Introduction
According to the most recent global estimates in
2012, ~307,000 and ~3,000 prostate cancer (PCa)-associated
mortalities were reported globally and in Ukraine, respectively,
and 1,111,700 and ~7,000 novel cases were diagnosed, respectively
(1,2). Although the advent of relatively
non-invasive prostate-specific antigen (PSA) testing has notably
improved PCa diagnosis, its routine usage remains controversial,
since a false positive diagnosis rate of PCa, over-treatment, and
excessive medical spending has been reported (3–5). It has
been reported that up to 75% of patients with elevated PSA level
(>4 ng/µl) do not have any prostatic malignancy, although at
least 1/3 of aforementioned patients undergo subsequent invasive
follow-up evaluation, including prostate biopsy (6). Additionally, ~25% of individuals with
normal PSA level exhibit biopsy evidence for PCa (7,8). Another
important limitation of a canonical PSA assay is that it is neither
a reliable discriminator between prostatic cancer and benign
hyperplasia nor a precise staging indicator of PCa (4,5).
Therefore, the development of an accurate, discriminative, and
cost-efficient non-invasive PCa diagnostic tool is required.
Previous studies involving high-throughput
techniques, including genome-wide sequencing, failed to identify a
common genetic driver event (e.g. specific point mutation) in PCa
tumorigenesis (9,10). None of the recurrent mutations have
been reported to appear in 40–50% of PCa cases, including
transmembrane serine protease 2-ERG fusion and phosphatase
and tensin homolog (PTEN) deletion (10). Additionally, when examined for
alterations in DNA sequences, PCa demonstrates comparatively high
clonal heterogeneity or even distinct genomic origin, complicating
the use of mutation hotspots as tumor biomarkers (11). Instead it has been indicated that
epigenetic alterations, including cytosine base followed
immediately by a guanine base island methylation, occur in a number
of loci in 80–90% of PCa cases, including methylation of
glutathione S-transferase π 1 (GSTP1) gene promoter, may
drive the neoplastic transformation and would be a preferable
target for prostate cancer diagnosis and its biological potential
assessment (11–13).
It has been previously proposed that the
free-floating DNA fragments originating from the apoptotic/necrotic
malignant cells in bodily fluids may be used for the detection of
cancer biomarkers (14). This
approach, also referred to as liquid biopsy, would be particularly
beneficial when performed on urine samples, due to the maximal
non-invasive requirements for its collection, which notably
improves patient's compliance and safety (15). Urine contains notable amounts of
cell-free DNA (UcfDNA) with a concentration of up to 250 ng/ml and
consisting of the following two size category fragments: Long
(>1 kb), which are primarily cell-associated, including from the
exfoliated epithelium; and short (150–250 bp), which are
predominantly non-cell associated and originate from urogenital
tract per se or circulation (16,17).
This observation potentially makes UcfDNA the optimal source of
data for diagnosing urogenital system cancer types. The prostate,
whose lumen is continuously connected to the urogenital tract via
prostatic sinuses, may be the optimal organ for investigation by
means of UcfDNA analysis, which was successfully demonstrated by a
number of studies (18–22).
In an effort to extend the list of biomarkers
applicable for non-invasive PCa detection, the methylation profile
of 17 cancer-associated genes was examined using the approach of
UcfDNA analysis in the urine from patients with prostate cancer.
From a functional perspective, the genes investigated in the
present study are considered well-established tumor suppressors
from earlier reports and participate in PCa pathogenesis [forkhead
box P1 (FOXP1), FOXP3, FOXP4, hypermethylated in
cancer 1 (HIC1), zinc finger protein of the cerebellum 4
(ZIC4), PTEN, cadherin 1 (CDH1),
O-6-methylguanine-DNA methyltransferase (MGMT) and leucine
rich repeat containing 3B (LRRC3B)] or are known to be
associated with other malignancies [adenomatosis polyposis coli 2
(APC2), homeobox A9 (HOXA9), Wnt family member 7A
(WNT7A) and N-Myc downstream-regulated gene 4 protein
(NDRG4)] (23,24). The protein products of FOXP1,
FOXP3, FOXP4, ZIC4 and HOXA9 are members of three
families of transcription factors, including Forkhead, Zic, and
HOX, which are broadly involved in the processes of tissue
morphogenesis and cell differentiation (13,22,25). A
number of genes encode the extracellular and intracellular
signaling proteins, including ligands (WNT7A), binding
factors (APC2) and enzymes (NDRG4 and PTEN),
are known to take part in embryonic development and cell cycle
regulation as well (26–28). E-cadherin encoded by the CDH1
gene is a key adhesion molecule in the epithelial tissues crucial
for the formation of adherent junctions, whose disruption results
in tumor metastasis (29). The gene
MGMT, which encodes the pivotal reparation enzyme MGMT, has
been extensively implicated in the neoplastic transformation, due
to the increased mutation rate following its silencing (29). The product of the LRRC3B gene
is a 29-kDa membrane-bound protein, whose function, to the best of
our knowledge, has yet to be defined; however, it has been reported
to participate in the tumorigenesis of a number of human cancer
types, including clear cell renal cell carcinoma (30).
In the present study this initial gene set was
analyzed and 13 genes demonstrating a statistically significant
increase in methylation frequency in the PCa group, compared with
controls, were selected. A final panel of 6 genes, including
APC2, CDH1, FOXP1, LRRC3B, WNT7A, and ZIC4, was
formed based on zero/low methylation level in controls, with
significant moderate-to-strong correlation with tumor stage, and no
significant correlation with patient's age. Within the panel, the
number of genes methylated (NGM) was observed to increase
monotonically from control samples to highly developed and
metastatic types of cancer, providing a simple and cost-efficient
method to identify tumor stage using the NGM value in the urine
sample.
