Introduction
Non-small cell lung cancer (NSCLC) was the leading
cause of cancer-related mortality worldwide in 2011 (1). Advances in chemotherapy, targeted
therapy and immunotherapy have prolonged survival in the last
decade (2) Recently, the
implementation of immune checkpoint inhibitors programmed cell
death protein 1 (PD-1) and/or programmed death ligand 1 has
advanced the treatment options (2).
Although those developments and achievements have provided
convicting data allow immunotherapy to be included in the treatment
of NSCLC, a considerable population experience recurrence or are
refractory to those agents; this may be partly due to immune
tolerance and immune microenvironment resistance occurrence.
However, the insufficiency of T cell distribution and/or T cell
exhaustion have been studied more extensively; the supplementary
effective T cells were able to enhance the interactions between T
cells and tumor cell through cytokines release and T cell recovery
(3).
Adoptive cellular immunotherapy (ACT), the delivery
of ex vivo activated cellular products, including dendritic
cells (DCs), natural killer (NK) cells or T cells, is a
personalized approach, which has demonstrated promising results in
melanoma (3,4) and a variety of other cancer types
(5–15). The combination of ex
vivo-expanded DCs, potent stimulators of tumor-specific T cell
responses, with cytokine-induced killer cells (CIKs), lymphocytes
with an NK/T-cell phenotype, results in the formation of a cell
infusion called DC-CIK (5). The
autologous adoptive cellular immunotherapy has certain benefits
including the relative feasibility of cell collection from the
patient induvial and T cell expansion in vitro, during which
CD8+ cytotoxic T cells are harvested. It has previously
been reported that DC-CIK infusions, alone or when combined with
chemotherapy, improved the clinical outcome of patients with
advanced cancer (12,16).
The DC-CIK product is comprised of various T cell
subpopulations post-induction with the presence of cytokine
stimulation. The final cell products may comprise effector T cells
and, to a lesser extent, regulatory T cells (Tregs) and suppressive
macrophage populations, all of which have the potential to impact
clinical therapeutic outcome (12,16). The
quantitation of these anti-cancer cell subpopulations, rather than
total cell count prior to iv infusion, should be addressed before
the infusions to collect the data required to qualify the culture
system. The efficient cytotoxic T cell infusion may be able to
predict the clinical responses (12,16). In
the present study, the T cell subsets within the DC-CIK infusion
and the association of changes in their frequency during ex
vivo culture with progression-free survival (PFS) and overall
survival (OS) time of patients with advanced NSCLC who were treated
with ACT were analyzed in order to aid in the optimization of
DC-CIK immunotherapy.
Patients and methods
Patients and study design
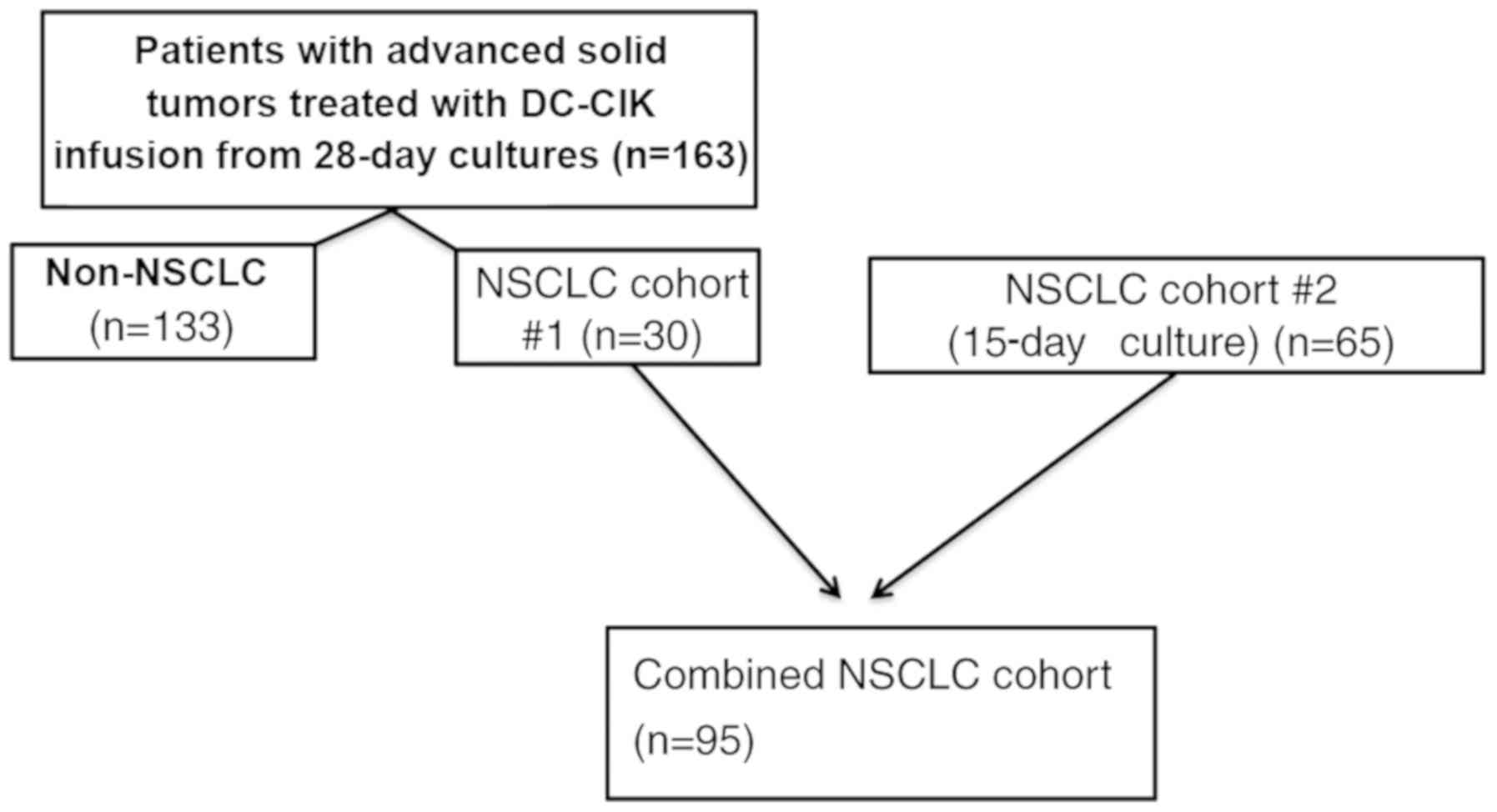
Data for the present study were derived from two
cohorts of patients treated at the Capital Medical University
Cancer Center, Beijing Shijitan Hospital (Beijing, China) between
September 2012 and June 2015 according to protocols approved by The
Regional Ethics Review Board of Capital Medical University Cancer
Center (Beijing, China) and to the ethical principles for medical
research involving human subjects of The Declaration of Helsinki.
