Introduction
Neuroblastoma is the most common extracranial tumor
in infants and accounts for 8–10% of all childhood tumors.
Neuroblastoma resulted in ~15% of pediatric cancer-associated
mortalities in 2010. The majority of cases with neuroblastoma (90%)
are diagnosed before the age of 5 years and 30% of these cases are
present within the first year. The median age of diagnosis is 22
months (1). Neuroblastoma is an
embryonal tumor derived from precursor cells of the sympathetic
nervous system. It is extremely heterogeneous with clinical
presentations ranging from spontaneous remission to rapid tumor
progression and mortality (2).
Neuroblastoma prognosis varies greatly. Children with neuroblastoma
exhibit marked variability in outcome when the disease is
categorized by age, stage and biological characteristics (3). Patients with low- and intermediate-risk
neuroblastoma, characterized by favorable stages and age at time of
diagnosis <1 year, have a 5-year overall survival >90%
following chemotherapy and surgical resection. By contrast, the
5-year overall survival for high-risk neuroblastoma, characterized
by features such as metastasis, age at diagnosis >1 year and
upregulated MYCN, is 40–50% despite intensive treatment
protocols that include chemotherapy, surgery, radiation and stem
cell transplantation (4,5). The biological behavior of neuroblastoma
is influenced by specific factors which are also outcome
predictors. These include patient-associated factors, including age
at the time of diagnosis and tumor-associated factors, including
histology, tumor stage, molecular and cytogenetic features
(6). Tissue factor (TF) is the
primary cellular initiator of blood coagulation and is a modulator
of cancer angiogenesis (7).
Hypercoagulability in cancer patients is closely associated with
tumor progression (8). Cancer is
associated with a 4-fold increase in thrombosis risk, with
chemotherapy further increasing the risk (9). A number of risk factors. including
leukocyte and platelet counts and circulating levels of tissue
factor (TF), P-selectin and D-dimer are also implicated (10).
Cancer cells and their vascular stroma often exhibit
procoagulant properties. An example of this is the deregulation of
TF expression, as TF is an important coagulation factor in the
extrinsic coagulation pathway (11).
Previous studies revealed TF to be a contributing factor in
intracellular signaling events through the TF cytoplasmic domain.
TF activates protease-activated receptors (PAR) 1 and 2 via the
TF/factor VIIa/factor Xa signalling pathway (12). Previous studies revealed a link
between tumor cell-associated procoagulant function and cancer
biology, as well as TF expression and poor patient outcome in
hepatocellular, ovarian and gastric carcinoma (13–15). The
present study investigated the association of TF expression with
tumor pathology, stage and outcome in patients with
neuroblastoma.
Materials and methods
Patients
A total of 40 patients with neuroblastoma treated at
the Pediatric Oncology Unit of Zagazig University Hospital
(Zagazig, Egypt) between January 2008 and December 2015 were
enrolled in the current study. The mean follow-up period was 29.1
months. The mean age of patients was, 2.29±1.67 years (age range, 5
months to 5.5 years). There were 24 (60%) males and 16 (40%)
females. All patients were treated according to the Children
Oncology Group risk-based protocol (16). Relevant laboratory data, including
complete blood count, liver and kidney function tests, serum
electrolytes, lactate dehydrogenase, and vanillyl mandelic and
homovanillic acid levels, were collected from patient medical
records.
Clinical data
Data collected included patient age at diagnosis,
sex, clinical symptoms and associated conditions at diagnosis. Data
from computed tomography of the chest and abdomen, magnetic
resonance imaging of the spine and bone and metaiodobenzylguanidine
(1311-MIBG) scans were used in the present study. Tumor
data collected included tumor site, metastasis, stage according to
the International Neuroblastoma Risk Group Staging System (INRGSS)
(17), histopathology according to
the International Neuroblastoma Pathology Classification (18) and MYCN expression status
according to the recommendation of the European Neuroblastoma
Quality Assurance Group (19).
Immunohistochemistry protocol
All samples were untreated biopsy specimens from
primary tumors and were examined independently by two pathologists
who were blinded to the collected data. Tissue samples were fixed
in formalin and embedded in paraffin blocks according to standard
procedures (20). Glass slides were
cleaned with 95% ethanol, treated with subbing solution (0.5%
gelatin in warm deionized water) and air-dried. Alternatively,
pretreated slides were used. The tissue samples were cut into
4–6-µm thick sections and mounted onto slides. The sections were
subsequently deparaffinized in xylene and rehydrated in a
descending alcohol series: The sections were washed in 100% ethanol
twice for 10 min each time, followed by two 10 min washes in 95%
ethanol and one 1 min wash in deionized water with stirring. The
heating temperature of the antigen retrieval step was 95–100°C. The
sections were incubated with 0.1% hydrogen peroxidase in deionized
water for 5–10 min at room temperature and rinsed in distilled
water. Blocking with 2–5% normal serum (Santa Cruz Biotechnology,
Inc.) for 30 min at room temperature was performed to reduce
background staining. Endogenous peroxidase activity was blocked
using 0.5–3% hydrogen peroxide. Expression of TF was determined by
incubating with an anti-TF mouse monoclonal antibody (cat no.
sc-80952; 100 mg/ml; Santa Cruz Biotechnology, Inc.) for 1 h at
room temperature. Primary antibody incubation was followed by
incubation with a horseradish peroxidase-conjugated anti-mouse IgG
secondary antibody (1:100; cat. no. HAF007; R&D systems, Inc.)
for 1 h at room temperature. Sections were counterstained using
hematoxylin for 30 sec at room temperature. Sections incubated with
2–5% normal mouse serum (Santa Cruz Biotechnology, Inc.) instead of
the primary antibody were used as a control. Neoplastic cells were
considered positive when they revealed cytoplasmic or membrane
staining.
