Apoptosis is a complex process that is regulated by
the interaction between pro- and anti-apoptotic proteins. It has
been revealed that the upregulation of anti-apoptotic proteins
prevents apoptosis and allows the proliferation of cancer cells
(6,8).
Apoptosis repressor with caspase recruitment domain
(ARC), an endogenous anti-apoptotic protein, is abundantly
expressed in terminally differentiated cells such as neurons, and
cardiomyocytes (10). However,
previous studies have reported that ARC is upregulated in several
different types of human cancer and promotes tumor cell survival,
and contributes to tumor invasion, metastasis and chemoresistance
(9,11). The present review summarizes the
function of ARC with a particular emphasis on the role of ARC in
cancer.
ARC serves a protective role against cellular stress
and is involved in homeostasis and development (24,25). In
a rabbit model of cardiac regional ischemia following reperfusion,
rabbits overexpressing ARC exhibited decreased apoptosis in the
left ventricle (24). In a murine
pulmonary hypertension model, the vascular remodeling capacity of
ARC-deficient mice was diminished and accompanied by enhanced
apoptosis and decreased proliferation in response to chronic
hypoxia-induced pulmonary hypertension (25). In chick embryo myocardial
cardiomyocytes, ARC overexpression protected cells from oxidative
injury and promoted their survival (26). H9c2 cells overexpressing ARC were
significantly more resistant to hypoxia-induced apoptosis (27). In addition, enforced ARC
overexpression significantly prevented doxorubicin (DOX)-induced
cardiotoxicity (28). Overexpression
of ARC remarkably suppressed whole cell Kv currents
(IK(V)) in transfected H9c2 cells following treatment
with staurosporine, an apoptosis inducer that increased
IK(V) in wild-type cells and induced apoptosis (29). These data suggest that ARC protects
myocardial cells from stress-induced apoptosis as well as chemical
toxicity.
In the liver, ARC overexpression completely blocked
the hepatocyte apoptosis or necrosis regulated via the Fas cell
surface death receptor (Fas)/tumor necrosis factor (TNF) signaling
pathway (30,31). Furthermore, ARC overexpression
protected muscle fibers from apoptosis induced by mechanical stress
or oxidative damage (32).
Inhibition of ARC promoted cell death by increasing the production
of pro-apoptotic factors, decreasing the stability of mitochondrial
membranes and increasing the level of reactive oxygen species (ROS)
following neomycin injury (23).
ARC prevented β-cell apoptosis induced by several
cell death inducers, including endoplasmic reticulum (ER) stress
and palmitate, while depletion of ARC in isolated islets
significantly increased apoptosis in palmitate-treated cells
(22). ARC expression was previously
documented in testicular tissue, regulating apoptosis of primary
spermatocytes (20). It was reported
that ARC antagonized the adverse effect of zoldronate in
osteoblasts, promoted osteogenic growth and differentiation of
osteoblasts, and decreased apoptosis (33).
In addition to its antiapoptotic role, ARC
participates in the regulation of cell differentiation and
proliferation. Overexpression of ARC suppressed myogenic
differentiation via caspase inhibition (34), whereas the absence of ARC altered
fiber-type distribution, resulting in muscle atrophy and decreased
force-generating capacity (35).
Russell et al (36)
demonstrated that mutations, which alter the post-translational
modification of ARC, may lead to myoclonus.
High levels of ARC expression have previously been
reported in several different types of cancer, including primary
human breast cancer, cervical carcinoma, gastric cancer and colon
adenocarcinoma (14,37–39).
Furthermore, the expression levels were not only associated with
different types of cancer, but also with sex, age, and tumor grade
and size (37). Additional reports
revealed that ARC is highly expressed in non-small cell lung cancer
cells, PC-3 prostate cancer cells and renal cell carcinoma cells
(40–42).
ARC expression in cancer cells has been associated
with increased chemoresistance. High expression levels of ARC in
breast cancer cells promoted tumor growth, invasion and metastasis,
and augmented chemoresistance (9,43). A
high expression level of ARC in colorectal cancer liver metastasis
enhanced the anti-apoptotic ability of colorectal cancer (37). ARC is highly expressed in newly
diagnosed acute myeloid leukemia (AML) samples and has been
demonstrated to decrease apoptosis induced by cytarabine and other
agents. ARC may therefore contribute to drug resistance and
increase survival time of leukemia cells (44–46). The
high expression of cytoplasmic and nuclear ARC in nasopharyngeal
carcinoma tissues are correlated with advanced local invasion.
Overexpression of ARC in NPC 6-10B cells plays an important role in
X-radiation and cisplatin resistance, and prolongs NPC cells
survival by blocking the activation of casapse-8 and casapse-2
(47).
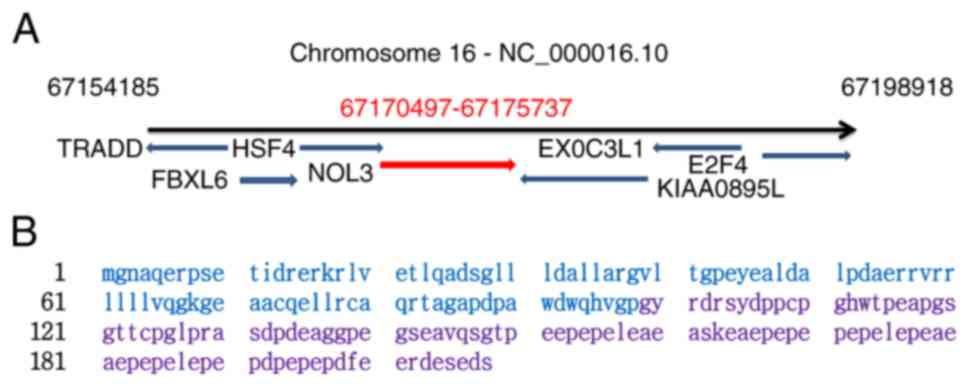
The expression level of ARC in tissues and cells is
regulated by a number of factors, including post-transcriptional
splicing, modification and degradation (43,52,53).
