Introduction
Multiple myeloma (MM) is a lymphoid malignancy
characterized by clonal expansion of malignant plasma cells
producing/secreting monoclonal protein (M-protein) as whole
immunoglobulin molecules or light chain immunoglobulins only.
Parameters correlating with negative outcome include IgA subtype of
M-protein or light-chain multiple myeloma (LCMM), advanced disease
stage according the International Staging System (ISS), high serum
ß2-microglobulin and lactate dehydrogenase (LDH), low
serum albumin and unfavorable cytogenetics, such as: t(4;14),
t(14;16), and del17p (1,2). Treatment of MM has evolved during the
last two decades which has resulted in unprecedented improvement of
patients' outcome with increase of the median survival from 3 to 4
years to 7–8 years (3). This
advancement started in the 1990s with the implementation of
high-dose therapy followed by autologous stem cell transplantation
(HDT/ASCT), however the most prominent effect for survival is
associated with the introduction of novel targeted agents,
including immunomodulatory drugs (IMIDs) and proteasome inhibitors
(PIs) (4). The principles of MM
first line therapy depend on the patients' eligibility for HDT/ASCT
which is still recommended as a standard of care for patients
younger than 65–70 years without prohibitive comorbidities that
achieve at least a partial response for the induction therapy
(1,5). The efficacy of induction regimen is of
key significance since the outcome after HDT/ASCT is highly related
to the depth of response after induction therapy. Another important
aspect is good tolerability and no negative impact on stem cell
mobilization. Nowadays, three drug bortezomib-based regimens are
generally recommended as a standard induction therapy for
transplant-eligible patients with newly diagnosed MM (1,5–7). The choice between different regimens
depends on drug availability in particular countries, their
toxicity profile and local preferences. The objective of this
retrospective analysis was to evaluate efficacy and safety of VTD
regimen in newly diagnosed MM patients eligible for HDT/ASCT in
routine clinical practice.
Materials and methods
Patients
In this retrospective analysis, we collected the
data of transplant-eligible patients with measurable MM who
received initial treatment with VTD regimen. Analytical work-flow
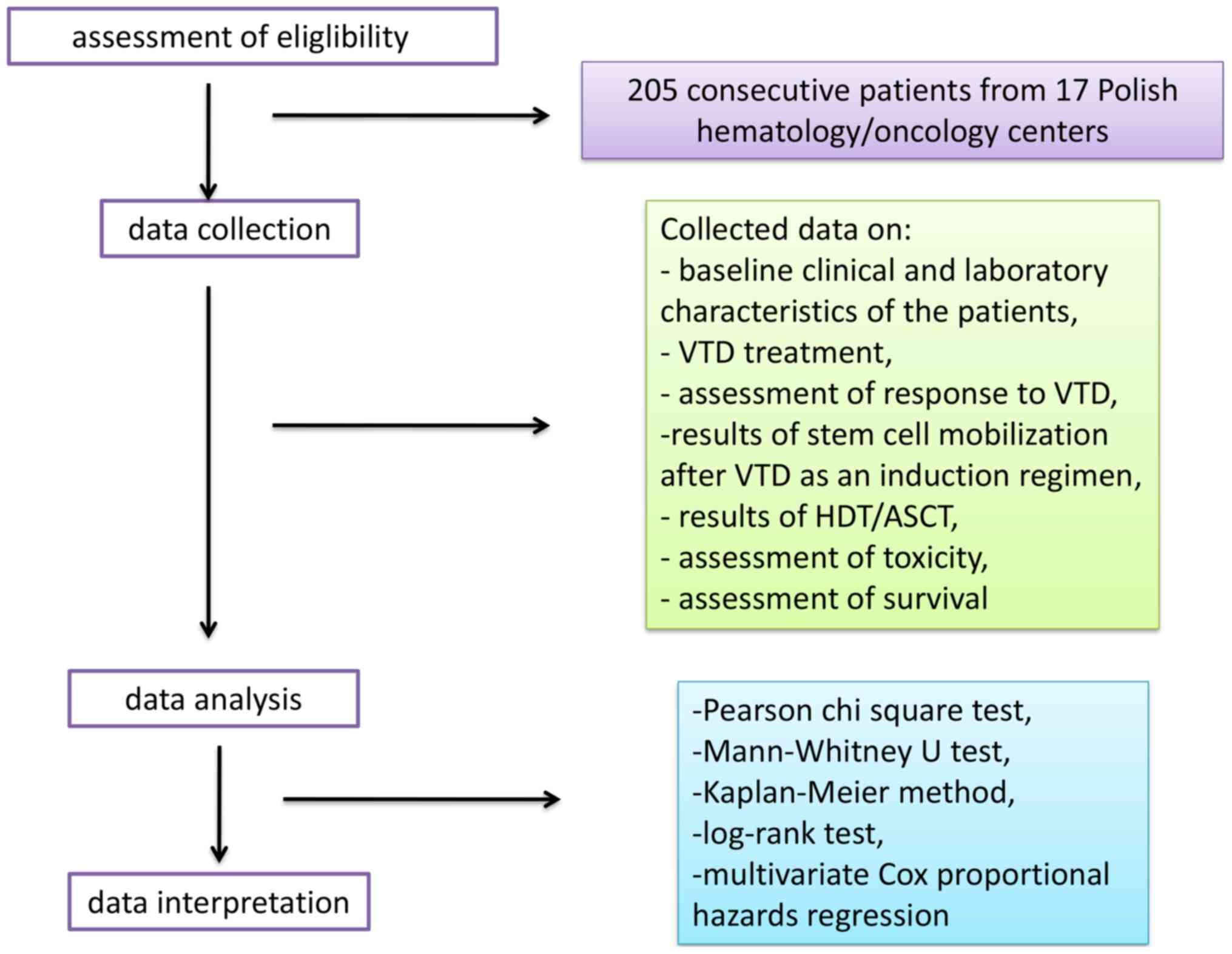
taken by research group during the study is shown in Fig. 1.
The following inclusion criteria for the study were
defined: Previously untreated, newly-diagnosed MM, age 18–70 years,
ECOG performance status <2, patient's compliance with the given
instructions, life expectancy ≥12 months.
Key exclusion criteria were: Plasma cell leukaemia,
light-chain amyloidosis, grade ≥2 peripheral neuropathy (PN) or
neuropathic pain, severe comorbidities, cardiac insufficiency (New
York Heart Association (NYHA) >2 grade or left ventricular
ejection fraction <60%, known human-immunodeficiency virus (HIV)
infection, pregnant, breast-feeding or lactating women.
Treatment
VTD regimen recommended by the Polish Myeloma Study
Group was as follows: Bortezomib at a dose of 1.3 mg/m2
administered subcutaneously (SC) or intravenous (IV) on days 1, 4,
8, and 11; dexamethasone at a dose 20–40 mg on days 1–4 and 9–12;
and thalidomide at a dose of 100–200 mg/day administered orally.
Each cycle should be repeated every 21 days for up to 4–6 cycles
(8). The analysis included medical
records of patients who received at least one cycle of VTD.