Materials and methods
Patients sample collection
The present study was approved by the local Ethics
Committee of the Institute of Molecular Biology and Genetics of the
National Academy of Sciences of Ukraine (approval no. 18/4). Urine
samples were collected between May and October 2017 from 64
individuals, including 31 patients diagnosed with PCa and receiving
treatment at the Institute of Urology NAMSU, and 33 patients who
were diagnosed as disease-free controls. Patient's detailed
information is presented in Table I.
None of the patients with PCa or control individuals underwent
radical prostatectomy or any type of pharmacological treatment
prior to sampling. All patients provided written informed consent
to participate in the present study. In each case the diagnosis was
further confirmed and the tumor was graded according to the Gleason
scoring system on prostate biopsy followed by histological
examination (31). The
Tumour-node-metastasis (TNM) staging system was used to classify
PCa cases according to their development and malignancy (32). Within the cancer group, individuals
were categorized with localized, including T1 and
T2N0M0/NxM0
(n=5 and n=9, respectively) PCa, locally-advanced, including
T3N0M0 (n=10) PCa, and metastatic,
including
T2NxM1/N1M1
and T3N1M1 (n=3 and n=4,
respectively) PCa.
 | Table I.Clinical characteristics of
patients. |
Table I.
Clinical characteristics of
patients.
| Variables | PCa, n=31 (%) | Control, n=33 |
|---|
| Age, years |
|
Median | 66 | 62 |
|
Range | 29–82 | 37–88 |
| Tumor stage (TNM
classification) |
|
T1 | 5 (16) | – |
|
T2N0M0/NxM0 | 9 (29) | – |
|
T3N0M0 | 10 (32) | – |
|
T2NxM1/N1M1 | 3 (10) | – |
|
T3N1M1 | 4 (13) | – |
| PSA |
|
Median | 37.7 | 2.0 |
|
Range | 5.9–223.0 | 0.2–4.1 |
| Gleason score |
| 6 | 10 (32) | – |
| 7 | 11 (36) | – |
| 8 | 3 (10) | – |
| 9 | 2 (6) | – |
| 9 | 5 (16) | – |
|
Unknown | 5 (16) | – |
UcfDNA isolation
Voided urine (50 ml) was harvested from patients
following prostate massage on the previous day of definitive
surgery. Each sample was spun at 3,000 × g for 10 min at room
temperature, the supernatant was removed, and the pellet was washed
twice with 1X PBS. The resultant pellet was cryopreserved at −80°C.
Genomic DNA was extracted using a Quick-gDNA MiniPrep kit (Zymo
Research Corp., Irvine, CA, USA), according to the manufacturer's
protocol. The quality of isolated DNA was checked by 3% agarose gel
electrophoresis. For the DNA concentration and purity measurements,
a spectrophotometer ND-2000 (NanoDrop Technologies; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was utilized.
Bisulfite treatment and
methylation-specific real-time polymerase chain reaction (MSP)
Extracted UcfDNA was subjected to bisulfite
conversion using an EZ DNA Methylation kit (Zymo Research Corp.),
according to manufacturer's protocol. MSP was conducted using 34
pairs of forward and reverse primers of methylated or unmethylated
type. Nucleotide sequences are presented in Table II. All primers were designed with
MethPrimer 2.0 online software (The Li Lab, Beijong, China;
http://www.urogene.org/methprimer) and
their performance was evaluated using 6% polyacrylamide gel
electrophoresis. The size of the polymerase chain reaction products
was within the range of 87–263 bp. Each reaction mix contained 2.5
µl 10X DreamTaq buffer (Thermo Fisher Scientific, Inc.), 0.3 mM
primers, 100 ng UcfDNA previously subjected to bisulfite conversion
and nuclease-free water to a final volume of 25 µl. MSP was
performed using thermocycler CFX96 Real-Time system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), according to the following
protocol: Initial 12-min incubation at 95°C, followed by 40 cycles
of denaturation for 15 sec at 95°C, annealing for 30 sec at 60°C
and extension for 30 sec at 72°C.
 | Table II.Primers used for methylation-specific
polymerase chain reaction. |
Table II.
Primers used for methylation-specific
polymerase chain reaction.
