Introduction
Gastric cancer is one of the most common malignant
tumors worldwide with an occurance rate of 10.79% (1). Gastric cancer is the fifth leading
cause of cancer-associated mortality in both the male and female
population worldwide with a mortality rate of 8.8% (2). Despite the continuous development of
comprehensive diagnosis and treatment technologies in recent years,
the 5-year survival rate for patients with advanced gastric cancer
is still >30% (3–5). The reasons for this are complex, and
one of the most important issues is that gastric cancer cells are
prone to survival and migration/invasion (6–8).
Therefore, it is worthwhile to explore the mechanism of gastric
cancer cell survival and migration/invasion for early intervention,
late treatment and improvement of treatment outcomes.
Endoplasmic reticulum (ER) is the primary site of
protein folding, modification and assembly, as well as
intracellular Ca2+ storage in eukaryotic cells (9,10). Under
stressed conditions, misfolded or unfolded protein aggregation and
imbalances in Ca2+ levels in the ER lumen occur, and the
cell enters a state termed ER stress (11–13). If
the stress persists or the stress damage exceeds the ability of
cell survival and protection, the ER stress-dependent apoptosis
pathway is activated, leading to apoptosis (14–16).
Recent studies have suggested that ER stress-mediated cell
migration/invasion is closely associated with the occurrence and
development of gastric cancer (17–20).
However, the initiator of ER stress that regulates gastric cancer
cell survival and migration/invasion remains unknown.
Yes-associated protein (YAP) is involved in the
regulation of cell proliferation, organ development and the
occurrence of tumors (21–23). Previous studies have demonstrated
that YAP is abnormally expressed in breast, ovarian and other types
of cancer, and its expression levels are associated with stage and
prognosis of patients with tumors (24–27).
Upregulation of YAP has been observed in gastric cancer and is
associated with the clinicopathological characteristics of patients
with gastric cancer (28,29). In addition, YAP integrates ER stress
to control liver size and tumorigenesis, suggesting a potential
connection between YAP and ER stress (29,30).
Therefore, the present study hypothesized that YAP may reduce
gastric cancer cell survival and migration through the activation
of ER stress.
Materials and methods
Cell culture and treatments
The gastric cancer MKN-28/74 cells and normal
gastric GES-1 cells were purchased from the American Type Culture
Collection. The MKN28 cell line has been reported as
cross-contaminated with MKN74; thus, it is referred to as MKN-28/74
throughout the present study (31).
MKN-28/74 cells were cultured in RPMI-1640 medium (Nacalai Tesque,
Inc.) supplemented with 10% fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences) at 37°C in a 5% CO2 humidified
incubator; GES-1 cells were cultured in DMEM (HyClone; GE
Healthcare Life Sciences) containing 10% FBS (HyClone; GE
Healthcare Life Sciences) at 37°C in a 5% CO2 humidified
incubator (32). Tunicamycin (TM;
100 nM; Sigma-Aldrich; Merck KGaA) and 4-phenylbutyrate (10 mM;
Sigma-Aldrich; Merck KGaA), the agonist and antagonist for ER
stress, respectively, were added to the medium for 12 h. MKN-28/74
cell were pre-treated with PD98059 (10 µM) for 24 h at 37°C.
Transfection
To evaluate the functional role of YAP, small
interfering (si)RNA was used to knockdown its expression. siYAP
(5′-GCGACATTCAGGGUGACUAUU−3′) and non-targeting sequences (siCtrl;
5′-UUCUCCGAACGUGUCACGU-3′) were purchased from GenePharma Co., Ltd.
(33). A total of 20 nM siYAP or
siCtrl was used to transfect MKN-28/74 cells (2×106
cells/well) with Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.) for 48 h in 6-well plates, and the transfection
efficiency was determined by western blotting.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the MKN-28/74 cells
using an RNeasy kit (Beyotime Institute of Biotechnology) and
reverse transcribed using One-step RT-PCR kit (cat. no., AE311-02;
Beijing Transgen Biotech Co., Ltd.) at 37°C for 30 min according to
the manufacturer's protocol (34).
qPCR was performed using the SYBR Green RT-PCR kit (Takara Bio,
Inc.) according to the manufacturer's protocol. The thermocycling
conditions were as follows: 95°C for 5 min; followed by 40 cycles
of 95°C for 40 sec, 60°C for 30 sec and 72°C for 30 sec. GAPDH was
selected as an internal control. The following primers were used
for PCR: YAP forward, 5′-AAGGCTTGACCCTCGTTT-3′ and reverse,
5′-CTGCTGCTGCTGGTTTGA-3′; and GAPDH forward,
5′-GTCAACGGATTTGGTCGTATTG-3′ and reverse,
5′-CATGGGTGGAATCATATTGGAA-3′. Fold-changes in mRNA expression were
calculated using the 2−ΔΔCq method (35).
