Introduction
Solid tumors are composed of parenchyma and stroma,
which participate in forming cancer-stroma interactions that favor
tumor invasive growth in the tumor microenvironment (TME) (1). Recently, some studies have focused on
the cellular composition of the TME and suggested that the
interactions between cancer cells and stroma may be crucial for
progression of various types of carcinomas (2–5). Cancer
progression is recognized as the product of evolving crosstalk
between cancer cells and various stromal constituents, including
the extracellular matrix (6) and
fibroblasts. These stromal fibroblasts, which compose the major
stromal compartment (7), are often
termed myofibroblasts or cancer-associated fibroblasts (CAFs).
These cells play an extremely central role in the sophisticated
process of tumor-stroma interactions and subsequent carcinogenesis
(8–12). Many researchers have already
identified the ‘reactive stroma’ as an inducer that helps to create
a growth-promoting TME, which is accompanied by modified
extracellular matrix components (13), elevated angiogenesis (14,15),
modified fibroblasts (CAFs), etc. In the microenvironment of
cancers including those in the oral and maxillofacial regions, CAFs
play a primary role in cancer development and progression at all
stages (16–18). The overall effects of CAFs and
altered extracellular matrix in cancers are still poorly
understood. Additionally, tumor-associated macrophages (TAMs), the
specific type of macrophages present in cancer stroma, are involved
in tumor growth and metastasis. These stromal-specific fibroblasts
and macrophages acquire abnormalities by interacting with cancer
cells, along with gene expression and various biological properties
in normal tissues (13). Tumor blood
vessels also exist in TME and nourish not only cancer cells but
also stromal cells. In this way, the cancer-associated stroma
promotes cancer development and progression in many ways.
Cancer-stroma interactions depend on the organs in
which the cancer develops, and the whole picture is now being
revealed in several types of cancer (19). However, few articles have focused on
cancer-stroma interactions in oral squamous cell carcinoma (OSCC),
and the cellular composition of the stroma in OSCC has not been
fully elucidated in detail.
Recent studies have reported that bone
marrow-derived cells (BMDCs) act as not only a source of cells for
tissue regeneration (20,21) but also as participants in tumor
invasion and proliferation (22). In
normal tissue, BMDCs differentiate into various kinds of cells
(23,24) in normal head and neck sites such as
intestinal mucosal epithelium and salivary glands (25,26).
Furthermore, BMDCs are thought to be recruited from bone marrow
adjacent to injured tissue during wound healing and cancer
progression. In tumors, circulating BMDCs possess the capability of
differentiating into vascular endothelial cells, myofibroblasts,
etc., (27–29). Thus, BMDCs can differentiate into
various cell types that constitute the stroma of the tumor. As
described above, BMDCs are closely involved in tumorigenesis as
well as cancer progression.
Although the multilineage differentiating ability of
BMDCs has already been explored, the distribution, characteristics,
and precise roles of BMDCs in tumorigenesis and cancer invasion of
OSCC remain unclear. Thus, in this study, we investigated the
locations and characteristics of stromal cells and how BMDCs affect
cancer development and invasion of OSCC. We used a mouse bone
marrow transplantation (BMT) model that was created by grafting
bone marrow cells from green fluorescent protein (GFP) mice into
BALB-c nu-nu mice. This GFP-transplanted mouse model allows
tracking of BMDCs via tracing of GFP-positive cells. Then, these
recipient mice were transplanted with a human oral cancer cell line
(HSC-2 cells) injected into their head.
Materials and methods
Mouse strains and cell lines
All animal experiments were performed in accordance
with relevant guidelines and regulations and were approved by the
institutional committees at Okayama University (OKU-2017406). A
total of sixteen female mice (4 C57BL/6-BALB-C-nu/nu-GFP transgenic
mice and 12 BALB-c nu-nu mice) were used. The HSC-2 human oral
squamous cell carcinoma cell line was purchased from JRCB Cell Bank
and cultured in Dulbecco's modified Eagle's medium (DMEM)
(Invitrogen) with 10% fetal bovine serum (FBS) and 100 U/ml
antimycotic-antibiotic at 37°C in a humidified atmosphere with 5%
CO2.
BMT model
BMT was carried out according to a standard protocol
as described previously (30). Bone
marrow cells from GFP mice were collected by introducing DMEM
(Invitrogen) into the marrow space. Cells were resuspended in
Hanks' Balanced Salt Solution (Invitrogen) in a volume of
approximately 1.0×107 cells/0.20 ml. Subsequently,
8-week-old female nude recipient mice underwent 10 Gy of lethal
whole-body irradiation, and resuspended bone marrow cells were
injected into the tail vein of recipient mice. The bone marrow in
the tibial epiphysis was examined with GFP immunohistochemistry
(IHC) 4 weeks after transplantation.
Head lesion tumor mouse model
In this study, we used oral squamous cell carcinoma
cell line: HSC-2, because we have already known that HSC-2 induced
abundant stroma in previous study (31). Four weeks after BMT, 12-week-old
BALB-c nu-nu female mice that underwent GFP BMT were injected
subcutaneously into the head with 1.0×106 HSC-2 cells.
In implantation of HSC-2, mice were anesthetized intraperitoneally
with ketamine hydrochloride (75 mg/kg body weight), medetomidine
hydrochloride (0.5 mg/kg body weight) and we confirmed whether mice
were anesthetize atipamezole hydrochloride (1 mg/kg body weight)
was injected subcutaneously when awakening. This anesthesia
protocol has been improved with reference to Laboratory Animal
Anaesthesia, 3rd edition, and were approved by the institutional
committees at Okayama University (OKU-2017406). We confirmed mice
anesthesia whether mice could return to lying face down when mice
were laid supine position. Since this cancer cells injection in
head lesion did not impacted negatively in the wellbeing of the all
mice, twenty-eight days later, all mice were sacrificed by
isoflurane excess inhalation anesthesia (concentration more than
5%). Then we verified cardiac arrest by palpation and dislocated
the cervical spine of the mice according to AVMA Guideline for
Euthanasia of Animal 2013 Edition. And the specimens were harvested
for analysis. We established humane endpoints such as eating and
drinking problems, symptoms of distress (self-harm, abnormal
posture, respiratory problems), long-term appearance abnormalities
without signs of recovery (diarrhea, bleeding, dirt on vulva),
rapid weight loss (more than 20% in several days), and the cancer
tissue size to 3 cm or more. The experiment was discontinued if the
mice's pain was judged to be intolerable and the mice were
euthanized.
Histological examination
All embedded tissues were fixed in 4%
paraformaldehyde for 12 h and then decalcified in 10% EDTA for 3
weeks. Tissues were processed and embedded in paraffin wax via
routine histological preparation and sectioned at 5-µm thickness.
The sections were used for hematoxylin-eosin (H&E) staining,
IHC, and double-fluorescent IHC.
Immunohistochemistry
IHC was carried out using the antibodies detailed in
Table I. Following antigen
retrieval, sections were treated with 10% normal serum for 30 min,
and then incubated with primary antibodies at 4°C overnight. The
immunoreactive site was identified via the avidin-biotin complex
method (Vector Lab).
 | Table I.Antibodies used in
immunohistochemistry. |
Table I.
