-1 expression was reduced in solid
tumors, which was supported by previous bioinformatics analysis
(21) To investigate the fundamental
mechanisms of DLC-1, pancreatic cancer cell lines with reduced
DLC-1 expression levels were utilized, revealing that an
upregulation of the DLC-1 gene may affect the cell cycle and
invasive capacity of pancreatic cancer cells. DLC-1 was therefore
indicated as a potential therapeutic target for the treatment of
pancreatic cancer.
Materials and methods
Plasmid construction
The full-length DLC-1 sequence was cloned into
lentiviral vector PCDH-puro (Addgene, Inc.), following restriction
endonuclease digestion with XbaI and Not I-HF (New
England BioLabs, Inc.). The T4 DNA ligase (New England BioLabs,
Inc.,) was used to ligate the fragment and vector. For detailed
plasmid construction; two miR30-targeted shRNAs (HP_260153 and
HP_255554) were subcloned from the pSM2 RNAi codex library vector
into the MSCV-SV40-GFP vector (Addgene, Inc.), in addition to a
constitutively active Rho A gene sequence (RhoAV14). Full-length
mouse DLC-1 was amplified from a RIKEN cDNA (M5C1068G17; http://www.riken.jp/en/) and cloned into the
MSCV-PGK-PIG vector, which harbors a 6×Myc N-terminal tag. Myc was
cloned into pWZL-Neo (Cell Biolabs, Inc.) (11). The vectors (2 µg/ml in PBS) were
transiently transfected into 293T cells (1×105 cells)
using Lipofectamine® 2000 (20 µl
Lipofectamine® in 5 ml cell culture medium) (Invitrogen;
Thermo Fisher Scientific, Inc.,) according to the manufacturer's
instructions. Following a 72 h incubation, the supernatant was
harvested by centrifugation at 13,000 × g, and clarified using a
0.22 µm filter (EMD Millipore). Antibiotic selection was
subsequently conducted using 1 µg/ml puromycin (22,23).
Cell lines and tissue samples
293T cells and a range of pancreatic cancer cell
lines (BxPC-3, SW1990, AsPC-1, PANC-1, Capan1, CFPAC-1, HPAC,
Hs766T and PSN1) were purchased from the American Type Culture
Collection, and cultured in Dulbecco's modified Eagle's medium
(Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with FBS
[10% (v/v), HyClone; GE Healthcare Life Sciences]. The cells were
incubated at 37°C with 5% CO2. Pancreatic cancer tissues
and adjacent tissues (≥5×5 cm2) from 35 patients were
collected from the Shanghai Dongfang hospital (Shanghai, China)
between January 2015 and January 2016. The present study included
15 male patients (mean age, 58 years; age range, 46–72 years) and
20 female patients (mean age, 62 years; age range, 49–78 years).
The present study investigated patients with pancreatic cancer.
Patients with more than one type of cancer were excluded from the
present study.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the cells and tissues
using TRIzol® Reagent (Invitrogen; Thermo Fisher
Scientific, Inc.,), according to the manufacturer's protocol
(24,25). RT-qPCR was performed using the
SingleShot™ SYBR® Green Cell Lysis RT-qPCR Kit (Bio-RAD
Laboratories, Inc.; cat. no. 1725095) following the manufacturer's
instructions. In each reaction, 5 ng cDNA and 300 nM primers were
used to a final volume of 10 µl. The PCR reactions were conducted
with CFX96 Connect apparatus (Bio-Rad Laboratories, Inc.,) using
the following thermocycling conditions: 95°C for 5 min, followed by
40 cycles at 95°C for 10 sec, and 56°C for 40 sec. After each
application, a melting curve assessment was carried out to confirm
successful amplification. The primer sequences were as follows:
DLC-1 forward, 5′-CCGCCTGAGCATCTACGA-3′, and reverse,
5′-TTCTCCGACCACTGATTGACTA-3′; GAPDH forward,
5′-CATGAGAAGTATGACAACAGCCT-3′, and reverse,
5′AGTCCTTCCACGATACCAAAGT-3. The results were quantified using the
2−ΔΔCq method (26–28) with
GAPDH employed as a reference.
Western blotting
The transfected cell lines were lysed using the
M-PER Mammalian Protein Extraction Reagent (Pierce; Thermo Fisher
Scientific, Inc.,) and the total protein was quantified with using
a bicinchoninic acid assay. The proteins (30 µg/well) were resolved
by SDS-PAGE using a 5% gel (Invitrogen; Thermo Fisher Scientific,
Inc.), followed by transfer onto nitrocellulose membranes
(Invitrogen; Thermo Fisher Scientific, Inc.). The membranes were
blocked using 5% non-fat milk (Santa Cruz Biotechnology, Inc.) at
20°C overnight and probed with anti-DLC-1 (1:500; cat. no. 612020;
BD Biosciences) and GAPDH (1:5,000; cat. no. mAbcam 9484; Abcam)
mouse monoclonal antibodies for 1.5 h at room temperature. The
membrane was then washed three times with 1X TBST, for 10 min each.
The membranes were then incubated with bovine anti-mouse
IgG-horseradish peroxidase (HRP) secondary antibody (1:2,000; cat.
no. sc-2380; Santa Cruz Biotechnology, Inc.,) at room temperature
for 1 h, followed by washing three times with 1X TBST for 10 min
each. The bands were visualized on X-ray film using the ChemiScope
6000 imaging system and ECL Western Blotting Substrate kit
(Abcam).
