Introduction
Lung cancer is one of the most common types of
malignant cancer and the leading cause of cancer-associated death
in Taiwan, United States and the European Union (1,2). Wang
et al (3) have shown that the
5-year overall survival (OS) rate for all patients with lung cancer
in Taiwan is 15.9%, being relatively lower in patients with
late-stage lung cancer (4.9% in stage IV). For a large proportion
of patients, the disease is initially diagnosed at a later stage of
cancer, and drug treatment is required for long-term survival
improvement. Typically, systemic therapy is used in patients with
advanced or recurring disease following initial definitive
treatment. The therapeutic regimen used depends on the stage of the
cancer, the molecular characteristics of the tumour and the
patient's overall medical condition. Advances in drug treatments
have increased the OS of patients with lung cancer and may control
tumour-associated symptoms without adversely affecting the
patients' overall quality of life (QoL) (4–6).
Prior to the development of epidermal growth factor
receptor (EGFR)-tyrosine kinase inhibitor (TKI) therapy,
chemotherapy with platinum-based doublets was administered to
patients with stage IV non-small-cell lung cancer (NSCLC). Several
meta-analyses have demonstrated the benefits of platinum-based
doublet chemotherapy (7). The
double-drug treatment strategy also results in improved OS rates
compared with a single-drug chemotherapy regimen (8). Platinum-based doublets are usually
combined with a third-generation cytotoxic drug, such as
gemcitabine, vinorelbine or taxane; incorporation of pemetrexed and
other drugs into individual treatment schedules may also be
considered (9). Single- and
double-drug chemotherapy has shown benefits in some elderly
patients (>70 years) (10).
Compared with patients who received supportive care alone, patients
treated with vinorelbine exhibited improved 1-year OS rates and a
significantly improved QoL, with acceptable toxic effects (7). Furthermore, EGFR mutation (+) patients
treated with EGFR-TKIs (such as, gefitinib, erlotinib or afatinib)
exhibited a higher response rate, longer progression-free survival
(PFS) and an improved QoL compared with patients treated with
standard platinum-based doublet chemotherapy (11,12).
The aim of lung cancer drug treatment is to control
symptoms and improve the OS of patients. In Taiwan, platinum-based
doublet chemotherapy and EGFR-TKIs are frequently used as a
first-line treatment combined with third-generation cytotoxic
combinations [Third generation Cytotoxic Combination (TCC),
including paclitaxel, vinorelbine, gemcitabine and docetaxel], or
as a monotherapy in select patients. A better suited drug treatment
may be associated with improved outcomes. The aim of the present
retrospective cohort study was to determine the real-world
prescription rates of the various drugs used to treat patients with
lung cancer in Taiwan and analyse the outcomes.
Materials and methods
Data source and patient
definition
The present study is a retrospective,
population-based study using claims records from the entire
National Health Insurance Database (NHID) of Taiwan between 2010
and 2015. NHID contains details of all the citizens in Taiwan
(23,492,074 in 2015). NHID of Taiwan is a publicly available
database through formal application and approved by the Health and
Welfare Data Science Centre at The Ministry of Health and Welfare,
Taiwan (https://dep.mohw.gov.tw/DOS/np-2500-113.html). The
International Classification of Diseases, 9th Revision (ICD-9);
Clinical Modification code (ICD-9 code 162) and catastrophic
illness certificate (ICD-9 code 162) were used to select patients
with lung cancer. This method of using a catastrophic illness
certificate as the diagnostic criteria for lung cancer is strict
and reliable. Patients who did not have a medical record of lung
cancer with ambulatory (expenditures due to visits) and inpatient
(expenditures due to admissions) care in the year 2010 were defined
as lung cancer patients during 2011–2015 and were enrolled in this
study as treatment-naive lung cancer patients. The TNM Staging of
lung cancer and EGFR gene mutation status were identified according
to the linked cancer registration file (13). Cancer registration file was founded
in 1979. Hospitals with >50-bed capacity, which are able to
provide care for outpatients and hospitalized patients with cancer
were recruited and reported all newly diagnosed cases of malignant
neoplasms to the registry. Staging and treatment details were
required to report for specific cancers in 2002 and Site-specific
factors (EGFR, Kras and other risk factors) were first included in
2012. Since the present study was between 2010 and 2015,
information on EGFR mutation from certain patients may be
missing.
Research ethics approval
The protocol used in the present study was approved
by the Joint Institutional Review Board Taiwan R.O.C. (Protocol
Number: 14-S-007). As this study was a retrospective database
analysis study, it does not require informed consent according to
local legislation issued by the Ministry of Health and Welfare in
Taiwan (14).
Assessment
The primary objective of the present study was to
determine the first-line drug treatment pattern in treatment-naive
patients with lung cancer. The medications used for treating
patients with lung cancer were in accordance with the Anatomical
Therapeutic Chemical (ATC) classification (15). The use of antineoplastic and
immunomodulating agents (ATC code, L) was monitored in these
patients. The agents were categorized into five classes:
Platinum-based compounds, third-generation cytotoxin combinations
(paclitaxel, vinorelbine, gemcitabine and docetaxel; TCC),
monotherapy (single third-generation cytotoxin; paclitaxel,
vinorelbine, gemcitabine and docetaxel), EGFR-TKIs (afatinib,
gefitinib and erlotinib) and others (other agents or combinations
which were not included in the other groups). Following diagnosis
of lung cancer, the first-line treatment was defined as the first
anti-neoplastic agent prescription and its combination with other
drugs within 90 days. Patients who underwent surgery were
identified by their cancer registration file. The second-line
treatment was identified as addition of one or more new
anti-neoplastic agents following the first-line treatment. The code
for neutropenia was ICD-9:288.0; for thrombocytopenia, ICD-9:287.4;
Nausea and vomiting, ICD-9:787.0; neuropathy, ICD-9:357.3; rash,
ICD-9:782.1; diarrhoea, ICD-9:787.9; nail disorders, ICD-9:703.8–9;
and finger and toe disorders ICD-9:681.0–1.
Data analyses
SAS 9.1 (SAS Institute Inc.) was used for data
analyses. The variable measures were identified based on the
criteria described above. Frequencies/percentages were used to
describe categorical variables. A log-rank test was used to compare
the Kaplan-Meier curves from different treatment groups. OS for
first- and second-line treatments were calculated from the first
day of drug administration of first- and second-line treatments,
respectively, until death. Cox regression analysis was used to
identify the independent risk factors.