Antibodies used in
immunohistochemistry.
| Primary
antibody | Immunized
animal | Antigen
retrival | Dilution | Supplier (catalogue
no.) |
|---|
| GFP | Rabbit | 0.1% trysin at
37°C, 5 min | 1:1,000 | MBL (598) |
|
| Goat | Heated in 0.01
mol/l citrate buffer for 1 min | 1:200 | Abcam (ab6673) |
| CD11b | Rabbit | Heated in 0.01
mol/l citrate buffer for 1 min | 1:1,000 | Abcam
(ab133357) |
| CD31 | Rat | 0.1% trysin at
37°C, 5 min | 1:500 | Abcam
(ab56299) |
| Vimentin | Rabbit | Heated in 0.01
mol/l citrate buffer for 1 min | 1:100 | Abcam
(ab16700) |
| α-SMA | Rabbit | Heated in 0.01
mol/l citrate buffer for 1 min | 1:100 | Abcam (ab5694) |
Double-fluorescent IHC staining
Double-fluorescent IHC for GFP-CD11b, GFP-CD31,
GFP-Vimentin, and GFP-Alpha smooth muscle actin (α-SMA) was
performed using GFP monoclonal antibodies (Abcam). The secondary
antibodies applied are detailed in Table II. Antibodies were diluted with Can
Get SignalA (TOYOBO). After antigen retrieval, sections were
treated with Block Ace (DS Pharma Bio-medical) for 30 min at room
temperature. Specimens were incubated with primary antibodies at
4°C overnight and then incubated with secondary antibodies (1:200)
for 1 h at room temperature. After the reaction, the specimens were
stained with 1 mg/ml DAPI (Dojindo Laboratories).
 | Table II.Antibodies used in double-fluorescent
immunohistochemistry. |
Table II.
Antibodies used in double-fluorescent
immunohistochemistry.
| Secondary
antibody | Immunized
animal | Fluorescent
dye | Supplier (catalogue
no.) |
|---|
| Anti Goat IgG | Donkey | Alexa Flour
568 | Thermo
(A11057) |
|
| Donkey | Alexa Flour
488 | Thermo
(A11055) |
| Anti Rat IgG | Donkey | Alexa Flour
488 | Thermo
(A21209) |
| Anti Rabbit
IgG | Donkey | Alexa Flour
568 | Thermo
(A10042) |
|
| Donkey | Alexa Flour
488 | Thermo
(A21441) |
Cell counting
Cell counting was performed in three areas (Fig. 1A): the skin side (Fig. 1B), the center part of the tumor
(Fig. 1C), and the bone side
(Fig. 1D). The subsequent
histological analysis as well as findings of their characteristics
were made according to these divisions. In each areas, cells were
counted in five randomly chosen fields from selected regions
(magnification, ×200). Then, three members in our group manually
and counted the numbers of GFP(+) cells as well as double-positive
cells using Image J1.47v [developed by Wayne Rasband, the National
Institute of Health (NHS)]. In each areas, we measured the GFP(+)
cells in cancer stroma and we calculated the ratio of GFP(+) cells
in cancer stroma to determine the average of the 3 areas. The
obtained average value was compared in each group, the rate of
GFP(+) cells were compared in 3 areas.
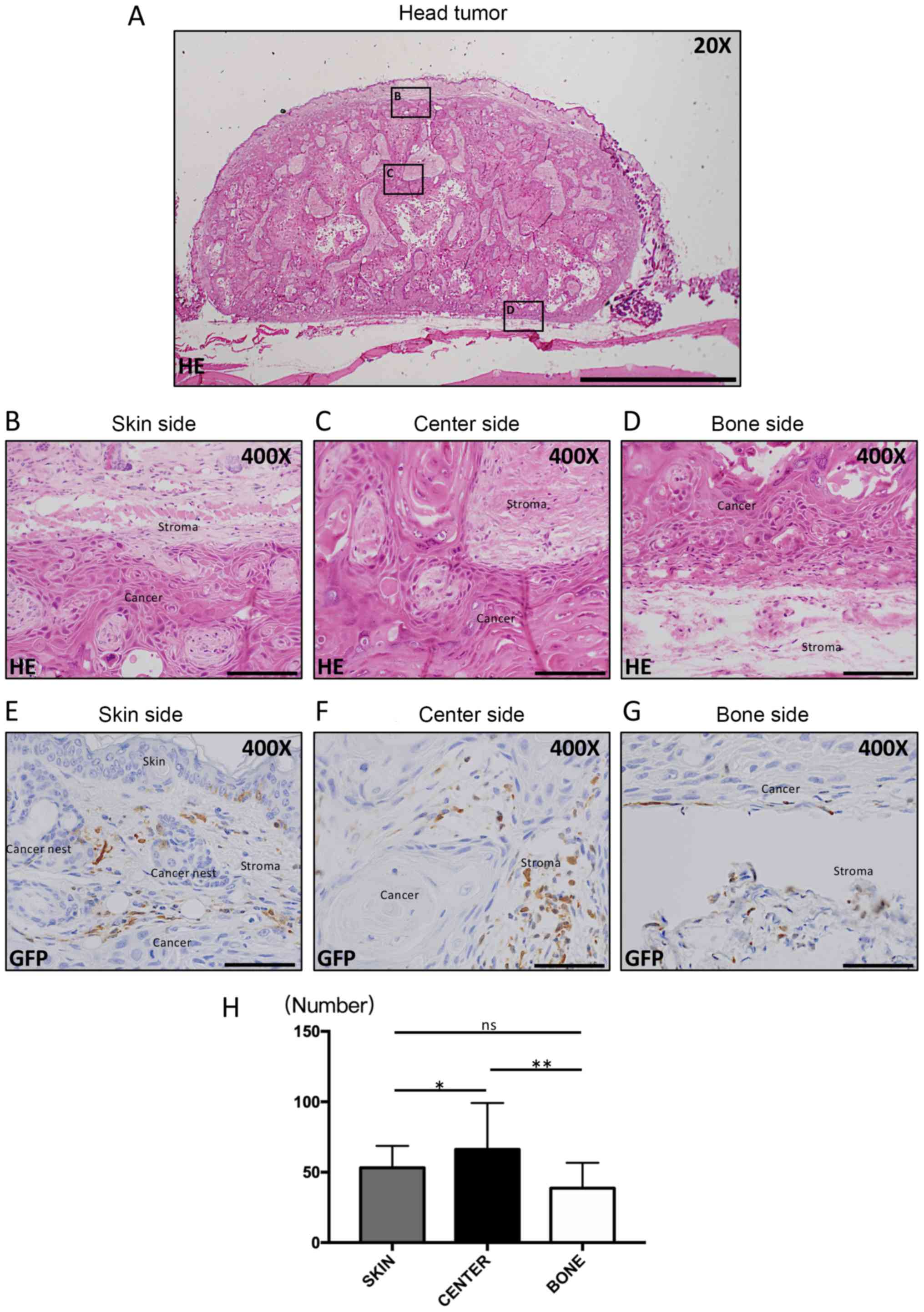 | Figure 1.Histopathological appearances and IHC
for GFP. Hematoxylin-eosin staining was performed. (A) Loupe image
of head cancer tissue (Scale bar, 2 mm; magnification, ×20). Round
or spindle-shaped cancer cells were observed in (B) the skin side,
(C) the center side and (D) the bone side of the tumor. Scale bars
in (B-D), 100 µm (magnification, ×400). GFP IHC staining was
subsequently performed. GFP-positive cells were mononuclear cells
and round or dendritic cells, and were demonstrated on the (E) skin
side, (F) center side and (G) bone side of the tumor. Scale bars in
(E-G), 1,500 µm (magnification, ×400). (H) The number of
GFP-positive cells was significantly higher in the center side of
the tumor compared with the skin and bone side. *P<0.01 and
**P<0.001 as indicated. IHC, immunohistochemistry; GFP, green
fluorescence protein; ns, no significance. |
Statistical analysis
All values are the mean ± standard deviation.
