Introduction
A large variety of plant-based nutrients and
phytochemicals consumed by humans has long been considered to be
associated with human health, and has even proved to reduce the
risk of inflammation (1–3) and illnesses such as diabetes (4), cardiovascular (5), neurodegenerative (6), microbial-related diseases (7,8), and
certain types of cancer (9–13). Cancer continues to be a major health
challenge, constituting the second-leading cause of death
worldwide, despite intensive and extensive research that has
revealed much about its biology in last few decades (14,15). In
parallel, considerable progress in anti-cancer therapies has been
made, allowing the cure of cancer patients and helping to prolong
their survival rate. Despite such advances in its early detection,
and improvements in treatment and prevention, cancer remains a
major challenge in terms of morbidity and mortality. Therefore,
enormous scientific and commercial endeavors have been made to
discover further anti-cancer agents. In view of this, natural
products that have been studied for a long time, have been found to
have pharmacologic activity, and have proved to be safe with
long-term exposure (16). Some of
these plant-extracted products are currently available on the
pharmaceutical market as antioxidants or scavengers, and are used
to counteract reactive oxygen species (ROS), the triggers for
various types of human cancer (17).
ROS, produced either endogenously or by exogenous stimuli, can
damage DNA, proteins, and lipids, which can lead to the
transformation of normal cells into cancer cells through the
mutation of key genes (18). Cancer
initiation and progression can also occur due to an unbalanced
redox equilibrium, an inherent defense system of cells that
endogenously generate and scavenge ROS, leading to increased DNA
damage, prevention of cell apoptosis, and consequently, to a higher
rate of cell survival (19,20). Given this, the excessive production
of intracellular ROS has been targeted by antioxidants as
therapeutic agents to prevent or suppress the development or
propagation of cancer cells (21).
Many plants have been found to have significant ROS-scavenging
activity (antioxidant activity), which is associated with
cytotoxicity (antiproliferative activity) toward cancer cells, and
thus could be used as therapeutic and preventive agents (22–24).
This association is clear in the observations that curcumin, a
natural polyphenol derived from the rhizome of turmeric, and
quercetin, an anti-oxidant derived from fruits and vegetables, have
been shown to have potent free radical-scavenging and cytotoxic
activity (25,26).
As cancer cells have developed the capability to
escape apoptosis through a number of mechanisms-cellular
transformation, apoptosis dysregulation, proliferation, migration,
angiogenesis, and metastasis. Patients with cancer have been
treated and managed by conventional surgery, chemotherapy, or
radiotherapy. However, researchers have begun to find encouraging
clinical results pointing to the value of plant-based products in
cancer treatment, and physicians have started to use these
medications. They support, and even strengthen, various systems of
the body that are under stress due to chemical toxicity or
traumatic events. In contrast to chemotherapy, which often induces
a number of undesired toxic side effects, natural therapies, such
as the use of plant-derived products, may have the capability to
reduce some of these toxicities (27). Plants and other natural products have
for a long time been the main source of anti-tumor drug candidates.
Many of the anticancer drugs used today, such as vinblastine,
vincristine, Paclitaxel, and camptothecin, are based mainly on
natural drugs (14,28).
ROS are unstable species that pair up their odd free
electrons by attacking healthy cells, causing a loss of cell
structure and/or function (29). The
impaired cells are key contributors to degenerative diseases such
as cancer, inflammation, immune system weakening, liver disease,
brain dysfunction, cardiovascular conditions, diabetes, and renal
failure (30). Therefore,
antioxidants/free radical-scavenging agents are vital to
controlling the damaging effect of free radicals in the human body
(31). As a result, verifying the
type of correlation between free radical-scavenging of plant
extracts and their cytotoxicity to cancer cells is an issue of
great importance. The purpose of this study is to evaluate the free
radical-scavenging of dozens of plant-based extracts, as well as
their cytotoxicity to the HepG2 cell line (liver cancer). To the
best of our knowledge to date, the correlation between free
radical-scavenging and cytotoxicity has not yet been tested on a
large scale nor reported in scientific journal. To explore and
possibly verify this correlation, we tested fifty-seven methanolic
extracts derived from regional plants that see heavy use as food
and as traditional medicines.
Finding the type of correlation between free
radical-scavenging of plant extracts and their cytotoxicity could
be helpful in high-throughput screening projects that search for
cytotoxic natural products. If a positive correlation exists, then,
it may not imply that a change in the value of one parameter will
cause a change in the value of the other parameter.
Materials and methods
Materials and cells
All plants that were used in this study were
purchased from Al-Alim Ltd. (Medicinal Herb Center) or from the
local market (those that are labeled with a symbol a in
Table I). All our research involving
wild-type species are not at risk of extinction and not registered
in the endangered species flora list. The gallic acid, DPPH, and
the solvents were purchased from Sigma. HepG2 liver cancer cell
line was purchased from the American Tissue Culture Collection
(ATCC; catalog no. HB-8065; passage 05–10). Eagle's minimum
essential medium (EMEM), fetal bovine serum, antibiotics, and the
XTT kit were purchased from Biological Industries.
 | Table I.A list of medicinal and edible plants
that were used in the current study and their yield of methanolic
extraction, EC50 for free radical scavenging, percentage
of inhibition at a concentration of 250 µg/ml of plant extract and
4-EC50 cytotoxicity for the most active plant. |
Table I.
A list of medicinal and edible plants
that were used in the current study and their yield of methanolic
extraction, EC50 for free radical scavenging, percentage
of inhibition at a concentration of 250 µg/ml of plant extract and
4-EC50 cytotoxicity for the most active plant.