Results
Sample description
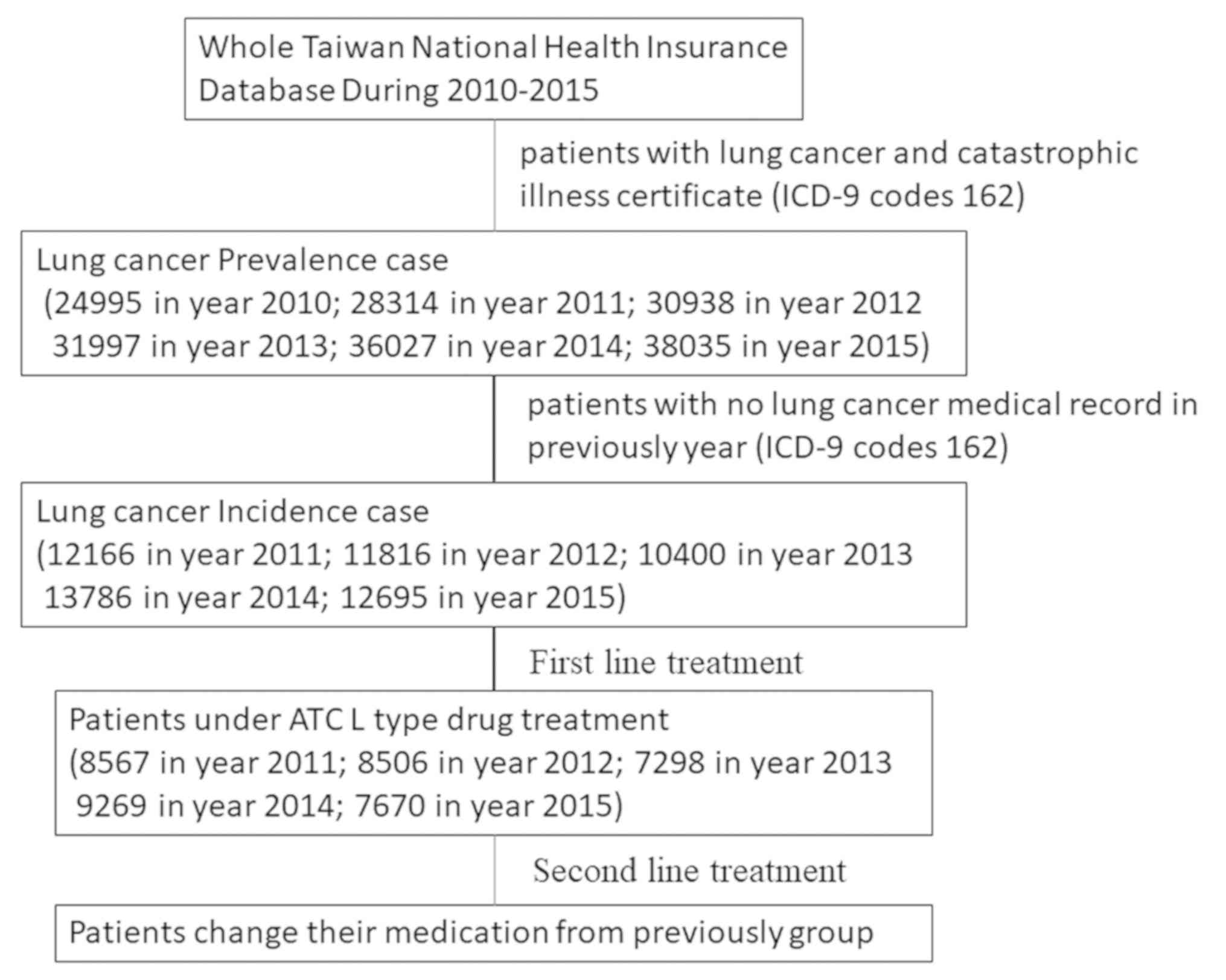
In 2015, the population of Taiwan was ~23.4 million,
of which 38,035 patients had lung cancer and there were 12,695 new
cases of lung cancer in Taiwan. According to the National Health
Insurance Database (NHID), lung cancer prevalence in Taiwan
increased from 0.11 to 0.16% between 2010 and 2015, while the
annual incidence density increased from 0.0524 to 0.0540% (Table I; Fig.
1).
 | Table I.Prevalence and incidence of patients
with lung cancer in Taiwan. |
Table I.
Prevalence and incidence of patients
with lung cancer in Taiwan.
| Year | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|
| Total
beneficiaries | 23,162,123 | 23,224,912 | 23,315,822 | 23,373,517 | 23,433,753 | 23,492,074 |
| Prevalence, no.
(%) | 24,995 (0.11%) | 28,314 (0.12%) | 30,938 (0.13%) | 31,997 (0.14%) | 36,027 (0.15%) | 38,035 (0.16%) |
| Incidence, no.
(%) | – | 12,166 (0.0524%) | 11,816 (0.0507%) | 10,400 (0.0445%) | 13,786 (0.0588%) | 12,695 (0.0540%) |
| Treated with an ATC L
type drug, no. (%) | – | 8,567 (70.42%) | 8,506 (71.99%) | 7,298 (70.17%) | 92,69 (67.23%) | 7,670 (60.42%) |
First-line anti-neoplastic drug
prescriptions in patients with lung cancer
Among the treatment-naive patients with lung cancer,
7,298–9,269 patients per year (60.42–71.99%) were administered
anti-neoplastic and immuno-modulating drugs (Fig. 2; Table
SI). Platinum-based doublet chemotherapy was the most
frequently prescribed first-line treatment (47.75% in 2011 and
39.97% in 2015), followed by EGFR-TKI therapy (21.00% in 2011 and
32.48% in 2015), other drugs (~20%), monotherapy (5.59% in 2011 and
5.15% in 2015) and TCC therapy (0.71% in 2011 and 0.37% in 2015)
(Fig. 2; Table SI).
