Introduction
Although glioma is the most common type of primary
brain malignancy in adults, it is a rare disease that only affects
6 cases out of 100,000 people worldwide (1). Despite the low incidence rate, this
disease is considered a major cause of cancer-associated mortality
worldwide, due to the unacceptably high mortality rate. Glioma
causes seizures, headaches and progressive neurological disease,
but also leads to changes in personality and behavior (2). Tumor metastasis is the major cause of
failure in the treatment of glioma (3). The survival time of patients with
metastatic glioma can be significantly prolonged with proper
chemotherapy or radiation therapy (4). Therefore, proper treatment based on the
existence of tumor metastasis is critical.
Hypoxia-inducible factor 1-α (HIF1α) is a
transcriptional regulator that serves a central role in the
regulation of cellular and developmental responses to hypoxia
(5,6). It has been well established that
genetic alternations or hypoxia in cancer cells can lead to the
altered expression of HIF1α, and dysregulation of HIF1α further
promotes the development of cancer (7). It has been revealed that inhibition of
HIF1α signaling may serve as a promising target for cancer therapy
(8). AWPPH is a recently identified
lncRNA that, to date, has known functions only in bladder cancer
(9) and hepatocellular carcinoma
(10). Thus, the aim of the present
study was to determine the involvement of lncRNA AWPPH in glioma.
The present study revealed that AWPPH may contribute to the
metastasis of glioma. The actions of AWPPH in the metastasis of
glioma are likely to be achieved through the upregulation of
HIF1α.
Materials and methods
Cell lines and human specimens
Hs 683 (ATCC® HTB-138™) and CCD-25Lu
(ATCC® CCL-215™) human glioma cell lines were purchased
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). Cells from the two cell lines were cultured in
ATCC-formulated Eagle's minimum essential medium (catalog no.
30-2003) supplemented with 10% fetal bovine serum (FBS).
Plasma specimens were obtained from 66 patients (40
men and 24 women; age range, 54–72 years; mean age 64.4±5.1 years)
with glioma and 42 healthy volunteers (28 men and 14 women; age
range, 54–71 years; mean age, 64.2±5.0 years). The 66 patients with
glioma were diagnosed and treated in the Chinese People's
Liberation Army Rocket Force General Hospital (Beijing, China)
between January 2014 and March 2018. Among those patients, 32 had
non-metastatic glioma and 34 had metastatic glioma. Inclusion
criteria were: i) Patients diagnosed by pathological examinations;
ii) patients completely understood the experimental protocol and
provided written informed consent. Exclusion criteria were: i)
Patients who had other diseases present as well; ii) patients who
were treated by any strategies within 3 months before admission.
The 42 healthy volunteers received physical examinations in the
Chinese People's Liberation Army Rocket Force General Hospital
during the same period. No significant differences in age, sex or
living habits were identified among the non-metastatic glioma,
metastatic glioma and control groups. The basic information of the
three patient groups is presented in Table I. The present study was approved by
the Ethics Committee of the Chinese People's Liberation Army Rocket
Force General Hospital. All patients completely understood the
experiment protocol and provided written informed consent.
 | Table I.Basic information for the three groups
of participants. |
Table I.
Basic information for the three groups
of participants.
| Characteristics | Non-metastatic
glioma | Metastatic
glioma | Healthy control |
|---|
| Cases, n | 32 | 34 | 42 |
| Sex |
| Male,
n | 15 | 19 | 20 |
| Female,
n | 17 | 15 | 22 |
| Lifestyle |
| Smoking,
n (%) | 14 (43.8%) | 16 (47.1%) | 19 (45.2%) |
| Alcohol
consumption, n (%) | 17 (53.1%) | 17 (50.0%) | 19 (45.2%) |
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following total RNA extraction from plasma and Hs
683 and CCD-25Lu cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), reverse transcription
was performed to synthesize cDNA. A SYBR® Green
Quantitative RT-qPCR kit (Sigma-Aldrich; Merck KGaA) was used to
prepare all PCR systems. The PCR conditions were: 57 sec at 95°C,
followed by 40 cycles of 16 sec at 95°C and 38 sec at 57.5°C. The
primers used in the PCR were: Forward, 5′-CTGGATGGTCGCTGCTTTTTA-3′
and reverse, 5′-AGGGGGATGAGTCGTGATTT-3′ for human AWPPH; forward,
5′-TCCATTATGAGGCTGACCATC-3′ and reverse,
5′-CCATCCTCAGAAAGCACCATA-3′ for human HIF1α; forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′
for β-actin. All data were normalized using the 2−ΔΔCq
method (11).
