Introduction
Hypoxia, which commonly occurs in the majority of
solid tumors, is associated with resistance to anticancer
therapies, including chemotherapy and radiotherapy (1,2). Cancer
cells become resistant to anticancer therapies during hypoxia due
to their adaptation to lower oxygen levels, which alters the gene
expression of metabolic enzymes partially mediated by
hypoxia-inducible factors 1α and 2α (3). In addition to transcriptional
regulation, hypoxia strongly suppresses the translation rate by
inhibiting the expression of eukaryotic initiation factors 2 and 4F
(4). The endoplasmic reticulum (ER)
stress and mTOR signaling pathways serve key roles in the
hypoxia-mediated inhibition of eukaryotic initiation factors
(4).
Despite the well-known role of hypoxia in the
resistance to anticancer therapies, its roles in cancer immunity,
to the best of our knowledge, have not been extensively studied.
Recent studies have demonstrated that hypoxia promotes cancer
progression by impairing anticancer immunity via various mechanisms
(5–8). Cancer cells in hypoxic regions release
molecules that induce the differentiation of tumor-associated
macrophages (TAM) into immunosuppressive phenotypes, including
M2-type TAMs, or recruit myeloid-derived suppressor cells (5,6). ER
stress in cancer cells, which can also be induced by hypoxia, and
low nutrient supply can be transmitted to dendritic cells and
impair their ability to prime CD8+ T cells (7,8). These
findings suggest that hypoxia is generally associated with the
suppression of anticancer immunity.
Different anticancer therapies result in different
types of cancer cell death in terms of immunogenicity. Cancer cell
death caused by doxorubicin or irradiation is strongly immunogenic,
whereas cancer cell death caused by cisplatin is poorly immunogenic
(9). The cell surface exposure of
calreticulin has been identified as a key feature in determining
immunogenic cell death (9).
Activation of the ER stress signaling pathway is involved in the
cell surface exposure of calreticulin (10).
Hypoxia induces ER stress in cancer cells. The ER
stress signaling pathway is one of the main mechanisms underlying
the increased cell surface exposure of calreticulin, a marker of
immunogenic cell death (9,10). The present study investigated the
role of ER stress induced by hypoxia in the immunogenicity of
cancer cells. It was identified that hypoxia increased the cell
surface exposure of calreticulin in an ER stress-dependent manner,
resulting in enhanced immunogenicity of cancer cells. Compared with
the efficient increase in the exposure of cell surface calreticulin
induced by hypoxia, the cell surface exposure of CD47, an
anti-phagocytic signal that antagonizes the activity of
calreticulin in phagocytosis, was not efficiently induced by
hypoxia. Therefore, hypoxia may enhance the immunogenicity of
cancer cells in addition to inducing an immunosuppressive cancer
microenvironment. These results may be useful in understanding the
role of hypoxia in cancer immunity to design effective anticancer
immunotherapies.
Materials and methods
Cell culture
MCF7 and MDA-MB-231 cells were purchased from the
American Type Culture Collection. Both cells were cultured in
Dulbecco's Modified Eagle medium (DMEM) supplemented with 10% FBS,
2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin at
37°C in a 5% CO2 atmosphere and irradiation were
performed as described previously (11,12). The
4TO7 cells were established by Dr Fred R. Miller at Wayne State
University and were cultured in DMEM supplemented with 10% FBS, 2
mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin at 37°C
in a 5% CO2 atmosphere (13). A total of 5×106 cells were
seeded in 150 mm dishes and following 36 h, cells of ~70%
confluence were exposed to 2 µM doxorubicin (Sigma-Aldrich; Merck
KGaA) or 300 µM cisplatin (Sigma-Aldrich; Merck KGaA) at 37°C for
24 h. A total of 5×106 cells were seeded in 150 mm
dishes and following 36 h, cells of ~70% confluence were irradiated
with 10 Gy at room temperature and harvested via
fluorescence-activated cell sorting (FACS) analysis 24 h later.
Cells were exposed to hypoxia at 37°C for 48 h in an anaerobic
system (Thermo Fisher Scientific, Inc.) using mixed gases (1%
O2, 5% CO2 and N2 balance). The
oxygen concentration was monitored using an O2 sensor
(New Cosmos Electric Co., Ltd.) prior to hypoxia treatment. A total
of 5×106 cells were seeded in 150 mm dishes and
following 36 h, cells of ~70% confluence were treated with 1 mM
tauroursodeoxycholic acid (TUDCA; Sigma-Aldrich; Merck KGaA) or 5
mM 4-phenylbutyrate (4-PBA; Sigma-Aldrich; Merck KGaA) at 37°C for
30 min prior to hypoxia treatment.
