Introduction
Incidence of hepatocellular carcinoma (HCC) ranks
5th place among all malignancies worldwide (1). Due to the high rate of extrahepatic and
intrahepatic metastases by the time of initial diagnosis, prognosis
of HCC patients is usually poor and the recurrence rate is high,
making HCC a major cause of cancer-related deaths (2). Therefore, how to prevent and treat
cancer metastasis is still a major task in the treatment of
cancers, such as HCC (3). Genetic
factors are the major players in HCC and a large number of tumor
suppressors and oncogenes are involved (4,5).
However, the molecular mechanism of the pathogenesis of HCC remains
unclear, leading to failures in clinical treatment and
prevention.
Long (>200 nt) non-coding RNAs (lncRNAs) have no
or limited potential for protein coding (6). LncRNAs play their roles through the
regulation of downstream genes (6).
Aberrant expression of lncRNAs in human body may lead to
dysregulated expression of certain genes involved in human diseases
(7), such as oncogenes and tumor
suppressors in cancers (8,9). In human cancers including HCC, lncRNAs
regulate downregulate tumor suppressors and oncogenes to promote or
inhibit cancer development (8,9).
Therefore, regulation of the expression of key lncRNA regulators
may contribute to the recovery of cancer patients (10). It has been reported that novel
mitotically-associated long non-coding RNA (MANCR) is involved in
malignant breast cancer (11). In
breast cancer, MANCR affects cell division and genomic stability to
promote the progression of cancer. However, the involvement of
MANCR in HCC is unknown. MicroRNA (miR)-122a is a key player in a
number of types of diseases including HCC (12–16).
miR-122a is downregulated in HCC and predicted poor survival
(16). An inverse correlation
between MANCR and miR-122a was observed from the transcriptome
analysis data (data not shown). Therefore, miR-122a may have
interactions with MANCR in HCC. The present study therefore
investigated the involvement of MANCR in HCC and explored its
interactions with miR-122a.
Materials and methods
Research subjects
This is a prospective study. From January 2010 to
April 2013, the present study included 68 patients (39 males and 29
females, aged 38 to 69 years, mean age 48.4±5.7 years) who were
diagnosed as HCC through histopathological examinations in the
Central Hospital of Wuhan. All the patients were enrolled according
to strict inclusion and exclusion criteria. Inclusion criteria: i)
First diagnosis; ii) no abnormal function of other organs observed.
Exclusion criteria: i) Other clinical conditions other than HCC
were observed; ii) any therapies received within 3 months after
admission. Based on the staging criteria proposed by AJCC (17), there were 16, 14, 20 and 18 cases at
stage I, II, III and IV, respectively. Among these patients, 39
were combined with liver fibrosis. All patients had signed informed
consent before the study and the Ethics Committee of the Central
Hospital of Wuhan had approved this study before admission.
Follow-up
All patients were followed up for 5 years after
admission. Follow-up was performed either by outpatient visit or
phone call once per 1–2 months. Patients who were lost during
follow-up, who died of other diseases or accidents were
excluded.
Specimen collection and cell
lines
Liver biopsy was performed to collect HCC tissues
and non-HCC tissues (within 2 cm around tumors) were collected from
each patient. All specimens were confirmed by at least 3
experiences pathologists.
SNU-398 and SNU-182 human HCC cell lines (American
Type Culture Collection) were used. The culture medium was RPMI
1640 medium (Sigma-Aldrich; Merck KGaA) with 10% fetal bovine serum
(FBS; Sigma-Aldrich; Merck KGaA). Culture conditions were 5%
CO2 and 37°C.
RNA interaction prediction
The interaction between miR-122a and MANCR was
predicted by using IntaRNA (version 2.0; http://rna.informatik.uni-freiburg.de/IntaRNA/Input.jsp).
The long sequence was MANCR and the short sequence was miR-122a.
All other parameters were default.