Western blotting
The MKN28/74 cells (5×106) was
homogenized and sonicated in a lysis buffer (Beyotime Institute of
Biotechnology). Protein concentrations were detected using a BCA
Protein Quantification kit, according to the manufacturer's
protocol. The proteins (50 µg) were separated by 10% SDS-PAGE and
then transferred onto polyvinylidene difluoride membranes. The
membrane was blocked with 5% non-fat dry milk for 1 h at room
temperature and incubated with specific primary antibodies
overnight at 4°C. The primary antibodies used were as follows: YAP
(1:1,000; Cell Signaling Technology, Inc.; cat. no. 14074),
pro-caspase-3 (1:1,000; Abcam; cat. no. ab13847), cleaved caspase-3
(1:1,000; Abcam; cat. no. ab49822), glucose-regulated protein 78
kDa (GRP78; 1:1,000; Abcam; cat. no. ab21685), GADPH (1:1,000;
Abcam; cat. no. ab8245), pro-caspase-12 (1:1,000; Abcam; cat. no.
ab8117), cleaved caspase-12 (1:1,000; Cell Signaling Technology,
Inc.; cat. no. 2202), C/EBP homologous protein (CHOP; 1:1,000;
Abcam; cat. no. ab11419), ERK (1:1,000; Cell Signaling Technology,
Inc.; cat. no. 4695), phosphorylated (p-)ERK (1:1,000; Cell
Signaling Technology, Inc.; cat. no. 4370). The blots were detected
with an enhanced chemiluminescence substrate kit (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
bands were scanned and quantified by ImageJ version 1.47 software
(National Institutes of Health) (36).
Immunofluorescence staining
Following transfection treatment, the MKN28/74 cell
(0.5×106 cells/well) were fixed with 3.7%
paraformaldehyde for 10 min at room temperature and subsequently
blocked with 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) in
PBS for 1 h at room temperature. Cells were incubated with primary
antibodies for 4 h at room temperature. The primary antibodies used
were YAP (1:500; Cell Signaling Technology, Inc.; cat. no. 14074)
and CHOP (1:500; Abcam; cat. no. ab11419). DAPI (5 mg/ml;
Sigma-Aldrich; Merck KGaA) was used to stain the nuclei at room
temperature for 3 min. A total of 5 randomly selected fields of
view were used per smaple and images were captured with a laser
confocal microscope (magnification, ×600; TcS SP5; Leica
Microsystems, Inc.).
Cell invasion and migration
Following transfection treatment, cell invasion was
analyzed using a Transwell chamber assay as previously described
(37). Briefly, cells
(1×106 cells/well) were suspended in RPMI-1640 medium
containing 10% FBS and seeded into the upper chambers.
Cell migration was analyzed using a wound-healing
assay and cells were cultured with RPMI-1640 medium in 12-well
plates. Once cells reached >80% confluency, a sterile pipette
tip was used to evenly scratch the 12-well plate. Following cell
attachment, a straight line was gently scratched in the cell layer
with a 200 µl pipette tip, and the cells were washed with PBS (pH
7.4) three times. The relative wound closure was imaged under a
light microscope (magnification, ×100; Leica Microsystems, Inc.) at
0 and 24 h. The wound was measured using ImageJ 1.74v software
(National Institutes of Health).
MTT assay and terminal
deoxynucleotidyl transferase-mediated dUTP nick end labeling
(TUNEL)
The MKN-28/74 cells were seeded into 96-well plates
at 8×103 cells/well and incubated overnight. Following
transfection treatment, MTT (5 mg/ml) was added to each well and
incubated for 4 h. The insoluble formazan was collected and
dissolved in dimethylsulfoxide, and the optical density value was
measured with a scanning spectrophotometer at a wavelength of 570
nm.
The TUNEL assay was used for the detection of
apoptosis. A one-step TUNEL kit (Beyotime Institute of
Biotechnology) was used for TUNEL staining. The MKN-28/74 cells
(1×106 cells) were incubated with fluorescein-dUTP
(Invitrogen; Thermo Fisher Scientific, Inc.) to stain the apoptotic
cell nuclei and with DAPI (5 mg/ml) to stain all cell nuclei at
room temperature for 3 min. Images were captured with a laser
confocal microscope (magnification, ×600; TcS SP5; Leica
Microsystems, Inc.). The number of TUNEL-positive cells was
calculated by counting at least five random fields of view as the
ratio of the experimental samples to the control samples
(untransfected cells).
Statistical analysis
All analyses were performed with SPSS 20.0 software
(IBM Corp.). Experiments were repeated three times and data are
presented as the means ± standard error of the mean. Statistical
analyses were performed using one-way analysis of variance with the
Bonferroni test for post hoc comparisons. P<0.05 was considered
to indicate a statistically significant difference.