Flow cytometry analysis
For the analysis of cell surface exposure of
calreticulin and CD47, the cells were harvested, washed with PBS
and stained with anti-calreticulin antibody (cat. no. 12238; 1:100;
Cell Signaling Technology, Inc.), fluorescein-labeled anti-rabbit
immunoglobulin G (IgG; cat. no. 554020; 1 µg/ml; BD Biosciences) or
AlexaFluor® 647-anti-CD47 antibodies (cat. no. 563584; 1
µg/ml; BD Biosciences). For determination of the total cell
expression of calreticulin, the cells were harvested, permeabilized
with Cytofix/Cytoperm (BD Biosciences), and stained with
anti-calreticulin antibody and fluorescein-labeled anti-rabbit IgG.
The cells were analyzed on a FACS Aria (Becton, Dickinson and
Company) using FACSDiva software v6.1.3 (BD Biosciences) to
evaluate cell surface exposure and total cell expression.
Western blot analysis
Protein samples were prepared using extraction
buffer [50 mM Tris-HCl, pH 7.4, 250 mM NaCl, 5 mM EDTA, 0.5% NP40,
10 mM NaF, 1 mM Na3VO4, 1 mM DTT, 1 mM PMSF
and 1X Protease Inhibitor Cocktail (cat. no. 11697498001; Merck
KGaA)] and concentrations of protein samples were measured using
Bio-Rad Protein assays (cat. no. 5000006; Bio-Rad Laboratories,
Inc.). Protein samples (10 µg) were separated on a 4–15% gradient
SDS-PAGE gel (cat. no. 456-1084; Bio-Rad Laboratories, Inc.) and
transferred to a PVDF membrane (cat. no. 03-010-040-001; Roche
Diagnostics GmbH). The membrane was blocked with 10% skimmed milk
(cat. no. 90002-594; Difco; BD Biosciences) in PBST (PBS with 0.1%
Tween) at room temperature (RT) for 30 min and probed with
anti-calreticulin (cat. no. 12238; 1:1,000 in 10% skimmed milk in
PBST; Cell Signaling Technology, Inc.) and anti-actin (cat. no.
A2228; 1:3,000 in 10% skip milk in PBST; Sigma-Aldrich; Merck KGaA)
antibodies at RT for 2 h. This was followed by probing with
HRP-conjugated anti-rabbit (cat. no. sc-2004; 1:1,000 in 10% skip
milk in PBST; Santa Cruz Biotechnology, Inc.) and HRP-conjugated
anti-mouse (cat. no. sc-2005; 1:1,000 in 10% skip milk in PBST;
Santa Cruz Biotechnology, Inc.) secondary antibodies at RT for 1 h.
The membrane was incubated with chemiluminescent reagent (ECL
Select Western Blottng Detection Reagent; cat. no. RPN2235; GE
Healthcare) and then subjected to analysis with a Fusion Fx5 image
analyzer (Fusion-CAPT software, Vilber Lourmat).
Animal experiments
Animal studies were conducted under specific
pathogen-free conditions (temperature, 22±3°C; humidity: 50±20 RH;
illumination, 150–300 Lux; light/dark cycle, 8 am-8 pm; access to
food and water, available anytime) and approved by the Ethics
Committee on the Use and Care of Animals of the Dongnam Institute
of Radiological and Medical Sciences (Busan, Republic of Korea;
approval no. DIRAMS AEC-2015-008). Female Balb/c mice n=120) were
purchased from Japan SLC, Inc. and were used for each experiment
(age, 6 weeks; weight, 15–20 g; n=12 per group). Following various
treatments (10 Gy irradiation, doxorubicin, cisplatin and culture
under hypoxic conditions), 1×106 4TO7 cells were
subcutaneously injected into the left thighs. At 7 days
post-injection, 5×105 live 4TO7 cells were injected into
the right thighs. Tumor volume was calculated using the equation:
Volume=width2 × length ×0.52. Mice with tumor sizes
<150 mm3 were considered tumor-free.