| Scientific name
(part of the plant) | The extract yield
(%) | EC50 of
free radical scavenging (µg/ml) | % Inhibition at
concentrations of 250 µg/ml of (%) | EC50 of
cytotoxicity (µg/ml) |
|---|
| Vitis vinifera
(leaf) | 6.32 | 4.63 | 13 |
|
| Stevia
rabaudiana (leaf) | 19.38 | 9.77 | 12 |
|
| Rosmarinus
officinalis (leaf) | 11.72 | 3.45 | 95 | 131 |
| Rubus idaeus
(leaf) | 4.82 | 4.25 | 22 |
|
| Punuca granatum
(fruit peel) | 38.02 | 87.30 | 49 |
|
| Origanum vulgare
(leaf) | 10.00 | 1.67 | 90 | 180 |
| Vitex
agnus-castus (seeds) | 3.60 | 166.43 | 99 | 42 |
| Thymus vulgaris
(leaf) | 11.63 | 1.98 | 33 |
|
| Mentha piperita
(leaf) | 10.97 | 1.68 | 30 |
|
| Melissa
officinalis (leaf) | 9.70 | 0.28 | 36 |
|
| Urtica
urens/pilulifera (leaf) | 7.33 | 220.92 | 0 |
|
| Orea europaea
(leaf) | 25.20 | 2.98 | 30 |
|
| Camelia sinensis
(leaf) | 11.26 | 54.2 | 33 |
|
| Cynara
cardunculus (leaf) | 20.15 | 17.61 | 95 | 152 |
| Foeniculurn
vulgare (seeds) | 4.06 | 26.74 | 0 |
|
| Petroselinum
crispum (leaf) | 18.78 | 282.70 | 0 |
|
| Pelargonium spp
(leaf) | 11.30 | 2.83 | 53 |
|
| Lippia
citriodora (leaf) | 8.33 | 4.14 | 92 | 197 |
| Ocimum basilicum
(leaf) | 11.22 | 8.73 | 3 |
|
| Sumac (ripe
fruit) | 30.44 | <0.5 | 66 |
|
| Zingiber
officinale (root) | 4.32 | 81.0 | 95 | 109 |
| Cinnamomum
aromaticum (bark) | 4.26 | 1.67 | 100 | 162 |
| Cuminum cyminum
(seeds) | 10.14 | 23.90 | 10 |
|
| Portulaca
oleracea (leaf and stem) | 10.08 | 47.33 | 14 |
|
| Centaurea (leaf
and stem) | 12.10 | 249.97 | 18 |
|
| Scolymus
maculatus (leaf and stem) | 3.82 | 259.03 | 0 |
|
| Cichorium
intybus (leaf) | 15.08 | 83.96 | 0 |
|
|
Malvaa (leaf) | 14.77 | <0.5 | 0 |
|
| Allium cepa
(leaf) | 8.15 | 215.20 | 0 |
|
| Corchorus
olitorius (leaf) | 11.23 | 10.64 | 0 |
|
| Gundelia
tournefortiia
(stem) | 10.44 | 140.79 | 0 |
|
| Hyssopus
(leaf) | 3.60 | 13.10 | 0 |
|
| Green tea
(leaf) | 14.73 | 0.38 | 0 |
|
| Petroselinum
(leaf and stem) | 6.96 | 35.97 | 0 |
|
| Thymus capitatus
(leaf) | 9.28 | 2.12 | 37 |
|
| Foeniculurn
vulgare (leaf and stem) | 4.76 | 20.86 | 33 |
|
| Melissa
officinalis (leaf) | 8.80 | 24.0 | 0 |
|
| Petroselium
(leaf) | 17.55 | 381.20 | 4 |
|
| Laurus nobilis
(leaf) | 12.74 | 8.92 | 93 | 182 |
| Salvia
officinalis (leaf) | 9.22 | 267.92 | 90 | 142 |
| Cymbopogon
citratus (leaf) | 11.54 | 9.80 | 24 |
|
| Linum
usitatissimum (seeds) | 0.40 | 4,523.5 | 0 |
|
| Avena sativa
(seeds) | 22.00 | 635.51 | 1 |
|
| Ceratonia
siliqua (ripe fruit) | 25.06 | 130.9 | 12 |
|
| Origanum
syriacum (leaf) | 13.53 | 1.84 | 15 |
|
| Camomile (leaf
and flowers) | 16.25 | 29.00 | 7 |
|
| Salvia hispanica
(seeds) | 0.50 | 3,736.5 | 4 |
|
|
Crocusa (seeds) | 0.96 | 765.2 | 31 |
|
| Vitex
agnus-castusa (stem + leaf) | 12.00 | 266.51 | 0 |
|
| Marrubium
vulgarea
(leaf) | 2.56 | 51.3 | 0 |
|
| Ficus
religiosaa
(stem) | 4.22 | 215.92 | 23 |
|
| Lepidium
sativuma
(seeds) | 0.84 | 2,529.8 | 0 |
|
| Angelica
sylvestrisa
(leaf) | 10.10 | 2.00 | 3 |
|
| Gentianaa
(leaf) | 30.76 | 396.03 | 0 |
|
| Pelargonium
sp.a (stem) | 3.88 | 146.5 | 3 |
|
|
Eryngiuma (stem) | 4.32 | 253.31 | 0 |
|
| Humulus
lupupusa
(leaf) | 12.36 | 4.22 | 3 |
|
Extraction of plants
To perform the extraction, one gram of dried plant
material was packed in a tube, soaked with 10 milliliters of
methanol, sonicated for 75 min at 40°C, and then left in for
3 h to cool down. After complete extraction, the methanolic extract
solution was filtered with Whatman paper, grade 1, dried under
vacuum, weighed, and then dissolved by DMSO at a concentration of
100 mg/ml. The extract was kept at 4°C until it was used.
Free radical scavenging (FRS)
The FRS of the methanolic extracts of the various
plants was measured by microdilution using the DPPH assay protocol,
with slight modifications (32,33). The
microdilution of DPPH was performed using two-fold serial dilution
in ddH2O. The tests were carried out in 96-well,
flat-bottomed plates. 100 µl of ethanolic DPPH solution (200 ppm)
was added to 100 µl of the plant extract at the concentration
stated in Table I. The mixture was
then shaken and allowed to settle for 30 min in the dark at room
temperature. The absorbance of the solution was measured at 517 nm
and converted into a percentage of FRS using the following
equation:
FRS%=100*{1-[(Asample-Ablank_1))/(Acontrol-Ablank_2)]}
where
Asample is the absorbance of the
mixture (of plant extract and DPPH)
Ablank-1 is the absorbance of the
plant extract
Acontrol is the absorbance of the
ethanolic solution of DPPH, and Ablank-2 is the
absorbance of ethanol.
Gallic acid at a concentration of 100 µg/ml was used
as a positive control. FRS was expressed in terms of the
EC50 (the amount of antioxidant necessary to decrease
the initial DPPH absorbance by 50%). The EC50 value for
each extract was determined by extracting the value from the
equation for the linear part of the graph. We substituted 50% for
the y value, while calculating the concentration value of
the x-axis.
Cell culture and cytotoxicity
assay
HepG2 cells were cultured in EMEM medium
supplemented with 10% fetal bovine serum and 100 U/ml of penicillin
streptomycin (Biological Industries). The cells were cultured at
37°C in an incubator with 5% CO2. The cytotoxic effect
of the extracts on the cells was assessed using a cell
proliferation kit (XTT-based). In short, 2.5×103 HepG2
cells were seeded into each well of a 96-well plate and cultured
for 24 h. The cells were treated with various concentrations of
plant extract (0, 10, 20, 30, 40, 50, 100, 200, 300, 400 and 500
µg/ml) for 48 h and then incubated with XTT reagents for 3 h at
37°C, and absorbance was measured at 450 nm. The mean absorbance of
non-treated cells served as the reference value for calculating the
percentage of cellular viability. The assay was carried out in
triplicate. Culture medium without cells was used as a background
control (blank) and was subtracted from the other measurements.
