Pancreas carcinoma is one of the most malignant
diseases, associated with late and difficult diagnosis and really
short survival after diagnosis. Although in most countries
effective radiological and other examination methods can be
reached, the early diagnosis of pancreas cancer is difficult
(1–4). Pituitary adenylate cyclase activating
polypeptide (PACAP) was first isolated as a hypothalamic
neuropeptide acting on the pituitary cAMP release (5,6). The
peptide is composed of 38 amino acid residues (PACAP38) and has a
shorter form, with only 27 amino acids (PACAP27) (7). Subsequent studies have shown that PACAP
is distributed in the entire body, with highest concentrations in
the central nervous system and endocrine glands, but it is also
present in the cardiovascular, urogenital and gastrointestinal
systems (8–13). PACAP has a diverse array of functions
via specific PAC1 receptor and VPAC1 and 2 receptors shared with
vasoactive intestinal peptide, as well as non-receptorial
mechanisms (13,14).
PACAP and its receptors have also been shown in
several exocrine glands. The lacrimal gland is innervated by a rich
PACAP-ergic fiber plexus (15) and
PAC1 receptors are responsible for the activation of tear secretion
(16,17). Mammary and salivary glands are also
innervated by PACAP-ergic nerves (18–20). In
the salivary glands, PACAP induces secretion (21), and enhances protein production while
inhibits Ca2+ channels (22–24). The
exocrine pancreas is histologically similar to serous salivary
glands, and the presence of PACAP has also been shown in the
exocrine pancreas, where it stimulates acinar lipase secretion
(25). Endocrine pancreas, composed
of the islets of Langerhans, expresses very high levels of the
peptide, similarly to other endocrine glands. Intrainsular PACAP
plays a regulatory role in insulin and glucagon secretion and is
implied in glucose homeostasis. Pancreatic PACAP has also been
implicated in the regulation of beta cell proliferation (26).
Under pathological conditions, a few studies have
dealt with changes in PACAP and receptor expression. Previous
studies showed that pancreatic over-expression of PACAP increases
in cerulein-induced inflammation leading to acute pancreatitis in a
mouse model (27). PACAP, along with
its receptors, has been shown to be involved in cell proliferation
and differentiation both under normal circumstances and in
tumourous transformation (28–33). For
some tumour cells, PACAP acts as a growth factor (30), while it inhibits growth of others
(34). Whether it stimulates growth
of pancreatic tumour cells, it is not known at present, however, a
PACAP-response gene associated with proliferation and stress
response has been described in pancreatic carcinoma (35). Stimulative role of tumour genesis of
PACAP is proven by stimulation of c-Fos as well as c-Jun
transcription, and PACAP strongly induces proliferation of the rat
pancreatic carcinoma cell line AR4-2J via interaction with the
G-protein coupled type 1 PACAP/VIP (PV1) receptor (36). PACAP and PAC1 receptor display
specific alterations in several different tumour types, such as
thyroid papillary carcinoma and testicular cancer (37,38). It
is not known how expression of the peptide and its specific
receptor changes in pancreatic cancer. Therefore, the aim of the
present study was to investigate whether there is a change in the
expression of PACAP and its PAC1 receptor in pancreas
adenocarcinoma.
A five-year-long period (September 2012-February
2017) was investigated. Preoperative and perioperative data of
patients operated in our Department of Surgery because of
pancreatic ductal carcinoma were collected. Operation type as well
as histological findings, grading, and margin resection were
investigated from the pathological tissue samples after diagnosis
and treatments had been made (Ethical permission number:
PTE/83069/2018).
After data collection new histological sections were
made and prepared for further specific histological examination of
PACAP and PAC1 receptor expression. Two-µm-thick paraffin sections
fixed in 4% buffered formalin were processed for
immunohistochemical staining. Sections were stained using standard
immunohistochemistry with human anti-PACAP antibody (dilution of
1:200; Peninsula, CA, USA) and with human PAC1 receptor antibody
raised in rabbit (dilution of 1:200; Sigma-Aldrich, Budapest,
Hungary). Immunohistochemical staining was performed with EnVision
FLEX Visualization Systems for Dako Omins (Dako, Denmark),
similarly to our earlier descriptions (37). Liquid fast-red substrate kit (Abcam,
UK) was used as a chromogen for the immunohistochemical staining.
Pathological analysis was performed by an expert pathologist, using
a semi-quantitative approach to evaluate the immunohistochemical
staining intensity between no staining, weak, medium and strong
staining. By omitting the primary antiserum, we performed a method
control, which resulted in no staining. Well-identified structures,
like insular cells, nerve elements of the myenteric plexus and
intramural ganglia, served as positive control, as both PACAP and
PAC1 receptor are known to be expressed in the insula and PACAP has
been described in the nerve elements. Tumour cell staining
intensity was compared to that of tumour-free tissue in the same
pancreas tissue in a semi-quantitative way.