RNA extractions and reverse
transcription-quantitative (RT-q)PCR
Expression of MANCR was detected according to the
following steps: TRIzol reagent (Thermo Fisher Scientific, Inc.)
was used to extract total RNAs from tissue specimens and in
vitro cultivated cells, following by reverse transcription
performed using Expand™ Reverse Transcriptase (Sigma-Aldrich; Merck
KGaA) to synthesize cDNA. The reaction conditions were: 25°C for 5
min, 55°C for 20 min and 80°C for 10 min. After that, PCR
SYBR® Green Quantitative RT-qPCR kit (Sigma-Aldrich;
Merck KGaA) was used to prepare PCR reaction systems with cDNA as
template and 18s rRNA as endogenous control. The reaction
conditions were: 95°C for 1 min, followed by 40 cycles of 95°C for
10 sec and 55°C for 20 sec, and 72°C for 30 sec. Expression of
miR-122a was detected through following steps: mirVana miRNA
Isolation kit (Thermo Fisher Scientific) was used to extract
miRNAs, following by reverse transcription performed using TaqMan
MicroRNA Reverse Transcription kit (Thermo Fisher Scientific.,
lnc.). The reaction conditions were: 25°C for 5 min, 52°C for 20
min and 80°C for 10 min. After that, Applied Biosystems™ TaqMan™
MicroRNA Assay (Thermo Fisher Scientific) was used to prepare PCR
reaction systems with U6 as endogenous control. The primer
sequences were: MANCR forward, 5′-CAATACCACAATTGCAATC-3′; MANCR
reverse, 5′-CATGTTCTTCCTCATATGGA-3′; 18S rRNA forward,
5′-GTAACCCGTTGAACCCCATT-3′; 18S rRNA reverse,
5′-CCATCCAATCGGTAGTAGCG-3′; miR-122 forward,
5′-GCTCGACCTCTCTATGGGC-3′; and miR-122 reverse primer of and U6
primers were included in the kit. The 2−ΔΔCq method
(18) was used to perform all data
normalizations.
Transient transfection
Scrambled negative control miRNA
(5′-UCAGCUUGCGAGCAGUCGAAA-3′) and MISSION® microRNA
Mimic hsa-miR-122a (5′-UGGAGUGUGACAAUGGUGUUUG-3′) were purchased
from Sigma-Aldrich (Merck KGaA). MANCR-expression pcDNA3.1 vectors
and empty vectors were provided by Sangon Biotech Co., Ltd.
Transient transfections were performed using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Doses of vectors and
miRNAs were 10 and 40 nM, respectively. Two controls were included
in this experiment. They are control (non-transfection) and
negative control (empty vector or control miRNA transfection).
Cells were harvested at 24 h after transfection to perform
subsequent experiments.
Transwell migration and invasion
assay
Cells were haversted at 24 h after transfection to
prepare single cell suspensions (4×104 cells/ml) using
non-serum RPMI 1640 medium. The upper Transwell chamber was coated
one day before invasion assay with Matrigel (cat. no. 356234; 300
µg/ml; EMD Millipore). Cell suspensions were added into the upper
chamber, while RPMI 1640 medium (20% FBS) was added into the lower
chamber. The plate was incubated for 2 h, followed by upper chamber
membrane staining at room temperature with 0.5% crystal violet
(Sigma-Aldrich, Merck KGaA) for 1.5 h. Invading and migrating cells
were observed and counted under an light microscope aat ×40
magnification.
Statistical analysis
The mean values of 3 biological replicates were
calculated. All statistical analyses were performed using GraphPad
Prism 6 software (GraphPad Software, Inc.) Paired t-test was used
for comparisons between HCC and non-HCC tissues. Analysis of
variance (one-way) and Tukey's test were used for multi-group
comparisons. Linear regression was performed to analyze the
correlations between MANCR and miR-122a. Based on Youden's index
(19) and expression levels of MANCR
in HCC tissues, patients were divided into high (n=31) and low
(n=37) groups. Survival curves were plotted and compared using the
Kaplan-Meier (K-M) method and log rank t test, respectively. A
χ2 test was used to analyze the correlations between
patients' clinical data. P<0.05 was considered to indicate a
statistically significant difference.