Results
YAP is upregulated in gastric cancer
MKN-28/74 cells and promotes cell survival
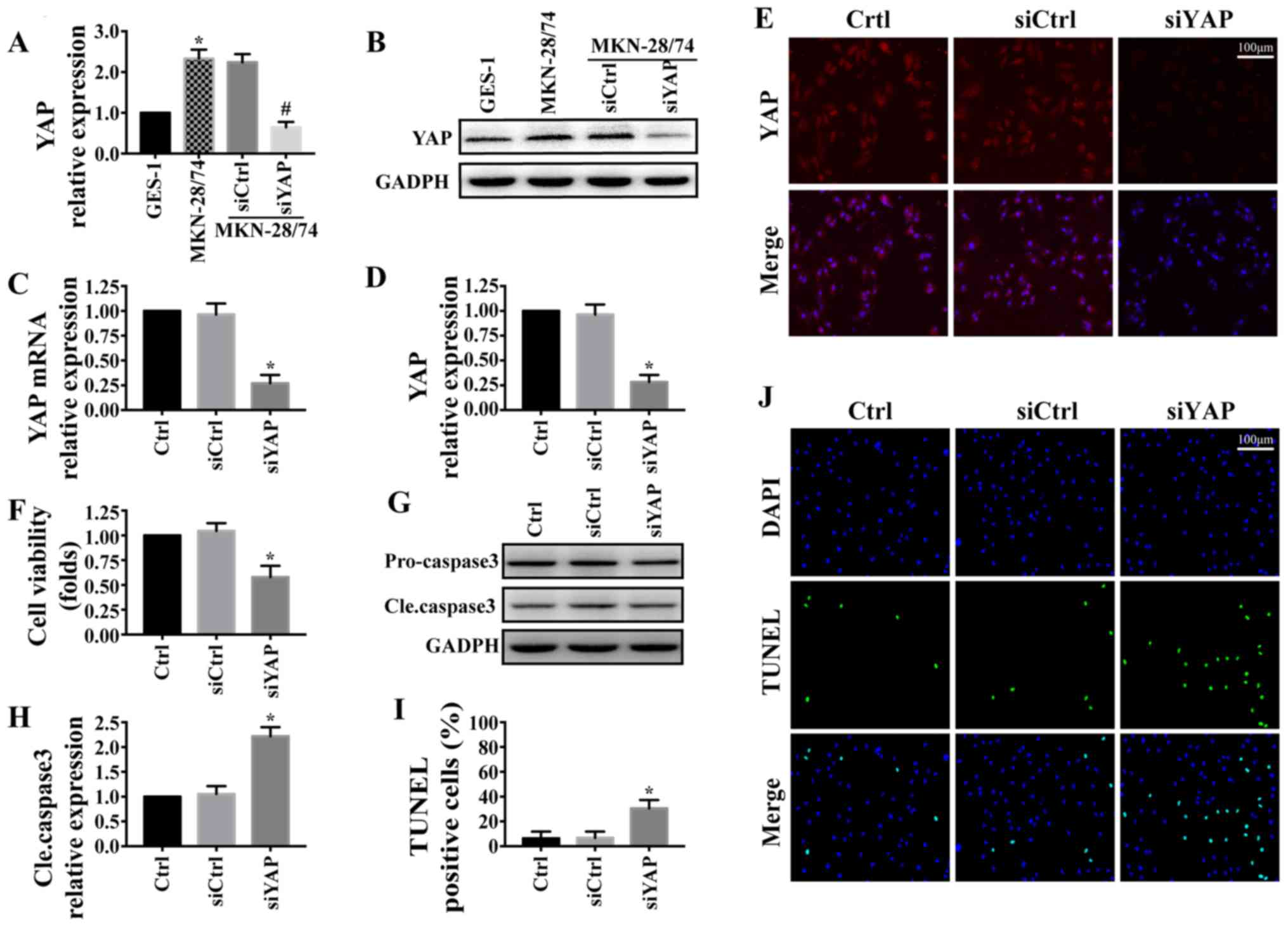
The expression levels of YAP were detected by
western blotting in MKN-28/74 gastric cancer cells and GES-1 normal
gastric cells. The results demonstrated that YAP was significantly
upregulated in gastric cancer MKN-28/74 cells compared with GES-1
cells (Fig. 1A and B). To confirm
the role of YAP in the progression gastric cancer, siYAP was
transfected into MKN-28/74 cells to knockdown the expression of
YAP. The transfection efficiency was detected by western blotting
(Fig. 1A and B), RT-qPCR (Fig. 1C) and immunofluorescence (Fig. 1D and E). The results demonstrated
that siYAP, but not siCtrl, significantly inhibited the expression
of YAP in gastric cancer MKN-28/74 cells compared with
untransfected cells. The effect of YAP on MKN-28/74 cell viability
was investigated. The results of the MTT assay demonstrated that
YAP knockdown significantly reduced the viability of MKN-28/74
cells (Fig. 1F). In addition, the
inhibition of YAP expression increased the expression of cleaved
caspase-3 (Fig. 1G and H) and the
number of TUNEL-positive cells (Fig. 1I
and J) in gastric cancer MKN-28/74 cells. These results
suggested that YAP was upregulated in gastric cancer MKN-28/74
cells and promoted cell survival by inhibiting apoptosis.
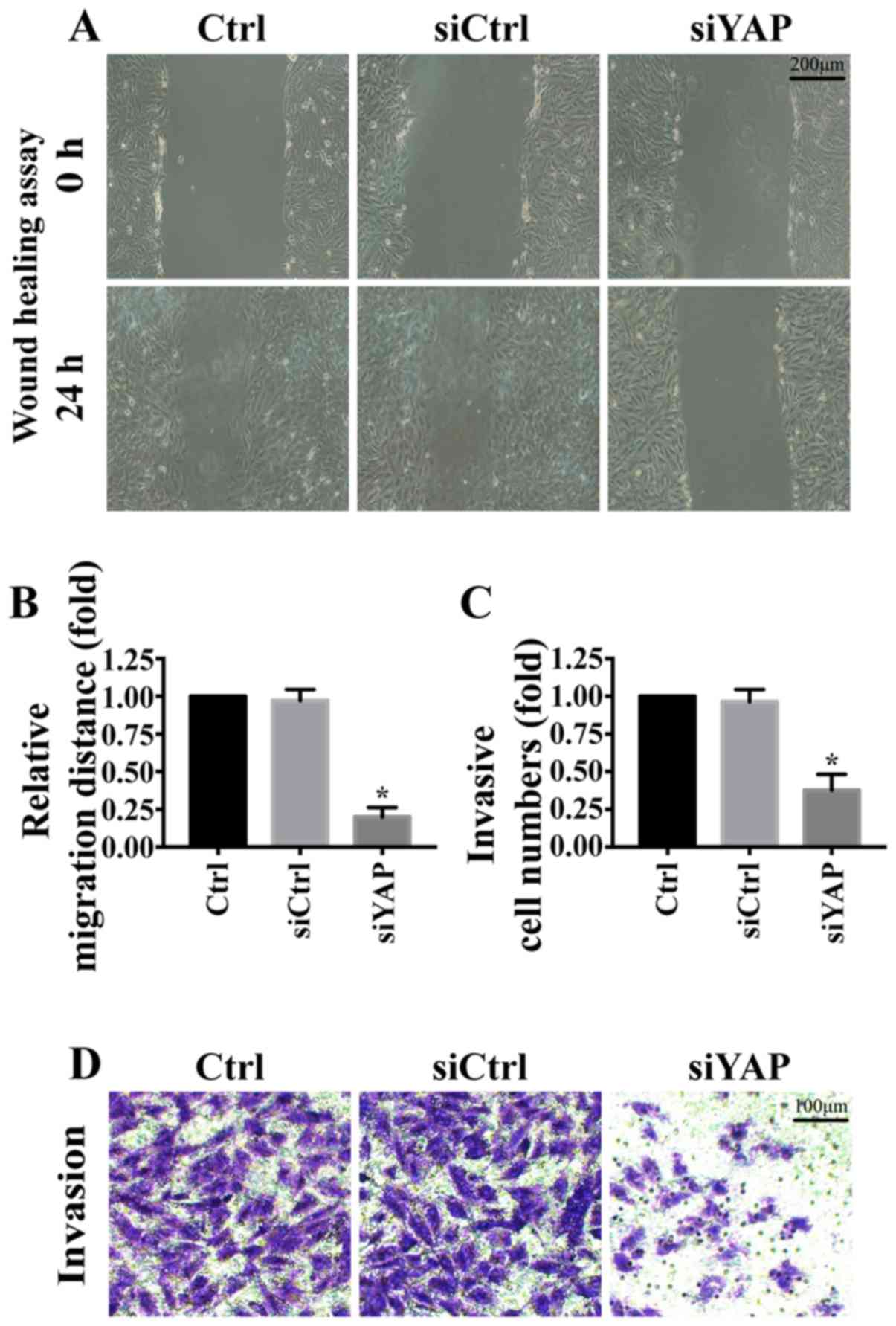