Concomitant medications included bisphosphonates, antiviral
prophylaxis and/or antibiotics in accordance with local practice
and deep venous thrombosis (DVT) prophylaxis with enoxaparin or
acetylsalicylic acid (ASA). In patients achieving at least a
partial response (PR) according to International Myeloma Working
Group (IMWG) guidelines (9),
peripheral blood hematopoietic stem cells (PBSC) were then
mobilized according to the center's experience and practice. The
minimal target yield was 2×106 CD34+ cells/kg
for one transplant. The assumption was to obtain a sufficient
number of CD34+ cells for two transplants. Following induction
therapy and effective mobilization, patients proceeded to ASCT. The
use of a conditioning regimen was left at the discretion of each
center, as was the decision to conduct a single or tandem ASCT and
the application of maintenance therapy. After transplantation,
patients were followed every 12 weeks until disease progression,
and then every 12 weeks for survival and subsequent therapies.
The response rates to the induction therapy as well
as to the consolidation with HDT/ASCT, progression-free survival
(PFS) and toxicity of the treatment were evaluated. Effectiveness
of mobilization after VTD-induction therapy was also analyzed. The
rresponse to the therapy was evaluated according to the updated
IMWG uniform response criteria (9).
Adverse events (AEs) were graded by the Common Terminology Criteria
for Adverse Events, version 4.03. [http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf].
Statistical analysis
The influence of categorized parameters on the
response to the treatment and toxicity was performed using the
Pearson chi square test and exact Fisher test. Associations between
response and patient characteristics for continuous parameters were
evaluated using the Mann-Whitney U test. Survival curves were
estimated by the Kaplan-Meier method and the log-rank test was
applied for comparison. PFS was defined as the time from
introducing the treatment to the last date on which the disease
activity was assessed, including death from any reason. Follow-up
time was short to achieve reliable results concerning overall
survival. The influence of independent variables on patient
survival was tested by univariate and multivariate Cox proportional
hazards regression. A multi-parameter analysis of factors
predicting response to the treatment was also performed.
Results
Patients
Between June 2015 and June 2017, a cohort of 205
consecutive patients qualified for HDT/ASCT were treated with VTD
as induction therapy in 17 Polish hematology/oncology centers. The
median age of patients was 59 years (range: 34–70 years). Advanced
clinical stage according ISS (International Staging System)
(10) was found in 88 patients
(43%). R-ISS (11) was evaluated in
only in 90 patients and stage III was found in 20 patients (22.2%).
Non-IgG type of M-protein was found in 83 patients (40.5%).
Cytogenetics was evaluated by FISH in 100 patients, with
abnormalities found in 38 patients (38%), including 20 patients
with adverse prognostic lesions, such as: t(4;14), t(11;14) and
abnormalities of 17 chromosome. Polyneuropathy was present in 4
patients (1.9%) before the start of VTD therapy. Baseline clinical
and laboratory characteristics of the patients are outlined in
Table I.
 | Table I.Clinical characteristics of patients
treated with the VTD regimen. |
Table I.
Clinical characteristics of patients
treated with the VTD regimen.
| Parameter | Value |
|---|
| Sex, n (%) |
|
|
Female | 97 (47.3) |
|
Male | 108 (52.7) |
| ISS
stagea, n (%) |
|
| 1 | 71 (34.6) |
| 2 | 45 (22.4) |
| 3 | 88 (43.0) |
| R-ISS
stageb, n (%) |
|
| 1 | 22 (24.4) |
| 2 | 47 (52.2) |
| 3 | 20 (22.2) |
| Protein M type, n
(%) |
|
|
IgG | 118 (57.6) |
|
IgA | 41 (20.0) |
| Light
chain MM | 39 (19.0) |
|
IgM | 3 (1.45) |
| Monoclonal light
chain, n (%) |
|
|
Kappa | 113 (55.1) |
|
Lambda | 88 (42.9) |
|
Non-secretory | 4 (1.95) |
| Albumin, g/l,
median; (min-max) | 3.6; (1.8–5.2) |
| β-2-microglobulin,
mg/l, median; (min-max) | 4.1;
(1.58–60.0) |
| Creatinine, mg/dl,
median; (min-max) | 0.92;
(0.37–10.57) |
| GFR acc. MDRD
formula, ml/min, median; (min-max) | 60; (53–150) |
| Hemoglobin, g/dl,
median; (min-max) | 10.9;
(6.0–17.3) |
| Neutrophils, G/l,
median; (min-max) | 3.2;
(0.91–10.1) |
| Platelets, G/l,
median; (min-max) | 206; (54–552) |
| Calcium, mmol/l,
median; (min-max) | 2.39;
(1.8–4.77) |
| Monoclonal protein,
g/dl, median; (min-max) | 3.59; (0–14.5) |
| Plasma cells, bone
marrow, % median; (min-max) | 37; (0–95) |
| Cytogenetics, del
17p, t(4;14) t(14;16) | 21/100 |
Treatment
The data on the drug dosage were available for 184
patients (90%). All the patients started the therapy with the
standard dose of bortezomib (1.3 mg/m2, in days 1, 4, 8,
11). Bortezomib was administered SC in 154 patients (75.1%), IV in
41 patients (20%) and 10 patients received bortezomib both routes.
In 170 patients (92.9%), thalidomide was administered in a dose of
100 mg/d and in 11 patients in the dose of 200 mg/d. In 91 patients
(44.3%) the dose of dexamethasone was 160 mg/cycle, and in 90
patients (43.9%)-320 mg/cycle. In 90 patients (43.9%) VTD was given
in 21-day cycles and in 115 patients in 28-day cycles (56%). During
therapy, all patients received anti-DVT prophylaxis. LMWH
(low-molecular weight heparin) was used in 119 patients (58%) and
ASA was used in 70 patients (34.1%). Antiviral prophylaxis with
acyclovir was used routinely in all of patients. The median number
of cycles was 6 (range 1–8), 43 patients received 3–4 cycles (21%),
107 patients received 5–6 cycles (52%) and 47 patients received 7–8
cycles (23%).
Assessment of response
All 205 patients were available for the evaluation
of response. ORR was 94.6%, (n=194) including 32.7% of ≥CR and
67.8% of ≥VGPR. sCR was achieved in 4.9% of patients, (n=10), CR in
27.8% (n=57), VGPR in 35.1% (n=72) and PR in 27.3% of patients
(n=56). Stable disease was observed in 2.4% of patients (n=5) and
disease progression in 2.4% of patients (n=5). In patients with
high-risk cytogenetics there was a lower rate of CR and sCR as
compared to the group without these abnormalities (23.8% vs. 30.4
and 0% vs. 10.1%), but not statistically significant (P>0.05).
There was no significant relationship between pre-treatment
laboratory parameters, steroids dose (160 vs. 320 mg), thalidomide
dose (200 vs. 100 mg), bortezomib dose and route of administration
and the achievement of response for the therapy (P>0.05).