| Genes | Forward 5′-3′ | Reverse 5′-3′ | Product size
(bp) |
|---|
| APC2 |
| M |
ATTTCGGGTCGGGATTTTC |
GCTTACGTACAACTAAACTAACG | 135 |
| U |
GTTGTTTGTATTTGTTTGTTTTTGA |
AAACATAACCTTAAACTCCCCACT | 136 |
| CDH1 |
| M |
GGTTTTGACGTCGAGAGTTATAC |
TACGTAAATTCCAAAAAATATCGTT | 211 |
| U |
TTTGGTTTTGATGTTGAGAGTTATATG |
TACATAAATTCCAAAAAATATCATT | 214 |
| FOXP1 |
| M |
CGGAGTTCGGAAAATTTAAATACGT |
GTCTCGAAAAAACGAAAACCGA | 87 |
| U |
TGGAGTTTGGAAAATTTAAATATGT |
TCATCTCAAAAAAACAAAAACCAAA | 89 |
| FOXP2 |
| M |
CGTTTTTTCGAGGAGAGGTAGTTTC |
GCGCGCGTATTATTAACAATACG | 101 |
| U |
TGTTTTTTTGAGGAGAGGTAGTTTT |
ACACACATATTATTAACAATACAAA | 103 |
| FOXP3 |
| M |
GGATAGGGTAGTTAGTTTTCGGAAC |
GAATACGCCGAACTTCATCGA | 93 |
| U |
ATAGGGTAGTTAGTTTTTGGAATGA |
ACCAAATACACCAAACTTCATCAAC | 94 |
| FOXP4 |
| M |
TTCGTAGTTATTCGTAGTTTAGGTTTAGTC |
TCGCGAACTAAAAACTCCGT | 120 |
| U |
TTGTAGTTATTTGTAGTTTAGGTTTAGTTG |
TCCTCACAAACTAAAAACTCCATCC | 122 |
| HIC1 |
| M |
TTTTATTAGTAATTTAATTCGAATAGCGTC |
AACCGCAATCCTAAAAATCG | 138 |
| U |
TATTAGTAATTTAATTTGAATAGTGTTGG |
TACAAAACCACAATCCTAAAAATCAC | 140 |
| HOXA9 |
| M |
ATCACCTAATAAATTAACCGACG |
TCGGATTATTAATAGCGTGC | 101 |
| U |
TGTAGTTTTTAGTTTAAGGTGATGG |
AATAATAATAATACACCACAACAAA | 100 |
| LRRC3B |
| M |
GGTGCGAGGAAGGTAGGC |
ACCAATACCTCGCCGACG | 222 |
| U |
TGGTGTAAGGTAAGGTGTAGTTGT |
AAACAAAAACAAAAAAAATCAAC | 217 |
| MGMT |
| M |
CGTTTGTAGTTGAGTAAGTATGAGTTTAG |
AAACGACCCTAAATTCATCGAAAA | 263 |
| U |
GTTTTGGATATGTTGGGATAGTTTG |
ACACCTAAAAAACACTTAAAACACA | 261 |
| NDRG4 |
| M |
GGTATTTTAGTCGCGTAGAAGGC |
GTACCCGCGTAAATTTAACGAA | 119 |
| U |
GTTAGATAGGTGGGTTTTGTAGATG |
CAAATCAAAACTAAAACAAAAACAC | 120 |
| PLCL2 |
| M |
GTATTTTTTTTCGGGAGAGTAAGTC |
CCAAAAACGACTAAAAATAAACGAT | 105 |
| U |
TTTTTTTGGGAGAGTAAGTTGG |
CCAAAAACAACTAAAAATAAACAAT | 100 |
| PTEN |
| M |
TTTTTTTATTTCGTTGTCGTCGT |
TTAACGATAACTAATACCCCTCGC | 155 |
| U |
TTTTTTTTATTTTGTTGTTGTTGT |
TTAACAATAACTAATACCCCTCACT | 156 |
| UBE2E2 |
| M |
ATTAGACGGTTCGTAGGGGATATTTC |
ATATCCGTACAAATCGCAAACTCGA | 180 |
| U |
GAGATTGAGATTATGGTGAAATTTT |
ACCCAAACTAAAATACAATAACACA | 181 |
| VHL |
| M |
TTATTCGGGAGGTTGAGGCGAGAC |
CGCAAAAAAATCCTCCAACACCGTAA | 103 |
| U |
AGGTAGGATATATTTAGGGTGATGT |
ACTCCAACCTAAACAACAAAACAA | 105 |
| WNT7A |
| M |
CGAAACCGTCTATCGATACG |
GTAGTTCGGCGTCGTTTTAC | 179 |
| U |
TTTTTTGATGTATATTAGGTTTGT |
CTAAACCACACTACCACAATTTCAA | 178 |
| ZIC4 |
| M |
GTTGTAGCGATAAGGTAGGAGTTTC |
CCACTTTAACGAAATAAAAATCGAT | 202 |
| U |
TGTAGTGATAAGGTAGGAGTTTTGG |
CCACTTTAACAAAATAAAAATCAAT | 200 |
Statistical analysis
Statistical analysis was performed using STATISTICA
7.0 software (StatSoft, Inc., Tulsa, OK, USA). Since the data
obtained were not normally distributed, it was analyzed with a
non-parametric approach. P<0.05 was considered to indicate a
statistically significant difference. The comparison of PCa and
control groups for the methylation frequency in the 17 genes was
conducted with Mann-Whitney U-test. The Spearman's rank correlation
coefficient with Bonferroni correction for multiple hypothesis
testing was used to calculate correlation between methylation rate
and patient's age or tumor stage. For this evaluation, the
variables were measured on the ordinal scale. In the correlation
analysis with regards to age, patients with PCa and controls were
involved, while during the correlation analysis with regards to
stage, only patients with cancer were taken into account. A
resulting R-value >0.3 along with P<0.05 was considered to
indicate a positive correlation between the values examined. The
comparison of the control and five cancer groups after the NGM
value was calculated was performed using the Kruskal-Wallis test
with Conover's post-hoc analysis adjusted by the Benjamini-Hochberg
false discovery rate method. The results are presented as the means
± standard error of the mean.
Results
Examination of the methylation status
of 17 genes in PCa and control groups
The methylation frequency of 17 gene promoters was
evaluated in urine samples from patients with PCa and the control
group (Table III). The increase in
methylation frequency in tumor samples was the lowest for
FOXP2 (3.03–6.45%) and the highest for ZIC4
(6.06–58.06%) genes, compared with controls. Among the panel
analyzed, 8 genes, including WNT7A, LRRC3B, FOXP3, FOXP4, CDH1,
HOXA9, NDRG4 and PTEN, were not methylated in all of the
control samples. However, aforementioned genes were indicated to be
methylated to different extents in prostate tissues from patients
with PCa. On the contrary, 9 genes, including Von Hippel-Lindau
tumor suppressor (VHL), FOXP1, FOXP2, APC2, ZIC4,
phospholipase C like 2 (PLCL2), HIC1, ubiquitin
conjugating enzyme E2 (UBE2E2) and MGMT were
identified to be methylated in control and cancer groups. A
statistically significant difference was indicated in the
methylation status of the following 13 genes: WNT7A, LRRC3B,
FOXP1, FOXP3, FOXP4, APC2, ZIC4, CDH1, HOXA9, NDRG4, PTEN, MGMT
and HIC1. All these genes, except HIC1, had a
methylation frequency between 0–12% in the control samples, while
in the PCa samples methylation frequency was between 13–58%. The
HIC1 gene demonstrated a notable methylation frequency even
in control samples (~39%) and for this reason HIC1 was
excluded in subsequent analyses. The difference in methylation
status of VHL, FOXP2, PLCL2, and UBE2E2 genes was
indicated to be statistically insignificant between cancer and
control individuals (Table
III).