Results
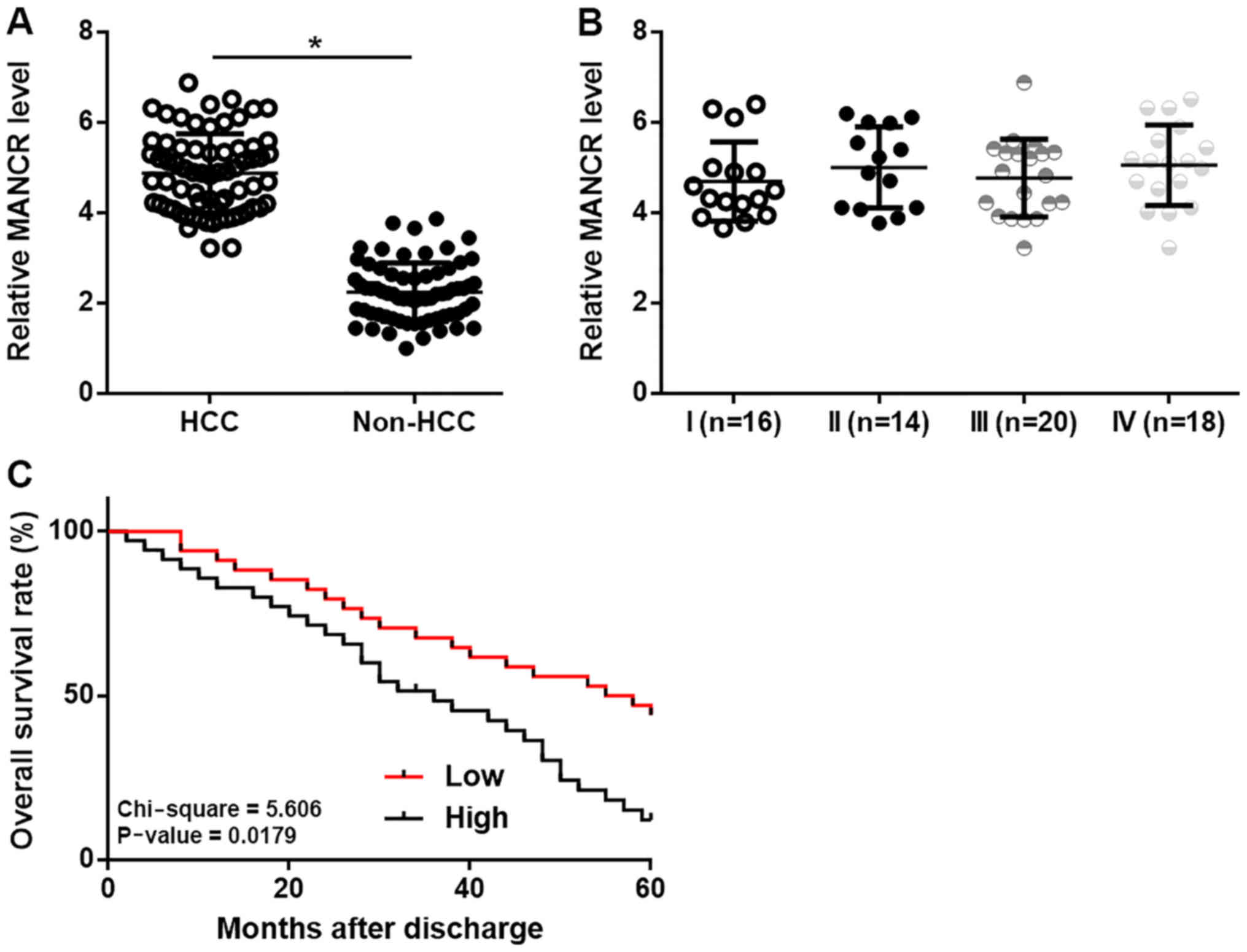
MANCR is upregulated in HCC and
predicted survival
RT-qPCR experiments were performed to investigate
the differential expression of MANCR in HCC and non-HCC tissues.