Statistical analysis
Each experiment was repeated at least twice. Results
are expressed as the mean ± SD. To determine statistical
significance, the data were analyzed using SPSS statistical
software for Windows (version 18.0; SPSS, Inc.). Student's t-test
(unpaired) was performed to determine significant differences
between two groups, and one-way analysis of variance with Dunnett's
post hoc test for multiple comparisons was performed to determine
significant differences among more than two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
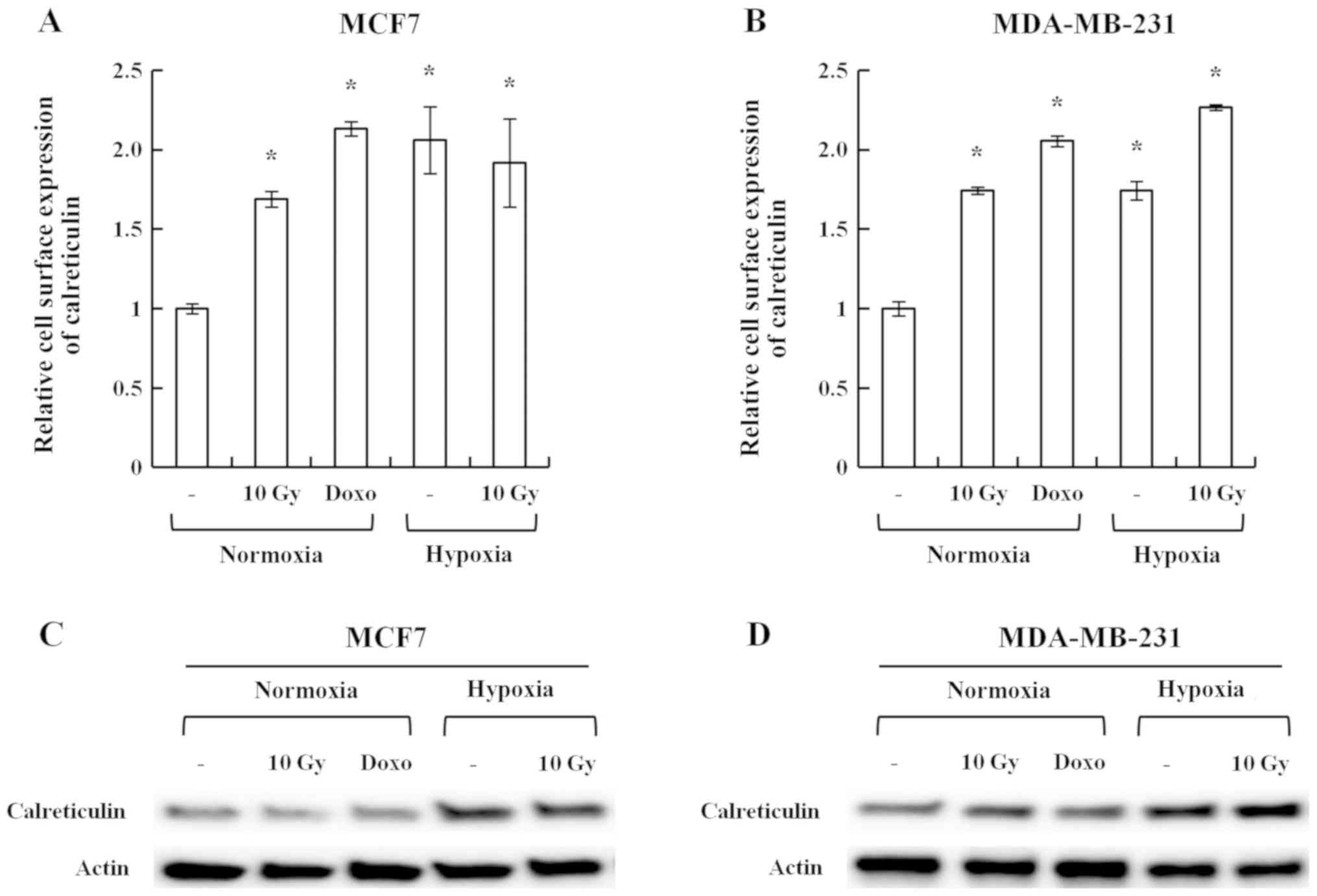
Hypoxia increases the cell surface
exposure of calreticulin in human and mouse breast cancer cell
lines in an ER stress-dependent manner
To investigate whether hypoxia induced the cell
surface exposure of calreticulin, the human breast cancer cell
lines MCF7 and MDA-MB-231 were cultured under 1% O2
hypoxic conditions. Irradiation (10 Gy) and doxorubicin, which are
known inducers of the cell surface exposure of calreticulin, were
used as positive controls (9,10).
Hypoxia, irradiation and doxorubicin induced the cell surface
exposure of calreticulin in MCF7 and MDA-MB-231 cells (Fig. 1A and B). Western blot analysis
suggested that hypoxia not only induced cell surface exposure of
calreticulin but also its total expression (Fig. 1C and D).
Furthermore, the present study investigated a mouse
breast cancer cell line to determine a similar pattern of results.
Using 4TO7, a balb/c-derived breast cancer cell line, it was
identified that hypoxia induced both the cell surface exposure and
total expression of calreticulin in 4TO7 cells, which was similar
to the effects observed in human breast cancer cell lines (Fig. 2A). The cell surface exposure of
calreticulin has been reported to be ER stress-dependent (10). Consistent with the previous study,
the chemical chaperones TUDCA and 4-PBA decreased hypoxia-induced
cell surface exposure of calreticulin (Fig. 2B). These results suggest that hypoxia
induced ER stress, which resulted in enhanced cell surface exposure
of calreticulin.
