Introduction
According to the 2018 global cancer statistics,
colorectal cancer (CRC) has the third highest incidence rate of all
types of cancers worldwide; it also has the second highest
mortality rate (1). In USA, it has
been estimated that there will be >140,000 new CRC cases and
23,380 deaths 2019, accounting for ~1 in 7 cancer cases and deaths
(2).
Adjuvant therapy is typically selected by
pathological and clinical staging as two of the prognostic
predictors (3). Vascular invasion
(VI) is prevalent in CRC; it has been reported that ~23% of CRC and
VI cases are combined in postoperative pathology (4). Therefore, accurate identification of VI
involvement in patients with CRC is crucial for prognosis and
treatment strategy decisions.
VI is a strong prognostic indicator in CRC (3). A previous study has shown that there is
a significant association between VI and metastasis and a high rate
of recurrence in univariate analysis and multivariable analysis
(5). In the Union for International
Cancer Control, stage I rectal cancer (T1, T2 N0), VI is a
high-risk factor for disease progression and recurrence (6). In addition, VI is associated with poor
loco-regional outcomes, which are potentially valuable predictors
of local recurrence in rectal cancers (7). A previous study demonstrated that VI is
also associated with reduced overall survival rate and disease-free
survival rates, therefore serving as a strong prognostic marker
(8). In the clinical practice
guidelines published by the National Comprehensive Cancer Network
(NCCN) (3), VI is regarded as a
high-risk factor for systemic recurrence and stage III or high-risk
stage II.
It is recommended that patients with CRC with VI
receive adjuvant therapy (9). VI is
a strong prognostic indicator, and a predictor of CRC metastasis
and recurrence (5). A better
understanding of VI supports the decision of whether adjuvant
therapy is required, determining the adequacy of surgical
resection, and selecting the optimal treatment (8). Vascular endothelial growth factor
(VEGF) and its expressed products Fms related tyrosine kinase-1 and
kinase insert domain receptor, regulate endothelial cell
proliferation, migration, invasion, survival and branching
morphogenesis, which are reported to be associated with VI
(10,11). However, the practicality of these
biomarkers is limited (10,11).
Since the first reported clinical application of a
nomogram in 1928 (12), nomograms
have attracted increased attention. A nomogram is a user-friendly
graphical prediction model with strong clinical application
(13–15). Users can build the nomogram to obtain
points assigned to each predicted factor at the top of the scale
(13–15). Through this, the total points can be
transformed to predict the possible risk of a specific event for
patients in the lowest scale. To date, nomograms have been widely
used in the diagnosis and prognostic prediction of a variety of
malignancies, such as Ewing sarcoma and thymoma prognosis (14,15). The
use of some notional diagrams has even been considered to assess
the efficacy of chemotherapy in prognostic prediction (13). However, to the best of our knowledge,
no nomogram is available for the preoperative prediction of VI in
CRC.
In the present study, a nomogram with clinical
features for the individualized preoperative prediction of VI in
patients with CRC was developed and validated. The goodness of fit,
differentiation and clinical application value of the nomogram were
evaluated. To the best of our knowledge, this is the first nomogram
to predict preoperative VI in patients with CRC, which can provide
preoperative optimization strategies for selected patients.
Patients and methods
Study population
The present retrospective analysis was approved by
the Ethics and Human Subject Committee of Affiliated Tumor Hospital
of Guangxi Medical University. According to the specific inclusion
and exclusion criteria, the present study recruited 989 patients
with CRC between August 2013 and April 2018 in the Affiliated Tumor
Hospital of Guangxi Medical University. The inclusion criteria
consisted of the following: i) Pathological confirmation of CRC in
patients; ii) primary tumor resection had been performed; and iii)
the status of VI was obtainable in the postoperative pathological
report. The exclusion criteria included the following: i)
Preoperative therapy involving radiotherapy, chemotherapy or
chemoradiotherapy; ii) patients currently suffering from other
cancer diseases; and iii) the presence of hereditary non-polyposis
colon cancer or familial adenomatous polyposis. The corresponding
demographic and preoperative clinical parameters, such as age, sex,
body mass index (BMI), first-degree relatives' tumor history, blood
routine examination, serum immunoglobulin level, tumor primary
site, computed tomography (CT)-based on T stage (CT T stage) or N
stage (CT N stage), preoperative histologic grade and tumor gross
type, were collected. Weight change was obtained by self-reporting
within the last three months prior to diagnosis and measured every
week after hospitalization.
In total, 664 patients, including 389 male and 275
female patients, with complete information were enrolled. All 664
patients were randomly divided into two independent datasets at a
ratio of 7:3 based on a computer-generated random number (training
datasets: 468 cases; and validation datasets: 196 cases). T and N
stages were determined on the basis of the 7th edition of The
American Joint Committee on Cancer, Cancer Staging Manual (16).
Feature selection
Least absolute shrinkage and selection operator
(LASSO) is a penalized regression method that estimates the
regression coefficients by maximizing the log-likelihood function,
while restraining the sum of the absolute values of the regression
coefficients (17). Regression
coefficients estimated by LASSO are sparse, and many components are
exactly 0. Therefore, LASSO automatically deletes unnecessary
covariates. The LASSO logistic regression algorithm is used for
determining the regression of high-dimensional data, which is
applied in many fields, including in genome-wide association
studies, when it is difficult to find significant genetic factors
with expected statistical significance in a large amount of data.
The LASSO method can be used to screen out significant genetic
factors with expected statistical significance, to produce a number
of algorithms (18). The present
study employed the LASSO logistic regression algorithm in the
training dataset to select the most diagnostically predictive
features. All of the categorical variables were transformed into
dummy variables. The status of VI served as the dependent variable.
The suitable tuning parameter (λ) for LASSO logistic regression was
determined using cross-validation. LASSO logistic regression was
performed by package ‘glmnet’ function of ‘glmnet’ package. A
minimum λ was used for features selection. Features with non-zero
coefficients at the optimal were selected by the LASSO logistic
regression algorithm. Finally, the multiple logistic regression was
performed using the diagnostic features selected by LASSO in the
training dataset to construct the prediction model. The evaluation
of the prediction model was performed in the validation
dataset.