Data of 19 patients (7 male, 12 female) were chosen
to be investigated (mean age were 69.6 years; 54 to 74 years).
Seven patients had Grade 2, 13 patients Grade 3 adenocarcinoma in
the pancreas head, with icterus and significant weight loss. Five
patients were operated by conventional Whipple operation, 14
patients underwent pylorus preserving pancreatoduodenectomy (PPPD).
In every case operation was followed by a three-day-long Intensive
Care Unit (ICU) observation. After ICU observation and further care
in normal surgery unit all patients were emitted. The histological
result of the resected pancreas tissue showed Grade 2
adenocarcinoma in 11 patients, Grade 3 adenocarcinoma in 7 cases,
and mucinous adenocarcinoma in 1 patient. Tumour staging in all
cases was pT3. Lymph node staging was N0 in 5 cases, the other
specimens showed N1 stage. Resection margin was not affected (R0
resection) in 9 cases, samples from 7 patients showed narrow
resection margin, 1 sample showed perineural invasion on ductus
choledochus, another sample showed tumour cell infiltration on the
wall of veins. In case of one patient, the tumour involved the
common hepatic artery and portal vein (R2 resection).
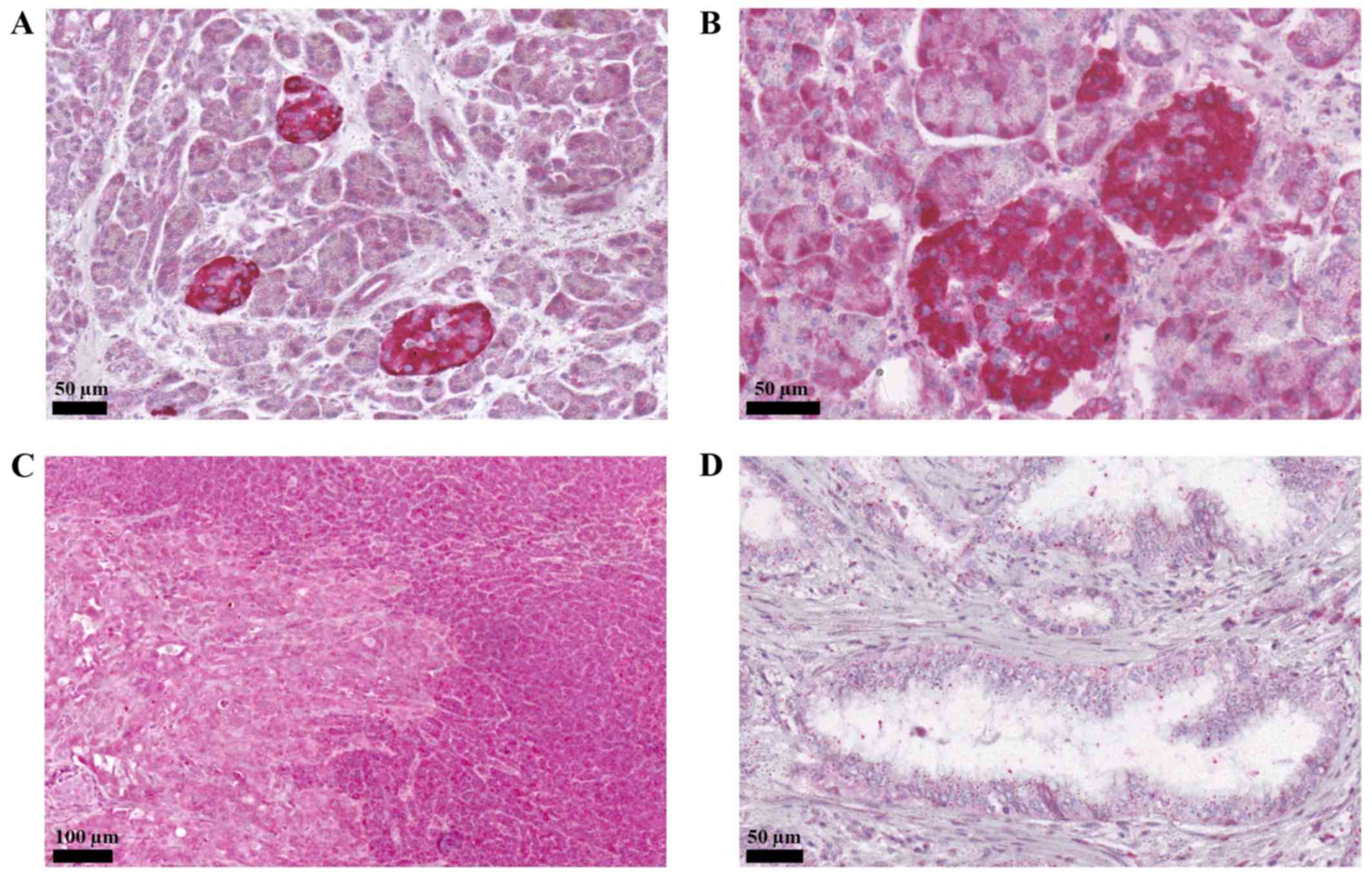
The immunohistochemical staining showed that PAC1
was expressed in both the exocrine and endocrine parts of the
pancreas, in accordance with earlier descriptions. We also
confirmed the particularly strong staining of the pancreatic islets
(Fig. 1A and B). In the
adenocarcinoma, receptor staining was markedly weaker. In tissue
samples, the border between tumourous and normal pancreas was also
shown by the different staining intensity for the PAC1 receptor
(Fig. 1C and D). Nerve elements did
not show receptor positivity.