Model assessments
Parameters such as the Matthews correlation
coefficient (MCC), accuracy, the precision enrichment factor, and
the area under the ROC curve (AUC) were used to assess the quality
of the cytotoxicity/free radical-scavenging correlation models.
Equation 1. Matthews correlation coefficient
(MCC).
MCC=(PN)-(PfNf)(N+Nf)(N+Pf)(P+Nf)(P+Pf)
where
P, N, Pf and Nf
are the numbers of true positive, true negative, false positive,
and false negative predictions, respectively. A perfect prediction
gives MCC=1.0, while a random performance gives MCC=0.0. MCC=−1.0
indicates a completely erroneous prediction.
Equation 2. Accuracy.
Accuracy=(P + N)/(P + N
+ Pf + Nf)
Equation 3. Precision.
Precision=P/(P +
Pf)
Equation 4. Enrichment factor.
EF=TFRS/TRS
where
TFRS is the % of actives when
using the FRS threshold criterion, and TRS is the
% of actives by random selection.
Statistical analysis
All statistical analyses were conducted using Excel
spreadsheet software (v16.0; Microsoft). The quality of correlation
between any two parameters was evaluated using a regression
analysis based on the value of the coefficient of determination
(R2). Reliability decreases with a decrease in the
R2 value (R2=1 means completely reliable,
while R2=0 means completely unreliable).
Results and Discussion
Fifty-seven edible medicinal plants were studied by
measuring their free radical-scavenging (by DPPH assay) and their
cytotoxic activity (by XTT assay). All results are summarized in
Table I. The average yield of
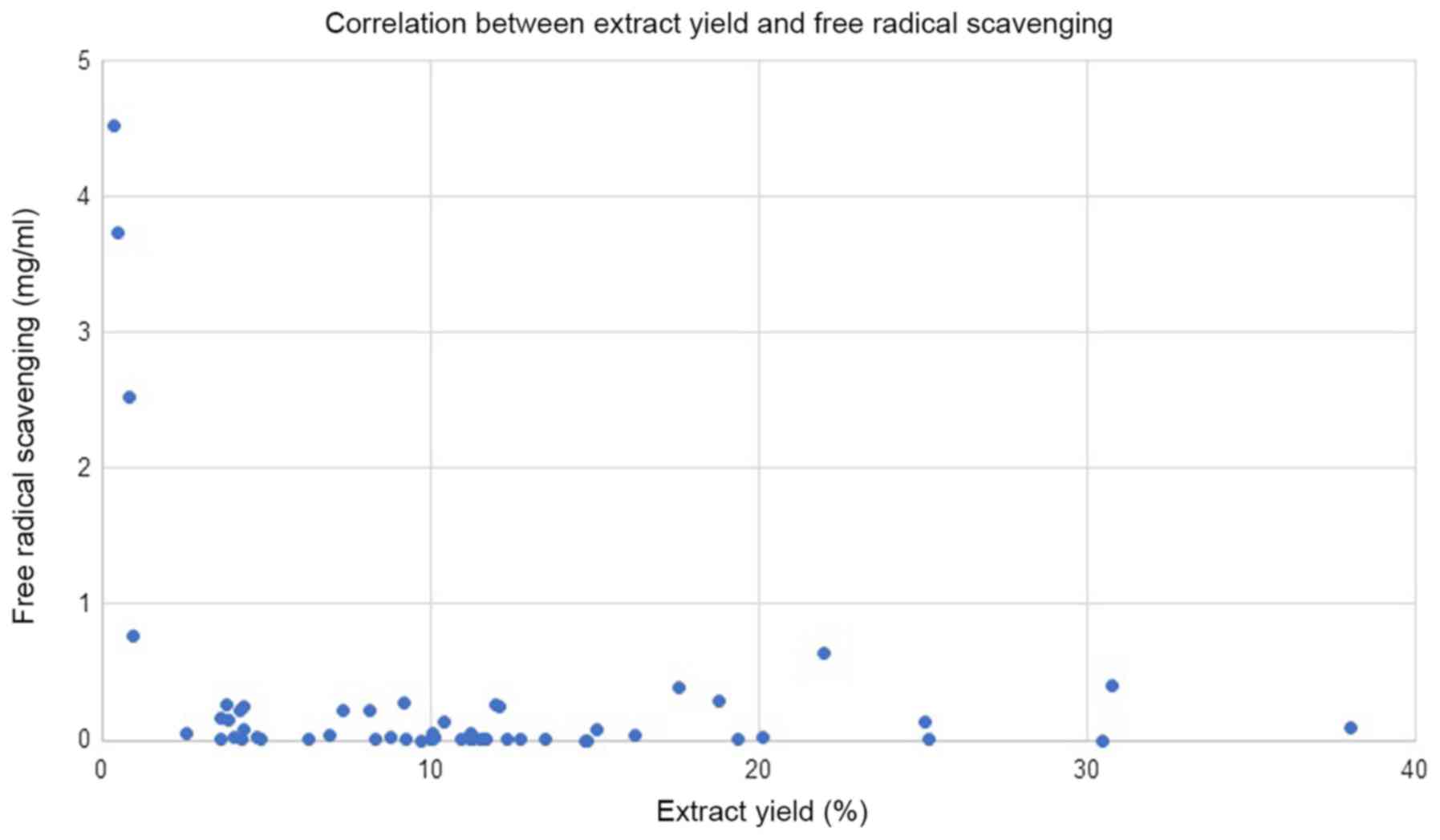
extraction by methanol was 11.2%, and as shown in Fig. 1, no correlation was detected between
the % of extraction yield and free radical scavenging. However, the
four plants that gave a % of yield of <1% (Linum
usitatissimum, Salvia hispanica, Lepidium sativum and
Crocus) possess the lowest free radical-scavenging activity
(EC50 values, of 4,523, 3,736, 2,529 and 765 µg/ml,
respectively). A review of column 3 of Table I reveals that Malva, sumac,
Melissa officinalis, and green tea have the highest content
of antioxidants, with EC50 values of free
radical-scavenging of <0.5 µg/ml. Cytotoxicity was first
verified by screening all extracts for their activity, using one
concentration of 250 µg/ml; column 4 depicts the % of inhibition at
this concentration. The extracts that gave a % of inhibition above
90% were tested in the second round at lower concentrations in dose
response manner to extract their EC50 values. The
results are summarized in column 5. Nine extracts were found to
have EC50 values of <250 µg/ml (Rosmarinus
officinalis, Origanum vulgare, Vitex agnus-castus, Cynara
cardunculus, Lippia citriodora, Zingiber officinale, Cinnamomum
aromaticum, Laurus nobilis, and Salvia officinalis).