Statistical analysis was performed using one-way ANOVA and Tukey's
tests. P<0.05 was considered to indicate a statistically
significant difference. All calculations were made using PASW
Statistics 18 (SPSS Inc.).
Results
Histopathological characteristics of
the OSCC-transplanted mouse model
On twenty-eight day, the cancer cells were
successful engraftment and the largest tumor mass diameter was 15
mm and multiple tumors and metastasis were not observed in our
study. From H&E staining results, HSC-2 cancer cells grew with
the stroma around and in the center of cancer tissues. In the
center part of cancer tissues, keratinization, which is a typical
finding of well-differentiated squamous cell carcinoma, was
observed (Fig. 1A). The cancer
tissues were composed of loosely arranged spindle-shaped or
round-shaped, relatively small cancer cells, which made contact
with stromal cells on the skin side (Fig. 1B). Similarly, cancer cells were round
or polygonal in shape in the center area. However, cancer cells
along the periphery of the cancer stroma appeared to be polymorphic
in shape, bigger, and had a darker nucleus and tighter connections
(Fig. 1C). Interestingly, cancer
cells seemed to be distributed around the front layers, and the
most atypical cells were in the bone side of the tumor (Fig. 1D).
GFP-positive cell location and cell
shape
Abundant GFP-positive cells infiltrated the TME of
the three areas. The majority of them were located along the
peripheral sides of the cancer parenchyma and seemed to parallel
the tumor in the skin side (Fig. 1E)
and bone side (Fig. 1G), which may
represent the front layers. On the other hand, in the central part
of the cancer tissue, GFP-positive cells were not parallel to the
cancer nest, but accumulated in the central part of the cancer
stroma (Fig. 1F). GFP-positive cells
were round or spindle-shaped in the skin side (Fig. 1E), whereas they appeared rounder and
larger in the center side (Fig. 1F).
Intriguingly, they were thinner and smaller in the bone side
(Fig. 1G).
GFP-positive cell counting
analysis
The number of GFP-positive cells in the three areas
was counted (Fig. 1H). The number of
GFP-positive cells in the center side was significantly higher than
in the other two sides (P<0.05).
Vimentin location and cell shape
The cancer stroma consists of mesenchymal cells, and
thus, we performed staining for Vimentin, which is a popular marker
of these cells. Almost all stromal cells were Vimentin positive in
the three areas. In the skin side, the GFP-positive cells were
spindle- and round-shaped, and were distributed in the front layers
(Fig. 2A). On the other hand,
spindle-shaped cells were seen along the peripheral edges of cancer
nests in the center side, and these cells were obviously larger in
size (Fig. 2B). In the bone side,
most of the positive cells were spherical or dendritic, and were
the smallest in the three areas. These cells morphologically
resembled GFP-positive cells (Fig.
2C).
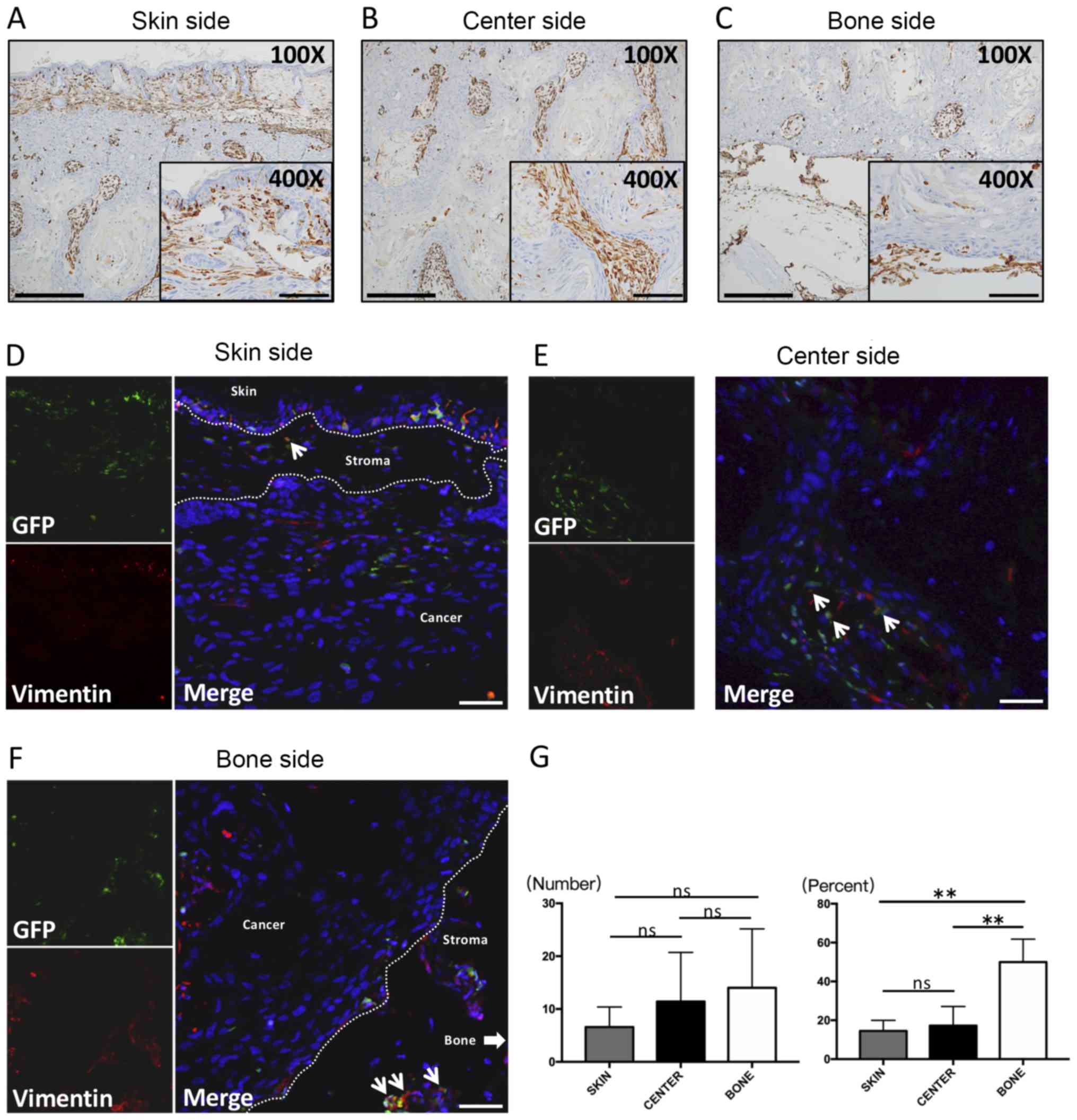 | Figure 2.IHC and immunofluorescence of
Vimentin. IHC staining for Vimentin was performed on the (A) skin
side, (B) center side and (C) bone side. All Vimentin cells were
present in the stroma, including on the cancer side.