YAP is associated with MKN-28/74 cell
migration and invasion
The role of YAP in MKN-28/74 cell migration and
invasion was further investigated. Knockdown of YAP significantly
reduced wound closure rates in the wound-healing assay (Fig. 2A and B). In addition, compared with
the control group, knockdown of YAP reduced the invasive ability of
gastric cancer MKN-28/74 cells (Fig. 2C
and D). These results suggested that YAP promoted MKN-28/74
cell migration and invasion.
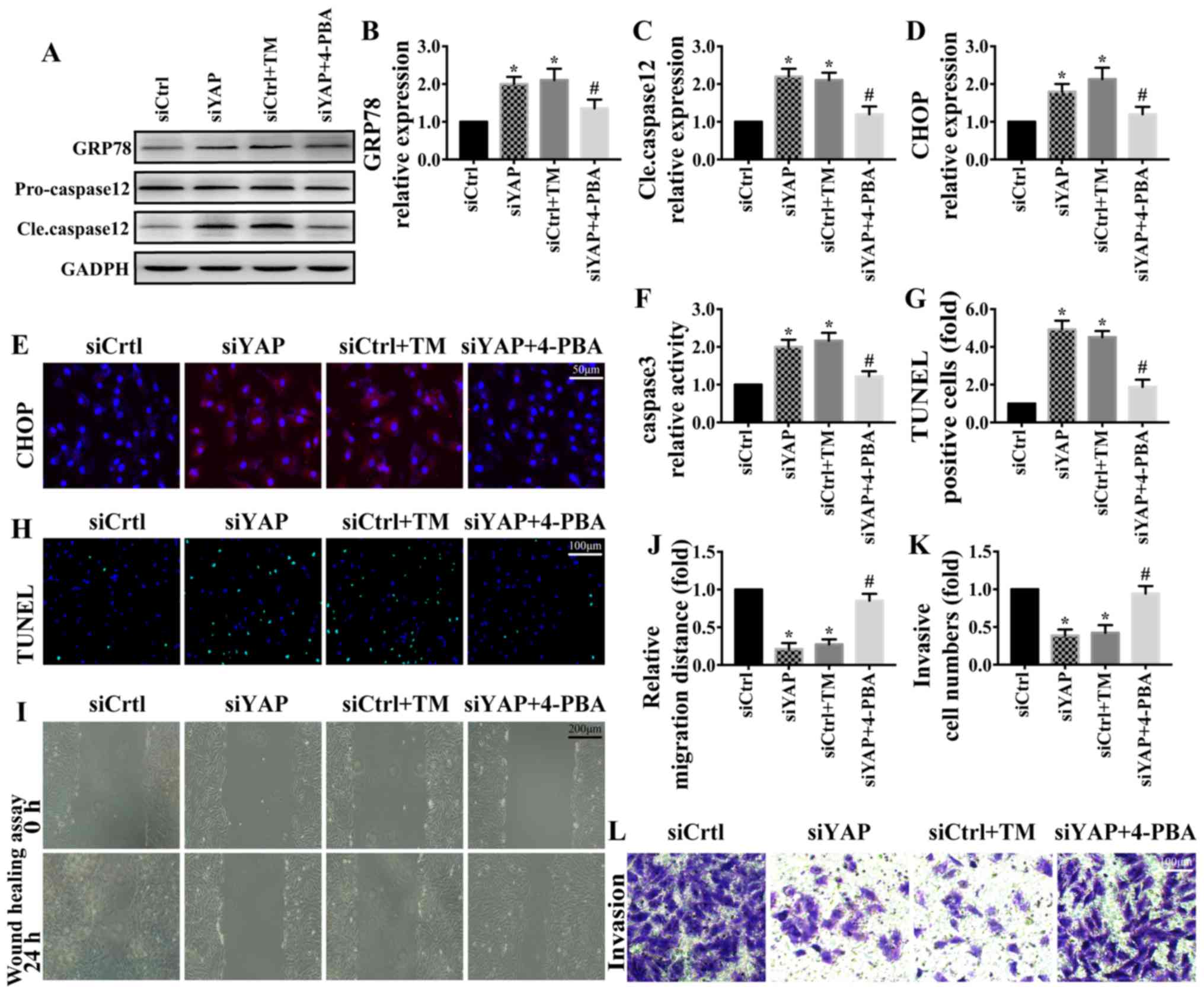
YAP promotes MKN-28/74 cell survival
and migration/invasion through the inhibition of ER stress
ER stress serves a critical role in the progression
of cancer (38,39). To determine the underlying mechanism
by which YAP may regulate gastric cancer MKN-28/74 cell survival
and metastasis, the present study focused on ER stress. TM, the
activator of ER stress, was used to induce ER stress in MKN-28/74
cells transfected with siCtrl. 4-phenylbutyrate (4-PBA), the
inhibitor of ER stress, was used to inhibit ER stress in
YAP-knockdown MKN-28/74 cells. Western blotting (Fig. 3A-C) and immunofluorescence (Fig. 3D and E) were used to determine the
changes in ER stress markers. Compared with the siCtrl group,
knockdown of YAP contributed to the upregulation of GRP78, CHOP and
cleaved caspase-12; similar results were observed following TM
