Introduction
Tumor metastasis poses a key challenge in the
treatment of almost all types of human malignancy (1). Once metastasis occurs, surgical
resection is an unsuitable treatment strategy, and chemotherapy and
radiation therapy can only be administered to prolong the survival
of patients but not cure the disease (2). Glioma, a type of cancer, develops from
glial cells of the brain or spine and accounts for >80% of all
cases of malignant brain tumor (3).
Different types of glioma may cause different consequences
depending on the tumor location (3).
Typical clinical symptoms may include seizures, cranial nerve
disorders, vomiting, headaches and visual loss (4,5). ‘Drop
metastases’ to the spinal cord frequently occurs in patients with
glioma (6). The prevention, early
identification and treatment of metastasis are major challenges in
overcoming glioma.
Epithelial-mesenchymal transition (EMT) serves
pivotal roles in tumor metastasis (7). As a key factor of EMT, transforming
growth factor (TGF)-β signaling has been reported to serve a role
in metastasis of different cancer types (8). Previously, TGF-β has been considered as
a target for the treatment of numerous types of cancer, including
glioma (9). TGF-β signaling in
certain cases functions via interactions with long noncoding RNAs
(lncRNAs), which are a subgroup of noncoding RNAs comprising
>200 nucleotides and serve critical functions in cancer biology
(10). lncRNA AWPPH, a newly
identified lncRNA, promotes the progression of hepatocellular
carcinoma (11) and bladder cancer
(12) via different pathways. The
present study identified that AWPPH may promote glioma cell
invasion and migration by activating TGF-β signaling.
Materials and methods
Patients
A total of 68 patients with glioma were diagnosed
and treated at Beijing Shijitan Hospital (Beijing, China) between
March 2014 and January 2018. Among these patients, 48 were included
in the present study according to the inclusion and exclusion
criteria. The inclusion criteria were as follows: i) Patient
diagnosed pathologically using tumor biopsies; ii) patient treated
for the first time; and iii) patient willing to participate. The
exclusion criteria were as follows: i) Patient diagnosed with
another type of severe disease; ii) patient received treatment
prior to admission; and iii) patient >70 years. The 48 patients
included 28 males and 20 females with a mean age of 47.2±7.1 years
(range, 27–68 years). In addition, a total of 38 healthy controls
were included in the physical health examination center of the same
hospital during the same time period to match the age and sex
distributions of patients. The control group included 22 males and
16 females with a mean age of 46.2±5.1 years (range, 25–69 years).
No significant differences in age or sex were identified between
the two groups. The Ethics Committee of Beijing Shijitan Hospital
(Beijing, China) approved the present study and all participants
provided written informed consent.
Specimen collection
Tumor tissue samples (100–150 mg) were collected
from patients with glioma during tumor biopsies for pathological
examination. Blood was extracted from the elbow vein of patients
and healthy controls on the day of admission. Blood was kept in a
BD Vacutainer® Plasma Preparation Tube for 30 min,
followed by centrifugation at 1,600 × g at room temperature for 20
min to collect plasma.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and in
vitro cultivated cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). In
cases of TGF-β1 treatment, cells were first incubated with 5 or 10
ng/ml TGF-β1 (Sigma-Aldrich; Merck KGaA) for 24 h at 37°C prior to
RNA extractions. Total RNA was transcribed into complementary DNA
(cDNA) by RT using the SuperScript III Reverse Transcriptase kit
(Thermo Fisher Scientific, Inc.). The reaction conditions were as
follows: 50°C for 20 min and 80°C for 5 min. SYBR® Green
Real-Time PCR Master mix (Thermo Fisher Scientific., Inc.) was used
to prepare PCR reactions. The following primers were used in the
PCR reactions: Human lncRNA AWPPH forward,
5′-CTGGATGGTCGCTGCTTTTTA-3′ and reverse,
5′-AGGGGGATGAGTCGTGATTT-3′; and β-actin forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′.
The PCR reaction conditions were as follows: 45 sec at 95°C,
followed by 40 cycles of 12 sec at 95°C and 38 sec at 56°C. PCR
reactions were performed on CFX96 Touch™ Real-Time PCR Detection
System (Bio-Rad). Data were evaluated using the 2−ΔΔCq
method (13) to normalize the AWPPH
expression level to the endogenous control β-actin.
Enzyme-linked immunosorbent assay
(ELISA)
Plasma levels of TGF-β1 were measured using an human
TGF-β1 ELISA kit (cat. no. DY240, R&D Systems, Inc.,
Minneapolis, MN, USA), according to the manufacturer's protocol.