Vectors, small interfering (si)RNAs
and cell transfection
AWPPH and HIF1α expression pIRSE2 vectors and empty
pIRSE2 vectors were purchased from GeneCopoeia, Inc.. HIF1α siRNA
(catalog no. AM16708) and Silencer® Negative Control #1
siRNA (catalog no. AM4611) were purchased from Thermo Fisher
Scientific, Inc. Cell transfection was performed using
Lipofectamine® 2000 Transfection Reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) with vectors at a concentration of
10 nM and siRNAs at 50 nM. In this experiment, the control was the
cells without transfection and the negative control was the cells
transfected with empty vectors or Silencer® Negative
Control #1 siRNA. Overexpression of AWPPH and HIF1α as well as
HIF1α siRNA silencing was confirmed using RT-qPCR. An
overexpression rate of 210–250% and a downregulation rate of 30–50%
was achieved prior to continuing experiments. Cells were
transfected 24 h prior to subsequent experiments.
Transwell migration and invasion
assay
Following transfection, cell migration and invasion
rates were measured using a Transwell migration and invasion assay.
The assays were performed according to the same protocol except
that the upper chamber was pre-coated with Matrigel (catalog no.
356234; EMD Millipore) prior to the invasion assay. Briefly, single
cell suspensions were prepared (5×104 cells/ml of medium
supplemented with 1% FBS). The upper chamber was filled with 0.1 ml
single cell suspension and culture medium containing 20% FBS was
added to the lower chamber. Cells were cultured for 12 h, followed
by membrane staining with 0.5% crystal violet (Sigma-Aldrich; Merck
KGaG) for 25 min at room temperature. Invading and migrating cells
were observed and counted using a light microscope (magnification,
40×).
Western blot analysis
Following total protein extraction using
radioimmunoprecipitation assay solution (Thermo Fisher Scientific,
Inc.) and protein quantification using BCA assay (Thermo Fisher
Scientific, Inc.), SDS-PAGE (12% gel) was performed with 20 µg
denatured protein in each well. Following gel transfer onto
polyvinylidene difluoride membranes, blocking was performed with 5%
skimmed milk in PBS for 1 h at room temperature. Subsequently,
membranes were incubated with primary antibodies against HIF1α
first (rabbit anti-human, 1:1,400; ab216842; Abcam, Cambridge, UK)
and GAPDH (rabbit anti-human, 1:1,400; ab9485; Abcam) at 4°C
overnight, followed by incubation with horseradish peroxidase
immunoglobulin secondary antibody (goat anti-rabbit, 1:1,300;
MBS435036; MyBioSource) for 1 h at room temperature. Signals were
developed using Pierce enhanced chemiluminescent Western Blot
substrate (Thermo Fisher Scientific, Inc.). Signals were detected
using a MYECL™ Imager (Thermo Fisher Scientific, Inc.) and
normalized using ImageJ software (version 1.6; National Institutes
of Health).