Their EC50 values are 131, 180, 42, 152, 197, 109, 162,
182 and 142 µg/ml, respectively. Three other extracts [Punuca
granatum (fruit peel), Pelargonium spp (leaf), Sumac (ripe
fruit)] have EC50 values close to 250 µg/ml, where
treatment with 250 µg/ml inhibit viability of liver cancer cells by
49, 53 and 66%, respectively.
Rules-based analysis using Matthew's correlation
coefficient (MCC) scores and enrichment factors as criteria for the
evaluation of the models' efficiency revealed that the plant
extracts whose EC50 for free radical scavenging ≤10
µg/ml showed some degree of enrichment toward more cytotoxicity
(Table II). The values for the
enrichment factor, the MCC, accuracy, and precision are 2.6, 0.28,
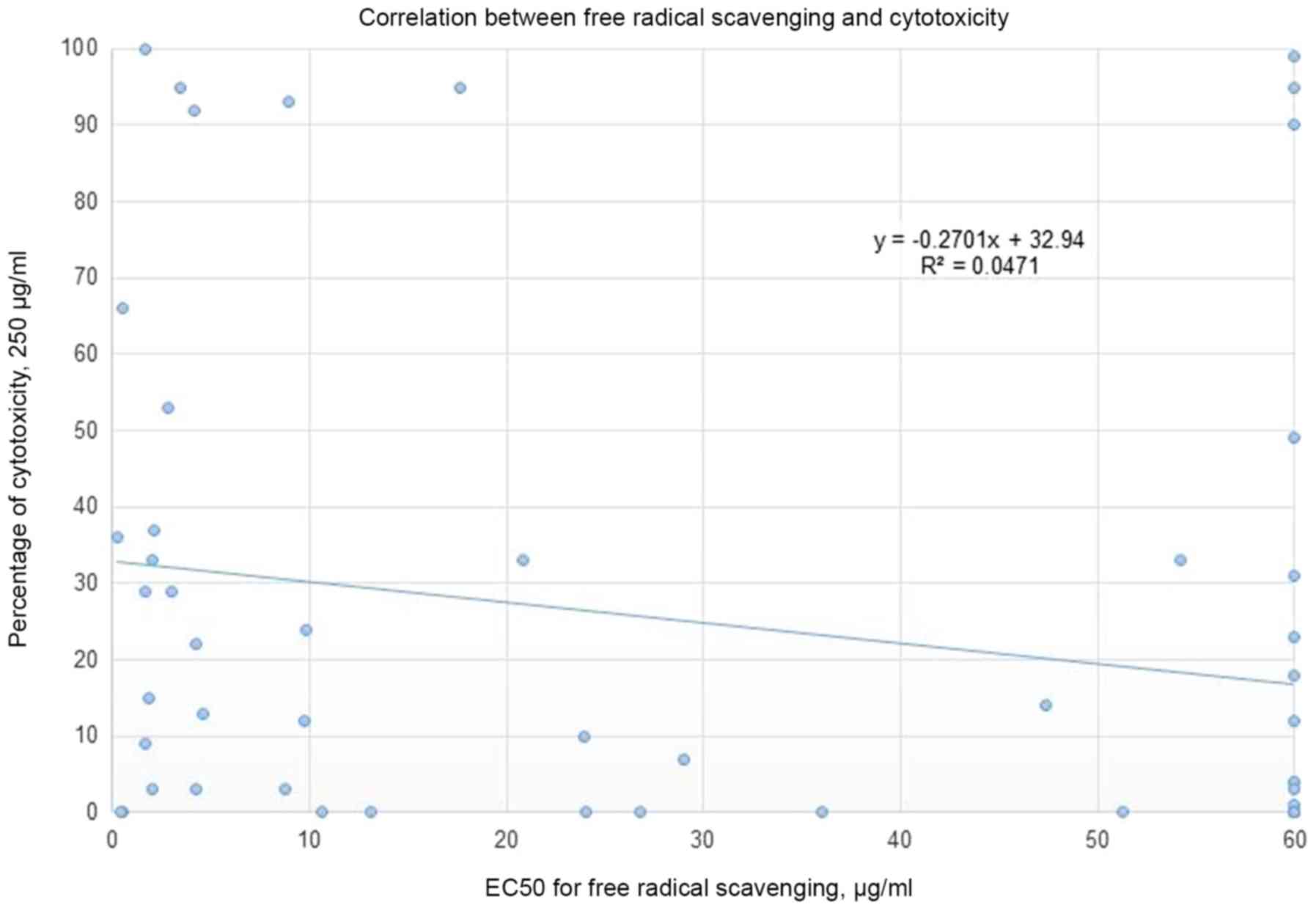
0.67 and 0.5, respectively. No correlation was detected between the
% of cytotoxicity (using a concentration of 250 µg/ml of plant
extract) and the EC50 for free radical scavenging
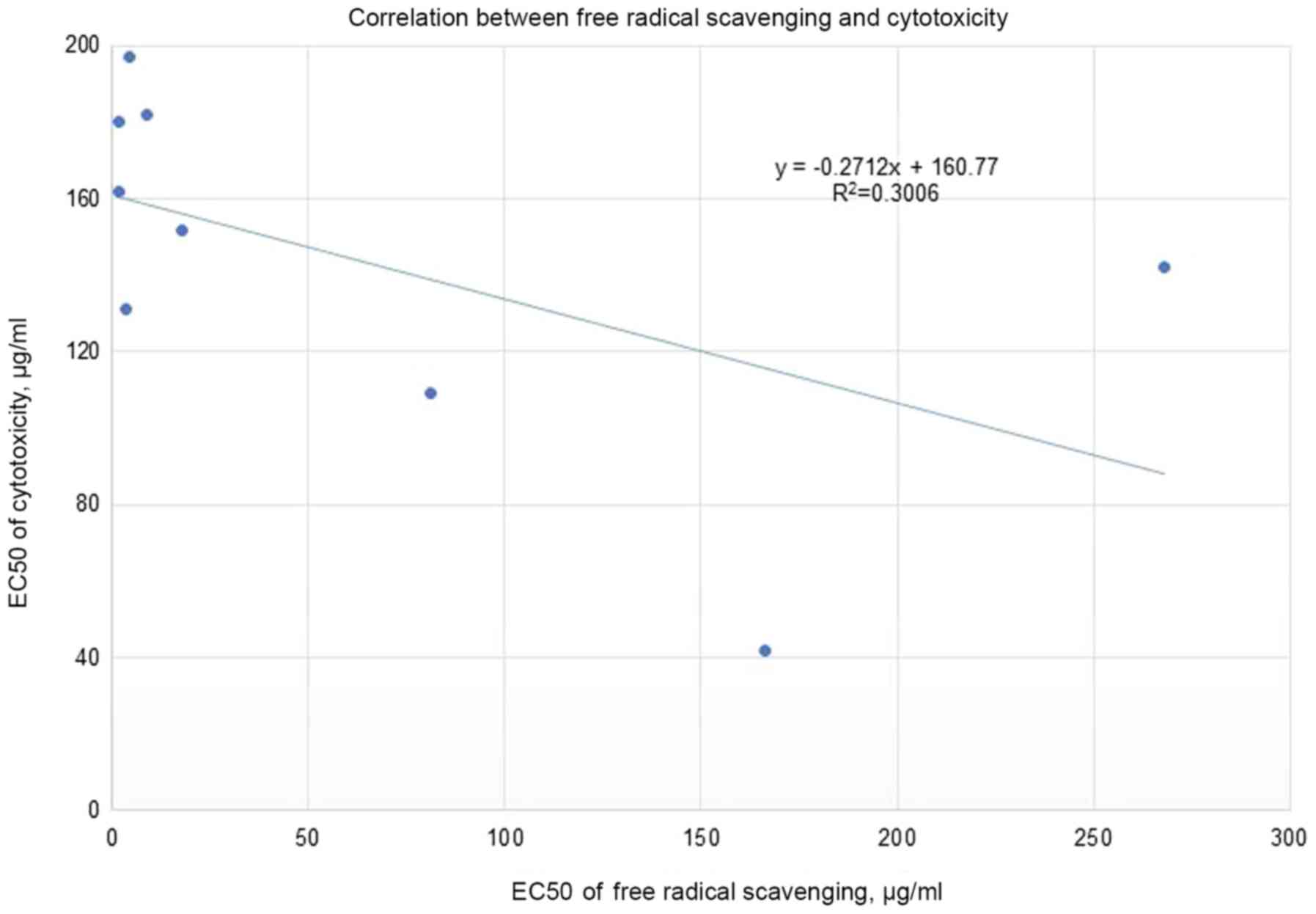
(Fig. 2). Moreover, the correlation
between the EC50 for cytotoxicity and the
EC50 for free radical scavenging for the nine most
cytotoxic plant extracts (Fig. 3)
tends slightly toward the negative. The most cytotoxic plant
extract (Vitex agnus-castus) showed, by several orders of
magnitude, free radical-scavenging less than seven other extracts
(Rosmarinus officinalis, Origanum vulgare, Cynara cardunculus,
Lippia citriodora, Zingiber officinale, Cinnamomum aromaticum,
and Laurus nobilis) out of the nine most cytotoxic plants.
The obtained results show that differences in cytotoxic activities
among the extracts are not mainly accredited to the level of