Plasma levels of TGF-β1 were normalized for platelet activation
using PF4 ELISA using a human PF4 ELISA Kit (ab100628, Abcam).
Cell culture and transfection
Two human glioma cell lines CCD-25Lu and Hs 683 were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). Cells were cultured in ATCC-formulated Eagle's
Minimum Essential medium (cat. no. 30-2003; ATCC) supplemented with
10% fetal bovine serum (FBS, ATCC). AWPPH cDNA was purchased from
Sangon (Shanghai, China) and inserted into the EcoR1-linearized
pIRSE2 vector (Clontech Laboratories, Inc., Mountainview, CA, USA).
AWPPH cDNA cloned using aforementioned RNA samples. Following the
same reverse transcriptions, PCR amplification was performed using
Phusion® High-Fidelity DNA Polymerase (NEB, USA) through
following thermal conditions: 1 min at 95°C, followed by 30 cycles
of 12 sec at 95°C, 10 sec at 55°C and 2 min at 72°C. Cloning primer
sequences were as follows: AWPPH forward,
5′-ACAAGTATGAAGAGAATGTCGG-3′ and reverse,
5′-AGATTTTTCCCACATGTGATTTTATTTC-3′. Lipofectamine® 2000
reagent (cat. no. 11668-019; Invitrogen; Thermo Fisher Scientific,
Inc.) was first mixed with 10 nM vector (empty vector was a
negative control, NC) and kept at room temperature for 30 min to
allow the formation of reagent-vector complexes. Subsequently, the
complexes were mixed with CCD-25Lu and Hs 683 cells and kept at
37°C for 6 h to allow transfection. The media was then immediately
replaced with fresh cell culture medium to maintain cells.
Overexpression of AWPPH was confirmed by RT-qPCR prior to
subsequent experimentation.
In vitro cell migration and invasion
assays
In vitro cell migration and invasion
abilities of CCD-25Lu and Hs 683 cells were detected by Transwell
migration and invasion assays. In cases of TGF-β inhibitor
treatment, cells were incubated with 100 nM TGF-β inhibitor
LY2109761 (Sigma-Aldrich; Merck KGaA) for 24 h at 37°C prior to
use. For the migration assay, 0.1 ml 1% cell suspension (1% FBS,
ATCC) containing 3×103 cells was added to the upper
Transwell chamber and RPMI-1640 medium (Thermo Fisher Scientific,
Inc.) containing 20% FBS (ATCC) was added to the lower chamber.
Cells were incubated at room temperature for 2 h. The membranes
were then washed and stained with 0.5% crystal violet
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 15 min at room
temperature. Cells were counted under an light microscope (40×).
Five visual fields were selected from each membrane. For the