Comparison between HCC tissues and non-HCC tissues using a paired
t-test showed that MANCR was significantly upregulated in HCC
tissues compared with in non-HCC (P<0.05; Fig. 1A). However, no significant
differences in the expression levels of MANCR in HCC tissues were
found among patients with different clinical stages (Fig. 1B). Based on Youden's index and
expression levels of MANCR in HCC tissues, patients were divided
into high (n=31) and low (n=37) MANCR level groups. Survival curves
were plotted and compared using K-M method and log rank t test,
respectively. It was observed that the overall survival rate of
high MANCR level group was significantly decreased compared with
low MANCR level group (Fig. 1C). The
χ2 test showed that MANCR is not associated with liver
fibrosis, gender and age (P>0.05).
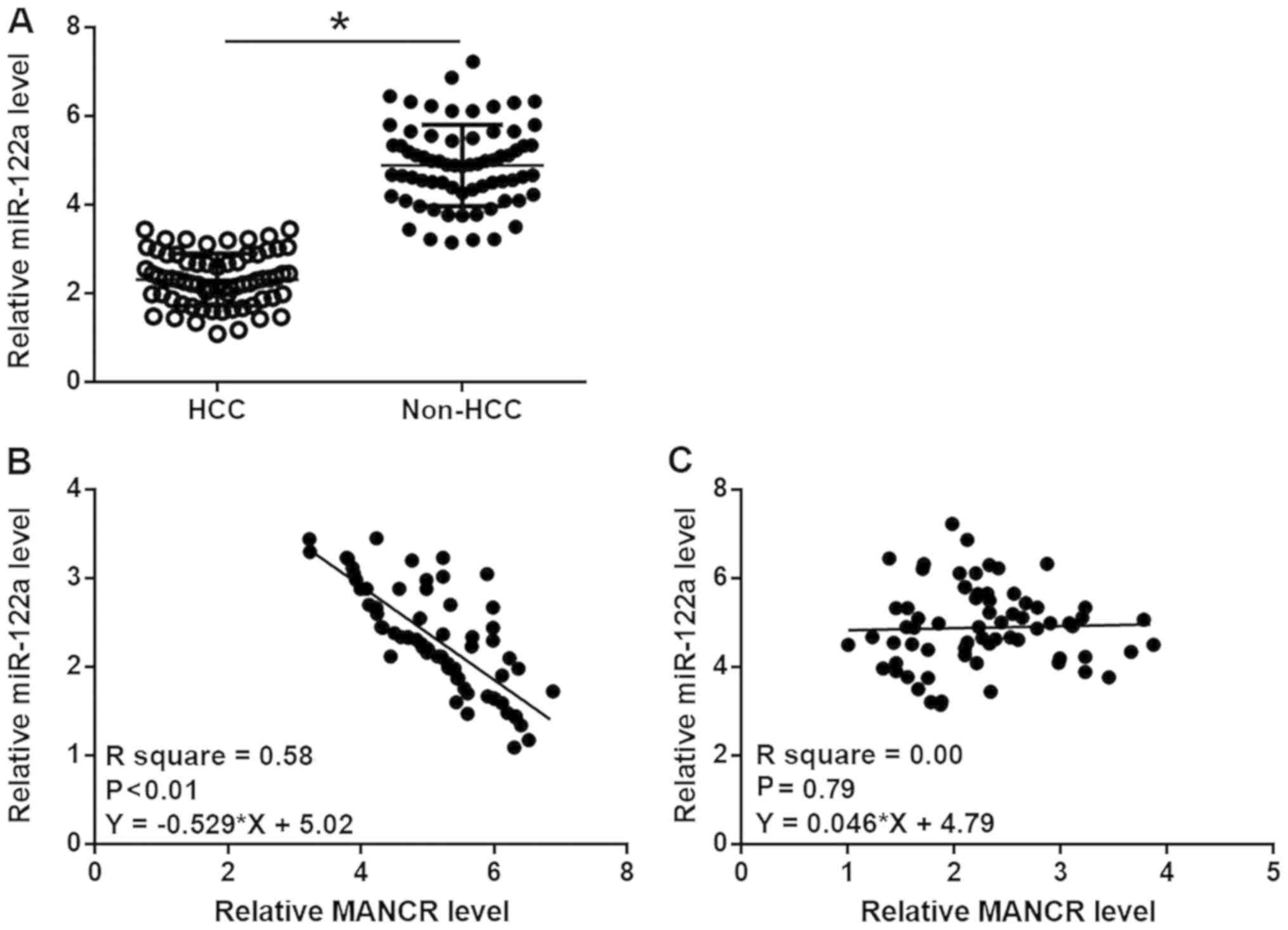
MiR-122a is inversely correlated with
MANCR in HCC
RT-qPCR experiments were also performed to
investigate the differential expression of miR-122a in HCC and
non-HCC tissues. Comparison between HCC tissues and non-HCC tissues
using a paired t test showed that miR-122a was significantly
downregulated in HCC tissues compared with in non-HCC (P<0.05;
Fig. 2A). Linear regression was
performed to analyze the correlations between MANCR and miR-122a.