Nomogram construction and performance
assessment
The prediction model was constructed in the training
dataset, which used features selected by the LASSO algorithm, using
a multivariate logistic regression model. All of the selected
features entered the multivariate logistic regression model and the
coefficient of each feature was calculated. The predicted index of
each patient was calculated by the ‘predict’ function, based on the
model constructed in the training dataset. A nomogram was
formulated according to the resultants of the multivariable
analyses, which incorporated the selected features. The goodness of
fit between the observed value and the predicted value was examined
by the calibration curve and tested using the Hosmer-Lemeshow test,
which is a statistical test for goodness of fit for logistic
regression models and used frequently in risk prediction models
(19). An ideal calibration curve
perfectly fits the 45-degree reference line. The predictive
discrimination of the nomogram was evaluated using the receiver
operating characteristic (ROC) curve and the area under the curve
(AUC). In the logistic regression model, the value of the AUC was
the same as the concordance index (c-index). An AUC of 1.0 was
determined, indicating perfect discrimination of the nomogram.
Validation of the nomogram
To assess the performance of the nomogram, the
constructed nomogram was validated in the validation dataset. The
predicted value of each patient in this dataset was calculated
based on the formula constructed in the training dataset. The ROC
and AUC were used to evaluate the predictive discrimination of the
nomogram in the validation dataset. The calibration curve and
Hosmer-Lemeshow test were used to assess the goodness of fit of the
nomogram in the aforementioned dataset.
Decision curve analysis (DCA)
The DCA method was employed to evaluate the clinical
usefulness of the nomogram through quantitative training and
verification, and compared with treat-all-patients scheme or the
treat-none scheme to predict the net benefit under the dataset's
different threshold probabilities (20). The treat-none scheme assumed no
patient had a disease and the treat-all-patients scheme assumed all
patients had a disease.
Statistical analysis
Statistical analysis was performed using R version
3.4.0 and RStudio (Version 1.1.447) (21,22).
LASSO logistic regression analysis was carried out with the
‘glmnet’ software package (version 2.0–16; http://cran.r-project.org/web/packages/glmnet/index.html).
In addition, the multivariate logistic regression analysis,
nomogram building and calibration plots were conducted using the
‘rms’ package (version 5.1–3.1; http://cran.r-project.org/web/packages/rms/). The DCA
and Hosmer-Lemeshow test were performed with the functions, ‘dca.R’
and ‘HLtest.R,’ respectively. P<0.05 was considered to indicate
a statistically significant difference.
Results
Clinical characteristics
A total of 664 patients were included in the
analysis; >200 clinical parameters were collected in the present
study. Patient demographics and pathologic parameters are listed in
Table I. The average age of the