 | Figure 2.Percentage of patients treated with
each of the various first-line treatments. The first line
treatments were categorized into five groups: Platinum-based; TCC;
monotherapy, EGFR-TKIs and others. EGFR-TKI, epidermal growth
factor receptor-tyrosine kinase inhibitor. TCC, third-generation
cytotoxin combinations (paclitaxel, vinorelbine, gemcitabine and
docetaxel); monotherapy, single third-generation cytotoxin:
Paclitaxel, vinorelbine, gemcitabine and docetaxel; EGFR-TKIs,
afatinib, gefitinib and erlotinib; others, other agents or
combination which did not fit into any of the categories. |
First-line clinical assessments
To determine the efficacy of different treatments
and distinguish the patients' lung cancer stages, each patient's
survival data was linked with their cancer registry file. Some of
the cancer registration files lacked information, and as such, the
final number of patients used for further staging and survival
analysis was less than the total number of patients. Table II shows the patients' demographic
data. A total of 38,100 patients underwent surgery or chemotherapy:
Group 1 (n=5,077), only surgery; group 2 (n=7,392), surgery with
chemotherapy; and group 3 (n=25,631), only chemotherapy or
EGFR-TKIs. In group 3, 10,588 patients underwent platinum-based
doublet chemotherapy, 179 TCC therapy, 771 monotherapy, 8,008
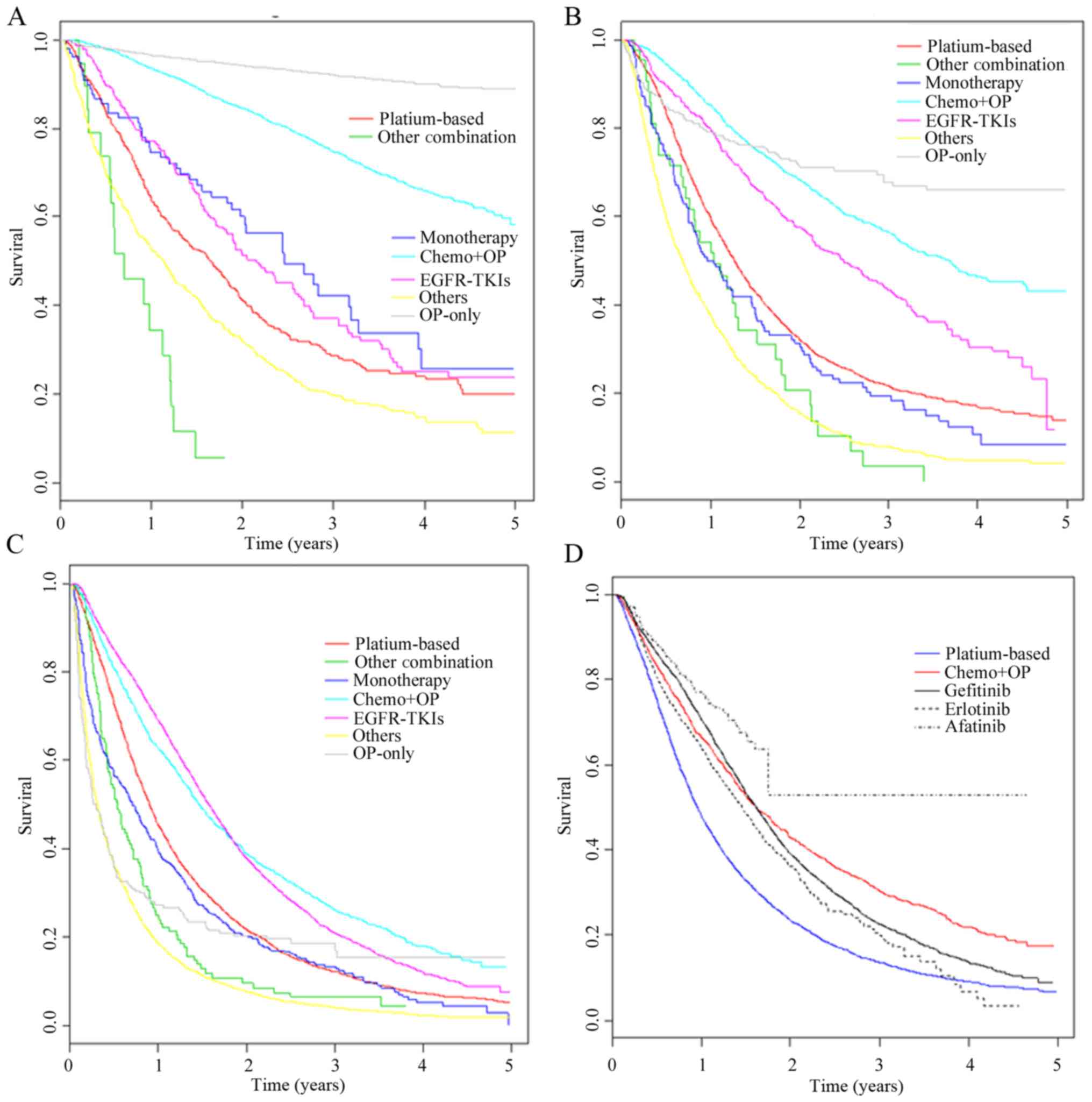
EGFR-TKI therapy and 6,085 other treatments (Table II). Patients with stage I and II
lung cancer in surgery and surgery plus chemotherapy showed
significantly improved OS benefits compared with other treatment
groups (Fig. 3A; P<0.001).
However, the surgery did not significantly improve OS in patients
with stage IV lung cancer (Fig. 3C).
EGFR-TKI therapy showed limited survival benefits for patients with
stage I and II lung cancer, but compared to other treatments, OS
was significantly increased with EGFR-TKI therapy in late-stage
lung cancer (III and IV) (Fig. 3B and
C).
 | Table II.Clinicopathological characteristics of
patients treated with different first-line treatments for lung
cancer. The EGFR expression status, lung cancer staging and grade
were identified by linking with the cancer registration file. |
Table II.
Clinicopathological characteristics of
patients treated with different first-line treatments for lung
cancer. The EGFR expression status, lung cancer staging and grade
were identified by linking with the cancer registration file.
|
|
|
| Without surgery |
|---|
|
|
|
|
|
|---|
| Variable | Surgery only (%) | Surgery plus
chemotherapy (%) | Platinum-based
compounds (%) | TCC (%) | Monotherapy (%) | EGFR-TKIs (%) | Others (%) |
|---|
| Patient number | 5,077 | 7,392 | 10,588 | 179 | 771 | 8,008 | 6,085 |
| Sex |
|
|
|
|
|
|
|
| Male | 2,254 (44.4) | 4,134 (55.9) | 7,884 (74.5) | 136 (76.0) | 521 (67.6) | 3,140 (39.2) | 4,329 (71.2) |
|
Female | 2,823 (55.6) | 3,255 (44.0) | 2,691 (25.4) | 43 (24.0) | 250 (32,4) | 4,857 (60.7) | 1,747 (28.7) |
|
Unknown |
| 3 (0.1) | 13 (0.1) | 0 | 0 | 11 (0.1) | 9 (0.1) |
| EGFR mutation |
|
|
|
|
|
|
|
|
Negative | 582 (11.5) | 1,443 (19.5) | 3,226 (30.5) | 52 (29.1) | 256 (33.2) | 313 (3.9) | 1,536 (25.2) |
|
Positive | 742 (14.6) | 2,213 (29.9) | 841 (7.9) | 4 (2.2) | 62 (8.0) | 6,801 (84.9) | 349 (5.8) |
|
Unknown | 3,753 (73.9) | 3,736 (50.6) | 6,521 (61.6) | 123 (68.7) | 453 (58.8) | 8,94 (11.2) | 4,200 (69.0) |
| TNM stage |
|
|
|
|
|
|
|
| 0 | 76 (1.4) | 75 (1.0) | 25 (0.2) | 0 | 3 (0.4) | 22 (0.3) | 25 (0.4) |
| I | 3,827 (75.4) | 2,812 (38.0) | 298 (2.8) | 7 (3.9) | 64 (8.3) | 184 (2.3) | 344 (5.7) |
| II | 334 (6.7) | 1,070 (14.5) | 321 (3.0) | 12 (6.7) | 35 (4.5) | 85 (1.1) | 318 (5.2) |
|
III | 268 (5.3) | 1,548 (20.9) | 2,747 (25.9) | 42 (23.5) | 141 (18.3) | 612 (7.6) | 1,195 (19.6) |
| IV | 210 (4.1) | 1,707 (23.1) | 6,989 (66.0) | 118 (65.9) | 504 (65.4) | 6,972 (87.1) | 3,965 (65.2) |