It was observed that miR-122a was inversely and significantly
correlated with MANCR in HCC tissues (P<0.01; Fig. 2B), but not in non-HCC tissues
(Fig. 2C).
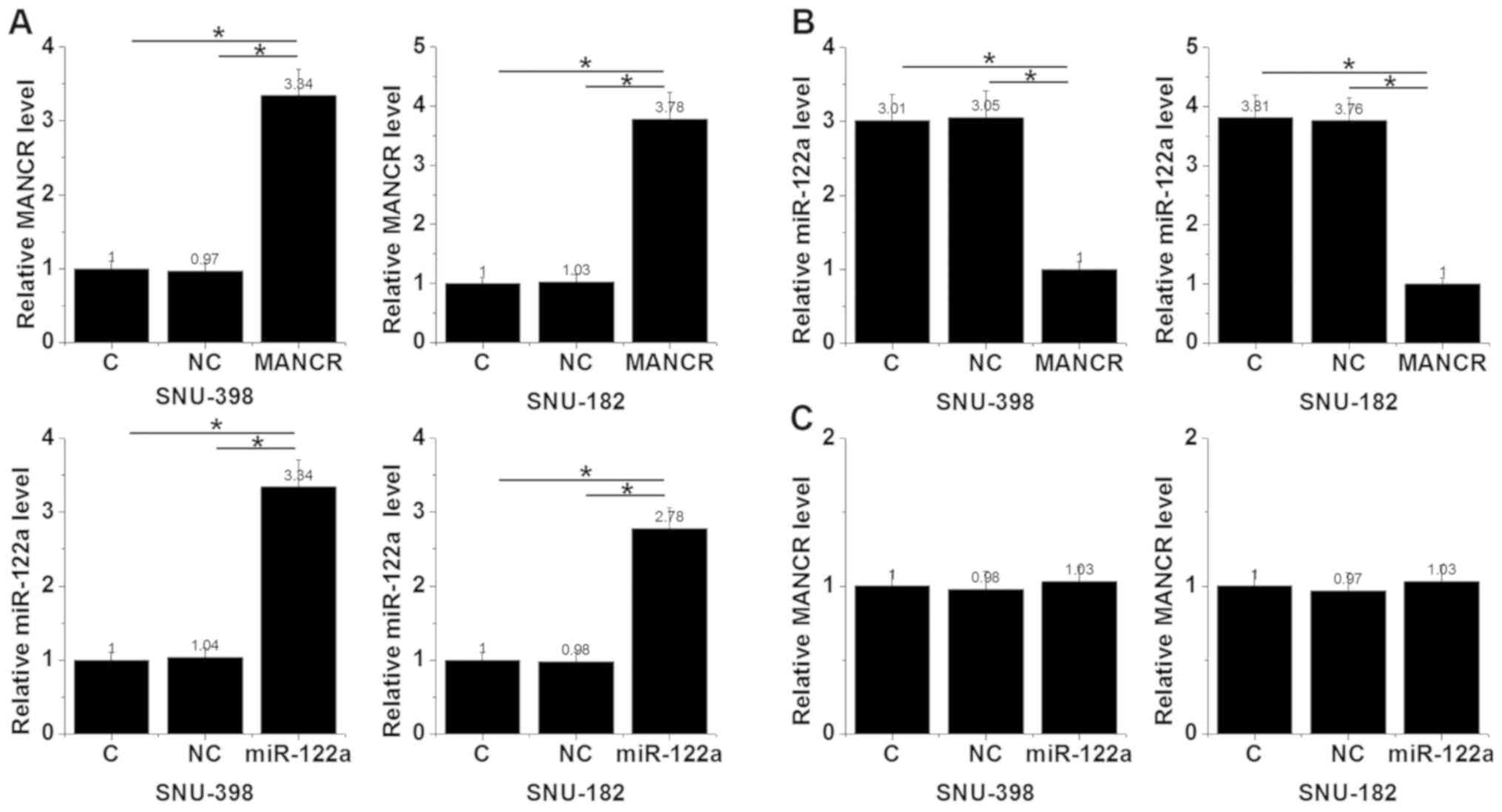
MANCR overexpression leads to
downregulation of miR-122a in HCC cells
To further investigate the interaction between MANCR
and miR-122a in HCC, MANCR-expression vectors and miR-122a mimics
were transfected into cells of both SNU-398 and SNU-182 cell lines,
followed by the detection of MANCR and miR-122a expression by
RT-qPCR. Comparing with the negative control (NC) and control (C)
groups, MANCR and miR-122a were significantly overexpressed in
cells of both cell lines at 24 h after transfection (P<0.05;
Fig. 3A). In addition, MANCR
overexpression in cells of these 2 cell lines resulted in the
significant downregulation of miR-122a (P<0.05; Fig. 3B), while miR-122a overexpression in
cells of these two cell lines showed no obvious effects on MANCR
expression (Fig. 3C).
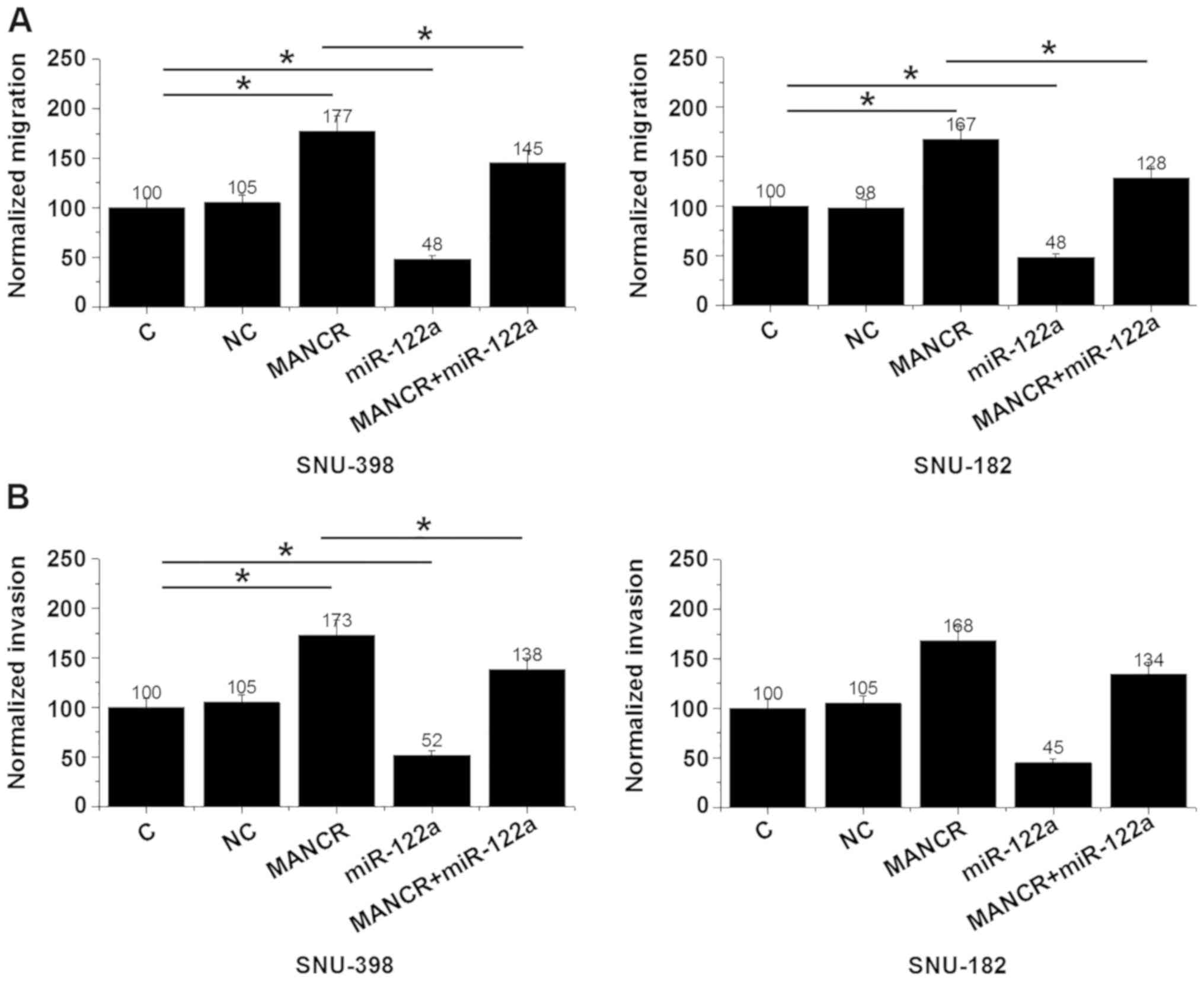
MANCR overexpression promotes HCC cell
migration and invasion through miR-122a
Transwell migration and invasion assays showed that,
compared with the NC and C groups, MANCR overexpression showed no