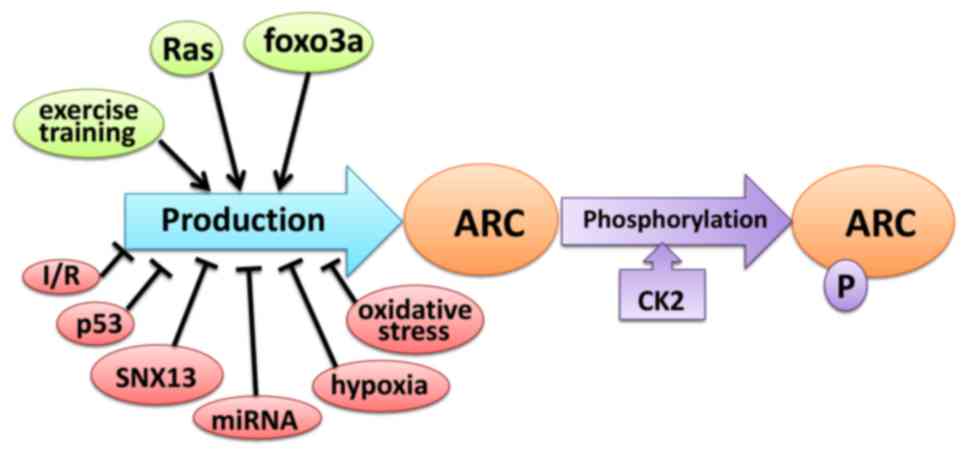
Rat sarcoma (Ras) controls ARC levels by
transcriptional regulation and maintaining protein stability. Ras
activates the Nol3 promoter in a MEK/ERK-dependent manner
and significantly increases the production of ARC mRNA.
Furthermore, Ras prevents ARC degradation via the
ubiquitin-proteasome pathway (43).
The transcription factor fork head box O3 (FOXO3A) expressed in
cardiomyocytes and skeletal muscle cells activates ARC expression
by directly binding and trans-activating its promoter (52). ARC protein levels in muscle are
increased as a result of endurance training; exercise resulted in a
37.5% increase in ARC protein levels (53) (Fig.
2).
A previous study revealed that the ARC protein had a
molecular weight of ~34 kDa in human skeletal muscle and various
adherent human cancer cell lines, and 38 kDa in human lymphoma cell
lines (63). McMillan et al
(64) revealed that ARC extracted
from normotensive rats had a molecular weight of ~30 kDa, compared
with 32 kDa in a spontaneously hypertensive rat. This shift in
molecular weight suggested that the ARC protein may undergo
post-translational modifications in different and cell types. Li
et al (62) reported that ARC
mRNA levels did not differ between cardiac tissues extracted from
rats with heart failure and controls or between SNX13-deficient
neonatal rat ventricular myocytes and normal cardiomyocytes,
suggesting that the regulation of ARC expression begins at the
posttranscriptional level. Dowds et al (65) revealed that during the serum
withdrawal stage in an in vitro culture, post-translational
regulation increased ARC stability and lead to its accumulation in
the nucleus, which promotes cell survival (Fig. 2). Phosphorylation is a functional
post-transcriptional modification and Li et al (66) revealed that casein kinase 2 (CK2), is
a serine/threonine protein kinase that phosphorylates ARC at
threonine 149. T149 phosphorylation directs ARC to the mitochondria
and allows ARC to exert its anti-apoptotic effects (Fig. 2).