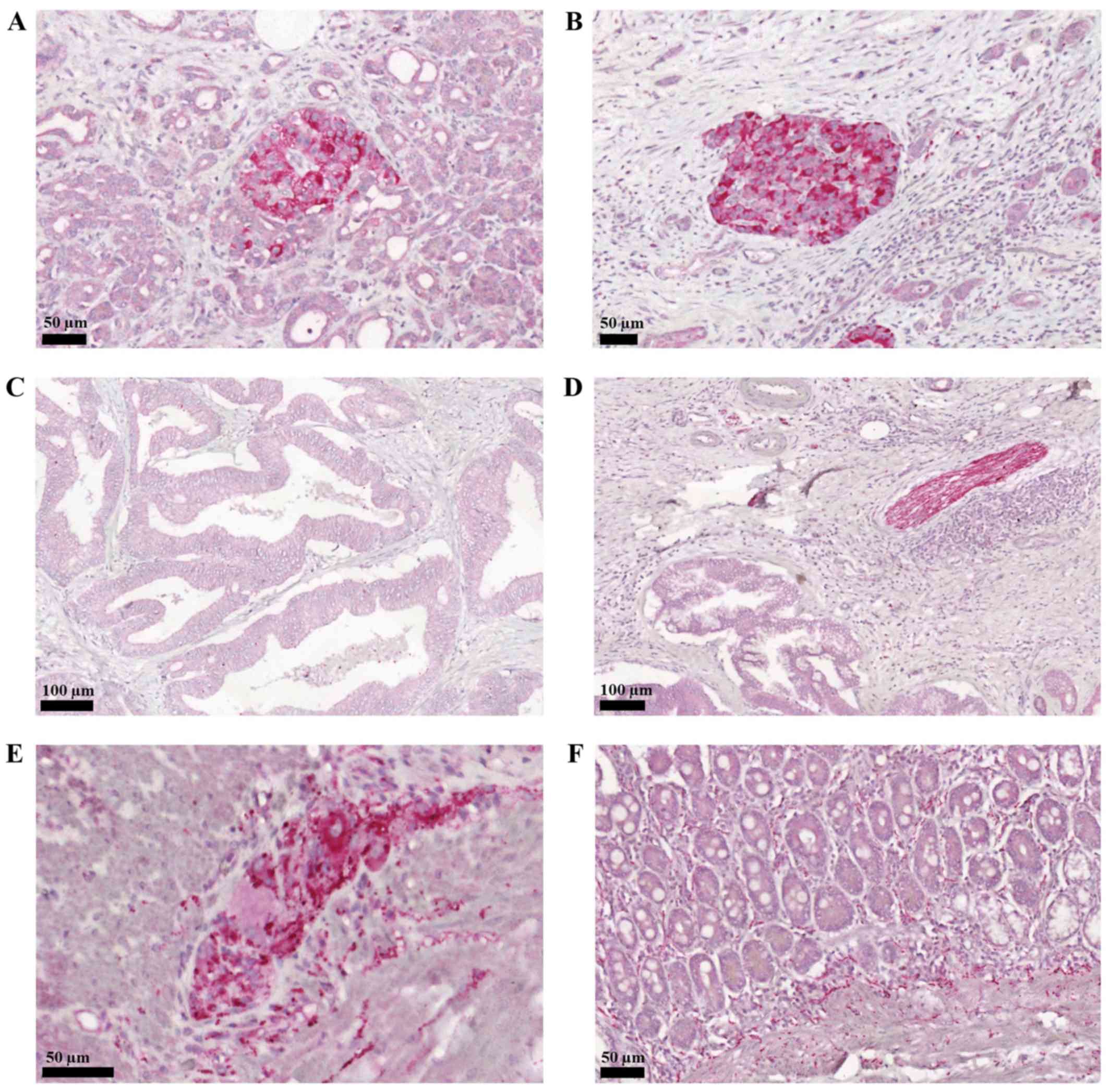
PACAP staining, on the other hand, was weaker in the
exocrine part, and again very strong in the endocrine islets
(Fig. 2A and B). Similarly to the
PAC1 receptor staining, PACAP expression was also weaker in the
adenocarcinoma parts of the tissue samples (Fig. 2C). Neither PACAP nor PAC1 receptor
expression showed correlation with the tumour outcome. In contrast
to the absence of PAC1 receptor, PACAP was also expressed in the
intrapancreatic nerves (Fig. 2D) and
ganglionic cells (Fig. 2E and H).