significant effects on HCC cell proliferation (data not shown), but
led to significantly promoted cell migration (P<0.05; Fig. 4A) and invasion (P<0.05; Fig. 4B). miR-122a overexpression led to
significantly inhibited migration (P<0.05; Fig. 4A) and invasion (P<0.05; Fig. 4B) of HCC cells and attenuated the
effects of MANCR overexpression.
Discussion
It has been reported that MANCR participates in
breast cancer (11). The present
study first proved that MANCR may also be involved in HCC. The
current study also showed that MANCR was upregulated in HCC and
MANCR overexpression may promote HCC cell migration and invasion by
downregulating miR-122a.
The function of miR-122a has been reported in
several human diseases (12–15). It has been shown that miR-122a is
likely involved in Warburg-like effects of hepatocyte deficient in
mice (12). In another study,
miR-122a was proved to participate in hepatitis C virus infection
(13), which is related to a number
of liver diseases (14), such as HCC
(15). The present study showed that
miR-122a was downregulated in HCC. The results of the current study
are consistent with the report of Zhang et al (16), which reported the downregulation of
miR-122 in HCC and its prognostic values for HCC. It was also found
that miR-122a overexpression led to the inhibited migration and
invasion of HCC cells. Therefore, miR-122a may play an oncogenic
role in HCC. MANCR participates in breast cancer by affecting cell
division and genomic stability (11), which are involved in all types of
cancer (20), indicating the
involvement of MANCR in other types of cancer, such as HCC.
Interestingly, the present study showed that MANCR was upregulated
in HCC tissues and overexpression of MANCR led to promoted
migration and invasion of HCC cells, but showed no significant
effects on cancer cell proliferation. Therefore, MANCR may play
different roles in different types of cancer.
LncRNAs play their roles through the regulation of
downstream genes at posttranscriptional and translational levels or
through epigenetic pathways (6).
Previous studies showed that lncRNAs may serve as miRNA sponges to
downregulated tumor suppression and oncogenic miRNAs in cancer
biology (21,22). The present study showed that MANCR
can downregulate miR-122a in HCC cells. However, no promising
target of miR-122a was found on the MANCR sequence. Therefore,
lncRNAs may regulate the expression of miRNAs through other
mechanisms. More studies are still needed.