Gene clip expression-profiling and
heatmap analysis of DLC-1
The Samr package for R software (version 3.6.1,
http://www.r-project.org/) was used to
detect the differences in DLC-1 expression between normal and
cancer tissues (29). As a threshold
for screening differential genes, delta=1 and fold change >2
were used. Additionally, limma was selected to ensure the
difference between normal and disease tissues could be well
characterized, and the threshold was set as adj.P.Val =0.05 and
fold change >2 (30). The genes
that were identified to be differential by both algorithms were
selected. The MiRWalk2.0 database (mirTarBase v6; http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/)
was selected for investigating the target gene. Pearson rank
correlation was used to study the significant correlation between
the different samples (31).
Flow Cytometry
Lentivirus-infected SW1990 pancreatic cancer cells
were inoculated into 6-well plates and gently homogenized into a
single cell suspension. The cells were washed twice with PBS, and
the supernatant discarded each time. The cells were fixed with 3 ml
100% ethanol (−20°C) at 4°C for 1 h, and washed with PBS prior to
staining with 0.4 ml propidium iodide (PI; 0.5% PI in PBS; 0.1%
Triton X-100) at room temperature for 10 min. Cell cycle analysis
was conducted using a flow cytometer and borders were defined for
different phases of the cell cycle (G0/G1,
G2, S, and M) as previously described (25,32).
Transwell assay
Transwell assays were performed as previously
described (33). A total of
5×104 cells/well were resuspended in serum-free DMEM
(Invitrogen; Thermo Fisher Scientific, Inc.). in a 24-well plate,
and incubation was performed at 37°C (5% CO2) for 24 h.
The cells were then fixed with 4% polymethyl alcohol and stained
with Coomassie brilliant blue (1 g/ml; Sigma-Aldrich; Merck KGaA).
The intensity of each sample was determined by assessing the
absorbance at 580 nm (34).
Animal model
Female C57BL/6J mice (4–7 weeks of age, ~20 g each)
were purchased from Changzhou Cavens Laboratory Animal Co., Ltd.
The mice were raised at room temperature with a 12 h alternating
light/dark cycle, and freely available food and water. The mice
were shaved for the convenience of tumor cell implantation.
Briefly, 5×105 SW1990 cells (either transfected with
DLC-1 or not) were implanted via intradermal injection into the
skin on the mouse's back; each test group contained 5–7 mice and
untreated mice were used as the negative control group. Tumor size
was recorded daily and assessed as the product of two orthogonal
diameters. The mice were sacrificed when the tumor reached 150
mm2. A mixture of oxygen (0.5–1.0 l/min) and isoflurane
was used to anesthetize the mice; 3–5% isoflurane was used for 3–7
min, followed by 1–3% for maintenance (a further 5–10 min).
Following anesthesia, cervical dislocation was performed to ensure
successful euthanasia.
Statistical analysis
SPSS 21.0 software and Graphpad Prism version 6.02
(GraphPad Software, Inc.,) were used for statistical analysis.
One-way ANOVA followed by Tukey's post hoc test was employed, and
P<0.05 was considered to indicate a statistically significant
difference. The data are presented as the mean ± standard error of
the mean, and all experiments were conducted in quadruplicate (i.e.
cell culture), or 5–7 mice per animal study group. A total of 21
mice were used in the present study.
Results
DLC-1 expression level is reduced in
pancreatic cancer tissues
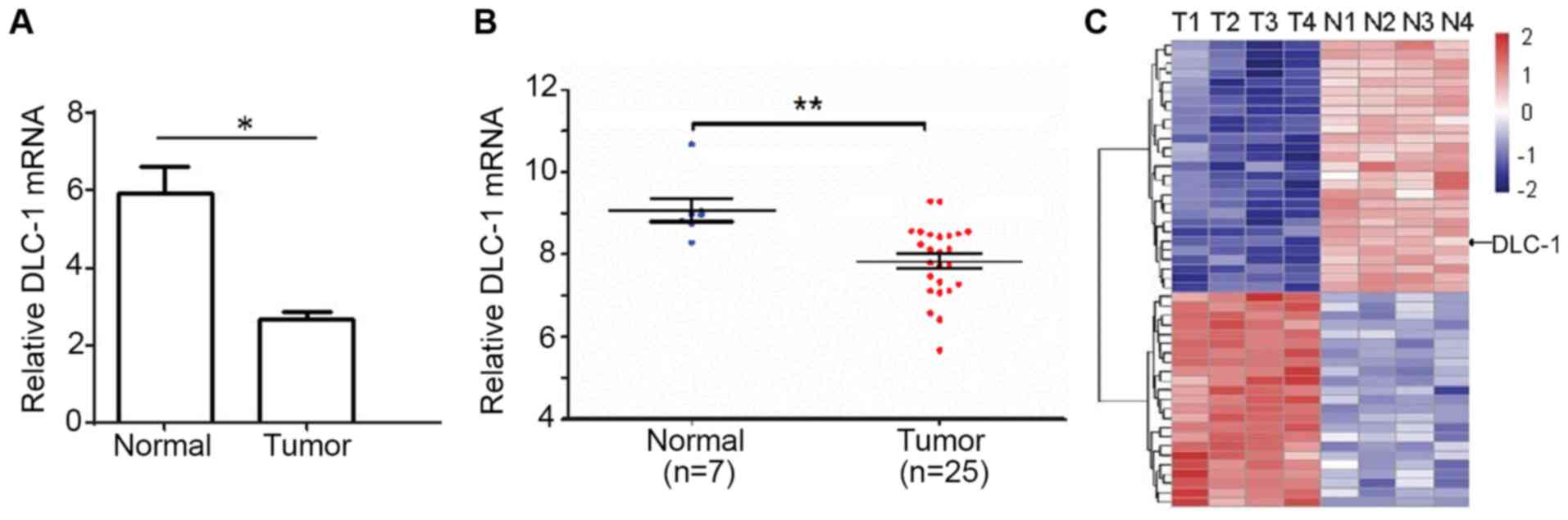
Using RT-qPCR, the expression level of DLC-1 was
investigated in the pancreatic cancer and adjacent normal tissues
of 35 patients. This indicated that the relative DLC-1 mRNA
expression level in the cancerous tissues was 5.97±0.47, which was
significantly higher than that of the adjacent normal tissues
(2.35±0.21; P<0.05; Fig. 1A).
