Introduction
Bladder cancer is the ninth most prevalent type of
cancer worldwide (1). In 2012, there
were 430,000 new cases of bladder cancer and 165,000 deaths as a
result of the disease worldwide (2),
with >60% of new bladder cancer cases and approximately one half
of deaths occurring in less developed regions (2). The clinical treatment of bladder cancer
is challenged by tumor metastasis in patients with advanced stages
of the disease (3). Although
surgical resection usually results in satisfactory outcomes in
patients with early stage bladder cancer (4), high postoperative recurrence rates lead
to unacceptably high mortality rates (5). Therefore, preventing recurrence
following treatment is critical for the survival of patients with
bladder cancer.
Rho-associated protein kinase 2 (ROCK2) is a member
of the protein kinase A/G/C family of serine-threonine kinases
involved in cell movement by regulating the cytoskeleton (6). Due to the regulatory roles in cellular
behavior, ROCK2 has been demonstrated to be a key player in human
cancer (7), and the inhibition of
ROCK2 is considered a promising therapeutic mechanism for cancer
treatment (8). ROCK2 participates in
the development of cancer through interactions with multiple
factors, including long non-coding RNAs (lncRNAs) (9), which are a group of non-protein coding
RNAs involved in almost all critical aspects of cancer biology
(10). Long intergenic non-protein
coding RNA 1638 (LINC01638) promotes breast cancer (11), while its roles in other human
diseases are currently unknown. The preliminary RNA-sequencing data
of the present study revealed the overexpression of LINC01638 in
bladder cancer tissues and its inverse correlation with ROCK2 mRNA
(data not shown), indicating the potential role of LINC01638 in
bladder cancer. The aim of the present study was to investigate the
potential interaction between LINC01638 and ROCK1 in bladder
cancer.
Materials and methods
Patients and cell lines
A total of 88 patients with bladder cancer from The
Second People's Hospital of Liaocheng (Linqing, China), The
People's Hospital of Liqing (Linqing, China) and Shanxi Provincial
People's Hospital Urology Surgery (Taiyuan, China) between March
2013 and March 2015 were analyzed in the present study. The
inclusion criteria were as follows: i) Bladder cancer confirmed by
pathological biopsy; ii) stage I or II bladder cancer; iii)
patients appropriate for surgical resection (based on their health
conditions and clinical stages); and iv) patients who had complete
3-year follow-up data. The exclusion criteria were as follows: i)
Patients diagnosed with multiple diseases; ii) patients that were
treated prior to admission; and iii) patients that failed to
complete the follow-up or died before the diagnosis of recurrence.
The 88 patients included 60 males and 28 females, and age ranged
from 36–67 years, with a mean age of 45.4±4.3 (standard deviation)
years. In addition, 68 healthy volunteers that had received
systemic physiological examinations in The Second People's Hospital
of Liaocheng, The People's Hospital of Liqing or Shanxi Provincial
People's Hospital Urology Surgery during the same time period were
included to serve as the control (C) group. The C group included 47
males and 21 females, and age ranged from 33–69 years, with a mean
age of 46.2±5.1 (standard deviation) years. The patient and C
groups all had similar age and sex distributions (revealed using
χ2 analysis). This study was approved by the Ethics
Committee of The Second People's Hospital of Liaocheng, The
People's Hospital of Liqing and Shanxi Provincial People's Hospital
Urology Surgery. All patients included in the study provided
written informed consent.
The two human bladder cancer cell lines HT-1197 and
HT-1376 (American Type Culture Collection; ATCC) were used in the
present study to perform all in vitro cell experiments.
Cells were cultivated in Eagle's minimum essential medium
containing 10% FBS (both ATCC) at 37°C in a humidified incubator
with 5% CO2.
Follow-up and specimens
Fasting blood (5 ml) was extracted from the elbow
vein of each participant 1 day after admission (before treatment).
All patients with bladder cancer received surgical resection and
were followed up for 3 years after discharge. Fasting blood (5 ml)
was extracted from patients on the day of discharge, or on the day
of the diagnosis of recurrence or at the end of follow-up in cases
of non-recurrence (NR). Plasma samples were prepared by
centrifuging the blood samples in EDTA tubes at room temperature
for 10 min at 1,200 × g.
Reverse transcription-quantitative PCR
(RT-qPCR)
To detect the expression of LINC01638 and ROCK2
mRNA, total RNA was extracted from plasma, and HT-1197 and HT-1376
cells using TRIzol® reagent (Thermo Fisher Scientific,
Inc.), RT was performed using SuperScript III Reverse Transcriptase
kit (Thermo Fisher Scientific, Inc.) at the following conditions:
50°C for 20 min and 80°C for 10 min. qPCR were prepared using
SYBR® Green PCR Master Mix (Thermo Fisher Scientific,
Inc.) and the following thermocycling conditions were used: Initial
denaturation at 95°C for 1 min; 40 cylcles of 95°C for 10 sec and
58°C for 40 sec. Primers for LINC01638, ROCK2 and endogenous
control GAPDH were synthesized by Invitrogen (Thermo Fisher
Scientific, Inc.). The following primer sequences were used: ROCK2
forward, 5′-CTAGGCCGGGCGAAG-3 and reverse
5′-TCCAGCTTCCTCTGACGAC-3′; LINC01638 forward,
5′-AATACATCAGCACTGTTGCCTTT-3′ and reverse
5′-CTCCATACATACATCTCCAAAAAGT-3′; GAPDH forward,
5′-AAGGTGAAGGTCGGAGTCAA-3′ and reverse 5′-AATGAAGGGGTCATTGATGG-3′.