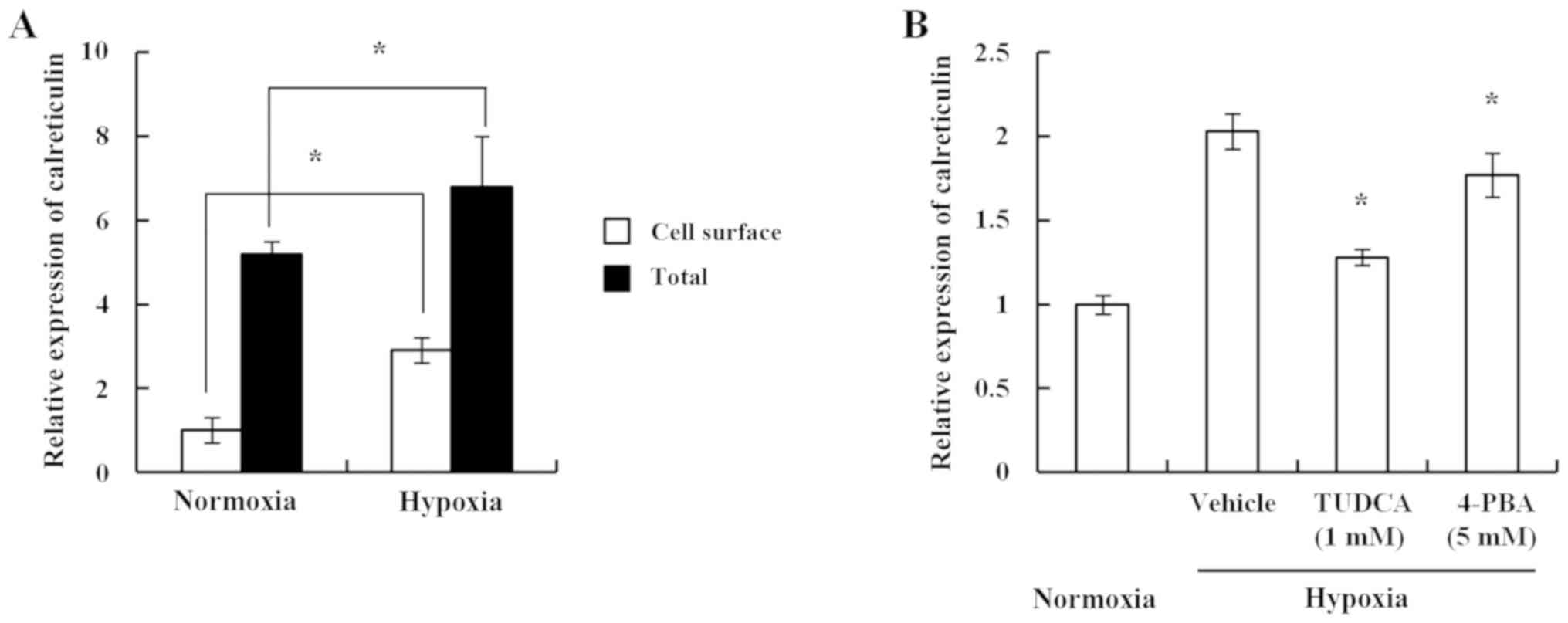 | Figure 2.Hypoxia induces the cell surface
exposure of calreticulin in a 4TO7 mouse breast cancer cell line in
an endoplasmic reticulum stress-dependent manner. (A) 4TO7 cells
were treated as indicated (hypoxia, 1% O2). At 24 h
post-treatment, the cells were analyzed by FACS using an
anti-calreticulin antibody. For the analysis of cell surface
exposure, cells were harvested and stained with anti-calreticulin
antibody and fluorescein-labeled anti-rabbit IgG. For total cell
expression of calreticulin, the cells were harvested and
permeabilized with Cytofix/Cytoperm, followed by staining with
anti-calreticulin antibody and fluorescein-labeled anti-rabbit IgG.
The relative exposure is shown as the mean MFI ± SD. Compared with
the normoxia groups, both cell surface exposure and total
expression of calreticulin in the hypoxia groups were statistically
significant. *P<0.05, as indicated. (B) 4TO7 cells were cultured
in hypoxic conditions, with or without chemical chaperones. Cells
were analyzed by FACS using an anti-calreticulin antibody. The
relative exposure is shown as the mean MFI ± SD. *P<0.05 vs.