Fig. 1A indicates the mean mRNA
expression level of DLC-1 in normal or patient tissues. In addition
to the average level, the present study also investigated the mRNA
expression levels of DLC-1 in each patients' normal and tumor
tissue samples, where a statistical difference was observed between
the two groups (P<0.01; Fig. 1B).
Only 32 samples (7 normal and 25 cancer tissue samples) were used
in this test since some samples were damaged during the process or
there was only a limited amount of the sample available. Thus, the
clinical data indicated that the normal tissues exhibited a higher
expression level of DCL-1 mRNA, and that the expression of DLC-1
was downregulated in cancer tissues.
The expression level of DCL-1 in pancreatic cancer
and adjacent normal tissues was then assessed using gene clip
expression-profiling analysis; this confirmed that the expression
of DLC-1 in pancreatic cancer tissues was lower than that of
adjacent healthy tissue (Fig 1C).
Collectively, these data suggested downregulated DLC-1 gene
expression in tumor tissues compared with normal adjacent tissues,
indicating that a reduced DLC-1 expression level is associated with
pancreatic cancer progression.
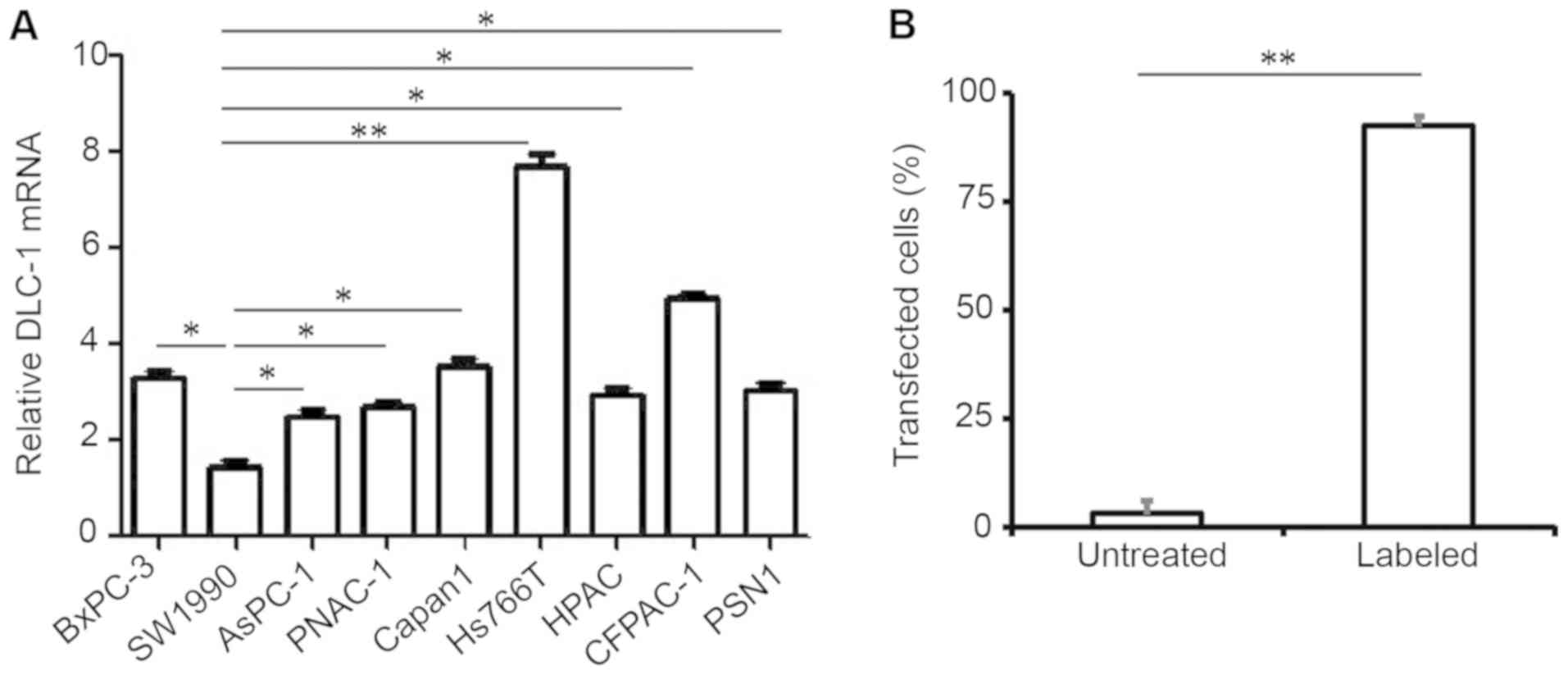
DLC-1 gene expression is reduced in
pancreatic cancer cell lines
After confirming the reduced expression of the DLC-1
gene in patient tissues (Fig. 1),
which was supported by clinical information in the existing
literature (10,11,14,17) the
expression level of DLC-1 in different pancreatic cell lines was
investigated using RT-qPCR. BxPC-3, SW1990, AsPC-1, PANC-1
(ATCC® CRL-1469™), Capan1, CFPAC-1, HPAC, Hs766T and
PSN1 cells were analyzed due to their reduced expression levels of
DLC-1. The results indicated that SW1990 pancreatic cancer cells
exhibited the lowest DLC-1 expression level, and that Hs766T cells
expressed the highest level (Fig.