 | Table III.Methylation status of 17 genes
determined in urine. |
Table III.
Methylation status of 17 genes
determined in urine.
|
| Methylation
frequency, % | Age correlation
(R-value) |
|
|---|
|
|
|
|
|
|---|
| Genes | Control | PCa | U-test
(P-value) | Control | PCa | Stage correlation
for PCa group (R-value) |
|---|
|
APC2a | 12.12 | 35.48 | 0.03a | 0.21 | 0.23 | 0.32b |
|
CDH1a | 0.00 | 48.39 |
<0.01a | 0.00 | 0.24 | 0.63b |
|
FOXP1a | 9.09 | 58.06 |
<0.01a | 0.22 | −0.20 | 0.41b |
| FOXP2 | 3.03 | 6.45 | 0.81 | 0.04 | 0.07 | −0.05 |
| FOXP3 | 0.00 | 32.26 | 0.03a | 0.00 | −0.13 | 0.03 |
| FOXP4 | 0.00 | 12.90 | 0.03a | 0.00 | −0.24 | 0.16 |
| HIC1 | 39.39 | 90.32 |
<0.01a | 0.06 | −0.10 | 0.47b |
| HOXA9 | 0.00 | 35.48 |
<0.01a | 0.00 | −0.09 | −0.01 |
|
LRRC3Ba | 0.00 | 19.35 | 0.01a | 0.00 | −0.06 | 0.31b |
| MGMT | 3.03 | 38.71 |
<0.01a | −0.20 | −0.18 | 0.01 |
| NDRG4 | 0.00 | 32.26 |
<0.01a | 0.00 | 0.12 | 0.20 |
| PLCL2 | 24.24 | 38.71 | 0.22 | 0.36b | −0.18 | 0.22 |
| PTEN | 0.00 | 22.58 |
<0.01a | 0.00 | 0.06 | 0.05 |
| UBE2E2 | 3.03 | 9.68 | 0.28 | 0.18 | 0.11 | −0.20 |
| VHL | 6.06 | 22.58 | 0.06 | −0.30b | 0.23 | 0.37b |
|
WNT7Aa | 0.00 | 41.94 |
<0.01a | 0.00 | 0.14 | 0.50b |
|
ZIC4a | 6.06 | 58.06 |
<0.01a | −0.08 | 0.22 | 0.41b |
The formation of the 6-gene diagnostic/prognostic
panel based on correlation analysis. The correlation between the
methylation of the considered genes and tumor stage was evaluated
using the Spearman's rank test. A positive correlation was
identified in the following 14 genes: VHL, WNT7A, LRRC3B, FOXP1,
FOXP3, FOXP4, APC2, ZIC4, PLCL2, CDH1, HIC1, NDRG4, PTEN and
MGMT. The genes FOXP3, FOXP4, NDRG4, PTEN and
MGMT were excluded from subsequent analyses, due to a weak
correlation with the disease stage. Furthermore, VHL was
also excluded due to low statistical significance (P>0.05),
following Bonferroni correction, in addition to a number of genes,
including FOXP3, FOXP4 and PTEN, indicating a weak
correlation. In contrast, 3 genes, FOXP2, HOXA9 and
UBE2E2, indicated negative values of correlation
coefficient. However, they were also rejected due to the low
correlation with tumor stage.
Since PCa is a relatively slow-growing cancer, the
actual age-associated methylation status dynamics may be
misinterpreted as the respective alterations caused by tumor
progression (6). Therefore, the
correlation between patient's age with methylation of the gene
panel investigated was also assessed (Table III). In the control group, 8/17
genes indicated no correlation with age (WNT7A, LRRC3B, FOXP3,
FOXP4, CDH1, HOXA9, NDRG4 and PTEN), 8 demonstrated weak
correlation (VHL, FOXP1, FOXP2, APC2, ZIC4, HIC1, UBE2E2 and
MGMT) and 1 exhibited moderate correlation (PLCL2).
In the cancer group all genes were indicated to be weakly
correlated with age. All 10 genes that indicated moderate
correlation with tumor stage (VHL, WNT7A, LRRC3B, FOXP1, FOXP3,
FOXP4, APC2, ZIC4, CDH1 and HIC1) demonstrated a notably
weaker correlation with age (Table
III).
Subsequently, the genes WNT7A, LRRC3B, FOXP1,
APC2, ZIC4 and CDH1 were selected to form a panel for
PCa diagnosis in urine samples, according to the following four
criteria: Low methylation frequency in control samples,
statistically significant increase of methylation frequency in
patients with PCa, notable correlation with PCa progression, and 0
or weak correlation with age.