Immunohistochemistry
interpretation
TF expression was visualized with an Axioskop 40
optical microscope (Carl Zeiss AG) at a magnification of ×100, and
analyzed using Image-Pro Plus image analysis software (version 7;
Media Cybernetics, Inc.). Each slide was examined by two
pathologists independently, and quantitative analysis of
immunohistochemical expression of TF was performed using the method
outlined by Sierko et al (20). This method pertains to cancer cell
percentage with positive staining and staining intensity. Values
from 0–4 were assigned to cancer cell percentage with positive
staining. These values, referred to as the A value, were as
follows: 0, no staining; 1, ≤10; 2, 11–50; 3, 51–75 and 4, >75%.
Staining intensity values, referred to as the B value were assigned
from 0–3 and were as follows: 0, no staining; 1, weak; 2, medium
and 3, strong. The immunoreactive score (IRS) was calculated by
multiplying the A and B values. The IRS value corresponded with TF
expression and was assigned the following values: 1, negative; 2,
weak; 3, medium and 4, strong. The IRS was assessed for cancer
cells and tumor vascular endothelial cells that expressed TF as
detected by optical microscopy (at least 20 high power
fields/sample; magnification, ×100).
Statistical analysis
SPSS software (version 15.0; SPSS, Inc.) was used
for data handling and statistical analyses. Data are presented as
the mean ± standard deviation for quantitative variables.
Categorical variables are expressed as number (%). Variables were
compared using Chi-squared test, Student's t-test, one-way ANOVA
and Kruskal-Wallis tests, respectively. Fisher's least significant
difference test was used as a post hoc test. The Kaplan-Meier
method, log-rank, Breslow and Tarone-Ware tests were for survival
curve analysis. The cumulative survival probability (St)
is the proportion of patients surviving (or remaining event-free)
past interval t and it is computed as follows. Firstly, the
proportion of participants surviving past time 0 (the starting
time) is defined as S0=1 (all participants alive or
event-free at time zero or the study start). The proportion
surviving past each subsequent interval is computed using
principles of conditional probability. The probability that a
participant survives past interval 1 is
S1=p1. The probability that a participant
survives past interval 2 means that they had to survive past
interval 1 and through interval 2: S2=P (survive past
interval 2)=P (survive through interval 2) × P (survive past
interval 1), or S2=p2 × S1. In
general, St+1=pt+1 × St. Death was
used as the event of interest and the status at serial time (points
of assessment during follow-up) was either 1=event of interest
(death) or 0=censored. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical data
Of the 40 patients, 22 (55%) were >18 months old,
and 18 (45%) were ≤18 months old (mean, 2.29±1.67 years; range, 5
months to 5.5 years). A total of 24 patients (60%) were male while
16 patients (40%) were female (Table
I). The primary tumor sites included the abdomen (34/40; 85%),
mediastinum (4/40; 10%) and neck (2/40; 5%). The bone marrow was
the most common site of metastasis (22/40; 55%) followed by bone
(20/40; 50%) and the eye (14/40; 35%) patients. Patients had >1
metastatic site. A total of 24 patients (60%) were diagnosed at
stage IV, 12 patients (30%) at stage III and 4 patients (10%) at
stage II. Histology was favorable for 20 out of 40 patients (50%).
MYCN amplification was reported in 18 (45%) patients, while
no amplification was observed in 22 (55%) cases. Risk
stratification revealed that 30 (75%) cases were classified as high
risk, 6 (15%) were intermediate risk and 4 (10%) were classified as
low risk.
 | Table I.Clinical and pathological
characteristics of the patients. |
Table I.
Clinical and pathological
characteristics of the patients.