All patients provided informed consent prior to participation in
the study. All eligible participants were included; the patients
included were diagnosed with NSCLC (n=95), metastatic breast
(n=30), colon (n=29), pancreatic (n=19), advanced gastric (n=20)
and other types of cancer (n=35). The first cohort comprised 163
patients recruited between September 2012 and December 2014 with
advanced cancer (including 30 with NSCLC) for whom CIK products
were generated ex vivo from autologous peripheral blood
mononuclear cells (PBMCs); the PBMCs were expanded ex vivo
over a 28-day period. The data obtained from the 28-day expansion
was analyzed as the preliminary condition trial and subjected to
optimization to determine the cell harvest time. Based on these,
the culture protocol was adjusted and the second cohort was
recruited, which comprsed 65 patients with NSCLC for whom CIK
products were generated ex vivo over a 15-day period at the
same hospital between January 2015 and June 2015. Subsequently, 30
patients with NSCLC from the first cohort plus the second group of
65 patients with NSCLC were combined into an additional NSCLC
cohort (n=95) to evaluate the impact of T cell subsets during the
ex vivo generation of DC-CIKs on clinical outcome in a
homogeneous group (Fig. 1).
Participants were required to meet the following inclusion
criteria: Histologically or cytologically confirmed, unresectable,
locally advanced or metastatic solid malignancy, planned treatment
with multi-cycle ACT, aged 18–80 years and adequate hematological
and organ function based on white blood cell count and normal
values of liver and kidney function tests. Eastern Cooperative
Oncology Group Performance Status (ECOG-PS) of 0–2 (17) and no previous history of
immunotherapy. Exclusion criteria consisted of the following: A
serious, uncontrolled medical condition or a psychiatric disorder
that would limit the ability of the patient to comply with study
requirements.
PBMC collection and CIK
generation
PBMCs were collected as described previously
(18,19). Briefly, patients received 5 µg/kg/day
of granulocyte-macrophage colony-stimulating factor (GM-CSF; Chugai
Pharmaceutical Co., Ltd.) subcutaneously until the level of
mononuclear cells in peripheral blood reached 1.5×109/l
and subsequently underwent apheresis. Patients were eligible for
all standard anti-cancer treatments and apheresis was performed
after chemotherapy. PBMCs were separated by a COBE Spectra cell
separator (Terumo BCT, Inc.) until CD34+ cells reached a
threshold of 4.5×106/kg. All collected cells were frozen
at −80°C until required for the DC-CIK infusion. For DC generation
preparation, the collected PBMCs were placed into a flask for 2 h
to attach to the walls; adherent cells (2–3×106) were
cultured in DC medium (X–VIVO Lonza Group, Ltd.) medium for 7 days
at 37°C with 5% CO2 with interleukin (IL)-4 (1,000 U/ml;
Amoytop Biotech Co., Ltd.), TNF-α (20 ng/ml; R&D Systems, Inc.)
and GM-CSF (800 U/ml; Amoytop Biotech Co., Ltd.). For CIK
generation, PBMCs (2–3×108) were cultured at 37°C in AIM
V medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% heat-inactivated patient's autologous plasma or human AB plasma
obtained from the Beijing Shijitan Hospital Blood Bank and the
recombinant cytokines IL-2 (2,000 U/ml; Sihuan Pharmaceutical
Holdings Group, Ltd.), interferon-γ (1,000 U/ml; cat. no. TL-105;
Beijing T&L Biotechnology Co., Ltd.) and anti-CD3 antibody (1.7
ml/ml; cat. no. TL-101; Beijing T&L Biotechnology Co., Ltd.).
Half of the medium was replaced with fresh AIM V containing IL-2
(2,000 IU/ml) every other day. After 7 days, the autologous DCs
were mixed with cultured CIKs at a ratio of 1:100, and resulting
DC-CIKs from each mixture (2×109) were subsequently
collected for infusion at day 15 or 28, at which time points the
resulting cells were harvested for treatment or analysis, as
described in the following text.
Flow cytometry analysis of ex vivo
expanded DC-CIKs
Various subpopulations within the cell products
(PBMCs prior to culture and cultured DC-CIKs) were identified by
flow cytometry, as previously described (11), using the following
fluorochrome-conjugated antibodies: CD3 PerCP-Cy5.5, CD4 FITC, CD8
FITC, CD25 PE, CD28 PE, CD56 PE (all Beckman Coulter, Inc.), PD-1
PE, lymphocyte-activation gene 3 (LAG-3) PE, tumor necrosis factor
receptor superfamily member 9 (4-1BB; CD137) PE and T cell
immunoglobulin and mucin protein 3 (TIM-3) PeCy-7 (all BioLegend,
Inc.). Three-color flow cytometry analysis was performed on a
Cytomics FC500 flow cytometer with CXP analysis software (Beckman
Coulter, Inc.).