PCa detection and determination of the
tumor stage by ‘number of genes methylated’ (NGM) approach
For the interpretation of the results obtained with
the 6-gene panel, the ‘number of genes methylated’ (NGM) value
ranging from 0 to 6 was introduced. With the cut-off value
established at 2, the panel provided PCa detection with 78%
sensitivity and 100% specificity. In the control group NGM values
were 0 for 24 patients and 1 for 9 patients, while none of them had
≥2 methylated genes (Table IV). The
NGM values of patients with PCa were uniformly distributed within
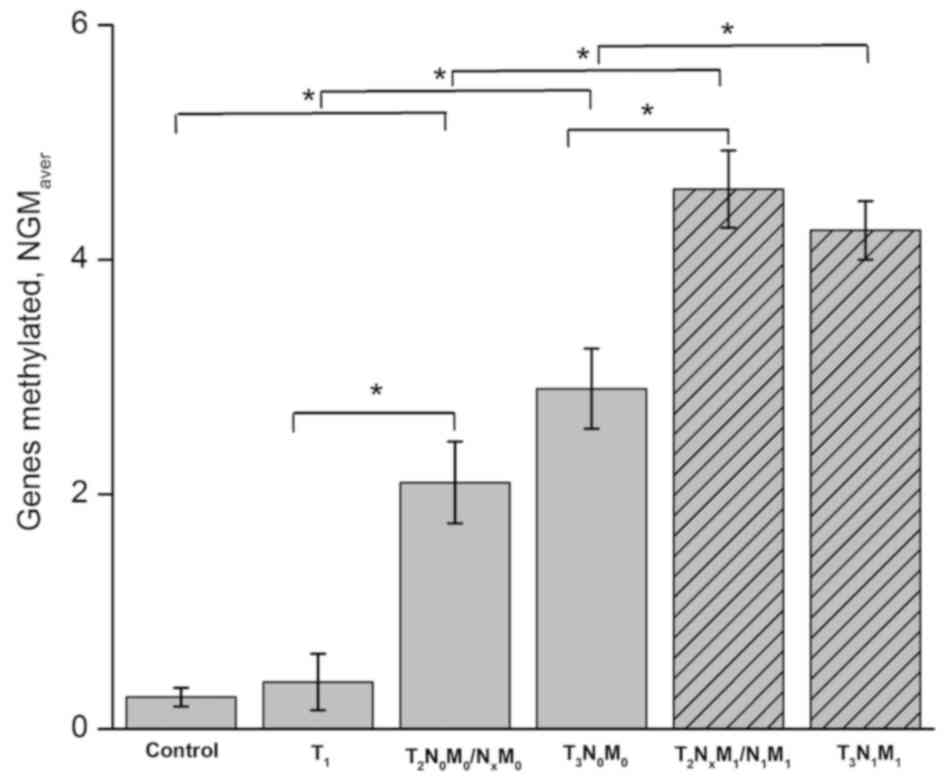
the range of 0–5 reflecting the tumor stage. The mean NGM value
determined for each PCa stage group (NGMaver) was
indicated to almost monotonously rise from 0.27–4.25 along with
tumor progression and/or increase of its metastatic potential
(Fig. 1). In particular, in the
group with the least developed T1 stage cancer the NGM
values overlapped with those in controls (0–1); however, it
included more NGM 1 values, reaching 40% of the total number
(Table V). Among the patients with
non-metastatic PCa of T2/T3 stages, 15/19
patients indicated NGM values between 2–4, while only 4 patients
had NGM values between 0–1, as indicated in the control and
T1 groups. Furthermore, none of the patients with
metastatic cancer of T2/T3 stages
demonstrated an NGM value <4, and in a number of cases an NGM
value of 5 was reached.
 | Table IV.The methylation of genes from 6-gene
panel in PCa and controls. |
Table IV.
The methylation of genes from 6-gene
panel in PCa and controls.
|
| Patients (n) |
|---|
|
|
|
|---|
| Number of genes
methylated | Control | PCa |
|---|
| 0 | 24 | 3 |
| 1 | 9 | 6 |
| 2 | – | 6 |
| 3 | – | 4 |
| 4 | – | 9 |
| 5 | – | 3 |
| 6 | – | – |
| Total | 33 | 31 |
 | Table V.The number of genes methylated values
in patients with different stages of prostate cancer. |
Table V.
The number of genes methylated values
in patients with different stages of prostate cancer.
|
| T1 |
T2N0M0/NxM0 |
T3N0M0 |
T2NxM1/N1M1 |
T3N1M1 |
|---|
|
|
|
|
|
|
|
|---|
| Panel genes
methylated | Samples. n | % | Samples. n | % | Samples. n | % | Samples. n | % | Samples. n | % |
|---|
| 0 | 3 | 60 | – | – | – | – | – | – | – | – |
| 1 | 2 | 40 | 3 | 33 | 1 | 10 | – | – | – | – |
| 2 | – | – | 3 | 33 | 3 | 30 | – | – | – | – |
| 3 | – | – | 2 | 22 | 2 | 20 | – | – | – | – |
| 4 | – | – | 1 | 12 | 4 | 40 | 1 | 33 | 3 | 75 |
| 5 | – | – | – | – | – | – | 2 | 67 | 1 | 25 |
| 6 | – | – | – | – | – | – | – | – | – | – |
| Total | 5 |
| 9 |
| 10 |
| 3 |
| 4 |
|
The NGM approach was further tested
for its prognostic potential
Although the resolving power of this method was not
adequate to discriminate between all 5 PCa and 1 control groups, it
provided the discrimination between at least three categories of
cases: controls and early cancer (T1), developed cancer
without metastases
(T2N0M0/NxM0
and T3N0M0) and developed
metastatic PCa
(T2NxM1/N1M1
and T3N1M1) (Fig. 1). The NGMaver of the
control and T1 groups was 0.27±0.07 and 0.40±0.24,
respectively, with no significant difference between the two
groups, but with significant differences determined between
neighboring groups without metastases,
T2N0M0/NxM0
and T3N0M0, (P=0.02 and P=0.0009
between T1/T2 and T1/T3
groups, respectively). The latter groups also demonstrated an
NGMaver of 2.10±0.35 and 2.90±0.34, respectively, and
could have not been statistically separated from each other, but
differed from metastatic groups
T2NxM1/N1M1
and T3N1M1 (P=0.0009 and P=0.001
comparing non-metastatic T2 and respective groups with
metastases; P=0.01 and P=0.02 comparing non-metastatic
T3 and the respective groups with metastases). Finally,
the patients with invasive cancer
T2NxM1/N1M1
and T3N1M1 exhibited
NGMaver of 4.60±0.33 and 4.25±0.25, respectively, and
exhibited no statistically significant difference between each
other. Collectively, these data indicated that NGM value could be
used to discriminate between PCa of three types: Early
T1 cancer; T2/T3 cancer without
metastases; and the highly invasive metastatic
T2/T3 cancer stages.
