Introduction
The status of hormone receptors (HRs), including
estrogen receptor (ER) and progesterone receptor (PR), in breast
cancer is crucial for predicting patient responsiveness to
endocrine therapy (1,2). The majority of breast tumors (~70%)
highly express hormone receptor (HR+). Patients with HR+ tumors
have better disease-specific survival and overall survival (OS)
compared with those with HR-negative (HR-) tumors (1,2).
Previously, HR positivity was assessed using
immunohistochemistry (IHC) scoring with a ≥10% cutoff value for
nuclear staining of tumor epithelial cells (3). However, the 2010 guidelines from the
College of American Pathologists and American Society of Clinical
Oncology changed the IHC cut-off value for determining HR
positivity from 10% to 1% of stained cells (4). This has led to the creation of a
subclass of low HR+ (1–10%) tumors in breast cancer. This new
subclass has been reported to have beneficial impact on patients'
response to antiestrogen therapy (2,5,6).
It has been demonstrated that, although
triple-negative breast cancer (TNBC) is more sensitive to
chemotherapy than HR+ breast cancer, its prognosis remains poor
(7). Previous studies reported that
low HR-positive tumors (low HR+) have more aggressive features and
poorer prognosis compared with high HR+ tumors (6,8).
Numerous studies have reported that tumors with ER expression
<10% are likely to exhibit biological behaviors similar to those
from ER negative (ER-) tumors (5,6,9). However, to the best of our knowledge,
studies comparing the neoadjuvant chemotherapy (NAC) response
between low ER+ breast cancer tumors and other types of breast
cancer remain limited (10,11). In addition, there is no consistency
in the choice of endocrine therapy for the treatment of low
HR+ breast cancer.
The present study compared low HR+ tumors with HR+
and HR- tumors in order to understand the clinical characteristics
and prognosis of patients with breast cancer following NAC
treatment. To do so, the pathological response to NAC in these
three types of tumors was determined. The effect of various
endocrine treatment regimens on the prognosis of patients with
breast cancer and low HR+ expression was subsequently
investigated.
Materials and methods
Patient selection
The present study was approved by the Ethics
Committee of Zhejiang Cancer Hospital. The medical records of 1,194
patients with stages IIA-IIIC primary breast cancer who received
NAC at the Zhejiang Cancer Hospital in China between January, 2007
and December, 2017 were examined retrospectively. All patients
enrolled had undergone a core needle biopsy and subsequent surgery
prior to and following NAC treatment. Patients with incomplete or
inconsistent IHC data were excluded. Other exclusion criteria were:
i) Patients with stage IV breast cancer, bilateral breast cancer,
inflammatory breast cancer or diagnosed with another primary
cancer; ii) patients who did not complete the standard NAC regimen;
and iii) patients who received radiation therapy prior to surgery.
The pathological stage of tumors was assessed according to the
American Joint Committee on Cancer (AJCC) 8th Staging System
(12). The intensity of HR nuclear
staining was divided into three groups and defined as negative, low
positive and positive for <1%, 1–10% and >10% of nuclear
staining, respectively. The following conditions were defined as
low HR+: Low ER+/low PR+, low ER+/PR-, and ER-/low PR+. The
therapeutic response of patients was investigated according to
alterations in tumor size that was determined by radiographic
assessment or clinical examination, as documented in the patient
medical records. The pathologic complete response (pCR) was defined
as the absence of invasive tumor in the breast resection specimen
and regional lymph nodes following surgery.
Patients had received NAC under various regimens.
The most common were anthracycline and cyclophosphamide followed by
paclitaxel (EC-T, daily injection, 21 days per cycle, 8 cycles
total) (13) or a combination of
three drugs (Paclitaxel/anthracycline/cyclophosphamide, TEC, daily
injection, 21 days per cycle, 6 cycles total) (14). Trastuzumab was routinely administered
to patients with human epidermal growth factor receptor-2 (HER-2)
positivity as an anti-HER therapy. Tamoxifen or aromatase
inhibitors were used as postoperative adjuvant endocrine
therapeutic agents for patients with HR+ tumors.
Statistical analysis
The expression data of HR staining from IHC analysis
were divided into three groups (HR+, low HR+ and HR-) and analyzed
as categorical variables. χ2 test was used to examine
the association between HR expression and the clinicopathological
factors of patients. Kaplan-Meier analysis was performed to
investigate the disease-free survival (DFS) and overall survival
(OS) of patients and a log-rank test was conducted to determine
significant differences. Patient features with P<0.1 in
univariate analysis were used for multivariate analysis, which was
used to determine differences in prognosis between the HR+, low HR+
and HR- groups. Forward conditional logistic regression was also
performed. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS software version 24 (IBM Corp.).