Treatment scheme
Patients received DC-CIK cell therapy (median,
5.2×109 CIKs) in scheduled 21-day treatment cycles,
specifically on days 15, 17 and 19 of each cycle. Patients could
receive >2 cycles (one cycle refers 3 infusions) dependent upon
the physician's decision when combined with chemotherapy. Since
adoptive cell immunotherapy was combined with the existing standard
anti-cancer treatments, the present study focused on the ex
vivo expansion parameter acquisition; the clinical treatment
options were determined by the attending physicians. A total of 50
patients received chemotherapy including paclitaxel plus cisplatin
(n=10), gemcitabine plus cisplatin (n=15), docetaxel plus cisplatin
(n=9), pemetrexed plus cisplatin (n=14) and Tegafur Gimeracil
Oteracil Potassium (S1) plus cisplatin (n=2) prior to the DC-CIK
infusions during each cycle.
Statistical analysis
Continuous variables are expressed as the mean ± SD
and were compared using two-tailed unpaired Student's t-tests.
Multiple subgroup comparisons were performed using ANOVA followed
by Tukey's post hoc test. Categorical variables were compared using
χ2 or Fisher's exact test. The predictive performance of
T cell subtypes was measured using receiver operating
characteristic (ROC) curve analysis and the area under the curve
(AUC). AUCs were also used to evaluate different T cell subtypes
using the Hanley and McNeil method (20). The impact of the combination of T
cell subtypes on the clinical outcome of patients with NSCLC who
received treatment, was analyzed using a Cox regression model. The
independent risk factors found to be significantly related to
survival at multivariate analysis were entered into the Cox model.
The sum of the relative risks that impacted the hazard function was
used in the Cox model to predict the prognosis of the patients.
Statistical analyses were performed with SPSS version 18.0 (SPSS,
Inc.) and ROC curve analysis was conducted using MedCalcV.11.0.3.0
(MedCalc Software bvba), GraphPad Prism version 5.04 (GraphPad
Software, Inc.) and SPSS version 21.0 (IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference in
all analyses.
Results
Patient characteristics
For data analysis, two study groups were created
(Fig. 1). Firstly, 163 patients with
advanced cancer (including 30 with NSCLC) were assessed to
determine when the number of cultured CIKs peaked Among these 95
NSCLC patients, 45 received DC-CIK cell therapy alone and 50
received DC-CIKs combined with chemotherapy. Their characteristics
are listed in Table I.
 | Table I.Demographics and baseline
characteristics of patients (n=95). |
Table I.
Demographics and baseline
characteristics of patients (n=95).
|
| Therapy group |
|
|---|
|
|
|
|
|---|
| Variable | DC-CIK alone | DC-CIK combined
with CT | P-value |
|---|
| Cases, n | 45 | 50 |
|
| Age (years; mean ±
SD) | 61.2±8.1 | 60.8±9.3 | 0.753 |
| Sex |
|
| 0.805 |
|
Female | 29 | 31 |
|
|
Male | 16 | 19 |
|
| ECOG-PS |
|
| 0.402 |
| 0 | 29 | 28 |
|
| 1 | 16 | 22 |
|
| TNM staging |
|
| 0.297 |
|
III | 10 | 7 |
|
| IV | 35 | 43 |
|
| Previous adjuvant
chemotherapy |
|
| 0.858 |
|
Yes | 17 | 18 |
|
| No | 28 | 32 |
|
| Histopathological
type |
|
| 0.825 |
|
Adenocarcinoma | 31 | 35 |
|
|
Squamous carcinoma | 14 | 15 |
|
| T cell
subtypes |
|
Post/pre
CD4+CD25+CD127+ T lymphocytes |
|
| 0.269 |
|
>0.6 | 21 | 29 |
|
|
≤0.6 | 24 | 21 |
|
|
Post/pre
CD8+CD28+ T lymphocytes |
|
| 0.204 |
|
>2.2 | 22 | 18 |
|
|
≤2.2 | 23 | 32 |
|
| Disease
control |
|
| 0.001a |
|
Stable | 10 | 28 |
|
|
Progressive | 35 | 22 |
|
DC-CIK phenotype during ex vitro
culture expands in a time dependent manner
Among the 163 patients for whom the DC-CIKs were
cultured for 28 days, the number of CIKs peaked at 15±2.16 days
followed by a slight decrease (Fig. 2E
and F). CIKs were successfully expanded ex vivo, with a
median fold expansion of 32.7 (range, 3.5–64.2) by day 15. The
percentages of CD3+, CD3+CD4+,
CD3+CD8+, CD8+CD28+,
CD8+4-1BB+, CD8+LAG-3+
and CD8+TIM-3+ cells also reached a peak on
day 14 (Fig. 2); however, the
percentage of Tregs
(CD4+CD25+CD127+) decreased after
day 7 of culture (Fig. 2C). In
addition, the percentages of B cells (CD19+) and NK
cells (CD3−CD16+/CD56+) decreased,
whereas NK T cells
(CD3+CD16+CD56+) increased by day
28 (Fig. 2B). The expression levels
of 4-1BB, LAG-3 and TIM-3 on CD4+ and CD8+ T
cells increased between days 7 and 14 before decreasing (Fig. 2E and F).