Statistical analysis
GraphPad Prism software (version 6; GraphPad
Software, Inc., La Jolla, CA, USA) was used for the statistical
analyses. All data were expressed as the mean ± standard deviation
and compared using a one-way analysis of variance followed by a
Tukey test. The diagnostic value of AWPPH for glioma was analyzed
using the receiver operating characteristic (ROC) curve analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
AWPPH is upregulated in metastatic
glioma but not in non-metastatic glioma
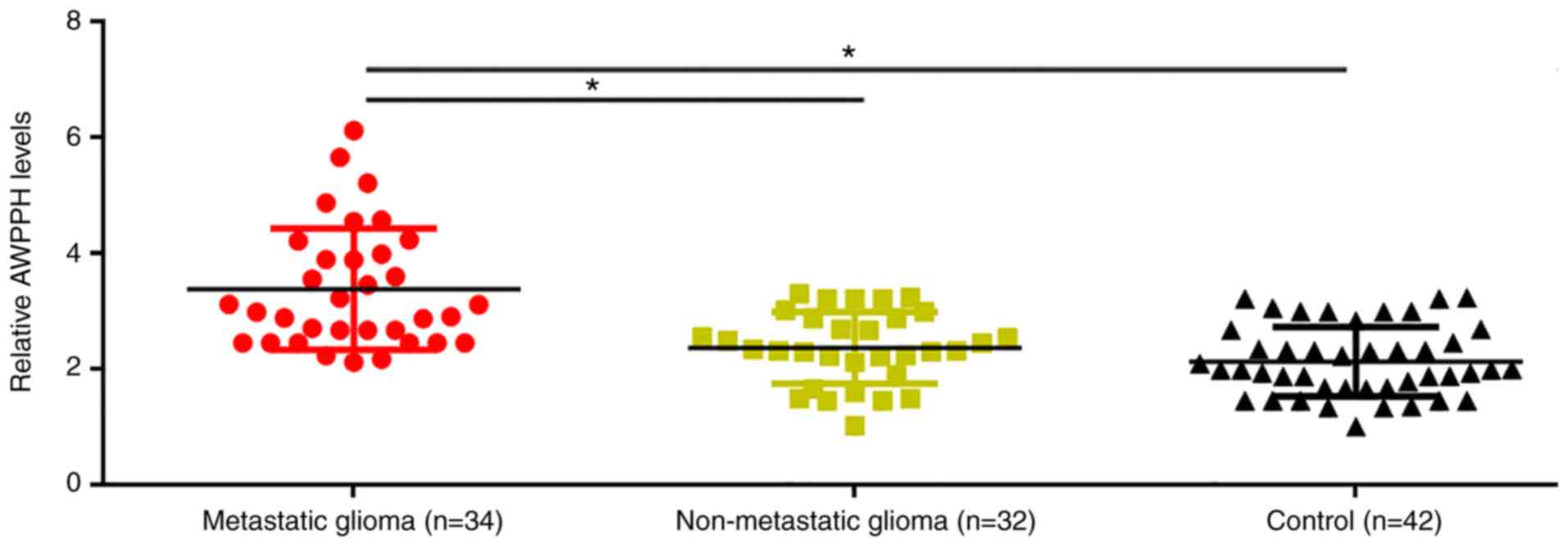
Plasma levels of AWPPH in patients with metastatic
glioma, patients with non-metastatic glioma and healthy controls
were determined using RT-qPCR. Compared with patients with
non-metastatic glioma and healthy controls, significantly increased
plasma levels of AWPPH were observed in patients with metastatic
glioma (P<0.05; Fig. 1). However,
no significant differences in plasma levels of AWPPH were
identified between patients with non-metastatic glioma and healthy
controls (Fig. 1).