Discussion
The present study identified 13 tumor suppressor
genes undergoing aberrant methylation in prostate cancer, where 4
of these genes have no prior reported association with PCa
pathogenesis (24–26). Furthermore, the present study
proposes the diagnostic and prognostic panel of 6 genes, which may
be used for non-invasive PCa detection and prognosis by
non-invasive UcfDNA analysis.
Additionally, the correspondence of polymerase chain
reaction data obtained from tissue samples and urine was examined.
Despite the fact that the present study did not question the
reliability of MSP analysis conducted on urine samples without
examining the tumor per se, previous aforementioned advanced
techniques made it possible to detect methylation events in urine,
regardless of apparent UcfDNA fragmentation and its comparatively
low amount (20). In a similar
investigation, including the parallel examination of PCa tumor
samples, it was demonstrated that a positive signal for a specific
biomarker could be registered from urine in 85% of cases compared
with the solid tissue samples (21).
Furthermore, the urine was indicated to be the most amenable source
of UcfDNA for cancer detection if compared with other types of
bodily fluids, including blood and semen (21).
Novel genes associated with PCa were examined and
the data of the present study indicated that the genes APC2,
HOXA9, WNT7A and NDRG4 are associated with PCa
pathogenesis. To the best of our knowledge, this has never been
demonstrated on a DNA sequence alteration level or in epigenetic
events. No methylation was indicated in HOXA9, WNT7A and
NDRG4 genes in any of the control samples, while APC2
was methylated in 12% of the control samples. In the PCa samples
methylation frequencies of aforementioned genes reached 35, 42, 32
and 35%, respectively. It should be noted that the actual
methylation rate of these genes in PCa may be even higher when
determined in the tumor samples themselves, due to seemingly
increased DNA integrity and amount in solid tissue, compared with
the UcfDNA pool (17). Further
detailed investigation is required to clarify the significance of
these genes for PCa pathogenesis.
Therefore, the present study proposed a 6-gene panel
consisting of APC2, CDH1, FOXP1, LRRC3B, WNT7A and
ZIC4 allowing PCa detection with 78% sensitivity and 100%
specificity. In a number of previous studies, other individual
genes or their respective panels were considered as candidate PCa
biomarkers for urine-based polymerase chain reaction diagnostics
(20–21,33–35). In
the present study, promoter hypermethylation in the GSTP1
gene, the most frequent molecular event in PCa pathogenesis
occurring in 80–90% of its cases, was detected in only 27% of urine
samples, while the respective biopsy samples indicated methylation
frequency of 79%, which is consistent with previous reports
(12,20). Although the sensitivity of this test
appeared to be inadequate for clinical application, it demonstrated
the practical feasibility of urine polymerase chain reaction/MSP
examination for PCa diagnosis (20).
In another study, it was reported that the detection rate in UcfDNA
analysis may be increased notably by selecting more appropriate
experimental conditions: When using a DNA isolation kit developed
for blood/tissue samples, the study reported GSTP1 promoter
hypermethylation in only 36% of urine samples from patients with
PCa, but switching to a viral kit allowed the detection of this
molecular event in 76% of cases investigated (21). Furthermore, the use of differential
display code 3 (DD3PCA3), a gene expressing a
non-coding RNA highly specific for prostate tissue, was proposed as
a non-invasive PCa detection method. The diagnostic test based on
the quantitative determination of its transcripts in urine was
reported to exhibit 67% sensitivity and a 90% negative predictive
value (33). In another study, the
use of the p16, p14ARF, MGMT and
GSTP1 gene combination, instead of focusing on a single
gene, provided a diagnostic test with 87% sensitivity along with
100% specificity (34).
Additionally, a gene panel consisting of GSTP1, Ras
association domain family member 1A, retinoic acid receptor β 2 and
APC1 was proposed for detection of localized PCa, yielding a
sensitivity and accuracy of 86 and 89%, respectively (35).
The main advantage of the 6-gene panel proposed in
the present study over the aforementioned is the prognostic value
it may bear, as the tumor stage could be at least roughly
determined based on a NGM value between 0–6. NGMaver
values calculated in the cohort rose monotonically from the control
group, 0.27, to groups with T2 and T3
metastatic cancer types, 4.6 and 4.25, respectively. This indicated
an increased probability of identifying malignant and metastatic
tumor types in a patient with an increased individual NGM value.