| Variable | Number of patients
(%) |
|---|
| Age |
|
|
Mean | 2.29±1.67
years |
|
Range | 5 months-5.5
years |
| ≤18
months | 18 (45) |
| >18
months | 22 (55) |
| Gender |
|
|
Males | 24 (60) |
|
Females | 16 (40) |
| Primary tumor
site |
|
|
Abdomen | 34 (85) |
|
Mediastinum | 4 (10) |
|
Neck | 2 (5) |
| Metastatic
sites |
|
| Bone
marrow | 22 (55) |
|
Bone | 20 (50) |
|
Eye | 14 (35) |
|
Liver | 4 (10) |
| Stages |
|
| Stage
I | 0 (0) |
| Stage
II | 4 (10) |
| Stage
III | 12 (30) |
| Stage
IV | 24 (60) |
| Pathology |
|
|
Favorable Shimada | 20 (50) |
|
Unfavorable Shimada | 20 (50) |
| MYCN proto-oncogene
bHLH transcription factor status |
|
|
Amplified | 18 (45) |
|
Non-amplified | 22 (55) |
| Risk
stratification |
|
|
Low | 4 (10) |
|
Intermediate | 6 (15) |
|
High | 30 (75) |
| Tissue factor
expression |
|
|
Positive (High) | 6 (15) |
|
Positive (Moderate) | 10 (25) |
|
Positive (Weak) | 14 (35) |
|
Negative | 10 (25) |
Immunohistochemical analysis
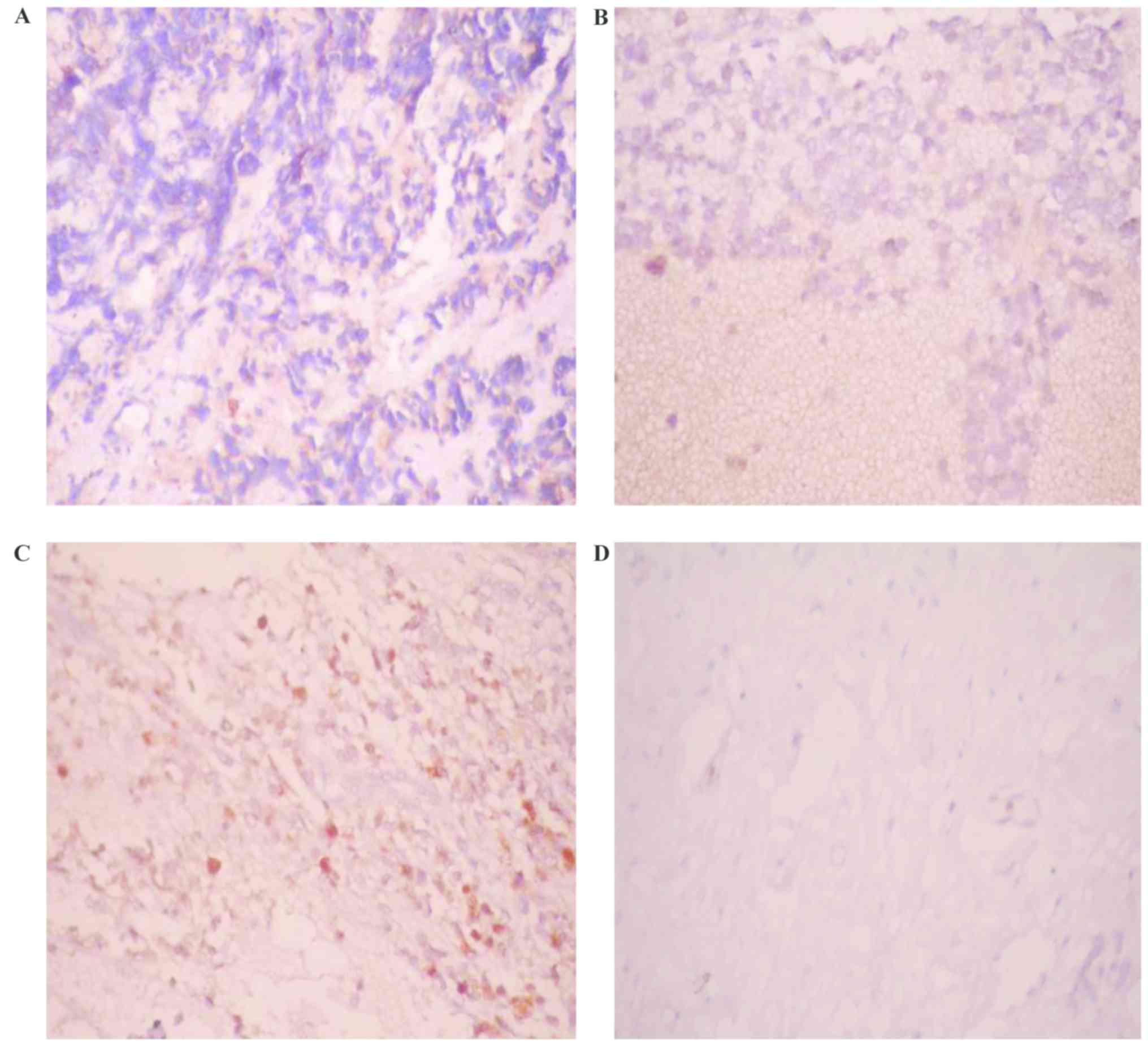
Varying levels of TF expression were observed in 30
out of 40 cases (75%), namely, weak intensity in 14 (35%), moderate
intensity in 10 (25%) and high intensity in 6 (15%), while 10 (25%)
cases showed no TF expression (Table
I; Fig. 1). In the present
study, 60% of unfavorable ‘Shimada pathology’ was detected in
patients with positive TF expression, while only 20% was detected
in patients with negative TF expression. This difference was
statistically insignificant (P>0.05; Table II).
 | Table II.Association between tissue factor
expression and tumor stage, pathology, risk stratification and
patient outcome. |
Table II.
Association between tissue factor
expression and tumor stage, pathology, risk stratification and
patient outcome.