antioxidants but could also be associated with the inhibitory
effects via other signaling pathways.
 | Table II.MCC scores and enrichment factors
were utilized as criteria for evaluating the models. All
calculations are based on the assumption that a % of cytotoxicity
≥30%, at a concentration of 250 µg/ml of plant extract, is
considered active (a true positive); otherwise, it is considered
inactive. One third of the tested plants (nineteen extracts) showed
activity ≥30% cytotoxicity. |
Table II.
MCC scores and enrichment factors
were utilized as criteria for evaluating the models. All
calculations are based on the assumption that a % of cytotoxicity
≥30%, at a concentration of 250 µg/ml of plant extract, is
considered active (a true positive); otherwise, it is considered
inactive. One third of the tested plants (nineteen extracts) showed
activity ≥30% cytotoxicity.
|
| EC50
cutoff of FRS (≤) |
|---|
|
|
|
|---|
| Criteria | 10 µg/ml | 50 µg/ml | 250 µg/ml | No limit |
|---|
| No. active plants
(true positives)a | 11 | 13 | 17 | 19 |
| No. inactive plants
(false positives)b | 11 | 19 | 28 | 38 |
| No. inactive plants
(true negatives)c | 27 | 19 | 10 | – |
| No. active plants
(false negatives)d | 8 | 6 | 2 | – |
| Precision | 0.5 | 0.41 | 0.38 | 0.34 |
| Accuracy | 0.67 | 0.56 | 0.47 | 0.34 |
| Enrichment
factor | 1.5 | 1.22 | 1.13 | 1.0 |
| MCC | 0.280 | 0.175 | 0.183 | 0.0 |
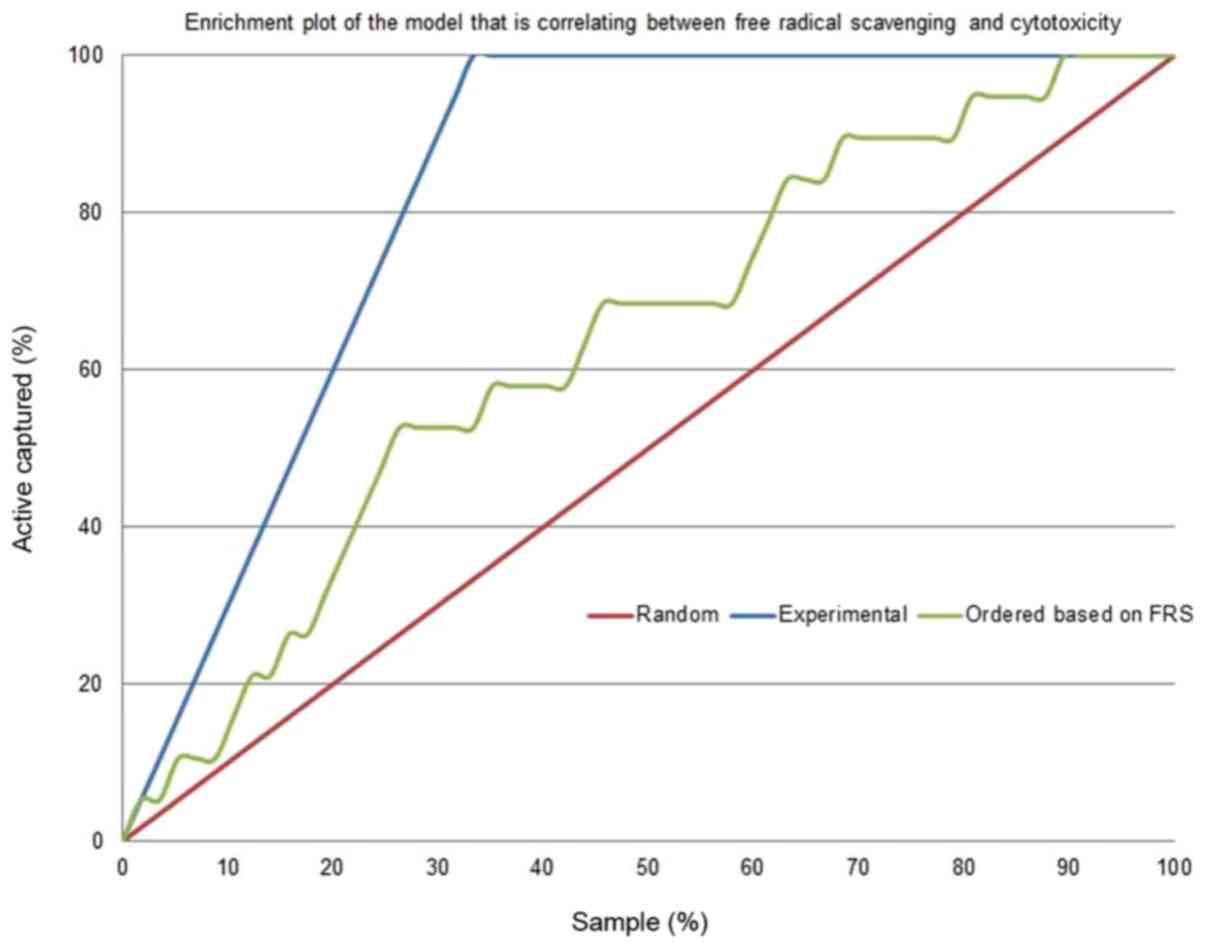
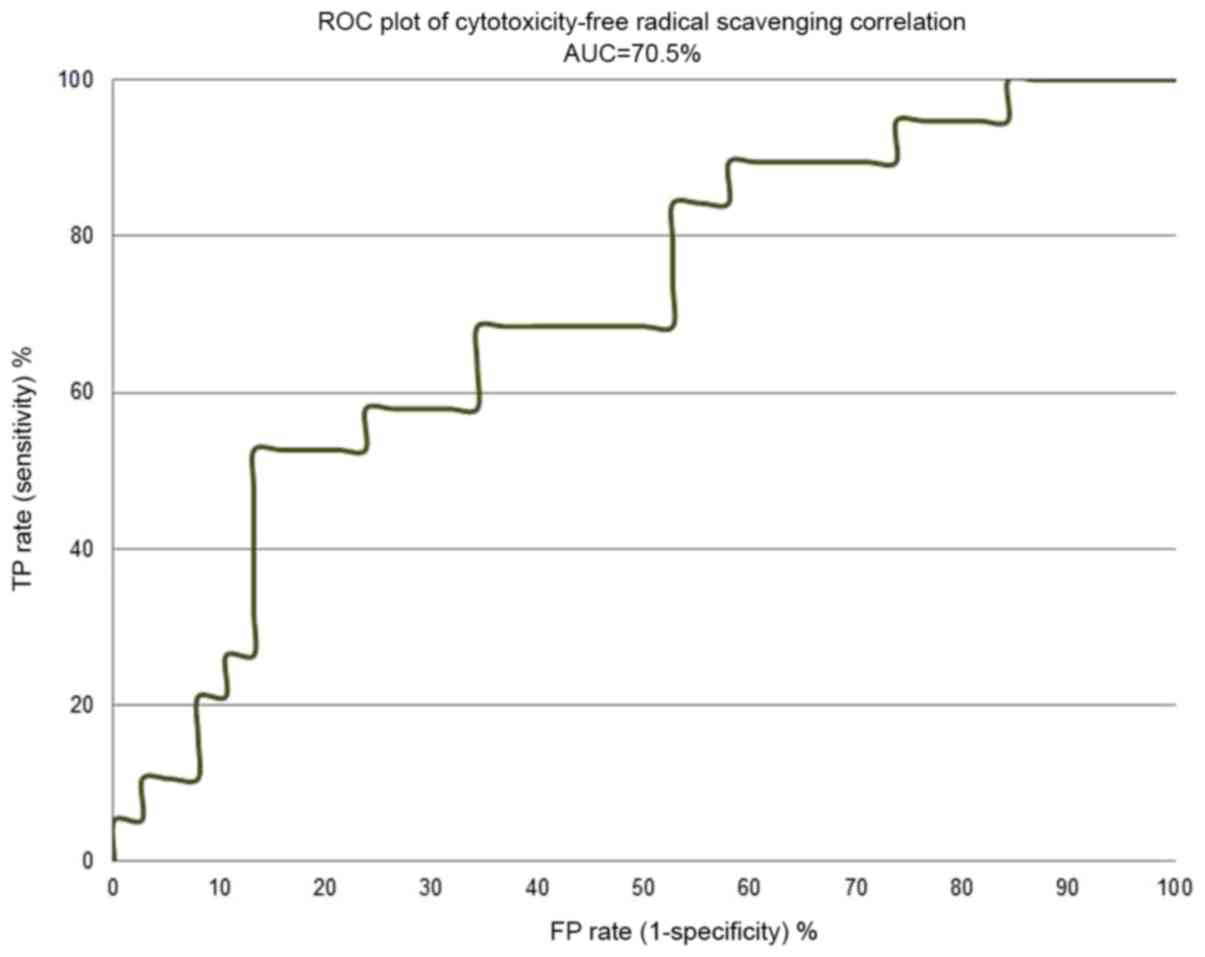
Figs. 4 and 5 depict the enrichment plot and the
receiver operating characteristic (ROC) plot for the
cytotoxicity/free radical-scavenging correlation model. It is worth
