Introduction
Tumor metastasis is a principal challenge in the
treatment of cancer and it is one of the leading causes of
cancer-associated mortalities (1,2).
Prevention and treatment of tumor metastasis is a principal task in
the clinical treatment of cancer (3). Bladder cancer is the second most common
malignant tumor of the genitourinary tract and it affects >2
million patients worldwide (4). It
was predicted that the incidence of bladder cancer may
significantly increase in the near future due to an increasing
exposure to risk factors and an aging population (4). Treatment outcomes of patients with
non-metastatic bladder cancer are generally satisfactory (5); however, survival of patients with
metastatic bladder cancer remains poor (6). At present, early diagnosis and
treatment are crucial for a positive prognosis.
The phosphatase and tensin homolog (PTEN) signaling
pathway is a well-studied tumor suppression pathway in different
types of human malignancies (7). The
signaling transduction of PTEN inhibits cancer development and
progression through multiple mechanisms, including promoting cancer
cell apoptosis, and inhibiting cancer cell proliferation, migration
and invasion (7). Activation of the
PTEN signaling pathway is hypothesized to be a promising
therapeutic axis for the clinical treatment of cancer (8). Inactivation of the PTEN signaling
pathway, which is commonly observed during cancer development, is
associated with the invasive nature of bladder cancer (9). It is frequently observed that microRNA
(miRNA) interactions result in PTEN signal transduction in cancer
development (10,11). Expression of PTEN in hepatocellular
cancer was regulated by miRNA-21 (10) and miRNA-205 directly targeted PTEN to
regulate the radiotherapy resistance of human nasopharyngeal
carcinoma cells (11).
miRNA-34a (miR-34a) serves as a tumor suppressor
gene in different types of cancer (12). In bladder cancer, miR-34a inhibits
cancer cell proliferation, migration and invasion through multiple
pathways (13,14). Prior to the present study,
preliminary screening of bladder cancer-associated miRNAs using
microarray data additionally demonstrated that miR-34a was
downregulated in this disease, suggesting a possible involvement of
miR-34a in bladder cancer (data not shown). In the present study,
miR-34a was downregulated in bladder cancer and overexpression of
miR-34a decreased the metastatic capacity of cells in vitro
by activating PTEN signaling, and inhibiting cancer cell migration
and invasion.
Materials and methods
Patients
A total of 172 patients were diagnosed and treated
at The China-Japan Friendship Hospital (Beijing, China) between May
2015 and October 2017. The present study included 52 individuals
out of these patients according to specific inclusion and exclusion
criteria. The inclusion criteria were as follows: i) Patients
diagnosed through bladder biopsies; ii) patients diagnosed and
treated for the first time; and iii) patients willing to
participate. The exclusion criteria were as follows: i) Patients
complicated with other malignancies; ii) patients with other
bladder lesions; iii) patients received treatment prior to
admission; and iv) patients and/or their families refused to
participate. The patients included 38 males and 14 females, age
ranged between 25 and 66 years, with a mean age of 45.3±7.2 years.
At the same time, 124 individuals with suspected bladder lesions
received bladder biopsies at The China-Japan Friendship Hospital.
Patients with bladder lesions were excluded, resulting in 56
remaining individuals. Among these 56 individuals, 30 males and 11
females were included in the final study to match the age and sex
distributions of the patient group. Bladder biopsies and plasma
samples were obtained from the specimen library of The China-Japan
Friendship Hospital. The present study was approved by The Ethics
Committee of The China-Japan Friendship Hospital, and all patients
and healthy volunteers signed informed consent.
Cell lines, cell culture and
transfection
The present study included two urinary bladder
cancer cell lines HT-1197 and HT-1376. The two cell lines were
purchased from The American Type Culture Collection (ATCC). Cells
were cultured with Eagle's minimum essential medium (EMEM; ATCC)
containing 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA)
in an incubator at 37°C with 5% CO2. MISSION®
miRNA mimics hsa-miR-34a (5′-UGGCAGUGUCUUUAGCUGGUUGU-3′) and
MISSION® miRNA negative control 1
(5′-UGUGUGGGCAUUCUACUACACU-3′) (both from Sigma-Aldrich; Merck
KGaA) were transfected into 5×105 cells at a dose of 50
nM using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Untransfected cells were used as control cells
and cells transfected with negative control 1 miRNA were used as
the negative control cells. Subsequent experiments were performed
at 24 h post-transfection.