Following Whipple operation, resected parts of the duodenum were
also examined. We could confirm earlier descriptions regarding the
presence of PACAP and its specific receptor in the duodenum.
Myenteric and submucosal plexi were strongly stained for PACAP,
including inter- and intramuscular as well as lamina propria nerve
fibers and ganglionic cells in the myenteric plexus (Fig. 2F).
In the present study we analysed normal and
tumourous pancreas tissues within the same samples for PACAP and
PAC1 receptor immunostaining. We observed a diminished expression
for both the peptide and its specific receptor in the
adenocarcinoma compared to the normal tissue, independent from
tumour grade.
Several growth factors play an important role in
pancreatic organogenesis and are also involved later in
tumourgenesis. Among others, fibroblast growth factor (FGF) has
been shown to be involved in the regulation of tumourous cell
growth and differentiation in the pancreas (39). FGF receptor IIIb and IIIc play
critical roles in the epithelio-mesenchymal transition in spite of
no expression in the ductal cells but showing very high expression
levels in the islets (40,41). It has been demonstrated that
increased nerve growth factor (NGF) expression correlates with
poorer prognosis, increased inflammation and pain (42). The involvement of transforming growth
factor beta (TGF beta) is unquestionable in tumour growth,
including that of the pancreas (43). Overexpression of epidermal growth
factor (EGF) occurs in the majority of ductal adenocarcinomas of
the pancreas and is associated with poorer prognosis (44–46). The
increased insulin-like growth factor expression has been found to
be correlated with increased risk of pancreatic cancer (47). There is a continuous, urgent need for
novel diagnostic markers for pancreatic cancer (48). The currently available markers have
low sensitivity and specificity. Personalized treatment approaches
call for more prognostic and treatment-predictive biomarkers
(48–50).
PACAP, as a growth factor, plays an important role
in the development of the nervous system and several peripheral
organs (51–53). It is not surprising, therefore, that
certain tumour types also express alterations in PACAP and/or
receptor expression. Certain tumours show overexpression of the
PACAP-ergic system, while others lack PACAP signalling. In
vitro studies have demonstrated that PACAP is able to stimulate
or inhibit tumour growth, depending on various factors, such as
tumour type, differentiation stage, origin or environmental
circumstances (54). For example,
PACAP inhibits cell survival in retinoblastoma cells (34), reduces invasiveness in glioblastoma
cells (55) and inhibits tumour
growth in cervical carcinoma (56).
On the other hand, it stimulates cell proliferation in an
osteosarcoma cell line (57) and
increases the number of viable cells in a colon tumour cell line
(58). Even within the same cell
line, different effects can be observed depending on exposure time,
concentration and other circumstances. This dual effect has been
described in a prostate cancer cell line, where short exposure to
PACAP induces cell proliferation, while long-term exposure induces
proliferation arrest (59). In a
human retinoblastoma cell line, nanomolar concentrations of PACAP
do not affect cell viability, while higher concentrations decrease
cell survival (34).
PACAP/VIP receptors are known to play a leading role
in cancer genesis and the VIP/PACAP receptors are expressed in the
most frequently occurring human tumours (breast, prostate, ductal
carcinoma of the pancreas, lung, colon, stomach, liver, and urinary
bladder, lymphomas and meningioma). In these cases the receptors
are predominantly VPAC1 type. On the other hand leiomyomas
predominantly express VPAC2 receptors, whereas paraganglioma,
pheochromocytoma, and endometrial carcinomas preferentially express
PAC1 receptors (60). Recent studies
have shown that VIP/PACAP-receptor expression can be found in only
65% of pancreatic ductal carcinomas (30). Both VPAC1 and 2 receptors have been
identified in pancreatic tumour samples (30). Overexpression of these receptors
(61) explains the attempts for the
clinical use of radiolabelled VIP-analogues in various cancer
types, including pancreas adenocarcinoma (30,62,63).
However, contradictory data have also been published, as according
to the observations of Hessenius and coworkers (62), no imaging was seen with
radiolabeled-VIP-analogues in pancreatic cancer patients, and in
vitro binding studies in these tumours did not confirm
overexpression of VPAC1. We found PAC1 receptor expression in the
exocrine pancreas in nearly all cases, but very weak expression in
the tumourous parts. Changes in PACAP expression have been shown in
a few tumours by radioimmunoassay and immunohistochemistry
(64). In earlier studies, we
described lower PACAP tissue levels in lung, kidney and colon
cancer, but higher levels in prostate cancer (64,65). A
changed staining pattern has been described in different human
testicular cancers (38) and in
human thyroid papillary carcinoma (37). In the present study we observed that
PACAP expression was weak in normal tissues in the exocrine
pancreas, and nearly absent in the adenocarcinoma parts of the
tissue samples. The limitation of our study is that we cannot draw
final conclusion at the moment whether the reduction of PACAP and
PAC1 receptor expression is a consequence of the adenocarcinoma
development or the reduced PACAP signaling plays a role in
pancreatic carcinogenesis. This should be further explored in
future studies.