Results
Clinical and pathological
characteristics of tumors
The median age of the enrolled patients was 50 years
(range, 21–75 years), and 9.1% of patients were ≤35 years old. In
the present study, 301 (52.8%) patients had HR+ tumors, 209 (36.7%)
patients had HR- tumors and 60 (10.5%) patients had low HR+ tumors
(Table I). The median follow-up
duration for the 570 patients included in this analysis was 48.98
months (range, 22.37–93.73 months). The rate of patients who
successfully completed the follow up was 92% (n=525); 45 patients
discontinued contact during follow-up.
 | Table I.Comparison of clinicopathological
characteristics according to HR expression level in patients with
primary breast cancer. |
Table I.
Comparison of clinicopathological
characteristics according to HR expression level in patients with
primary breast cancer.
|
| HR positive | Low HR
positive | HR negative |
|
|---|
|
|
|
|
|
|
|---|
| Patient
characteristics | Number | % | Number | % | Number | % | P-value |
|---|
| Total | 301 | 52.81 | 60 | 10.53 | 209 | 36.67 |
|
| Age, years |
|
|
|
|
|
|
|
|
Median | 48 |
| 51 |
| 50 |
|
|
|
≤35 | 30 | 10.0 | 8 | 3.3 | 14 | 6.7 | 0.22 |
|
>35 | 271 | 90.0 | 52 | 86.7 | 195 | 93.3 |
|
| Menopausal
status |
|
|
|
|
|
|
|
|
Premenopausal | 181 | 60.1 | 27 | 45.0 | 99 | 47.4 | 0.01 |
|
Postmenopausal | 120 | 39.9 | 33 | 55.0 | 110 | 52.6 |
|
| Histology |
|
|
|
|
|
|
|
|
IDC | 279 | 92.7 | 58 | 96.7 | 200 | 95.7 | 0.23 |
|
Others | 21 | 7.0 | 2 | 3.3 | 8 | 3.8 |
|
|
Missing | 1 | 0.3 | – | – | 1 | 0.5 |
|
| Nuclear grade |
|
|
|
|
|
|
|
| I | 11 | 3.7 | – | – | – | – | <0.001 |
| II | 97 | 32.2 | 9 | 15.0 | 36 | 17.2 |
|
|
III | 47 | 15.6 | 12 | 20.0 | 53 | 25.4 |
|
|
Missing | 146 | 48.5 | 39 | 65.0 | 120 | 57.4 |
|
| AJCC stage |
|
|
|
|
|
|
|
|
IIA | 69 | 22.9 | 9 | 15.0 | 33 | 15.8 | 0.09 |
|
IIB/IIA | 174 | 57.8 | 34 | 56.7 | 132 | 63.2 |
|
|
IIIB/IIIC | 58 | 19.3 | 17 | 28.3 | 44 | 21.1 |
|
| Ki-67
expression |
|
|
|
|
|
|
|
|
≤14% | 80 | 26.6 | 6 | 10.0 | 17 | 8.1 | <0.001 |
|
>14% | 203 | 67.4 | 50 | 83.3 | 186 | 89.0 |
|
|
Missing | 18 | 6.0 | 4 | 6.7 | 6 | 2.9 |
|
| Her-2
expression |
|
|
|
|
|
|
|
|
Negative | 200 | 66.4 | 32 | 53.3 | 97 | 46.4 | <0.001 |
|
Equivocal | 37 | 12.3 | 4 | 6.7 | 14 | 6.7 |
|
|
Positive | 62 | 20.6 | 24 | 40.0 | 98 | 46.9 |
|
|
Missing | 2 | 0.7 | – | – | – | – |
|
| Therapeutic
evaluation |
|
|
|
|
|
|
|
|
cCR | 32 | 10.6 | 9 | 15.0 | 47 | 22.5 | 0.22 |
|
cPR | 192 | 63.8 | 41 | 68.3 | 103 | 49.3 |
|
|
cSD | 75 | 24.9 | 9 | 15.0 | 53 | 25.4 |
|
|
cPD | 2 | 0.7 | 1 | 1.7 | 6 | 2.9 |