|
Unknown | 362 (7.1) | 180 (2.5) | 208 (2.1) |
| 24 (3.1) | 133 (1.7) | 238 (3.9) |
| Pathologic
grade |
|
|
|
|
|
|
|
| 1 | 1,353 (26.6) | 631 (8.5) | 207 (2.0) | 5 (2.8) | 38 (4.9) | 297 (3.7) | 163 (2.7) |
| 2 | 2,392 (47.1) | 3,034 (41.0) | 1,508 (14.2) | 23 (12.8) | 170 (22.0) | 1,544 (19.3) | 919 (15.1) |
| 3 | 609 (12.0) | 1,647 (22.4) | 1,964 (26.6) | 42 (23.5) | 132 (17.1) | 997 (12.5) | 1,052 (17.3) |
| 4 | 60 (1.2) | 104 (1.4) | 71 (0.7) |
| 6 (0.8) | 10 (0.1) | 50 (0.8) |
|
Other | 663 (13.1) | 1,976 (26.7) | 6,838 (64.5) | 109 (60.9) | 425 (55.1) | 5,160 (64.4) | 3,901 (64.1) |
| Area |
|
|
|
|
|
|
|
|
North | 2,684 (52.9) | 3,423 (46.3) | 4,661 (44.0) | 58 (32.4) | 241 (31.3) | 3,441 (43.0) | 2,888 (47.5) |
|
Central | 1,133 (22.3) | 1,831 (24.8) | 2,591 (24.5) | 63 (35.2) | 257 (33.3) | 1,874 (23.4) | 1,528 (25.1) |
|
South | 1,154 (22.7) | 1,941 (26.3) | 2,990 (28.2) | 53 (29.6) | 256 (33.2) | 2,453 (30.6) | 1,450 (23.8) |
|
East | 80 (1.6) | 173 (2.3) | 297 (2.8) | 5 (2.8) | 17 (2.2) | 200 (2.5) | 163 (2.7) |
| Outer
Islands or unknown | 26 (0.5) | 24 (0.3) | 36 (0.3) |
| 0 | 40 (0.5) | 56 (0.9) |
| Age |
|
|
|
|
|
|
|
|
<40 | 133 (2.6) | 152 (2.1) | 243 (2.3) | 0 | 14 (1.8) | 122 (1.5) | 42 (0.7) |
|
40–49 | 513 (10.1) | 703 (9.5) | 1,016 (9.6) | 7 (3.9) | 36 (4.7) | 584 (7.3) | 122 (2.0) |
|
50–59 | 1,310 (25.8) | 1,842 (24.9) | 2,535 (23.9) | 17 (9.5) | 101 (13.1) | 1,586 (19.8) | 402 (6.6) |
|
60–69 | 1,565 (30.8) | 2,295 (31.0) | 3,311 (31.3) | 41 (22.9) | 137 (17.8) | 1,920 (24.0) | 831 (13.7) |
|
70–79 | 1,174 (23.1) | 1,883 (25.5) | 2,754 (26.0) | 78 (43.6) | 295 (38.3) | 2,251 (31.9) | 2,088 (34.3) |
|
>80 | 382 (7.6) | 517 (7.0) | 729 (6.9) | 36 (20.1) | 188 (24.4) | 1,545 (19.3) | 2,600 (42.7) |
| Mean ± standard
deviation | 62.9±11.6 | 66.3±11.4 | 63.4±11.3 | 71.1±9.8 | 70.3±12.2 | 67.4±12.6 | 75.7±10.8 |
| Side effects |
|
|
|
|
|
|
|
|
Neutropenia | 21 (0.4) | 510 (6.9) | 1,505 (14.2) | 11 (6.1) | 30 (3.9) | 303 (3.8) | 247 (4.1) |
|
Thrombocytopenia | 3 (<0.1) | 24 (0.3) | 63 (0.6) | 0 |
| 16 (0.2) | 7 (0.1) |
|
Nausea/vomiting | 67 (1.3) | 589 (8.0) | 1,058 (10.0) | 4 (2.2) | 32 (4.2) | 627 (7.8) | 192 (3.2) |
|
Neuropathy |
| 26 (0.4) | 32 (0.3) | 0 | 0 | 16 (0.2) | 8 (0.1) |
|
Rash | 6 (0.1) | 50 (0.7) | 74 (0.7) | 8 (4.5) | 3 (0.4) | 92 (1.1) | 13 (0.2) |
|
Diarrhea | 29 (0.6) | 234 (3.2) | 303 (2.9) |
| 20 (2.6) | 367 (4.6) | 98 (1.6) |
| Nail
disorders | 3 (<0.1) | 11 (0.1) | 7 (<0.1) |
| 15 (1.9) | 22 (0.3) | 3 (<0.1) |
| Finger
and toe disorders | 14 (0.3) | 183 (2.5) | 117 (1.1) | 0 |
| 450 (5.6) | 28 (0.5) |
The survival differences between the three EGFR-TKIs
used (gefitinib, erlotinib and afatinib) were also determined. The
OS rate for patients treated with gefitinib was significantly
higher compared with erlotinib and platinum-based doublet
chemotherapy (Fig. 3D). For
afatinib, the sample size was too small to calculate the median
survival rates. The detailed OS, median survival and hazard ratios
are presented in Tables SII and
SIII.
Results of the multivariate Cox hazards regression
analysis for patients treated with different first-line treatments
for lung cancer are shown in Table
III. Overall, elderly patients with lung cancer (OR=1.01–1.03
compared with <65) and those with late-stage lung cancer
(OR=1.03–17.29 for stage IV compared with stage I; OR=0.65–4.16 for
stage III compared with stage I; OR=0.79–2.42 for stage II compared
with stage I) had a poor OS, and women exhibited improved OS rates
compared to men (OR=0.50–0.73 compared with men).
 | Table III.Multivariate Cox proportional hazards
regression analysis for overall survival in patients treated with
different first-line treatments for lung cancer. |
Table III.
Multivariate Cox proportional hazards
regression analysis for overall survival in patients treated with
different first-line treatments for lung cancer.