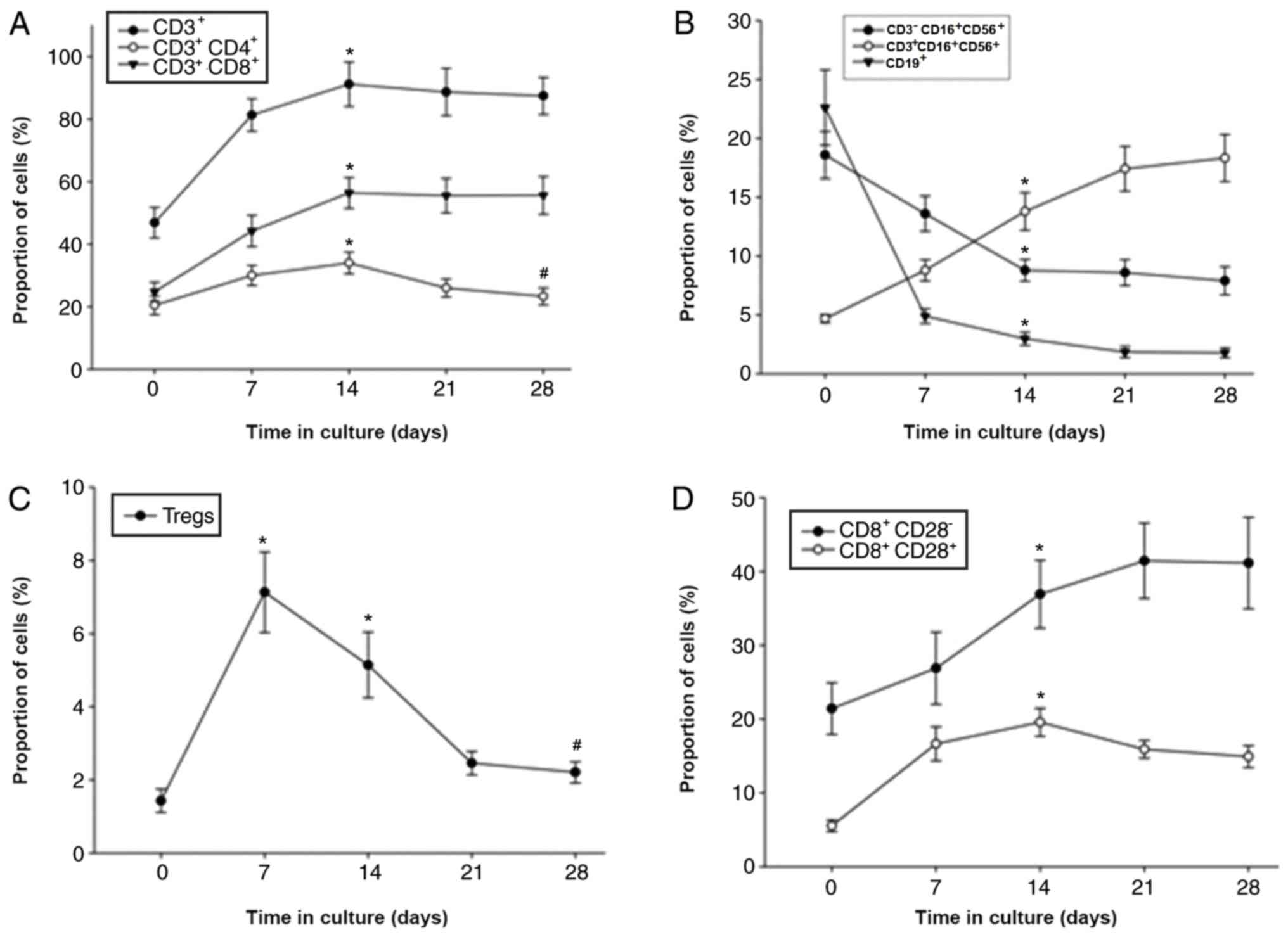 | Figure 2.Measurements of the expanded
population percentages of various cytokine-induced killer cell
groups during ex vivo culture. Six sub-groups are presented
according to T cell subtypes. (A-F) Changes in the proportion of
(A) CD3+, CD3+CD4+ and
CD3+CD8+; (B)
CD3−CD16+CD56+,
CD3+CD16+CD56+ and
CD19+; (C) Tregs; (D) CD8+CD28−
and CD8+CD28+; (E)
CD4+TIM-3+, CD4+LAG-3+
and CD4+4-1BB+; and (F)
CD8+LAG-3+, CD8+4-1BB+
and CD8+TIM-3+. Multiple subgroup comparisons
were performed using ANOVA. *P<0.05 vs. day 0;
#P<0.05 vs. day 15. IFN-γ, interferon-γ; TNF-α, tumor
necrosis factor-α; IL-2, interleukin-2; LAG-3,
lymphocyte-activation gene 3; 4-1BB, tumor necrosis factor receptor
superfamily member 9; TIM-3, T cell immunoglobulin and mucin
protein 3. |
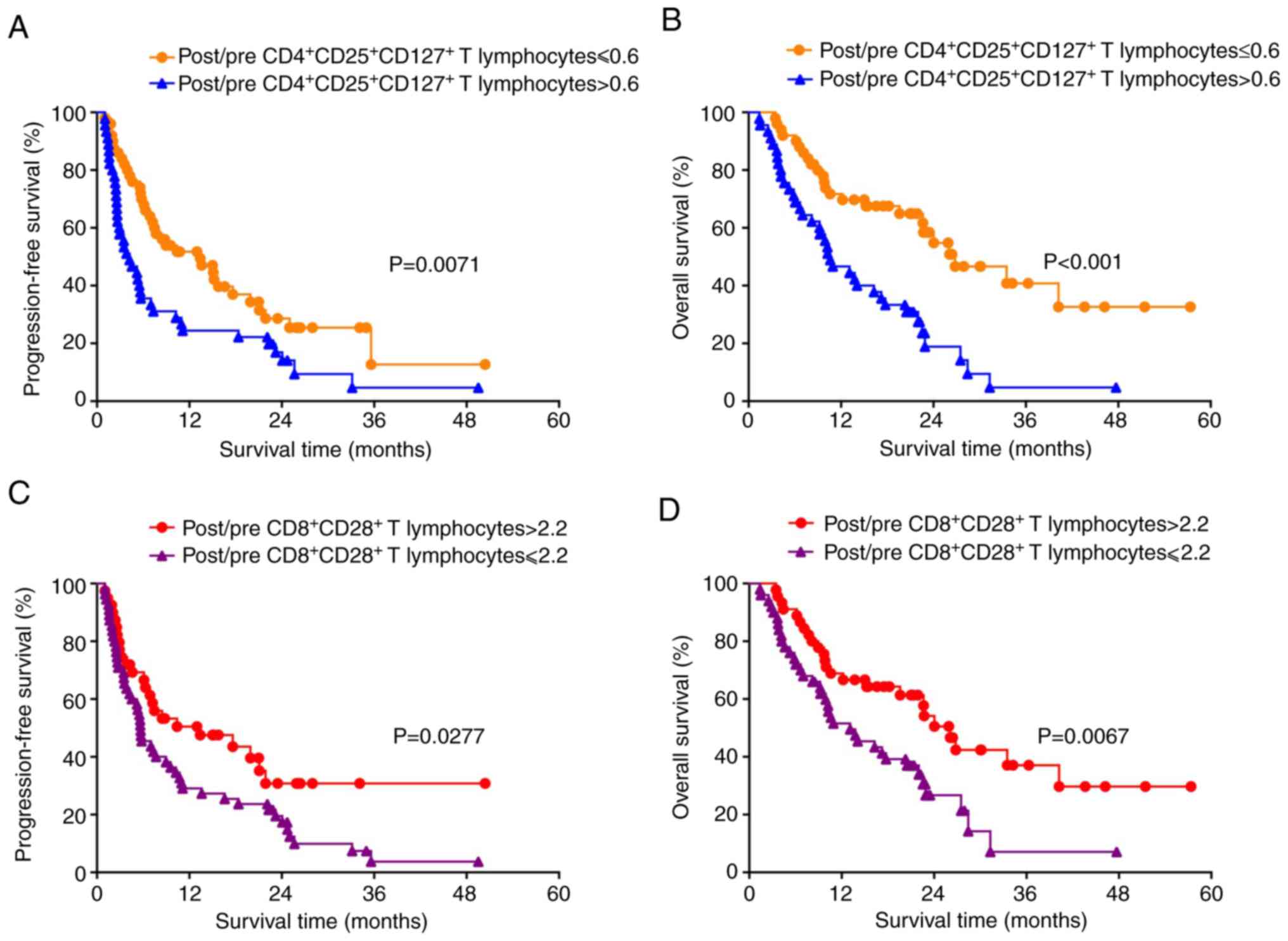
Alterations in frequency of
CD4+CD25+CD127+ and
CD8+CD28+ T cells after ex vivo expansion for
15 days as predictors for the efficacy of ACT in patients with
NSCLC
Having identified day 15 as the time point of
maximum CIK expansion, the ratios (pre-culture vs. day 15 of ex
vivo expansion) of the various T cell subsets within the DC-CIK
infusion were then compared in the 95 patients with NSCLC.
Specifically, post/pre-culture ratios of 0.6 and 2.2 were
determined as the cut-off values of
CD4+CD25+CD127+ and
CD8+CD28+ T cells, respectively. Patients
with a post/pre-culture
CD4+CD25+CD127+ Treg ratio ≤0.6
were identified to have significantly favorable PFS (P=0.0071;
Fig. 3A) and OS (P<0.001;
Fig. 3B) compared with those with
higher rations of these cells. Patients with post/pre-culture
CD8+CD28+ T lymphocyte ratio >2.2 had
significantly favorable PFS (P=0.0277; Fig. 3C) and OS (P=0.0067; Fig. 3D) compared with those with ratios
≤2.2. Subsequently, ROC analysis was performed to confirm the
optimal cut-off value (Fig. 4).