2A).
To investigate the impact of the DLC-1 gene on
pancreatic cancer progression, a lentiviral vector expressing a
GFP-labeled DLC-1 sequence was constructed and subsequently
transfected into SW1990 pancreatic cancer cells, indicating a
transfection efficiency of >92% (Fig.
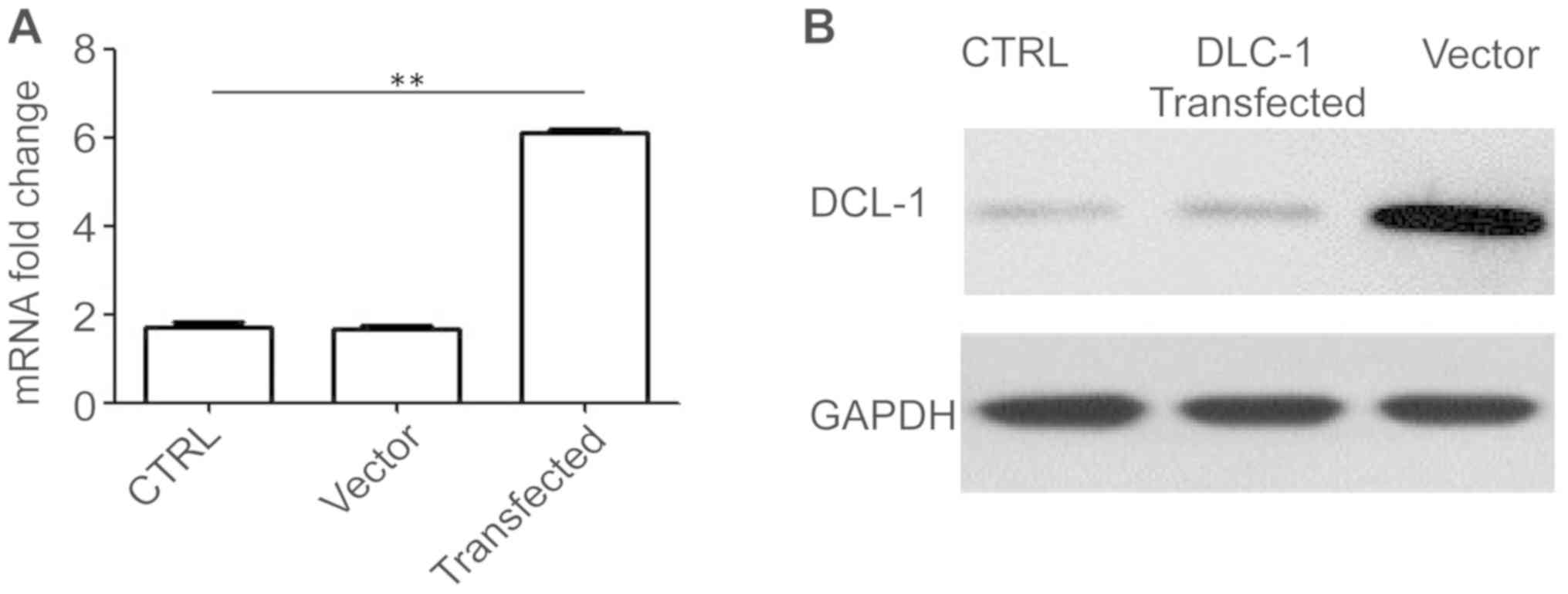
2B). The relative expression of DLC-1 mRNA was also determined
using RT-qPCR. DCL-1 transfection resulted in a signigficant
increase in DCL-1 mRNA expression level compared with the control
groups (Fig. 3A). A similar trend
was also confirmed for the DCL-1 protein expression level using
western blotting, where DCL-1 lentiviral transfection increased the
level of DCL-1 protein expression compared with the control groups
(Fig. 3B). This confirmed that
transfection of the DCL-1 gene into SW1990 pancreatic cancer cells
increased the relative expression levels of DLC-1 mRNA and protein
compared with the control groups.