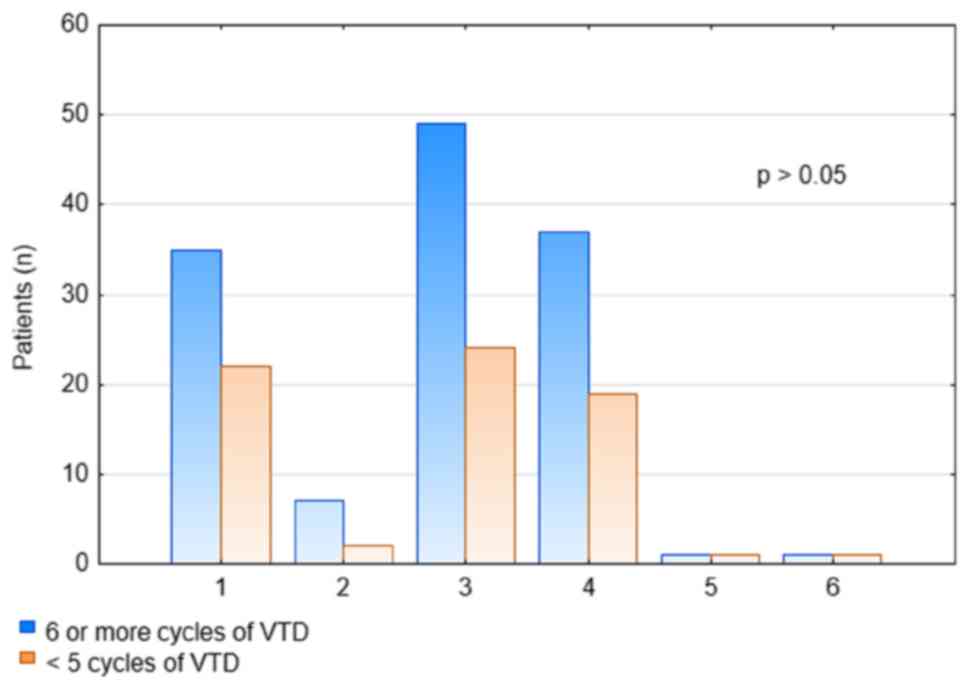
Response rates were higher after ≥6 cycles of VTD as compared to
the response rates after the 4th cycle, but the difference was not
statistically significant (P>0.05; Fig. 2).
Assessment of survival
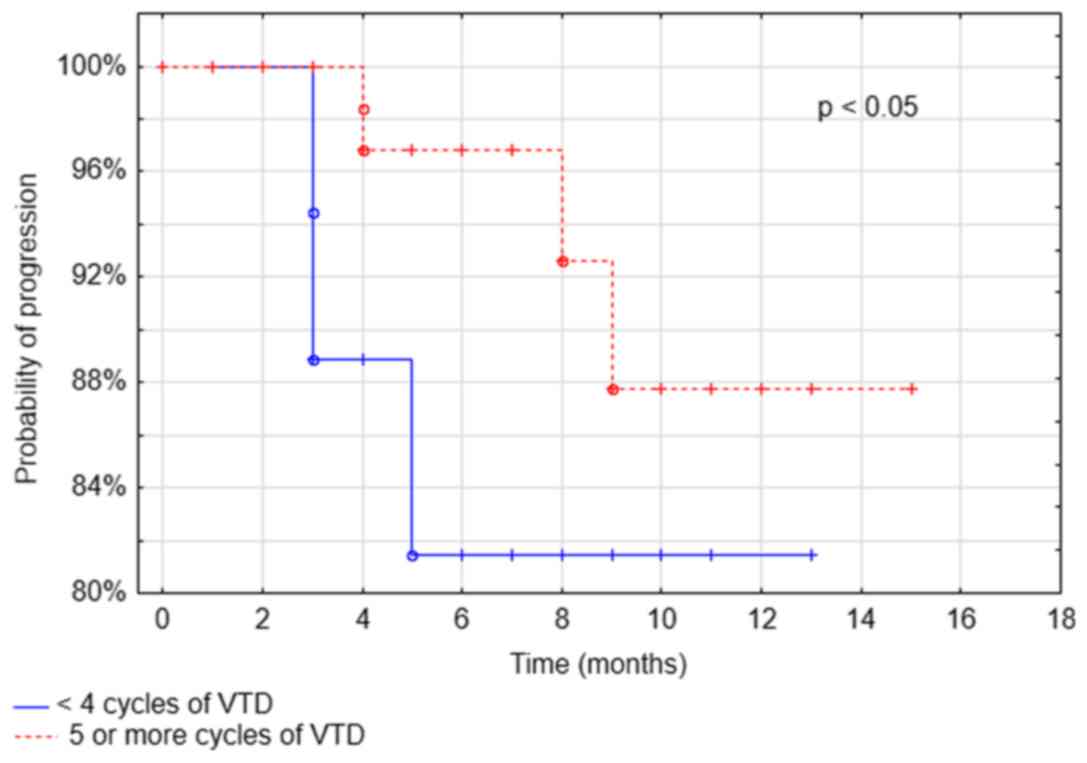
After the median follow-up of 18 months, PFS was
83.1%. The only parameter associated with longer PFS was the number
of administered VTD cycles >4 (P<0.05; Fig. 3). In patients with renal failure PFS
was similar to the patients with normal renal function.
Toxicity
The most common non-hematological grade adverse
event was PN that occurred in 44.5% of patients (Table II). The incidence of PN was similar
in patients receiving bortezomib IV (39%-all grades and 2.44%
-severe) and SC (45.5%-all grades and 4.55% -severe). VZV
(varicella zoster virus) reactivation was observed in 3.9% (8/205)
of patients. The rates of hematological adverse events are
presented in Table III.
 | Table II.Non-hematological toxicity of the VTD
regimen. |
Table II.
Non-hematological toxicity of the VTD
regimen.
|
| 1–2 grade adverse
events | 3–4 grade adverse
events |
|---|
|
|
|
|
|---|
| Non-hematological
toxicity | Number, n | Percentage
(n=205) | Number, n | Percentage
(n=205) |
|---|
| Polyneuropathy |
| Grade
1 | 36 | 17.6 | 10 | 4.9 |
| Grade
2 | 45 | 33.0 |
|
|
| Infections | 8 | 4.0 | 2 | 1.0 |
| Thrombosis | 7 | 3.4 | 1 | 0.5 |
| Pulmonary
embolism | – | – | 2 | 1.0 |
| Constipation | 2 | 1.0 | – | – |
| Skin
alterations | 2 | 1.0 | 3 | 1.5 |
 | Table III.Hematological toxicity of VTD
regimen. |
Table III.
Hematological toxicity of VTD
regimen.
|
| 1–2 grade adverse
events | 3–4 grade adverse
events |
|---|
|
|
|
|
|---|
| Hematological
toxicity | Number, n | Percentage
(n=205) | Number, n | Percentage
(n=205) |
|---|
| Neutropenia | 1 | 0.5 | 5 | 2.5 |
|
Thrombocytopenia | 4 | 2.0 | 1 | 0.5 |
| Anemia | 12 | 6.0 | – | – |
Bortezomib dose was reduced in 42 patients (20.5%).
PN was the main reason of bortezomib dose reduction (33 patients;
78.6%; grade 2 neuropathy with pain-23 patients, grade 3–10
patients), among the other reasons there were: Skin changes in 2
patients (4.7%), lung fibrosis in 2 patients (4.7%). Severe
thrombocytopenia and neutropenia were the reason of dose reduction
only in 1 patient each. Bortezomib treatment was discontinued in 26
patients (12.7%). The main reasons for discontinuation were adverse
events (88.4%), including PN in 9 patients (34.6%), thrombotic
complications (thrombosis, pulmonary embolism) in 5 patients
(19.2%). Thalidomide treatment was discontinued in 3 patient (1.5%)
due to polyneuropathy. In 8 (3.9%) patients with grade 3
polyneuropathy and 3 (1.5%) patients with grade 2 polyneuropathy,
the dose of thalidomide was reduced to 50 mg.