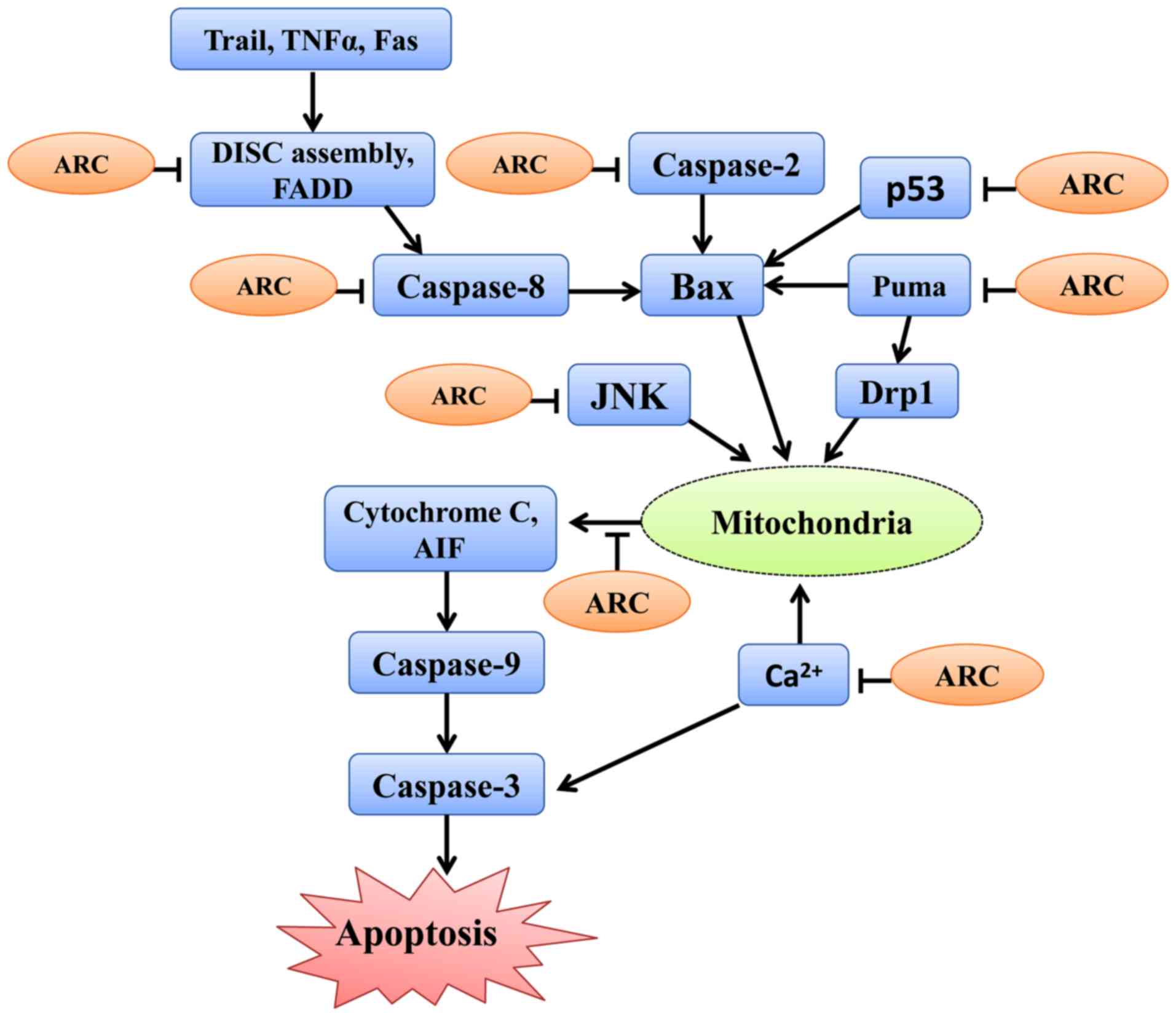
Apoptosis is controlled by an extrinsic pathway that
originates from cell-surface receptors, and an intrinsic pathway
that involves the mitochondria and ER (1). The extrinsic pathway is mediated by
death receptors (67) and
death-associated domains (68). The
intrinsic pathway is activated by varied stimuli, including
metabolic, oxidative, proteotoxic stress and ROS (69). The extrinsic and intrinsic pathways
activate caspases, which cleave multiple cellular proteins to
induce cell apoptosis (70). ARC
exhibits its cytoprotective and anti-apoptotic effects by
inhibiting the extrinsic and intrinsic apoptotic pathways.
ARC inhibits the extrinsic pathway by interacting
with Fas and Fas associated via the death domain. This subsequently
prevents the homotypic interactions required for the assembly of
the death-inducing signaling complex, which is important for the
activation of caspases and further apoptotic signaling (14). A previous study revealed that ARC
selectively interacts with the apoptosis initiator caspase-2 and
caspase-8 and attenuates death receptor-induced apoptosis,
involving the Fas cell surface death receptor, TNF receptor
superfamily member 1A and TNF receptor superfamily member 25, or
adaptor-induced apoptosis, involving TNFRSF1A associated via death
domain and CASP8 and FADD like apoptosis regulator (10). Ekhterae et al (29) suggested that upregulation of ARC
decreases IK(V) density and inhibits the
staurosporine-induced IK(V) increase in cardiomyocytes,
thereby enhancing survival and attenuating apoptosis by inhibiting
Kv channel activity (29)
(Fig. 3, Table I).
ARC antagonizes the intrinsic pathway by interacting
with apoptosis-associated proteins (11). The binding of ARC and Bax, which can
inhibit Bax activation and accumulation in the mitochondria
(15), increases the Bcl2 apoptosis
regulator/Bax ratio (71,72), stabilizes the mitochondrial membrane
and prevents cytochrome c release (27,73,74).
Furthermore, ARC is also involved in the regulation of
mitochondrial dynamics. For instance, ARC interacts with PUMA via
its N terminus, and suppresses PUMA-mediated translocation of
density regulated re-initiation and release factor in mitochondria,
preventing mitochondrial fission and subsequent apoptosis (11).
miR-532-3p negatively regulates ARC expression and
sensitizes cardiomyocytes to DOX-induced mitochondrial fission and
apoptosis (75). It has been
reported that ARC participates in cardioprotection and suppresses
apoptosis caused by hypoxia and reoxygenation by preventing
cytochrome c release from the mitochondria in a caspase-independent
pattern (27,76). Furthermore, ARC overexpression
prevented oxidative stress-induced cell apoptosis by protecting
mitochondrial function independently of caspase inhibition,
suggesting that ARC may act on a mitochondrial level (56).