invasion assay, the upper Transwell chamber was pre-coated with
Matrigel (cat. no. 356234; EMD Millipore, Billerica, MA, USA).
Western blot analysis
Radioimmunoprecipitation assay lysis solution
(Thermo Fisher Scientific, Inc.) was used for protein extraction
from CCD-25Lu and Hs 683 cells and protein concentrations were
measured by BSA assay. Subsequently, 10% SDS-PAGE was performed
with 25 µg denatured protein in each well. Following protein
transfer to polyvinylidene difluoride membranes, blocking was
performed with 5% skimmed milk at room temperature for 2 h. After
three washes with TBS and 0.3% Tween (TBST) buffer, the membranes
were incubated with rabbit anti-human primary antibodies against
TGF-β1 (1:1,500; cat. no. ab92486; Abcam, Cambridge, UK) and GAPDH
(1:1,200; cat. no. ab9485; Abcam) at 4°C overnight. The membranes
were then washed three times with TBST buffer, followed by
incubation with goat anti-rabbit IgG-horseradish peroxidase
secondary antibody (1:1,000; cat. no. MBS435036; MyBioSource, Inc.,
San Diego, CA, USA) for 2 h at room temperature. Subsequently, the
membranes were washed three times with TBST buffer and the Pierce
ECL Western Blotting Substrate (Thermo Fisher Scientific, Inc.) was
used to develop signals. Signals were scanned using MYECL™ Imager
(Thermo Fisher Scientific, Inc.). TGF-β1 expression was normalized
to that of GAPDH using ImageJ 1.6 software (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
GraphPad Prism 6 (GraphPad Software, Inc., La Jolla,
CA, USA) was used for all statistical analysis. Data of 3
biological replicates are presented as the mean ± standard
deviation. A Student's t-test was used for comparisons between two
groups and one-way analysis of variance followed by Tukey's test
was used for comparisons among multiple groups. Pearson's
correlation coefficient was used for correlation analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
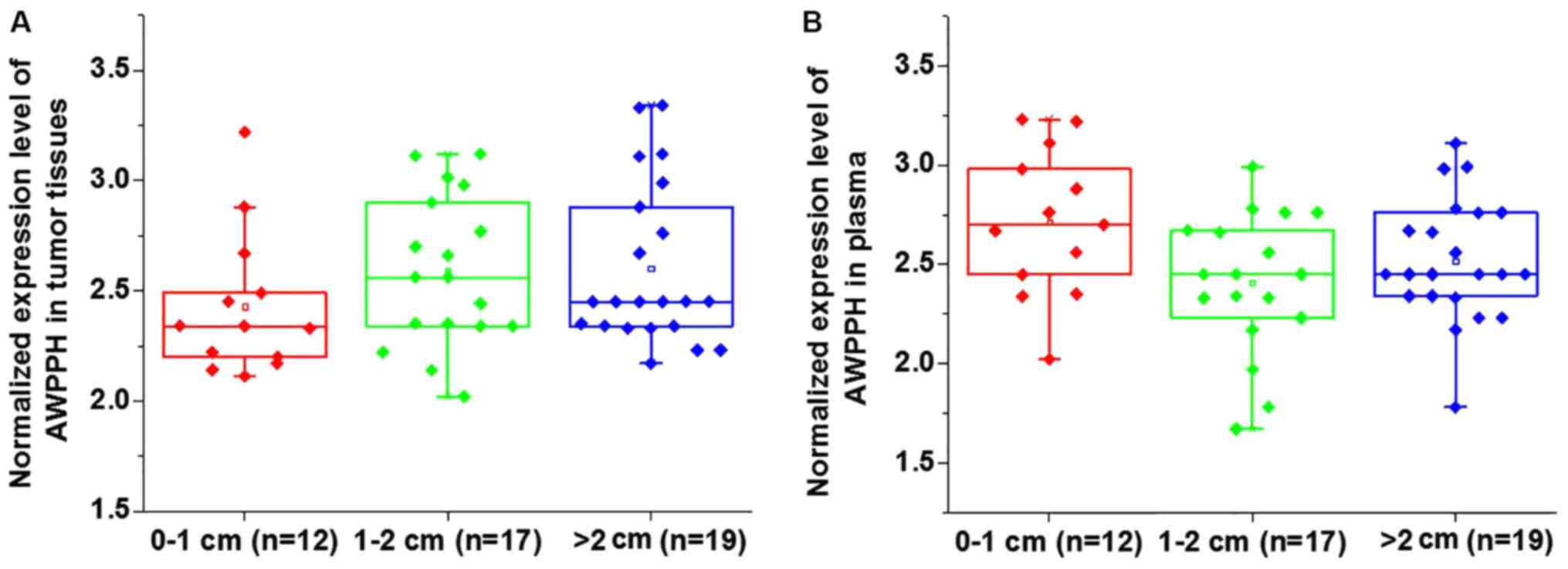
Comparison of AWPPH expression in
patients with different tumor sizes
Based on the diameter of primary tumors, 12 patients
presented with a tumor diameter between 0–1 cm, 17 patients had a
tumor diameter between 1–2 cm and 19 patients presented with a
tumor diameter >2 cm. No significant differences in the
expression of AWPPH were identified in tumor tissues (Fig. 1A) and the plasma (Fig. 1B) among patients with different tumor
sizes (P>0.05).