Overexpression of AWPPH distinguishes
patients with metastatic glioma from patients with non-metastatic
glioma and healthy controls
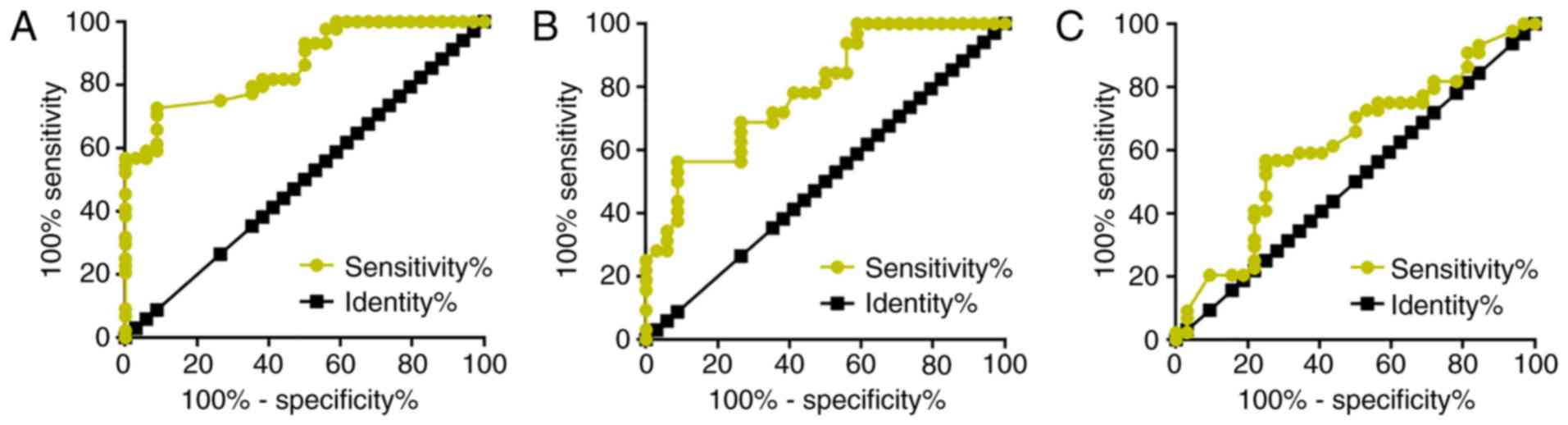
The diagnostic value of AWPPH for glioma was
analyzed by ROC curve analysis. For metastatic glioma with healthy
controls as references, the area under the curve (AUC) was 0.8640
[standard error, 0.03950; 95% confidence interval (CI),
0.7865–0.9414; P<0.0001; Fig.
2A]. For metastatic glioma with patients with non-metastatic
glioma as references, the AUC was 0.7881 (standard error, 0.05451;
95% CI, 0.6813–0.8950; P<0.0001; Fig.
2B). For non-metastatic glioma with healthy controls as
references, the AUC was 0.6186 (standard error, 0.06578; 95% CI,