ARC interacts with protein kinase RNA-like ER kinase
and binds inositol-requiring protein 1α, which prevents C/EBP
homologous protein induction, diminishes the ER stress response and
blocks Ca2+ release from ER (22). Furthermore, ARC not only blocks
Ca2+ release but also binds Ca2+ through its
C-terminal P/E-rich domain. Therefore, ARC suppresses intracellular
Ca2+ increase and further prevents Ca2+
mediated apoptosis (16).
The proline-glutamic acid-rich region of ARC could
bind with and negatively regulate p53 by inhibiting its
transcriptional function, tetramerization and causing its
cytoplasmic localization, thereby preventing p53 transfer into the
nucleus and abrogate p53-induced apoptosis (17).
ARC binds with and prevents the activation of JUN
N-terminal kinase (JNK), inhibiting cell death dependent on the JNK
signaling pathway (30,77,78). ARC
blocks acetaminophen- induced hepatic damage by antagonizing the
JNK signaling pathway and preventing ROS production (77). ARC decreases amyloid-induced JNK
phosphorylation, and ARC upregulation decreases β-cell apoptosis
induced by activation of the JNK signaling pathway (78). Furthermore, ARC blocks Fas- and
TNF-regulated cell death by JNK-dependent or independent pathways
(30) (Fig. 3, Table
I).
Increased ARC expression has been documented in
several types of cancer cells, including colon cancers (6), lung cancer (8), leukemia (44), glioblastoma (80). ARC upregulation contributes to
chemotherapy and radiation resistance (11,81).
Consistent with its role as an anti-apoptotic protein, ARC
upregulation is associated with cancer grade and poor prognosis.
For example, patients with AML with high ARC expression levels
exhibited a poor chemotherapeutic response (82), while those with low ARC expression
levels had an increased survival time (45), suggesting that ARC may have
prognostic and therapeutic value in AML (46). ARC promotes multiple aspects of
breast carcinogenesis, such as tumorigenesis, invasion, metastasis
and chemoresistance. Therefore, ARC may serve as a novel
therapeutic target for the development of future breast cancer
therapies (9).
The antiapoptotic capacity of ARC may be regulated
by certain miRNAs and enzymes. miR-185 targets ARC, decreases its
anti-apoptotic function and increases apoptosis in gastric cancer
cells (39). Phosphorylation of ARC
by CK2 contributes to chemotherapy resistance by inhibiting
DOX-induced apoptosis; whereas, CK2 inhibitors increase the
sensitivity of cancer cells to DOX by inhibiting the
phosphorylation of ARC (83). These
data suggest that ARC can be used a novel drug target in cancer
treatment (84).
In summary, ARC is an important regulator of
apoptosis and is associated with several human diseases,
particularly cancer. Therefore, ARC may serve as a novel drug
target in cancer treatment. Furthermore, miRNAs and enzyme
inhibitors may target and prevent ARC from exerting its
anti-apoptotic function directly or indirectly (13).
Apoptosis is an important regulator of tissue and
developmental homeostasis. Furthermore, apoptosis is associated
with the pathogenesis of a large number of diseases, including
autoimmune diseases, viral infection, degenerative disorders and
cancer (85,86). Cancer results in significant
morbidity and mortality and is a significant public health problem
worldwide (87–89). The currently used chemotherapeutic
drugs are cytotoxic agents that have various side effects (90). Therefore, there is a requirement for
the development of targeted and more effective treatment options
with fewer side effects. ARC has been revealed to decrease cell
death in various different types of cell by binding and
inactivating components of the apoptosis pathways. Upregulation of
ARC is highly associated with tumorigenesis and chemotherapy
resistance; therefore, inhibiting the expression of ARC in cancer
cells may increase the efficacy of anti-cancer drugs (45). Future studies are required to
investigate how to effectively deliver targeted ARC inhibitors for
the treatment of cancer.
Not applicable.
The present study was supported by the National
Science Foundation of China (grant nos. 81741173 and 31430041).
All data generated or analyzed during this study are
included in this published article.
ZY, LA and PL designed the review and edited the
manuscript. ZY, QL and YA wrote the manuscript. XC, ZQL, ZL and JG
collected and analyzed data.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adhihetty PJ, Ljubicic V and Hood DA:
Effect of chronic contractile activity on SS and IMF mitochondrial
apoptotic susceptibility in skeletal muscle. Am J Physiol
Endocrinol Metab. 292:E748–E755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crews L, Patrick C, Adame A, Rockenstein E
and Masliah E: Modulation of aberrant CDK5 signaling rescues
impaired neurogenesis in models of Alzheimer's disease. Cell Death
Dis. 2:e1202011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang K, Long B, Zhou LY, Liu F, Zhou QY,
Liu CY, Fan YY and Li PF: CARL lncRNA inhibits anoxia-induced
mitochondrial fission and apoptosis in cardiomyocytes by impairing
miR-539-dependent PHB2 downregulation. Nat Commun. 5:35962014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qiao G, Li Z, Minto AW, Shia J, Yang L,
Bao L, Tschopp J, Gao JX, Wang J, Quigg RJ and Zhang J: Altered
thymic selection by overexpressing cellular FLICE inhibitory
protein in T cells causes lupus-like syndrome in a BALB/c but not
C57BL/6 strain. Cell Death Differ. 17:522–533. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rampino N, Yamamoto H, Ionov Y, Li Y,
Sawai H, Reed JC and Perucho M: Somatic frameshift mutations in the
BAX gene in colon cancers of the microsatellite mutator phenotype.