noting that a fully random model should yield an AUC value around
0.5. The area under the curve (AUC) that was attained for the
current model, as shown in Fig. 5,
is 0.705, which means that the model is very poor, indicating a
very weak correlation between cytotoxicity and free radical
scavenging. The enrichment plot that is shown in Fig. 4 illustrates how quickly cytotoxic
extracts of plants can be identified when they are sorted according
to their free radical scavenging activity. If the enrichment plot
of the proposed model is close to the perfect model, it indicates
high prioritization power. A close look reveals that the shape of
the figure fits well with the conclusions drawn from the detailed
analysis of Table II, which
disclosed that plant extracts with an EC50 of free
radical scavenging ≤10 µg/ml display some degree of enrichment
toward more cytotoxicity. At this point, the model line is closer
to the experimental line than to the random line.
In mid-October 2018, the electronic database PubMed
was searched using the scientific names of the demonstrably
cytotoxic plants disclosed herein and the keyword
anticancer. Eight out of the nine cytotoxic plant extracts
were reported to be active against cancer cell lines. As well, half
of the eight were reported as active against HepG2, while the rest
are reported here for the first time as showing activity against
HepG2. Rosmarinus officinalis (34,35) and
its components, the phenolic compound rosmarinic acid (36) and the abietane diterpenoid sageone
(37), were reported to show
anticancer properties, but had not been tested on HepG2.