|
|
|
| Without
surgery |
|---|
|
|
|
|
|
|---|
| Variable | Surgery only, HR
(95% CI) | Surgery plus
chemotherapy, HR (95% CI) | Platinum-based
compounds, HR (95% CI) | TCC, HR (95%
CI) | Monotherapy, HR
(95% CI) | Target therapy, HR
(95% CI) | Others, HR (95%
CI) |
|---|
| Sex |
|
|
|
|
|
|
|
|
Female | 0.50
(0.41–0.60) | 0.61
(0.56–0.66) | 0.61
(0.58–0.64) | 0.69
(0.46–1.04) | 0.73
(0.61–0.87) | 0.76
(0.72–0.890) | 0.67
(0.63–0.71) |
| Age |
|
|
|
|
|
|
|
|
≥65 | 1.03
(1.03–1.05) | 1.02
(1.02–1.03) | 1.01
(1.01–1.02) | 1.01
(0.99–1.03) | 1.01
(1.00–1.02) | 1.02
(1.01–1.02) | 1.01
(1.01–1.02) |
| TNM Stage (ref,
stage I) |
|
|
|
|
|
|
|
| II | 2.42
(1.85–3.16) | 1.56
(1.39–1.78) | 1.17
(0.95–1.43) | 0.79
(0.27–2.33) | 1.85
(1.03–3.30) | 1.40
(1.00–1.96) | 1.51
(1.27–1.80) |
|
III | 4.16
(3.21–5.38) | 2.08
(1.86–2.34) | 1.31
(1.11–1.53) | 0.65
(0.25–1.70) | 2.39
(1.56–3.64) | 1.04
(0.83–1.30) | 1.69
(1.47–1.95) |
| IV | 17.29
(13.81–21.64) | 5.35
(4.81–5.96) | 2.09
(1.79–2.44) | 1.03
(0.42–2.56) | 3.52
(2.37–5.22) | 1.88
(1.53–2.30) | 3.00
(2.63–3.43) |
Efficacy of first-line treatment for
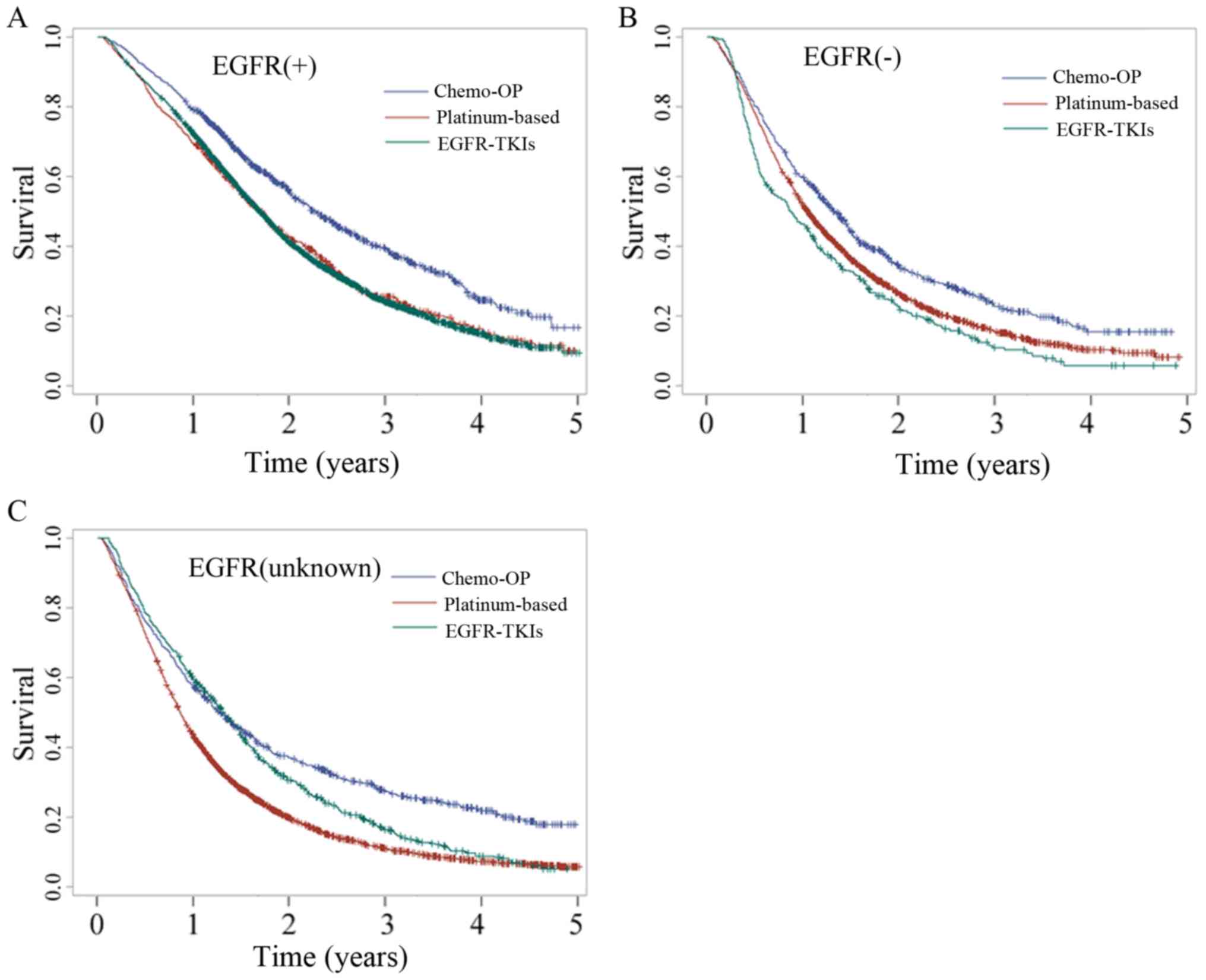
patients with EGFR mutations
The EGFR mutation status was used to select patients
with lung cancer who may exhibit an improved response to EGFR-TKI
therapy. The activity of EGFR-TKIs in EGFR wild-type or unknown
lung cancer patients still requires investigation. Patients with
stage IIIB and IV lung cancer who underwent surgery with
chemotherapy, platinum-based doublet chemotherapy or EGFR-TKI
therapy were selected and their OS analysed. In patients with EGFR
mutation (+), the OS was improved compared with patients with EGFR
mutation (−) and EGFR mutation (unknown) in all the treatment
groups (Fig. 4; Tables SIV and SV). The efficacies of platinum-based
doublet chemotherapy and EGFR-TKI therapy were similar in EGFR
mutation (+) patients (20.7 vs. 20.3 months; P=0.82), whereas
patients with EGFR mutation (−) who underwent platinum-based
doublet chemotherapy had significantly improved OS compared with
patients who underwent EGFR-TKI therapy (12.53 vs. 10.47 months;
P=0.008). In addition, EGFR mutation (unknown) patients who
underwent EGFR-TKI therapy exhibited improved OS compared with
patients who underwent platinum-based doublet chemotherapy (15.83
vs. 10.4 months; P<0.0001). These results suggest that EGFR-TKIs
should not be used in patients with EGFR mutation (−).
Second-line clinical assessment
The benefits of different second-line treatments
were assessed. Current guidelines recommend both chemotherapy and
EGFR-TKIs, and the Taiwan National Health Insurance (NHI) system
allows the use of both. Table IV
shows the patients' demographic data. A total of 24,248 patients
with lung cancer underwent second-line treatment. Pemetrexed
(n=4,962) for chemotherapy and erlotinib (n=3,901) for targeted
EGFR-TKI therapy were the most frequently prescribed. The median
survival time for patients treated with gefitinib was 16.23 months,
for pemetrexed it was 11.73 months and for the remainder of the
drugs it was ~7 months (ranging between 6.73 and 8.2 months)
(Fig. 5 and Tables SVI and SVII). These results suggest that as a
second-line treatment, gefitinib resulted in improved OS compared
with other chemotherapeutic drugs. Results of the multivariate Cox
hazards regression analysis for different second-line treatments
for lung cancer are shown in Table
V. The efficacy of gefitinib was superior to other medication
as second-line treatment for lung cancer. This result was similar
for the first-line treatments.
 | Table IV.Clinicopathological characteristics
of patients treated with different second-line treatments for lung
cancer. EGFR expression status was identified through linking with
the cancer registration file. |
Table IV.