|
| T stage |
|
|
|
|
|
|
|
|
T0/1 | 26 | 8.6 | 8 | 13.3 | 16 | 7.7 | 0.10 |
| T2 | 211 | 70.1 | 37 | 61.7 | 126 | 60.3 |
|
| T3 | 26 | 8.6 | 7 | 11.7 | 44 | 21.1 |
|
| T4 | 37 | 12.3 | 8 | 13.3 | 23 | 11.0 |
|
|
Missing | 1 | 0.3 | – | – | – | – |
|
| N stage |
|
|
|
|
|
|
|
| N0 | 60 | 19.9 | 6 | 10.0 | 38 | 18.2 | 0.05 |
| N1 | 177 | 58.8 | 34 | 56.7 | 120 | 57.4 |
|
| N2 | 37 | 12.3 | 10 | 16.7 | 28 | 13.4 |
|
| N3 | 27 | 9.0 | 10 | 16.7 | 23 | 11.0 |
|
Overall, HR+ tumors were detected more frequently in
premenopausal women (60.13%) compared with other subtypes (low HR+,
45.0% and HR-, 47.4%; P=0.006). A total of 537 (94.2%) patients had
invasive ductal carcinoma, and among the other patients, 14 (2.5%)
had invasive lobular carcinoma, 11 (1.9%) had invasive
micropapillary carcinoma, four (0.7%) had mucinous breast carcinoma
and four (0.7%) had metaplastic breast carcinoma. The majority of
patients had stage II (59.8%) or III (40.2%) disease. In addition,
there were 374 (65.6%) T2 tumors and 331 (58.1%) N1 tumors based on
the National Comprehensive Cancer Network (NCCN) guidelines
(15). Furthermore, the 11 (3.7%)
patients who had nuclear grade (NG) I tumors were included in the
HR+ group. Compared with HR+ tumors, low HR+ tumors were not
significantly different with regards to the histopathological type,
AJCC stage and T stage. In addition, compared with HR+ tumors, HR-
and low HR+ tumors exhibited higher NG (NG III of 20 and 25.4%,
respectively vs. 15.6%; P<0.001), upregulated Ki-67 expression
(83.3 and 89.0%, respectively vs. 67.4%; P<0.001), increased
HER-2 expression (40.0 and 46.9%, respectively vs. 20.6%;
P<0.001) and higher N stage (N2/N3 stages of 33.3 and 24.4%,
respectively vs. 21.3%; P=0.046). However, there was no difference
between the clinicopathological characteristics of HR- tumors and
low HR+ tumors (P>0.05).
A total of 424 (74.4%) patients presented a positive
clinical response (complete response and partial response) to NAC
based on the Response Evaluation Criteria In Solid Tumors (Table I). The rates of CR for HR+, low HR+
and HR- tumors were 10.6, 15.0 and 22.5%, respectively; however, no
statistically significant difference between the three groups was
observed (P=0.219).
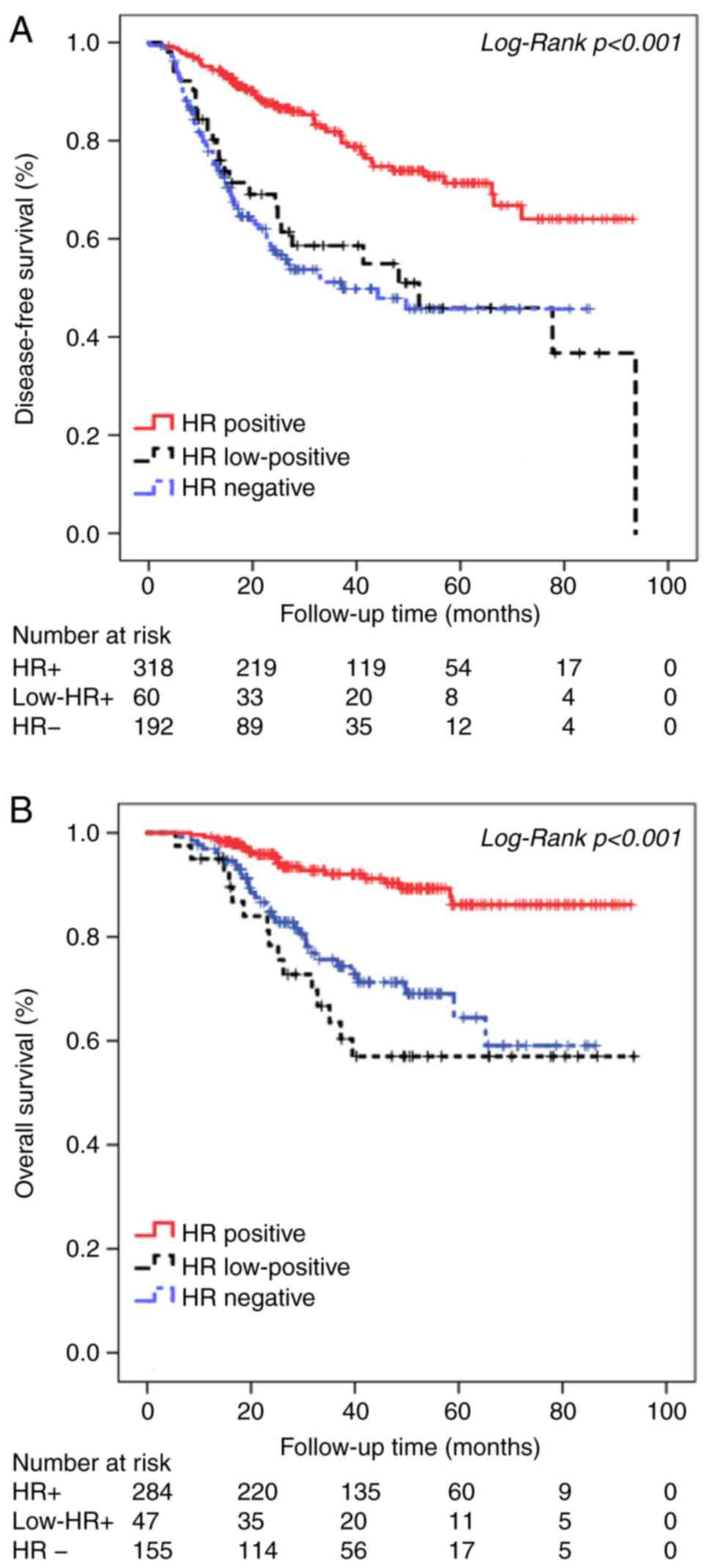
Survival analysis outcomes
Univariate analysis for DFS and OS in the HR+, HR-
and low HR+ tumors groups was performed using Kaplan-Meier survival
analysis. The survival curve for low HR+ tumors was located between
that of HR+ and HR− tumors. Patients with low
HR+ tumors had worse DFS and OS (Fig.