included patients was 59.2 years (range, 17–87 years). In total,
314 patients had were diagnosed with rectal cancer, and 350
patients were diagnosed with colon cancer at the Affiliated Tumor
Hospital of Guangxi Medical University. Of the 664 total patients,
139 had a history of first-degree relatives with tumors, while 525
patients did not. The average weight loss over the last 3 months
was 1.73 kgk (range, −3–16 kgk). In addition, these patients were
identified as VI-positive (203 cases) in the postoperative
pathology report.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Factor | n | % |
|---|
| Age, years |
|
|
|
17–30 | 14 | 2.1 |
|
31–45 | 69 | 10.4 |
|
46–60 | 259 | 39.0 |
|
>60 | 322 | 48.5 |
| Sex |
|
|
|
Male | 389 | 58.6 |
|
Female | 275 | 41.4 |
| Body mass index,
kg/m2 |
|
|
|
≤18.4 | 73 | 11.0 |
|
18.5–23.9 | 431 | 64.9 |
|
24-27.9 | 136 | 20.5 |
|
≥28 | 24 | 3.6 |
| Primary site |
|
|
|
Rectum | 314 | 47.3 |
|
Colon | 350 | 52.7 |
| Weight loss,
kg |
|
|
|
<3 | 474 | 71.4 |
|
3–6 | 132 | 19.9 |
|
>6 | 56 | 8.4 |
| First-degree
relatives' tumor history |
|
|
| No | 525 | 79.1 |
|
Yes | 139 | 20.9 |
| CT T Stage |
|
|
| T1 | 10 | 1.5 |
| T2 | 70 | 10.5 |
| T3 | 200 | 30.1 |
| T4 | 384 | 57.8 |
| CT N Stage |
|
|
| N0 | 371 | 55.9 |
| N1 | 190 | 28.6 |
| N2 | 103 | 15.5 |
|
Differentiation |
|
|
|
Well | 25 | 3.8 |
|
Moderately | 537 | 80.9 |
|
Poorly | 102 | 15.4 |
| Tumor gross
type |
|
|
|
Ulceration | 337 | 50.8 |
|
Infiltrative | 43 | 6.5 |
|
Ulceration and
Infiltrative | 40 | 6.0 |
|
Protruded | 239 | 36.0 |
|
Other | 5 | 0.8 |
| Tumor distance from
anus, cm |
|
|
|
<5 | 65 | 9.8 |
|
5–10 | 165 | 24.8 |
|
11–15 | 73 | 11.0 |
|
>15 | 361 | 54.4 |
| Perineural
invasion |
|
|
| No | 329 | 49.5 |
|
Yes | 335 | 50.5 |
| Vascular
invasion |
|
|
| No | 461 | 69.4 |
|
Yes | 203 | 30.6 |
| Lymphovascular
invasion |
|
|
| No | 429 | 64.6 |
|
Yes | 235 | 35.4 |
Feature selection
The LASSO logistic regression method was employed to
select the most significant prediction features in the prediction
model. The present study performed feature selection based on the
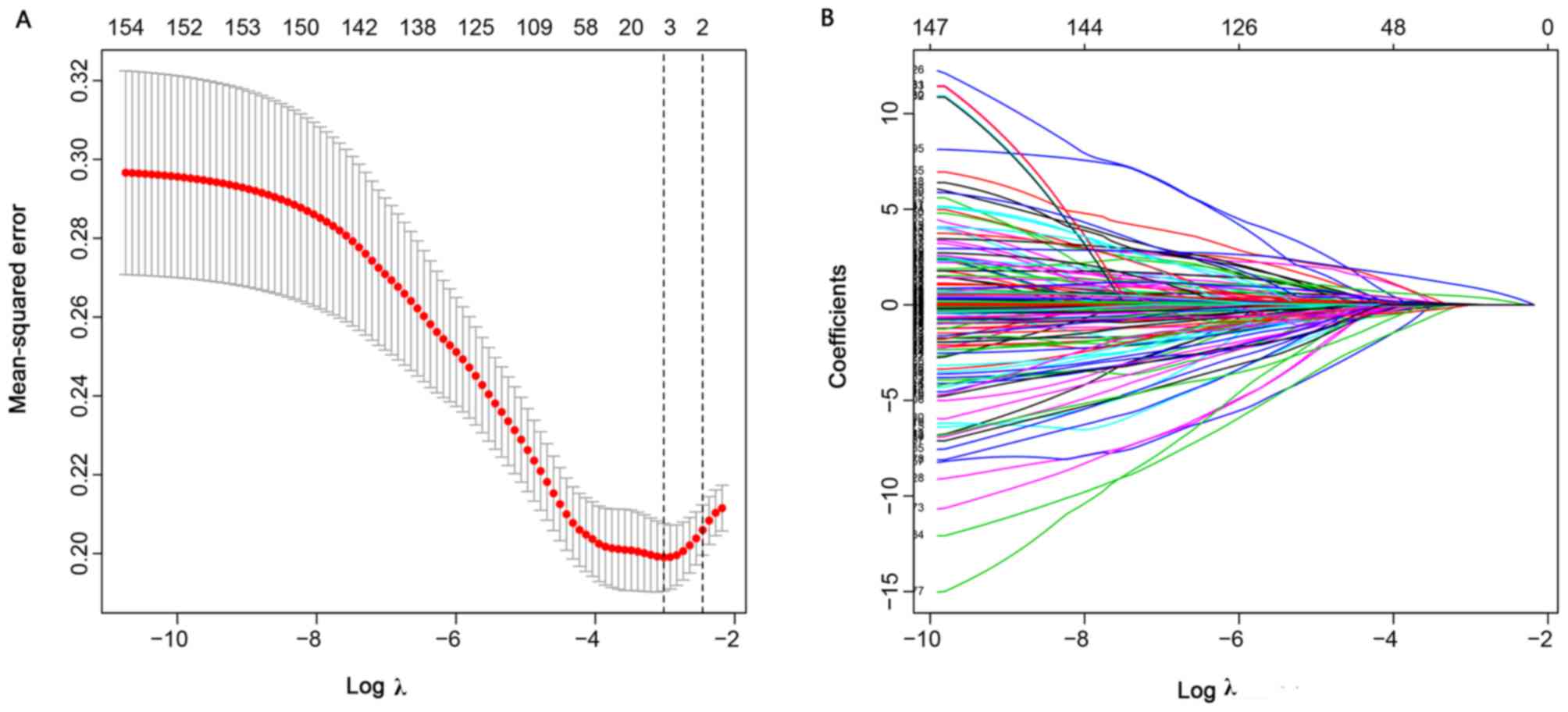
training dataset. Overall, 154 features were used in the LASSO
logistic regression. In addition, 4 features with non-zero
coefficients were selected by the LASSO logistic regression
algorithm with an optimal λ of 0.048 (Fig. 1A and B). The 4 features included
differentiation, CT-based N stage (CT N stage), hemameba and tumor
distance from the anus (cm).
Nomogram construction and performance
assessment
The 4 features selected by LASSO logistic regression
were included in the multivariate logistic regression modeling. As
shown in Table II, multivariate
logistic regression identified poor differentiation
(P=4.66×10−3), CT-based N1/N2 stage
(P=4.48×10−6) and hemameba (P=0.02) as independent
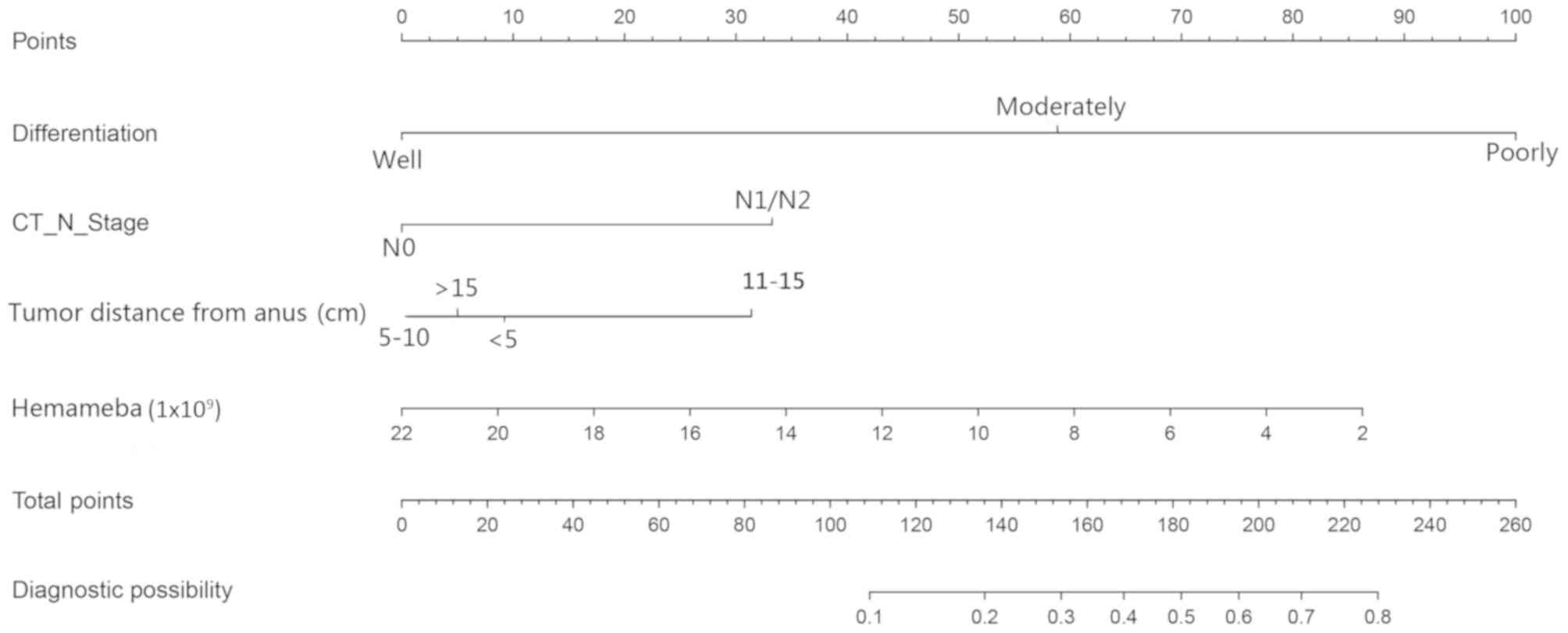
impact factors of VI. The present study constructed a nomogram
based on the features (Fig. 2). A
nomogramn can indicate the points assigned to each variable by the
top of the scale. The summation of each point (the total points)
can be transformed to predict the possible risk of VI for patients
at the lowest scale.