In summary, we found that both PACAP and PAC1
receptor expression is markedly decreased in human pancreatic
ductal adenocarcinoma tissue samples, while staining remained
strong in the endocrine islets. This suggests that decrease or lack
of the PAC1 receptor/PACAP signalling may contribute to tumour
growth and/or differentiation, details of which must be further
explored.
Not applicable.
The present study was supported by the following
grants (grant nos. GINOP-2.3.2-15-2016-00050 ‘PEPSYS’,
MTA-TKI14016; NKFIH K119759, Bolyai Scholarship,
EFOP-3.6.3-VEKOP-16-2017-00009, EFOP-3.6.1.-16-2016-00004
Comprehensive Development for Implementing Smart Specialization
Strategies at the University of Pécs; New Excellence Program,
UNKP-16-4-IV, TAMOP 4.2.4.A/2-11-1-2012-0001,
EFOP-3.6.2-VEKOP-16-15 2017-00008, ‘The role of neuro-inflammation
in neurodegeneration: from molecules to clinics’, and Higher
Education Institutional Excellence Programme of the Ministry of
Human Capacities in Hungary, within the framework of the
20765-3/2018/FEKUTSTRAT FIKPII; NAP2017-1.2.1-NKP-2017-00002).
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
The patients' data collection was performed by SF,
ZV, VV, OK and DK. The histological sections were produced by BK,
which were subsequently stained and examined by DR, OK, DT and AB.
Figures were produced by DT. The manuscript was written by SF, DR,
OK and DK.
Data collection was permitted by Local Ethic
Committee of University Pecs (use of patient data system of the
Clinical Centre of University Pecs) (Permission number
PTE/83069/2018).
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Pancreatic Cancer. Cancer Research UK, .
https://www.cancerresearchuk.org/about-cancer/pancreatic-cancerMarch
21–2019
|
|
2
|
Klaiber U, Leonhardt CS, Strobel O, Tjaden
C, Hackert T and Neoptolemos JP: Neoadjuvant and adjuvant
chemotherapy in pancreatic cancer. Langenbecks Arch Surg.
403:917–932. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ling S, Feng T, Jia K, Tian Y and Li Y:
Inflammation to cancer: The molecular biology in the pancreas
(Review). Oncol Lett. 7:1747–1754. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frampas E, David A, Regenet N, Touchefeu
Y, Meyer J and Merla O: Pancreatic carcinoma: Key-points from
diagnosis to treatment. Diagn Interv Imaging. 97:1207–1223. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirabayashi T, Nakamachi T and Shioda S:
Discovery of PACAP and its receptors in the brain. J Headache Pain.
19:282018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyata A, Arimura A, Dahl RR, Minamino N,
Uehara A, Jiang L, Culler MD and Coy DH: Isolation of a novel 38
residue-hypothalamic polypeptide which stimulates adenylate cyclase
in pituitary cells. Biochem Biophys Res Commun. 164:567–574. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyata A, Jiang L, Dahl RD, Kitada C, Kubo
K, Fujino M, Minamino N and Arimura A: Isolation of a neuropeptide
corresponding to the N-terminal 27 residues of the pituitary
adenylate cyclase activating polypeptide with 38 residues
(PACAP38). Biochem Biophys Res Commun. 170:643–648. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ojala J, Tooke K, Hsiang H, Girard BM, May
V and Vizzard MA: PACAP/PAC1 expression and function in micturition
pathways. J Mol Neurosci. 68:357–367. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reglodi D, Illes A, Opper B, Schafer E,
Tamas A and Horvath G: Presence and effects of pituitary adenylate
cyclase activating polypeptide under physiological and pathological
conditions in the stomach. Front Endocrinol (Lausanne). 9:902018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lajko A, Meggyes M, Fulop BD, Gede N,
Reglodi D and Szereday L: Comparative analysis of decidual and
peripheral immune cells and immune-checkpoint molecules during
pregnancy in wild-type and PACAP-deficient mice. Am J Reprod
Immunol. 80:e130352018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parsons RL and May V: PACAP-induced PAC1
receptor internalization and recruitment of endosomal signaling
regulate cardiac neuron excitability. J Mol Neurosci. 68:340–347.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sarszegi Z, Szabo D, Gaszner B, Konyi A,
Reglodi D, Nemeth J, Lelesz B, Polgar B, Jungling A and Tamas A:
Examination of pituitary adenylate cyclase-activating polypeptide
(PACAP) as a potential biomarker in heart failure patients. J Mol
Neurosci. 68:368–376. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reglodi D and Tamas A: Pituitary Adenylate
Cyclase Activating Polypeptide-PACAPCurr Topics Neurotox Springer
Int. Switzerland: pp. 1–840. 2016
|
|
14
|
Vaudry D, Falluel-Morel A, Bourgault S,
Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H,
Galas L and Vaudry H: Pituitary adenylate cyclase-activating
polypeptide and its receptors: 20 years after the discovery.