1) compared with patients with HR+ tumors, which was similar to
that of HR- patients. The median DFS was 72.71±2.42, 53.57±5.97 and
48.18±3.16 months for the HR+, low HR+ and HR- groups, respectively
(χ2=43.59; P<0.001). The median OS was 85.11±1.73,
63.92±5.92 and 65.24±3.17 months for the HR+, low HR+ and HR-
groups, respectively (χ2=28.31; P<0.001). The 5-year
DFS for patients with HR+, low HR+ and HR- tumors was 71.3, 45.9
and 45.7%, respectively, and the 5-year OS for patients with HR+,
low HR+ and HR- tumors was 86.2, 57.0 and 64.4%, respectively.
The multivariate Cox proportional hazards model was
used to determine differences in prognosis between the HR+, low HR+
and HR- tumors groups, excluding potential survival confounding
factors, including age, menopausal status, histological type, NG,
AJCC staging, and tumor and lymph node staging. As presented in
Table II, patients with HR+ tumors
had significantly better DFS and OS compared with patients with low
HR+ tumors (DFS, P=0.018; OS, P=0.019). In addition, patients with
HR- tumors had similar DFS and OS to patients with low HR+ tumors
(data not shown).
 | Table II.Cox regression analysis of patient
survival outcomes according to HR expression level in patients with
primary breast cancer. |
Table II.
Cox regression analysis of patient
survival outcomes according to HR expression level in patients with
primary breast cancer.
| A, Disease-free
survival |
|---|
|
|---|
|
| Univariate
Analysis | Multivariate
Analysis |
|---|
|
|
|
|
|---|
| HR status | Hazard Ratio | 95% CI | P-value | Hazard Ratio | 95% CI | P-value |
|---|
| Positive | 1 |
| <0.001 | 1 |
| <0.001 |
| Low-positive | 2.60 | 1.60–4.24 | 0.006 | 2.82 | 2.10–3.86 | 0.018 |
| Negative | 3.04 | 2.13–4.35 | <0.001 | 2.94 | 1.75–5.00 | <0.001 |
|
| B, Overall
survival |
|
|
| Univariate
Analysis | Multivariate
Analysis |
|
|
|
|
| HR
status | Hazard
Ratio | 95% CI | P-value | Hazard
Ratio | 95% CI | P-value |
|
| Positive | 1 |
| <0.001 | 1 |
| 0.001 |
| Low-positive | 4.57 | 2.34–8.92 | <0.001 | 4.76 | 4.11–5.56 | 0.019 |
| Negative | 3.28 | 1.87–5.74 | <0.001 | 4.76 | 2.08–11.11 | <0.001 |