Reverse transcription
quantitative-polymerase chain reaction (RT-qPCR)
Extraction of miRNA from biopsies and in
vitro cultivated cells was performed using the miRNeasy kit
(Qiagen China Co., Ltd.) under the following thermal conditions:
55°C for 10 min and 80°C for 10 min. cDNA was synthesized using the
miScript II RT kit (Qiagen China Co., Ltd.). miR-34a expression was
detected by RT-qPCR using the miScript SYBR Green PCR kit (Qiagen
China Co., Ltd.) with RNU6 miRNA as the endogenous control. The
primer sequences were: miR-34a forward,
5′-TGGGCATCTCTCGCTTCATCTTCCC-3′; miR-34a reverse,
5′-GTGCTGGGGAGAGGCAGGACAG-3′; U6 forward,
5′-TCGCTTCGGCAGCACATATACT-3′; and U6 reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′. The thermocycling conditions were:
95°C for 55 sec, followed by 40 cycles of 95°C for 10 sec, 55°C for
10 sec and 72°C for 30 min. Data quantifications were normalized
using the 2−ΔΔCq method (15).
Transwell cell migration and Matrigel
invasion assays
Following transfection, expression of miR-34a was
detected by RT-qPCR and subsequent experiments were performed only
if the rate of overexpression of miR-34a was ≥200%. Cell migration
ability was evaluated using a transwell cell migration assay kit
(BD Biosciences). Cell suspensions were prepared with a final cell
density of 4×104 cells/ml. The upper chamber was filled
with 4×103 cells in 0.1 ml cell suspension and 1% FBS
was added. The lower chamber was filled with EMEM supplemented with
20% FBS. Cells were cultured in an incubator at 37°C with 5%
CO2 for 6 h. Membranes were collected and stained with
0.5% crystal violet (Sigma-Aldrich; Merck KGaA) at 25°C for 15 min.
For the invasion assays, the upper chamber membrane was pre-coated
with Matrigel (EMD Millipore). Invading and migrating cells were
counted using an optical microscope (Olympus Corporation;
magnification, ×40).
Western blotting
Radioimmunoprecipitation assay buffer (Thermo Fisher
Scientific, Inc.) was used to extract the total protein from
bladder cancer cells, according to the manufacturer's protocol. The
protein concentration was measured using a bicinchoninic acid
assay. Subsequent to denaturing, protein samples (30 µg/well) were
separated by SDS-PAGE on a 10% gel and subsequently transferred to
polyvinylidene fluoride membranes for blocking in 5% skimmed milk
for 2 h at room temperature. Rabbit anti-human primary antibodies
against PTEN (1:1,000; cat. no. ab32199; Abcam) and GAPDH (1:1,000;
cat. no. ab32199; Abcam) were used for an overnight incubation at
4°C. On the following day, the membranes were incubated with goat
anti-rabbit immunoglobulin G-horseradish peroxidase secondary
antibody (1:1,000; cat. no. MBS435036; MyBioSource, Inc.) for 1 h
at room temperature. Membranes were developed using enhanced
chemiluminescence reagent (Sigma-Aldrich; Merck KGaA) and detected
using a MYECL™ Imager (Thermo Fisher Scientific, Inc.). Data
normalization was performed using ImageJ (v1.34; National
Institutes of Health).
Statistical analysis
All data were analyzed using GraphPad Prism 6
(GraphPad Software, Inc.). All experiments were repeated 3 times.
miR-34a expression levels were compared between patients and
controls using a Mann Whitney U test. Cell migration and invasion
data are presented as the mean ± standard deviation and were
compared either by unpaired t-test (between two groups) or a
one-way analysis of variance with a post hoc Tukey's test (among
multiple groups). Associations between miR-34a expression and the
clinicopathological data of the patients with bladder cancer were
analyzed by a χ2 test. P<0.05 was considered to
indicate a statistically significant difference.