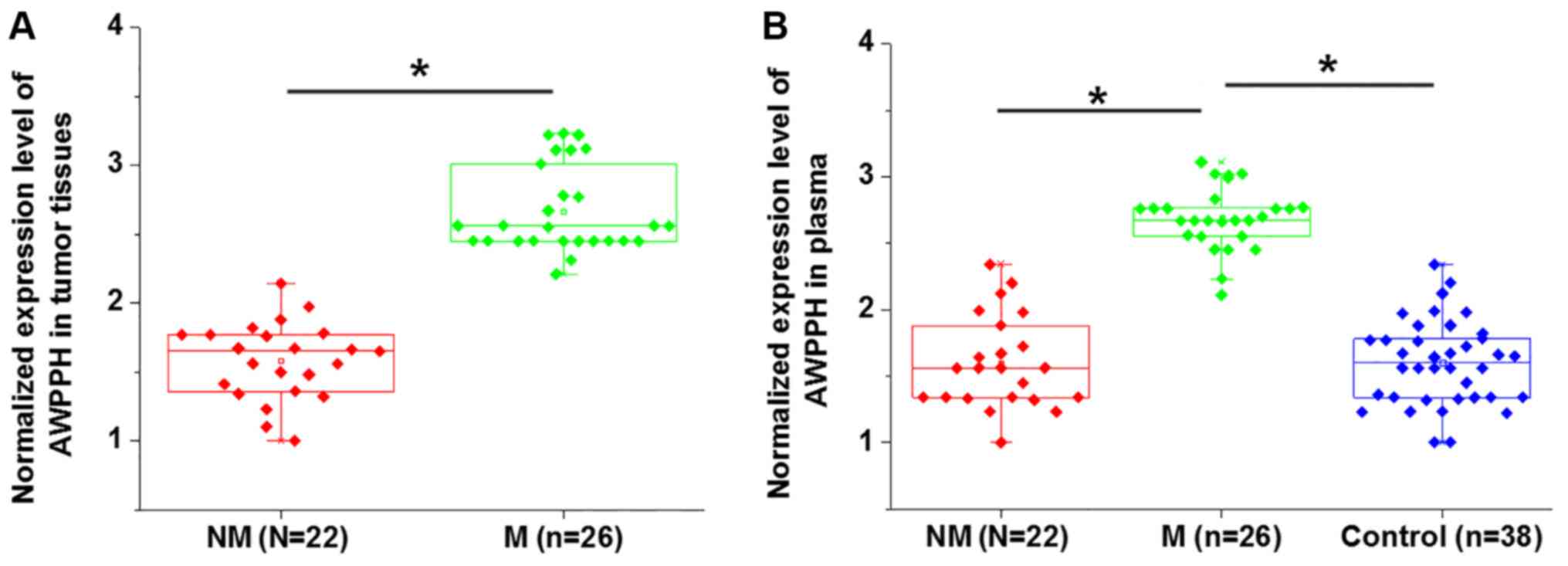
Comparison of AWPPH expression in
patients with or without distant tumor metastasis
Distant tumor metastasis was observed in 22
patients. As presented in Fig. 2,
the expression levels of AWPPH in tumor tissues (Fig. 2A) and plasma (Fig. 2B) were significantly higher in
patients with metastatic glioma compared with patients with
non-metastatic glioma (P<0.05). In addition, the plasma levels
of AWPPH were significantly increased in patients with metastatic
glioma compared with the controls and non-metastatic patients,
while no significant differences in the plasma levels of AWPPH were
identified between the non-metastatic glioma and control groups
(P<0.05; Fig. 2B).
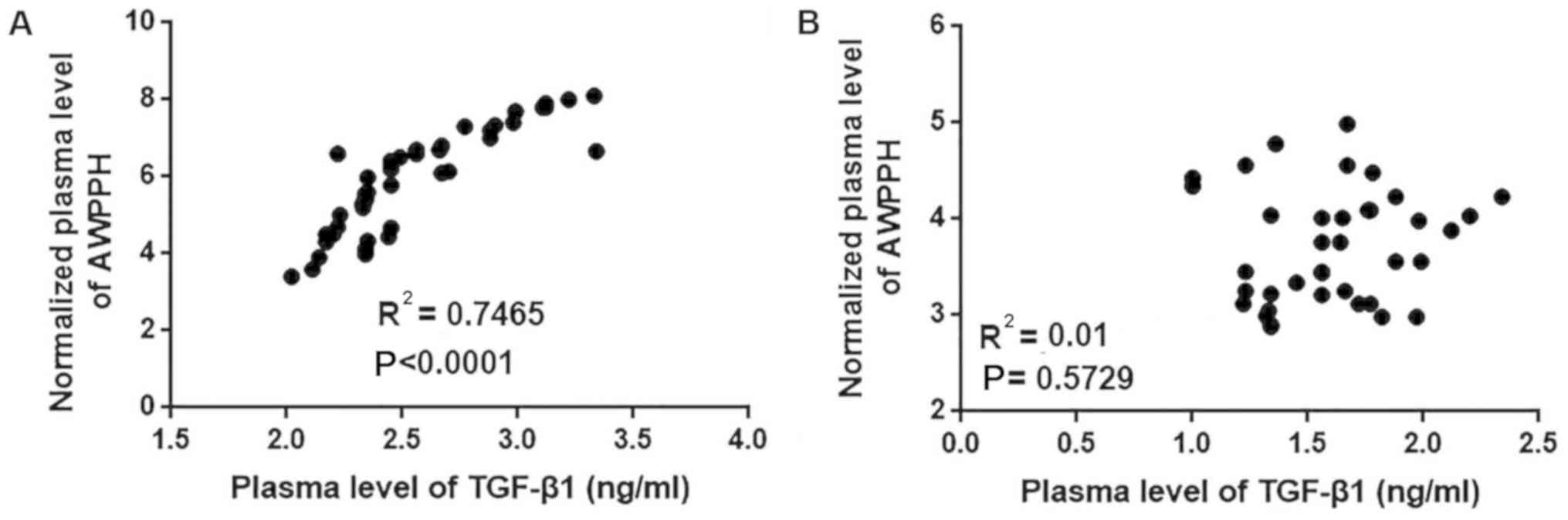
Correlation between plasma levels of
AWPPH and TGF-β1 in patients with glioma and healthy controls
The current data suggest that AWPPH is likely
involved in the regulation of tumor metastasis but not tumor growth
in glioma. TGF-β signaling serves pivotal roles in the metastasis
of different types of cancer, including glioma (14). Therefore, the correlation between the
plasma levels of AWPPH and TGF-β1 was analyzed using all plasma
samples. As presented in Fig. 3, the
plasma levels of AWPPH were positively correlated with the plasma
levels of TGF-β1 in patients with glioma (P<0.0001; Fig. 3A) but not in healthy controls
(P=0.5927; Fig. 3B).