treatment in the siCtrl group. However, the upregulation of ER
stress markers was partially reversed by 4-PBA (Fig. 3A-E). These results suggested that YAP
knockdown was associated with ER stress. In addition, ER stress
activation was associated with apoptosis activation (Fig. 3F-H) and the inhibition of
migration/invasion (Fig. 3I-L). By
contrast, inhibiting ER stress with 4-PBA in YAP-knockdown cells
promoted cell survival and invasion. These results indicated that
YAP promoted gastric cancer MKN-28/74 cell survival and
migration/invasion through the regulation of ER stress.
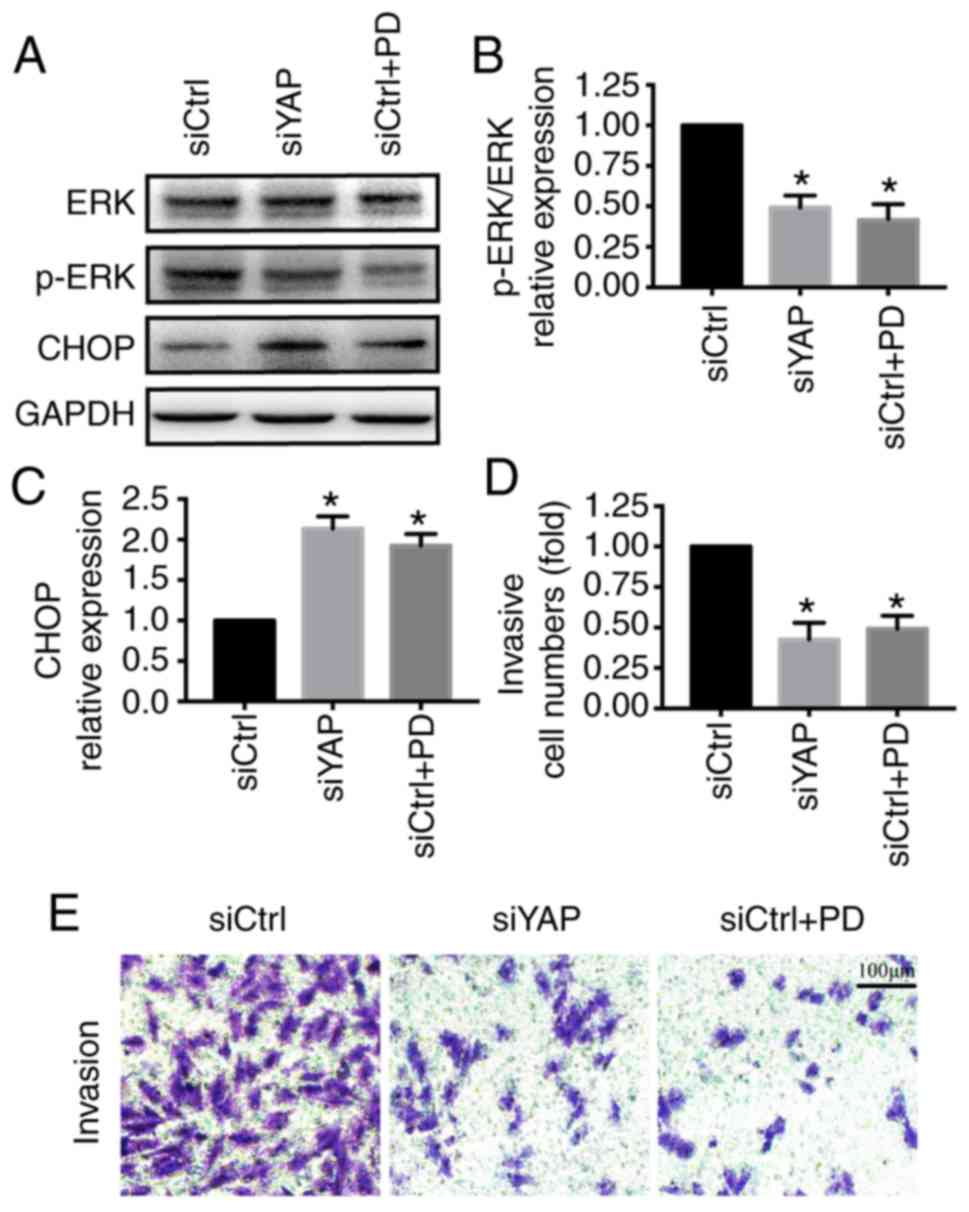
YAP regulates ER stress via the ERK
pathway
Finally, experiments were performed to determine how
YAP inhibited ER stress. The ERK pathway has been reported to be
involved in YAP-associated functions and ER stress inhibition
(40,41). PD98059, an inhibitor of the ERK
pathway, was used to inhibit the ERK pathway in MKN-28/74 cells
transfected with siCtrl. The activation of the ERK pathway was
assessed by western blotting. Compared with the control group, YAP
knockdown inhibited ERK phosphorylation, similar to PD98059
treatment (Fig. 4A). The inhibition
of the ERK pathway by siYAP promoted the activation of ER stress as
indicated by the upregulation of CHOP and reduced cell invasion
(Fig. 4). These results suggested
that the ERK pathway may contribute to the YAP-induced ER stress
inhibition.