Clinicopathological characteristics
of patients treated with different second-line treatments for lung
cancer. EGFR expression status was identified through linking with
the cancer registration file.
| Variable | Afatinib (%) | Docetaxel (%) | Erlotinib (%) | Etoposide (%) | Gefitinib (%) | Gemcitabine
(%) | Paclitaxel (%) | Pemetrexed (%) | Vinorelbine
(%) |
|---|
| Patient number | 109 | 4,427 | 3,901 | 599 | 1,230 | 3,298 | 1,669 | 4,962 | 4,051 |
| Sex |
|
|
|
|
|
|
|
|
|
|
Male | 43 (39.4) | 2,644 (59.7) | 2,513 (64.4) | 374 (62.4) | 533 (43.3) | 1,945 (59.0) | 966 (57.9) | 2,189 (44.1) | 2,239 (55.3) |
|
Female | 66 (60.6) | 1,773 (40.3) | 1,381 (35.6) | 225 (37.6) | 694 (56.7) | 1,342 (41.0) | 699 (42.1) | 2,757 (55.9) | 1,801 (44.7) |
| EGFR mutation |
|
|
|
|
|
|
|
|
|
|
Negative | 4 (3.7) | 1,202 (27.1) | 1,518 (38.9) | 137 (22.9) | 225 (18.3) | 834 (25.3) | 423 (25.3) | 888 (17.9) | 881 (21.7) |
|
Positive | 67 (61.5) | 1,376 (31.1) | 521 (13.4) | 117 (19.5) | 459 (37.3) | 1,005 (30.5) | 465 (27.9) | 2,938 (59.2) | 1,408 (34.8) |
|
Unknown | 38 (34.8) | 1,849 (41.8) | 1,862 (47.7) | 345 (57.6) | 546 (44.4) | 1,459 (44.2) | 781 (46.8) | 1,136 (22.9) | 1,762 (43.5) |
| Age |
|
|
|
|
|
|
|
|
|
|
<40 | 4 (3.7) | 127 (2.9) | 104 (2.7) | 28 (4.7) | 18 (1.5) | 122 (3.7) | 68 (4.1) | 124 (2.5) | 108 (2.7) |
|
40–49 | 12 (11.0) | 551 (12.4) | 430 (11.0) | 100 (16.7) | 110 (8.9) | 411 (12.5) | 225 (13.5) | 592 (11.9) | 410 (10.1) |
|
50–59 | 31 (28.4) | 1,304 (29.5) | 989 (25.4) | 168 (28.0) | 299 (24.3) | 922 (28.0) | 483 (28.9) | 1,407 (28.4) | 1,009 (24.9) |
|
60–69 | 31 (28.4) | 1,328 (30.0) | 1,042 (26.7) | 176 (29.4) | 314 (25.5) | 949 (28.8) | 479 (25.6) | 1,439 (29.0) | 1,099 (27.1) |
|
70–79 | 27 (24.8) | 941 (21.2) | 1,010 (25.9) | 97 (16.2) | 352 (28.6) | 702 (21.2) | 329 (19.7) | 1,081 (21.8) | 1,022 (25.2) |
|
>80 | 4 (3.7) | 176 (4.0) | 326 (8.3) | 30 (5.0%) | 137 (11.1) | 192 (5.8) | 85 (5.1) | 319 (6.4) | 403 (10.0) |
| Mean ±
standard deviation | 61.5±11.6 | 61.0±11.1 | 63.1±12.0 | 59.3±12.1 | 61.2±11.8 | 60.5±11.7 | 61.9±11.6 | 60.5±11.7 | 61.9±11.6 |
| Side effect |
|
|
|
|
|
|
|
|
|
|
Neutropenia | 7 (6.4) | 304 (6.9) | 168 (4.3) | 59 (9.8) | 51 (4.1) | 182 (5.5) | 96 (5.8) | 336 (6.8) | 255 (6.3) |
|
Thrombocytopenia |
| 12 (0.3) | 7 (0.2) |
| 3 (0.2) | 16 (0.5) | 6 (0.4) | 16 (0.3) | 12 (0.3) |
|
Nausea/vomiting |
| 276 (6.2) | 192 (4.9) | 34 (5.7) | 73 (5.9) | 140 (4.2) | 71 (4.3) | 422 (8.5) | 192 (4.7) |
|
Neuropathy |
| 13 (0.3) | 16 (0.4) |
| 4 (0.3) | 8 (0.2) | 6 (0.4) | 13 (0.3) | 6 (0.1) |
|
Rash |
| 29 (0.7) | 26 (0.7) | 7 (1.2) | 6 (0.5) | 22 (0.7) | 10 (0.6) | 44 (0.9) | 27 (0.7) |
|
Diarrhea |
| 146 (3.3) | 100 (2.6) | 15 (2.5) | 37 (3.0) | 78 (2.4) | 36 (2.2) | 161 (3.2) | 113 (3.8) |
| Nail
disorders | 0 | 9 (0.2) | 4 (0.2) | 10 (1.7) | 48 (3.9) | 4 (0.1) | 22 (1.3) | 9 (0.2) | 8 (0.2) |
| Finger
and toe disorders | 9 (8.3) | 69 (1.6) | 98 (2.5) |
|
| 48 (1.5) |
| 137 (2.8) | 61 (1.5) |
 | Table V.Multivariate Cox proportional hazards
regression analysis for overall survival in different second-line
treatment of the lung cancer patients. |
Table V.
Multivariate Cox proportional hazards
regression analysis for overall survival in different second-line
treatment of the lung cancer patients.