Results
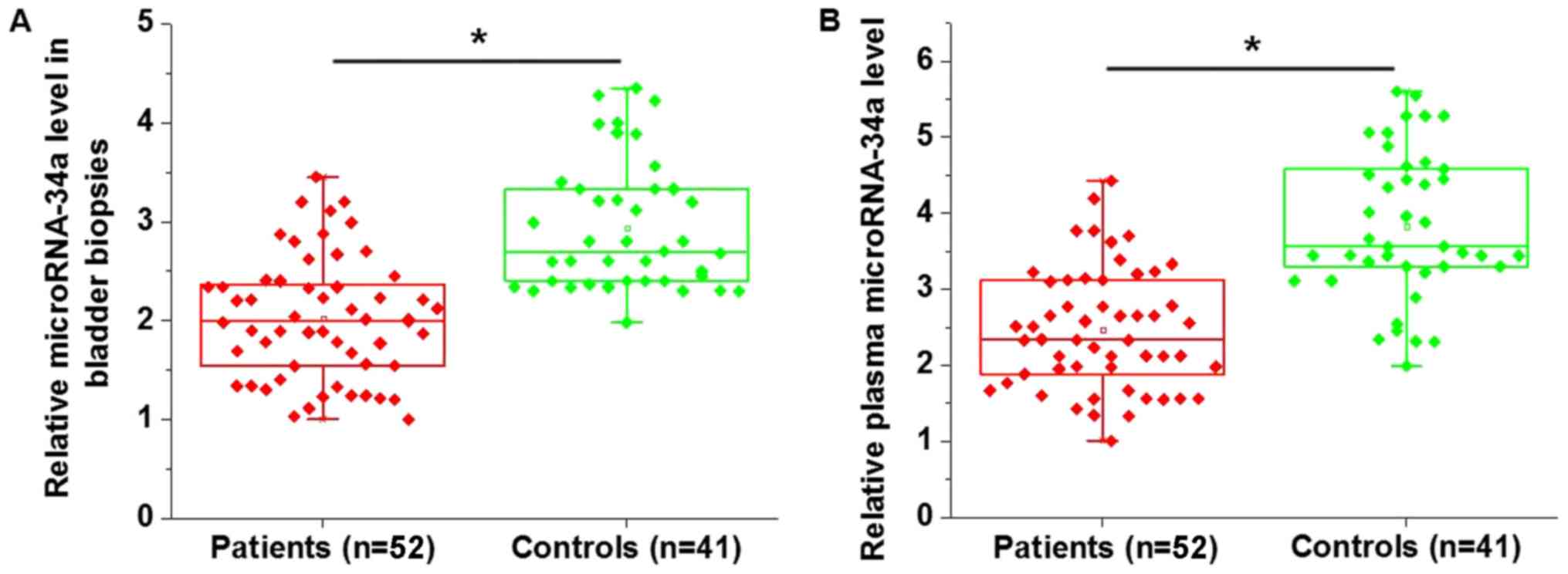
miR-34a expression is significantly
downregulated in patients with bladder cancer compared with healthy
controls in bladder biopsies and plasma
Differential expression of specific genes between
patients and healthy controls may suggest the involvement of those
genes in the corresponding diseases. The expression of miR-34a in
the bladder biopsies and plasma of patients with bladder cancer and
healthy controls were measured. miR-34a expression was
significantly downregulated in patients with bladder cancer in
bladder biopsies (P<0.05; Fig.
1A) and plasma (P<0.05; Fig.
1B) compared with the healthy controls.
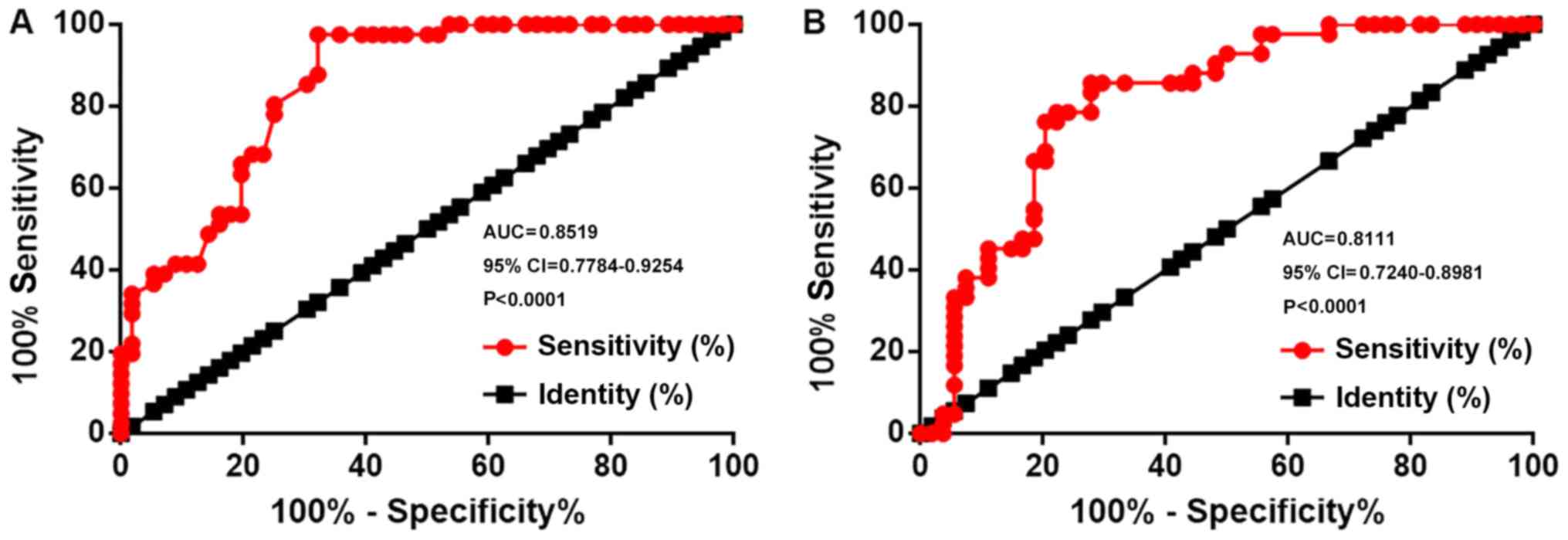
Downregulation of miR-34a
distinguishes between patients with bladder cancer and healthy
controls
Receiver operating characteristic curve analysis was
performed to evaluate the diagnostic value of miR-34a expression
for bladder cancer. For miR-34a expression in bladder biopsies, the
area under the curve (AUC) was 0.8519, with a standard error of
0.03748 and a 95% confidence interval (CI) of 0.7784–0.9254
(P<0.0001; Fig. 2A). For plasma
miR-34a, the AUC was 0.8111, with a standard error of 0.04439 and
95% CI of 0.7240–0.8981 (P<0.0001; Fig. 2B).