hypoxia-vehicle group. 4-PBA, 4-phenylbutyrate; FACS,
fluorescence-activated cell sorting; IgG, immunoglobulin G; MFI,
median fluorescence intensity; TUDCA, tauroursodeoxycholic
acid. |
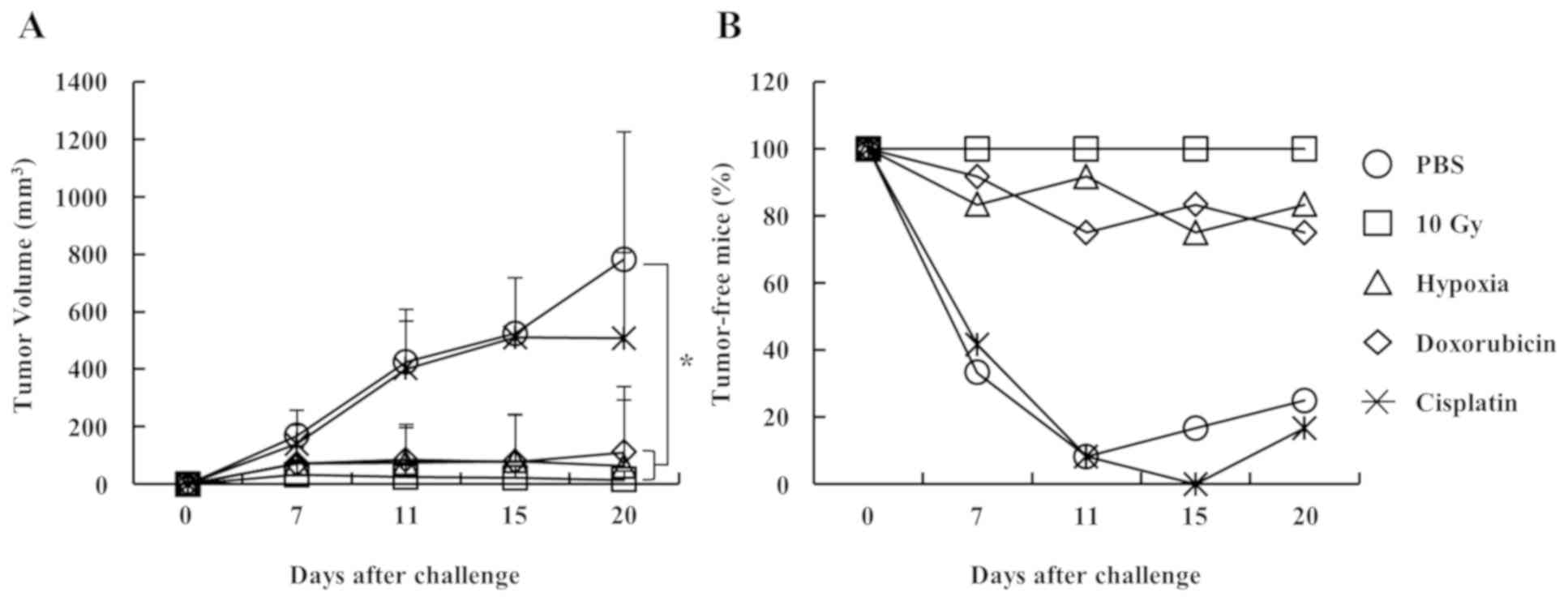
Hypoxia-induced cell surface exposure
of calreticulin is associated with enhanced immunogenicity of the
4TO7 mouse breast cancer cell line
To investigate whether the hypoxia-induced cell
surface exposure of calreticulin enhanced anticancer
immunogenicity, a mouse experiment was performed. The results
demonstrated that ~60% of 4TO7 cells died after 2 days of culture
under 1% O2 hypoxic conditions, based on annexin V and
propidium iodide staining analysis (data not shown). The left
thighs of 6-week-old mice were subcutaneously injected (vaccinated)
with 1×106 dying 4TO7 cells. At 7 days post-injection,
the right thighs were subcutaneously injected (challenged) with
5×105 live 4TO7 cells to induce tumor growth. Tumor
growth in the right thighs of mice vaccinated with hypoxia-treated
4TO7 cells was inhibited as efficiently as in the groups exposed to
irradiation (10 Gy) and doxorubicin. However, tumor growth was not
suppressed in mice vaccinated with 4TO7 cells treated with PBS and
cisplatin, which is a poor inducer of immunogenic cell death
(Fig. 3A). Additionally, analysis of
tumor-free mice (mice with tumor sizes <150 mm3 were
considered tumor-free since, empirically, it is very difficult to
measure the tumor volume below 150 mm3) demonstrated
that the tumor growth in the hypoxia group was inhibited as
efficiently as in the irradiation (10 Gy) and doxorubicin groups
(Fig. 3B). These results suggest
that hypoxia may have induced immunogenic cell death by enhancing
the cell surface exposure of calreticulin.