Pharmacol Rev. 61:283–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elsås T, Uddman R and Sundler F: Pituitary
adenylate cyclase-activating peptide-immunoreactive nerve fibers in
the cat eye. Graefes Arch Clin Exp Ophthalmol. 234:573–580. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gaal V, Mark L, Kiss P, Kustos I, Tamas A,
Kocsis B, Lubics A, Nemeth V, Nemeth A, Lujber L, et al:
Investigation of the effects of PACAP on the composition of tear
and endolymph proteins. J Mol Neurosci. 36:321–329. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakamachi T, Ohtaki H, Seki T, Yofu S,
Kagami N, Hashimoto H, Shintani N, Baba A, Mark L, Lanekoff I, et
al: PACAP suppresses dry eye signs by stimulating tear secretion.
Nat Commun. 7:120342016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pedersen AM, Dissing S, Fahrenkrug J,
Hannibal J, Reibel J and Nauntofte B: Innervation pattern and Ca2+
signalling in labial salivary glands of healthy individuals and
patients with primary Sjögren's syndrome (pSS). J Oral Pathol Med.
29:97–109. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Skakkebaek M, Hannibal J and Fahrenkrug J:
Pituitary adenylate cyclase activating polypeptide (PACAP) in the
rat mammary gland. Cell Tissue Res. 298:153–159. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tobin G, Asztély A, Edwards AV, Ekström J,
Håkanson R and Sundler F: Presence and effects of pituitary
adenylate cyclase activating peptide in the submandibular gland of
the ferret. Neuroscience. 66:227–235. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matoba Y, Nonaka N, Takagi Y, Imamura E,
Narukawa M, Nakamachi T, Shioda S, Banks WA and Nakamura M:
Pituitary adenylate cyclase-activating polypeptide enhances saliva
secretion via direct binding to PACAP receptors of major salivary
glands in mice. Anat Rec (Hoboken). 299:1293–1299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Calvert PA, Heck PM and Edwards AV:
Autonomic control of submandibular protein secretion in the
anaesthetized calf. Exp Physiol. 83:545–556. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kamaishi H, Endoh T and Suzuki T: Multiple
signal pathways coupling VIP and PACAP receptors to calcium
channels in hamster submandibular ganglion neurons. Auton Neurosci.
111:15–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mirfendereski S, Tobin G, Håkanson R and
Ekström J: Pituitary adenylate cyclase activating peptide (PACAP)
in salivary glands of the rat: Origin, and secretory and vascular
effects. Acta Physiol Scand. 160:15–22. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmidt WE, Seebeck J, Höcker M,
Schwarzhoff R, Schäfer H, Fornefeld H, Morys-Wortmann C, Fölsch UR
and Creutzfeldt W: PACAP and VIP stimulate enzyme secretion in rat
pancreatic acini via interaction with VIP/PACAP-2 receptors:
Additive augmentation of CCK/carbachol-induced enzyme release.
Pancreas. 8:476–487. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sakurai Y, Shintani N, Hayata A, Hashimoto
H and Baba A: Trophic effects of PACAP on pancreatic islets: A
mini-review. J Mol Neurosci. 43:3–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hamagami K, Sakurai Y, Shintani N, Higuchi
N, Ikeda K, Hashimoto H, Suzuki A, Kiyama H and Baba A:
Over-expression of pancreatic pituitary adenylate
cyclase-activating polypeptide (PACAP) aggravates cerulein-induced
acute pancreatitis in mice. J Pharmacol Sci. 110:451–458. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jung S, Yi L, Jeong D, Kim J, An S, Oh TJ,
Kim CH, Kim CJ, Yang Y, Kim KI, Lim JS and Lee MS: The role of
ADCYAP1, adenylate cyclase activating polypeptide, as a methylation
biomarker for the early detection of cervical cancer. Oncol Rep.
25:245–252. 2011.PubMed/NCBI
|
|
29
|
Moody TW, Chan D, Fahrenkrug J and Jensen
RT: Neuropeptides as autocrine growth factors in cancer cells. Curr
Pharm Des. 9:495–509. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moody TW, Nuche-Berenguer B and Jensen RT:
Vasoactive intestinal peptide/pituitary adenylate cyclase
activating polypeptide, and their receptors and cancer. Curr Opin
Endocrinol Diabetes Obes. 23:38–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moody TW and Jensen RT: PACAP and
cancerPituitary Adenylate Cyclase Activating Polypeptide-PACAP.