|
| Tissue factor
expression |
|
|---|
|
|
|
|
|---|
| Variable | Negative (n=10)
(%) | Positive (n=30)
(%) | P-value |
|---|
| Stage |
|
|
|
| II | 0 (0.0) | 4 (13.3) | 0.64 |
|
III | 4 (40) | 8 (26.7) |
|
| IV | 6 (60) | 18 (60) |
|
| Risk
stratification |
|
|
|
|
Low | 0 (0.0) | 4 (13.3) | 0.16 |
|
Intermediate | 4 (40) | 2 (6.7) |
|
|
High | 6 (60) | 24 (80) |
|
| Pathology |
|
|
|
|
Unfavorable | 2 (20) | 18 (60) | 0.3 |
|
Favorable | 8 (80) | 12 (40) |
|
| Outcome |
|
|
|
|
Mortality | 0 (0.0) | 18 (60) | 0.002 |
|
Cured | 10 (100) | 6 (20) |
|
| Under
treatment | 0 (0.0) | 6 (20) |
|
| Age |
|
|
|
| ≤18
months | 6 (60) | 12 (40) | 0.79 |
| >18
months | 4 (40) | 18 (60) |
|
| Gender |
|
|
|
|
Male | 8 (80) | 16 (53.3) | 0.59 |
|
Female | 2 (20) | 14 (46.7) |
|
| MYCN proto-oncogene
bHLH transcription factor status |
|
|
|
|
Amplified | 1 (10) | 17 (56.7) | 0.01 |
|
Non-amplified | 9 (90) | 13 (43.3) |
|
There was a significant association between TF
expression and patient outcome. All patients with negative TF
expression survived, whereas 18 (60%) patients with positive TF
expression succumbed, 6 (20%) patients survived while another 6
(20%) were still undergoing treatment during the study period
(P<0.05; Table II). The mean
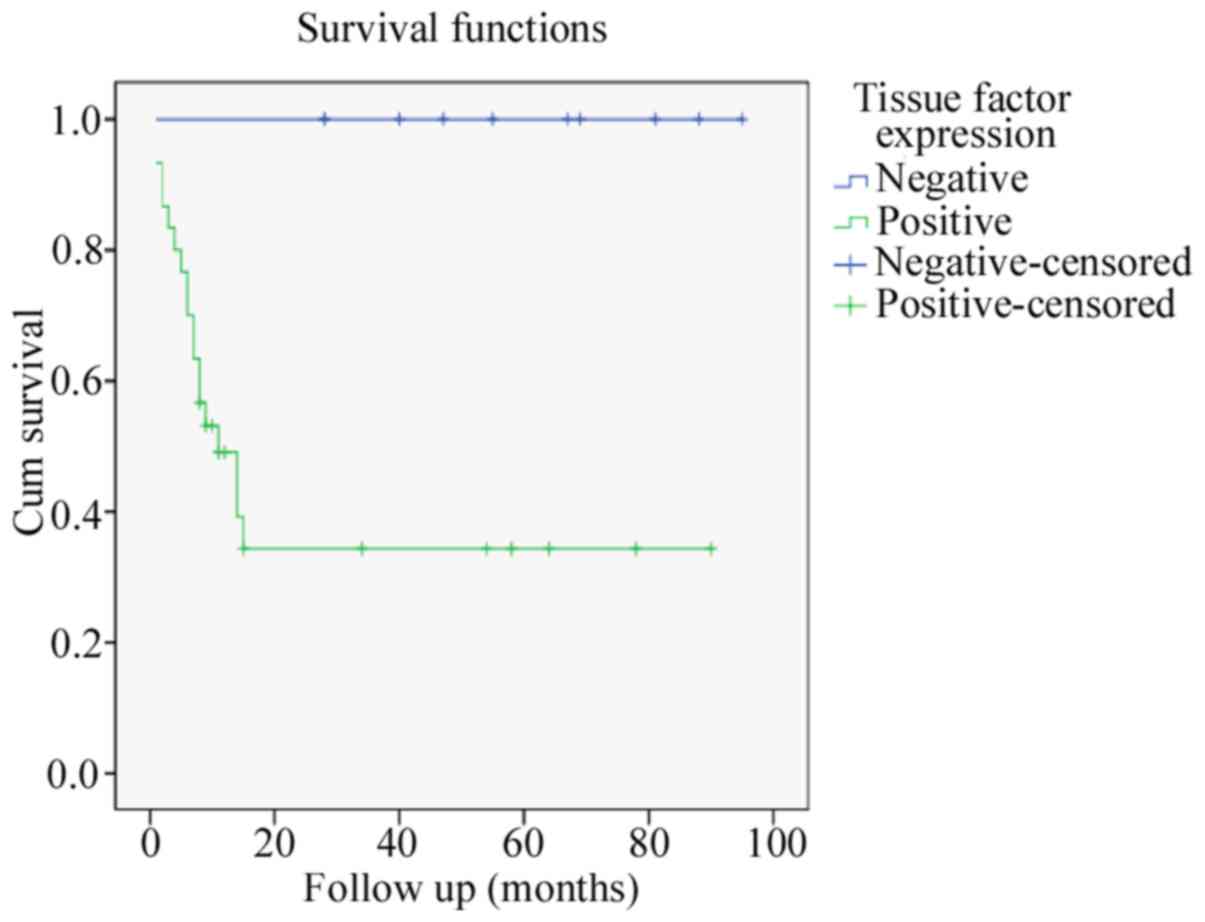
follow-up period was 29.1 months. The mean survival time for
patients with negative TF expression was 59.8 months as opposed to
18.87 months for those with positive TF expression (P<0.05;
Table III). All patients with
negative tissue factor expression survived and completed their
treatment successfully. The mean survival time is based on the last
follow up point of the study and ranged from 28–95 months. Survival
distributions for varying levels of TF expression were tested, and
results revealed a significant difference between patients with and
without TF expression (P<0.05; Table
IV). There were no mortalities reported in patients with
negative TF expression (Table IV;
Fig. 2). In the present study, no
association was observed between TF intensity staining and
variables including sex, age, clinical symptoms, tumor stage, risk
stratification, pathology, MYCN amplification and outcome
(P>0.05; Table SI). Similarly,
no significant link was identified between patient outcome and sex,
age, clinical symptoms, tumor stage, risk stratification, pathology
and MYCN amplification (P>0.05). Patients who succumbed
to the disease were found to have a higher percentage of
MYCN amplification, however, this did not reach a
statistically significant level (P=0.08). The present study
revealed a significant association between TF expression and
MYCN amplification. Only 10% of cases with negative TF
expression showed MYCN amplification, as opposed to 56.7% of
cases with positive TF expression (P=0.01; Table II).
 | Table III.Mean survival time for patients with
and without tissue factor expression. |
Table III.