On the basis of the exact Fisher test, it was found
that there was no significant relationship between the dose of
steroids (160 vs. 320 mg), the dose of thalidomide (200 vs. 100
mg), route of bortezomib administration (SC vs. IV), duration of
cycle (21 days vs. 28 days), and toxicity of VTD therapy
(P>0.05). However, it was shown that in the group of patients in
whom the dose of bortezomib was reduced, polyneuropathy was
statistically significantly more frequent (P<0.05; Table IV). No correlation between the
number of treatment cycles, dose of thalidomide and occurrence of
polyneuropathy and other adverse events (P>0.05; Table IV). In 3 patients, treatment was
discontinued because of the lack of efficacy. Six patients died
during the median 8.56 months follow-up (range 2.8–24.9). There
were three early deaths and the reasons were sepsis (2 patients
after 2 and 3 cycles of VTD, respectively) and heart failure (1
patient after 6 cycles of VTD). One patient died from pneumonia
before CD34+ cell mobilization, one patient from sepsis after ASCT
(+59 day) and one due to MM progression (19 months after VTD
completion).
 | Table IV.Assessment of non-hematological and
hematological adverse events incidence during VTD therapy. |
Table IV.
Assessment of non-hematological and
hematological adverse events incidence during VTD therapy.
|
| Occurrence |
|
|---|
|
|
|
|
|---|
| Analyzed
parameter | Yes (%) | No (%) | P-value |
|---|
| Polyneuropathy |
| Number
of VTD cycles |
|
≥6 | 9.7 | 55.6 | >0.05 |
|
<5 | 6.8 | 27.8 |
|
|
Duration of cycle |
|
21 days | 9.3 | 34.6 | >0.05 |
|
28 days | 7.3 | 48.8 |
|
| Initial
dose of thalidomide |
|
200 mg | 1.6 | 4.4 | >0.05 |
|
100 mg | 14.3 | 78.6 |
|
|
<100 mg | 0.55 | 0.55 |
|
|
Bortezomib dose reduction |
|
Yes | 16.6 | 3.9 | <0.05 |
|
No | 0.0 | 79.5 |
|
| Infection |
| Number
of VTD cycles |
|
≥6 | 2.9 | 62.4 | <0.05 |
|
<5 | 2.0 | 32.7 |
|
|
Duration of cycle |
|
21 days | 2.9 | 41.0 | >0.05 |
|
28 days | 2.0 | 54.1 |
|
| Initial
dose of thalidomide |
|
200 mg | 0.0 | 11.0 | >0.05 |
|
100 mg | 4.0 | 165 |
|
|
<100 mg | 0.0 | 2.0 |
|
|
Bortezomib dose reduction |
|
Yes | 2.0 | 18.5 | >0.05 |
|
No | 2.9 | 76.6 |
|
| Thrombosis |
| Number
of VTD cycles |
|
≥6 | 2.9 | 62.4 | >0.05 |
|
<5 | 1.0 | 33.7 |
|
|
Duration of cycle |
|
21 days | 2.0 | 41.9 | >0.05 |
|
28 days | 2.0 | 54.1 |
|
| Initial
dose of thalidomide |
|
200 mg | 0.6 | 5.5 | >0.05 |
|
100 mg | 1.6 | 91.2 |
|
|
<100 mg | 0.0 | 1.1 |
|
|
Bortezomib dose reduction |
|
Yes | 1.5 | 19.0 | >0.05 |
|
No | 2.4 | 77.1 |
|
| Pulmonary
embolism |
| Number
of VTD cycles |
|
≥6 | 1.0 | 64.4 | >0.05 |
|
<5 | 0.0 | 34.6 |
|
|
Duration of cycle |
|
21 days | 0.0 | 43.9 | >0.05 |
|
28 days | 1.0 | 55.1 |
|
| Initial
dose of thalidomide |
|
200 mg | 0.0 | 6.0 | >0.05 |
|
100 mg | 1.1 | 91.8 |
|
|
<100 mg | 0.0 | 1.1 |
|
|
Bortezomib dose reduction |
|
Yes | 0.0 | 20.5 | >0.05 |
|
No | 1.0 | 78.5 |
|
| Constipations |
| Number
of VTD cycles |
|
≥6 | 1.0 | 64.4 | >0.05 |
|
<5 | 0.0 | 34.6 |
|
|
Duration of cycle |
|
21 days | 0.0 | 43.9 | >0.05 |
|
28 days | 1.0 | 55.1 |
|
| Initial
dose of thalidomide |
|
200 mg | 0.0 | 6.0 | >0.05 |
|
100 mg | 0.0 | 92.9 |
|
|
<100 mg | 0.0 | 1.1 |
|
|
Bortezomib dose reduction |
|
Yes | 0.0 | 20.5 | >0.05 |
|
No | 1.0 | 79.5 |
|
| Skin
alterations |
| Number
of VTD cycles |
|
≥6 | 1.0 | 64.4 | >0.05 |
|
<5 | 1.5 | 33.1 |
|
|
Duration of cycle |
|
21 days | 1.0 | 38.1 | >0.05 |
|
28 days | 1.5 | 54.6 |
|
| Initial
dose of thalidomide |
|
200 mg | 1.1 | 4.8 | >0.05 |
|
100 mg | 0.5 | 91.6 |
|
|
<100 mg | 0.0 | 0.0 |
|
|
Bortezomib dose reduction |
|
Yes | 0.5 | 20.0 | >0.05 |
|
No | 2.0 | 77.5 |
|
| Neutropenia |
| Number
of VTD cycles |
|
≥6 | 2.4 | 63.0 | >0.05 |
|
<5 | 0.5 | 34.1 |
|
|
Duration of cycle |
|
21 days | 1.0 | 42.9 | >0.05 |
|
28 days | 2.0 | 54.1 |
|
| Initial
dose of thalidomide |
|
200 mg | 0.55 | 5.5 | >0.05 |
|
100 mg | 0.55 | 92.3 |
|
|
<100 mg | 0.0 | 1.1 |
|
|
Bortezomib dose reduction |
|
Yes | 0.0 | 20.5 | >0.05 |
|
No | 2.9 | 76.6 |
|
|
Thrombocytopenia |
| Number
of VTD cycles |
|
≥6 | 0.5 | 64.9 | >0.05 |
|
<5 | 1.5 | 33.2 |
|
|
Duration of cycle |
|
21 days | 2.0 | 41.9 | >0.05 |
|
28 days | 0.0 | 56.1 |
|
| Initial
dose of thalidomide |
|
200 mg | 1.1 | 4.9 | >0.05 |
|
100 mg | 0.6 | 92.3 |
|
|
<100 mg | 0.0 | 1.1 |
|
|
Bortezomib dose reduction |
|
Yes | 0.5 | 20.0 | >0.05 |
|
No | 1.5 | 78.0 |
|
|
Anemia |
|
Number of VTD
cycles |
|
≥6 | 2.9 | 62.4 | >0.05 |
|
<5 | 2.9 | 31.8 |
|
|
Duration of cycle |
|
21 days | 4.4 | 39.5 | >0.05 |
|
28 days | 1.5 | 54.6 |
|
| Initial
dose of thalidomide |
|
200 mg | 3.3 | 2.7 | >0.05 |
|
100 mg | 1.1 | 91.8 |
|
|
<100 mg | 0.0 | 1.1 |
|
|
Bortezomib dose reduction |
|
Yes | 1.5 | 19.0 | >0.05 |
|
No | 4.4 | 75.1 |
|
Results of stem cell mobilization
after VTD as an induction regimen
Hematopoietic stem cell mobilization was performed
at the time of analysis in 146 patients (71%). In 63.7% of patients
(n=93) one apheresis allowed the number of stem cells sufficient
for transplantation to be obtained. In 20% of patients (n=29) two
apheresis were performed. The median yield of CD34+ cells was
12.6×106/kg (max 70×106/kg) which was
sufficient for two transplantations in the majority of
patients.