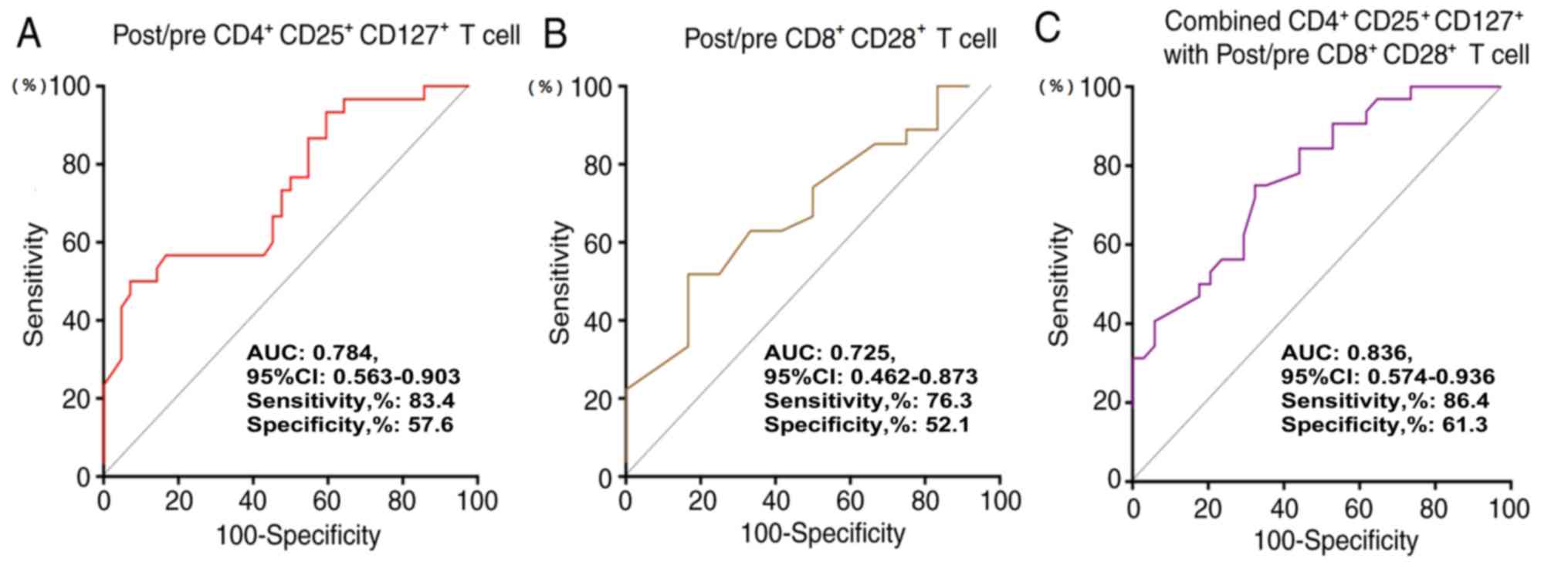
Prognostic performance of T cell
subtypes in patients with advanced NSCLC
The performance of the post/pre-culture
CD4+CD25+CD127+ Treg ratios,
post/pre-culture CD8+CD28+ T cell ratios and
the combined ratio of these T cell subtypes was evaluated to
determine whether these ratios could predict different clinical
outcomes in patients with NSCLC (Fig.
4). The analysis demonstrated that the combination of these T
cell subtypes was a valuable marker in predicting the OS of
patients with NSCLC (AUC, 0.836; 95% CI, 0.574–0.936; sensitivity,
86.4%; specificity, 61.3%). Details are provided in Fig. 4.
Risk factors associated with clinical
outcomes
Cox proportional hazard models were used to quantify
the prognostic significance of risk factors following multivariate
adjustment. A multivariate analysis was performed to assess the
factors that demonstrated significant effects. Following adjustment
for competing risk factors (ECOG-PS, TNM stage and infusion
cycles), a post/pre-culture
CD4+CD25+CD127+ Treg ratio
>0.6, post/pre-culture CD8+CD28+ T
lymphocyte ratio ≤2.2 and treatment with the DC-CIK infusion
combined with chemotherapy remained independent predictors of PFS
and OS (Table II).
 | Table II.Multivariate Cox proportional hazard
regression analysis of patient demographic and clinical
characteristics and survival. |
Table II.
Multivariate Cox proportional hazard
regression analysis of patient demographic and clinical
characteristics and survival.
|
| PFS | OS |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| ECOG-PS, 2 | 1.032
(0.783–1.231) | 0.462 | 0.923
(0.872–1.253) | 0.188 |
| TNM stage, IV | 1.059
(0.718–1.629) | 0.521 | 0.936
(0.783–1.258) | 0.894 |
| Post/pre
CD4+CD25+CD127+ T lymphocytes
>0.6 | 1.574
(1.381–2.932) | 0.017 | 1.859
(1.136–2.264) | 0.006 |
| Post/pre
CD8+CD28+ T lymphocytes ≤2.2 | 1.834
(1.524–3.187) | 0.011 | 2.732
(1.774–5.673) | 0.002 |
| Infusion
cycles | 1.103
(0.851–1.253) | 0.724 | 0.972
(0.761–1.354) | 0.758 |
| DC-CIK combined
with CT | 0.436
(0.168–0.579) | 0.001 | 0.343
(0.257–0.857) | 0.035 |
Discussion
ACT with CIKs has demonstrated antitumor activity
against bulky metastases in patients with various solid tumors
(21,22); however, the DC-CIK infusion generated
in the present study contained a variety of T cell subtypes.
Improvements in efficacy require further engineering of the cell
infusion to include additional cells with potent anti-tumor
activity (CD8+ effector T cells) and fewer cells with
immunosuppressive activity (including Tregs). Therefore, a detailed
analysis of the number and phenotype of T cells within the DC-CIK
infusion administered to patients with NSCLC was performed. In
order to identify the optimal culture time for harvesting the CIKs,
a total of 163 patients with advanced solid tumors were recruited
for apheresis and subsequent DC-CIK immunotherapy. The number of
CIKs gradually increased until day 15 during the culture period,
followed by a slight decline by day 28. The percentages of
CD3+, CD3+CD4+,
CD3+CD8+ and CD8+CD28+
cells peaked at day 15. Therefore, CIKs cultured for 15 days were
chosen to be administered to the patients.
CD4+CD25+ Tregs maintain the
balance between immune activation and tolerance (23,24),
preventing autoimmune disease (25).
Tregs are also thought to facilitate tumor progression by
suppressing adaptive immunity against tumors. Treg cell depletion
in transplantable, carcinogen-induced, and autochthonous tumor
models has demonstrated increased anti-tumor immune responses
(26,27). A total of 95 patients with NSCLC with
a post/pre-culture CD4+CD25+CD127+
T lymphocyte ratio of ≤0.6 were identified to have significantly
improved OS and PFS compared with those with a post/pre-culture
CD4+CD25+CD127+ Treg ratio
>0.6.