 | Table II.Multivariable logistic regression
analysis of the selected clinical features in the training set. |
Table II.
Multivariable logistic regression
analysis of the selected clinical features in the training set.
| Variable | Odds ratio (95%
CI) | P-value |
|---|
|
Differentiation |
|
|
|
Well | 1 |
|
|
Moderately | 5.92
(1.17–108.09) | 0.09 |
|
Poorly | 20.52
(3.77–384.19) |
4.66×10−3 |
| CT N Stage |
|
|
| N0 | 1 |
|
|
N1/N2 | 2.73
(1.78–4.22) |
4.48×10−6 |
| Tumor distance from
anus, cm |
|
|
|
<5 | 1 |
|
|
5–10 | 0.76
(0.34–1.73) | 0.50 |
|
11–15 | 1.95
(0.81–4.81) | 0.14 |
|
>15 | 0.88
(0.42–1.89) | 0.74 |
| Hemameba | 0.88
(0.79–0.97) | 0.02 |
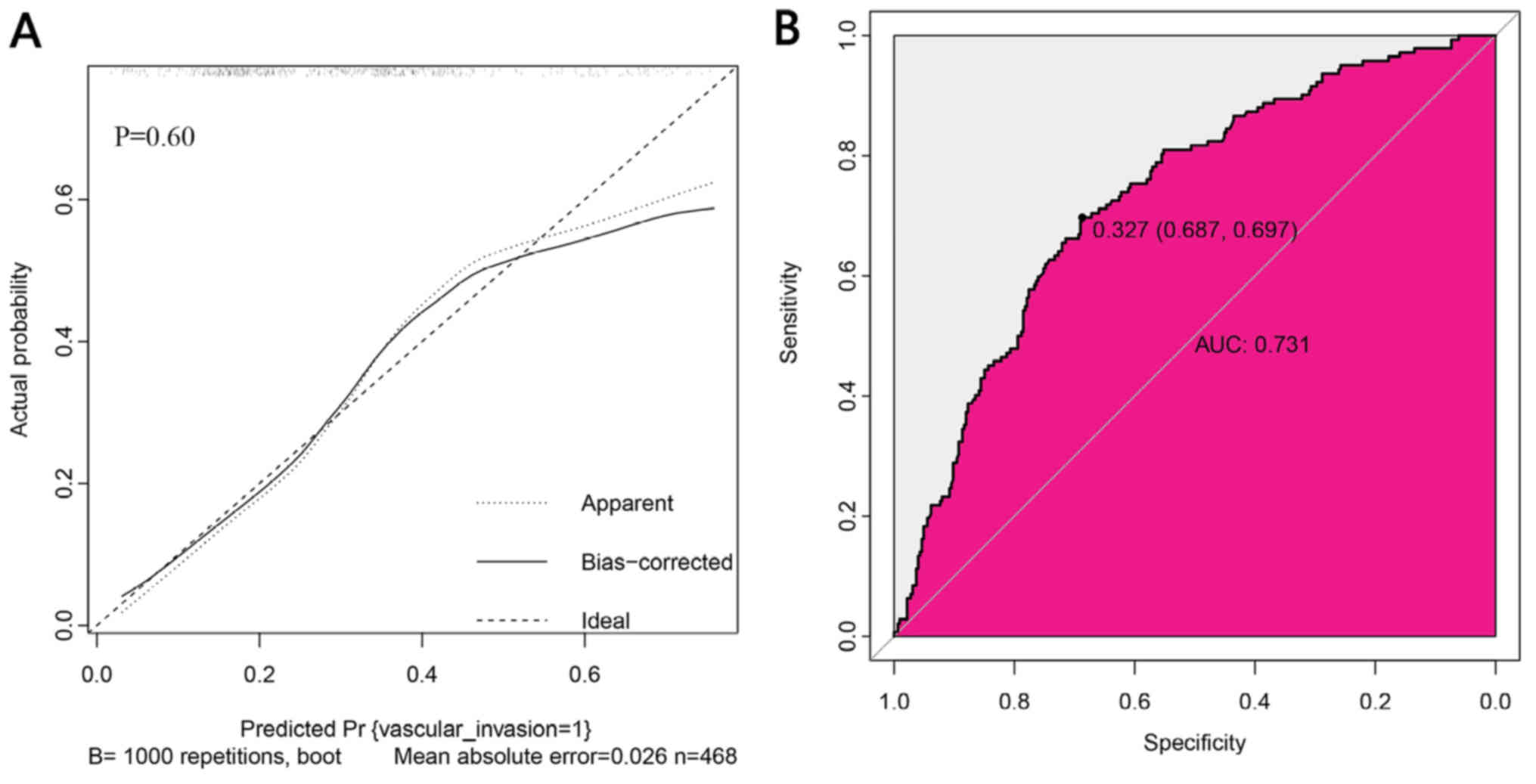
The calibration plot of the nomogram for the
probability of VI demonstrated a good agreement between prediction
and observation in the training data set (Fig. 3A). The P-value for the
Hosmer-Lemeshow test was 0.60 (Fig.
3A), which indicated that there was no departure from a perfect
fit. The AUC for the prediction nomogram was 0.731 in the training
dataset (Fig. 3B).