| Variable | Afatinib, HR (95%
CI) | Docetaxel, HR (95%
CI) | Erlotinib, HR (95%
CI) | Etoposide, HR (95%
CI) | Gefitinib, HR (95%
CI) | Gemcitabine, HR
(95% CI) | Paclitaxel, HR (95%
CI) | Pemetrexed, HR (95%
CI) | Vinorelbine, HR
(95% CI) |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
|
Female | 0.69 | 0.79 | 0.62 | 0.90 | 0.77 | 0.71 | 0.89 | 0.78 | 0.81 |
|
| (0.17–2.82) | (0.73–0.85) | (0.58–0.67) | (0.74–1.10) | (0.67–0.88) | (0.65–0.77) | (0.79–0.99) | (0.73–0.84) | (0.75–0.87) |
| Age |
|
|
|
|
|
|
|
|
|
|
≥65 | 1.01 | 1.01 | 1.01 | 1.01 | 1.02 | 1.00 | 0.99 | 1.01 | 1.00 |
|
| (0.96–1.06) | (1.00–1.01) | (1.00–1.01) | (0.99–1.01) | (1.01–1.02) | (1.00–1.01) | (0.99–1.00) | (1.00–1.01) | (1.00–1.01) |
| TNM Stage (ref,
stage I) |
|
|
|
|
|
|
|
|
|
| II | 1.83 | 1.07 | 1.20 | 1.62 | 1.45 | 1.09 | 0.99 | 1.20 | 1.41 |
|
| (0.11–30.55) | (0.82–1.39) | (0.92–1.58) | (0.87–2.99) | (0.96–2.20) | (0.79–1.51) | (0.65–1.51) | (0.92–1.56) | (1.05–1.89) |
|
III | 6.67 | 1.25 | 1.25 | 1.41 | 1.86 | 1.26 | 1.32 | 1.41 | 1.58 |
|
| (0.75–59.02) | (1.01–1.55) | (1.02–1.54) | (0.82–2.41) | (1.34–2.58) | (0.99–1.62) | (0.95–1.83) | (1.15–1.73) | (1.26–1.99) |
| IV | 6.49 | 1.59 | 1.69 | 1.90 | 2.80 | 1.74 | 1.61 | 1.75 | 2.30 |
|
| (0.51–83.40) | (1.30–1.94) | (1.39–2.06) | (1.13–3.18) | (2.07–3.80) | (1.37–2.21) | (1.19–2.20) | (1.46–2.10) | (1.85–2.86) |
Discussion
The present study is one of few retrospective cohort
studies examining first-line treatment for treatment-naive lung
cancer patients using a national sample (16). Using data from the NHID, it was
demonstrated that first-line treatments used in Taiwan are similar
to that recommended by the American Society of Clinical Oncology
(ASCO) guidelines for patients with stage IV NSCLC (17). The majority of new cases of lung
cancer are treated with platinum-based doublet chemotherapy or
EGFR-TKI therapy as initial treatment (39.91 and 32.48%,
respectively, in 2015). Based on the data from NHID, the use of
EGFR-TKIs for lung cancer treatment has increased over time, while
that of other therapies has decreased. The results of the present
study suggest that EGFR-TKIs as a first-line treatment is less
efficacious compared with chemotherapy when used to treat patients
with stage III and IV treatment-naive lung cancer. There is little
benefit of treating patients with stage I and II lung cancer and no
benefit for treating patients with stage III and IV with EGFR
mutation (−) with EGFR-TKIs. In addition, direct comparison of
EGFR-TKIs and other chemotherapy drugs used as second-line
treatment showed that gefitinib was superior to the other
drugs.
ASCO guidelines recommend platinum-based doublet
chemotherapy for patients with NSCLC without EGFR or ALK mutations
and with improved ability to take care of themself (17); however, a meta-analysis showed that
1-year survival was not significantly increased in patients treated
with platinum-based doublet chemotherapy compared with patients
treated with TCC therapy (18).
Based on the data from NHID, median survival in patients with stage
IV lung cancer who underwent platinum-based doublet chemotherapy
was 11 months, which was higher compared with patients who
underwent other types of chemotherapy (6.6 months with TCC therapy,
8.67 months with monotherapy and 3.77 months with others). This
inconsistency in our results and previously published data may be
due to different stage responses to various therapies, as the
median survival in patients with stage III lung cancer was not
significantly different between platinum-based doublet chemotherapy
and TCC therapy [15.47 months (14.77–16.03) vs. 12.6 months
(8.80–15.97)].
According to the Taiwan NHI system, patients with
locally advanced NSCLC who fail to respond to chemotherapy as a
first-line treatment (with or without EGFR mutation) are prescribed
EGFR-TKIs. A few randomised studies comparing gefitinib with
erlotinib prescription, showed that the median PFS in the gefitinib
group was higher compared with the erlotinib group (4.9 months vs.
3.1 months; 95% CI=1.3–8.5 vs. 0.0–6.4) (19). In another cohort study in Taiwan,
previously treated EGFR-TKIs naïve NSCLC patients administered with
gefitinib had longer PFS and OS times compared with patients
administered erlotinib (20). In the
present study, OS for 9 commonly used second-line treatments for
patients with lung cancer, three EGFR-TKIs (afatinib, gefitinib and
erlotinib) and six chemotherapy drugs (docetaxel, etoposide,
gemcitabine, paclitaxel, pemetrexed and vinorelbine) were assessed.
Among the 24,292 lung cancer patients who received second-line
treatment, 5,237 (21.56%) were administered EGFR-TKIs. The results
of the present study demonstrated that patients whom had previously
been administered gefitinib had improved OS time. There are several
possible explanations for the superior therapeutic effects of
gefitinib compared with erlotinib. Erlotinib is more likely to be
prescribed to patients with lung cancer with a higher severity of
disease, such as those with cachexia and increased intracranial
pressure (20). EGFR mutation status
may also serve a crucial role in patients with lung cancer.
Clinical evidence involving a comparison between chemotherapy and
EGFR-TKIs as second-line treatment has yielded contrasting results
which may be due to inconsistent inclusion criteria with, without,
or with mixed EGFR mutation status (21–23).
Claims-based analyses have several limitations, such
as incomplete data and the possibility of coding errors or
omissions. To better understand the information on lung cancer,
data on lung cancer patients with catastrophic illness certificates
(ICD-9 code 162) was linked with the cancer registry file (ICD-O-3,
C33-C34, lung, bronchus and trachea). However, not all the relevant
information was available on the initial staging of lung cancer. A
high percentage (>90%) of patients with stage IV lung cancer and
treated with EGFR-TKI therapy as a first line treatment underwent
molecular testing for EGFR mutation, and the majority of these
tested positive. However, <50% of patients with lung cancer
undergoing platinum-based doublet chemotherapy underwent molecular
testing to determine EGFR mutation status. Therefore, the results
of the present study may not be generalizable, as lung cancer
staging and EGFR gene mutation status may differ across countries.
Performance status (PS) and smoking history may have an impact on
outcomes and therapeutic strategies. There was no information on PS
and smoking history listed in the cancer registry files.
Drug-induced lung injuries was an adverse event during lung cancer
treatment. There is no specific code for drug-induced lung
injuries, including interstitial lung disease in ICD-9. The most
similar coding was ‘respiratory conditions due to other specified
external drugs (508.0)’. To protect personal privacy, the Health
and Welfare Data Science Centre only allows exporting results with
more than two cases in each event. As there were results for
patients with respiratory conditions with no more than two cases in
each group, it was not possible to obtain and analyse this data. In
future studies, data from medical charts may be used to examine the
factors which may affect outcomes and therapeutic strategies.
The results of the present study suggested that
patients with stage III or IV lung cancer undergoing first-line
EGFR-TKI therapy may show improved OS; however, patients with stage
I and II lung cancer may only exhibit smaller benefits and patients
with stage III and IV EGFR mutation (−) patients may not benefit at
all. The efficacy of two first-generation EFGR-TKIs may not be the
same. Gefitinib may be more effective than erlotinib in
treatment-naive and previously treated patients with lung cancer.