CD28 is a co-stimulatory molecule that serves
multiple roles in the activation, proliferation and survival of T
cells (28,29). CD8+CD28+ T
cells are found in the tumor microenvironment and in the
circulation of patients with cancer. Both active and suppressive
antitumor immune responses have been ascribed to
CD8+CD28+ T cell populations (30,31). It
was found that CD8+CD28+ T cells were
significantly increased after CIK expansion and were associated
with PFS and OS in the treated patients with NSCLC. Specifically,
patients with a post/pre-culture CD8+CD28+ T
lymphocyte ratio >2.2 had significantly improved OS and PFS
compared with those with a post/pre-culture
CD8+CD28+ T lymphocyte ratio of ≤2.2.
In our previous report, the role of DC-CIK infusion
in patients with NSCLC was determined using a non-randomized
control study design; the results demonstrated that the
incorporation of DC-CIK into the standard anti-cancer treatment
exhibited benefits for clinical responses (32). Therefore, the present study was
performed to further analyze the expanded cell phenotype variations
that may impact the cell yield. The results of the present study
demonstrated that patients with a post/pre-culture
CD8+CD28+ T lymphocyte ratio >2.2 and
post/pre-culture CD4+CD25+CD127+
Treg ratio ≤0.6 exhibited significantly longer PFS and OS time.
The present study has certain limitations. First, 50
of the patients with NSCLC received chemotherapy prior to the
DC-CIK infusions; the number of patients receiving DC-CIK alone was
too low to allow subgroup analysis in the current study. Secondly,
despite assessing the effect of changes in the major lymphocyte
subsets within the DC-CIK infusion during ex vivo culture on
clinical outcome, other cellular components or polymorphisms in
cytokines or their receptors on the cells within the DC-CIK
infusion may have potentially affected the outcome. Larger numbers
of treated patients are required to assess these impacts.
Nonetheless, the present study supports the hypothesis that further
ex vivo manipulations of the DC-CIKs may contribute to the
development of a consistent cell therapy product with greater
antitumor activity.
Acknowledgements
The authors would like to thank Ms. Yanhua Yan and
Ms. Meisheng Liu, Cancer Immunotherapy Research Center, Beijing
Shijitan Hospital, Capital Medical University Cancer Centre,
Beijing, China, for their technical contribution.
Funding
This work was supported by the Enhancement Funding
of Lab of Therapeutic Cancer Vaccine (grant no. 2019-JS01), Beijing
Shijitan Hospital, Capital Medical University Cancer Center.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JR and HKL conceived and designed the study. GQ, MAM
and JR drafted and critically revised the manuscript for important
intellectual content. MAM participated in the study design and data
management GQ performed statistical analysis. LH, GQ, XW, XZ, JW
and AH contributed to data acquisition and interpretation. JR and
HKL supervised the study.
Ethics approval and consent to
participate
Patient data was used in the present study according
to the ethical principles for medical research involving human
subjects of The Declaration of Helsinki. The study protocols were
approved by The Regional Ethics Review Board of Capital Medical
University Cancer Center (Beijing, China), and all patients
provided written informed consent prior to participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosenberg SA, Restifo NP, Yang JC, Morgan
RA and Dudley ME: Adoptive cell transfer: A clinical path to
effective cancer immunotherapy. Nat Rev Cancer. 8:299–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Y, Zhu Y, Zhao E, He X, Zhao L, Wang
Z, Fu X, Qi Y, Ma B, Song Y and Gao Q: Autologous cytokine-induced
killer cell immunotherapy may improve overall survival in advanced
malignant melanoma patients. Immunotherapy. 9:1165–1174. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peng H, Yao M, Fan H, Song L, Sun J, Zhou
Z, Du Y, Lu K, Li T, Yin A, et al: Effects of autologous
cytokine-induced killer cells infusion in colorectal cancer
patients: A prospective study. Cancer Biother Radiopharm.
32:221–226. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai XR, Li X, Lin JX, Wang TT, Dong M,
Chen ZH, Jia CC, Hong YF, Lin Q and Wu XY: Autologous
transplantation of cytokine-induced killer cells as an adjuvant
therapy for hepatocellular carcinoma in Asia: An update
meta-analysis and systematic review. Oncotarget. 8:31318–31328.
2017.PubMed/NCBI
|
|
7
|
Ryu JI, Han MH, Cheong JH, Kim JM and Kim
CH: Current update of adoptive immunotherapy using cytokine-induced
killer cells to eliminate malignant gliomas. Immunotherapy.
9:411–421. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kong DS, Nam DH, Kang SH, Lee JW, Chang
JH, Kim JH, Lim YJ, Koh YC, Chung YG, Kim JM and Kim CH: Phase III
randomized trial of autologous cytokine-induced killer cell
immunotherapy for newly diagnosed glioblastoma in Korea.
Oncotarget. 8:7003–7013. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chung MJ, Park JY, Bang S, Park SW and
Song SY: Phase II clinical trial of ex vivo-expanded
cytokine-induced killer cells therapy in advanced pancreatic
cancer. Cancer Immunol Immunother. 63:939–946. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hontscha C, Borck Y, Zhou H, Messmer D and
Schmidt-Wolf IG: Clinical trials on CIK cells: First report of the
international registry on CIK cells (IRCC). J Cancer Res Clin
Oncol. 137:305–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ren J, Gwin WR, Zhou X, Wang X, Huang H,
Jiang N, Zhou L, Agarwal P, Hobeika A, Crosby E, et al: Adaptive T
cell responses induced by oncolytic Herpes Simplex
Virus-granulocyte macrophage-colony-stimulating factor therapy
expanded by dendritic cell and cytokine-induced killer cell
adoptive therapy. Oncoimmunology. 6:e12645632017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang N, Qiao G, Wang X, Morse MA, Gwin
WR, Zhou L, Song Y, Zhao Y, Chen F, Zhou X, et al: Dendritic
Cell/Cytokine-induced killer cell immunotherapy combined with S-1
in patients with advanced pancreatic cancer: A prospective study.