The 2−ΔΔCq method (12)
was used to normalize the expression of LINC01638 and ROCK2 to
endogenous control GAPDH.
Vectors and cell transfection
Vectors expressing LINC01638 or ROCK2 and empty
vectors were constructed by Sangon Biotech Co., Ltd using the
pcDNA3.1 vector as the primary vector. ROCK2 small interfering
(si)RNA (human; cat. no. sc-29474) and negative control (NC) siRNA
(cat. no. sc-37007) were purchased from Santa Cruz Biotechnology,
Inc. Lipofectamine® 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to perform all cell transfections
with 10 nM vectors or 50 nM siRNA and using aforementioned cell
lines at a density of 1×106 cells in 2 ml cell
suspension. Treatment with Lipofectamine® 2000 reagent
alone was used as the C group and transfection with empty vectors
or NC siRNA was used as the NC group. Cells were used for
subsequent experiments when LINC01638 and ROCK2 overexpression
rates were >200% and ROCK2 knockdown rate was <50%, only. The
time interval between transfection and subsequent experimentation
was 24 h.
In vitro migration and invasion
assay
Following transfection, cell suspensions were
prepared and cell density was adjusted to 5×104 cells/ml
using serum-free cell culture medium (as aforementioned). Cell
suspensions were transferred to the upper chamber at 0.1 ml per
well, while the lower chamber was filled with cell culture medium
containing 20% FBS (Sigma-Aldrich; Merck KGaA). Cells were cultured
for 2 h, followed by staining of the upper chamber membrane with
0.5% crystal violet (Sigma-Aldrich; Merck KGaA) for 20 min at room
temperature. Cell migration and invasion assays were performed in
the same way except that the upper chamber was pre-coated with
Matrigel (cat. no. 356234; Corning Incorporated) prior to the
invasion assay. Invading and migrating cells were observed under a
light microscope (×40 magnification) and 5 visual fields were
selected to count cells.
Western blot analysis
To detect the expression levels of the ROCK2
protein, total protein was extracted using RIPA solution (Thermo
Fisher Scientific, Inc.), protein concentration was determined by
bicinchoninic acid assay and all protein samples were separated via
SDS-PAGE (10% gel) electrophoresis (30 µg per well) following
denaturation. Following this, the proteins were transferred to PVDF
membranes, which were blocked in 5% skimmed milk in PBS membrane at
room temperature for 2 h. Subsequently, membranes were further
incubated with primary antibodies against rabbit anti-human ROCK2
(1:1,200; cat. no. ab71598; Abcam) and anti-GAPDH (1:1,200; cat.
no. ab9485; Abcam) for 12 h at 4°C, followed by incubation with
IgG-horseradish peroxidase secondary antibody (1:1,200; cat. no.
MBS435036; MyBioSource, Inc.) for 2 h at room temperature. Signals
were developed using ECL (Sigma-Aldrich; Merck KGaA) and analyzed
using ImageJ software v1.46 (National Institutes of Health).
Statistical analysis
All experiments were performed in 3 independent
biological replicates and data are expressed as the mean ± standard
deviation. Comparisons between patients and controls were performed
using an unpaired t-test. Comparisons between the pre-treatment and
follow-up data within the same group were performed using a paired
t-test. Comparisons among multiple groups were performed using
one-way ANOVA and Tukey's test. Receiver operating characteristic
(ROC) curve analysis was performed to evaluate the diagnostic value
of plasma LINC01638 and ROCK2 mRNA for bladder cancer. Correlation
between the expression levels of LINC01638 and ROCK2 were performed
using Pearson's correlation coefficient. P<0.05 was considered
to indicate a statistically significant difference.
Results
Plasma LINC01638 and ROCK2 mRNA is
upregulated in patients with bladder cancer
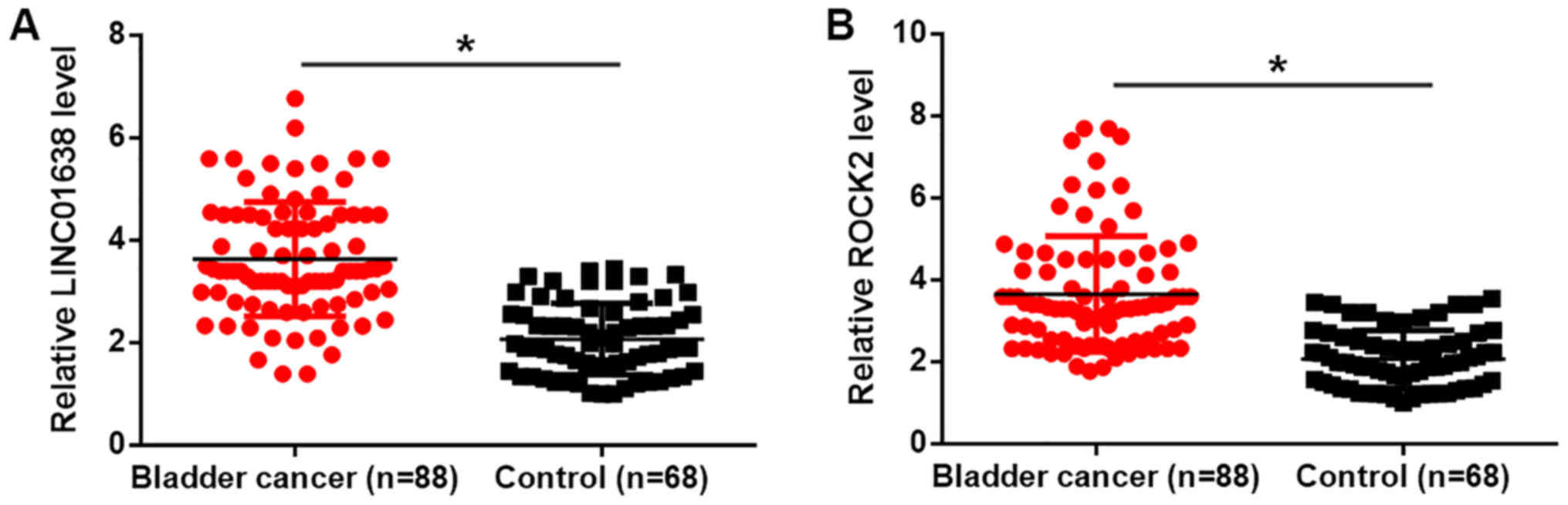
RT-qPCR was performed to detect the plasma LINC01638
and ROCK2 mRNA in patients with bladder cancer and healthy
controls. As presented in Fig. 1,
compared with healthy controls, plasma LINC01638 (Fig. 1A) and ROCK2 mRNA (Fig. 1B) were significantly higher in
patients with bladder cancer (P<0.05). This data suggests that
overexpression of LINC01638 and ROCK2 may have a role in bladder
cancer.