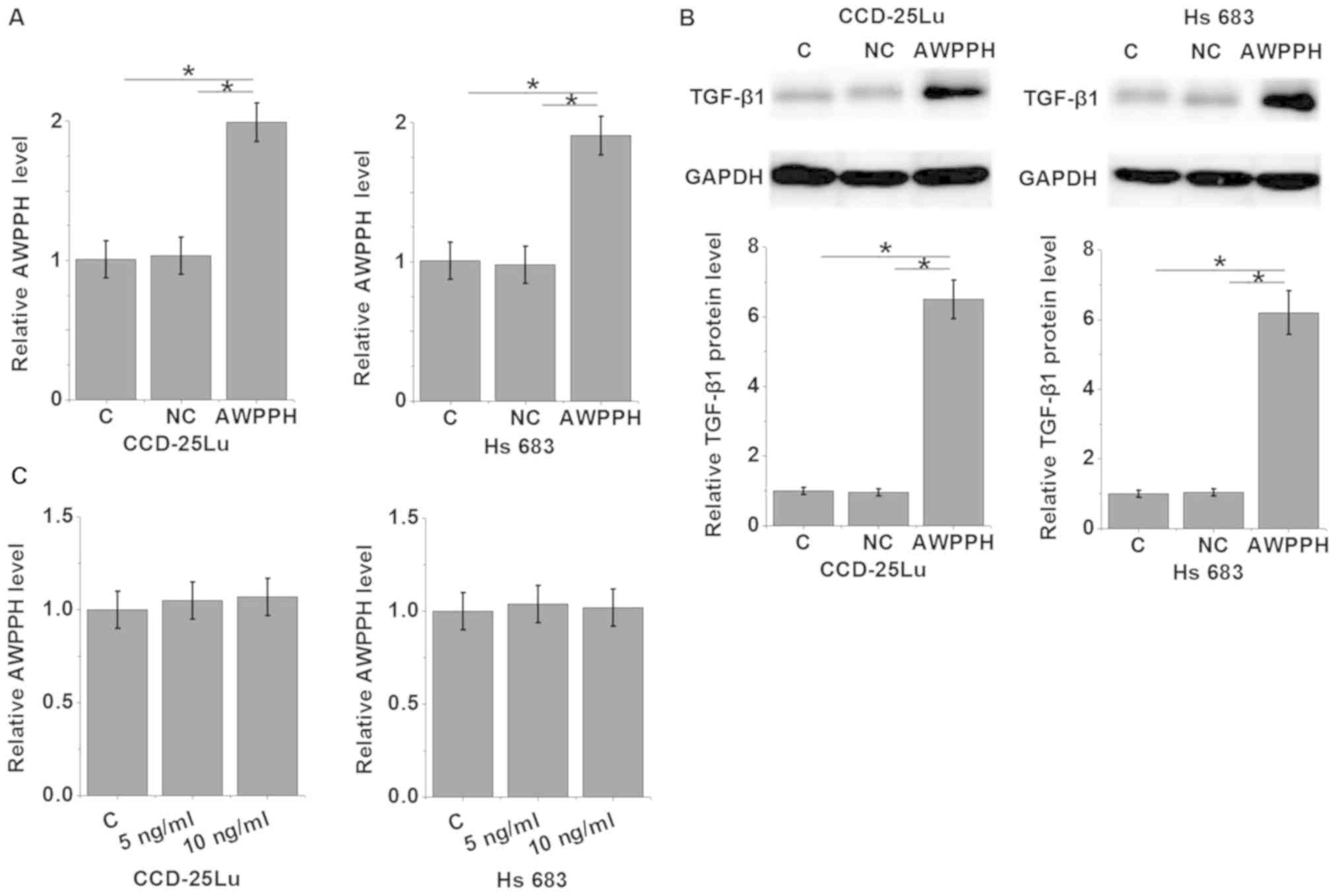
AWPPH overexpression promotes the
expression of TGF-β1 in glioma cells
To further investigate the association between AWPPH
and TGF-β1 in glioma, an AWPPH expression vector was transfected
into the human glioma cell lines CCD-25Lu and Hs 683 (P<0.05;
Fig. 4A), and the expression of
TGF-β1 was detected by western blot analysis (P<0.05; Fig. 4B). The results revealed that compared
with control group and negative control group, AWPPH overexpression
significantly promoted the expression of TGF-β1 in CCD-25Lu and Hs
683 cells (P<0.05; Fig. 4B). By
contrast, treatment with 5 or 10 ng/ml TGF-β1 (Sigma-Aldrich; Merck
KGaA) demonstrated no significant effects on AWPPH expression in
CCD-25Lu and Hs 683 cells (P>0.05; Fig. 4C).