These 4 features were used in the construction of
the nomogram (Fig. 2). Each feature
corresponds to a specific point by drawing a straight line up to
the point axis. After the summation of the points has been plotted
on the master axis, which represents the probability of VI, it is
drawn directly down to the diagnostic axis. For example, 1 patient
with CRC was recorded to have poor differentiation (100 points), a
CT N stage of N1 (32 points), a tumor distance from the anus <5
cm (10 points), and the hemameba count was 10×109 (51
points). In this example, the total points equated to equals 193,
and the VI probability is ~60%. According to the 50% threshold for
this patient, they are high-risk and neoadjuvant chemotherapy
should be considered.
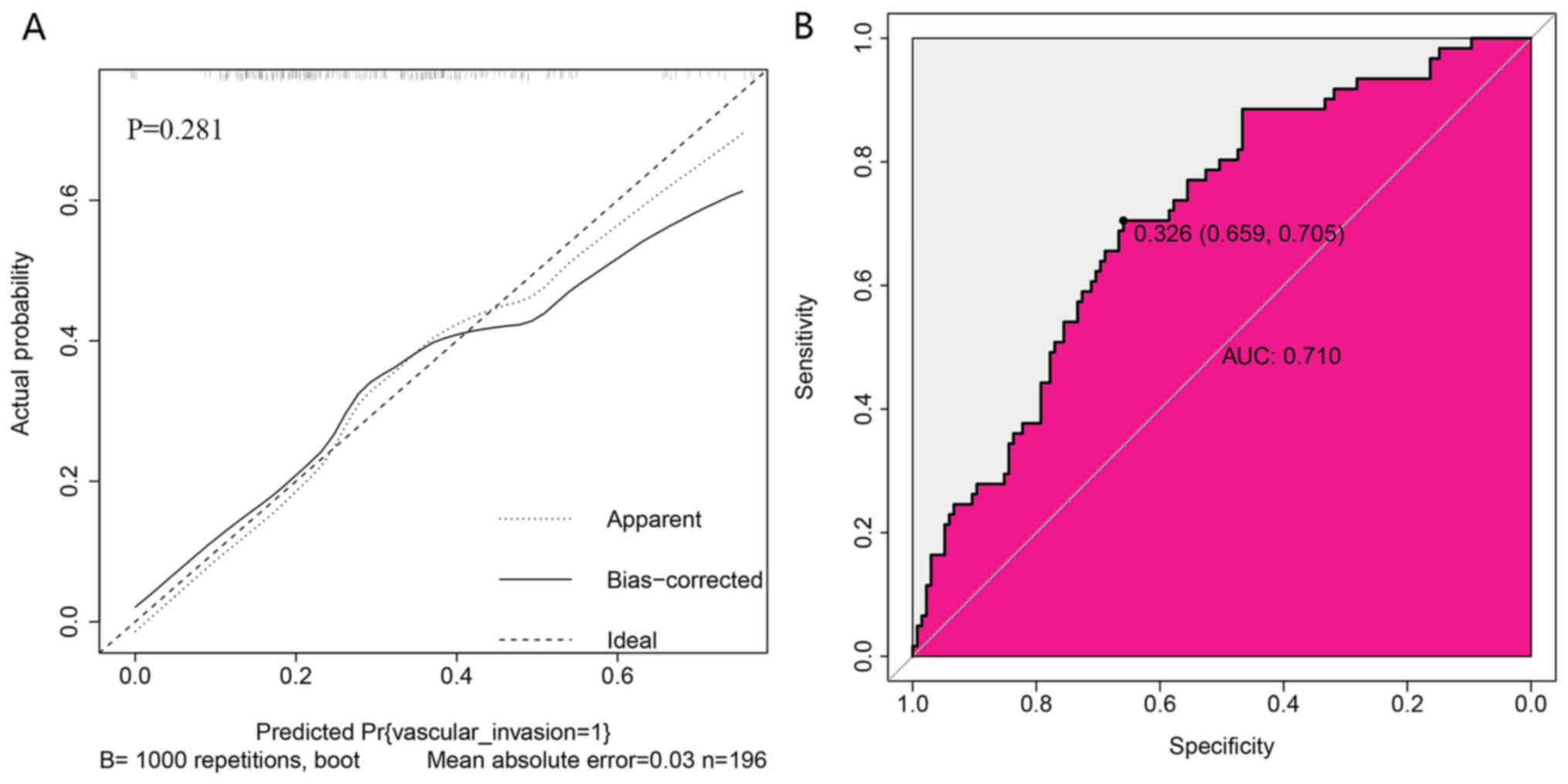
Validation of the nomogram
The present study observed good identification
(Fig. 4B) and good calibration
(Fig. 4A) in the validation dataset.
The nomogram exhibited an AUC of 0.710 (Fig. 4B). Validation of the calibration
curve exhibited good concordance between the predicted probability
and actual probability. The Hosmer-Lemeshow test yielded a
non-significant statistic (P=0.281; Fig.
4A).