Most commonly used protocols before HSCT
mobilization were cytosine arabinoside (n=53, 36.3%) and
cyclophosphamide (n=63; 43.1%). The other protocols included:
Etoposide (n=11; 7.5%); G-CSF alone (n=11; 7.5%); cyclophosphamide
with etoposide (n=7; 4.8%) and plerixafor (n=1; 0.8%). The median
yield of CD34+ cells was the highest in patients mobilized with
cytosine arabinoside. Protocols used before HSCT mobilization and
their efficacy are presented in Table
V. In three patients that needed a second procedure of stem
cell mobilization, protocols with cytosine arabinoside, G-CSF and
plerixafor were used and the number of HSCT sufficient for the
transplant was obtained (median 3.56×106/kg; range
3.02–4.1) Table V.
 | Table V.Efficacy of the protocols used prior
to HSCT mobilization. |
Table V.
Efficacy of the protocols used prior
to HSCT mobilization.
|
| Yield of
CD34+ cells (×106/kg) |
|---|
|
|
|
|---|
| Mobilization
protocol | Median | Minimum | Maximum |
|---|
| CPX+G-CSF | 9.9 | 3.1 | 30.0 |
| VEP +G-CSF | 18.0 | 6.5 | 55.0 |
| ID-AraC+G-CSF | 20.0 | 3.1 | 70.0 |
| G-CSF alone | 7.2 | 4.6 | 17.0 |
Results of HDT/ASCT after VTD as an
induction regimen
HDT/ASCT was performed at the time of analysis in
128 patients with MEL 200 (melphalan 200 mg/m2) protocol
as conditioning regimen in 87.5% of patients. The other
conditioning protocols included: MEL 140 (8.6%) and TMI (total
marrow irradiation) with 12Gy (2.3%); treosulfan+MEL (0.8%);
TBI+MEL (0.8%).
The median number of transplanted CD34+ cells was
5.4×106/kg (range: 3.1–10.6×106/kg). The
median time to reach ANC (absolute neutrophil count) >0.5 G/l
and PLT (platelets) count >20 G/l were 11 and 12 days,
respectively. The most common grade ≥3 adverse events observed
after HDT/ASCT were infections noted in 28 patients (21.9%),
including gastrointestinal tract infections in 9 patients (7%),
mucositis in 7 patients (5.5%), neutropenic fever in 7 patients
(5.5%) and sepsis in 3 patients (2.3%). CMV (cytomegalovirus)
infection was observed in 2 patients (1.6%).
Evaluation of response 100 days after HDT/ASCT was
performed in 104 patients. Comparing the response rates after
HDT/ASCT to the responses after the induction, there was an
increase of ≥CR (50% vs. 32.7%) and ≥VGPR (85.6% vs. 67.8%). CR
rate increased from 27 to 35.6% and sCR rate increased from 4.9 to
14.4% (Fig. 4).
Tandem HDT/ASCT was performed in 21 patients with
suboptimal response to the first course of HDT. Conditioning
protocols included: MEL200 in 15 patients and MEL140 in 4 patients,
TMI in 2 patients, achieving significant improvement in the quality
of response (Pearson test, P<0.001) with increase of CR to 52.4%
(11 patients).
Discussion
In the era of modern therapies, consolidation with
HDT/ASCT is still considered as a standard of first line treatment
in eligible MM patients since evidence from phase 3 studies
demonstrated the improvement in the depth of response and
progression-free survival (12,13).
Three-drug regimens containing PI and IMID were shown to be
superior than three-drug regimens with either PI or IMID as well as
than two-drug regimens in the terms of response rates and survival
(14–20). Currently in Poland, VTD is the most
common induction regimen used in about 85% of patients, while VCD
being used only in the minority of them. Our data show that high
response rates with ≥VGPR rate of 67.8% could be achieved with VTD
in clinical practice being in line with the data from clinical
trials where at least VGPR ranged from 49 to 69% (14–16,21,22).
However, in phase 3 clinical trial IFM2013-04, at
least VGPR was achieved in 66.3% of patients after 4 cycles of VTD
(16) and in retrospective
case-matched analysis by Cavo et al (23). in 64% of patients treated with 3
cycles of VTD regimen. The median number of cycles in our study was
6, so, in clinical practice a higher number of VTD cycles was
needed to achieve results comparable to the clinical studies. There
are a few possible explanations of these discrepancies. First,
there were differences in study groups characteristics, like, for
example a higher percentage of the patients with a more advanced
stage of MM in the group in our study as compared to the IFM2013-04
trial. What would be even more important, in our retrospective
analysis, cytogenetics profile was unknown in about half of the
patients and as it demonstrated in so far published data,
bortezomib could only partially overcome the adverse prognostic
impact associated with high risk cytogenetics. The results of the
IFM group study showed that it could eliminate the poor risk
prognosis of t(4;14) (24), however
the data concerning del(17p) remain unsatisfactory (14,22,25–29).
Another reason might be probably the lower median dose intensity
caused by more arbitrary approach to dose reduction and
discontinuation than in clinical trials. The rate of ≥CR after the
induction with VTD in our study was 32.7% that was much higher than
in the IFM2013-04 trial (16) and
Italian case-matchedanalysis (23).
It was comparable though to the results obtained in the Spanish
PETHEMA Group study after 6 cycles of VDT (22), where a significant proportion of
patients achieved CR during the three final cycles. As it was
demonstrated by the results of IFM 2005-01 trial, achieving at
least VGPR before transplant was associated with longer PFS
(30). So, it might be useful
continuing the therapy to increase the quality of response
especially in patients with adverse cytogenetics or in patients in
which cytogenetics is unknown, like it happens in clinical
practice, since achievement of less than VGPR was a stronger
predictor for progression than cytogenetics (30). According to the most recent ESMO
guidelines 4 to 6 cycles of induction regimen is recommended before
HDT/ASCT (1). As much as 23% of
patients in our retrospective analysis received more than 6 cycles
of VTD. As we think, this could have been related to non-medical
conditions, like delayed patients' decision on undergoing HDT/ASCT
or extended time of waiting for transplantation procedure. Lower
doses of dexamethasone (160 vs. 320 mg) as well as thalidomide (100
vs. 200 mg) were as effective as the higher ones confirming once
again that lower, less toxic doses should be preferred (31).
Among the regimens used in induction treatment in MM
patients eligible for HDT/ASCT, VRD (bortezomib, lenalidomid,
dexamethasone) is considered as very effective both in the context
of response rates (32), as well as
the benefit in OS (VRd vs. Rd) that was not was observed in case of
VTD (17). However, lenalidomide is
not yet approved in the first-line therapy of patients eligible to
HDT/ASCT and the much higher cost of VRD compared to VTD would be
an issue in some countries.