Science. 257:967–969. 1997. View Article : Google Scholar
|
|
7
|
Mitra A, Basak T, Datta K, Naskar S,
Sengupta S and Sarkar S: Role of α-crystallin B as a regulatory
switch in modulating cardiomyocyte apoptosis by mitochondria or
endoplasmic reticulum during cardiac hypertrophy and myocardial
infarction. Cell Death Dis. 4:e5822013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Favaloro B, Allocati N, Graziano V, Di
Ilio C and De Laurenzi V: Role of apoptosis in disease. Aging
(Albany NY). 4:330–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Medina-Ramirez CM, Goswami S, Smirnova T,
Bamira D, Benson B, Ferrick N, Segall J, Pollard JW and Kitsis RN:
Apoptosis inhibitor ARC promotes breast tumorigenesis, metastasis,
and chemoresistance. Cancer Res. 71:7705–7715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koseki T, Inohara N, Chen S and Núñez G:
ARC, an inhibitor of apoptosis expressed in skeletal muscle and
heart that interacts selectively with caspases. Proc Natl Acad Sci
USA. 95:5156–5160. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang JX, Li Q and Li PF: Apoptosis
repressor with caspase recruitment domain contributes to
chemotherapy resistance by abolishing mitochondrial fission
mediated by dynamin-related protein-1. Cancer Res. 69:492–500.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stoss O, Schwaiger FW, Cooper TA and Stamm
S: Alternative splicing determines the intracellular localization
of the novel nuclear protein Nop30 and its interaction with the
splicing factor SRp30c. J Biol Chem. 274:10951–10962. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jang TH, Kim SH, Jeong JH, Kim S, Kim YG
and Park HH: Crystal structure of caspase recruiting domain (CARD)
of apoptosis repressor with CARD (ARC) and its implication in
inhibition of apoptosis. Sci Rep. 5:98472015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nam YJ, Mani K, Ashton AW, Peng CF,
Krishnamurthy B, Hayakawa Y, Lee P, Korsmeyer SJ and Kitsis RN:
Inhibition of both the extrinsic and intrinsic death pathways
through nonhomotypic death-fold interactions. Mol Cell. 15:901–912.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gustafsson AB, Tsai JG, Logue SE, Crow MT
and Gottlieb RA: Apoptosis repressor with caspase recruitment
domain protects against cell death by interfering with Bax
activation. J Biol Chem. 279:21233–21238. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jo DG, Jun JI, Chang JW, Hong YM, Song S,
Cho DH, Shim SM, Lee HJ, Cho C, Kim DH and Jung YK: Calcium binding
of ARC mediates regulation of caspase 8 and cell death. Mol Cell
Biol. 24:9763–9770. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Foo RS, Nam YJ, Ostreicher MJ, Metzl MD,
Whelan RS, Peng CF, Ashton AW, Fu W, Mani K, Chin SF, et al:
Regulation of p53 tetramerization and nuclear export by ARC. Proc
Natl Acad Sci USA. 104:20826–20831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Engidawork E, Gulesserian T, Yoo BC,
Cairns N and Lubec G: Alteration of caspases and apoptosis-related
proteins in brains of patients with Alzheimer's disease. Biochem
Biophys Res Commun. 281:84–93. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quadrilatero J and Bloemberg D: Apoptosis
repressor with caspase recruitment domain is dramatically reduced
in cardiac, skeletal, and vascular smooth muscle during
hypertension. Biochem Biophys Res Commun. 391:1437–1442. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jewgenow K, Neubauer K, Blottner S, Schön
J, Wildt DE and Pukazhenthi BS: Reduced germ cell apoptosis during
spermatogenesis in the teratospermic domestic cat. J Androl.
30:460–468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sasson R, Rimon E, Dantes A, Cohen T,
Shinder V, Land-Bracha A and Amsterdam A: Gonadotrophin-induced
gene regulation in human granulosa cells obtained from IVF
patients. Modulation of steroidogenic genes, cytoskeletal genes and
genes coding for apoptotic signalling and protein kinases. Mol Hum
Reprod. 10:299–311. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McKimpson WM, Weinberger J, Czerski L,
Zheng M, Crow MT, Pessin JE, Chua SC Jr and Kitsis RN: The
apoptosis inhibitor ARC alleviates the ER stress response to
promote β-cell survival. Diabetes. 62:183–193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guan M, Fang Q, He Z, Li Y, Qian F, Qian
X, Lu L, Zhang X, Liu D, Qi J, et al: Inhibition of ARC decreases
the survival of HEI-OC-1 cells after neomycin damage in vitro.