Clinical usefulness of the
nomogram
Predicted probability of VI could be obtained from
the nomogram. With the DCA based on 664 patients, the present study
performed decision-making based on the evaluation for improvement
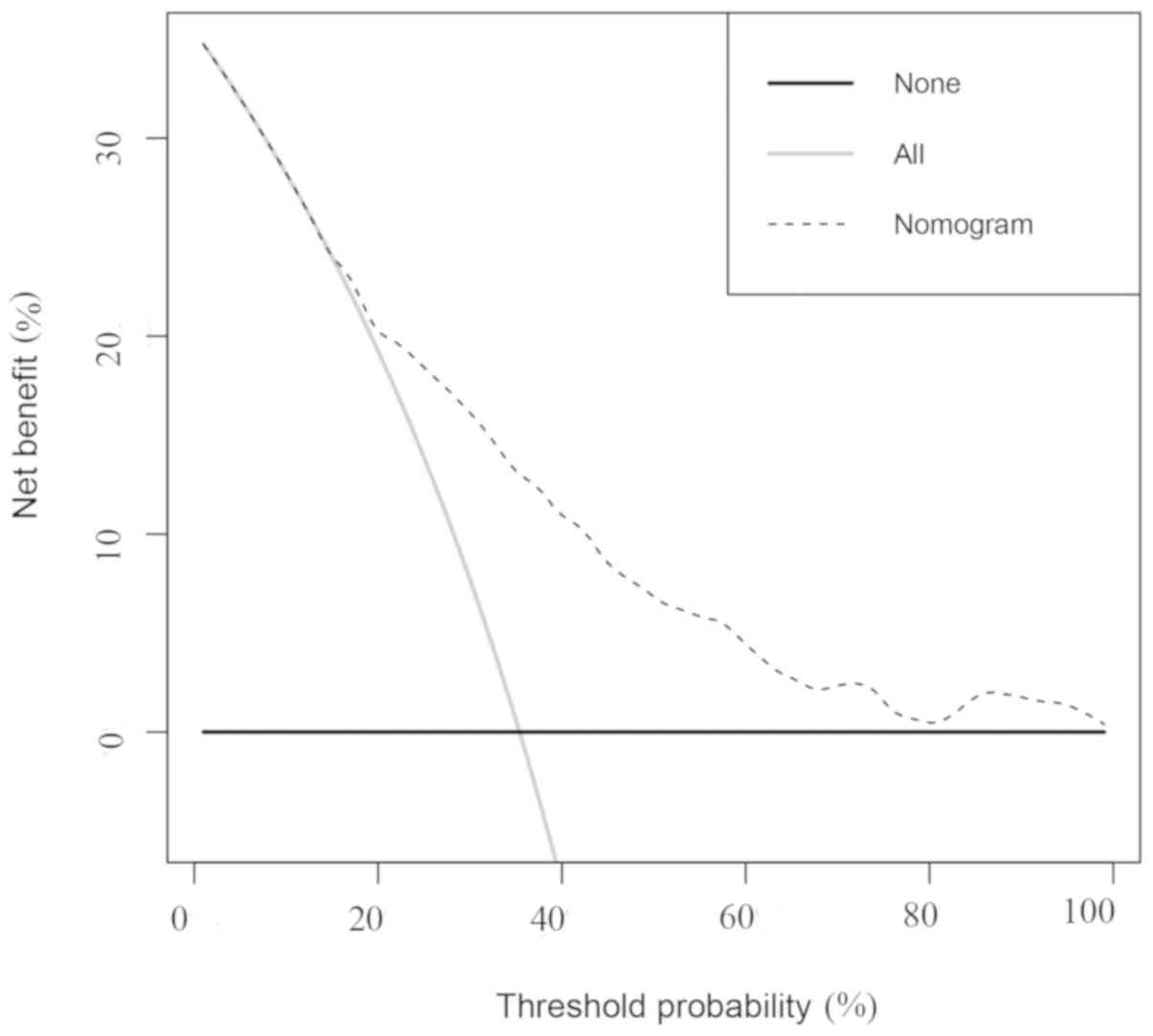
of the nomogram. As shown in Fig. 5,
the DCA curve indicated that if the probability of producing VI by
the nomogram is >20 and <70%, it is more beneficial to
predict VI with the treat-all-patients scheme or the treat-none
scheme. For example, with a 60% probability of VI, the nomogram
increases the net benefit by 4.4% of the treat-all-patients scheme
or the treat-none scheme. This suggests the nomogram is clinically
useful.
Discussion
In the present study, the single preoperative
clinical features of a nomogram for the prediction of VI in
patients with CRC, combined with clinical features was constructed
and validated. Nomograms have high prediction accuracy and
reliability. To the best of our knowledge, this is the first
preoperative predictive tool for patients with CRC, who are at a
high-risk of VI, in addition to facilitating the preoperative
optimization strategy for this group. Although magnetic resonance
imaging (MRI) and CT are the main diagnostic methods recommended by
the NCCN guidelines for preoperative clinical staging, they are
also the main diagnostic basis for the differential diagnosis of
VI. However, due to the limitations of these imaging techniques, CT
and MRI need to accurately identify VI, especially small vessel
invasion; however, technical problems remain, affecting the staging
and prognosis (23,24). A previous reported that the accuracy
of CT recognition of VI is 30.9%, and the accuracy of MRI
recognition of VI is 54% (23,24).
However, the accuracy of CT and MRI in the identification of VI is
associated with the clinical experience of doctors, therefore this
accuracy rate may be even lower (23,24).
However, the present nomogram revealed that the AUC value of VI is
0.731, which is of high sensitivity and specificity. Both have
their own advantages, however the combination of the two can better
identify VI (23,24). VI is a fundamental determinant of
solid tumor progression, which is a strong prognostic indicator in
CRC (25). A number of studies have
demonstrated that VI is a negative prognostic index for the
survival of patients undergoing radical resection of CRC (3,9,16).
The tumor microenvironment (TME) is a complex system
composed of cells, cytokines and extracellular matrix (26). VI is associated with the influence of
TME (26,27). Tumor cell proliferation requires
nutrients and energy, and vessels are important channels for
providing the above (27). The
secretion of VEGF, fibroblast growth factor, angiopoietin-like
proteins and the corresponding inflammatory cells by tumor cells
and other cells in the TME environment can promote the formation of
new vessels (28,29). This can lead to the recurrence and
metastasis of the tumor (27–29).
Therefore, VI-positive can mean the progression or recurrence of
the disease (30).
To construct a nomogram for the preoperative
prediction of VI in patients with CRC, the present study first
screened out the most significant predictive features using a LASSO
logistic regression. This method has been widely used in the
feature selection of high-dimensional data (18). It is more suitable than the linear
regression method to analyze the data of the selection study with
dichotomous fitness results, and it is regarded as an analytical
tool for the empirical study of multivariate selection (31).
The nomogram had favorable discrimination and
calibration, with a P-value for the Hosmer-Lemeshow test of 0.60
and an AUC of 0.731 in the training dataset. The results of the
nomogram were evaluated in the validation dataset, which indicated
that the nomogram had reasonable discrimination and good
calibration, with an AUC of 0.710 and a P-value 0.281 via the
Hosmer-Lemeshow test. The DCA curve showed that if the probability
of VI generated by the nomogram was >20 and <70%, the
prediction of VI will be more effective than either the treat-all
scheme or the treat-none scheme. Therefore, the preoperative
nomogram could be used as a clinical predictor of VI in patients
with CRC.