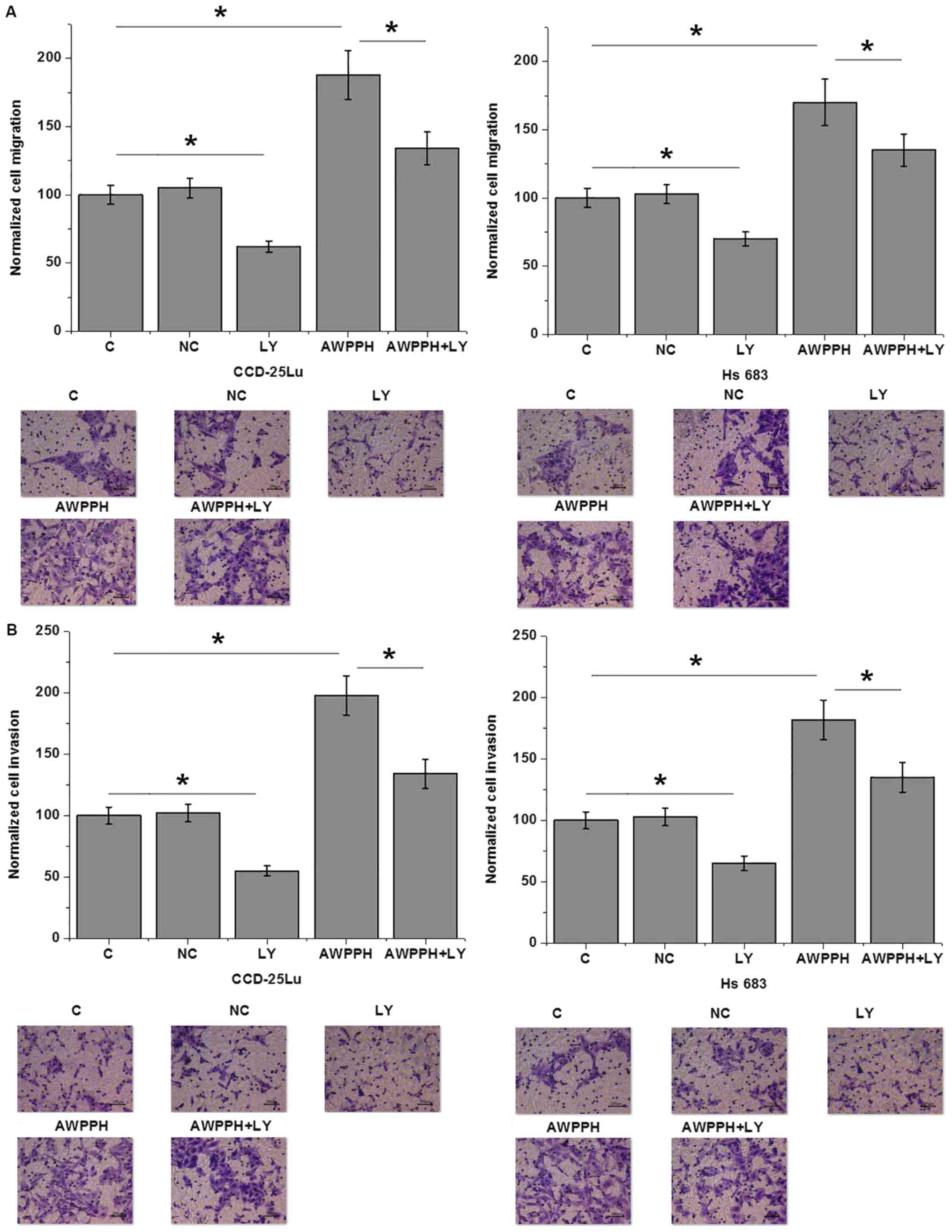
AWPPH overexpression promotes the
migration and invasion of glioma cells
The present data suggest that AWPPH is likely
involved in the metastasis of glioma. Therefore, Transwell
migration and invasion assays were performed to investigate the
effects of AWPPH on the migration and invasion of in vitro
cultured cells. As presented in Fig.