Oncotarget. 7:66647–66659. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chatterjee S, Bish LT, Jayasankar V,
Stewart AS, Woo YJ, Crow MT, Gardner TJ and Sweeney HL: Blocking
the development of postischemic cardiomyopathy with viral gene
transfer of the apoptosis repressor with caspase recruitment
domain. J Thorac Cardiovasc Surg. 125:1461–1469. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zaiman AL, Damico R, Thoms-Chesley A,
Files DC, Kesari P, Johnston L, Swaim M, Mozammel S, Myers AC,
Halushka M, et al: A critical role for the protein apoptosis
repressor with caspase recruitment domain in hypoxia-induced
pulmonary hypertension. Circulation. 124:2533–2542. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu L, Xi Z, Guo R, Liu S, Yang S, Liu D,
Dong S and Guo D: Exogenous ARC down-regulates caspase-3 expression
and inhibits apoptosis of broiler chicken cardiomyocytes exposed to
hydrogen peroxide. Avian Pathol. 42:32–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ekhterae D, Lin Z, Lundberg MS, Crow MT,
Brosius FC III and Núñez G: ARC inhibits cytochrome c release from
mitochondria and protects against hypoxia-induced apoptosis in
heart-derived H9c2 cells. Circ Res. 85:e70–e77. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
An J, Li P, Li J, Dietz R and Donath S:
ARC is a critical cardiomyocyte survival switch in doxorubicin
cardiotoxicity. J Mol Med (Berl). 87:401–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ekhterae D, Platoshyn O, Zhang S,
Remillard CV and Yuan JX: Apoptosis repressor with caspase domain
inhibits cardiomyocyte apoptosis by reducing K+ currents. Am J
Physiol Cell Physiol. 284:C1405–C1410. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
An J, Harms C, Lättig-Tünnemann G, Sellge
G, Mandić AD, Malato Y, Heuser A, Endres M, Trautwein C and Donath
S: TAT-apoptosis repressor with caspase recruitment domain protein
transduction rescues mice from fulminant liver failure. Hepatology.
56:715–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kung G, Dai P, Deng L and Kitsis RN: A
novel role for the apoptosis inhibitor ARC in suppressing
TNFα-induced regulated necrosis. Cell Death Differ. 21:634–644.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abmayr S, Crawford RW and Chamberlain JS:
Characterization of ARC, apoptosis repressor interacting with CARD,
in normal and dystrophin-deficient skeletal muscle. Hum Mol Gene.
13:213–221. 2004. View Article : Google Scholar
|
|
33
|
Hu L, Han J, Yang X, Wang Y, Pan H and Xu
L: Apoptosis repressor with caspase recruitment domain enhances
survival and promotes osteogenic differentiation of human
osteoblast cells under Zoledronate treatment. Mol Med Rep.
14:3535–3542. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hunter AL, Zhang J, Chen SC, Si X, Wong B,
Ekhterae D, Luo H and Granville DJ: Apoptosis repressor with
caspase recruitment domain (ARC) inhibits myogenic differentiation.
FEBS Lett. 581:879–884. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mitchell AS, Smith IC, Gamu D, Donath S,
Tupling AR and Quadrilatero J: Functional, morphological, and
apoptotic alterations in skeletal muscle of ARC deficient mice.
Apoptosis. 20:310–326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Russell JF, Steckley JL, Coppola G, Hahn
AF, Howard MA, Kornberg Z, Huang A, Mirsattari SM, Merriman B,
Klein E, et al: Familial cortical myoclonus with a mutation in
NOL3. Ann Neurol. 72:175–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tóth C, Meinrath J, Herpel E, Derix J,
Fries J, Buettner R, Schirmacher P and Heikaus S: Expression of the
apoptosis repressor with caspase recruitment domain (ARC) in liver
metastasis of colorectal cancer and its correlation with DNA
mismatch repair proteins and p53. J Cancer Res Clin Oncol.
142:927–935. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mercier I, Vuolo M, Jasmin JF, Medina CM,
Williams M, Mariadason JM, Qian H, Xue X, Pestell RG, Lisanti MP
and Kitsis RN: ARC (apoptosis repressor with caspase recruitment
domain) is a novel marker of human colon cancer. Cell Cycle.
7:1640–1647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li Q, Wang JX, He YQ, Feng C, Zhang XJ,
Sheng JQ and Li PF: MicroRNA-185 regulates chemotherapeutic
sensitivity in gastric cancer by targeting apoptosis repressor with
caspase recruitment domain. Cell Death Dis. 5:e11972014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Toth C, Funke S, Nitsche V, Liverts A,
Zlachevska V, Gasis M, Wiek C, Hanenberg H, Mahotka C, Schirmacher
P and Heikaus S: The role of apoptosis repressor with a CARD domain
(ARC) in the therapeutic resistance of renal cell carcinoma (RCC):
The crucial role of ARC in the inhibition of extrinsic and
intrinsic apoptotic signalling. Cell Commun Signal. 15:162017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Q, Li A, Wang H and Wang J: Knockdown
of apoptosis repressor with caspase recruitment domain (ARC)
increases the sensitivity of human glioma cell line U251MG to
VM-26. Int J Clin Exp Pathol. 5:555–561. 2012.PubMed/NCBI
|
|
42
|
Gobe GC, Ng KL, Small DM, Vesey DA,
Johnson DW, Samaratunga H, Oliver K, Wood S, Barclay JL, Rajandram
R, et al: Decreased apoptosis repressor with caspase recruitment
domain confers resistance to sunitinib in renal cell carcinoma
through alternate angiogenesis pathways. Biochem Biophys Res
Commun. 473:47–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu L, Nam YJ, Kung G, Crow MT and Kitsis
RN: Induction of the apoptosis inhibitor ARC by Ras in human
cancers. J Biol Chem. 285:19235–19245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mak PY, Mak DH, Ruvolo V, Jacamo R,
Kornblau SM, Kantarjian H, Andreeff M and Carter BZ: Apoptosis
repressor with caspase recruitment domain modulates second
mitochondrial-derived activator of caspases mimetic-induced cell
death through BIRC2/MAP3K14 signalling in acute myeloid leukaemia.