Immunofluorescence double staining for Vimentin and GFP was
subsequently performed. Double-positive cells that were spindle or
round-shaped were exhibited in sections from the (D) skin side, (E)
center side and (F) bone side. (G) The number of Vimentin-positive
cells (left) and the percent of double-positive cells (right). No
significant differences in the number of Vimentin-positive cells
among the three areas were observed. However, the percent of
double-positive cells in the bone side was significantly higher
compared with the skin side and center side and the average percent
of the three areas was 27%. Thin arrows presented in (D-F) indicate
Vimentin (+) and GFP (+) double positive staining. The thick arrow
in (F) indicate the area of the bone side. Large scale bars in
(A-C), 200 µm (magnification, ×100); smaller scale bars in (A-C),
100 µm (magnification, ×400); Scale bars in (D-F), 100 µm
(magnification, ×200). **P<0.001 as indicated. IHC,
immunohistochemistry; GFP, green fluorescence protein; ns, no
significance. |
Vimentin double-fluorescent
staining
Spherical or dendritic-shaped Vimentin(+)GFP(+)
cells were observed in the skin side (Fig. 2D), center side (Fig. 2E), and bone side (Fig. 2F).
Vimentin cell counting analysis
No significant differences were found among the
three regions. However, Vimentin-positive cells tended to increase
in the following order: skin side, center side, bone side (Fig. 2G, left). Interestingly, the percent
of dual-positive cells in the bone side, which represents the front
layers of the bone invasion area, was significantly the highest
among the three areas of cancer (P<0.05; Fig. 2G, right). Also, the average percent
among the three areas was calculated as 27% (data shown in Fig. 2G, right).
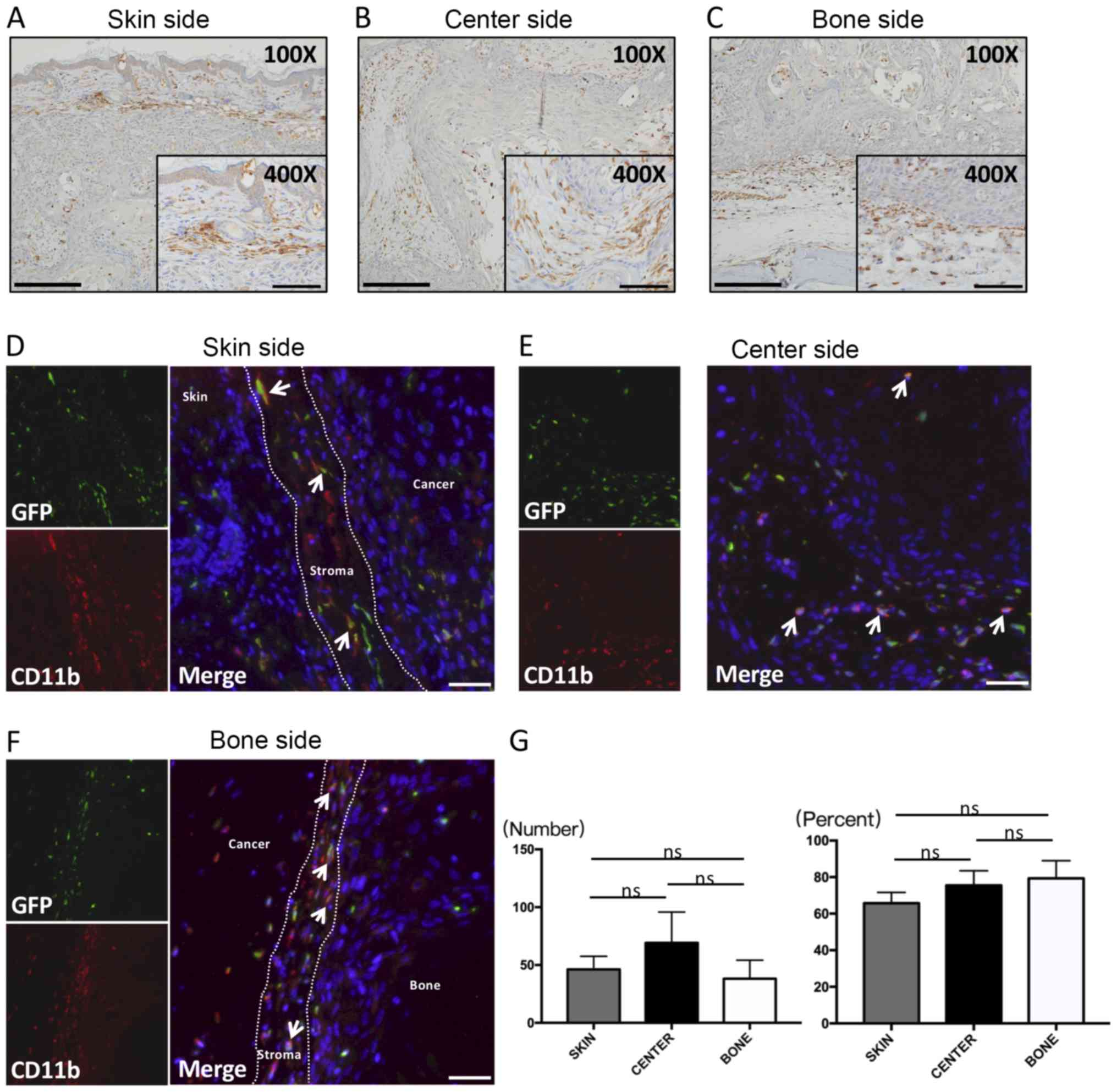
CD11b location and cell shape
CD11b is a marker of various kinds of monocytes,
especially macrophages, some of which are TAMs in tumors. Numerous
studies have reported that CD11b plays a role in invasion as well
as metastasis of cancer. Abundant CD11b-positive cells infiltrated
stromal tissues in the three areas. CD11b-positive cells were
spindle-shaped, located in parallel with each other, and scattered
along the front layers in the skin side (Fig. 3A) and bone side (Fig. 3C). Many spherical or dendritic-shaped
CD11b-positive cells were found in the stroma of the center side
(Fig. 3B), and were similar to
GFP-positive cells in shape. Moreover, CD11b-positive cells were
also observed around necrotic areas of the cancer center (Fig. 3B; ×100, black arrows).
CD11b double-fluorescent staining
Spindle-shaped GFP-positive cells co-expressing
CD11b were observed along the front layers mostly in the skin side
(Fig. 3D) and bone side (Fig. 3F). These cells had critical impacts
on cancer invasion. At the same time, these double-positive, round
or dendritic cells infiltrated necrotic areas of the cancer center
(Fig. 3E).
CD11b cell counting analysis
The accumulation of CD11b-positive cells tended to
be higher in the central area, although no significant difference
was observed (Fig. 3G, left). On the
other hand, the percent of CD11b(+)GFP(+) cells in the bone side,
which represented the front layers of the bone invasion area, was
the highest among the three areas of cancer, although the
difference was not significant (Fig.
3G, right).
CD31 location and cell shape
CD31 is a marker associated with angiogenesis and
labels vascular endothelial cells of blood vessels in cancer
tissues. Our findings were consistent with other experiments
showing that CD31 was positive in vascular endothelial cells that
formed lumens. The majority of the vessels in the skin side
(Fig. 4A) and bone side (Fig. 4C) had smaller and narrower cavities.
On the other hand, mature blood vessels with larger lumens and
thicker walls were found in the center side of the tumor (Fig. 4B). In addition, spherical or
dendritic cells with no evidence of lumen formation were CD31
positive. These spherical or dendritic CD31-positive cells were
located adjacent to the cancer invasive front both in the skin side
(Fig. 4A) and bone side (Fig. 4C).