The number of genes included in this 6-gene panel is increased,
compared with the 4-gene panels proposed by Hoque et al
(34) and Roupret et al
(35), which indicates a
disadvantage in terms of cost-efficiency; however, it is necessary
for distinguishing between PCa cases of different stages and
metastatic potential. It is also important that a 6-gene set is
amenable so that it can be applied in a single-tube multiplex
polymerase chain reaction screen, providing maximal cost-efficiency
and convenience. The cut-off NGM value of 2 was selected as a
diagnostic criterion of PCa, since the NGM values of individuals
without a diagnosed prostate malignancy and T1 cancer
extensively overlapped in the range of 0–1. The aforementioned
disadvantage is mitigated by the fact that prostate cancer at
T1 stage is usually a small localized slow-growing tumor
frequently left untreated, due to its asymptomatic nature, and the
additional age-associated health problems that the patient with PCa
may exhibit.
The main limitation of the present study is the
relatively small patient cohort, including 31 individuals with PCa,
while other studies of this type usually involve a broader cohort
of patients, including 67 PCa cases in the study by Salvi et
al (22), and 52 cases in the
study by Hoque et al (34).
Nevertheless, the number of patients involved in the present study
allowed the identification of methylation for 13 genes in PCa, and
the establishment of the correlation with tumor stage for 8 of them
with adequate statistical significance. Therefore, although the
6-gene panel proposed in the present study may not be yet
applicable in a clinical setting, it could still be potentially
used for this purpose following additional verification.
Furthermore, separate genes from the selected panel, whose
methylation frequency, according to the present data, was increased
in the PCa group, compared with controls, could be introduced in
other detecting or prognostic panels. The same applies for the
comparison of cancer subgroups with different PCa stages using the
NGM value parameter. Although the NGM value tends to rise with
tumor development, the application of this tool for clinical use
requires more precise determination of the correspondence between
its particular values and the PCa stage.
Furthermore, the prognostic potential of the NGM
approach has its own limitations. Clinical conclusions drawn can
only be of a probabilistic nature, according to the present data,
as NGM values obtained from patients with close PCa stages
partially overlapped. However, at more distant tumor stages, NGM
values significantly differed, including 0–1 at T1 and
4–5 at late metastatic stages. Therefore, it was demonstrated that
the NGM approach serves as a valuable tool for further development
of the panel proposed here or other gene panels designed for PCa
prognosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by Science and
Technology Center of Ukraine (grant no. 6056). The funding body had
no role in the study design, data collection, its analysis and
interpretation and article writing.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request
Authors' contributions
MVV, IVI, VMG and ROD provided clinical material
from patients with prostate cancer and control individuals. AGK and
KAN designed the initial panel of cancer-associated genes and
performed primer design. KAN and EER conducted the UcfDNA isolation
and its MSP analysis. LAS, BRS and VIK conducted data analysis and
wrote the article.
Ethics approval and consent to
participate
The study was approved by local Ethics Committee of
the Institute of Molecular Biology and Genetics of the National
Academy of Sciences of Ukraine (approval no. 18/4 from 15th July
2016). All patients provided written informed consent to take part
in the present study including further publication of the results
obtained.
Patient consent for publication
All patients provided written informed consent for
this study.
Competing interests
The authors declare no conflict of interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancer in Ukraine 2012–2013, . Bulletin of
National Cancer Registry of Ukraine №15. 17–10. 2017
|
|
3
|
Rao AR, Motiwala HG and Karim OM: The
discovery of prostate-specific antigen. BJU Int. 101:5–10.
2008.PubMed/NCBI
|
|
4
|
Nogueira L, Corradi R and Eastham JA:
Prostatic specific antigen for prostate cancer detection. Int Braz
J Urol. 35:521–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loeb S and Catalona WJ: Prostate-specific
antigen in clinical practice. Cancer Lett. 249:30–39. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Walter LC, Fung KZ, Kirby KA, Shi Y,
Espaldon R, O'Brien S, Freedland SJ, Powell AA and Hoffman RM:
Five-year downstream outcomes following prostate-specific antigen
screening in older men. JAMA Intern Med. 173:866–873. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thompson IM, Pauler DK, Goodman PJ, Tangen
CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford
ED, et al: Prevalence of prostate cancer among men with a
prostate-specific antigen level < or =4.0 ng per milliliter. N
Engl J Med. 350:2239–2246. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sunami E, Shinozaki M, Higano CS, Wollman
R, Dorff TB, Tucker SJ, Martinez SR, Mizuno R, Singer FR and Hoon
DS: Multimarker circulating DNA assay for assessing blood of
prostate cancer patients. Clin Chem. 55:559–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frank S, Nelson P and Vasioukhin V: Recent
advances in prostate cancer research: Large-scale genomic analyses
reveal novel driver mutations and DNA repair defects. F1000Res.
7(pii): F1000 Faculty Rev. –1173. 2018.
|
|
10
|
Barbieri CE, Bangma CH, Bjartell A, Catto
JW, Culig Z, Grönberg H, Luo J, Visakorpi T and Rubin MA: The
mutational landscape of prostate cancer. Eur Urol. 64:567–576.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Massie CE, Mills IG and Lynch AG: The
importance of DNA methylation in prostate cancer development. J
Steroid Biochem Mol Biol. 166:1–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee WH, Isaacs WB, Bova GS and Nelson WG:
CG island methylation changes near the GSTP1 gene in prostatic
carcinoma cells detected using the polymerase chain reaction: A new
prostate cancer biomarker. Cancer Epidemiol Biomarkers Prev.
6:443–450. 1997.PubMed/NCBI
|
|
13
|
Li LC: Epigenetics of prostate cancer.