Br J Haematol. 167:376–384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Carter BZ, Qiu YH, Zhang N, Coombes KR,
Mak DH, Thomas DA, Ravandi F, Kantarjian HM, Koller E, Andreeff M
and Kornblau SM: Expression of ARC (apoptosis repressor with
caspase recruitment domain), an antiapoptotic protein, is strongly
prognostic in AML. Blood. 117:780–787. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mak PY, Mak DH, Mu H, Shi Y, Ruvolo P,
Ruvolo V, Jacamo R, Burks JK, Wei W, Huang X, et al: Apoptosis
repressor with caspase recruitment domain is regulated by MAPK/PI3K
and confers drug resistance and survival advantage to AML.
Apoptosis. 19:698–707. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu P, Tang Y, He J, Qi L, Jiang W and Zhao
S: ARC is highly expressed in nasopharyngeal carcinoma and confers
X-radiation and cisplatin resistance. Oncol Rep. 30:1807–1813.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen LH, Jiang CC, Watts R, Thorne RF,
Kiejda KA, Zhang XD and Hersey P: Inhibition of endoplasmic
reticulum stress-induced apoptosis of melanoma cells by the ARC
protein. Cancer Res. 68:834–842. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jiang CC, Lucas K, Avery-Kiejda KA, Wade
M, deBock CE, Thorne RF, Allen J, Hersey P and Zhang XD:
Up-regulation of Mcl-1 is critical for survival of human melanoma
cells upon endoplasmic reticulum stress. Cancer Res. 68:6708–6717.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Carter BZ, Mak PY, Wang X, Tao W, Ruvolo
V, Mak D, Mu H, Burks JK and Andreeff M: An ARC-regulated
IL1β/Cox-2/PGE2/β-catenin/ARC circuit controls
leukemia-microenvironment interactions and confers drug resistance
in AML. Cancer Res. 79:1165–1177. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Huang W, Su G, Huang X, Zou A, Wu J, Yang
Y, Zhu Y, Liang S, Li D, Ma F and Guo L: Long noncoding RNA PCAT6
inhibits colon cancer cell apoptosis by regulating anti-apoptotic
protein ARC expression via EZH2. Cell Cycle. 18:69–83. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lu D, Liu J, Jiao J, Long B, Li Q, Tan W
and Li P: Transcription factor Foxo3a prevents apoptosis by
regulating calcium through the apoptosis repressor with caspase
recruitment domain. J Biol Chem. 288:8491–8504. 2003. View Article : Google Scholar
|
|
53
|
Kavazis AN, McClung JM, Hood DA and Powers
SK: Exercise induces a cardiac mitochondrial phenotype that resists
apoptotic stimuli. Am J Physiol Heart Circ Physiol. 294:H928–H935.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yaniv G, Shilkrut M, Lotan R, Berke G,
Larisch S and Binah O: Hypoxia predisposes neonatal rat ventricular
myocytes to apoptosis induced by activation of the Fas (CD95/Apo-1)
receptor: Fas activation and apoptosis in hypoxic myocytes.
Cardiovasc Res. 54:611–623. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nam YJ, Mani K, Wu L, Peng CF, Calvert JW,
Foo RS, Krishnamurthy B, Miao W, Ashton AW, Lefer DJ and Kitsis RN:
The apoptosis inhibitor ARC undergoes ubiquitin-
proteasomal-mediated degradation in response to death stimuli:
Identification of a degradation-resistant mutant. J Biol Chem.
282:5522–5528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Neuss M, Monticone R, Lundberg MS, Chesley
AT, Fleck E and Crow MT: The apoptotic regulatory protein ARC
(apoptosis repressor with caspase recruitment domain) prevents
oxidant stress-mediated cell death by preserving mitochondrial
function. J Biol Chem. 276:33915–33922. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Foo RS, Chan LK, Kitsis RN and Bennett MR:
Ubiquitination and degradation of the anti-apoptotic protein ARC by
MDM2. J Biol Chem. 282:5529–5535. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li X, Du N, Zhang Q, Li J, Chen X, Liu X,
Hu Y, Qin W, Shen N, Xu C, et al: MicroRNA-30d regulates
cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic
cardiomyopathy. Cell Death Dis. 5:e14792014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wu H, Huang T, Ying L, Han C, Li D, Xu Y,
Zhang M, Mou S and Dong Z: MiR-155 is involved in renal
ischemia-reperfusion injury via direct targeting of FoxO3a and
regulating renal tubular cell pyroptosis. Cell Physiol Biochem.
40:1692–1705. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li YZ, Lu DY, Tan WQ, Wang JX and Li PF:
p53 initiates apoptosis by transcriptionally targeting the
antiapoptotic protein ARC. Mol Cell Biol. 28:564–574. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Loan Le TY, Mardini M, Howell VM, Funder
JW, Ashton AW and Mihailidou AS: Low-dose spironolactone prevents
apoptosis repressor with caspase recruitment domain degradation
during myocardial infarction. Hypertension. 59:1164–1169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li J, Li C, Zhang D, Shi D, Qi M, Feng J,
Yuan T, Xu X, Liang D, Xu L, et al: SNX13 reduction mediates heart
failure through degradative sorting of apoptosis repressor with
caspase recruitment domain. Nat Commun. 5:51772014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Quadrilatero J and Rush JW: Evidence for a
pro-apoptotic phenotype in skeletal muscle of hypertensive rats.