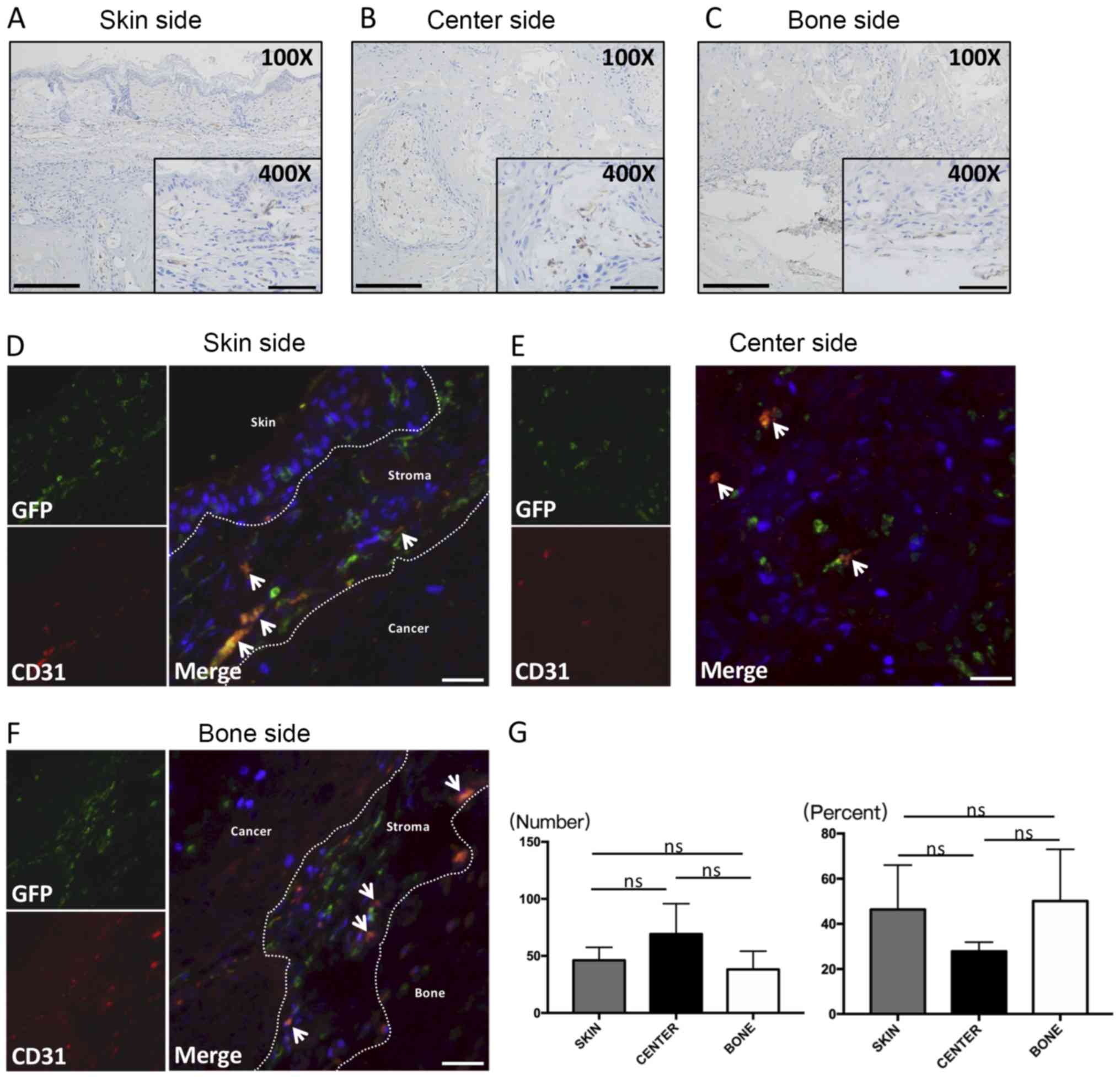 | Figure 4.IHC and immunofluorescence of CD31.
IHC features of CD31 are presented. Most of the CD31 cells are
observed along the front layers of the tumor in (A) the skin side
and (C) the bone side, whereas CD31 cells are distributed in the
center of the stroma in (B) the center side. The majority of the
positive cells were similar to lacunae vasorums. The lumens in the
center side appeared larger than those in the skin and bone sides.
Immunofluorescence double staining with CD31 and GFP was
subsequently performed. Double-positive cells are spindle-shaped or
rounded cells were observed in the (D) skin side, (E) center side
and (F) bone side. (G) The number of CD31-positive cells (left) and
the percent of double-positive cells (right) are presented. No
significant differences were identified. Thin arrows in (D-F)
represent CD31 (+) and GFP (+) double positive staining. Larger
scale bars in (A-C), 200 µm (magnification, ×100); smaller scale
bars in (A-C), 100 µm (magnification, ×400); scale bars in (D-F),
100 µm (magnification, ×200). IHC, immunohistochemistry; GFP, green
fluorescence protein; ns, no significance. |
CD31 double-fluorescent staining
In the skin side (Fig.
4D) and bone side (Fig. 4F),
abundant spherical double-positive cells were found to a higher
extent than in the center side (Fig.
4E).
CD31 cell counting analysis
The number of CD31-positive cells tended to be lower
in the peripheral sides such as the skin side and bone side than in
the central area of the cancer (Fig.
4G, left). However, the number of CD31(+)GFP(+) cells tended to
be higher in peripheral sides than in the central area of the
cancer (Fig. 4G, right).
α-SMA location and cell shape
α-SMA is considered to be a common marker of
myoepithelial cells, especially CAFs, in many cancer types
(32). Many α-SMA-positive cells
were detected in the three areas of the cancer. These cells were
arranged parallel to the front layers of the cancer in the skin
side (Fig. 5A) and bone side
(Fig. 5C). In the center side, most
of the α-SMA-positive cells had accumulated around the borders
between the cancer parenchyma and stroma, like a layer (Fig. 5B). The large majority of
α-SMA-positive cells were spindle-shaped cells with long
cytoplasmic extensions, and were obviously different from the shape
of GFP-positive cells in the three regions.