miR-34a expression is associated with
tumor metastasis; however, not with tumor size
Patients were divided into high- and low-level
groups according to the median levels of miR-34a in bladder
biopsies (1.99) and plasma (2.12). The associations between miR-34a
expression and the clinicopathological data of patients with
bladder cancer were analyzed by a χ2 test. The results
demonstrated that miR-34a expression levels in bladder biopsies
(Table I) were significantly
associated with the presence of tumor metastasis (P<0.05).
However, miR-34a expression levels were not associated with tumor
size, age, sex, smoking or drinking habits.
 | Table I.Association between microRNA-34a
expression in bladder biopsies and the clinicopathological data of
patients with bladder cancer. |
Table I.
Association between microRNA-34a
expression in bladder biopsies and the clinicopathological data of
patients with bladder cancer.
|
|
| MicroRNA-34a
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Cases | High | Low | χ2 | P-value |
|---|
| Sex |
|
|
| 0.39 | 0.53 |
| Male | 38 | 18 | 20 |
|
|
|
Female | 14 | 8 | 6 |
|
|
| Age, years |
|
|
| 1.23 | 0.27 |
|
>45 | 28 | 12 | 16 |
|
|
| ≤45 | 24 | 14 | 10 |
|
|
| Smoking |
|
|
| 0.75 | 0.39 |
| Yes | 33 | 15 | 18 |