5, compared with the control (C) group, AWPPH overexpression
significantly enhanced the migration (Fig. 5A) and invasion (Fig. 5B) of the human glioma cell lines
CCD-25Lu and Hs 683. In addition, treatment with 100 nM TGF-β
inhibitor LY2109761 (Sigma-Aldrich; Merck KGaA) significantly
inhibited cell migration and invasion compared with the control,
and significantly attenuated the effects of AWPPH overexpression on
the migration (Fig. 5A) and invasion
(Fig. 5B) of glioma cells.
Discussion
It is understood that the lncRNA AWPPH, a newly
identified lncRNA, promotes the progression of hepatocellular
carcinoma (11) and bladder cancer
(12) via interactions with
different signaling molecules. The key finding of the present study
is that AWPPH may serve an oncogenic role in glioma. The molecular
mechanism of AWPPH is likely associated with the activation of
TGF-β signaling.
In a study investigating hepatocellular carcinoma,
Zhao et al (11) identified
AWPPH as a novel lncRNA that is significantly upregulated in cancer
tissues compared with healthy liver tissues. In another study,
significantly upregulated expression levels of AWPPH were observed
in bladder cancer tissues and cells compared with normal bladder
tissues and cells, which indicates a potential oncogenic role of
AWPPH in bladder cancer. In contrast to the aforementioned
findings, the present study investigated the differential
expression of AWPPH in patients with different tumor sizes and
patients with or without distant tumor metastasis. This strategy
was selected as the expression levels of AWPPH were not
significantly different in numerous patients with glioma compared
with the healthy controls. In addition, a simple comparison between
healthy controls and patients with glioma is insufficient to reveal
the role of AWPPH in a specific process of tumor progression,
including tumor growth and metastasis. The present study revealed
that AWPPH is not differentially expressed in patients with
different tumor sizes. However, patients with metastatic glioma
exhibited significantly higher AWPPH expression levels compared
with patients with non-metastatic glioma. This suggests that AWPPH
is involved in the metastasis of glioma.
TGF-β signaling inhibits tumor growth at the initial
stage of cancer development but promotes tumor metastasis via EMT
at later stages (15). Differential
expression of TGF-β isoforms has also been observed in different
stages of glioma (16). It has been
reported that TGF-β may serve as a potential target for the
treatment of glioma (14). The cross
talk between TGF-β and lncRNAs has been extensively reported in
different types of malignancy (17–19).
However, to the best of our knowledge, studies regarding the
interactions between TGF-β and lncRNAs are relatively rare. The
present study identified a significant positive correlation between
the plasma levels of TGF-β1 and AWPPH in patients with glioma. In
addition, in vitro experiments demonstrated that AWPPH may
be an upstream activator of TGF-β1 in glioma. AWPPH overexpression
upregulated TGF-β1 expression in human glioma cells, while TGF-β1
treatment demonstrated no significant effects on AWPPH expression.
Additionally, treatment with the TGF-β inhibitor LY2109761
significantly inhibited cell migration and invasion, and attenuated
the effects of AWPPH overexpression. These data also suggest that
inhibition of endogenous TGF-β may serve as a therapeutic target
for glioma.
No significant correlation between plasma TGF-β1 and
AWPPH levels was observed in healthy controls. Therefore, the
present study did not include a normal human brain tissue cell line
as a control. This also suggests that the regulatory role of AWPPH
on TGF-β1 is glioma-specific or specific to disease conditions.
Notably, LINC00152, a homolog of AWPPH, promotes the invasion of
glioblastoma via a 3′-hairpin structure, which serves as a
protein-binding site (20). This is
consistent with the results of the present study.