Altered plasma levels of LINC01638 and
ROCK2 mRNA distinguish patients with bladder cancer from healthy
controls
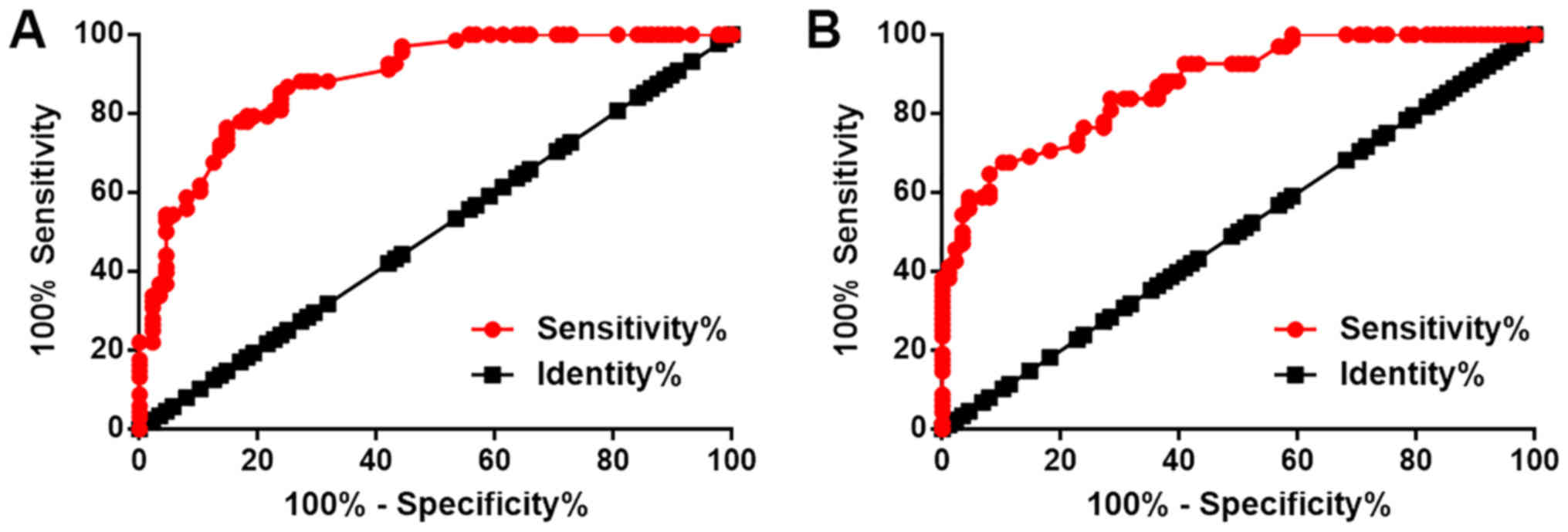
A ROC curve analysis was performed to evaluate the
diagnostic value of plasma LINC01638 and ROCK2 mRNA for bladder
cancer, with bladder cancer as true positive cases and healthy
controls as true negative cases. For plasma LINC01638, the area
under the curve was 0.88, with a standard error of 0.026 and 95%
confidence interval of 0.83–0.94 (Fig.
2A). For plasma ROCK2, area under the curve was 0.87, with a
standard error of 0.027 and 95% confidence interval of 0.82–0.93
(Fig. 2B). Therefore, plasma
LINC01638 and ROCK2 mRNA may serve as diagnostic biomarkers for
bladder cancer.