Mean survival time for patients with
and without tissue factor expression.
| Tissue factor
expression | n | Mean (months) | Standard deviation
(months) | Median
(months) | Minimum
(months) | Maximum
(months) | t-test | P-value |
|---|
| Negative tissue
factor expression | 10 | 59.80 | 24.087 | 61.00 | 28 | 95 | 4.64 | <0.001 |
| Positive tissue
factor expression | 30 | 18.87 | 24.176 | 9.00 | 1 | 90 |
|
|
| Total | 40 | 29.10 | 29.845 | 13.00 | 1 | 95 |
|
|
 | Table IV.Tests of equality of survival
distributions for the upregulated and normal expression levels of
tissue factor. |
Table IV.
Tests of equality of survival
distributions for the upregulated and normal expression levels of
tissue factor.
| Test | Chi-square
test | Degrees of
freedom | P-value |
|---|
| Log rank
(mantel-cox) | 10.048 | 1 | 0.002 |
| Breslow
(generalized wilcoxon) | 8.860 | 1 | 0.003 |
| Tarone-ware | 9.523 | 1 | 0.002 |
Discussion
TF is an evolutionary conserved glycoprotein that
serves an important role in the pathogenesis of cancer. TF affects
a variety of pathological processes, including tumor-associated
angiogenesis, thrombogenicity, tumor growth and metastasis
(21). Additionally, high levels of
TF expression were associated with increased expression of vascular
endothelial growth factor and vascularity as development of blood
vessels in tumors is required for tumor growth and spread (22).
Previous studies revealed that TF expression was
higher in patients with KRAS proto-oncogene GTPase and tumor
protein 53 mutations, suggesting that TF expression is a poor
prognostic factor (23,24). TF upregulation parallels the
expression of several mutant oncogenes including epidermal growth
factor receptor, erb-b2 receptor tyrosine kinase 2 and
promyelocytic leukemia-retinoic acid receptor α. Previous studies
have revealed that the upregulation of TF is associated with the
upregulation of the aforementioned oncogenes in colorectal cancer
cells, mammary glands, cutaneous tissue, astrocytes and blood
(23,24). Although upregulation of TF is often
detected on the surface of tumor endothelial cells, determining its
effect on prognosis and patient survival remains challenging
(25). To the best of our knowledge,
the present study is the first relating immunohistochemical
analysis of TF expression in children with neuroblastoma and its
role in tumor pathology, stage and patient outcome. The current
study revealed increased TF expression in 30/40 (75%) patients.
Similarly, Maciel et al (26), reported increased TF expression in
38/41 (88.3%) cases of nephroblastoma. In a previous study by
Abdulkadir et al (27), 73%
of patients diagnosed with prostate cancer had positive TF
expression, which was positively correlated with preoperative serum
prostate specific antigen levels. As demonstrated by Callander
et al (28), TF upregulation
is a characteristic marker of certain neoplasms. At least 60% of
epithelial neoplasms, and up to 100% of gastrointestinal
carcinomas, express high levels of TF. TF expression has been
detected in breast cancer, lung carcinomas, colon cancer and
gliomas (29–32). In the aforementioned malignancies, TF
is expressed by the tumor cells or adjacent stromal cells. This has
been correlated with tumor grading, metastasis and poor prognosis.
TF upregulation has been associated with tumorigenesis with high
procoagulant activity observed in tumor cells (29–32).
TF is involved in hematogenous metastasis where TF
levels in metastatic cells may be 1,000-fold higher than in
non-metastatic cells in leukemia and melanoma (33,34). TF
enhances metastasis by inducing a fibrin coating on malignant
cells, trapping the cells in the microvasculature, thereby aiding
metastasis. Additionally, thrombin activates platelets, leading to
the formation of platelet-fibrin thrombi in the microvasculature
(35). Clot formation around the
tumor cells in circulation prevents the removal of tumor cells by
natural-killer cells (35). TF
expression by the tumor and host cells initiates direct or indirect
signaling events that support tumor development by distinct
mechanisms. TF supports cell migration and cellular trafficking by
binding to the protein filamin-A, resulting in reorganization of
actin filaments and cell migration (36). Regulation of cell motility by binding
to its ligand factor VIIa is another pathway by which TF may
directly influence tumor metastasis (37). The present study revealed TF
expression in 18/24 (75%) patients with stage IV neuroblastoma, and
8/12 (67%) patients with stage III neuroblastoma. However, results
revealed no significant statistical association between TF
expression and neuroblastoma stages. The present study was based on
a small patient number with a high stage IV percentile (60%).
Previous studies have revealed an association
between TF expression and metastases in various types of cancer
including prostate cancer, human glioma, colorectal cancer, breast
cancer, hepatocellular carcinoma and pancreatic duct carcinoma
(27,32,38–41).
Additionally, it has been shown that TF serves a role in
tumor-associated angiogenesis and its expression levels are
associated with the metastatic potential of numerous hematological
malignancies (42). In the present
study, a significant association between TF expression and age and
sex of the patients was not observed. These results are similar to
previous studies in human non-small cell lung carcinoma, colorectal
carcinoma, hepatocellular carcinoma and pancreatic ductal
adenocarcinoma (43–46). As pathological symptoms of
neuroblastoma have been used to further analyze these tumors,
Shimada et al (18),
initially classified these tumors as favorable and unfavorable by
combining age with the extent of tumor differentiation, presence of
schwannian stroma components and cellular information from the
mitosis-karyorrhexis index. In the present study, 60% of
unfavorable ‘Shimada pathology’ was detected in patients with
positive TF expression, while only 20% was detected in patients
with negative TF expression. This difference was statistically
insignificant (P>0.05).