In conclusion, it was identified that AWPPH is
upregulated in glioma. Overexpression of AWPPH may promote the
migration and invasion of glioma cells by activating TGF-β
signaling. However, the present study is limited by a small sample
size. Studies with larger sample sizes are required to further
validate the current conclusions. In addition, we only demonstrated
possible AWPPH-TGF-β1 sequential signaling in glioma, whether this
interaction occurs in a direct or indirect manner requires further
investigation.
Acknowlegements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request
Authors' contributions
BD, ZX and ZH conceived and designed the study. BD,
ZX, BM, GZ, HH, FG, HS, ZL, WP performed the experiments. BD, ZX,
GZ, HH, FG, HS, ZL and WP wrote the paper. BD, ZX, BM, ZX, BM and
GZ reviewed and edited the manuscript. All authors read and
approved the manuscript.
Ethics approval and consent to
partcipate
The Ethics Committee of Beijing Shijitan Hospital
(Beijing, China)approved the present study and all participants
provided written informed consent.
Patient consent for publication
All individuals involved in this study provided
written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mishra MV, Andrews DW, Glass J, Evans JJ,
Dicker AP, Shen X and Lawrence YR: Characterization and outcomes of
optic nerve gliomas: A population-based analysis. J Neurooncol.
107:591–597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stark AM, van de Bergh J, Hedderich J,
Mehdorn HM and Nabavi A: Glioblastoma: Clinical characteristics,
prognostic factors and survival in 492 patients. Clin Neurol
Neurosurg. 114:840–845. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heerboth S, Housman G, Leary M, Longacre
M, Byler S, Lapinska K, Willbanks A and Sarkar S: EMT and tumor
metastasis. Clin Transl Med. 4:62015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Katsuno Y, Lamouille S and Derynck R:
TGF-β signaling and epithelial-mesenchymal transition in cancer
progression. Curr Opin Oncol. 25:76–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Joseph JV, Balasubramaniyan V, Walenkamp A
and Kruyt FA: TGF-β as a therapeutic target in high grade
gliomas-promises and challenges. Biochem Pharmacol. 85:478–485.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao X, Liu Y and Yu S: Long noncoding RNA
AWPPH promotes hepatocellular carcinoma progression through YBX1
and serves as a prognostic biomarker. Biochim Biophys Acta Mol
Basis Dis. 1863:1805–1816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu F, Zhang X, Yu Q, Han G, Diao F, Wu C
and Zhang Y: LncRNA AWPPH inhibits SMAD4 via EZH2 to regulate
bladder cancer progression. J Cell Biochem. 119:4496–4505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han J, Alvarez-Breckenridge CA, Wang QE
and Yu J: TGF-β signaling and its targeting for glioma treatment.
Am J Cancer Res. 5:945–955. 2015.PubMed/NCBI
|
|
15
|
Mirzaei H and Faghihloo E: Viruses as key
modulators of the TGF-β pathway; a double-edged sword involved in
cancer. Rev Med Virol. 28:2018.doi: 10.1002/rmv.1967. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kjellman C, Olofsson SP, Hansson O, Von
Schantz T, Lindvall M, Nilsson I, Salford LG, Sjögren HO and
Widegren B: Expression of TGF-beta isoforms, TGF-beta receptors,
and SMAD molecules at different stages of human glioma. Int J
Cancer. 89:251–258. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li C, Wan L, Liu Z, Xu G, Wang S, Su Z,
Zhang Y, Zhang C, Liu X, Lei Z and Zhang HT: Long non-coding RNA
XIST promotes TGF-β-induced epithelial-mesenchymal transition by
regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer.
Cancer Lett. 418:185–195. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Z, Dong M, Fan D, Hou P, Li H, Liu L,
Lin C, Liu J, Su L, Wu L, et al: LncRNA ANCR down-regulation
promotes TGF-β-induced EMT and metastasis in breast cancer.
Oncotarget. 8:67329–67343. 2017.PubMed/NCBI
|
|
19
|
Zhao JJ, Hao S, Wang LL, Hu CY, Zhang S,
Guo LJ, Zhang G, Gao B, Jiang Y, Tian WG and Luo DL: Long
non-coding RNA ANRIL promotes the invasion and metastasis of
thyroid cancer cells through TGF-β/Smad signaling pathway.
Oncotarget. 7:57903–57918. 2016.PubMed/NCBI
|
|
20
|
Reon BJ, Takao Real Karia B, Kiran M and
Dutta A: LINC00152 promotes invasion through a 3′-Hairpin structure
and associates with prognosis in glioblastoma. Mol Cancer Res.
16:1470–1482. 2018. View Article : Google Scholar : PubMed/NCBI
|