|
|
| No | 19 | 11 | 8 |
|
|
| Drinking |
|
|
| 0.79 | 0.38 |
| Yes | 35 | 19 | 16 |
|
|
| No | 17 | 7 | 10 |
|
|
| Tumor size |
|
|
| 0.69 | 0.41 |
| >3
cm | 27 | 12 | 15 |
|
|
| ≤3
cm | 25 | 14 | 11 |
|
|
| Distant
metastasis |
|
|
| 9.43 | 0.002 |
| Yes | 23 | 17 | 6 |
|
|
| No | 29 | 9 | 20 |
|
|
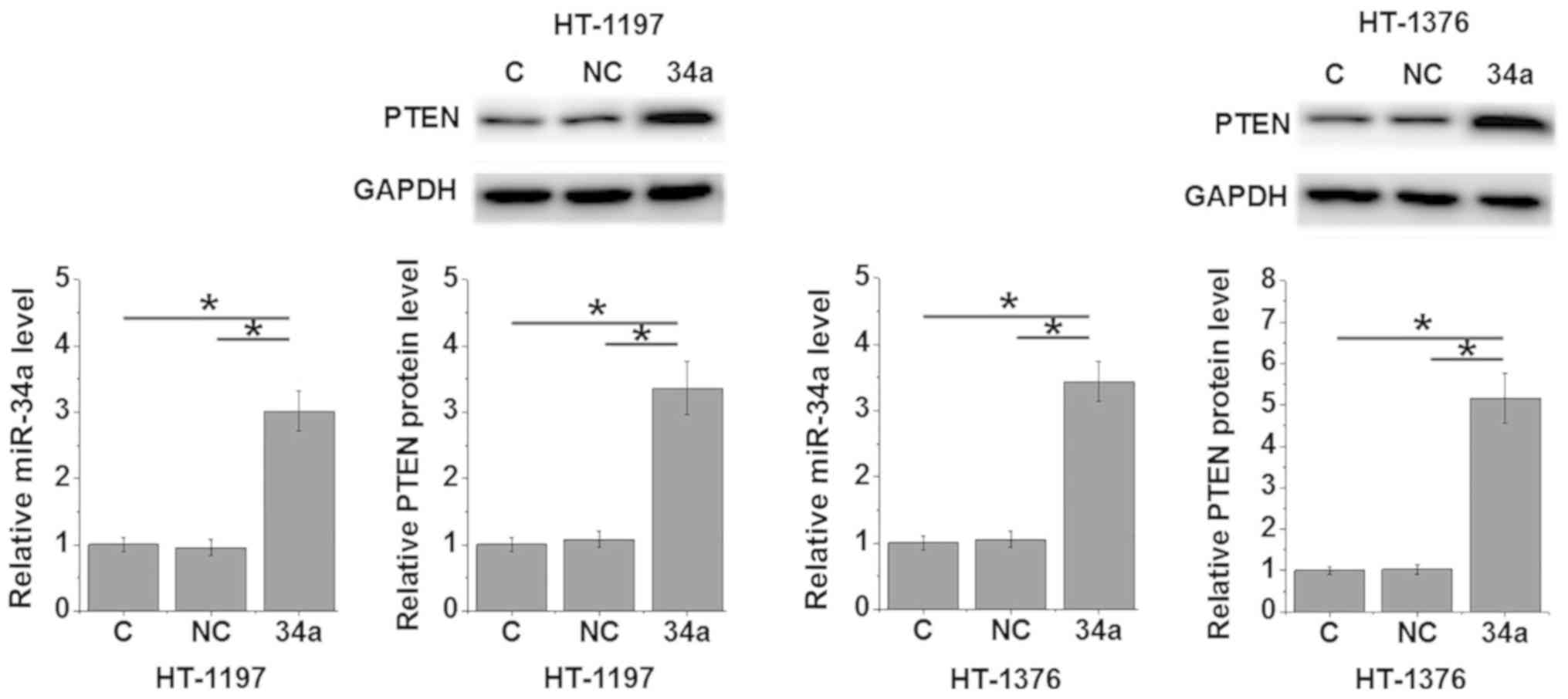
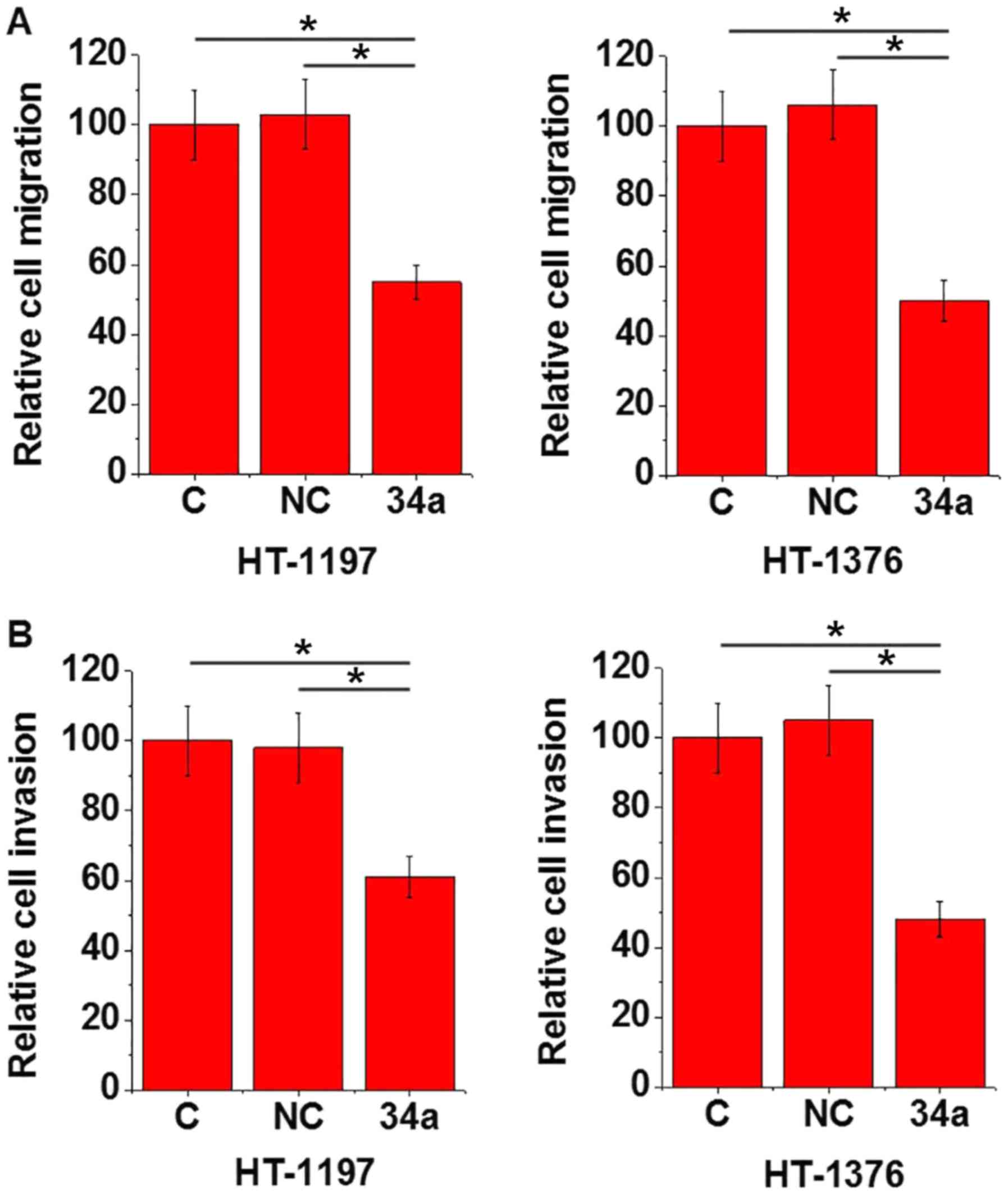
miR-34a overexpression promotes the
expression of PTEN in bladder cancer cells and promotes cell
migration and invasion
The data in Tables I
and II suggested that miR-34a may
be involved in the metastasis of bladder cancer. Inactivation of
the PTEN signaling pathway is closely associated with the invasive
nature of bladder cancer (9). In the
present study, miR-34a mimics were transfected into two bladder
cancer cell lines, HT-1197 and HT-1376, and the effects on PTEN
protein expression levels, and cell migration and invasion were
determined by western blotting, transwell migration and Matrigel
invasion assays, respectively. The results demonstrated that
compared with the control cells and the negative control cells,
cells with miR-34a overexpression demonstrated significantly
upregulated miR-34a and PTEN protein expression levels (P<0.05;
Fig. 3), and significantly decreased