Discussion
Previous studies have demonstrated that YAP is
essential for gastric cancer cell survival and migration/invasion
(42–44). However, the underlying mechanism
remains unclear. The present study proposes a novel underlying
mechanism by which YAP regulates gastric cancer MKN-28/74 cell
survival and metastasis. The results of the present study
demonstrated that: i) YAP was upregulated in gastric cancer
MKN-28/74 cells compared with normal gastric GES-1 cells; ii) YAP
promoted gastric cancer MKN-28/74 cell survival and
migration/invasion by inhibiting ER stress; iii) YAP may regulate
ER stress by activating the ERK pathway. The present study provides
a new target for the treatment of gastric cancer that may affect
cancer cell survival and metastasis. A limitation of the present
study was that only one gastric cancer cell line was used.
Additional cell lines will be used in our future study, to confirm
the results.
In eukaryotic cells, the ER is responsible for
protein synthesis and calcium storage; perturbations in the ER
function, a process termed ER stress, have been reported to be
involved in cancer initiation, growth and metastasis in the
majority of solid tumors (45,46).
However, the role of ER stress in tumorigenesis and development is
still controversial. Previous studies have demonstrated that ER
stress is a tumor suppressor, and the activation of ER stress
inhibits gastric cancer cell survival and migration (19,47,48).
However, a number of studies have suggested that ER stress can
promote tumor development (49,50).
Induction of ER stress protects gastric cancer cell apoptosis
during cisplatin and doxorubicin treatment via the p38 MAPK pathway
(51).
Recent studies have identified an association
between YAP and ER stress. The activated Hippo-YAP signaling
pathway promoted neuron survival in the TNFα-induced
microenvironment by inhibiting ER stress (52). In addition, downregulation of YAP
evoked ER stress and contributed to myocyte death in
isoproterenol-induced myocardial infarction (53). The results of the present study are
consistent with previous studies. However, the exact mechanism by
which YAP controls ER stress remains unknown. The results of the
present study suggested that YAP may inhibit ER stress via the ERK
pathway. Thus, these results provide valuable information on the
role of YAP and ER stress in tumorigenesis.
In the present study, the critical role of YAP in
the progression of gastric cancer was identified. A recent study
demonstrated that YAP regulates gastric cancer survival and
migration through SIRT1/Mfn2/mitophagy (42). The results of the present study
demonstrated that YAP may function via the ERK/ER stress pathway in
gastric cancer survival and metastasis. To the best of our
knowledge, this is the first identification of YAP functions
involved in ER stress and the ERK pathway in the development of
gastric cancer. However, in vivo experiments and clinical
data are required to support these results.
In conclusion, the results of the present study
identified the important role of YAP in gastric cancer cell
migration and survival. YAP promoted gastric cancer MKN-28/74 cell
survival and migration/invasion via the ERK/ER stress pathway.
These results suggested that the YAP/ERK/ER stress pathway may be a
potential target for the treatment of gastric cancer.
Acknowledgements
Not applicable.
Funding
This work was supported in part by Inner Mongolia
Autonomous Region Natural Science Foundation (grant no.,
2016MS0847), Scientific Research Planning Project of Health and
Family Planning Commission of Inner Mongolia Autonomous Region
(grant no., 201701048) and Science and Technology Innovation
Guidance Project of Inner Mongolia Autonomous Region (grant no.,
KCBJ2018021).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL and DM conceived and designed the study,
performed the experiments, analyzed and interpreted the data and
wrote the manuscript. PX, HW and YW were involved in data analysis
and interpretation.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening, and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang XY and Zhang PY: Gastric cancer:
Somatic genetics as a guide to therapy. J Med Genet. 54:305–312.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamashima C: Current issues and future
perspectives of gastric cancer screening. World J Gastroenterol.
20:13767–13774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric
Cancer. 20:1–19. 2017. View Article : Google Scholar
|
|
5
|
Sano T: Gastric cancer: Asia and the
world. Gastric Cancer. 20 (Suppl 1):S1–S2. 2017. View Article : Google Scholar
|
|
6
|
Rahman R, Asombang AW and Ibdah JA:
Characteristics of gastric cancer in Asia. World J Gastroenterol.
20:4483–4490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldenring JR: The AGA/Funderburg award in
gastric cancer: Twenty-five years of advances in gastric cancer
research. Gastroenterology. 152:1262–1266. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bekaii-Saab T and El-Rayes B: Identifying
and targeting cancer stem cells in the treatment of gastric cancer.