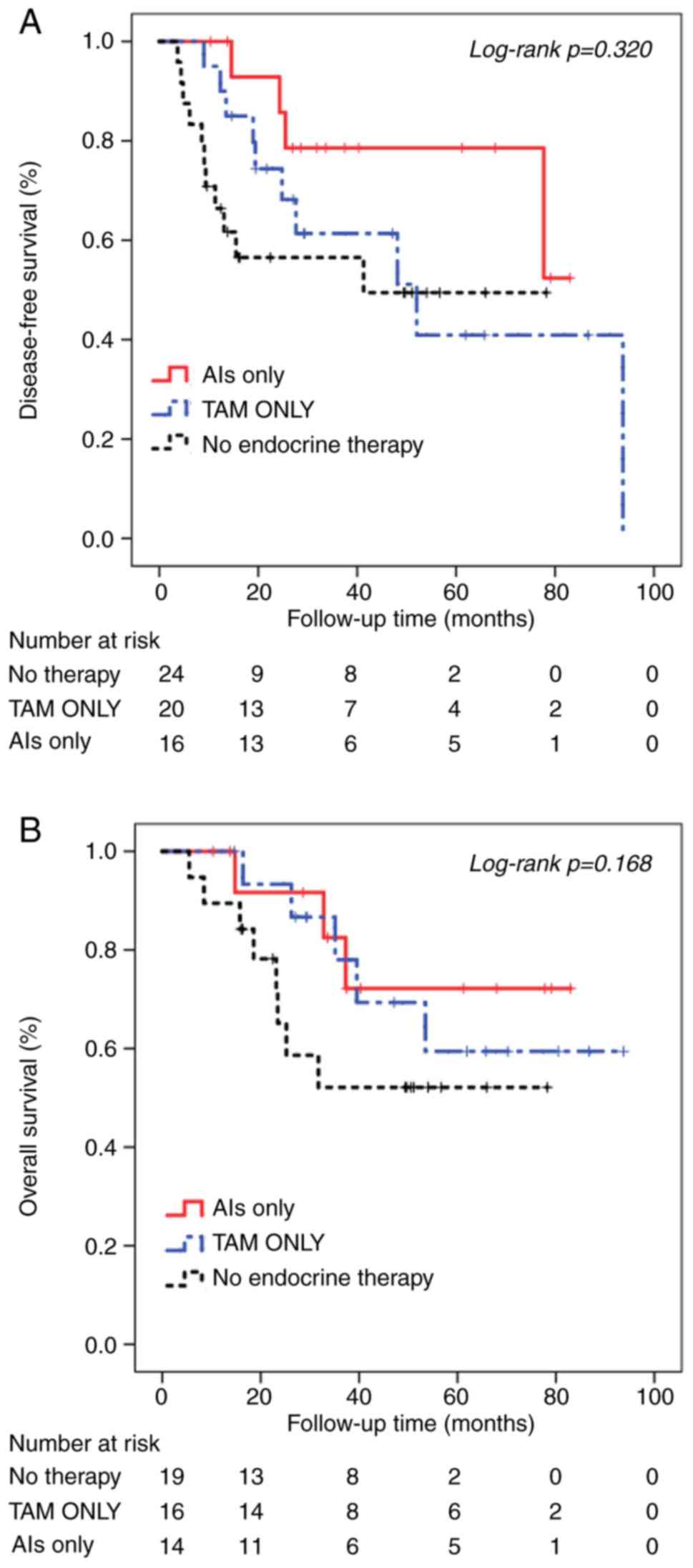
Among the 60 patients with low HR+ tumors, 24
(40.0%) patients did not receive postoperative adjuvant endocrine
therapy. The remaining 36 patients underwent different regimens of
endocrine therapy: 20 patients (33.3%) received tamoxifen and 16
patients (26.7%) were administered an aromatase inhibitor.
Univariate Kaplan-Meier analysis was performed of the low HR+
tumors group stratified according to the different endocrine
therapies administered. The results demonstrated that patients who
did not receive endocrine therapy had poorer DFS (45.44±7.20
months, Fig. 2A) and OS (50.21±7.31
months, Fig. 2B) compared with
patients who received endocrine therapy. Among patients who
received endocrine therapy, patients who received treatment with
the aromatase inhibitor exhibited better DFS (68.42±6.68 months)
compared with patients treated with tamoxifen (55.79±9.21 months).
However, there were no significant differences in the DFS and OS
between the three groups (DFS, χ2=2.28, P=0.320; OS,
χ2=3.56, P=0.168).
Discussion
The present study evaluated the clinicopathological
characteristics and prognosis of patients with low HR+ tumors
compared with patients with HR+ and HR- tumors. The results
demonstrated that 10.5, 52.8 and 36.7% of patients had low
HR+, HR+ and HR- tumors. As demonstrated in previous
studies, the clinical and biological features of low HR+ tumors
were similar to those of HR- tumors, which presented aggressive
biological behaviors (5,6). In addition, patients with low HR+
tumors of advanced stages presented an increased incidence of
aggressive phenotype. With regards to NG and the expression of
Ki-67 and HER-2, low HR+ tumors exhibited moderate characteristics
compared with the other two cohorts. The survival curve of patients
with low HR+ tumors was located between that of patients with HR+
and HR- tumors, which indicated poorer DFS and OS compared with
patients with HR+ tumors. It has been reported that increased
activity of the growth factor signaling pathways and upregulated
Ki-67 expression could be associated with the aggressiveness of low
HR+ tumors (16–19).