Biochem Biophys Res Commun. 368:168–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
McMillan EM, Graham DA, Rush JW and
Quadrilatero J: Decreased DNA fragmentation and apoptotic signaling
in soleus muscle of hypertensive rats following 6 weeks of
treadmill training. J Appl Physiol (1985). 113:1048–1057. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dowds TA and Sabban EL: Endogenous and
exogenous ARC in serum withdrawal mediated PC12 cell apoptosis: A
new pro-apoptotic role for ARC. Cell Death Differ. 8:640–648. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li PF, Li J, Müller EC, Otto A, Dietz R
and von Harsdorf R: Phosphorylation by protein kinase CK2: A
signaling switch for the caspase-inhibiting protein ARC. Mol Cell.
10:247–258. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Itoh N and Nagata S: A novel protein
domain required for apoptosis. Mutational analysis of human Fas
antigen. J Biol Chem. 268:10932–10937. 1993.PubMed/NCBI
|
|
69
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Parrish AB, Freel CD and Kornbluth S:
Cellular mechanisms controlling caspase activation and function.
Cold Spring Harb Perspect Biol. 5:2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pollack M and Leeuwenburgh C: Apoptosis
and aging: Role of the mitochondria. J Gerontol A Biol Sci Med Sci.
56:B475–B482. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Dirks A and Leeuwenburgh C: Apoptosis in
skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol.
282:R519–R527. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ha HJ and Park HH: Molecular basis for the
effect of the L31F mutation on CARD function in ARC. FEBS Lett.
591:2919–2928. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Dirks AJ and Leeuwenburgh C: Aging and
lifelong calorie restriction result in adaptations of skeletal
muscle apoptosis repressor, apoptosis-inducing factor, X-linked
inhibitor of apoptosis, caspase-3, and caspase-12. Free Radic Biol
Med. 36:27–39. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang JX, Zhang XJ, Feng C, Sun T, Wang K,
Wang Y, Zhou LY and Li PF: MicroRNA-532-3p regulates mitochondrial
fission through targeting apoptosis repressor with caspase
recruitment domain in doxorubicin cardiotoxicity. Cell Death Dis.
6:e16772015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li YZ, Liu XH, Zhu XM and Cai LR: ARC
contributes to the inhibitory effect of preconditioning on
cardiomyocyte apoptosis. Apoptosis. 12:1589–1595. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
An J, Mehrhof F, Harms C, Lättig-Tünnemann
G, Lee SL, Endres M, Li M, Sellge G, Mandić AD, Trautwein C and
Donath S: ARC is a novel therapeutic approach against
acetaminophen-induced hepatocellular necrosis. J Hepatol.
58:297–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Templin AT, Samarasekera T, Meier DT,
Hogan MF, Mellati M, Crow MT, Kitsis RN, Zraika S, Hull RL and Kahn
SE: Apoptosis repressor with caspase recruitment domain ameliorates
amyloid-induced β-cell apoptosis and JNK pathway activation.
Diabetes. 66:2636–2645. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Donath S, Li P, Willenbockel C, Al-Saadi
N, Gross V, Willnow T, Bader M, Martin U, Bauersachs J, Wollert KC,
et al: Apoptosis repressor with caspase recruitment domain is
required for cardioprotection in response to biomechanical and
ischemic stress. Circulation. 113:1203–1212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ziegler DS, Wright RD, Kesari S, Lemieux
ME, Tran MA, Jain M, Zawel L and Kung AL: Resistance of human
glioblastoma multiforme cells to growth factor inhibitors is
overcome by blockade of inhibitor of apoptosis proteins. J Clin
Invest. 118:3109–3122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Mercier I, Vuolo M, Madan R, Xue X,
Levalley AJ, Ashton AW, Jasmin JF, Czaja MT, Lin EY, Armstrong RC,
et al: ARC, an apoptosis suppressor limited to terminally
differentiated cells, is induced in human breast cancer and confers
chemo- and radiation-resistance. Cell Death Differ. 12:682–686.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Carter BZ, Mak PY, Chen Y, Mak DH, Mu H,
Jacamo R, Ruvolo V, Arold ST, Ladbury JE, Burks JK, et al:
Anti-apoptotic ARC protein confers chemoresistance by controlling
leukemia-microenvironment interactions through a NFκB/IL1β
signaling network. Oncotarget. 7:20054–20067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang J, Feng C, He Y, Ding W, Sheng J,
Arshad M, Zhang X and Li P: Phosphorylation of apoptosis repressor
with caspase recruitment domain by protein kinase CK2 contributes
to chemotherapy resistance by inhibiting doxorubicin induced
apoptosis. Oncotarget. 6:27700–27713. 2015.PubMed/NCBI
|
|
84
|
Damiano JS and Reed JC: CARD proteins as
therapeutic targets in cancer. Curr Drug Targets. 5:367–374. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Vaux DL and Strasser A: The molecular
biology of apoptosis. Proc Natl Acad Sci USA. 93:2239–2244. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Fidler MM, Bray F and Soerjomataram I: The
global cancer burden and human development: A review. Scand J
Public Health. 46:27–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Gorjánácz M: Nuclear assembly as a target
for anti-cancer therapies. Nucleus. 5:47–55. 2014. View Article : Google Scholar : PubMed/NCBI
|