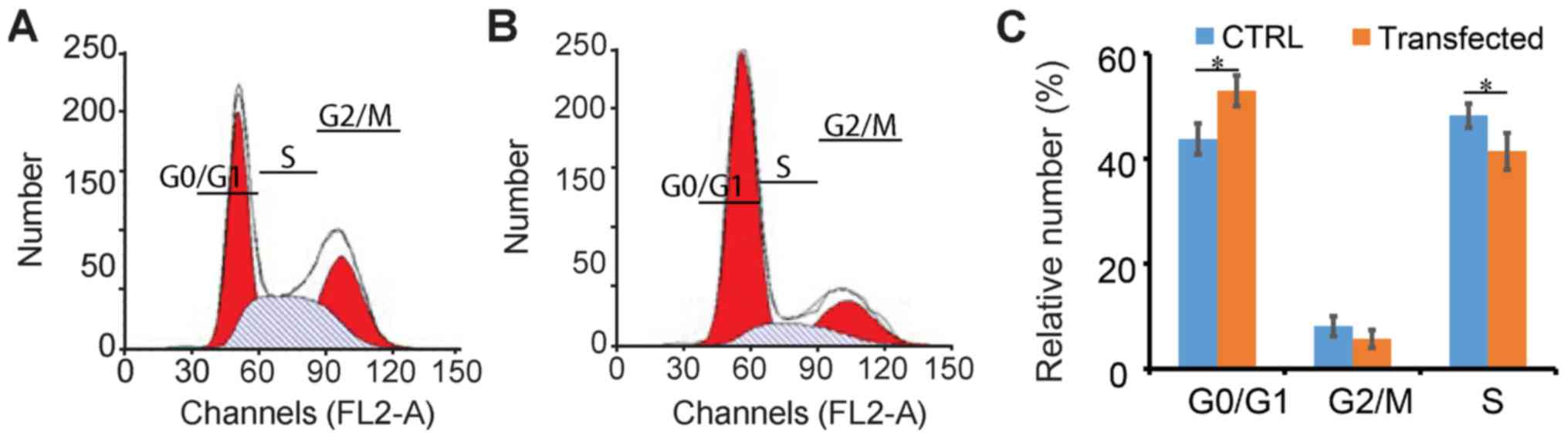
DLC-1 alters the cell cycle in SW1990
pancreatic cancer cells
After confirming the successful transfection and
expression of the DLC-1 gene in SW1990 pancreatic cancer cells, the
impact of DCL-1 on the cell cycle was flow cytometrically analyzed
(35). This indicated that in
DCL-1-transfected SW1990 cells, the cell cycle was moderately
inhibited (Fig. 4A and B); For the
control group, 43.2% cells were in the G0/G1
phase (Fig. 4C), whilst the number
in the transfected cells was 53.1%. Additionally, there were 9.2%
untransfected cells in the G2/M phase, and 5.7% DLC-1
transfected cells; 47.7 and 41.4% control and transfected cells
were in the S phase, respectively (Fig.
4C). Therefore, DLC-1 transfection resulted in slight
alterations to the cell cycle in SW1990 cells.
DLC-1 transfection influences the
invasive capacity of pancreatic cancer cells
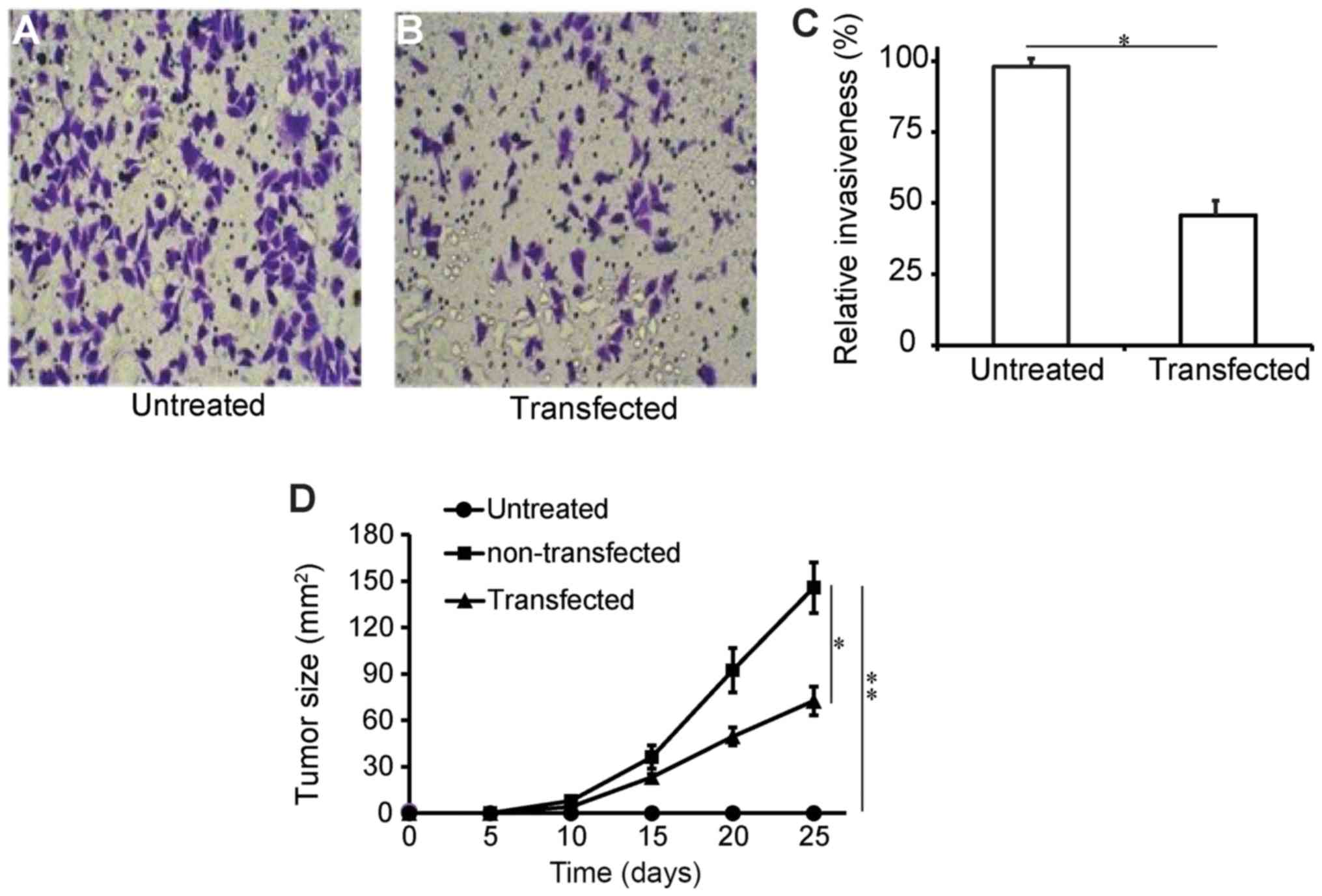
The impact of DLC-1 transfection on the invasive
capacity of SW1990 pancreatic cells was assessed using a tumor
invasion assay kit. Through fluorescence staining and microscopic
observation, it was identified that compared with the untreated
group, transfected cells exhibited significantly reduced staining
intensity, indicating lowered invasive capacity (Fig. 5A and B). It was revealed that the
transfected cells had a relative invasion capacity of 47% of the
untransfected group (Fig. 5C;
P<0.05). These data suggested that DLC-1 is associated with the
invasive capacity of pancreatic cancer. The effects of DLC-1
overexpression were also investigated using a mouse tumor model.
The longest tumor diameter was 16.8 mm, and multiple tumors were
not observed in any of the mice involved. Compared with the
untransfected cells, DCL-1-transfected cells exhibited less
progressive solid tumor growth, confirming that the DLC-1 is able
to reduce tumor invasion capacity (Fig.
5D). Conclusively, the data showed that DLC-1 was able to
retard in vivo tumor progression, suggesting a potential use
in pancreatic cancer gene therapy.
Discussion
Existing studies have focused on the
tumor-suppressing roles of DLC-1 in multiple types of cancer,
including the inhibition of tumor proliferation and metastasis
(11,12,16).
However, further investigation is required to ascertain the
tumor-suppressing mechanism of DLC-1 in the pathogenesis of
pancreatic cancer, from both a clinical aspect, and to confirm the
functions of this gene on a molecular level. Multiple studies have