Cancer. 123:1303–1312. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song S, Tan J, Miao Y and Zhang Q:
Crosstalk of ER stress-mediated autophagy and ER-phagy: Involvement
of UPR and the core autophagy machinery. J Cell Physiol.
233:3867–3874. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sarvani C, Sireesh D and Ramkumar KM:
Unraveling the role of ER stress inhibitors in the context of
metabolic diseases. Pharmacol Res. 119:412–421. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coppola-Segovia V, Cavarsan C, Maia FG,
Ferraz AC, Nakao LS, Lima MM and Zanata SM: ER stress induced by
tunicamycin triggers alpha-Synuclein oligomerization, dopaminergic
neurons death and locomotor impairment: A New model of Parkinson's
disease. Mol Neurobiol. 54:5798–5806. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rahmati M, Moosavi MA and McDermott MF: ER
stress: A therapeutic target in rheumatoid arthritis? Trends
Pharmacol Sci. 39:610–623. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith M and Wilkinson S: ER homeostasis
and autophagy. Essays Biochem. 61:625–635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koga T, Suico MA, Shimasaki S, Watanabe E,
Kai Y, Koyama K, Omachi K, Morino-Koga S, Sato T, Shuto T, et al:
Endoplasmic reticulum (ER) stress induces Sirtuin 1 (SIRT1)
expression via the PI3K-Akt-GSK3β signaling pathway and promotes
hepatocellular injury. J Biol Chem. 290:30366–30374. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mohamed E, Cao Y and Rodriguez PC:
Endoplasmic reticulum stress regulates tumor growth and anti-tumor
immunity: A promising opportunity for cancer immunotherapy. Cancer
Immunol Immunother. 66:1069–1078. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Papaioannou A and Chevet E: Driving Cancer
tumorigenesis and metastasis through UPR signaling. Curr Top
Microbiol Immunol. 414:159–192. 2018.PubMed/NCBI
|
|
18
|
Kim C and Kim B: Anti-cancer natural
products and their bioactive compounds inducing ER stress-mediated
apoptosis: A review. Nutrients. 10(pii): E10212018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang B, Han H, Fu S, Yang P, Gu Z, Zhou Q
and Cao Z: Dehydroeffusol inhibits gastric cancer cell growth and
tumorigenicity by selectively inducing tumor-suppressive
endoplasmic reticulum stress and a moderate apoptosis. Biochem
Pharmacol. 104:8–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen W, Zou P, Zhao Z, Chen X, Fan X,
Vinothkumar R, Cui R, Wu F, Zhang Q, Liang G and Ji J: Synergistic
antitumor activity of rapamycin and EF24 via increasing ROS for the
treatment of gastric cancer. Redox Biol. 10:78–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Panciera T, Azzolin L, Cordenonsi M and
Piccolo S: Mechanobiology of YAP and TAZ in physiology and disease.
Nat Rev Mol Cell Biol. 18:758–770. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Andl T, Zhou L, Yang K, Kadekaro AL and
Zhang Y: YAP and WWTR1: New targets for skin cancer treatment.
Cancer Lett. 396:30–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zanconato F, Cordenonsi M and Piccolo S:
YAP/TAZ at the roots of cancer. Cancer Cell. 29:783–803. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sorrentino G, Ruggeri N, Zannini A,
Ingallina E, Bertolio R, Marotta C, Neri C, Cappuzzello E, Forcato
M, Rosato A, et al: Glucocorticoid receptor signalling activates
YAP in breast cancer. Nat Commun. 8:140732017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xia Y, Chang T, Wang Y, Liu Y, Li W, Li M
and Fan HY: YAP promotes ovarian cancer cell tumorigenesis and is
indicative of a poor prognosis for ovarian cancer patients. PLoS
One. 9:e917702014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hall CA, Wang R, Miao J, Oliva E, Shen X,
Wheeler T, Hilsenbeck SG, Orsulic S and Goode S: Hippo pathway
effector Yap is an ovarian cancer oncogene. Cancer Res.
70:8517–8525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brown JS, O'Carrigan B, Jackson SP and Yap
TA: Targeting DNA repair in cancer: Beyond PARP inhibitors. Cancer
Discov. 7:20–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan Z, Tian Y, Zhang B, Zhang X, Shi H,
Liang Z, Wu P, Li R, You B, Yang L, et al: YAP signaling in gastric
cancer-derived mesenchymal stem cells is critical for its promoting
role in cancer progression. Int J Oncol. 51:1055–1066. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma L, Cui J, Xi H, Bian S, Wei B and Chen
L: Fat4 suppression induces Yap translocation accounting for the
promoted proliferation and migration of gastric cancer cells.
Cancer Biol Ther. 17:36–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu P, Xue J, Zhang ZJ, Jia YP, Tong YN,
Han D, Li Q, Xiang Y, Mao XH and Tang B: Helicobacter pylori VacA
induces autophagic cell death in gastric epithelial cells via the
endoplasmic reticulum stress pathway. Cell Death Dis. 8:32072017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yokozaki H: Molecular characteristics of
eight gastric cancer cell lines established in Japan. Pathol Int.