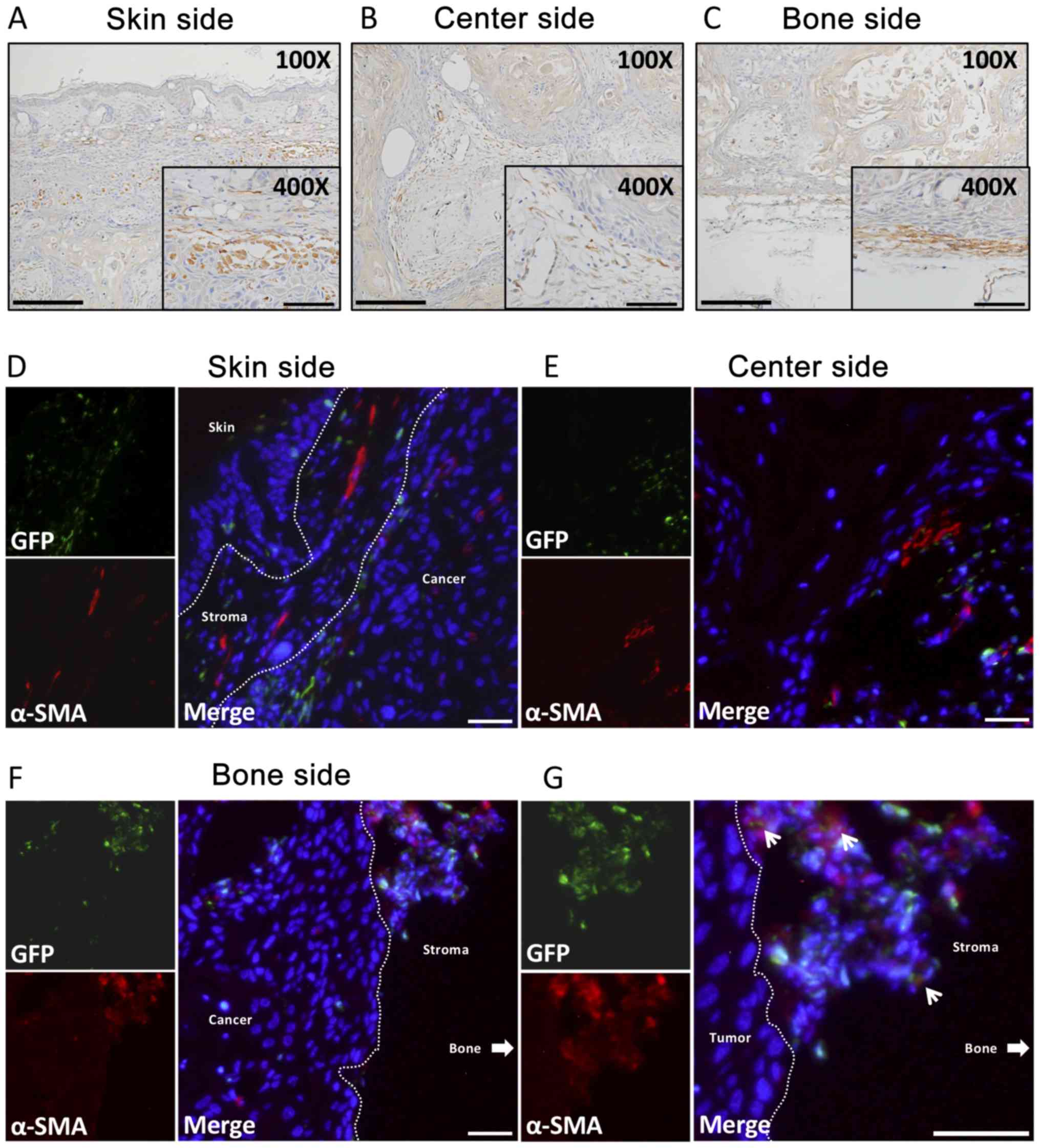 | Figure 5.IHC and immunofluorescence of α-SMA.
IHC features of α-SMA are presented. Positive cells were scattered
in different areas within (A) the skin side and (C) the bone side,
but were parallel the front-most layers. α-SMA-positive cells
accumulated around the cancer parenchyma in (B) the center side.
Immunofluorescence double staining with α-SMA and GFP was
subsequently performed. Almost no double-positive cells were
observed in (D) the skin side (square: skin area; triangle: stromal
area; asterisk: tumor area) and (E) the center side. However, a few
double-positive cells in (F) the bone side were observed. The area
of double-positive cells is magnified in (G). Thin arrows in (F)
represent α-SMA (+) and GFP (+) double positive staining. Thick
arrow in (F) represents the area of the bone side. Larger scale
bars in (A-C), 200 µm (magnification, ×100); smaller scale bars in
(A-C), 100 µm (magnification, ×400); scale bars in (D-F; F right
image), 100 µm (magnification, ×200); scale bar in (F; left image),
100 µm (magnification, ×400). IHC, immunohistochemistry; SMA,
smooth muscle actin; GFP, green fluorescence protein. |
α-SMA double-fluorescent staining
Almost no GFP-positive cells were also α-SMA
positive, both in the skin side (Fig.
5D) and center side (Fig. 5E).
In the bone side, however, a few α-SMA(+)GFP(+) cells were detected
(Fig. 5F) and had a round shape
(Fig. 5G).
Discussion
Our experiments showed that GFP-labeled BMDCs were
abundant in the cancer stroma of OSCC. Also, we evaluated the
distribution of various cellular constituents and morphological
evidence for multilineage differentiation derived from transplanted
BMDCs within the TME of OSCC using a BMT mouse model. The cancer
stroma can be obviously detected with Vimentin because OSCC is an
epithelial tumor and Vimentin is a popular marker of mesenchymal
cells. Thus, our results confirmed that the majority of stromal
cells were Vimentin positive in the cancer stroma. Interestingly,
Vimentin-positive cells tended to increase in the order of the skin
side, center side, and bone side. Recently, close associations
between Vimentin and cancer development and progression as well as
chemosensitivity were suggested by various gene profiling studies
(33–35). Also, Vimentin expression in
colorectal cancer stroma is correlated with shorter survival of
patients (36). Double
immunofluorescent staining showed that the percent of
Vimentin(+)GFP(+) cells was about 27%, indicating that many BMDCs
became incorporated into the cancer stroma and may play potential
roles in tumorigenesis. Additionally, the percent of Vimentin/GFP
double-positive cells in the bone side, which is the front layer of
the bone invasion area, was significantly the highest among the
three areas of the tumor. The stroma of OSCC contained
approximately 50% GFP-positive cells (BMDCs) in the bone side
(Fig. 2G, right). Compared with the
other two sides of the tumor, more GFP-positive cells had
accumulated in the tumor center, mostly within tumor stromal cells;
these cells were rounder and larger. Abundant GFP-labeled BMDCs
were previously described in gastric and colon cancer stroma
(37,38). Udagawa et al showed that the
rate of recruitment of BMDCs varies among different cancer types
(39). Lung carcinoma is composed of
30–40% non-tumor cells recruited from bone marrow. In contrast, the
same study showed that the recruitment was lower in a model of
osteosarcoma. Thus, our results indicated that BMDCs may
participate in cancer progression and development, especially the
process of cancer invasion, because BMDCs infiltrated into the
invasive front of the tumor. Furthermore, BMDCs were recruited by
the OSCC in the same proportion compared with other types of cancer
(Fig. 2G, right). In addition to
GFP-positive cells, we traced other important cell types in the
cancer stroma. Their characteristics and potential roles are
discussed further below.
CD11b is generally known as a marker of monocytes,
macrophages, and TAMs (40). TAMs
are involved in tumor growth and metastasis (41). In our results, CD11b-positive cells
were round or spherical-shaped near the necrotic areas in the
center side of the cancer, and more than half of CD11b-positive
cells were GFP positive (Fig. 3G,
right). Thus, these CD11b(+)GFP(+) cells were thought to be
macrophages that function to phagocytize necrotic tissues. On the
other hand, CD11b-positive cells that contacted the cancer
parenchyma in the skin side and bone side were spindle-shaped cells
that were situated parallel with each other and were scattered
along the front layers; more than half of these cells were GFP
positive (Fig. 3G, right).
Considering the characteristics of their distribution and shape,
CD11b-positive cells in the skin and bone side may represent TAMs.
Our results indicated that CD11b-positive cells may engulf necrotic
tissue in the center area of the cancer and participate in cancer
invasion around the peripheral areas of the cancer, especially in
the bone side. Therefore, BMDCs likely play a crucial role,
especially at the periphery of the cancer, as TAMs.