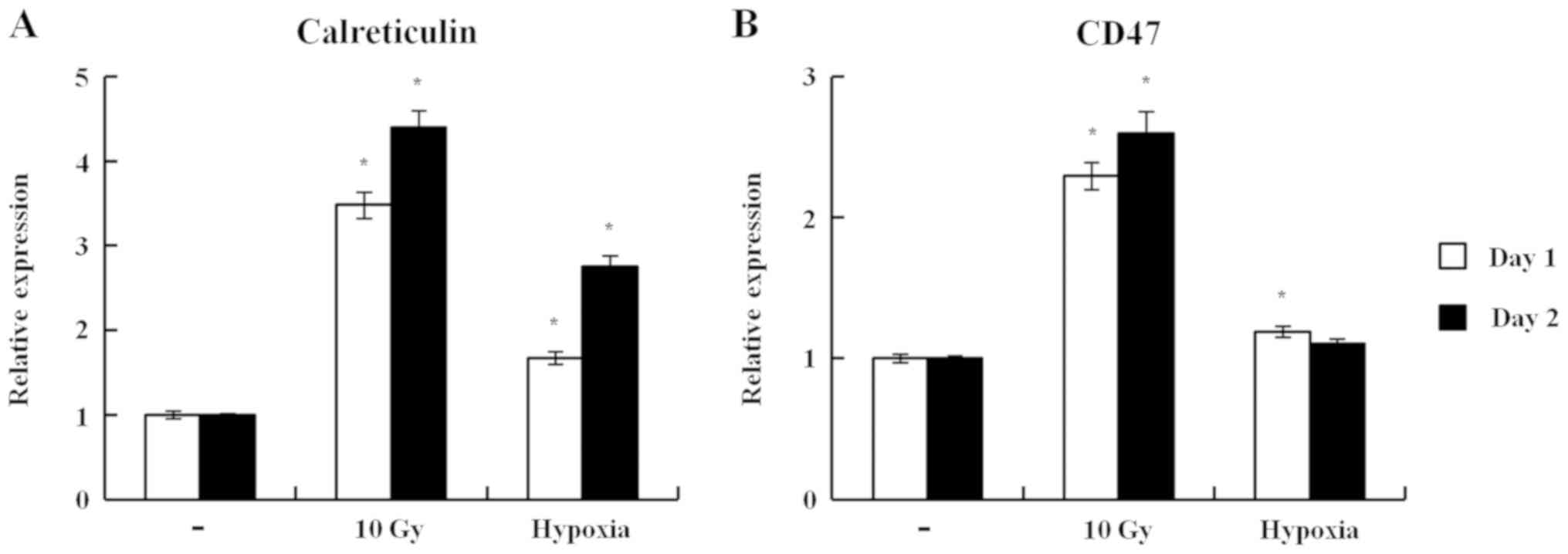
Hypoxia increases the cell surface
exposure of calreticulin but not CD47, an anti-phagocytic
signal
The cell surface exposure of calreticulin is an ‘eat
me’ signal, prompting the recognition and removal of dying tumor
cells by phagocytes (9,14). By contrast, the cell surface exposure
of CD47 works as a ‘do not eat me’ signal, which antagonizes the
function of calreticulin (9,14). Therefore, the present study examined
whether hypoxia modulated the cell surface exposure of CD47 in 4TO7
cells. Irradiation efficiently induced the cell surface exposure of
calreticulin and CD47 (4.4-fold for calreticulin and 2.6-fold for
CD47 vs. no treatment on day 2; Fig.
4). It is possible that the cell surface exposure of
calreticulin induced by irradiation was partially counteracted by
the enhanced cell surface exposure of CD47. Hypoxia also induced
the cell surface exposure of calreticulin but not that of CD47
(2.8-fold for calreticulin and 1.1-fold for CD47 vs. no treatment
on day 2; Fig. 4). Therefore,
calreticulin surface expression induced by hypoxia may work as an
‘eat me’ signal effectively, without being antagonized by CD47.
Overall, the results suggest that hypoxia may have induced
immunogenic cell death by enhancing the cell surface exposure of
calreticulin in an ER stress-dependent manner, although hypoxia
elicits numerous alterations in the cancer microenvironment, which
are unfavorable for the induction of antitumor immunogenicity.
Further investigations are required to elucidate the cumulative
effect of hypoxia-induced alterations in the cancer
microenvironment on the immunogenicity of cancer cells.
Discussion
Apoptotic cells are removed by phagocytic cells to
maintain homeostasis. Phosphatidylserine (PS) on the cell surface
of apoptotic cells is a well-known signal recognized by phagocytes
(15,16). It is referred to as the ‘eat me
signal’ (15,16). Gardai et al (14) have demonstrated that calreticulin
also acts as an ‘eat me signal’ for phagocytes. Unlike PS, which is
involved in anti-inflammatory and anti-immunogenic responses, the
exposure of calreticulin on the apoptotic cell surface induces
immunogenic cell death (9).