Clin Cancer Res. 23:5066–5073. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song QK, Ren J, Zhou XN, Wang XL, Song GH,
Di LJ, Yu J, Hobeika A, Morse MA, Yuan YH, et al: The prognostic
value of peripheral CD4+CD25+ T lymphocytes among early stage and
triple negative breast cancer patients receiving dendritic
cells-cytokine induced killer cells infusion. Oncotarget.
6:41350–41359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin M, Liang S, Jiang F, Xu J, Zhu W, Qian
W, Hu Y, Zhou Z, Chen J, Niu L, et al: 2003-2013, a valuable study:
Autologous tumor lysate-pulsed dendritic cell immunotherapy with
cytokine-induced killer cells improves survival in stage IV breast
cancer. Immunol Lett. 183:37–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Qi Y, Wang A, Ma B, Fu X, Zhao L
and Gao Q: Clinical effects of autologous cytokine-induced killer
cell-based immunotherapy in the treatment of endometrial cancer: A
case report and literature review. Onco Targets Ther. 10:4687–4690.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiao G, Wang X, Zhou L, Zhou X, Song Y,
Wang S, Zhao L, Morse MA, Hobeika A, Song J, et al: Autologous
dendritic cell-cytokine induced killer cell immunotherapy combined
with S-1 plus cisplatin in patients with advanced gastric cancer: A
prospective study. Clin Cancer Res. 25:1494–1504. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang H, Chen A, Wang T, Wang M, Ning X,
He M, Hu Y, Yuan L, Li S, Wang Q, et al: Detecting cell-in-cell
structures in human tumor samples by E-cadherin/CD68/CD45 triple
staining. Oncotarget. 6:20278–20287. 2015.PubMed/NCBI
|
|
19
|
Ren J, Di L, Song G, Yu J, Jia J, Zhu Y,
Yan Y, Jiang H, Liang X, Che L, et al: Selections of appropriate
regimen of high-dose chemotherapy combined with adoptive cellular
therapy with dendritic and cytokine-induced killer cells improved
progression-free and overall survival in patients with metastatic
breast cancer: Reargument of such contentious therapeutic
preferences. Clin Transl Oncol. 15:780–788. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanley JA: Receiver operating
characteristic (ROC) methodology: The state of the art. Crit Rev
Diagn Imaging. 29:307–335. 1989.PubMed/NCBI
|
|
21
|
Takimoto R, Kamigaki T, Okada S, Matsuda
E, Ibe H, Oguma E, Naitoh K, Makita K and Goto S: Efficacy of
adoptive immune-cell therapy in patients with advanced gastric
cancer: A retrospective study. Anticancer Res. 37:3947–3954.
2017.PubMed/NCBI
|
|
22
|
Shi G, Zhou C, Wang D, Ma W, Liu B and
Zhang S: Antitumor enhancement by adoptive transfer of tumor
antigen primed, inactivated MHC-haploidentical lymphocytes. Cancer
Lett. 343:42–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Joshi NS, Akama-Garren EH, Lu Y, Lee DY,
Chang GP, Li A, DuPage M, Tammela T, Kerper NR, Farago AF, et al:
Regulatory T cells in tumor-associated tertiary lymphoid structures
suppress Anti-tumor T cell responses. Immunity. 43:579–590. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Savage PA, Leventhal DS and Malchow S:
Shaping the repertoire of tumor-infiltrating effector and
regulatory T cells. Immunol Rev. 259:245–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dooms H, Wolslegel K, Lin P and Abbas AK:
Interleukin-2 enhances CD4+ T cell memory by promoting the
generation of IL-7R alpha-expressing cells. J Exp Med. 204:547–557.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bos PD, Plitas G, Rudra D, Lee SY and
Rudensky AY: Transient regulatory T cell ablation deters
oncogene-driven breast cancer and enhances radiotherapy. J Exp Med.
210:2435–2466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami
B, Axtell RC, Zhou G, Rajapaksa R, Green MR, Torchia J, Brody J, et
al: Depleting tumor-specific Tregs at a single site eradicates
disseminated tumors. J Clin Invest. 123:2447–2463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dumitriu IE: The life (and death) of CD4+
CD28(null) T cells in inflammatory diseases. Immunology.
146:185–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maly K and Schirmer M: Corrigendum to ‘The
Story of CD4 (+) CD28(−) T cells revisited: Solved or still
ongoing?’. J Immunol Res. 2015:2516572015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Filaci G, Fenoglio D, Fravega M, Ansaldo
G, Borgonovo G, Traverso P, Villaggio B, Ferrera A, Kunkl A, Rizzi
M, et al: CD8+ CD28-T regulatory lymphocytes inhibiting T cell
proliferative and cytotoxic functions infiltrate human cancers. J
Immunol. 179:4323–4334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Casado JG, Soto R, DelaRosa O, Peralbo E,
del Carmen Muñoz-Villanueva M, Rioja L, Peña J, Solana R and
Tarazona R: CD8 T cells expressing NK associated receptors are
increased in melanoma patients and display an effector phenotype.
Cancer Immunol Immunother. 54:1162–1171. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao Y, Qiao G, Wang X, Song Y, Zhou X,
Jiang N, Zhou L, Huang H, Zhao J, Morse MA, et al: Combination of
DC/CIK adoptive T cell immunotherapy with chemotherapy in advanced
non-small-cell lung cancer (NSCLC) patients: A prospective
patients' preference-based study (PPPS). Clin Transl Oncol.
21:721–728. 2019. View Article : Google Scholar : PubMed/NCBI
|