Numerous studies have investigated the concept of
low HR+ tumors (9,10,17);
however, the efficacy of NAC against low HR+ tumors remains to be
determined. In the majority of cases, this subtype are often not
considered in the clinical treatment of tumors, as patients are
divided into two categories: HR+ and HR-. The present study
identified a group of low HR+ tumors with a distinct
phenotype that were sensitive to NAC. Guarneri et al
(20) demonstrated that, although
the overall prognosis of TNBC is poor compared with that of breast
cancer luminal subtype, TNBC has a higher pCR rate following NAC
treatment. Furthermore, Carey et al (21) reported that there was no difference
in prognosis between patients with TNBC subtype and non-TNBC
subtypes who achieved pCR after NAC treatment. However, the
prognosis of TNBC subtype is significantly worse compared with
non-TNBC subtypes following non-pCR after NAC. compared with
non-triple-negative ones. The CREATE-X studies also reported that
in certain subgroups of breast cancer, including HER-2 positive and
TNBC types, increased pCR rate following NAC treatment could
benefit patient survival (7). The
present study evaluated the efficacy of chemotherapy in low HR+,
HR+ and HR- tumors following NAC treatment. The results
demonstrated that the pCR rates of the three groups following NAC
treatment were 10.63 (HR+), 15.00 (low HR+) and
22.49% (HR-). In addition, the pCR rate of the low HR+ group
was slightly increased compared with the HR+ group.
Regarding the response to NAC, the low HR+ cohort
appeared to have potentially benefited from postoperative enhanced
adjuvant chemotherapy regimens, including 6–8 cycles of
capecitabine.
To the best of our knowledge, only a few studies
have determined whether different endocrine therapy regimens:
Tamoxifen, aromatase inhibitor or combined treatment with ovarian
function suppression, affect the prognosis of patients with low HR+
tumors. The findings from the present study were consistent with
results from Yi et al (11)
that demonstrated that tumors with an ER-positivity rate of 1–9% do
not significantly benefit from endocrine therapy. However, the DFS
and OS of patients who received endocrine therapy had notably
improved compared with non treated patients (11). The St Gallen 2005 guidelines for the
primary therapy of early breast cancer (3) suggested the three following categories
for scoring ER status: i) Endocrine responsive, with strong ER
expression; ii) endocrine response uncertain, with low ER
expression; and iii) endocrine nonresponsive, with no ER
expression. These guidelines suggested that the endocrine
responsive group should receive endocrine therapy and adjuvant
chemotherapy; however, the distinction between ‘endocrine
responsive’ and ‘endocrine response uncertain’ was not determined
in the guidelines. It has been suggested that the loss of PR could
be considered as a marker of aberrant growth factor signaling and
was proposed as being associated with endocrine resistance
(22).
A recent meta-analysis reported that the recurrence
rate of breast cancer continued to rise over 5–20 years following
treatment and after 5 years of endocrine therapy, whereas the
cumulative risk may vary between 10 and 41% (23). The BIG19-8 (24) and ATAC (25) clinical studies have confirmed that
enhanced or prolonged endocrine therapy might be beneficial for the
survival of patients with a recurrence high risk. Furthermore, the
NCCN guidelines (version 3.2018) (26) recommended that some genomic assays
could have a prognostic value for screening patients with a high
risk of recurrence 0–10 years after surgery, including the 21-gene
Oncotype Dx assay (27), 70-gene
MammaPrint assay (28), PAM50
(Prosigna) (29), EPclin (12 gene,
EndoPredict) (30,31) and the Breast Cancer Index (32). Dowsett et al (33) reported a simpler predictive tool
called CTS5 for investigating endocrine therapy-enhancing
strategies that is based on the analysis of the clinicopathological
characteristics of patients with breast cancer. The results from
the present study suggested that the prognostic benefits of
postoperative adjuvant endocrine therapy may be restricted for low
HR+ patients; however, with the rational use of the
aforementioned effective tools to predict the risk of recurrence
following standard treatments in patients with high risk and poor
prognosis, including patients with low HR+ tumors, enhanced
endocrine therapy may be beneficial to patient survival.
This study presented certain limitations. This study
was a retrospective analysis, and the type of adjuvant treatment
was not administered on a randomized basis. Furthermore, patient
classification was not made according to treatment with
trastuzumab, since only 49.49% (98/198) of patients overexpressing
HER-2- received trastuzumab due to drug availability and
unfavorable health care policies.
In conclusion, the present study demonstrated that
patients with breast cancer and low HR+ tumors presented similar
clinicopathological characteristics to patients with HR- tumors.
Furthermore, patients with low HR+ tumors exhibited
poorer survival compared with patients with HR+ tumors. In
addition, no significant difference in the survival between
patients with low HR+ tumors and those with HR- tumors was
reported. These findings suggested that patients with low
HR+ tumors may benefit from postoperative intensive adjuvant
chemotherapy and endocrine therapy. Further investigation is
required to determine the underlying mechanism of low HR+
breast cancer. In addition, prospective clinical studies are
urgently needed to validate the importance of enhanced adjuvant
therapy for the prognosis of patients with low HR+ breast
cancer.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Zhejiang Medical
and Health Science and Technology Project, Zhejiang Provincial
Department of Health (grant no. 2017KY019).