In this retrospective analysis, PN grade 2 was
reported in 22% of patients and grade 3 in 4.9% of patients even
though the median number of cycles was 6. Therefore, grade 2–4 PN
(26.9%) was lower than expected basing on the results of clinical
trials (16,33,34). The
possible explanation is that in clinical practice the grading of
neuropathy was determined by the physicians caring for these
patients. Presumably hematologists tend to minimize or ‘down grade’
these complaints, especially in patients that benefit from the
therapy. Given the subjective nature of grading these symptoms, it
would perhaps more helpful to look at the number of patients who
had to be either dose reduced or who discontinued therapy due to
neuropathy (20.5%). This means that twice weekly bortezomib (given
IV or SC) in combination with thalidomide/dexamethasone leads to
significant neuropathy. However, PN was the reason for bortezomib
discontinuation only in 9/205 patients (4.4%). Thalidomide dose was
reduced even in fewer patients, (8/3.9%), though according to the
guidelines, the dose of thalidomide should be reduced first due to
its irreversibility. It is possible that the dose of 100 mg was
presumed to be low by the treating physicians and that is why they
reduced bortezomib first. These observations suggest that vigilance
for neuropathy should be higher in clinical practice since along
with prolongation of patients survival its negative influence of
quality of life is extremely important. It would be advisable to
include independent neurological assessment in patients'
management. While lenalidomide may not be available for first line
therapy an easy, less toxic (and perhaps more effective) solution
may be weekly bortezomib which has been shown to lead to less
neuropathy and could be potential option to improve
tolerability.
In contrast to the results of the phase 3 clinical
trial (35), but similar to our
previous retrospective analysis on VMP in clinical practice
(36) and also the observations of
the other authors (37–39) we did not observe the differences in
the rate of neuropathy between patients receiving bortezomib SC and
IV. Grade 3/4 non-hematological complications were rare, with
neutropenia observed only in 2.5% of patients similarly to the
Italian retrospective analysis by Cavo et al (23) (2%) and much lower than in the
IFM2013-04 trial 1(8.9%) (16). The
lower incidence of serious adverse events might result from earlier
decisions on dose reductions or treatment discontinuation in
clinical practice than in clinical trials.
In the majority of MM patients undergoing subsequent
stem cell mobilization, one apheresis procedure was sufficient to
obtain HSC for two transplants. These results confirm that VTD does
not negatively impact HSC collection (16,22,40),
even given at the higher number of cycles. In contrast,
lenalidomide was reported to adversely impair HSC harvest (41–43),
though in the more recent retrospective analysis a sufficient
number of stem cells was obtained in MM patients treated with
lenalidomide-based regimens (44).
In our study, cytosine arabinoside that is often used in Polish
transplantation centers allowed to achieve higher numbers of HSC
compared to cyclophosphamide, confirming the previous data from
retrospective analysis of the Polish Lymphoma Research Group
(45). Efficient method of HSC
mobilization was also G-CSF alone.
After HDT/ASCT there was an improvement in the depth
of response with increase rate of ≥VGPR, CR and sCR. As it was
demonstrated, achievement of ≥VGPR after transplant has a
significant effect for prolongation of PFS and OS (46–49).
Tandem ASCT allowed for a further increase of CR, similarly to the
previous reports (50,51). Neutrophils and platelets engraftment
after ASCT was not impaired in patients treated with VCT compared
to the other protocols used in induction (16,22,52).
This study has some limitations, such as the
differences in the regimen received by patients (21 vs. 28 day
cycles), thalidomide/dexamethasone dosing, bortezomib routes
administration and limited availability of cytogenetics/FISH tests
that result from retrospective data analysis.
One the other hand, these diversities reflect the
management of the MM patients in routine clinical practice. In
spite of VTD extensive evaluation in randomized comparative late
phase clinical trials, in ‘real world’ MM patient populations are
much more divergent (53) that
affects the clinical outcomes. This real-world analysis is very
important from the clinical point of view, the more so it is the
first of its kind in Poland.
In summary, VTD as induction regimen in MM patients
eligible to ASCT allowed high rates of response to be achieved in
routine clinical practice, though more cycles were needed to obtain
results like those in clinical trials. These data suggest that in
some patients it would be useful to give more than 4 cycles to
optimize the quality of response, especially as the treatment with
the median of 6 cycles was well tolerated and had no negative
impact on stem cell collection or hematopoiesis reconstitution
after ASCT. However, attention should be paid to more adequate
neuropathy assessment and appropriate bortezomib/thalidomide dose
reduction.
Acknowledgements
Not applicable.
Funding
The present study was funded by a research grant
obtained from the Medical University of Lublin, Poland (grant no.
DS174).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
IH, JM and AD designed the study, IH and JM analyzed
the data. DJ and AS performed the statistical analysis. IH and JM
performed the literature search and data extraction. IH and JM
wrote the manuscript. IH, JM, DJ, AJ, GC, KPG, LUZ, MS, ADS, AŚ,
AK, NG, MRa, AW, AP, AG, DD, TK, MRo, AWG, JDS, BP, AP, MDD, AS and
AD. contributed to the acquisition and interpretation of the
clinical data. IH, JM and AS reanalyzed the data and manuscript
after revision. AD critically revised the article for intellectual
content. All authors read the manuscript and approved the final
version.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moreau P, San Miguel J, Sonneveld P,
Mateos MV, Zamagni E, Avet-Loiseau H, Hajek R, Dimopoulos MA,
Ludwig H, Einsele H, et al: Multiple myeloma: ESMO Clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 28 (Suppl 4):iv52–iv61. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Munshi NC, Anderson KC, Bergsagel PL,
Shaughnessy J, Palumbo A, Durie B, Fonseca R, Stewart AK,
Harousseau JL, Dimopoulos M, et al: Consensus recommendations for
risk stratification in multiple myeloma: Report of the
International Myeloma workshop consensus panel 2. Blood.
117:4696–4700. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anderson KC: The 39th David A. Karnofsky
Lecture: Bench-to-bedside translation of targeted therapies in
multiple myeloma. J Clin Oncol. 30:445–452. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Palumbo A and Anderson K: Multiple
myeloma. N Engl J Med. 364:1046–1060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar SK, Callander NS, Alsina M,
Atanackovic D, Biermann JS, Chandler JC, Costello C, Faiman M, Fung
HC, Gasparetto C, et al: Multiple myeloma, version 3.2017, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
15:230–269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mateos MV, Ocio EM, Paiva B, Rosiñol L,
Martínez-López J, Bladé J, Lahuerta JJ, García-Sanz R and San
Miguel JF: Treatment for patients with newly diagnosed multiple
myeloma in 2015. Blood Rev. 29:387–403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rajkumar SV: Multiple myeloma: 2016 update
on diagnosis, risk-stratification, and management. Am J Hematol.
91:719–734. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dmoszynska A, Walter-Croneck A,
Usnarska-Zubkiewicz L, Stella-Holowiecka B, Walewski J, Charliński
G, Jedrzejczak W, Wiater E, Lech-Maranda E, Dytfeld D, et al:
Recommendations of polish myeloma group concerning diagnosis and
therapy of multiple myeloma and other plasmacytic dyscrasias for
2015. Acta Hematol Pol. 46:159–211. 2015.
|
|
9
|
Kyle RA and Rajkumar SV: Criteria for
diagnosis, staging, risk stratification and response assessment of
multiple myeloma. Leukemia. 23:3–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Greipp PR, San Miguel J, Durie BG, Crowley
JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H,
Kyle RA, et al: International staging system for multiple myeloma.