Previous studies have shown no significant
association between TF expression and favorable or unfavorable
nephroblastoma histology (26).
Similarly, no association between pathological grade and TF
expression in prostatic cancer has been found (47). However, a number of studies have
revealed an association between the histological grading of a tumor
and TF expression. Kakkar et al (48) reported that TF expression was
associated with histological grade in pancreatic cancer and a
significant linear trend was detected with stronger
immunoreactivity observed in poorly differentiated tumors. Similar
results have been documented in human glioma and breast cancer
(29,32).
The MYCN gene was the first oncogene revealed
to be amplified in solid malignancies and the only consistently
amplified gene in neuroblastoma (49). The MYCN gene has been used by
The Children's Oncology Group to assign newly diagnosed
neuroblastoma patients to a high-risk group requiring intensive
multimodal therapy (50). In the
present study, all patients were tested for MYCN
amplification. It was amplified in 18/40 (45%) patients and was not
amplified in 22/40 (55%) patients. There was an association between
MYCN amplification and TF expression, whereby only 10% of
those with negative TF expression had amplified MYCN, while
56.7% of those with positive TF expression had amplified
MYCN. The link between TF and MYCN expression may
improve the value of MYCN as a prognostic indicator in
children with neuroblastoma.
In the present study, TF expression was
significantly associated with patient outcome. All patients with
negative TF expression survived, whereas 18/30 (60%) cases with
positive TF expression succumbed to the disease. These results were
similar to a previous study where an association between TF
expression and prognosis in patients with nephroblastoma and
increased immunohistochemical detection of TF expression was the
most important risk factor for recurrence and mortality according
to bivariate and multivariate analysis (26). Furthermore, immunohistochemical TF
expression was an important independent predictor of mortality in a
cohort of patients with clear cell renal cell carcinoma (51). Rao et al (23) reported that TF expression was
markedly higher in patients with poor differentiation in colorectal
carcinoma based on Dukes' staging system, whereas patients with low
TF expression had longer disease-free survival and overall survival
compared with patients with high TF expression (23). Akashi et al (47) revealed that in patients with
metastatic prostate cancer treated with androgen-withdrawal
therapy, TF expression was not associated with therapeutic
response; however, patients with TF positive tumors had a shorter
survival rate compared with patients with TF negative tumors.
Hamada et al (32) did not
demonstrate a correlation between TF expression and patient outcome
in human glioma; however, an association with malignancy grade was
observed. In the present study, no significant association was
found between TF staining intensity and pathology, stage and age of
the patients. Nitori et al (46) reported that patients with pancreatic
duct carcinoma with negative TF expression had markedly improved
survival time compared with patients with positive TF expression.
Furthermore, increased levels of TF expression are an independent
risk factor for poor survival in patients with solid tumors such as
breast cancer, colorectal carcinoma, hepatocellular carcinoma and
pancreatic duct carcinoma (29,38,44–46).
However, more extensive and disease-specific clinical analysis is
warranted to establish whether tumor-associated TF levels as well
as changes in circulating TF, possess independent prognostic and
predictive utility (52). In
summary, TF is a unique molecule that may be explored further and
utilized in various ways in order to treat human cancer.
The present study had a number of limitations. A
limited number of patients were enrolled, however, this should be
read in light of the rarity of the disease. Additionally, the
present study focused on TF and its relationship with outcome and
other well-known risk factors. Future larger studies with a wide
scope that take into consideration all variables that may impact
outcome are required. In conclusion, the results of the present
study revealed that TF expression may be useful for the prognosis
of patients with neuroblastoma. The mechanisms underlying the
effects of TF expression on the progression of neuroblastoma and
patient outcome, as well as its potential for the development of
novel therapeutic strategies, require further investigation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LMS and THH designed the study. LMS participated in
the data analyses, manuscript writing and revision. THH performed
the statistical analysis and participated in manuscript writing and
revision. MZ, MF and MAB collected the clinicopathological data,
performed statistical analysis and participated in manuscript
writing. EAE performed the histopathological examinations and
analyzed MYCN upregulation and TF expression. AE performed
routine laboratory work for the patients. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the Faculty of Medicine, Zagazig University
(Zagazig, Egypt). The study was performed according to the Helsinki
Declaration as revised in 2000. Written informed consent was
obtained from the legal guardians of the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maris JM, Hogarty MD, Bagarteii R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goto S, Umehara S, Gerbing RB, Stram DO,
Brodeur GM, Seeger RC, Lukens JN, Matthay KK and Shimada H:
Histopathology (International Neuroblastoma Pathology
Classification) and MYCN status in patients with peripheral
neuroblastic tumors: A report from the Children's Cancer Group.