0.4896–0.7476; P=0.07899).
AWPPH overexpression leads to
upregulated HIF1α expression in glioma cells
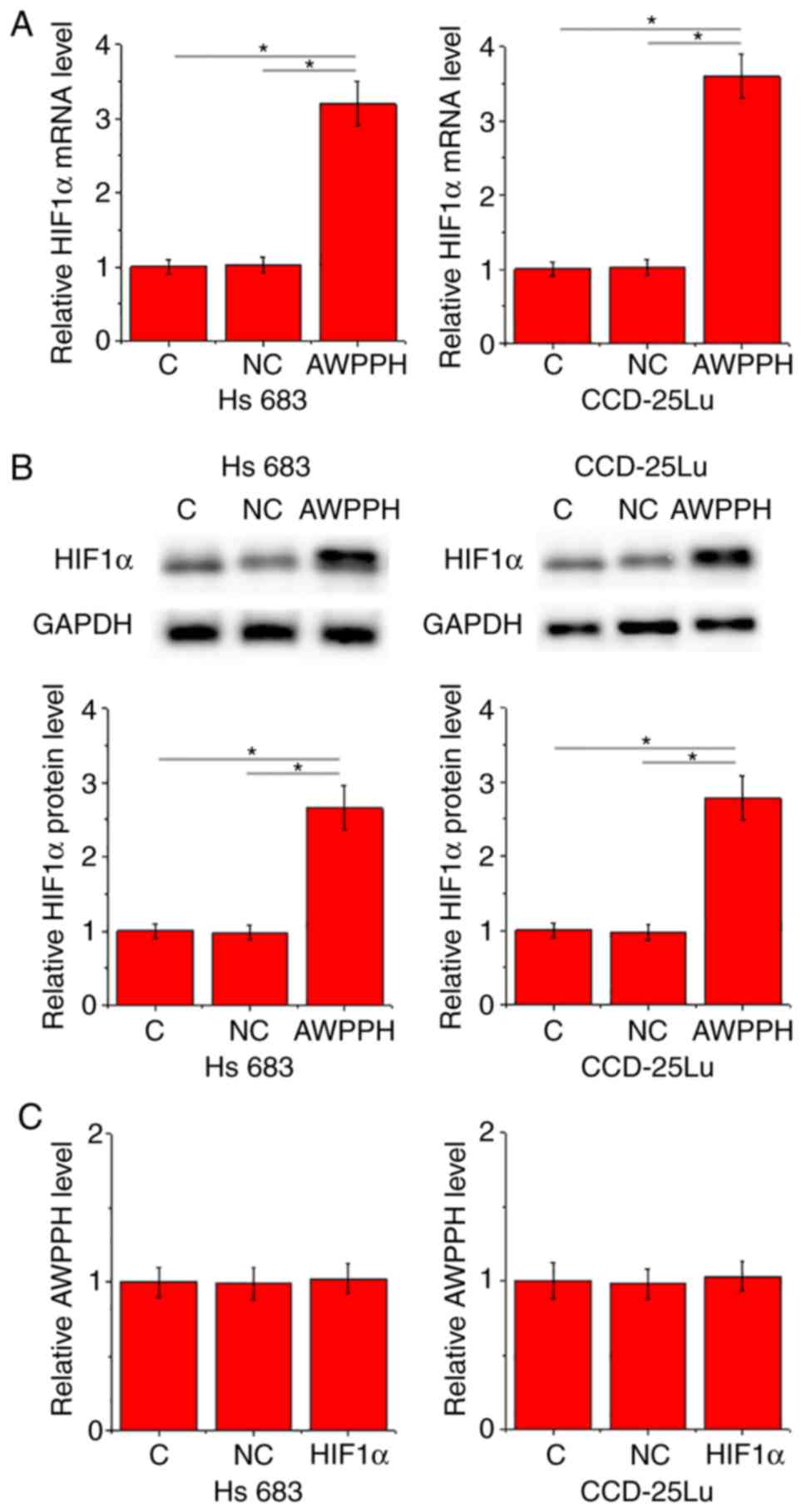
In the present study the interactions between AWPPH
and HIF1α were investigated in cells of the two human glioma cell
lines Hs 683 and CCD-25Lu. Compared with control cells (C) and
negative control cells (NC), overexpression of AWPPH led to
significantly promoted expression of HIF1α in the two cell lines at
the mRNA (P<0.05; Fig. 3A) and
protein (P<0.05; Fig. 3B) levels.
By contrast, compared with C and NC groups, there were no
significant changes in the expression level of AWPPH revealed in
cells with HIF1α overexpression (Fig.
3C).