Origanum vulgare (38) and
its main constituents (carvacrol, thymol, citral, and limonene)
have been tested on HepG2 cell line (liver cancer) and were
reported as active against cancer. Cynara cardunculus L. has
evidenced anticancer potential (39)
on triple-negative breast cancer (TNBC). It highlights the
antiproliferative effects of lipophilic extracts from the leaves
and florets of C. cardunculus L., and of their major
constituents, namely cynaropicrin and taraxasteryl acetate, against
MDA-MB-231 cells. A review article by Bahramsoltani (40) reported anticancer effects for
Lippia citriodora against human colon cancer (HT29) cells;
its extract enhances BAX (a pro-apoptotic gene) and reduces the
expression level of Bcl-2 (an anti-apoptotic gene). Zingiber
officinale extract significantly inhibited the proliferation of
HepG2 cells and induced apoptosis (41). Cinnamomum cassia (syn.
Cinnamomum aromaticum) extracts were reported to have
anticancer activity (42). Laurus
nobilis was reported as active against cancer cell lines
(43) such as HeLa cells, but its
activity on HepG2 had not been tested. Salvia officinalis
extracts were confirmed to have cytotoxic effects on HepG2 cells
(44). Recently, Kikuchi et
al (45). demonstrated that an
extract from the ripe fruit of Vitex angus-castus
(Vitex), might be a promising anticancer candidate. It was
the only scientific report to mention its cytotoxicity, which was
tested by its effects on HL-60 cells, but not on HepG2; no
phytochemicals were identified as the source of its cytotoxicity.
We are currently working on isolating and identifying its bioactive
chemical ingredients.
Since reactive oxygen species (ROS) are known to be
triggers of various human cancers, and antioxidants or scavengers
are used to counteract these dangerous species, we have raised a
question regarding the correlation between free radical scavenging
and the cytotoxicity of plant extracts. Free radical scavenging was
assessed by DPPH assay, while cytotoxicity was measured by XTT
assay. Nine extracts were found to be cytotoxic with
EC50 values of <250 µg/ml, and four others had a high
content of antioxidants, with EC50 values of free
radical scavenging of <0.5 µg/ml. Upon looking on the results
which were obtained from screening fifty-seven plants for their
cytotoxic activity, we concluded, from first inspection, that there
is no correlation between free radical scavenging and cytotoxicity.
However, an in-depth analysis of the results reveals that the
extracts of plants that had an EC50 for free radical
scavenging ≤10 µg/ml exhibited a certain enrichment toward more
cytotoxicity (enrichment factor of 1.5). We suggest checking
further the validity of the conclusions that are drawn from the
current study on other cancer cell-lines, and also by utilizing
aqueous or other organic solvents to perform the extraction. The
nine active extracts of plants disclosed here could be a source of
anticancer hits/lead phytochemicals, worth the effort it would take
to isolate and chemically identify them.
Acknowledgements
Not applicable.
Funding
This work was partially supported by the Al-Qasemi
Research Foundation and the Ministry of Science, Space and
Technology, Israel.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AR conceived the study, designed the experiments,
and contributed to writing and editing the manuscript. MF
interpreted the data and wrote the original manuscript. BAF and IR
collected the plants, performed their extraction and the run free
radical-scavenging experiments. MS designed and performed the
cytotoxicity experiments, interpreted the data and wrote the
original draft.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aswad M, Rayan M, Abu-Lafi S, Falah M,
Raiyn J, Abdallah Z and Rayan A: Nature is the best source of
anti-inflammatory drugs: Indexing natural products for their
anti-inflammatory bioactivity. Inflamm Res. 67:67–75. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frank A, Abu-Lafi S, Adawi A, Schwed JS,
Stark H and Rayan A: From medicinal plant extracts to defined
chemical compounds targeting the histamine H4 receptor: Curcuma
longa in the treatment of inflammation. Inflamm Res. 66:923–929.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zaid H, Raiyn J, Osman M, Falah M, Srouji
S and Rayan A: In silico modeling techniques for predicting the
tertiary structure of human H4 receptor. Front Biosci (Landmark
Ed). 21:597–619. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zeidan M, Rayan M, Zeidan N, Falah M and
Rayan A: Indexing natural products for their potential
anti-diabetic activity: Filtering and mapping discriminative
physicochemical properties. Molecules. 22:E15632017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sui YB, Liu L, Tian QY, Deng XW, Zhang YQ
and Li ZG: A retrospective study of traditional Chinese medicine as
an adjunctive therapy for patients with chronic heart failure.
Medicine (Baltimore). 97:e116962018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maiti P and Dunbar GL: Use of curcumin, a
natural polyphenol for targeting molecular pathways in treating
age-related neurodegenerative diseases. Int J Mol Sci.
19:E16372018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masalha M, Rayan M, Adawi A, Abdallah Z
and Rayan A: Capturing antibacterial natural products with in
silico techniques. Mol Med Rep. 18:763–770. 2018.PubMed/NCBI
|
|
8
|
Kacergius T, Abu-Lafi S, Kirkliauskiene A,
Gabe V, Adawi A, Rayan M, Qutob M, Stukas R, Utkus A, Zeidan M and
Rayan A: Inhibitory capacity of Rhus coriaria L. extract and
its major component methyl gallate on Streptococcus mutans
biofilm formation by optical profilometry: Potential applications
for oral health. Mol Med Rep. 16:949–956. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vallejo MJ, Salazar L and Grijalva M:
Oxidative stress modulation and ROS-mediated toxicity in cancer: A
review on in vitro models for plant-derived compounds. Oxid Med
Cell Longev. 2017:45860682017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chikara S, Nagaprashantha LD, Singhal J,
Horne D, Awasthi S and Singhal SS: Oxidative stress and dietary
phytochemicals: Role in cancer chemoprevention and treatment.
Cancer Lett. 413:122–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gavamukulya Y, Wamunyokoli F and El-Shemy
HA: Annona muricata: Is the natural therapy to most disease
conditions including cancer growing in our backyard? A systematic
review of its research history and future prospects. Asian Pac J
Trop Med. 10:835–848. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kapinova A, Stefanicka P, Kubatka P, Zubor
P, Uramova S, Kello M, Mojzis J, Blahutova D, Qaradakhi T, Zulli A,
et al: Are plant-based functional foods better choice against
cancer than single phytochemicals? A critical review of current
breast cancer research. Biomed Pharmacother. 96:1465–1477. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rayan A, Raiyn J and Falah M: Nature is
the best source of anticancer drugs: Indexing natural products for
their anticancer bioactivity. PLoS One. 12:e01879252017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tariq A, Sadia S, Pan K, Ullah I, Mussarat
S, Sun F, Abiodun OO, Batbaatar A, Li Z, Song D, et al: A
systematic review on ethnomedicines of anti-cancer plants.
Phytother Res. 31:202–264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raza A and Sood GK: Hepatocellular
carcinoma review: Current treatment, and evidence-based medicine.