In conclusion, MANCR was upregulated and miR-122a
was downregulated in HCC. MANCR may downregulate miR-122a to
participate in HCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and HL were responsible for the experiments,
analysis of the data, and the manuscript writing. JZ and SY
performed the laboratory work and literature research. MM and XW
performed the project management, research design and literature
research. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from the Ethics
Committee of the Central Hospital of Wuhan. The study followed the
tenets of the Declaration of Helsinki and informed written consent
was obtained from all patients of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hernaez R and El-Serag HB: Hepatocellular
carcinoma surveillance: The road ahead. Hepatology. 65:771–773.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zucman-Rossi J, Villanueva A, Nault JC and
Llovet JM: Genetic landscape and biomarkers of hepatocellular
carcinoma. Gastroenterology. 149:1226–1239.e4. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niu ZS, Niu XJ and Wang WH: Genetic
alterations in hepatocellular carcinoma: An update. World J
Gastroenterol. 22:9069–9095. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi X, Sun M, Liu H, Yao Y and Song Y:
Long non-coding RNAs: A new frontier in the study of human
diseases. Cancer Lett. 339:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spizzo R, Almeida MI, Colombatti A and
Calin GA: Long non-coding RNAs and cancer: A new frontier of
translational research? Oncogene. 31:4577–4587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qi P and Du X: The long non-coding RNAs, a
new cancer diagnostic and therapeutic gold mine. Mod Pathol.
26:155–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tracy KM, Tye CE, Ghule PN, Malaby HLH,
Stumpff J, Stein JL, Stein GS and Lian JB: Mitotically-associated
lncRNA (MANCR) affects genomic stability and cell division in
aggressive breast cancer. Mol Cancer Res. 16:587–598. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu HQ, Cheng ML, Lai JM, Wu HH, Chen MC,
Liu WH, Wu WH, Chang PM, Huang CF, Tsou AP, et al: Flux balance
analysis predicts Warburg-like effects of mouse hepatocyte
deficient in miR-122a. PLoS Comput Biol. 13:e10056182017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sakurai F, Furukawa N, Higuchi M, Okamoto
S, Ono K, Yoshida T, Kondoh M, Yagi K, Sakamoto N, Katayama K and
Mizuguchi H: Suppression of hepatitis C virus replicon by
adenovirus vector-mediated expression of tough decoy RNA against
miR-122a. Virus Res. 165:214–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lavanchy D: Evolving epidemiology of
hepatitis C virus. Clin Microbiol Infect. 17:107–115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanwal F, Hoang T, Kramer JR, Asch SM,
Goetz MB, Zeringue A, Richardson P and El-Serag HB: Increasing
prevalence of HCC and cirrhosis in patients with chronic hepatitis
C virus infection. Gastroenterology. 140:1182–1188.e1. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Li Y, Jiang W, Li Q and Lan Y:
The clinical significance of microRNA-122 in predicting the
prognosis of patients with hepatocellular carcinoma: A
meta-analysis validated by the Cancer Genome Atlas dataset.
Medicine (Baltimore). 98:e148102019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chun YS, Pawlik TM and Vauthey JN: 8th
edition of the AJCC cancer staging manual: Pancreas and
hepatobiliary cancers. Ann Surg Oncol. 25:845–847. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Böhning D, Böhning W and Holling H:
Revisiting Youden's index as a useful measure of the
misclassification error in meta-analysis of diagnostic studies.
Stat Methods Med Res. 17:543–554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen Z: Genomic instability and cancer: An
introduction. J Mol Cell Biol. 3:1–3. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang WC, Fu WM, Wong CW, Wang Y, Wang WM,
Hu GX, Zhang L, Xiao LJ, Wan DC, Zhang JF and Waye MM: The lncRNA
H19 promotes epithelial to mesenchymal transition by functioning as
miRNA sponges in colorectal cancer. Oncotarget. 6:22513–22525.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paraskevopoulou MD and Hatzigeorgiou AG:
Analyzing miRNA-lncRNA interactions. Methods Mol Biol.
1402:271–286. 2016. View Article : Google Scholar : PubMed/NCBI
|