Angiogenesis of tumors has critical impacts on
development of the tumor. The details of the contribution of BMDCs
to tumor angiogenesis are still unknown. However, bone
marrow-derived endothelial progenitor cells and tissue stem cells
have been identified (42).
Moreover, recent studies have provided increasing evidence that
postnatal neovascularization does not rely exclusively on sprouting
of preexisting vessels, but also involves bone marrow-derived
circulating endothelial precursors (43). In our study, about half of the
CD31-positive cells were derived from bone marrow in the cancer
stroma, and the number of CD31-positive cells tended to be higher
in the peripheral areas of the cancer compared to the center side.
However, the opposite trend was observed for CD31(+)GFP(+) cells.
Mature blood vessels with larger lumens and thicker walls were
found in the center side, providing compulsory nutrition for
tumorigenesis, compared with the peripheral sides of the cancer.
Therefore, BMDCs are involved in tumor angiogenesis, and especially
CD31(+)GFP(+) cells may participate in tumor angiogenesis in
invasive areas owing to the higher quantity of BMDCs in tumor
peripheral areas.
α-SMA is a popular marker of myoepithelial cells and
CAFs in tumors. We found many spindle-shaped α-SMA-positive cells
surrounding the cancer parenchyma. However, almost no
α-SMA(+)GFP(+) cells were seen. Therefore, in cancer stroma,
α-SMA-positive cells are derived from recipient tissue. Several
studies have explored the origins of CAFs, including resident
fibroblasts (44), smooth muscle
cells, endothelial cells, epithelial cells (through
epithelial-mesenchymal transition), fibrocytes, and BMDCs such as
mesenchymal stem cells (45,46). Moreover, another study discovered
that BMDCs may change to cancer-associated orthotopic
myofibroblasts by the education of gastric cells (37). Another study indicated that about 20%
of local CAFs were derived from mesenchymal stem cells present in
the bone marrow using a gastric inflammatory carcinogenesis model
(47). However, the methods of these
reports were different from our study method. In the previous
studies, only mesenchymal stem cells (MSCs) or adhesive cells of
bone marrow were transplanted, on the other hand, in our study we
transplanted all bone marrow cells. In previous studies, MSCs of
bone marrow differentiated into CAFs. However, the bone marrow
includes hematopoietic stem cells, mesenchymal stem cells, and
somatic pluripotent progenitor cells, and mesenchymal stem cells
only comprise 0.001–0.01% of cells in the bone marrow and
mesenchymal stem cells content is very low (48). Thus, it is possible that mesenchymal
stem cells failed to engraft following bone marrow transplantation
in this study, but our methods mimicked in vivo condition.
In terms of tumor stroma, there are almost two orientations,
generally. One of origins is bone marrow, the other is host tissues
around tumor. In our study, in oral squamous cell carcinoma, these
α-SMA(+) CAFs might originate mostly from host tissues rather than
BMDCs. Therefore, our results showed that unlike these studies, in
OSCC, CAFs originate mostly from the host tissue rather than BMDCs.
We discovered very few α-SMA(+)GFP(+) cells in the bone side after
amplification. However, these cells may not be CAFs because the
morphology of these cells was round, which distinguished them from
CAFs. Our experiments indicated that GFP-positive BMDCs may play an
important role in inducing CAFs because these GFP-positive cells
were distributed side by side with α-SMA-positive cells.
In conclusion, given all the findings we observed
from the GFP mouse BMT model, BMDCs may participate in the
processes of tumorigenesis and cancer development. The different
distributions and morphological characteristics provide authentic
evidence for the involvement of BMDCs in the development of cancer
via differentiation into various kinds of cells in the cancer
stroma, such as macrophages, fibroblasts, angioblasts, etc. Our
results suggest roles for these cells in tumorigenesis due to their
multilineage differentiation potential.
Acknowledgements
Not applicable.
Funding
The presen study was funded by Japanese Society for
Promotion of Science KAKENHI Grant-in-Aid for Scientific Research
(grant nos. 18K09789, 18K17224, 16K20577 and 19K19160).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CA and KT designed the experiments. HK, SY, MWO and
HO performed immunohistochemistry. MF and SS performed statistical
analysis. KN, HT, ZJN and HN collected the data and drafted the
manuscript. All authors approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were performed in accordance
with relevant guidelines and regulations, and were approved by the
institutional Committees of Okayama University (approval no.
OKU-2017406).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
α-SMA
|
alpha smooth muscle actin
|
|
BMDCs
|
bone marrow-derived cells
|
|
BMT
|
bone marrow transplantation
|
|
CAFs
|
cancer-associated fibroblasts
|
|
GFP
|
green fluorescent protein
|
|
HSC-2 cells
|
human squamous cell carcinoma-2
cells
|
|
H&E
|
hematoxylin-eosin
|
|
IHC
|
immunohistochemistry
|
|
OSCC
|
oral squamous cell carcinoma
|
|
TAMs
|
tumor-associated macrophages
|
|
TME
|
tumor microenvironment
|
References
|
1
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yashiro M and Hirakawa K: Cancer-stromal
interactions in scirrhous gastric carcinoma. Cancer Microenviron.
3:127–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fuyuhiro Y, Yashiro M, Noda S, Matsuoka J,
Hasegawa T, Kato Y, Sawada T and Hirakawa K: Cancer-associated
orthotopic myofibroblasts stimulates the motility of gastric
carcinoma cells. Cancer Sci. 103:797–805. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tripathi M, Billet S and Bhowmick NA:
Understanding the role of stromal fibroblasts in cancer
progression. Cell Adhes Migr. 6:231–235. 2012. View Article : Google Scholar
|
|
5
|
Harper J and Sainson RC: Regulation of the
anti-tumour immune response by cancer-associated fibroblasts. Semin
Cancer Biol. 25:69–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li H, Fan X and Houghton JM: Tumor
microenvironment: The role of the tumor stroma in cancer. J Cell
Biochem. 101:805–815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clark RAF: The molecular cell biology of
wound repairPlenum Press New York. 3. pp. 501996
|
|
8
|
Desmoulière A, Geinoz A, Gabbiani F and
Gabbiani G: Transforming growth factor-beta 1 induces alpha-smooth
muscle actin expression in granulation tissue myofibroblasts and in
quiescent and growing cultured fibroblasts. J Cell Biol.
122:103–111. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roberts AB, Sporn MB, Assoian RK, Smith
JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl
JH, et al: Transforming growth factor type beta: Rapid induction of
fibrosis and angiogenesis in vivo and stimulation of collagen
formation in vitro. Proc Natl Acad Sci USA. 83:4167–4171. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tjomsland V: Studies of the tumor
microenvironment: Local and systemic effects exerted by the
cross-talk between tumor and stroma cells in pancreatic cancer. PhD
dissertation. Linköping UniversityPublication no. 1219. Linköping;
Sweden: 2010
|
|
11
|
Bhowmick NA, Neilson EG and Moses HL:
Stromal fibroblasts in cancer initiation and progression. Nature.
432:332–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Wever O and Mareel M: Role of tissue
stroma in cancer cell invasion. J Pathol. 200:429–447. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Micke P and Ostman A: Exploring the tumour
environment: Cancer-associated fibroblasts as targets in cancer
therapy. Expert Opin Ther Targets. 9:1217–1233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhowmick NA, Chytil A, Plieth D, Gorska
AE, Dumont N, Shappell S, Washington MK, Neilson EG and Moses HL:
TGF-beta signaling in fibroblasts modulates the oncogenic potential
of adjacent epithelia. Science. 303:848–851. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mbeunkui F and Johann DJ Jr: Cancer and
the tumor microenvironment: A review of an essential relationship.