A total of 4 factors were finally identified by the
present nomogram: Differentiation, CT N stage, hemameba and tumor
distance from the anus. Previous reports have demonstrated that
these factors have an unusual impact on the prognosis of CRC
(32–34), however, to the best of our knowledge,
this is the first time they have been incorporated for modeling. A
previous study reported the association between VI and distant
metastasis and depth of invasion (32). As the differentiation level is
associated with the malignant degree of the tumor, this study
suggests that the degree of differentiation may be a reasonable
predictor of VI (3,32). Preoperative MRI examination is an
effective and convenient method for evaluating tumor staging, depth
of invasion and local metastasis (35).
Inflammatory markers include inflammatory cells,
various immune cells, cytokines, chemokines and pro-inflammatory
mediators, of which hemameba is the most significant indicator
(28,36). This is because hemameba is associated
with the pathogenesis of various inflammatory processes, including
allergies, parasitic diseases, bacterial and viral infections, and
tumor immune tissue damage (29,33,37).
Chronic inflammation can be caused by tumor proliferation (34). Hemameba accumulate in inflammatory
sites through blood circulation (28). Hemameba and their secreted products
promote the development and metastasis of tumors through the immune
response. For example, CCL 20/CCR 6 mediate organ selective liver
metastasis of colorectal cancer (29,36–39).
A previous tudy reported that ~20% of cancer-related
mortality is associated with inflammatory cells leading to cell
transformation and the enhancement of tumor cell invasion. White
blood cells are an important component of inflammatory cells
(27). Hemameba also play an
important role at different stages of tumor development, including
initiation, promotion, malignant conversion, invasion and
metastasis (34), which is the index
of tumor therapy. It can also be used to predict postoperative
infection in patients (40). A
previous study also reported that it is an independent risk factor
for surgical site infection (40).
The present study presented with several
limitations. First of all, due to the single center utilized for
retrospective research, potential selection bias is inevitable.
Secondly, although a genome classifier is a promising predictor, no
application of genome features has been considered. In addition,
the sample size of the validation set is small, which may affect
the credibility of the evaluation results to some extent. Finally,
the validation of the results using the same cohort of patients is
another potential limitation of the study, and a larger external
validation with multi-center and larger samples may the optimal
choice. Therefore, further efforts are required to collect
additional data and incorporate more impartial predictors to
improve the performance of the model.
In conclusion, this model has good discrimination
and calibration capability. It may be used in patients with CRC
prior to surgery as it predicted VI for the majority of patients
and it may help to provide accurate treatment options.
Acknowledgements
Not applicable.
Funding
The present study was supported by the
Self-financing Research Project of the Health and Family Planning
Commission of Guangxi Zhuang Autonomous Region (grant no.
Z2015607), Guangxi Medical and Health Appropriate Technology
Development and Promotion Application Project (grant no. S2017098)
and Guangxi Science and Technology Department Project (grant no.
GuikeAB16380202).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WT, LG, WX, XH and JL conceived and designed the
experiments. XH, JL, GW, FJ, SC, CZ, WX, WT, LG, WY, CL and ZL
performed the experiments. XH, JL and GW analyzed the data. XH, JL,
GW, FJ, SC, CZ, WX, WT, WY, CL and ZL contributed to the reagents,
materials and analysis tools used in the present study. XH, JL, WT
and WX wrote the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics and Human
Subject Committee of Affiliated Tumor Hospital of Guangxi Medical
University (approval no. LW2019020, Nanning, China). Due to the
retrospective design of the current study and patient
anonymization, the review board determined that informed consent
was not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Benson AB III, Venook AP, Cederquist L,
Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC,
Fichera A, et al: Colon cancer, version 1.2017, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
15:370–398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Betge J, Pollheimer MJ, Lindtner RA,
Kornprat P, Schlemmer A, Rehak P, Vieth M, Hoefler G and Langner C:
Intramural and extramural vascular invasion in colorectal cancer:
Prognostic significance and quality of pathology reporting. Cancer.
118:628–138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siddiqui MRS, Simillis C, Hunter C, Chand
M, Bhoday J, Garant A, Vuong T, Artho G, Rasheed S and Tekkis P: A
meta-analysis comparing the risk of metastases in patients with
rectal cancer and MRI-detected extramural vascular invasion
(mrEMVI) vs mrEMVI-negative cases. Br J Cancer. 116:1513–1519.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blumberg D, Paty PB, Picon AI, Guillem JG,
Klimstra DS, Minsky BD, Quan SH and Cohen AM: Stage I rectal
cancer: Identification of high-risk patients. J Am Coll Surg.
186:574–580. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bentzen SM, Balslev I, Pedersen M,
Teglbjaerg PS, Hanberg-Sørensen F, Bone J, Jacobsen NO, Sell A,
Overgaard J, Bertelsen K, et al: Time to loco-regional recurrence
after resection of Dukes' B and C colorectal cancer with or without
adjuvant postoperative radiotherapy. A multivariate regression
analysis. Br J Cancer. 65:102–107. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhangu A, Fitzgerald JE, Slesser A,
Northover JM, Faiz O and Tekkis P: Prognostic significance of
extramural vascular invasion in T4 rectal cancer. Colorectal Dis.
15:e665–e671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Benso AB, Venook AP, Al-Hawary MM,
Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D and
Engstrom PF: NCCN guidelines insights: Colon cancer, version
2.2018. J Natl Compr Canc Netw. 16:359–369. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaipainen A, Korhonen J, Pajusola K,
Aprelikova O, Persico MG, Terman BI and Alitalo K: The related
FLT4, FLT1, and KDR receptor tyrosine kinases show distinct
expression patterns in human fetal endothelial cells. J Exp Med.
178:2077–2088. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nguyen QD, Rodrigues S, Rodrigue CM, Rivat
C, Grijelmo C, Bruyneel E, Emami S, Attoub S and Gespach C:
Inhibition of vascular endothelial growth factor (VEGF)-165 and
semaphorin 3A-mediated cellular invasion and tumor growth by the
VEGF signaling inhibitor ZD4190 in human colon cancer cells and
xenografts. Mol Cancer Ther. 5:2070–2077. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Downs AW: Blood, A study in general
physiology. Can Med Assoc J. 19:754–756. 1928.
|
|
13
|
Massacesi C, Norman A, Price T, Hill M,
Ross P and Cunningham D: A clinical nomogram for predicting
long-term survival in advanced colorectal cancer. Eur J Cancer.