Front Biosci. 12:3377–3397. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wan JCM, Massie C, Garcia-Corbacho J,
Mouliere F, Brenton JD, Caldas C, Pacey S, Baird R and Rosenfeld N:
Liquid biopsies come of age: Towards implementation of circulating
tumour DNA. Nat Rev Cancer. 17:223–238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salvi S, Martignano F, Molinari C, Gurioli
G, Calistri D, De Giorgi U, Conteduca V and Casadio V: The
potential use of urine cell free DNA as a marker for cancer. Expert
Rev Mol Diagn. 16:1283–1290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zancan M, Galdi F, Di Tonno F, Mazzariol
C, Orlando C, Malentacchi F, Agostini M, Maran M, Del Bianco P,
Fabricio AS, et al: Evaluation of cell-free DNA in urine as a
marker for bladder cancer diagnosis. Int J Biol Markers.
24:147–155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su YH, Wang M, Brenner DE, Ng A, Melkonyan
H, Umansky S, Syngal S and Block TM: Human urine contains small,
150 to 250 nucleotide-sized, soluble DNA derived from the
circulation and may be useful in the detection of colorectal
cancer. J Mol Diagn. 6:101–107. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia Y, Huang CC, Dittmar R, Du M, Wang Y,
Liu H, Shenoy N, Wang L and Kohli M: Copy number variations in
urine cell free DNA as biomarkers in advanced prostate cancer.
Oncotarget. 7:35818–35831. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bryzgunova OE, Morozkin ES, Yarmoschuk SV,
Vlassov VV and Laktionov PP: Methylation-specific sequencing of
GSTP1 gene promoter in circulating/extracellular DNA from blood and
urine of healthy donors and prostate cancer patients. Ann N Y Acad
Sci. 1137:222–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cairns P, Esteller M, Herman JG,
Schoenberg M, Jeronimo C, Sanchez-Cespedes M, Chow NH, Grasso M, Wu
L, Westra WB and Sidransky D: Molecular detection of prostate
cancer in urine by GSTP1 hypermethylation. Clin Cancer Res.
7:2727–2730. 2001.PubMed/NCBI
|
|
21
|
Goessl C, Müller M, Heicappell R, Krause
H, Straub B, Schrader M and Miller K: DNA-based detection of
prostate cancer in urine after prostatic massage. Urology.
58:335–338. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Salvi S, Gurioli G, Martignano F, Foca F,
Gunelli R, Cicchetti G, De Giorgi U, Zoli W, Calistri D and Casadio
V: Urine cell-free DNA integrity analysis for early detection of
prostate cancer patients. Dis Markers. 2015:5741202015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takayama K, Suzuki T, Tsutsumi S, Fujimura
T, Takahashi S, Homma Y, Urano T, Aburatani H and Inoue S:
Integrative analysis of FOXP1 function reveals a tumor-suppressive
effect in prostate cancer. Mol Endocrinol. 28:2012–2024. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Calvo R, West J, Franklin W, Erickson P,
Bemis L, Li E, Helfrich B, Bunn P, Roche J, Brambilla E, et al:
Altered HOX and WNT7A expression in human lung cancer. Proc Natl
Acad Sci USA. 97:12776–12781. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bhatlekar S, Fields JZ and Boman BM: HOX
genes and their role in the development of human cancers. J Mol Med
(Berl). 92:811–823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Daly CS, Shaw P, Ordonez LD, Williams GT,
Quist J, Grigoriadis A, Van Es JH, Clevers H, Clarke AR and Reed
KR: Functional redundancy between Apc and Apc2 regulates tissue
homeostasis and prevents tumorigenesis in murine mammary
epithelium. Oncogene. 36:1793–1803. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Phin S, Moore MW and Cotter PD: Genomic
rearrangements of PTEN in prostate cancer. Front Oncol. 3:2402013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding W, Zhang J, Yoon JG, Shi D, Foltz G
and Lin B: NDRG4 is downregulated in glioblastoma and inhibits cell
proliferation. OMICS. 16:263–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang GH, Lee S, Lee HJ and Hwang KS:
Aberrant CpG island hypermethylation of multiple genes in prostate
cancer and prostatic intraepithelial neoplasia. J Pathol.
202:233–240. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kondratov AG, Stoliar LA, Kvasha SM,
Gordiyuk VV, Zgonnyk YM, Gerashchenko AV, Vozianov AF, Rynditch AV,
Zabarovsky ER and Kashuba VI: Methylation pattern of the putative
tumor-suppressor gene LRRC3B promoter in clear cell renal cell
carcinomas. Mol Med Rep. 5:509–512. 2012.PubMed/NCBI
|
|
31
|
Chen N and Zhou Q: The evolving Gleason
grading system. Chin J Cancer Res. 28:58–64. 2016.PubMed/NCBI
|
|
32
|
Schröder FH, Hermanek P, Denis L, Fair WR,
Gospodarowicz MK and Pavone-Macaluso M: The TNM classification of
prostate cancer. Prostate Suppl. 4:129–138. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hessels D, Klein Gunnewiek JM, van Oort I,
Karthaus HF, van Leenders GJ, van Balken B, Kiemeney LA, Witjes JA
and Schalken JA: DD3(PCA3)-based molecular urine analysis for the
diagnosis of prostate cancer. Eur Urol. 44:8–16. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hoque MO, Topaloglu O, Begum S, Henrique
R, Rosenbaum E, Van Criekinge W, Westra WH and Sidransky D:
Quantitative methylation-specific polymerase chain reaction gene
patterns in urine sediment distinguish prostate cancer patients
from control subjects. J Clin Oncol. 23:6569–6575. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rouprêt M, Hupertan V, Yates DR, Catto JW,
Rehman I, Meuth M, Ricci S, Lacave R, Cancel-Tassin G, de la Taille
A, et al: Molecular detection of localized prostate cancer using
quantitative methylation-specific PCR on urinary cells obtained
following prostate massage. Clin Cancer Res. 13:1720–1725. 2007.
View Article : Google Scholar : PubMed/NCBI
|