Reglodi D and Tamas A: Springer Int.; Switzerland: pp. 795–814.
2016, View Article : Google Scholar
|
|
32
|
Schulz S, Röcken C, Mawrin C, Weise W,
Höllt V and Schulz S: Immunocytochemical identification of VPAC1,
VPAC2, and PAC1 receptors in normal and neoplastic human tissues
with subtype-specific antibodies. Clin Cancer Res. 10:8235–8242.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schulz S, Mann A, Novakhov B, Piggins HD
and Lupp A: VPAC2 receptor expression in human normal and
neoplastic tissues: Evaluation of the novel MAB SP235. Endocr
Connect. 4:18–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wojcieszak J and Zawilska JB: PACAP38 and
PACAP6-38 exert cytotoxic activity against human retinoblastoma Y79
cells. J Mol Neurosci. 54:463–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schäfer H, Lettau P, Trauzold A, Banasch M
and Schmidt WE: Human PACAP response gene 1 (p22/PRG1):
Proliferation-associated expression in pancreatic carcinoma cells.
Pancreas. 18:378–384. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schäfer H, Zheng J, Gundlach F, Günther R
and Schmidt WE: PACAP stimulates transcription of c-Fos and c-Jun
and activates the AP-1 transcription factor in rat pancreatic
carcinoma cells. Biochem Biophys Res Commun. 221:111–116. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bardosi S, Bardosi A, Nagy Z and Reglodi
D: Expression of PACAP and PAC1 receptor in normal human thyroid
gland and in thyroid papillary carcinoma. J Mol Neurosci.
60:171–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakamura K, Nakamachi T, Endo K, Ito K,
Machida T, Oka T, Hori M, Ishizaka K and Shioda S: Distribution of
pituitary adenilate cyclase-activating polypeptide (PACAP) in the
human testis and in testicular germ cell tumours. Andrologia.
46:465–471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ndlovu R, Deng LC, Wu J, Li XK and Zhang
JS: Fibroblast growth factor 10 in pancreas development and
pancreatic cancer. Front Genet. 9:4822018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ishiwata T: Role of fibroblast growth
factor receptor-2 splicing in normal and cancer cells. Front Biosci
(Landmark Ed). 23:626–639. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu G, Xiong D, Xiao R and Huang Z:
Prognostic role of fibroblast growth factor receptor 2 in human
solid tumours: A systematic review and meta-analysis. Tumour Biol.
39:10104283177074242017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Saloman JL, Singhi AD, Hartman DJ,
Normolle DP, Albers KM and Davis BM: Systemic depletion of nerve
growth factor inhibits disease progression in a genetically
engineered model of pancreatic ductal adenocarcinoma. Pancreas.
47:856–863. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Melzer C, Hass R, von der Ohe J, Lehnert H
and Ungefroren H: The role of TGF-β and its crosstalk with
RAC1/RAC1b signaling in breast and pancreas carcinoma. Cell Commun
Signal. 15:192107. View Article : Google Scholar
|
|
44
|
Chiramel J, Backen AC, Pihlak R, Lamarca
A, Frizziero M, Tariq NU, Hubner RA, Valle JW, Amir E and McNamara
MG: Targeting the epidermal growth factor receptor in addition to
chemotherapy in patients with advanced pancreatic cancer: A
systematic review and meta-analysis. Int J Mol Sci. 18:E9092017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weiss GA, Rossi MR, Khushalani NI, Lo K,
Gibbs JF, Bharthuar A, Cowell JK and Iyer R: Evaluation of
phosphatidylinositol-3-kinase catalytic subunit (PIK3CA) and
epidermal growth factor receptor (EGFR) gene mutations in
pancreaticobiliary adenocarcinoma. J Gastrointest Onco. 4:20–29.
2013.
|
|
46
|
Luo G, Long J, Qiu L, Liu C, Xu J and Yu
X: Role of epidermal growth factor receptor expression on patient
survival in pancreatic cancer: A meta-analysis. Pancreatology.
11:595–600. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gong Y, Zhang B, Liao Y, Tang Y, Mai C,
Chen T and Tang H: Serum insulin-like growth factor axis and the
risk of pancreatic cancer: Systematic review and meta-analysis.