Plasma levels of LINC01638 and ROCK2
are positively correlated in bladder cancer patients but not in
healthy controls
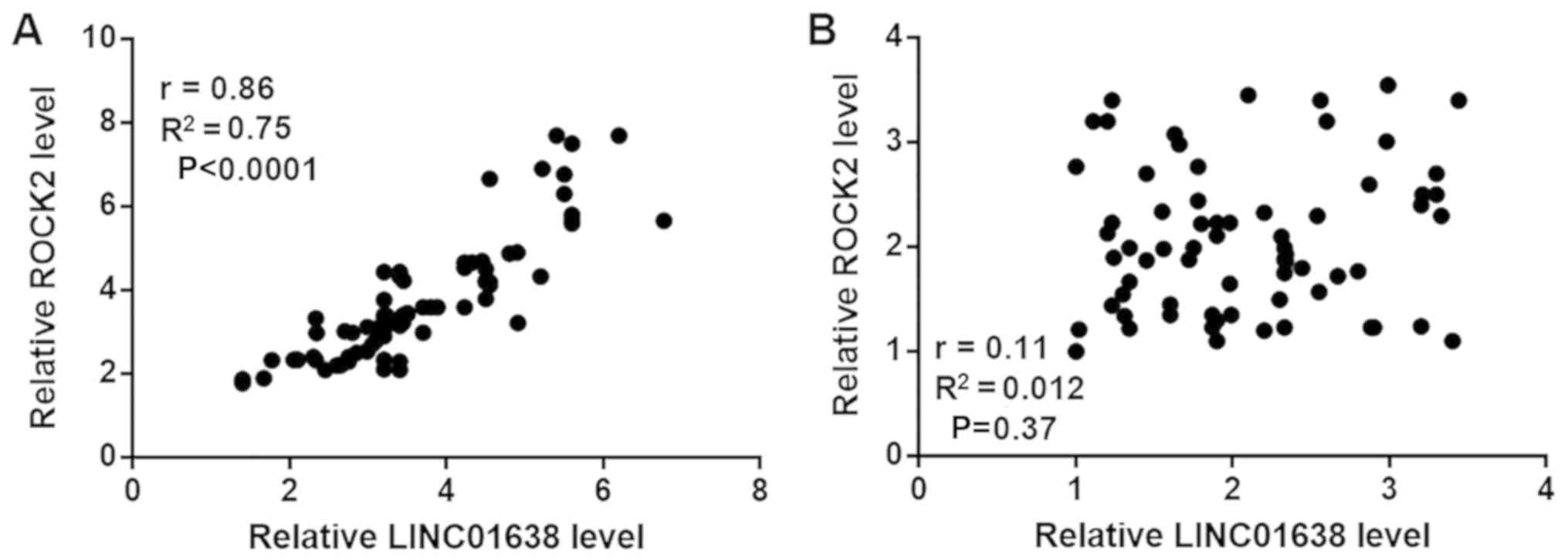
Correlations between the expression levels of
LINC01638 and ROCK2 were performed by Pearson's correlation
coefficient. As presented in Fig.
3A, the plasma levels of LINC01638 and ROCK2 were positively
and significantly correlated in patients with bladder cancer
(Fig. 3A). However, no significant
correlation was observed between the plasma levels of LINC01638 and
ROCK2 in healthy controls (Fig. 3B).
Therefore, there may be interaction between LINC01638 and ROCK2 in
bladder cancer, but not under normal physiological conditions.
LINC01638 and ROCK2 upregulation is
induced by distant recurrence (DR)
Based on the follow-up data, there were 16 cases of
DR, 12 cases of both distant and local recurrence (DLR), 14 cases