Cancer. 92:2699–2708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Irwin MS and Park JR: Neuroblastoma:
Paradigm for precision medicine. Pediatr Clin North Am. 62:225–256.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park JR, Bagatell R, London WB, Maris JM,
Cohn SL, Mattay KK and Hogarty M; COG Neuroblastoma Committee, :
Children's Oncology Group's 2013 blueprint for research:
Neuroblastoma. Pediatr Blood Cancer. 60:985–993. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brodeur GM: Molecular basis for
heterogeneity in human neuroblastomas. Eur J Cancer. 31A:505–510.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rak J, Milsom C and Yu J: Tissue factor in
cancer. Curr Opin Hematol. 15:522–528. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rickles FR: Mechanisms of cancer-induced
thrombosis in cancer. Pathophysiol Haemost Thromb. 35:103–110.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khorana AA, Kuderer NM, Culakova E, Lyman
GH and Francis CW: Development and validation of a predictive model
for chemotherapy-associated thrombosis. Blood. 111:4902–4907. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ay C, Dunkler D, Marosi C, Chiriac AL,
Vormittag R, Simanek R, Quehenberger P, Zielinski C and Pabinger I:
Prediction of venous thromboembolism in cancer patients. Blood.
116:5377–5382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mackman N: Triggers, targets and
treatments for thrombosis. Nature. 451:914–918. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Versteeg HH and Ruf W: Emerging insights
in tissue factor-dependent signaling events. Semin Thromb Hemost.
32:24–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaido T, Oe H, Yoshikawa A, Mori A, Arii S
and Imamura M: Tissue factor is a useful prognostic factor of
recurrence in hepatocellular carcinoma in 5-year survivors.
Hepatogastroenterology. 52:1383–1387. 2005.PubMed/NCBI
|
|
14
|
Han LY, Landen CN Jr, Kamat AA, Lopez A,
Bender DP, Mueller P, Schmandt R, Gershenson DM and Sood AK:
Preoperative serum tissue factor levels are an independent
prognostic factor in patients with ovarian carcinoma. J Clin Oncol.
24:755–761. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamashita H, Kitayama J, Ishikawa M and
Nagawa H: Tissue factor expression is a clinical indicator of
lymphatic metastasis and poor prognosis in gastric cancer with
intestinal phenotype. J Surg Oncol. 95:324–331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matthay KK, Villablanca JG, Seeger RC,
Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT,
Brodeur GM, et al: Treatment of high-risk neuroblastoma with
intensive chemotherapy, radiotherapy, autologous bone marrow
transplantation, and 13-cis-retinoic acid. Children's Cancer Group.
N Engl J Med. 341:1165–1173. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Castleberry RP, Pritchard J, Ambros P,
Berthold F, Brodeur GM, Castel V, Cohn SL, De Bernardi B,
Dicks-Mireaux C, Frappaz D, et al: The international neuroblastoma
risk groups (INRG): A preliminary report. Eur J Cancer.
33:2113–2116. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimada H, Ambros IM, Dehner LP, Hata J,
Joshi VV and Roald B: Terminology and morphologic criteria of
neuroblastic tumors: Recommendations by the International
Neuroblastoma Pathology Committee. Cancer. 86:349–363. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Theissen J, Boensch M, Spitz R, Betts D,
Stegmaier S, Christiansen H, Niggli F, Schilling F, Schwab M, Simon
T, et al: Heterogeneity of the MYCN oncogene in neuroblastoma. Clin
Cancer Res. 15:2085–2090. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sierko E, Wojtukiewicz MZ, Zimnoch L and
Kisiel W: Expression of tissue factor pathway inhibitor (TFPI) in
human breast and colon cancer tissue. Thromb Haemost. 103:198–204.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eisenreich A, Bolbrinker J and Leppert U:
Tissue factor: A conventional or alternative target in cancer
therapy. Clin Chem. 62:563–570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khorana AA, Ahrendt SA, Ryan CK, Francis
CW, Hruban RH, Hu YC, Hostetter G, Harvey J and Taubman MB: Tissue
factor expression, angiogenesis, and thrombosis in pancreatic
cancer. Clin Cancer Res. 13:2870–2875. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rao B, Gao Y, Huang J, Gao X, Fu X, Huang
M, Yao J, Wang J, Li W, Zhang J, et al: Mutations of p53 and K-ras
correlate TF expression in human colorectal carcinomas: TF
downregulation as a marker of poor prognosis. Int J Colorectal Dis.
26:593–601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu JL, May L, Lhotak V, Shahrzad S,
Shirasawa S, Weitz JI, Coomber BL, Mackman N and Rak JW: Oncogenic
events regulate tissue factor expression in colorectal cancer
cells: Implications for tumor progression and angiogenesis. Blood.
105:1734–1741. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Staton CA, Chetwood ASA, Cameron IC, Cross
SS, Brown NJ and Reed MW: The angiogenic switch occurs at the
adenoma stage of the adenoma carcinoma sequence in colorectal
cancer. Gut. 56:1426–1432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maciel EO, Carvalhal GF, da Silva VD,
Batista EL Jr and Garicochea B: Increased tissue factor expression
and poor nephroblastoma prognosis. J Urol. 182:1594–1599. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abdulkadir SA, Carvalhal GF, Kaleem Z,
Kisiel W, Humphrey PA, Catalona WJ and Milbrandt J: Tissue factor
expression and angiogenesis in human prostate carcinoma. Hum
Pathol. 31:443–447. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Callander NS, Varki N and Rao LV:
Immunohistochemical identification of tissue factor in solid
tumors. Cancer. 70:1194–1201. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ueno T, Toi M, Koike M, Nakamura S and
Tominaga T: Tissue factor expression in breast cancer tissues: Its
correlation with prognosis and plasma concentration. Br J Cancer.
83:164–170. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sawada M, Miyake S, Ohdama S, Matsubara O,
Masuda S, Yakumaru K and Yoshizawa Y: Expression of tissue factor
in non-small-cell lung cancers and its relationship to metastasis.