Availability of data and material
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YD designed the study, analyzed and interpreted the
data, prepared figures, wrote the manuscript and supervised the
study. KD analyzed and interpreted the data. XD and DZ developed
the methodology and revised the manuscript. HY provided technical
support, assisted in developing the methodology and revised the
manuscript. XH and WM prepared tables and figures, performed
database research and analyzed and interpreted the data. KY and XY
provided and prepared histological sections from patients with
breast cancer. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Administration
Ethics Committee of Zhejiang Cancer Hospital and conducted in
accordance with the Principles of Helsinki Declaration. Patient
consent was not required because of the retrospective nature of the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AJCC
|
American Joint Committee on Cancer
|
|
DFS
|
disease-free survival
|
|
ER
|
estrogen receptor
|
|
HER-2
|
human epidermal growth factor receptor
2
|
|
HR
|
hormone receptor
|
|
NAC
|
neoadjuvant chemotherapy
|
|
NG
|
nuclear grade
|
|
pCR
|
pathological complete response
|
|
PR
|
progesterone receptor
|
|
OS
|
overall survival
|
|
TNBC
|
triple negative breast cancer
|
References
|
1
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), : Effects of chemotherapy and
hormonal therapy for early breast cancer on recurrence and 15-year
survival: An overview of the randomised trials. Lancet.
365:1687–1717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldhirsch A, Glick JH, Gelber RD, Coates
AS, Thürlimann B and Senn HJ; Panel members, : Meeting highlights:
International expert consensus on the primary therapy of early
breast cancer 2005. Ann Oncol. 16:1569–1583. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American Society of Clinical Oncology/College Of
American Pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. J Clin Oncol. 28:2784–2795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gloyeske NC, Dabbs DJ and Bhargava R: Low
ER+ breast cancer: Is this a distinct group? Am J Clin Pathol.
141:697–701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raghav KP, Hernandez-Aya LF, Lei X,
Chavez-Macgregor M, Meric-Bernstam F, Buchholz TA, Sahin A, Do KA,
Hortobagyi GN and Gonzalez-Angulo AM: Impact of low
estrogen/progesterone receptor expression on survival outcomes in
breast cancers previously classified as triple negative breast
cancers. Cancer. 118:1498–1506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES,
Yokota I, Kuroi K, Im SA, Park BW, Kim SB, et al: Adjuvant
capecitabine for breast cancer after preoperative chemotherapy. N
Engl J Med. 376:2147–2159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reddy SM, Barcenas CH, Sinha AK, Hsu L,
Moulder SL, Tripathy D, Hortobagyi GN and Valero V: Long-term
survival outcomes of triple-receptor negative breast cancer
survivors who are disease free at 5 years and relationship with low
hormone receptor positivity. Br J Cancer. 118:17–23. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prabhu JS, Korlimarla A, Desai K,
Alexander A, Raghavan R, Anupama C, Dendukuri N, Manjunath S,
Correa M, Raman N, et al: A majority of low (1–10%) ER positive
breast cancers behave like hormone receptor negative tumors. J
Cancer. 5:156–165. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Landmann A, Farrugia DJ, Zhu L, Diego EJ,
Johnson RR, Soran A, Dabbs DJ, Clark BZ, Puhalla SL, Jankowitz RC,
et al: Low estrogen receptor (ER)-positive breast cancer and
neoadjuvant systemic chemotherapy: Is response similar to typical
ER-positive or ER-negative disease? Am J Clin Pathol. 150:34–42.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yi M, Huo L, Koenig KB, Mittendorf EA,
Meric-Bernstam F, Kuerer HM, Bedrosian I, Buzdar AU, 0Symmans WF,
Crow JR, et al: Which threshold for ER positivity? a retrospective
study based on 9639 patients. Ann Oncol. 25:1004–1011. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC cancer staging manual. New York: 2017,
View Article : Google Scholar
|
|
13
|
von Minckwitz G, Raab G, Caputo A, Schütte
M, Hilfrich J, Blohmer JU, Gerber B, Costa SD, Merkle E, Eidtmann
H, et al: Doxorubicin with cyclophosphamide followed by docetaxel
every 21 days compared with doxorubicin and docetaxel every 14 days
as preoperative treatment in operable breast cancer: The GEPARDUO
study of the german breast group. J Clin Oncol. 23:2676–2685. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bines J, Earl H, Buzaid AC and Saad ED:
Anthracyclines and taxanes in the neo/adjuvant treatment of breast
cancer: Does the sequence matter? Ann Oncol. 25:1079–1085. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: NCCN guidelines insights: Breast cancer,
Version 1.2017. J Natl Compr Canc Netw. 15:433–451. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prat A, Cheang MC, Martin M, Parker JS,
Carrasco E, Caballero R, Tyldesley S, Gelmon K, Bernard PS, Nielsen
TO and Perou CM: Prognostic significance of progesterone
receptor-positive tumor cells within immunohistochemically defined
luminal A breast cancer. J Clin Oncol. 31:203–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bae SY, Kim S, Lee JH, Lee HC, Lee SK, Kil
WH, Kim SW, Lee JE and Nam SJ: Poor prognosis of single hormone
receptor- positive breast cancer: Similar outcome as
triple-negative breast cancer. BMC Cancer. 15:1382015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cancello G, Maisonneuve P, Rotmensz N,
Viale G, Mastropasqua MG, Pruneri G, Montagna E, Iorfida M, Mazza
M, Balduzzi A, et al: Progesterone receptor loss identifies Luminal
B breast cancer subgroups at higher risk of relapse. Ann Oncol.