of local recurrence (LR) only and 46 cases of NR. Compared with the
level at discharge, the plasma levels of LINC01638 (Fig. 4A) and ROCK2 (Fig. 4B) were increased in the DR and DLR
groups (P<0.05), but not in LR and NR groups (day of the
diagnosis of recurrence or at the end of follow-up in cases of
non-recurrence). Therefore, LINC01638 and ROCK2 may specifically
participate in the DR of bladder cancer.
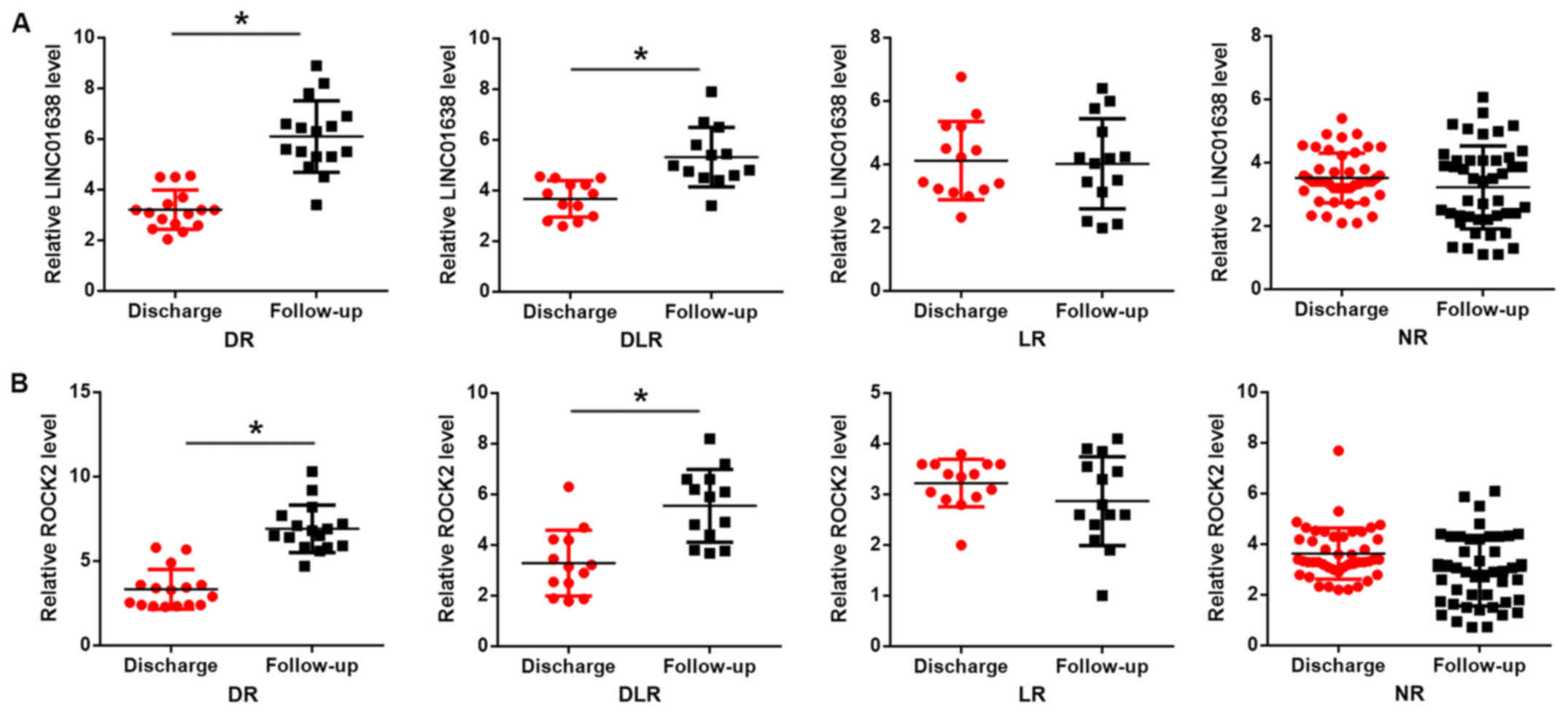 | Figure 4.LINC01638 and ROCK2 upregulation is
induced by DR. Reverse transcription-quantitative PCR was performed
to detect the expression levels of LINC01638 and ROCK2 in patients
with bladder cancer that had different recurrence conditions. The
results revealed that, compared with discharge level, plasma levels
of (A) LINC01638 and (B) ROCK2 were significantly increased in the