World J Gastroenterol. 20:4115–4127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ali H, Dixit S, Ali D, Alqahtani SM,
Alkahtani S and Alarifi S: Isolation and evaluation of anticancer
efficacy of stigmasterol in a mouse model of DMBA-induced skin
carcinoma. Drug Des Devel Ther. 9:2793–2800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ziech D, Franco R, Georgakilas AG,
Georgakila S, Malamou-Mitsi V, Schoneveld O, Pappa A and
Panayiotidis MI: The role of reactive oxygen species and oxidative
stress in environmental carcinogenesis and biomarker development.
Chem Biol Interact. 188:334–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Galaris D, Skiada V and Barbouti A: Redox
signaling and cancer: The role of ‘labile’ iron. Cancer Lett.
266:21–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jambunathan S, Bangarusamy D, Padma PR and
Sundaravadivelu S: Cytotoxic activity of the methanolic extract of
leaves and rhizomes of Curcuma amada Roxb against breast cancer
cell lines. Asian Pac J Trop Med. 7S1:S405–S409. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fuchs-Tarlovsky V: Role of antioxidants in
cancer therapy. Nutrition. 29:15–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chua MT, Tung YT and Chang ST: Antioxidant
activities of ethanolic extracts from the twigs of Cinnamomum
osmophloeum. Bioresour Technol. 99:1918–1925. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abu-Lafi S, Rayan B, Kadan S, Abu-Lafi M
and Rayan A: Anticancer activity and phytochemical composition of
wild Gundelia tournefortii. Oncol Lett. 17:713–717.
2019.PubMed/NCBI
|
|
24
|
Hwang YJ, Lee EJ, Kim HR and Hwang KA: In
vitro antioxidant and anticancer effects of solvent fractions from
Prunella vulgaris var. lilacina. BMC Complement Altern Med.
13:3102013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Radomska-Leśniewska DM, Hevelke A,
Skopiński P, Bałan B, Jóźwiak J, Rokicki D, Skopińska-Różewska E
and Białoszewska A: Reactive oxygen species and synthetic
antioxidants as angiogenesis modulators: Clinical implications.
Pharmacol Rep. 68:462–471. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boots AW, Haenen GR and Bast A: Health
effects of quercetin: From antioxidant to nutraceutical. Eur J
Pharmacol. 585:325–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kadan S, Rayan M and Rayan A: Anticancer
Activity of Anise (Pimpinella anisum L.) Seed Extract. Open
Nutraceuticals J. 6:1–5. 2013. View Article : Google Scholar
|
|
28
|
Zaid H, Rayan A, Said O and Saad B: Cancer
treatment by Greco-Arab and Islamic herbal medicine. Open
Nutraceuticals J. 3:203–213. 2010. View Article : Google Scholar
|
|
29
|
Uttara B, Singh AV, Zamboni P and Mahajan
RT: Oxidative stress and neurodegenerative diseases: A review of
upstream and downstream antioxidant therapeutic options. Curr
Neuropharmacol. 7:65–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Perrone S, Santacroce A, Longini M,
Proietti F, Bazzini F and Buonocore G: The free radical diseases of
prematurity: From cellular mechanisms to bedside. Oxid Med Cell
Longev. 2018:74830622018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abu-Lafi S, Rayan M, Masalha M, Abu-Farich
B, Al-Jaas H, Abu-Lafi M and Rayan A: Phytochemical composition and
biological activities of wild Scolymus maculatus L.
Medicines (Basel). 6:E532019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Masalha M, Abu-Lafi S, Abu-Farich B, Rayan
M, Issa N, Zeidan M and Rayan A: A new approach for indexing honey
for its heath/medicinal benefits: Visualization of the concept by
indexing based on antioxidant and antibacterial activities.
Medicines (Basel). 5:E1352018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moore J, Yousef M and Tsiani E: Anticancer
effects of rosemary (Rosmarinus officinalis L.) extract and
rosemary extract polyphenols. Nutrients. 8:E7312016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gonzalez-Vallinas M, Reglero G and Ramirez
de Molina A: Rosemary (Rosmarinus officinalis L.) extract as
a potential complementary agent in anticancer therapy. Nutr Cancer.
67:1221–1229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Swamy MK, Sinniah UR and Ghasemzadeh A:
Anticancer potential of rosmarinic acid and its improved production
through biotechnological interventions and functional genomics.
Appl Microbiol Biotechnol. 102:7775–7793. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shrestha S, Song YW, Kim H, Lee DS and Cho
SK: Sageone, a diterpene from Rosmarinus officinalis,
synergizes with cisplatin cytotoxicity in SNU-1 human gastric
cancer cells. Phytomedicine. 23:1671–1679. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Elshafie HS, Armentano MF, Carmosino M,
Bufo SA, De Feo V and Camele I: Cytotoxic activity of Origanum
Vulgare L. on hepatocellular carcinoma cell line HepG2 and
evaluation of its biological activity. Molecules. 22:E14352017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ramos PA, Guerra AR, Guerreiro O, Santos
SA, Oliveira H, Freire CS, Silvestre AJ and Duarte MF:
Antiproliferative Effects of Cynara cardunculus L. var.
altilis (DC) lipophilic extracts. Int J Mol Sci. 18:E632016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bahramsoltani R, Rostamiasrabadi P,
Shahpiri Z, Marques AM, Rahimi R and Farzaei MH: Aloysia
citrodora Palau (Lemon verbena): A review of phytochemistry and
pharmacology. J Ethnopharmacol. 222:34–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Elkady AI, Abu-Zinadah OA and Hussein R:
Crude flavonoid extract of medicinal herb Zingibar
officinale inhibits proliferation and induces apoptosis in
hepatocellular carcinoma cells. Oncol Res. 25:897–912. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin CY, Hsieh YH, Yang SF, Chu SC, Chen PN
and Hsieh YS: Cinnamomum cassia extracts reverses
TGF-β1-induced epithelial-mesenchymal transition in human lung
adenocarcinoma cells and suppresses tumor growth in vivo. Environ
Toxicol. 32:1878–1887. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Berrington D and Lall N: Anticancer
activity of certain herbs and spices on the cervical epithelial
carcinoma (HeLa) cell line. Evid Based Complement Alternat Med.
2012:5649272012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jiang Y, Zhang L and Rupasinghe HP:
Antiproliferative effects of extracts from Salvia
officinalis L. and Saliva miltiorrhiza Bunge on
hepatocellular carcinoma cells. Biomed Pharmacother. 85:57–67.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kikuchi H, Yuan B, Nishimura Y, Imai M,
Furutani R, Kamoi S, Seno M, Fukushima S, Hazama S, Hirobe C, et
al: Cytotoxicity of Vitex agnus-castus fruit extract and its
major component, casticin, correlates with differentiation status
in leukemia cell lines. Int J Oncol. 43:1976–1984. 2013. View Article : Google Scholar : PubMed/NCBI
|