J Clin Oncol. 23:3412–3420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palumbo A, Avet-Loiseau H, Oliva S,
Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S,
Lahuerta JJ, Facon T, et al: Revised international staging system
for multiple myeloma: A report from international myeloma working
group. J Clin Oncol. 33:2863–2869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Voorhees PM and Usmani SZ: The role of
high-dose melphalan and autologous stem cell transplant in the
rapidly evolving era of modern multiple myeloma therapy. Clin Adv
Hematol Oncol. 14:719–728. 2016.PubMed/NCBI
|
|
13
|
Hari P: Recent advances in understanding
multiple myeloma. Hematol Oncol Stem Cell Ther. 10:267–271. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cavo M, Tacchetti P, Patriarca F, Petrucci
MT, Pantani L, Galli M, Di Raimondo F, Crippa C, Zamagni E, Palumbo
A, et al: Bortezomib with thalidomide plus dexamethasone compared
with thalidomide plus dexamethasone as induction therapy before,
and consolidation therapy after, double autologous stem-cell
transplantation in newly diagnosed multiple myeloma: A randomised
phase 3 study. Lancet. 376:2075–2085. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moreau P, Avet-Loiseau H, Facon T, Attal
M, Tiab M, Hulin C, Doyen C, Garderet L, Randriamalala E, Araujo C,
et al: Bortezomib plus dexamethasone versus reduced-dose
bortezomib, thalidomide plus dexamethasone as induction treatment
before autologous stem cell transplantation in newly diagnosed
multiple myeloma. Blood. 118:5752–5758. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moreau P, Hulin C, Macro M, Caillot D,
Chaleteix C, Roussel M, Garderet L, Royer B, Brechignac S, Tiab M,
et al: VTD is superior to VCD prior to intensive therapy in
multiple myeloma: Results of the prospective IFM2013-04 trial.
Blood. 127:2569–2574. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Durie BG, Hoering A, Abidi MH, Rajkumar
SV, Epstein J, Kahanic SP, Thakuri M, Reu F, Reynolds CM, Sexton R,
et al: Bortezomib with lenalidomide and dexamethasone versus
lenalidomide and dexamethasone alone in patients with newly
diagnosed myeloma without intent for immediate autologous stem-cell
transplant (SWOG S0777): A randomised, open-label, phase 3 trial.
Lancet. 389:519–527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang A, Duan Q, Liu X, Ding K, Han Y, Zhu
W, Cai X, Wu J and Sun Z: (Bortezomib plus
lenalidomide/thalidomide)-vs. (bortezomib or
lenalidomide/thalidomide)-containing regimens as induction therapy
in newly diagnosed multiple myeloma: A meta-analysis of randomized
controlled trials. Ann Hematol. 91:1779–1784. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Landgren O and Iskander K: Modern multiple
myeloma therapy: Deep, sustained treatment response and good
clinical outcomes. J Intern Med. 281:365–382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang H, Zhou L, Peng L, Fu W, Zhang C and
Hou J: Bortezomib-thalidomide-based regimens improved clinical
outcomes without increasing toxicity as induction treatment for
untreated multiple myeloma: A meta-analysis of phase III randomized
controlled trials. Leuk Res. 38:1048–1054. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ludwig H, Viterbo L, Greil R, Masszi T,
Spicka I, Shpilberg O, Hajek R, Dmoszynska A, Paiva B, Vidriales
MB, et al: Randomized phase II study of bortezomib, thalidomide,
and dexamethasone with or without cyclophosphamide as induction
therapy in previously untreated multiple myeloma. J Clin Oncol.
31:247–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rosinol L, Oriol A, Teruel AI, Hernández
D, López-Jiménez J, de la Rubia J, Granell M, Besalduch J, Palomera
L, González Y, et al: Superiority of bortezomib, thalidomide, and
dexamethasone (VTD) as induction pretransplantation therapy in
multiple myeloma: A randomized phase 3 PETHEMA/GEM study. Blood.
120:1589–1596. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cavo M, Pantani L, Pezzi A, Petrucci MT,
Patriarca F, Di Raimondo F, Marzocchi G, Galli M, Montefusco V,
Zamagni E, et al: Bortezomib-thalidomide-dexamethasone (VTD) is
superior to bortezomib-cyclophosphamide-dexamethasone (VCD) as
induction therapy prior to autologous stem cell transplantation in
multiple myeloma. Leukemia. 29:2429–2431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Avet-Loisseau H, Leleu X, Roussel M,
Moreau P, Guerin-Charbonnel C, Caillot D, Marit G, Benboubker L,
Voillat L, Mathiot C, et al: Bortezomib plus dexamethasone
induction improves outcome of patients with t(4;14) myeloma but not
outcome of patients with del(17p). J Clin Oncol. 28:4630–4634.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mateos MV, Gutierrez NC, Martín-Ramos ML,
Paiva B, Montalbán MA, Oriol A, Martínez-López J, Teruel AI,
Bengoechea E, Martín A, et al: Outcome according to cytogenetic
abnormalities and DNA ploidy in myeloma patients receiving short
induction with weekly bortezomib followed by maintenance. Blood.
118:4547–4553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shaughnessy JD, Zhou Y, Haessler J, van
Rhee F, Anaissie E, Nair B, Waheed S, Alsayed Y, Epstein J, Crowley
J and Barlogie B: TP53 deletion is not an adverse feature in
multiple myeloma treated with total therapy 3. Br J Haematol.
147:347–351. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
An G, Acharya C, Deng S, Yi S, Xu Y, Qin
X, Sui W, Li Z, Shi L, Zang M, et al: Cytogenetic and clinical
marks for defining high-risk myeloma in the context of bortezomib
treatment. Exp Hematol. 43:168–176.e2. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Neben K, Lokhorst HM, Jauch A, Bertsch U,
Hielscher T, van der Holt B, Salwender H, Blau IW, Weisel K,
Pfreundschuh M, et al: Administration of bortezomib before and
after autologous stem cell transplantation improves outcome in
multiple myeloma patients with deletion 17p. Blood. 119:940–948.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sonneveld P, Salwender H, Van Der Holt B,
El Jarari L, Bertsch U, Blau LW, Zweegman S, Weisel KC, Vellenga E,
Pfreundschuh M, et al: Bortezomib induction and maintenance in
patients with newly diagnosed multiple myeloma: Long-term follow-up
of the HOVON-65/GMMG-HD4 trial. Blood. 126:272015.
|
|
30
|
Moreau P, Attal M, Pégourié B, Planche L,
Hulin C, Facon T, Stoppa AM, Fuzibet JG, Grosbois B, Doyen C, et
al: Achievement of VGPR to induction therapy is an important
prognostic factor for longer PFS in the IFM2005-01 trial. Blood.
117:3041–3044. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rajkumar SV, Jacobus S, Callander NS,
Fonseca R, Vesole DH, Williams ME, Abonour R, Siegel DS, Katz M and
Greipp PR; Eastern Cooperative Oncology Group, : Lenalidomide plus
high-dose dexamethasone versus lenalidomide plus low-dose
dexamethasone as initial therapy for newly diagnosed multiple
myeloma: An open-label randomised controlled trial. Lancet Oncol.