DR and DLR groups, but not in LR and NR groups. *P<0.05.
LINC01638, long intergenic non-protein coding RNA 1638; ROCK2,
Rho-associated, coiled-coil containing protein kinase 2; DR,
distant recurrence; DLR, distant and local recurrence; LR, local
recurrence; NR, non-recurrence. |
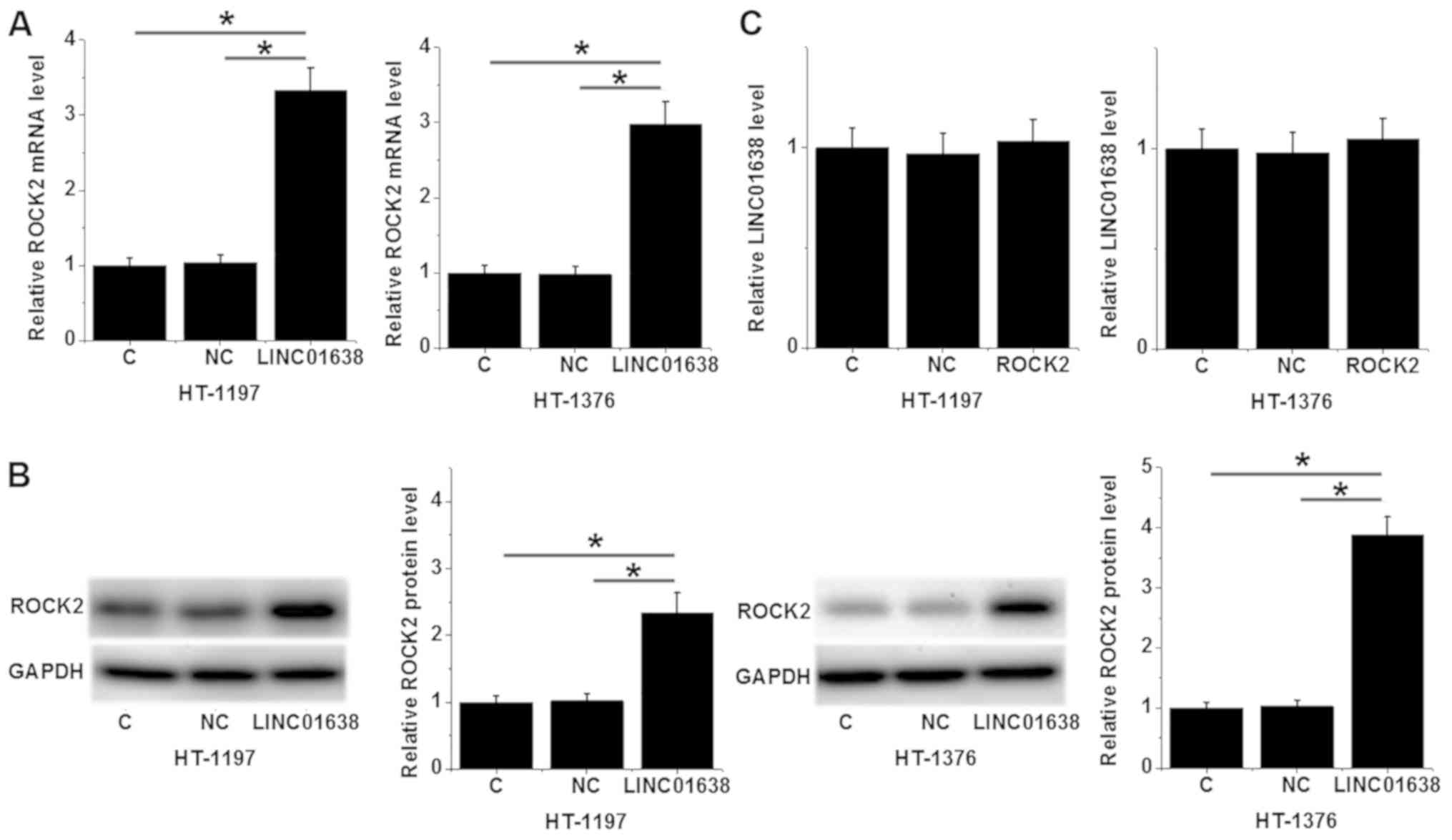
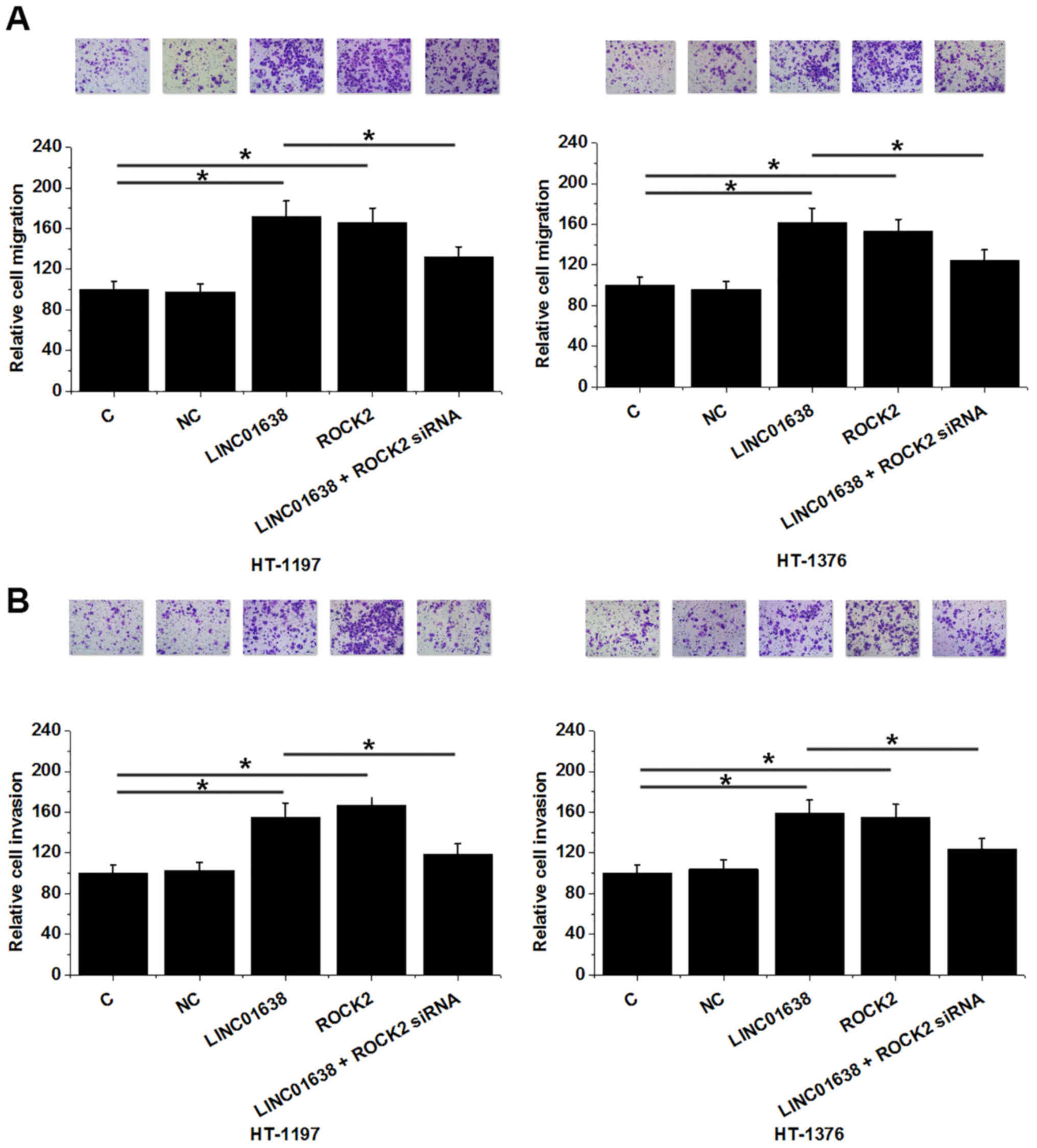
Overexpression of LINC01638 leads to
ROCK2 upregulation in bladder cancer cells
Compared with the C and NC, overexpression of
LINC01638 led to ROCK2 upregulation in the bladder cancer cell
lines HT-1197 and HT-1376 at the mRNA (P<0.05; Fig. 5A) and protein levels (P<0.05;
Fig. 5B). However, ROCK2
overexpression failed to significantly affect the expression of
LINC01638 (Fig. 5C). These data
suggest that LINC01638 may serve as an upstream positive regulator
of ROCK2 in bladder cancer.