reviewed the mechanisms or signaling pathways involved in
DLC-1-associated cancers (10,13,36). A
study showed that PKA modulated the Rho-GAP activity and tumor
suppressive capacity of DLC-1 (20),
while another study demonstrated that human DLC-1 interacts with
caveolin-1, identifying a caveolin-1 binding motif within DLC-1
(617FSWAVPKF624) (37). In the present study, the roles of
DLC-1 in the occurrence and development of pancreatic cancer were
investigated from both clinical and molecular aspects.
Specifically, RT-qPCR analysis confirmed that cancerous tissues
from patients with pancreatic cancer showed reduced expression
levels of DLC-1 compared with adjacent normal tissues. This
clinical information indicated that the reduced expression level of
DLC-1 was associated with pancreatic cancer progression. The role
of DLC-1 in tumors has been studied by multiple groups, focusing
predominantly on intracellular signaling pathways including Rho-Roc
and Wnt/β-catenin (23,38–40).
Multiple mechanistic studies have revealed the signaling pathways
that involve DLC-1. Multiple studies connect PKA with Rho-GAP
activity and DLC-1 function (19,39);
others have linked DLC-1 with caveolin-1, showing that reduced
DLC-1 expression levels frequently resulted in poor clinical
outcome in patients with lung cancer (41). In the present study, the focus was on
direct cellular and in vivo assessments to reveal clinical
trends associated with reduced DLC-1 gene expression in diseased
tissues. Furthermore, few studies had reported the mechanism of the
DLC-1 gene in the occurrence and development of pancreatic cancer;
thus in the present study, the roles of DLC-1 gene in pancreatic
cancer occurrence and development were assessed using clinical
samples and pancreatic cancer cell lines.
To further illustrate the role of DLC-1 genes in
pancreatic cancers, the relative expression level of DLC-1 was
determined in a number pancreatic cancer cell lines. Among these
cell lines, DLC-1 had the lowest expression level in SW1990 cells,
and this cell line was subsequently selected for further
investigation. SW1990 cells overexpressing DLC-1 were successfully
generated, which demonstrated that the increased expression of
DLC-1 altered the cell cycle, arresting a higher ratio of
pancreatic cells in the G0/G1 phases. This
indicated that DLC-1 was associated with tumor-suppression by
influencing the cell cycle. In the invasion capacity test, the
upregulation of DLC-1 reduced the invasiveness of pancreatic cancer
cells in mice bearing a pancreatic tumor.