cell migration and invasion (P<0.05; Fig. 4).
 | Table II.Association between plasma levels of
microRNA-34a and the clinicopathological characteristics of
patients with bladder cancer. |
Table II.
Association between plasma levels of
microRNA-34a and the clinicopathological characteristics of
patients with bladder cancer.
|
|
| MicroRNA-34a
expression |
|
|
|---|
|
|
|
|
|
|
| Clinicopathological
characteristics | Cases | High | Low | χ2 | P-value |
|---|
| Sex |
|
|
| 1.56 | 0.21 |
|
Male | 38 | 17 | 21 |
|
|
|
Female | 14 | 9 | 5 |
|
|
| Age, years |
|
|
| 2.79 | 0.10 |
|
>45 | 28 | 11 | 17 |
|
|
|
≤45 | 24 | 15 | 9 |
|
|
| Smoking |
|
|
| 0.08 | 0.77 |
|
Yes | 33 | 16 | 17 |
|
|
| No | 19 | 10 | 9 |
|
|
| Drinking |
|
|
| 0.09 | 0.77 |
|
Yes | 35 | 18 | 17 |
|
|
| No | 17 | 8 | 9 |
|
|
| Tumor size |
|
|
| 1.93 | 0.17 |
| >3
cm | 27 | 11 | 16 |
|
|
| ≤3
cm | 25 | 15 | 10 |
|
|
| Distant
metastasis |
|
|
| 6.31 | 0.11 |
|
Yes | 23 | 16 | 7 |
|
|
| No | 29 | 10 | 19 |
|
|
Discussion
The present study demonstrated that miR-34a may
serve as a tumor suppressor in bladder cancer. miR-34a may mediate
tumor suppression through the upregulation of the tumor suppressor,
PTEN, which may inhibit tumor cell migration and invasion.