Overexpression of LINC01638 promotes
bladder cancer cell migration and invasion through ROCK2
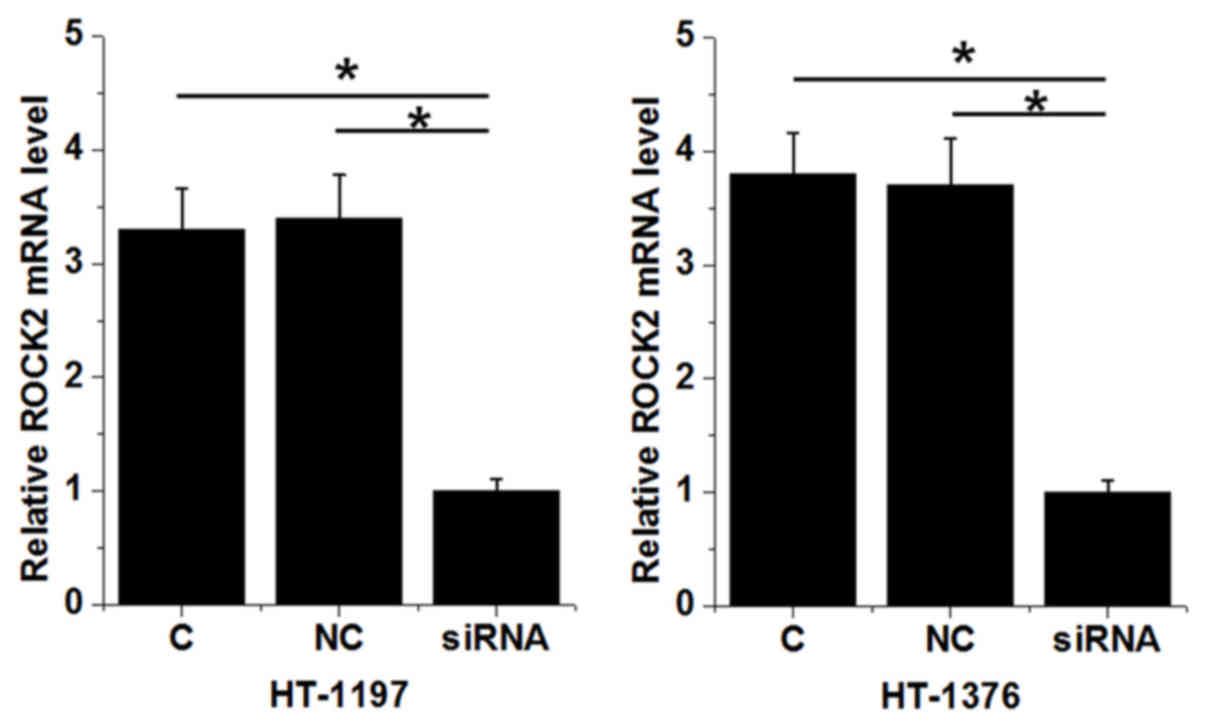
Successful knockdown of ROCK2 was established in the
two cell lines (P<0.05; Fig. 6).
Compared with C and NC, overexpression of LINC01638 and ROCK2
promoted the migration (Fig. 7A) and
invasion (Fig. 7B) of bladder cancer
cell lines HT-1197 and HT-1376 (P<0.05), while ROCK2 siRNA
silencing attenuated the effect of LINC01638 on cancer cell
migration and invasion. These data indicate that LINC01638 may
promote the migration and invasion of bladder cancer cells via
upregulation of ROCK2.
Discussion
The high recurrence rate is a major challenge in the
treatment of bladder cancer. The main result of the present study
is that LINC01638 appears to be involved in the DR of bladder
cancer. The actions of LINC01638 are likely mediated by ROCK2.
ROCK2 mediates cancer cell migration and invasion
(13), with downregulation of ROCK2
inhibiting tumor metastasis (14,15). To
the best of our knowledge, studies investigating the involvement of
ROCK2 in bladder cancer are rare. The present study, demonstrated
the upregulated expression of ROCK2 in patients with bladder cancer
and the effect of ROCK2 on bladder cancer cell migration and
invasion. These results indicate that ROCK2 may have an oncogenic
role in bladder cancer.
The present study demonstrated that LINC01638 may be
an oncogenic lncRNA in bladder cancer as LINC01638 and ROCK2 mRNA
were detected in plasma of patients with bladder cancer, which
indicated that LINC01638 and ROCK2 mRNA can be released into the
blood after synthesis, possibly from multiple organs but further
investigation is required. In addition, it was indicated that
LINC01638 is likely an upstream activator of ROCK2 in the
regulation of bladder cancer migration and invasion. Overexpression
of LINC01638 led to the upregulation of ROCK2 at the mRNA and
protein levels. It is known that lncRNAs can participate in cancer
biology by regulating oncogene or tumor suppressor expression at
the post-transcriptional and translational levels, such as
recruiting a regulatory protein complex or inhibiting the binding
of a transcriptional regulatory proteins, such as transcription
factors (10). Therefore, LINC01638
may affect ROCK2 mRNA degradation and possibly also ROCK2
translation, which in turn regulates the ROCK2 protein level. The
upregulated expression of LINC01638 and ROCK2 in patients with DR
suggest that LINC01638 may promote postoperative DR of bladder
cancer by promoting the expression of ROCK2. However, further in
vivo experiments, such as animal models, are required in order
to confirm this conclusion.