The current study did not involve the study of
DLC-1-related signaling pathways in pancreatic cancer, which is a
limitation of the work. Continued research will investigate the
impact of DLC-1 on increased Rho and Cdc42 activity (18,19), in
addition to the therapeutic effects of DLC-1 upregulation as a
potential method of gene therapy. Additional studies may also
include the deletion of DLC-1 to investigate its effect on the
progression of pancreatic cancer in a mouse model.
In conclusion, the present study indicated that a
reduced expression level of DLC-1 may promote the development of
pancreatic cancer. Alterations in DLC-1 expression are associated
with the pancreatic cancer cell cycle, apoptosis, invasion and
metastasis. Furthermore, patients with high expression levels of
DLC-1 may exhibit improved prognosis. The present study thus
suggested that the overexpression of DLC-1 may inhibit the
development of pancreatic cancer and lays the foundations for
screening targets for the treatment of pancreatic cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shanghai
Science Foundation (grant no. PWRD2015-10).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BC and MZX performed the experiments, and were
involved in the writing and discussion of the project. MX designed
the experiments, and contributed to writing and revising the
paper.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Tongji University. All patients enrolled in the
study signed a consent form for the use of their data and tissue
samples.
Patient consent for publication
All patients signed a consent form allowing the
publication of any data associated with their tissue samples.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sandhu V, Wedge DC, Bowitz Lothe IM,
Labori KJ, Dentro SC, Buanes T, Skrede ML, Dalsgaard AM, Munthe E,
Myklebost O, et al: The genomic landscape of pancreatic and
periampullary adenocarcinoma. Cancer Res. 76:5092–5102. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang MJ, Jang JY, Kwon W and Kim SW:
Clinical significance of defining borderline resectable pancreatic
cancer. Pancreatology. 18:139–145. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhosale P, Cox V, Faria S, Javadi S,
Viswanathan C, Koay E and Tamm E: Genetics of pancreatic cancer and
implications for therapy. Abdom Radiol (NY). 43:404–414. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Treves S, Feriotto G, Moccagatta L,
Gambari R and Zorzato F: Molecular cloning, expression, functional
characterization, chromosomal localization, and gene structure of
junctate, a novel integral calcium binding protein of
sarco(endo)plasmic reticulum membrane. J Biol Chem.
275:39555–39568. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou XL, Thorgeirsson SS and Popescu NC:
Restoration of DLC-1 gene expression induces apoptosis and inhibits
both cell growth and tumorigenicity in human hepatocellular
carcinoma cells. Oncogene. 23:1308–1313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan BZ, Jefferson AM, Baldwin KT,
Thorgeirsson SS, Popescu NC and Reynolds SH: DLC-1 operates as a
tumor suppressor gene in human non-small cell lung carcinomas.
Oncogene. 23:1405–1411. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ng IO, Liang ZD, Cao L and Lee TK: DLC-1
is deleted in primary hepatocellular carcinoma and exerts
inhibitory effects on the proliferation of hepatoma cell lines with
deleted DLC-1. Cancer Res. 60:6581–6584. 2000.PubMed/NCBI
|
|
9
|
Basak P, Dillon R, Leslie H, Raouf A and
Mowat MR: The deleted in liver cancer 1 (Dlc1) tumor suppressor is
haploinsufficient for mammary gland development and epithelial cell
polarity. BMC Cancer. 15:6302015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ullmannova-Benson V, Guan M, Zhou X,
Tripathi V, Yang XY, Zimonjic DB and Popescu NC: DLC1 tumor
suppressor gene inhibits migration and invasion of multiple myeloma
cells through RhoA GTPase pathway. Leukemia. 23:383–390. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xue W, Krasnitz A, Lucito R, Sordella R,
Vanaelst L, Cordon-Cardo C, Singer S, Kuehnel F, Wigler M, Powers
S, et al: DLC1 is a chromosome 8p tumor suppressor whose loss
promotes hepatocellular carcinoma. Gene Dev. 22:1439–1444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lahoz A and Hall A: DLC1: A significant
GAP in the cancer genome. Gene Dev. 22:1724–1730. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wuestefeld T and Zender L: DLC1 and liver
cancer: The Akt connection. Gastroenterology. 139:1093–1096. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song LJ, Liu Q, Meng XR, Li ShL, Wang LX,
Fan QX and Xuan XY: DLC-1 is an independent prognostic marker and
potential therapeutic target in hepatocellular cancer. Diagn
Pathol. 11:192016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shih YP, Takada Y and Lo SH: Silencing of
DLC1 upregulates PAI-1 expression and reduces migration in normal
prostate cells. Mol Cancer Res. 10:34–39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xue YZ, Wu TL, Wu YM, Sheng YY, Wei ZQ, Lu
YF, Yu LH, Li JP and Li ZS: DLC-1 is a candidate biomarker
methylated and down-regulated in pancreatic ductal adenocarcinoma.
Tumor Biol. 34:2857–2861. 2013. View Article : Google Scholar
|
|
17
|
Popescu NC and Goodison S: Deleted in
liver cancer-1 (DLC1): An emerging metastasis suppressor gene. Mol
Diagn Ther. 18:293–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qian X, Li G, Asmussen HK, Asnaghi L, Vass
WC, Braverman R, Yamada KM, Popescu NC, Papageorge AG and Lowy DR:
Oncogenic inhibition by a deleted in liver cancer gene requires
cooperation between tensin binding and rho-specific
GTPase-activating protein activities. Proc Natl Acad Sci USA.