AWPPH promotes glioma cell migration
and invasion possibly by upregulating HIFα
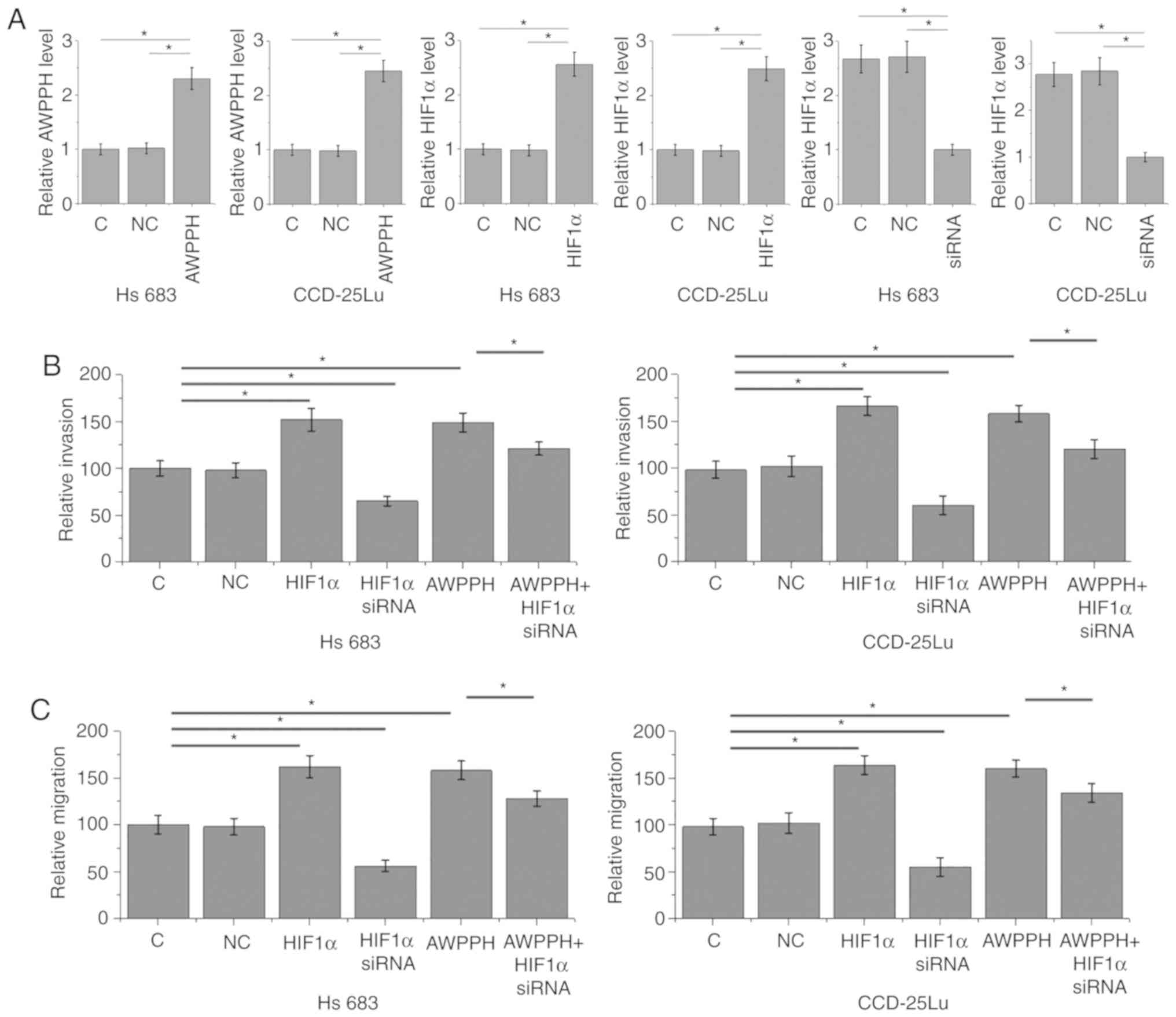
The aforementioned data indicated the involvement of
AWPPH in glioma metastasis. AWPPH and HIF1α overexpression, as well
as HIF1α siRNA silencing, were achieved following transfection
(P<0.05; Fig. 4A). Compared with
the C and NC groups, AWPPH and HIF1α overexpression significantly
promoted the invasion (Fig. 4B) and
migration (Fig. 4C) of cells of the
two human glioma cells lines Hs 683 and CCD-25Lu (P<0.05). In
addition, compared with glioma cells that only presented with AWPPH
overexpression, cells transfected with both AWPPH expression vector
and HIF1α siRNA demonstrated significantly decreased migration and
invasion rates, but they remained lower compared with those of the
C and NC groups.
Discussion
AWPPH is a characterized oncogenic lncRNA in bladder
cancer (9) and hepatocellular
carcinoma (10), although its
involvement in other diseases is currently unknown. The present
study revealed that AWPPH may contribute to the metastasis of
glioma. The actions of AWPPH in the metastasis of glioma are likely
to be achieved through the upregulation of HIF1α.