Therefore, anticancer therapies, which induce cell surface exposure
of calreticulin during apoptosis, lead to immunogenic cancer cell
death. However, calreticulin is also present on the cell surface of
live cells, which are not taken up by phagocytes, suggesting a role
of specific regulatory mechanisms in the process. CD47 has been
demonstrated to act as a ‘do not eat me’ signal (14). It prevents the uptake of
calreticulin-expressing live cells by phagocytes (14). Therefore, CD47 is used as an
anti-phagocytic signal in the immune evasion of cancer cells. An
anti-CD47 antibody has been developed to enhance anticancer
immunity by modulating the balance between pro- and anti-phagocytic
signals (17).
Calreticulin is a highly-conserved 46 kDa protein
predominantly located in the ER due to the presence of the ER
retrieval signal (KDEL) at the C-terminal (18). Calreticulin is a multifunctional
protein with Ca2+-binding and chaperone activities
important for numerous biological processes, including
Ca2+ homeostasis, cellular signaling and protein folding
(19–21). Since the Ca2+ signaling
pathway is important for T-cell receptor activation, calreticulin
contributes to the modulation of the T cell-mediated adaptive
immune response (22). Therefore,
calreticulin induces immune responses via extracellular and
intracellular signals. In addition to its role in immunogenic cell
death in anticancer therapies, calreticulin has been revealed to be
involved in a number of aspects of cancer biology, including cancer
cell proliferation, differentiation of neuroblastoma and cancer
cell migration (23).
Hypoxia is an important obstacle to anticancer
therapies since it induces a number of metabolic alterations
associated with resistance to apoptosis in cancer cells.
Alterations in cancer cells induced by hypoxia have also been
associated with immune evasion mechanisms (5–8).
Interleukin 10, transforming growth factor-β-β and vascular
endothelial growth factor secreted by cancer cells, and ER stress
induced in cancer cells under hypoxic conditions are associated
with immune suppression in the tumor microenvironment (5–8). The
results of the present study appear to be inconsistent with the
results of previous studies (5–8). One may
propose that hypoxia-induced alterations in cancer cells result in
evasion of immune surveillance and resistance to immune responses.
However, other alterations may lead to the immunogenic cell death
of cancer cells in a hypoxic microenvironment. Immune evasion or
resistance to immune responses may be determined by the cumulative
alterations induced by hypoxia. The present study revealed that
hypoxia induced immunogenic cell death of cancer cells in an ER
stress-dependent manner. This observation is supported by previous
studies suggesting that lysates derived from cancer cells cultured
at 5% O2 were improved sources of cancer vaccine antigen
than those obtained at 20% O2 (24,25).
Although these studies did not explore the mechanisms underlying
the phenomenon, they are similar to the findings of the present
study, suggesting that culture conditions at oxygen concentrations
<20% may enhance the immunogenicity of cancer cells. Future
studies may investigate whether the cell surface exposure of
calreticulin is induced in cancer cells cultured at 2–5% oxygen
concentrations, which is higher than the oxygen concentration used
in the present study. In summary, the results suggest that hypoxia
induced favorable and unfavorable alterations in terms of
anticancer immunity. Elucidation of the exact mechanisms may
facilitate the design of effective anticancer immunotherapies.
Acknowledgements
The 4TO7 cells were provided by Dr. Wook Jin at
Gachon University (Incheon, Korea).
Funding
The present study was supported by the Dongnam
Institute of Radiological and Medical Sciences grant funded by the
Korean government (MSIT) (grant no. 50491-2015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YKH, JSK, WSJ and CGL were involved in the
conceptualization of the study and in the methodology. YKH, GYP and
MJB performed the experiments. YKH and CGL were involved in data
analysis and the writing of the original draft. JSK and WSJ were
involved in the writing, reviewing and editing of the manuscript.
JSK, WSJ and CGL supervised the study. WSJ and CGL were involved in
funding acquisition. All aforementioned authors participated in the
conception and design of the study. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Animal studies were approved by the Ethics Committee
on the Use and Care of Animals of the Dongnam Institute of
Radiological and Medical Sciences (Busan, Republic of Korea;
approval no. DIRAMS AEC-2015-008).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Evans SM and Koch CJ: Prognostic
significance of tumor oxygenation in humans. Cancer Lett. 195:1–16.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Le QT, Denko NC and Giaccia AJ: Hypoxic
gene expression and metastasis. Cancer Metastasis Rev. 23:293–310.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Majmundar AJ, Wong WJ and Simon MC:
Hypoxia-inducible factors and the response to hypoxic stress. Mol
Cell. 40:294–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koumenis C and Wouters BG: ‘Translating’
tumor hypoxia: Unfolded protein response (UPR)-dependent and
UPR-independent pathways. Mol Cancer Res. 4:423–436. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gabrilovich D: Mechanisms and functional
significance of tumour-induced dendritic-cell defects. Nat Rev
Immunol. 4:941–952. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hao NB, Lü MH, Fan YH, Cao YL, Zhang ZR
and Yang SM: Macrophages in tumor microenvironments and the
progression of tumors. Clin Dev Immunol. 2012:9480982012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mahadevan NR, Anufreichik V, Rodvold JJ,
Chiu KT, Sepulveda H and Zanetti M: Cell-extrinsic effects of tumor
ER stress imprint myeloid dendritic cells and impair
CD8+ T cell priming. PLoS One. 7:e518452012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zanetti M, Rodvold JJ and Mahadevan NR:
The evolving paradigm of cell-nonautonomous UPR-based regulation of
immunity by cancer cells. Oncogene. 35:269–278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Obeid M, Tesniere A, Ghiringhelli F, Fimia
GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T,
Casares N, et al: Calreticulin exposure dictates the immunogenicity
of cancer cell death. Nat Med. 13:54–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Panaretakis T, Kepp O, Brockmeier U,
Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N,
Pierron G, van Endert P, et al: Mechanisms of pre-apoptotic
calreticulin exposure in immunogenic cell death. EMBO J.