36:2044–2052. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Q, Wu ZY and Lin ZQ: A nomogram to
predict prognosis in Ewing sarcoma of bone. J Bone Oncol.
15:1002232019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao M, Yin J, Yang X, Jiang T, Lu T,
Huang Y, Li M, Yang X, Lin M, Niu H, et al: Nomogram to predict
thymoma prognosis: A population-based study of 1312 cases. Thorac
Cancer. 10:1167–1175. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edge SB and Compton CC: The American Joint
Committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bickel PJ, Ritov Y and Tsybakov AB:
Simultaneous analysis of Lasso and dantzig selector. Ann Stat.
37:1705–1732. 2009. View Article : Google Scholar
|
|
18
|
Jiang Y, He Y and Zhang H: Variable
selection with prior information for generalized linear models via
the prior LASSO method. J Am Stat Assoc. 111:355–376. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paul P, Pennell ML and Lemeshow S:
Standardizing the power of the Hosmer-Lemeshow goodness of fit test
in large data sets. Stat Med. 32:67–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van Calster B, Wynants L, Verbeek JFM,
Verbakel JY, Christodoulou E, Vickers AJ, Roobol MJ and Steyerberg
EW: Reporting and interpreting decision curve analysis: A guide for
investigators. Eur Urol. 74:796–804. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
R Core Team, . R: A language and
environment for statistical computingR Foundation for Statistical
Computing; Vienna, Austria: 2012, ISBN 3-900051-07-0. http://www.R-project.org/
|
|
22
|
RStudio Team, . RStudio: Integrated
development for R. RStudio, Inc, Boston, MA. 2015, http://www.rstudio.com/
|
|
23
|
Jhaveri KS, Hosseini-Nik H, Thipphavong S,
Assarzadegan N, Menezes RJ, Kennedy ED and Kirsch R: MRI Detection
of extramural venous invasion in rectal cancer: Correlation with
histopathology using elastin stain. AJR Am J Roentgenol.
206:747–755. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao X, Yang SX, Song XH, Cui YC, Ye YJ and
Wang Y: Prognostic significance of computed tomography-detected
extramural vascular invasion in colon cancer. World J
Gastroenterol. 22:7157–7165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Angelucci A, Delle Monache S, Cortellini
A, Di Padova M and Ficorella C: ‘Vessels in the Storm’: Searching
for prognostic and predictive angiogenic factors in colorectal
cancer. Int J Mol Sci. 19(pii): E2992018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Egeblad M, Nakasone ES and Werb Z: Tumors
as organs: Complex tissues that interface with the entire organism.
Dev Cell. 18:884–901. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bahrami A, Khazaei M, Hassanian SM,
ShahidSales S, Joudi-Mashhad M, Maftouh M, Jazayeri MH, Parizade
MR, Ferns GA and Avan A: Targeting the tumor microenvironment as a
potential therapeutic approach in colorectal cancer: Rational and
progress. J Cell Physiol. 233:2928–2936. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carbone C, Piro G, Merz V, Simionato F,
Santoro R, Zecchetto C, Tortora G and Melisi D: Angiopoietin-like
proteins in angiogenesis, inflammation and cancer. Int J Mol Sci.
19(pii): E4312018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kounis NG, Soufras GD, Tsigkas G and
Hahalis G: White blood cell counts, leukocyte ratios, and
eosinophils as inflammatory markers in patients with coronary
artery disease. Clin Appl Thromb Hemost. 21:139–143. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Courtney ED, West NJ, Kaur C, Ho J, Kalber
B, Hagger R, Finlayson C and Leicester RJ: Extramural vascular
invasion is an adverse prognostic indicator of survival in patients
with colorectal cancer. Colorectal Dis. 11:150–156. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Janzen FJ and Stern HS: Logistic
regression for empirical studies of multivariate selection.
Evolution. 52:1564–1571. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fujii T, Sutoh T, Morita H, Yajima R,
Yamaguchi S, Tsutsumi S, Asao T and Kuwano H: Vascular invasion,
but not lymphatic invasion, of the primary tumor is a strong
prognostic factor in patients with colorectal cancer. Anticancer
Res. 34:3147–3151. 2014.PubMed/NCBI
|
|
33
|
Shalapour S and Karin M: Immunity,
inflammation, and cancer: An eternal fight between good and evil. J
Clin Invest. 125:3347–3355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singh R, Mishra MK and Aggarwal H:
Inflammation, immunity, and cancer. Mediators Inflamm.
2017:60273052017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Elmi A, Hedgire SS, Covarrubias D, Abtahi
SM, Hahn PF and Harisinghani M: Apparent diffusion coefficient as a
non-invasive predictor of treatment response and recurrence in
locally advanced rectal cancer. Clin Radiol. 68:e524–e531. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang WY, Berndt SI, Shiels MS, Katki HA,
Chaturvedi AK, Wentzensen N, Trabert B, Kemp TJ, Pinto LA,
Hildesheim A, et al: Circulating inflammation markers and
colorectal adenoma risk. Carcinogenesis. 40:765–770. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oliver-Baxter JM, Whitford HS, Turnbull DA
and Bond MJ: Effects of vitamin supplementation on inflammatory
markers and psychological wellbeing among distressed women: A
randomized controlled trial. J Integr Med. 16:322–328. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin WF, Lu JY, Cheng BB and Ling CQ:
Progress in research on the effects of traditional Chinese medicine
on the tumor microenvironment. J Integr Med. 15:282–287. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sagawa M, Yoshimatsu K, Yokomizo H, Yano
Y, Okayama S, Usui T, Yamaguchi K, Shiozawa S, Shimakawa T, Katsube
T, et al: Worse preoperative status based on inflammation and host
immunity is a risk factor for surgical site infections in
colorectal cancer surgery. J Nippon Med Sch. 84:224–230. 2017.
View Article : Google Scholar : PubMed/NCBI
|