The low diagnosis rate of bladder cancer in the
early stages limits the used of surgical resection, which is
currently the only radical treatment for solid bladder tumors
(15). Despite the efforts made to
develop of novel diagnostic approaches, such as biomarkers
(16) and imaging techniques
(17), the rate of early bladder
cancer diagnosis remains low. In the present study, it was
demonstrated that high levels of circulating LINC01638 and ROCK2 in
the plasma may be used to effectively distinguish patients with
early stage bladder cancer from healthy controls, indicating the
potential of LINC01638 and ROCK2 for use in the early diagnosis of
bladder cancer. However, these two factors should be combined with
other markers in order to increase the diagnostic specificity.
The present study only included two cancer cell
lines. Future studies should include additional cancer cell lines
in order to perform in-depth analysis of the correlation between
LINC01638 expression and cancer cell behavior.
It is worth noting that LINC01638 is a novel lncRNA,
with its functions currently only established in breast cancer
(11,18). In breast cancer, LINC01638 inhibits
speckle-type POZ protein-mediated c-Myc degradation to activate
metadherin-Twist1 signaling (11).
Similar interactions may exist in bladder cancer, and future
studies will explore this.
In conclusion, LINC01638 and ROCK2 have potential
oncogenic roles in bladder cancer. LINC01638 lncRNA may be
associated with postoperative DR of bladder cancer by upregulating
ROCK2.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY, XL and SW designed the experiments. SY, XL and
GH performed the experiments. KG and XZ collected and analyzed the
data. SW drafted the manuscript. All authors approved the
manuscript for publication.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The Second People's Hospital of Liaocheng, The People's Hospital of
Liqing and Shanxi Provincial People's Hospital Urology Surgery. All
patients included in the study provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Witjes JA, Compérat E, Cowan NC, De Santis
M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG and Sherif A;
European Association of Urology, : EAU guidelines on
muscle-invasive and metastatic bladder cancer: Summary of the 2013
guidelines. Eur Urol. 65:778–792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Naito S, Algaba F, Babjuk M, Bryan RT, Sun
YH, Valiquette L and de la Rosette J; CROES Narrow Band Imaging
Global Study Group, : The clinical research office of the
endourological society (CROES) multicentre randomised trial of
narrow band imaging-assisted transurethral resection of bladder
tumour (TURBT) versus conventional white light imaging-assisted
TURBT in primary non-muscle-invasive bladder cancer patients: Trial
protocol and 1-year results. Eur Urol. 70:506–515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Knowles MA and Hurst CD: Molecular biology
of bladder cancer: New insights into pathogenesis and clinical
diversity. Nat Rev Cancer. 15:25–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Riento K and Ridley AJ: Rocks:
Multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol.
4:446–456. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei L, Surma M, Shi S, Lambert-Cheatham N
and Shi J: Novel insights into the roles of Rho kinase in cancer.
Arch Immunol Ther Exp (Warsz). 64:259–278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rath N and Olson MF: Rho-associated
kinases in tumorigenesis: Re-considering ROCK inhibition for cancer
therapy. EMBO Rep. 13:900–908. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang Y, He Y, Zhang P, Wang J, Fan C, Yang
L, Xiong F, Zhang S, Gong Z, Nie S, et al: lncRNAs regulate the
cytoskeleton and related Rho/ROCK signaling in cancer metastasis.
Mol Cancer. 17:772018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang G, Lu X and Yuan L: lncRNA: A link
between RNA and cancer. Biochim Biophys Acta. 1839:1097–1109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo L, Tang H, Ling L, Li N, Jia X, Zhang
Z, Wang X, Shi L, Yin J, Qiu N, et al: LINC01638 lncRNA activates
MTDH-Twist1 signaling by preventing SPOP-mediated c-Myc degradation
in triple-negative breast cancer. Oncogene. 37:6166–6179. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang W, Zhou X and Wei M: MicroRNA-144
suppresses osteosarcoma growth and metastasis by targeting ROCK1
and ROCK2. Oncotarget. 6:10297–10308. 2015.PubMed/NCBI
|
|
14
|
Kroiss A, Vincent S, Decaussin-Petrucci M,
Meugnier E, Viallet J, Ruffion A, Chalmel F, Samarut J and Allioli
N: Androgen-regulated microRNA-135a decreases prostate cancer cell
migration and invasion through downregulating ROCK1 and ROCK2.
Oncogene. 34:2846–2855. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bellmunt J, Orsola A, Leow JJ, Wiegel T,
De Santis M and Horwich A; ESMO Guidelines Working Group, : Bladder
cancer: ESMO practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 25 (Suppl 3):iii40–iii48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmitz-Dräger BJ, Droller M, Lokeshwar
VB, Lotan Y, Hudson MA, van Rhijn BW, Marberger MJ, Fradet Y,
Hemstreet GP, Malmstrom PU, et al: Molecular markers for bladder
cancer screening, early diagnosis, and surveillance: The WHO/ICUD
consensus. Urol Int. 94:1–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng C, Lv Y, Zhong Q, Wang R and Jiang
Q: Narrow band imaging diagnosis of bladder cancer: Systematic
review and meta-analysis. BJU Int. 110:E680–E687. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu P, Tang H, Wu J, Qiu X, Kong Y, Zhang
L, Xie X and Xiao X: Linc01638 promotes tumorigenesis in
HER2+ breast cancer. Curr Cancer Drug Targets. 19:74–80.
2019. View Article : Google Scholar : PubMed/NCBI
|