24:661–668. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Braun L, Mietzsch F, Seibold P,
Schneeweiss A, Schirmacher P, Chang-Claude J, Peter Sinn H and
Aulmann S: Intrinsic breast cancer subtypes defined by estrogen
receptor signalling-prognostic relevance of progesterone receptor
loss. Mod Pathol. 26:1161–1171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guarneri V, Broglio K, Kau SW,
Cristofanilli M, Buzdar AU, Valero V, Buchholz T, Meric F,
Middleton L, Hortobagyi GN and Gonzalez-Angulo AM: Prognostic value
of pathologic complete response after primary chemotherapy in
relation to hormone receptor status and other factors. J Clin
Oncol. 24:1037–1044. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carey LA, Dees EC, Sawyer L, Gatti L,
Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML and Perou
CM: The triple negative paradox: Primary tumor chemosensitivity of
breast cancer subtypes. Clin Cancer Res. 13:2329–2334. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui X, Schiff R, Arpino G, Osborne CK and
Lee AV: Biology of progesterone receptor loss in breast cancer and
its implications for endocrine therapy. J Clin Oncol. 23:7721–7735.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pan H, Gray R, Braybrooke J, Davies C,
Taylor C, McGale P, Peto R, Pritchard KI, Bergh J, Dowsett M, et
al: 20-Year risks of breast-cancer recurrence after stopping
endocrine therapy at 5 Years. N Engl J Med. 377:1836–1846. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Breast International Group (BIG) 1–98
Collaborative Group and, ; Thurlimann B, Keshaviah A, Coates AS,
Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch
M, Gelber RD, et al: A comparison of letrozole and tamoxifen in
postmenopausal women with early breast cancer. N Engl J Med.
353:2747–2757. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cuzick J, Sestak I, Baum M, Buzdar A,
Howell A, Dowsett M and Forbes JF; ATAC/LATTE investigators, :
Effect of anastrozole and tamoxifen as adjuvant treatment for
early-stage breast cancer: 10-year analysis of the ATAC trial.
Lancet Oncol. 11:1135–1141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bevers TB, Helvie M, Bonaccio E, Calhoun
KE, Daly MB, Farrar WB, Garber JE, Gray R, Greenberg CC, Greenup R,
et al: Breast cancer screening and diagnosis, Version 3.2018, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
16:1362–1389. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paik S, Shak S, Tang G, Kim C, Baker J,
Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al: A
multigene assay to predict recurrence of tamoxifen-treated,
node-negative breast cancer. N Engl J Med. 351:2817–2826. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van't Veer LJ, Dai H, van de Vijver MJ, He
YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ,
Witteveen AT, et al: Gene expression profiling predicts clinical
outcome of breast cancer. Nature. 415:530–536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sestak I, Dowsett M, Zabaglo L,
Lopez-Knowles E, Ferree S, Cowens JW and Cuzick J: Factors
predicting late recurrence for estrogen receptor-positive breast
cancer. J Natl Cancer Inst. 105:1504–1511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Filipits M, Rudas M, Jakesz R, Dubsky P,
Fitzal F, Singer CF, Dietze O, Greil R, Jelen A, Sevelda P, et al:
A new molecular predictor of distant recurrence in ER-positive,
HER2-negative breast cancer adds independent information to
conventional clinical risk factors. Clin Cancer Res. 17:6012–6020.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Buus R, Sestak I, Kronenwett R, Denkert C,
Dubsky P, Krappmann K, Scheer M, Petry C, Cuzick J and Dowsett M:
Comparison of endopredict and EPclin with oncotype DX recurrence
score for prediction of risk of distant recurrence after endocrine
therapy. J Natl Cancer Inst. 108:2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jerevall PL, Ma XJ, Li H, Salunga R, Kesty
NC, Erlander MG, Sgroi DC, Holmlund B, Skoog L, Fornander T, et al:
Prognostic utility of HOXB13:IL17BR and molecular grade index in
early-stage breast cancer patients from the Stockholm trial. Br J
Cancer. 104:1762–1769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dowsett M, Sestak I, Regan MM, Dodson A,
Viale G, Thürlimann B, Colleoni M and Cuzick J: Integration of
clinical variables for the prediction of late distant recurrence in
patients with estrogen receptor-positive breast cancer treated with
5 years of endocrine therapy: CTS5. J Clin Oncol. 36:1941–1948.
2018. View Article : Google Scholar : PubMed/NCBI
|