Development of bladder cancer not only affects the
local tissue environment; however, additionally globally regulates
the expression levels of a larger set of genes, including miRNAs
(16). At present, numerous miRNAs
with altered expression patterns in bladder cancer exhibit
oncogenic or tumor suppressive properties. miRNA-146a-5p expression
levels were significantly upregulated in patients with bladder
cancer compared with healthy individuals, and the expression level
of this miRNA decreased following transurethral resection (17), suggesting a potential role as an
oncogenic biomarker for bladder cancer. In contrast, miRNA-144-5p
was downregulated in bladder cancer and the decreased expression
level of miRNA-144-5p in patients predicted poor survival (18). miR-34a, as a tumor suppressor, is
downregulated in the development and progression of a number of
types of cancer, such as liver and gastric cancer (12). In the present study, miR-34a
expression levels in bladder biopsies and plasma samples were
downregulated in patients with bladder cancer compared with the
healthy controls, suggesting a potential role of miR-34a as a tumor
suppressor in bladder cancer.
Treatment outcomes of cancer at advanced stages are
generally poor. At present, early diagnosis and treatment is
crucial for improving the prognosis of patients with cancer.
Alterations in gene expression affect the tumor microenvironment
(19), and thus, may be used to
guide the diagnosis of different types of cancer. In the present
study, downregulation of miR-34a in bladder biopsies and plasma may
be used to effectively distinguish between patients with bladder
cancer and the healthy controls. miR-34a expression levels were not
significantly associated with age, sex, smoking or drinking habits,
which are considered influencing factors on the expression levels
of certain miRNAs (20–22). Therefore, miR-34a may serve as a
reliable biomarker for the detection of bladder cancer. However,
miR-34a expression levels are altered in numerous types of human
diseases, such as in different types of cancer, such as lung cancer
and liver cancer (12). Therefore,
it is preferable to use multiple biomarkers to improve the accuracy
of the diagnosis.
miR-34a is involved in the regulation of tumor
growth of certain types of cancer (23); however, in the present study, no
significant association between miR-34a and tumor size was observed
in patients with bladder cancer, suggesting the possibility of
different routes of pathogenesis for different malignancies.
miR-34a may be involved in the regulation of tumor metastasis due
to the significant association between miR-34a expression levels
and the presence of distant tumor metastasis. The in vitro
cell migration and invasion assays additionally demonstrated the
inhibitory effect of miR-34a on bladder cancer cell migration and
invasion. PTEN signaling is involved in a tumor suppression pathway
and inactivation of PTEN signaling is commonly observed in
metastatic bladder cancer (9). In
the present study, PTEN expression levels were significantly
increased in bladder cancer cells following miR-34a overexpression.
However, the mechanisms of the regulatory effects of miR-34a on
PTEN or the intermediates between miR-34a and PTEN in this proposed
signaling transduction pathway are unknown and require further
study.
In conclusion, miR-34a is downregulated in bladder
cancer and has diagnostic values. miR-34a may be involved in
bladder cancer metastasis through upregulation of PTEN and
inhibiting cancer cell migration and invasion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZSD and XFZ designed experiments. ZSD, JFW, XC and
PYZ performed experiments. YHH, YSD and PXP analyzed data. ZSD, PXP
and XFZ drafted the manuscript. All authored approved this
manuscriptt.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of The China-Japan Friendship Hospital (Beijing, China).
All patients and healthy volunteers provided written informed
consent prior to their inclusion in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wan L, Pantel K and Kang Y: Tumor
metastasis: Moving new biological insights into the clinic. Nat
Med. 19:1450–1464. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ploeg M, Aben KK and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 27:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marcos-Gragera R, Mallone S, Kiemeney LA,
Vilardell L, Malats N, Allory Y and Sant M; EUROCARE-5 Working
Group, : Urinary tract cancer survival in Europe 1999–2007: Results
of the population-based study EUROCARE-5. Eur J Cancer.