28:578–590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee CG, Park GY, Han YK, Lee JH, Chun SH,
Park HY, Lim KH, Kim EG, Choi YJ, Yang K and Lee CW: Roles of
14-3-3η in mitotic progression and its potential use as a
therapeutic target for cancers. Oncogene. 32:1560–1569. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park GY, Han JY, Han YK, Kim SD, Kim JS,
Jo WS, Chun SH, Jeong DH, Lee CW, Yang K and Lee CG: 14-3-3 eta
depletion sensitizes glioblastoma cells to irradiation due to
enhanced mitotic cell death. Cancer Gene Ther. 21:158–163. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aslakson CJ and Miller FR: Selective
events in the metastatic process defined by analysis of the
sequential dissemination of subpopulations of a mouse mammary
tumor. Cancer Res. 52:1399–1405. 1992.PubMed/NCBI
|
|
14
|
Gardai SJ, McPhillips KA, Frasch SC,
Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg
PA, Michalak M and Henson PM: Cell-surface calreticulin initiates
clearance of viable or apoptotic cells through trans-activation of
LRP on the phagocyte. Cell. 123:321–334. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fadok VA, Bratton DL, Guthrie L and Henson
PM: Differential effects of apoptotic versus lysed cells on
macrophage production of cytokines: Role of proteases. J Immunol.
166:6847–6854. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoffmann PR, Kench JA, Vondracek A, Kruk
E, Daleke DL, Jordan M, Marrack P, Henson PM and Fadok VA:
Interaction between phosphatidylserine and the phosphatidylserine
receptor inhibits immune responses in vivo. J Immunol.
174:1393–1404. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chao MP, Jaiswal S, Weissman-Tsukamoto R,
Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB,
Raveh T, Park CY, et al: Calreticulin is the dominant
pro-phagocytic signal on multiple human cancers and is
counterbalanced by CD47. Sci Transl Med. 2:63ra942010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Michalak M, Corbett EF, Mesaeli N,
Nakamura K and Opas M: Calreticulin: One protein, one gene, many
functions. Biochem J. 344:281–292. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dedhar S: Novel functions for
calreticulin: Interaction with integrins and modulation of gene
expression? Trends Biochem Sci. 19:269–271. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leung-Hagesteijn CY, Milankov K, Michalak
M, Wilkins J and Dedhar S: Cell attachment to extracellular matrix
substrates is inhibited upon downregulation of expression of
calreticulin, an intracellular integrin alpha-subunit-binding
protein. J Cell Sci. 107:589–600. 1994.PubMed/NCBI
|
|
21
|
Raghavan M, Wijeyesakere SJ, Peters LR and
Del Cid N: Calreticulin in the immune system: Ins and outs. Trends
Immunol. 34:13–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Porcellini S, Traggiai E, Schenk U,
Ferrera D, Matteoli M, Lanzavecchia A, Michalak M and Grassi F:
Regulation of peripheral T cell activation by calreticulin. J Exp
Med. 203:461–471. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu YC, Weng WC and Lee H: Functional roles
of calreticulin in cancer biology. Biomed Res Int. 2015:5265242015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Olin MR, Andersen BM, Litterman AJ, Grogan
PT, Sarver AL, Robertson PT, Liang X, Chen W, Parney IF, Hunt MA,
et al: Oxygen is a master regulator of the immunogenicity of
primary human glioma cells. Cancer Res. 71:6583–6589. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Olin MR, Andersen BM, Zellmer DM, Grogan
PT, Popescu FE, Xiong Z, Forster CL, Seiler C, SantaCruz KS, Chen
W, et al: Superior efficacy of tumor cell vaccines grown in
physiologic oxygen. Clin Cancer Res. 16:4800–4808. 2010. View Article : Google Scholar : PubMed/NCBI
|