104:9012–9017. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qian X, Durkin ME, Wang D, Tripathi BK,
Olson L, Yang XY, Vass WC, Popescu NC and Lowy DR: Inactivation of
the Dlc1 gene cooperates with downregulation of p15INK4b and
p16Ink4a, leading to neoplastic transformation and poor prognosis
in human cancer. Cancer Res. 72:5900–5911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ko FC and Ping Yam JW: Regulation of
deleted in liver cancer 1 tumor suppressor by protein-protein
interactions and phosphorylation. Int J Cancer. 135:264–269. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ullmannova V and Popescu NC: Expression
profile of the tumor suppressor genes DLC-1 and DLC-2 in solid
tumors. Int J Oncol. 29:1127–1132. 2006.PubMed/NCBI
|
|
22
|
Dai ZS and Jin YL: Promoter methylation of
the DLC-1 gene and its inhibitory effect on human colon cancer.
Oncol Rep. 30:1511–1517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li GR, Du XL, Vass WC, Papageorge AG, Lowy
DR and Qian XL: Full activity of the deleted in liver cancer 1
(DLC1) tumor suppressor depends on an LD-like motif that binds
talin and focal adhesion kinase (FAK). Proc Natl Acad Sci USA.
108:17129–17134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Palombella S, Pirrone C, Cherubino M,
Valdatta L, Bernardini G and Gornati R: Identification of reference
genes for qPCR analysis during hASC long culture maintenance. PLoS
One. 12:e01709182017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang C, Wang J, Liu H and Fu Z: Tumor
suppressor DLC-1 induces apoptosis and inhibits the growth and
invasion of colon cancer cells through the wnt/beta-catenin
signaling pathway. Oncol Rep. 31:2270–2278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boulter N, Suarez FG, Schibeci S,
Sunderland T, Tolhurst O, Hunter T, Hodge G, Handelsman D,
Simanainen U, Hendriks E and Duggan K: A simple, accurate and
universal method for quantification of PCR. BMC Biotechnol.
16:272016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Larionov A, Krause A and Miller W: A
standard curve based method for relative real time PCR data
processing. Bmc Bioinformatics. 21:622005. View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tusher VG, Tibshirani R and Chu G:
Significance analysis of microarrays applied to the ionizing
radiation response. Proc Natl Acad Sci USA. 98:5116–5121. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Diboun I, Wernisch L, Orengo CA and
Koltzenburg M: Microarray analysis after RNA amplification can
detect pronounced differences in gene expression using limma. BMC
Genomics. 7:2522006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou X, Xu X, Wang J, Lin J and Chen W:
Identifying miRNA/mRNA negative regulation pairs in colorectal
cancer. Sci Rep. 5:129952015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang P, Qiao Y, Wang C, Ma L and Su M:
Enhanced radiation therapy with internalized polyelectrolyte
modified nanoparticles. Nanoscale. 6:10095–10099. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakano T, Kanai Y, Amano Y, Yoshimoto T,
Matsubara D, Shibano T, Tamura T, Oguni S, Katashiba S, Ito T, et
al: Establishment of highly metastatic KRAS mutant lung cancer cell
sublines in long-term three-dimensional low attachment cultures.
PLoS One. 12:e01813422017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Neuhoff V, Stamm R, Pardowitz I, Arold N,
Ehrhardt W and Taube D: Essential problems in quantification of
proteins following colloidal staining with coomassie brilliant blue
dyes in polyacrylamide gels, and their solution. Electrophoresis.
11:101–117. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Esmaeelian B, Benkendorff K, Johnston MR
and Abbott CA: Purified brominated indole derivatives from
dicathais orbita induce apoptosis and cell cycle arrest in
colorectal cancer cell lines. Mar Drugs. 11:3802–3822. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Braun AC and Olayioye MA: Rho regulation:
DLC proteins in space and time. Cell Signal. 27:1643–1651. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yam JW, Ko FC, Chan CY, Jin DY and Ng IO:
Interaction of deleted in liver cancer 1 with tensin2 in caveolae
and implications in tumor suppression. Cancer Res. 66:8367–8372.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim TY, Jackson S, Xiong Y, Whitsett TG,
Lobello JR, Weiss GJ, Tran NL, Bang YJ and Der CJ:
CRL4A-FBXW5-mediated degradation of DLC1 Rho GTPase-activating
protein tumor suppressor promotes non-small cell lung cancer cell
growth. Proc Natl Acad Sci USA. 110:16868–16873. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wong CC, Wong CM, Ko FC, Chan LK, Ching
YP, Yam JW and Ng IO: Deleted in liver cancer 1 (DLC1) negatively
regulates Rho/ROCK/MLC pathway in hepatocellular carcinoma. PLoS
One. 23:e27792008. View Article : Google Scholar
|
|
40
|
Cao X, Kaneko T, Li JS, Liu AD, Voss C and
Li SS: A phosphorylation switch controls the spatiotemporal
activation of rho GTPases in directional cell migration. Nat
Commun. 6:77212015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Du X, Qian X, Papageorge A, Schetter AJ,
Vass WC, Liu X, Braverman R, Robles AI and Lowy DR: Functional
interaction of tumor suppressor DLC1 and caveolin-1 in cancer
cells. Cancer Res. 72:4405–4416. 2012. View Article : Google Scholar : PubMed/NCBI
|