Cancer Chemother Pharmacol. 63:571–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Costea DE, Hills A, Osman AH, Thurlow J,
Kalna G, Huang X, Pena Murillo C, Parajuli H, Suliman S, Kulasekara
KK, et al: Identification of two distinct carcinoma-associated
fibroblast subtypes with differential tumor-promoting abilities in
oral squamous cell carcinoma. Cancer Res. 73:3888–3901. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Routray S, Sunkavali A and Bari KA:
Carcinoma-associated fibroblasts, its implication in head and neck
squamous cell carcinoma: A mini review. Oral Dis. 20:246–253. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naritani M, Inoue M, Raju R, Miyagi M,
Oshima M and Matsuka Y: Analysis of Bone marrow-derived mesenchymal
stem cell kinetics after short-term stimulation with tumor necrosis
factor-α (TNF-α). J Hard Tissue Biol. 28:99–108. 2019. View Article : Google Scholar
|
|
21
|
Badiavas EV, Abedi M, Butmarc J, Falanga V
and Quesenberry P: Participation of bone marrow derived cells in
cutaneous wound healing. J Cell Physiol. 196:245–250. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Houghton J, Stoicov C, Nomura S, Rogers
AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR and Wang TC:
Gastric cancer originating from bone marrow-derived cells. Science.
306:1568–1571. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Caplan AI: Mesenchymal stem cells. J
Orthop Res. 9:641–650. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krause DS, Theise ND, Collector MI,
Henegariu O, Hwang S, Gardner R, Neutzel S and Sharkis SJ:
Multi-organ, multi-lineage engraftment by a single bone
marrow-derived stem cell. Cell. 105:369–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsujigiwa H, Nishizaki K, Teshima T,
Takeda Y, Yoshinobu J, Takeuchi A, Orita Y, Sugata Y, Nagatsuka H
and Nagai N: The engraftment of transplanted bone marrow-derived
cells into the olfactory epithelium. Brain Res. 1052:10–15. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aghi M and Chiocca EA: Contribution of
bone marrow-derived cells to blood vessels in ischemic tissues and
tumors. Mol Ther. 12:994–1005. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lyden D, Hattori K, Dias S, Costa C,
Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et
al: Impaired recruitment of bone-marrow-derived endothelial and
hematopoietic precursor cells blocks tumor angiogenesis and growth.
Nat Med. 7:1194–1201. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bamba S, Lee CY, Brittan M, Preston SL,
Direkze NC, Poulsom R, Alison MR, Wright NA and Otto WR: Bone
marrow transplantation ameliorates pathology in interleukin-10
knockout colitic mice. J Pathol. 209:265–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kawai H, Tsujigiwa H, Siar CH, Nakano K,
Takabatake K, Fujii M, Hamada M, Tamamura R and Nagatsuka H:
Characterization and potential roles of bone marrow-derived stromal
cells in cancer development and metastasis. Int J Med Sci.
15:1406–1414. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Masui M, Okui T, Shimo T, Takabatake K,
Fukazawa T, Matsumoto K, Kurio N, Ibaragi S, Naomoto Y, Nagatsuka H
and Sasaki A: Novel midkine inhibitor iMDK inhibits tumor growth
and angiogenesis in oral squamous cell carcinoma. Anticancer Res.
36:2775–2781. 2016.PubMed/NCBI
|
|
32
|
Mhawech-Fauceglia P, Wang D, Samrao D, Kim
G, Lawrenson K, Meneses T, Liu S, Yessaian A and Pejovic T:
Clinical implications of marker expression of carcinoma-associated
fibroblasts (CAFs) in patients with epithelial ovarian carcinoma
after treatment with neoadjuvant chemotherapy. Cancer Microenviron.
7:33–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zajchowski DA, Bartholdi MF, Gong Y,
Webster L, Liu HL, Munishkin A, Beauheim C, Harvey S, Ethier SP and
Johnson PH: Identification of gene expression profiles that predict
the aggressive behavior of breast cancer cells. Cancer Res.
61:5168–5178. 2001.PubMed/NCBI
|
|
34
|
Mellick AS, Day CJ, Weinstein SR,
Griffiths LR and Morrison NA: Differential gene expression in
breast cancer cell lines and stroma-tumor differences in
microdissected breast cancer biopsies revealed by display array
analysis. Int J Cancer. 100:172–180. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peñuelas S, Noé V and Ciudad CJ:
Modulation of IMPDH2, survivin, topoisomerase I and vimentin
increases sensitivity to methotrexate in HT29 human colon cancer
cells. FEBS J. 272:696–710. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ngan CY, Yamamoto H, Seshimo I, Tsujino T,
Man-i M, Ikeda JI, Konishi K, Takemasa I, Ikeda M, Sekimoto M, et
al: Quantitative evaluation of vimentin expression in tumour stroma
of colorectal cancer. Br J Cancer. 96:986–992. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kasashima H, Yashiro M, Nakamae H, Masuda
G, Kinoshita H, Morisaki T, Fukuoka T, Hasegawa T, Sakurai K,
Toyokawa T, et al: Bone marrow-derived stromal cells are associated
with gastric cancer progression. Br J Cancer. 113:443–452. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ishii S, Tsuji S, Tsujii M, Kanazawa Y,
Nishida T, Iijima H, Yasumaru M, Irie T, Yamamoto K, Tsutsui S, et
al: Involvement of bone marrow-derived stromal cells in
gastrointestinal cancer development and metastasis. J Gastroenterol
Hepatol. 23 (Suppl 2):S242–S249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Udagawa T, Puder M, Wood M, Schaefer BC
and D'Amato RJ: Analysis of tumor-associated stromal cells using
SCID GFP transgenic mice: Contribution of local and bone
marrow-derived host cells. FASEB J. 20:95–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Colegio OR, Chu NQ, Szabo AL, Chu T,
Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC,
Phillips GM, et al: Functional polarization of tumour-associated
macrophages by tumour-derived lactic acid. Nature. 513:559–563.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Komohara Y, Jinushi M and Takeya M:
Clinical significance of macrophage heterogeneity in human
malignant tumors. Cancer Sci. 105:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Le Ricousse-Roussanne LS, Barateau V,
Contreres JO, Boval B, Kraus-Berthier L and Tobelem G: Ex vivo
differentiated endothelial and smooth muscle cells from human cord
blood progenitors home to the angiogenic tumor vasculature.
Cardiovasc Res. 62:176–184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Direkze NC, Forbes SJ, Brittan M, Hunt T,
Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR and
Wright NA: Multiple organ engraftment by bone-marrow-derived
myofibroblasts and fibroblasts in bone-marrow-transplanted mice.
Stem Cells. 21:514–520. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Quante M, Tu SP, Tomita H, Gonda T, Wang
SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, et al: Bone
marrow-derived myofibroblasts contribute to the mesenchymal stem
cell niche and promote tumor growth. Cancer Cell. 19:257–272. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ohgushi H and Caplan AI: Stem cell
technology and bioceramics: From cell to gene engineering. J Biomed
Mater Res. 48:913–927. 1999. View Article : Google Scholar : PubMed/NCBI
|