11:29–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Richardson PG, Weller E, Lonial E,
Jakubowiak AJ, Jagannath S, Raje NS, Avigan DE, Xie W, Ghobrial IM,
Schlossman RL, et al: Lenalidomide, bortezomib, and dexamethasone
combination therapy in patients with newly diagnosed multiple
myeloma. Blood. 116:679–686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tacchetti P, Terragna C, Galli M, Zamagni
E, Petrucci MT, Pezzi A, Montefusco V, Martello M, Tosi P, Baldini
L, et al: Bortezomib- and thalidomide-induced peripheral neuropathy
in multiple myeloma: Clinical and molecular analyses of a phase 3
study. Am J Hematol. 89:1085–1091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leiba M, Kedmi M, Duek A, Freidman T,
Weiss M, Leiba R, Nagler A and Avigdor A:
Bortezomib-cyclophosphamide-dexamethasone (VCD) versus
bortezomib-thalidomide-dexamethasone (VTD)-based regimens as
induction therapies in newly diagnosed transplant eligible patients
with multiple myeloma: A meta-analysis. Br J Haematol. 166:702–710.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moreau P, Pylypenko H, Grosicki S,
Karamanesht I, Leleu X, Grishunina M, Rekhtman G, Masliak Z, Robak
T, Shubina A, et al: Subcutaneous versus intravenous administration
of bortezomib in patients with relapsed multiple myeloma: A
randomised, phase 3, non-inferiority study. Lancet Oncol.
12:431–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hus I, Walter-Croneck A, Masternak A,
Jurczyszyn A, Usnarska-Zubkiewicz L, Bołkun Ł, Druzd-Sitek A, Rymko
M, Łętowska J, Lech-Marańda E, et al: Real-life experience with
bortezomib-based regimens in elderly patients with newly diagnosed
multiple myeloma and comorbidities: A Polish retrospective
multicenter study. Pol Arch Intern Med. 127:765–774.
2017.PubMed/NCBI
|
|
37
|
Goldschmidt H, Moreau P, Ludwig H,
Niesvizky R, Chng WJ, Joshua D, Weisel K, Spencer A, Orlowski RZ,
Feng S, et al: Carfilzomib-dexamethasone versus subcutaneous or
intravenous bortezomib in relapsed or refractory multiple myeloma:
Secondary analysis of the phase 3 ENDEAVOR study. Leuk Lymphoma.
59:1364–1374. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Merz M, Salwender H, Haenel M, Mai EK,
Bertsch U, Kunz C, Hielscher T, Blau IW, Scheid C, Hose D, et al:
Peripheral neuropathy associated with subcutaneous or intravenous
bortezomib in patients with newly diagnosed myeloma treated within
the GMMG MM5 phase III trial. Haematologica. 101:e485–e487. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Minarik J, Pavlicek P, Pour L, Pika T,
Maisnar V, Spicka I, Jarkovsky J, Krejci M, Bacovsky J, Radocha J,
et al: Subcutaneous bortezomib in multiple myeloma patients induces
similar therapeutic response rates as intravenous application but
it does not reduce the incidence of peripheral neuropathy. PLoS
One. 10:e01238662015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brioli A, Perrone G, Patriarca F, Pezzi A,
Nobile F, Ballerini F, Motta MR, Ronconi S, Tacchetti P, Catalano
L, et al: Successful mobilization of PBSCs predicts favorable
outcomes in multiple myeloma patients treated with novel agents and
autologous transplantation. Bone Marrow Transplant. 50:673–678.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kumar S, Dispenzieri A, Lacy MQ, Hayman
SR, Buadi FK, Gastineau DA, Litzow MR, Fonseca R, Roy V, Rajkumar
SV and Gertz MA: Impact of lenalidomide therapy on stem cell
mobilization and engraftment post-peripheral blood stem cell
transplantation in patients with newly diagnosed myeloma. Leukemia.
21:2035–2042. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Paripati H, Stewart AK, Cabou S, Dueck A,
Zepeda VJ, Pirooz N, Ehlenbeck C, Reeder C, Slack J, Leis JF, et
al: Compromised stem cell mobilization following induction therapy
with lenalidomide in myeloma. Leukemia. 22:1282–1284. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kumar S, Giralt S, Stadtmauer EA,
Harousseau JL, Palumbo A, Bensinger W, Comenzo RL, Lentzsch S,
Munshi N, Niesvizky R, et al: Mobilization in myeloma revisited:
IMWG consensus perspectives on stem cell collection following
initial therapy with thalidomide-, lenalidomide-, or
bortezomib-containing regimens. Blood. 114:1729–1735. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sinha S, Gertz MA, Lacy MQ, Dispenzieri A,
Hayman SR, Buadi FK, Dingli D, Micallef IN, Hogan WJ, Gastineau DA,
et al: Majority of patients receiving initial therapy with
lenalidomide-based regimens can be successfully mobilized with
appropriate mobilization strategies. Leukemia. 26:1119–1122. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Giebel S, Sadus-Wojciechowska M, Halaburda
K, Drozd-Sokolowska J, Wierzbowska A, Najda J, Mendrek W,
Sobczyk-Kruszelnicka M, Nowicki M, Holowiecki J and Czerw T:
Increased efficacy of intermediate-dose cytarabine + G-CSF compared
to DHAP + G-CSF for stem cell mobilization in patients with
lymphoma: An analysis by the polish lymphoma research group. Ann
Hematol. 95:263–269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Harousseau JL, Attal M and Avet-Loiseau H:
The role of complete response in multiple myeloma. Blood.
114:3139–3146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Child JA, Morgan GJ, Davies FE, Owen RG,
Bell SE, Hawkins K, Brown J, Drayson MT and Selby PJ; Medical
Research Council Adult Leukaemia Working Party, : High-dose
chemotherapy with hematopoietic stem cell rescue for multiple
myeloma. N Engl J Med. 348:1875–1883. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang M, Delasalle K, Feng L, Thomas S,
Giralt S, Qazilbash M, Handy B, Lee JJ and Alexanian R: CR
represents an early index of potential long survival in multiple
myeloma. Bone Marrow Transplant. 45:498–504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chanan-Kahn A and Giralt S: Importance of
achieving a complete response in multiple myeloma, and the impact
of novel agents. J Clin Oncol. 28:2612–2624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Attal M, Harousseau JL, Facon T, Guilhot
F, Doyen C, Fuzibet JG, Monconduit M, Hulin C, Caillot D,
Bouabdallah R, et al: Single versus double autologous stem-cell
transplantation for multiple myeloma. N Engl J Med. 349:2495–2502.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cavo M, Tosi P, Zamagni E, Cellini C,
Tacchetti P, Patriarca F, Di Raimondo F, Volpe E, Ronconi S,
Cangini D, et al: Prospective, randomized study of single compared
with double autologous stem-cell transplantation for multiple
myeloma: Bologna 96 clinical study. J Clin Oncol. 25:2434–2441.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Benyamini N, Avivi I, Dann EJ, Zuckerman
T, Lavi N and Katz T: Comparison of engraftment following different
stem cell mobilization modalities in patients with multiple myeloma
treated with a uniform induction regimen containing bortezomib,
cyclophosphamide and dexamethasone. Ann Hematol. 96:461–467. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Blimark CH, Turesson I, Genall A, Ahlberg
L, Björkstrand B, Carlson K, Forsberg K, Juliusson G, Linder O,
Mellqvist UH, et al: Outcome and survival of myeloma patients
diagnosed 2008–2015. Real-world data on 4904 patients from the
Swedish Myeloma Registry. Haematologica. 103:506–513. 2018.
View Article : Google Scholar : PubMed/NCBI
|