Nutrients. 9:E3942017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Loosen SH, Neumann UP, Trautwein C,
Roderburg C and Luedde T: Current and future biomarkers for
pancreatic adenocarcinoma. Tumour Biol. 39:10104283176922312017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yamaoka T, Ohba M and Ohmori T:
Molecular-targeted therapies for epidermal growth factor receptor
and its resistance mechanisms. Int J Mol Sci. 18:E24202017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Le N, Sund M and Vinci A; GEMS
collaborating group of Pancreas 2000, : Prognostic and predictive
markers in pancreatic adenocarcinoma. Dig Liver Dis. 48:223–230.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fulop BD, Sandor B, Szentleleky E,
Karanyicz E, Reglodi D, Gaszner B, Zakany R, Hashimoto H, Juhasz T
and Tamas A: Altered notch signaling in developing molar teeth of
pituitary adenylate cyclase-activating polypeptide
(PACAP)-deficient mice. J Mol Neurosci. 68:377–388. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu Z, Ohtaki H, Watanabe J, Miyamoto K,
Murai N, Sasaki S, Matsumoto M, Hashimoto H, Hiraizumi Y, Numazawa
S and Shioda S: Pituitary adenylate cyclase-activating polypeptide
(PACAP) contributes to the proliferation of hematopoietic
progenitor cells in murine bone marrow via PACAP-specific receptor.
Sci Rep. 6:223732016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sandor B, Fintor K, Reglodi D, Fulop DB,
Helyes Z, Szanto I, Nagy P, Hashimoto H and Tamas A: Structural and
morphometric comparison of lower incisors in PACAP-deficient and
wild-type mice. J Mol Neurosci. 59:300–308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zibara K, Zeidan A, Mallah K, Kassem N,
Awad A, Mazurier F, Badran B and El-Zein N: Signaling pathways
activated by PACAP in MCF-7 breast cancer cells. Cell Signal.
50:37–47. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Maugeri G, D'Amico AG, Reitano R, Magro G,
Cavallaro S, Salomone S and D'Agata V: PACAP and VIP inhibit the
invasiveness of glioblastoma cells exposed to hypoxia through the
regulation of HIFs and EGFR expression. Front Pharmacol. 7:1392016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lee JH, Lee JY, Rho SB, Choi JS, Lee DG,
An S, Oh T, Choi DC and Lee SH: PACAP inhibits tumour growth and
interferes with clusterin in cervical carcinomas. FEBS Lett.
588:4730–4739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Juhász T, Matta C, Katona É, Somogyi C,
Takács R, Hajdú T, Helgadottir SL, Fodor J, Csernoch L, Tóth G, et
al: Pituitary adenylate cyclase-activating polypeptide (PACAP)
signalling enhances osteogenesis in UMR-106 cell line. J Mol
Neurosci. 54:555–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Le SV, Yamaguchi DJ, McArdle CA, Tachiki
K, Pisegna JR and Germano P: PAC1 and PACAP expression, signaling,
and effect on the growth of HCT8, human colonic tumour cells. Regul
Pept. 109:115–125. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Farini D, Puglianiello A, Mammi C,
Siracusa G and Moretti C: Dual effect of pituitary adenylate
cyclase activating polypeptide on prostate tumour LNCaP cells:
Short- and long-term exposure affect proliferation and
neuroendocrine differentiation. Endocrinology. 144:1631–1643. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Reubi JC, Läderach U, Waser B, Gebbers JO,
Robberecht P and Laissue JA: Vasoactive intestinal
peptide/pituitary adenylate cyclase-activating peptide receptor
subtypes in human tumours and their tissues of origin. Cancer Res.
60:3105–3112. 2000.PubMed/NCBI
|
|
61
|
Hessenius C, Bäder M, Meinhold H, Böhmig
M, Faiss S, Reubi JC and Wiedenmann B: Vasoactive intestinal
peptide receptor scintigraphy in patients with pancreatic
adenocarcinomas or neuroendocrine tumours. Eur J Nucl Med.
27:1684–1693. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Raderer M, Kurtaran A, Yang Q, Meghdadi S,
Vorbeck F, Hejna M, Angelberger P, Kornek G, Pidlich J, Scheithauer
W and Virgolini I: Iodine-123-vasoactive intestinal peptide
receptor scanning in patients with pancreatic cancer. J Nucl Med.
39:1570–1575. 1998.PubMed/NCBI
|
|
63
|
Tang C, Biemond I, Offerhaus GJ, Verspaget
W and Lamers CB: Expression of receptors for gut peptides in human
pancreatic adenocarcinoma and tumour-free pancreas. Br J Cancer.
75:1467–1473. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tamas A, Javorhazy A, Reglodi D, Sarlos
DP, Banyai D, Semjen D, Nemeth J, Lelesz B, Fulop DB and Szanto Z:
Examination of PACAP-like immunoreactivity in urogenital tumour
samples. J Mol Neurosci. 59:177–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Szanto Z, Sarszegi Z, Reglodi D, Nemeth J,
Szabadfi K, Kiss P, Varga A, Banki E, Csanaky K, Gaszner B, et al:
PACAP immunoreactivity in human malignant tumour samples and
cardiac diseases. J Mol Neurosci. 48:667–673. 2012. View Article : Google Scholar : PubMed/NCBI
|