The development of glioma led to altered signaling
transduction of multiple molecular pathways in the human body
(12). The dysregulation of lncRNAs
may serve as a mediator between signaling pathways to participate
in the different aspects of the development and progression of
glioma (13). Certain lncRNAs, such
as lncRNA taurine upregulated gene 1 (14), have lower expression levels in glioma
tumor tissues compared with in healthy tissues, and overexpression
of those lncRNAs inhibits tumor progression, indicating the role
they serve as a tumor suppressor in glioma. In contrast, certain
lncRNAs, such as lncRNA activated by transforming growth factor β,
serve an oncogenic role in glioma and demonstrate an upregulated
expression pattern (15). However,
all those lncRNAs appear to be involved in the growth and
metastasis of glioma, and lack the potential to predict a certain
stage of glioma, such as tumor metastasis. In bladder cancer,
upregulation of AWPPH was observed prior to the occurrence of tumor
metastasis (stage Ta-T1) (9). The
study on hepatocellular carcinoma did not distinguish between the
expression of AWPPH prior to and following tumor metastasis
(10). In contrast with the
aforementioned studies, the present study revealed upregulated
expression of AWPPH only in metastatic glioma, not in
non-metastasis glioma. Therefore, AWPPH may participate only in the
metastasis of glioma. The present study provided novel insights
into the pathogenesis of glioma, and suggests that tumor growth and
metastasis may require the involvement of different cellular
factors. Therefore, inhibition of tumor growth and metastasis in
clinical trials should have different targets.
Proper treatment strategies designed based on
accurate clinical stages are critical for the treatment of cancer.
Even though the survival of patients with metastatic brain tumors
is generally poor, proper treatment strategies can still
significantly prolong survival time (16,17). In
the present study, it was demonstrated that the overexpression of
AWPPH can allow the differentiation of patients with metastatic
glioma from patients with non-metastatic glioma and healthy
controls. However, the present study failed to demonstrate the
differentiation of patients with non-metastatic glioma from healthy
controls. Therefore, upregulation of plasma AWPPH may serve as a
biomarker for the metastasis of glioma. It has been well
established that HIF1α may participate in cancer biology through
interactions with different lncRNAs (18,19).
Activation of HIF1α promotes cancer metastasis, and inhibition of
HIF1α has been proven to be a promising target for the treatment of
cancer (20,21). The results of the present study
indicated that AWPPH may be an upstream activator of HIF1α in the
regulation of migration and invasion of glioma cells under
non-hypoxic condition; however, only the sequential signaling of
AWPPH-HIF1α in glioma was reported. Whether this regulation is
direct or indirect is still unknown.
The present study did not include in vivo
studies; therefore, future studies should attempt to establish
animal models for glioma to further confirm the conclusions of the
present study. In addition, future studies should try to use double
fluorescence reporter enzyme systems to verify whether lncRNA AWPPH
is a direct regulator of HIF1α mRNA.
In conclusion, AWPPH may specifically participate in
the metastasis of glioma through the upregulation of HIF1α.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TZ and BZ designed the experiments. TZ and FW
performed all of the experiments. YL and LY collected and analyzed
data. TZ and BZ drafted the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Chinese People's Liberation Army Rocket Force General Hospital.
All patients completely understood the experiment protocol and
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ricard D, Idbaih A, Ducray F, Lahutte M,
Hoang-Xuan K and Delattre JY: Primary brain tumours in adults.
Lancet. 379:1984–1996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Catt S, Chalmers A and Fallowfield L:
Psychosocial and supportive-care needs in high-grade glioma. Lancet
Oncol. 9:884–891. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wolff JE, Driever PH, Erdlenbruch B,
Kortmann RD, Rutkowski S, Pietsch T, Parker C, Metz MW, Gnekow A
and Kramm CM: Intensive chemotherapy improves survival in pediatric
high-grade glioma after gross total resection: Results of the
HIT-GBM-C protocol. Cancer. 116:705–712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsang DS, Murphy ES, Ezell SE, Lucas JT
Jr, Tinkle C and Merchant TE: Craniospinal irradiation for
treatment of metastatic pediatric low-grade glioma. J Neurooncol.