Gefitinib also improved survival compared with other frequently
used chemotherapy drugs. Additional randomised control trials are
required to confirm this finding.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Mackay Medical
College (Sanzhi, Taiwan; grant nos. MMC-1071E03 and
MMC-1081B27).
Availability of data and materials
The present study is based on data from the Centre
for Health and Welfare Data Science Centre in Ministry of Health
and Welfare (H103065). The ownership of the data used in the
present study belong to the National Health Insurance Research
Database (NHIRD) of Taiwan and cannot be made publicly available
due to legal restrictions. However, the data are available through
formal application to the Health and Welfare Data Science Centre at
Ministry of Health and Welfare, Taiwan (https://dep.mohw.gov.tw/DOS/np-2500-113.html) and
require a signed affirmation regarding data confidentiality. The
authors have no special privilege of access to the database.
Authors' contributions
CC collected and analysed the data, and wrote the
manuscript.
Ethics approval and consent to
participate
The protocol used in the present study was approved
by the Joint Institutional Review Board Taiwan R.O.C. (Protocol
Number: 14-S-007).
Patient consent for publication
Not applicable.
Competing interests
The author declares that they have no competing
interests.
References
|
1
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang BY, Huang JY, Cheng CY, Lin CH, Ko J
and Liaw YP: Lung cancer and prognosis in Taiwan: A
population-based cancer registry. J Thorac Oncol. 8:1128–1135.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Douillard JY, Rosell R, De Lena M,
Carpagnano F, Ramlau R, Gonzáles-Larriba JL, Grodzki T, Pereira JR,
Le Groumellec A, Lorusso V, et al: Adjuvant vinorelbine plus
cisplatin versus observation in patients with completely resected
stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine
International Trialist Association [ANITA]): A randomised
controlled trial. Lancet Oncol. 7:719–727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gilligan D, Nicolson M, Smith I, Groen H,
Dalesio O, Goldstraw P, Hatton M, Hopwood P, Manegold C, Schramel
F, et al: Preoperative chemotherapy in patients with resectable
non-small cell lung cancer: Results of the MRC LU22/NVALT 2/EORTC
08012 multicentre randomised trial and update of systematic review.
Lancet. 369:1929–1937. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim E, Harris G, Patel A, Adachi I,
Edmonds L and Song F: Preoperative versus postoperative
chemotherapy in patients with resectable non-small cell lung
cancer: Systematic review and indirect comparison meta-analysis of
randomized trials. J Thorac Oncol. 4:1380–1388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
NSCLC Meta-Analyses Collaborative Group, :
Chemotherapy in addition to supportive care improves survival in
advanced non-small-cell lung cancer: A systematic review and
meta-analysis of individual patient data from 16 randomized
controlled trials. J Clin Oncol. 26:4617–4625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Delbaldo C, Michiels S, Syz N, Soria JC,
Le Chevalier T and Pignon JP: Benefits of adding a drug to a
single-agent or a 2-agent chemotherapy regimen in advanced
non-small-cell lung cancer: A meta-analysis. JAMA. 292:470–484.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li M, Zhang Q, Fu P, Li P, Peng A, Zhang
G, Song X, Tan M, Li X, Liu Y, et al: Pemetrexed plus platinum as
the first-line treatment option for advanced non-small cell lung
cancer: A meta-analysis of randomized controlled trials. PLoS One.
7:e372292012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Effects of vinorelbine on quality of life
and survival of elderly patients with advanced non-small-cell lung
cancer. The Elderly Lung Cancer Vinorelbine Italian Study Group. J
Natl Cancer Inst. 91:66–72. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han JY, Park K, Kim SW, Lee DH, Kim HY,
Kim HT, Ahn MJ, Yun T, Ahn JS, Suh C, et al: First-SIGNAL:
First-line single-agent iressa versus gemcitabine and cisplatin
trial in never-smokers with adenocarcinoma of the lung. J Clin
Oncol. 30:1122–1128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carter BW, Lichtenberger JP III,
Benveniste MK, de Groot PM, Wu CC, Erasmus JJ and Truong MT:
Revisions to the TNM staging of lung cancer: Rationale,
significance, and clinical application. Radiographics. 38:374–391.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
http://regulation.cde.org.tw/data/downloadfile.php?sid=910
|
|
15
|
Skrbo A, Begovic B and Skrbo S:
Classification of drugs using the ATC system (Anatomic,
Therapeutic, Chemical Classification) and the latest changes. Med
Arh. 58 (1 Suppl 2):S138–S141. 2004.(In Bosnian).
|
|
16
|
Liang YH, Shao YY, Liao BC, Lee HS, Yang
JC, Chen HM, Chiang CJ, Cheng AL and Lai MS: Cytotoxic chemotherapy
as first-line therapy for advanced non-small-cell lung cancer in
Taiwan: Daily practice. J Cancer. 7:1515–1523. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Masters GA, Temin S, Azzoli CG, et al:
Systemic therapy for stage IV non-small-cell lung cancer: American
Society of Clinical Oncology Clinical Practice Guideline Update. J
Clin Oncol. 33:3488–3515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
D'Addario G, Pintilie M, Leighl NB, Feld
R, Cerny T and Shepherd FA: Platinum-based versus
non-platinum-based chemotherapy in advanced non-small-cell lung
cancer: A meta-analysis of the published literature. J Clin Oncol.
23:2926–2936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim ST, Uhm JE, Lee J, Sun JM, Sohn I, Kim
SW, Jung SH, Park YH, Ahn JS, Park K and Ahn MJ: Randomized phase
II study of gefitinib versus erlotinib in patients with advanced
non-small cell lung cancer who failed previous chemotherapy. Lung
Cancer. 75:82–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang CH, Lee CH, Ko JC, Chang LY, Lee MC,
Wang JY and Yu CJ: Gefitinib or erlotinib in previously treated
non-small-cell lung cancer patients: A cohort study in Taiwan.
Cancer Med. 6:1563–1572. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim ES, Hirsh V, Mok T, Socinski MA,
Gervais R, Wu YL, Li LY, Watkins CL, Sellers MV, Lowe ES, et al:
Gefitinib versus docetaxel in previously treated non-small-cell
lung cancer (INTEREST): A randomised phase III trial. Lancet.
372:1809–1818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ciuleanu T, Stelmakh L, Cicenas S,
Miliauskas S, Grigorescu AC, Hillenbach C, Johannsdottir HK,
Klughammer B and Gonzalez EE: Efficacy and safety of erlotinib
versus chemotherapy in second-line treatment of patients with
advanced, non-small-cell lung cancer with poor prognosis (TITAN): A
randomised multicentre, open-label, phase 3 study. Lancet Oncol.
13:300–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garassino MC, Martelli O, Broggini M,
Farina G, Veronese S, Rulli E, Bianchi F, Bettini A, Longo F,
Moscetti L, et al: Erlotinib versus docetaxel as second-line
treatment of patients with advanced non-small-cell lung cancer and
wild-type EGFR tumours (TAILOR): A randomised controlled trial.
Lancet Oncol. 14:981–988. 2013. View Article : Google Scholar : PubMed/NCBI
|