51:2217–2230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mahmoud-Ahmed AS, Suh JH, Kupelian PA,
Klein EA, Peereboom DM, Dreicer R and Barnett GH: Brain metastases
from bladder carcinoma: Presentation, treatment and survival. J
Urol. 167:2419–2422. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang BH and Liu LZ: PI3K/PTEN signaling
in angiogenesis and tumorigenesis. Adv Cancer Res. 102:19–65. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keniry M and Parsons R: The role of PTEN
signaling perturbations in cancer and in targeted therapy.
Oncogene. 27:5477–5485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Puzio-Kuter AM, Castillo-Martin M, Kinkade
CW, Wang X, Shen TH, Matos T, Shen MM, Cordon-Cardo C and
Abate-Shen C: Inactivation of p53 and Pten promotes invasive
bladder cancer. Genes Dev. 23:675–680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu C, Liang Z, Huang J, Zhao R, Su C, Wang
S, Wang X, Zhang R, Lee MH and Yang H: MiR-205 determines the
radioresistance of human nasopharyngeal carcinoma by directly
targeting PTEN. Cell Cycle. 11:785–796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li XJ, Ren ZJ and Tang JH: MicroRNA-34a: A
potential therapeutic target in human cancer. Cell Death Dis.
5:e13272014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun H, Tian J, Xian W, Xie T and Yang X:
miR-34a inhibits proliferation and invasion of bladder cancer cells
by targeting orphan nuclear receptor HNF4G. Dis Markers.
2015:8792542015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang C, Yao Z, Zhu M, Ma X, Shi T, Li H,
Wang B, Ouyang J and Zhang X: Inhibitory effects of microRNA-34a on
cell migration and invasion of invasive urothelial bladder
carcinoma by targeting Notch1. J Huazhong Univ Sci Technolog Med
Sci. 32:375–382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dyrskjøt L, Ostenfeld MS, Bramsen JB,
Silahtaroglu AN, Lamy P, Ramanathan R, Fristrup N, Jensen JL,
Andersen CL, Zieger K, et al: Genomic profiling of microRNAs in
bladder cancer: miR-129 is associated with poor outcome and
promotes cell death in vitro. Cancer Res. 69:4851–4860. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sasaki H, Yoshiike M, Nozawa S, Usuba W,
Katsuoka Y, Aida K, Kitajima K, Kudo H, Hoshikawa M, Yoshioka Y, et
al: Expression level of urinary MicroRNA-146a-5p is increased in
patients with bladder cancer and decreased in those after
transurethral resection. Clin Genitourin Cancer. 14:e493–e499.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsushita R, Seki N, Chiyomaru T,
Inoguchi S, Ishihara T, Goto Y, Nishikawa R, Mataki H, Tatarano S,
Itesako T, et al: Tumour-suppressive microRNA-144-5p directly
targets CCNE1/2 as potential prognostic markers in bladder cancer.
Br J Cancer. 113:282–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Q, Hu B, Hu X, Kim H, Squatrito M,
Scarpace L, deCarvalho AC, Lyu S, Li P, Li Y, et al: Tumor
evolution of glioma-intrinsic gene expression subtypes associates
with immunological changes in the microenvironment. Cancer Cell.
33:1522018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Balaraman S, Schafer JJ, Tseng AM,
Wertelecki W, Yevtushok L, Zymak-Zakutnya N, Chambers CD and
Miranda RC: Plasma miRNA profiles in pregnant women predict infant
outcomes following prenatal alcohol exposure. PLoS One.
11:e01650812016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzuki K, Yamada H, Nagura A and Ohashi K:
Association of cigarette smoking with serum microRNA expression
among middle-aged Japanese adults. Fujita Med J. 2:1–5. 2016.
|
|
22
|
Jung HJ, Lee KP, Milholland B, Shin YJ,
Kang JS, Kwon KS and Suh Y: Comprehensive miRNA profiling of
skeletal muscle and serum in induced and normal mouse muscle
atrophy during aging. J Gerontol A Biol Sci Med Sci. 72:1483–1491.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bayraktar R, Ivan C, Bayraktar E,
Kanlikilicer P, Kabil NN, Kahraman N, Mokhlis HA, Karakas D,
Rodriguez-Aguayo C, Arslan A, et al: Dual suppressive effect of
microRNA-34a on the FOXM1/eEF2-kinase axis regulates
triple-negative breast cancer growth and invasion. Clin Cancer Res.
24:4225–4241. 2018. View Article : Google Scholar : PubMed/NCBI
|