Br J Cancer. 79:472–477. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shigemori C, Wada H, Matsumoto K, Shiku H,
Nakamura S and Suzuki H: Tissue factor expression and metastatic
potential of colorectal cancer. Thromb Haemost. 80:894–898. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hamada K, Kuratsu J, Saitoh Y, Takeshima
H, Nishi T and Ushio Y: Expression of tissue factor correlates with
grade of malignancy in human glioma. Cancer. 77:1877–1883. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rickles FR, Hair GA, Zeff RA, Lee E and
Bona RD: Tissue factor expression in human leukocytes and tumor
cells. Thromb Haemost. 74:391–395. 1995.PubMed/NCBI
|
|
34
|
Amirkhosravi A, Meyer T, Chang JY, Amaya
M, Siddiqui F, Desai H and Francis JL: Tissue factor pathway
inhibitor reduces experimental lung metastasis of B16 melanoma.
Thromb Haemost. 87:930–936. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Palumbo JS, Talmage KE, Massari JV, La
Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M and Degen JL:
Platelets and fibrin(ogen) increase metastatic potential by
impeding natural killer cell-mediated elimination of tumor cells.
Blood. 105:178–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abe K, Shoji M, Chen J, Bierhaus A, Danave
I, Micko C, Casper K, Dillehay DL, Nawroth PP and Rickles FR:
Regulation of vascular endothelial growth factor production and
angiogenesis by the cytoplasmic tail of tissue factor. Proc Natl
Acad Sci USA. 96:8663–8668. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dorfleutner A, Hintermann E, Tarui T,
Takada Y and Ruf W: Cross-talk of integrin alpha3beta1 and tissue
factor in cell migration. Mol Biol Cell. 15:4416–4425. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Seto S, Onodera H, Kaido T, Yoshikawa A,
Ishigami S, Arii S and Imamura M: Tissue factor expression in human
colorectal carcinoma: Correlation with hepatic metastasis and
impact on prognosis. Cancer. 88:295–301. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou JN, Ljungdahl S, Shoshan MC,
Swedenborg J and Linder S: Activation of tissue-factor gene
expression in breast carcinoma cells by stimulation of the RAF-ERK
signaling pathway. Mol Carcinog. 21:234–243. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Poon RT, Lau CP, Ho JW, Yu WC, Fan ST and
Wong J: Tissue factor expression correlates with tumor angiogenesis
and invasiveness in human hepatocellular carcinoma. Clin Cancer
Res. 9:5339–5345. 2003.PubMed/NCBI
|
|
41
|
Ueda C, Hirohata Y, Kihara Y, Nakamura H,
Abe S, Akahane K, Okamoto K, Itoh H and Otsuki M: Pancreatic cancer
complicated by disseminated intravascular coagulation associated
with production of tissue factor. J Gastroenterol. 36:848–850.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Versteeg HH, Spek CA, Peppelenbosch MP and
Richel DJ: Tissue factor and cancer metastasis: The role of
intracellular and extracellular signaling pathways. Mol Med.
10:6–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Koomägi R and Volm M: Tissue-factor
expression in human non-small-cell lung carcinoma measured by
immunohistochemistry: Correlation between tissue factor and
angiogenesis. Int J Cancer. 79:19–22. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lykke J and Nielsen HJ: The role of tissue
factor in colorectal cancer. Eur J Surg Oncol. 29:417–422. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Poon RT, Lau CP, Cheung ST, Yu WC and Fan
ST: Quantitative correlation of serum levels and tumor expression
of vascular endothelial growth factor in patients with
hepatocellular carcinoma. Cancer Res. 63:3121–3126. 2003.PubMed/NCBI
|
|
46
|
Nitori N, Ino Y, Nakanishi Y, Yamada T,
Honda K, Yanagihara K, Kosuge T, Kanai Y, Kitajima M and Hirohashi
S: Prognostic significance of tissue factor in pancreatic ductal
adenocarcinoma. Clin Cancer Res. 11:2531–2539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Akashi T, Furuya Y, Ohta S and Fuse H:
Tissue factor expression and prognosis in patients with metastatic
prostate cancer. Urology. 62:1078–1082. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kakkar AK, Lemoine NR, Scully MF, Tebbutt
S and Williamson RC: Tissue factor expression correlates with
histological grade in human pancreatic cancer. Br J Surg.
82:1101–1104. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schwab M, Shimada H, Joshi V and Brodeur
GM: Neuroblastic tumors of adrenal glandand sympathetic nervous
systemPathology and Genetics of Tumors of the Nervous System.
Kleihues P and Cavenee WK: World Health Organization, IARC; Lyon:
pp. 153–161. 2000
|
|
50
|
Maris JM, Weiss MJ, Guo C, Gerbing RB,
Stram DO, White PS, Hogarty MD, Sulman EP, Thompson PM, Lukens JN,
et al: Loss of heterozygosity at 1p36 independently predicts for
disease progression but not decreased overall survival probability
in neuroblastoma patients: A Children's Cancer Group study. J Clin
Oncol. 18:1888–1899. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Silva DD, Noronha JA, Silva VD and
Carvalhal GF: Increased tissue factor expression is an independent
predictor of mortality in clear cell carcinoma of the kidney. Int
Braz J Urol. 40:499–506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zwicker Jl, Furie BC and Furie B:
Cancer-associated thrombosis. Crit Rev Oncol Hematol. 62:126–136.
2007. View Article : Google Scholar : PubMed/NCBI
|