50:767–777. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang XR, Wang SY, Sun W and Wei C:
Isoliquiritigenin inhibits proliferation and metastasis of MKN28
gastric cancer cells by suppressing the PI3K/AKT/mTOR signaling
pathway. Mol Med Rep. 18:3429–3436. 2018.PubMed/NCBI
|
|
33
|
Noto A, De Vitis C, Pisanu ME, Roscilli G,
Ricci G, Catizone A, Sorrentino G, Chianese G, Taglialatela-Scafati
O, Trisciuoglio D, et al: Stearoyl-CoA-desaturase 1 regulates lung
cancer stemness via stabilization and nuclear localization of
YAP/TAZ. Oncogene. 36:4573–4584. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu P, Hu S, Jin Q, Li D, Tian F, Toan S,
Li Y, Zhou H and Chen Y: Ripk3 promotes ER stress-induced
necroptosis in cardiac IR injury: A mechanism involving calcium
overload/XO/ROS/mPTP pathway. Redox Biol. 16:157–168. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li R, Yang HQ, Xi HL, Feng S and Qin RH:
Inhibition of CDH17 gene expression via RNA interference reduces
proliferation and apoptosis of human MKN28 gastric cancer cells.
Int J Oncol. 50:15–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ye H, Wang WG, Cao J and Hu XC: SPARCL1
suppresses cell migration and invasion in renal cell carcinoma. Mol
Med Rep. 16:7784–7790. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu SH, Lee WJ, Lai DW, Wu SM, Liu CY,
Tien HR, Chiu CS, Peng YC, Jan YJ, Chao TH, et al: Honokiol confers
immunogenicity by dictating calreticulin exposure, activating ER
stress and inhibiting epithelial-to-mesenchymal transition. Mol
Oncol. 9:834–849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yan Y, Liu S, Li M, Zhao Y, Shao X, Hang M
and Bu X: Recombinant Newcastle disease virus expressing human
IFN-λ1 (rL-hIFN-λ1)-induced apoptosis of A549 cells is connected to
endoplasmic reticulum stress pathways. Thorac Cancer. 9:1437–1452.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kennedy D, Mnich K, Oommen D, Chakravarthy
R, Almeida-Souza L, Krols M, Saveljeva S, Doyle K, Gupta S,
Timmerman V, et al: HSPB1 facilitates ERK-mediated phosphorylation
and degradation of BIM to attenuate endoplasmic reticulum
stress-induced apoptosis. Cell Death Dis. 8:e30262017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Yuan J, Zhang X, Yan F, Huang M,
Wang T, Zheng X and Zhang M: Angiomotin promotes the malignant
potential of colon cancer cells by activating the YAP-ERK/PI3K-AKT
signaling pathway. Oncol Rep. 36:3619–3626. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yan H, Qiu C, Sun W, Gu M, Xiao F, Zou J
and Zhang L: Yap regulates gastric cancer survival and migration
via SIRT1/Mfn2/mitophagy. Oncol Rep. 39:1671–1681. 2018.PubMed/NCBI
|
|
43
|
Jiao S, Wang H, Shi Z, Dong A, Zhang W,
Song X, He F, Wang Y, Zhang Z, Wang W, et al: A peptide mimicking
VGLL4 function acts as a YAP antagonist therapy against gastric
cancer. Cancer Cell. 25:166–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Qiao Y, Chen J, Lim YB, Finch-Edmondson
ML, Seshachalam VP, Qin L, Jiang T, Low BC, Singh H, Lim CT and
Sudol M: YAP regulates actin dynamics through ARHGAP29 and promotes
metastasis. Cell Rep. 19:1495–1502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cubillos-Ruiz JR, Bettigole SE and
Glimcher LH: Tumorigenic and immunosuppressive effects of
endoplasmic reticulum stress in cancer. Cell. 168:692–706. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dumartin L, Alrawashdeh W, Trabulo SM,
Radon TP, Steiger K, Feakins RM, di Magliano MP, Heeschen C,
Esposito I, Lemoine NR and Crnogorac-Jurcevic T: ER stress protein
AGR2 precedes and is involved in the regulation of pancreatic
cancer initiation. Oncogene. 36:3094–3103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen PP, Ma XY, Lin Q, Xu HL, Shi HX,
Zhang HY, Xiao J, Geng FN and Zhao YZ: Kangfuxin promotes apoptosis
of gastric cancer cells through activating ERstress and autophagy.
Mol Med Rep. 16:9043–9050. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim JL, Lee DH, Na YJ, Kim BR, Jeong YA,
Lee SI, Kang S, Joung SY, Lee SY, Oh SC and Min BW: Iron
chelator-induced apoptosis via the ER stress pathway in gastric
cancer cells. Tumour Biol. 37:9709–9719. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hersey P and Zhang XD: Adaptation to ER
stress as a driver of malignancy and resistance to therapy in human
melanoma. Pigment Cell Melanoma Res. 21:358–367. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ye J and Koumenis C: ATF4, an ER stress
and hypoxia-inducible transcription factor and its potential role
in hypoxia tolerance and tumorigenesis. Curr Mol Med. 9:411–416.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Feng R, Zhai WL, Yang HY, Jin H and Zhang
QX: Induction of ER stress protects gastric cancer cells against
apoptosis induced by cisplatin and doxorubicin through activation
of p38 MAPK. Biochem Biophys Res Commun. 406:299–304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hou S, Wang L and Zhang G: Mitofusin-2
regulates inflammation-mediated mouse neuroblastoma N2a cells
dysfunction and endoplasmic reticulum stress via the Yap-Hippo
pathway. J Physiol Sci. 69:697–709. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ashokkumar R, Jamuna S, Sakeena Sadullah
MS and Niranjali Devaraj S: Vitexin protects isoproterenol induced
post myocardial injury by modulating hipposignaling and ER stress
responses. Biochem Biophys Res Commun. 496:731–737. 2018.
View Article : Google Scholar : PubMed/NCBI
|