134:317–324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci
USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iyer NV, Kotch LE, Agani F, Leung SW,
Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY
and Semenza GL: Cellular and developmental control of O2
homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev.
12:149–162. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masoud GN and Li W: HIF-1α pathway:
Role, regulation and intervention for cancer therapy. Acta Pharm
Sin B. 5:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ranasinghe WK, Baldwin GS, Bolton D,
Shulkes A, Ischia J and Patel O: HIF1α expression under normoxia in
prostate cancer-which pathways to target? J Urol. 193:763–770.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu F, Zhang X, Yu Q, Han G, Diao F, Wu C
and Zhang Y: LncRNA AWPPH inhibits SMAD4 via EZH2 to regulate
bladder cancer progression. J Cell Biochem. 119:4496–4505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao X, Liu Y and Yu S: Long noncoding RNA
AWPPH promotes hepatocellular carcinoma progression through YBX1
and serves as a prognostic biomarker. Biochim Biophys Acta Mol
Basis Dis. 1863:1805–1816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ceccarelli M, Barthel FP, Malta TM,
Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh
A, Pagnotta SM, et al: Molecular profiling reveals biologically
discrete subsets and pathways of progression in diffuse glioma.
Cell. 164:550–563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y, Wu JJ, Lin XB, Bao Y, Chen ZH,
Zhang CR, Cai Z, Zhou JY, Ding MH, Wu XJ, et al: Differential
lncRNA expression profiles in recurrent gliomas compared with
primary gliomas identified by microarray analysis. Int J Clin Exp
Med. 8:5033–5043. 2015.PubMed/NCBI
|
|
14
|
Li J, Zhang M, An G and Ma Q: LncRNA TUG1
acts as a tumor suppressor in human glioma by promoting cell
apoptosis. Exp Biol Med (Maywood). 241:644–649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma CC, Xiong Z, Zhu GN, Wang C, Zong G,
Wang HL, Er Bian B and Zhao B: Long non-coding RNA ATB promotes
glioma malignancy by negatively regulating miR-200a. J Exp Clin
Cancer Res. 35:902016.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Owonikoko TK, Arbiser J, Zelnak A, Shu HK,
Shim H, Robin AM, Kalkanis SN, Whitsett TG, Salhia B, Tran NL, et
al: Current approaches to the treatment of metastatic brain
tumours. Nat Rev Clin Oncol. 11:203–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parrish KE, Sarkaria JN and Elmquist WF:
Improving drug delivery to primary and metastatic brain tumors:
Strategies to overcome the blood-brain barrier. Clin Pharmacol
Ther. 97:336–346. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin A, Li C, Xing Z, Hu Q, Liang K, Han L,
Wang C, Hawke DH, Wang S, Zhang Y, et al: The LINK-A lncRNA
activates normoxic HIF1α signalling in triple-negative breast
cancer. Nat Cell Biol. 18:213–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang Y, Zhu X, Xu Y, Tang Q, Huang Z, Zhao
Z, Lu J, Song G, Xu H, Deng C and Wang J: Energy stress-induced
lncRNA HAND2-AS1 represses HIF1α-mediated energy metabolism and
inhibits osteosarcoma progression. Am J Cancer Res. 8:526–537.
2018.PubMed/NCBI
|
|
20
|
El-Naggar AM, Veinotte CJ, Cheng H,
Grunewald TG, Negri GL, Somasekharan SP, Corkery DP, Tirode F,
Mathers J, Khan D, et al: Translational activation of HIF1α by YB-1
promotes sarcoma metastasis. Cancer Cell. 27:682–697. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Q, Li Y, Li J, Ma Y, Dai W, Mo S, Xu Y,
Li X and Cai S: FBW7 suppresses metastasis of colorectal cancer by
inhibiting HIF1α/CEACAM5 functional axis. Int J Biol Sci.
14:726–735. 2018. View Article : Google Scholar : PubMed/NCBI
|