Introduction
Almost one-fifth of new cancer cases among American
males are prostate cancer (PCa), and ~10% of these led to death in
2018 (1). Despite many efficacious
treatments for localized PCa, such as radical prostatectomy (RP) or
external-beam radiotherapy, 27–53% of patients exhibit biochemical
recurrence (BCR), which may cause an increased risk of distant
metastasis and mortality (2).
Furthermore, the current predictive models of PCa following surgery
have demonstrated limitations and ineffectiveness in daily clinical
practice. PCa is a heterogeneous and multifocal cancer, which
suggests that patients with PCa with the same prostate-specific
antigen (PSA) levels and tumor stage may have a different prognosis
(3). Notably, there are no critical
predictive methods available to accurately evaluate PCa recurrence
or predict the prognosis of individuals. New methods are therefore
required to predict BCR in patients with PCa and to provide an
effective method for personalized treatment for patients with
PCa.
Numerous studies have shown that during mitosis,
disfunction or destabilization of centromeres results in chromosome
deletion, anomaly separation and aneuploidy, which may also lead to
chromosome instability and cancer development (4–7).
Centromere protein A (CENP-A) is a crucial centromere protein that
is required to maintain the functional centromere and ensure
chromosomes separate correctly during mitosis (8) However, many studies have shown that the
abnormal expression of CENP-A may lead to chromosome instability
and cancer progression (5,6). Further research on centromere
disfunction may aid the identification of a new mechanism involved
in cancer formation and progression (7).
As the CENP-A chaperone, Holliday junction
recognition protein (HJURP) mediates CENP-A deposition at
centromeres (9). An increasing body
of evidence suggests that HJURP is expressed in normal cells, and
also abnormally expressed in cancer cell lines (10,11).
Notably, HJURP is overexpressed in lung, ovarian, breast cancer and
glioblastoma cells and is associated with poor prognosis (11). Therefore, HJURP may be used as a
tumor detection tool for a variety of types of cancer (12). Research indicates that using small
interfering RNA against HJURP in cancer cells results in chromosome
instability and contributes to cell cycle arrest (13). Findings have demonstrated that the
regulation of HJURP is vital for promoting genome stability
(6). HJURP has also been considered
as a new therapeutic target of some anticancer drugs (13). Thus, these findings suggest that
HJURP might have a crucial role in the generation and progression
of cancer.
To the best of our knowledge, no study to date has
assessed the expression pattern of HJURP in PCa. Therefore, the aim
of the present study was to analyze the expression of HJURP in PCa
and to assess its association with clinicopathological data.
Materials and methods
Clinical samples
This study was approved by the Research Ethics
Committee of Guangzhou First People's Hospital (Guangzhou, China).
All patients provided written informed consent. The specimens were
handled and made anonymous according to ethical and legal
standards.
All patient information and clinical features in
this study are presented in Table I.
For reverse transcription quantitative polymerase chain reaction
(RT-qPCR) analysis, a total of 22 PCa and 10 benign prostate
tissues were used, which were collected and frozen during RP and
transurethral resection of the prostate between January 2012 and
December 2016 at Guangzhou First People's Hospital. No patients
recruited in this study had received chemotherapy or radiotherapy
prior to surgery. Furthermore, immunohistochemical analysis was
performed on tissue microarrays (TMAs), which included PCa tissues
(n=99) and benign prostate tissues (n=81). The TMAs were obtained
from Shanghai Outdo Biotech Co., Ltd., (cat. no. HPro-Ade180PG-01).
Owing to some sample tissues falling off during immunohistochemical
analysis and others being defective or missing related clinical
information, 40 samples were excluded and 140 (65 PCa and 75 benign
prostate tissues) samples were subsequently used. Furthermore, an
online PCa dataset (Taylor dataset) from http://cbio.mskcc.org/cancergenomics/prostate/
was downloaded for statistical analysis (14). The Taylor dataset contains 150 PCa
and 21 benign prostate tissues with detailed clinical
information.
 | Table I.Detailed clinical information of the
patients included in the study. |
Table I.
Detailed clinical information of the
patients included in the study.
| Clinical
features | RT-qPCR |
Immunohistochemistry | Taylor Dataset |
|---|
| Benign tissue, n | 10 | 81 | 29 |
| Prostate cancer,
n | 22 | 99 | 150 |
| Age in years, mean ±
SD | 72.27±6.94 | 70.71±8.00 | 58.34±7.07 |
|
<66 | 4 | 26 | 25 |
| ≥66 | 18 | 73 | 125 |
| Serum
prostate-specific antigen, n |
|
|
|
| <4
ng/ml | 9 | – | 24 |
| 4–10
ng/ml | 3 | – | 81 |
| ≥10
ng/ml | 10 | – | 42 |
| Gleason score, n |
|
|
|
| ≤6 | 8 | 26 | 41 |
| 7 | 3 | 44 | 76 |
| ≥8 | 11 | 28 | 22 |
| Pathological stage,
n |
|
|
|
| T2 | 22 | 70 | 86 |
|
≥T3A | 0 | 29 | 55 |
RNA extraction and reverse RT-qPCR
analysis
Total RNA was extracted from cultured prostate cells
(~5×106 cells) with the RNeasy mini kit (Qiagen,
Germany). The Invitrogen SuperScript III First-Strand System
(Invitrogen; Thermo Fisher Scientific, Inc.) was used for RT with
random primers (Hexadeoxyribonucleotide mixture; pd (N)6; cat. no.
3801; Takara Biotechnology Co., Ltd., Dalian, China). Reverse
transcription was performed as follows: 37°C for 15 min and 85°C
for 5 sec. mRNA expression was detected using SYBR Green PCR mix
(Toyobo Co., Ltd.) and normalized to β-actin as the internal
control. The following primers were used: HJURP forward,
5′-CACAAAGCCATCAAGCATCATC-3′ and reverse,
5′-TCAGAGCAGGGTATGAAGTTCT-3′; and β-actin forward,
5′-AGCGAGCATCCCCCAAAGTT-3′ and reverse, 3′-GGGCACGAAGGCTCATCATT-5′.
QPCR was performed on a MyiQ.2 Two-Color Real-Time qPCR Detection
System (Bio-Rad Laboratories, Inc.), and the thermocycling
conditions were as follows: 48°C for 30 min, 95°C for 1 min,
followed by 40 cycles at 95°C for 15 sec, 52°C for 30 sec and 72°C
for 30 sec. All assays were carried out in triplicate. CT values
were determined using the IQ5 software (Bio-Rad Laboratories,
Inc.). Relative quantification of target mRNA expression was
evaluated using the 2−ΔΔCq method (15). The mean ± standard deviation (SD) was
calculated from three independent experiments.
Immunohistochemistry
Immunohistochemistry and the immunoreactivity scores
(IRS) were used to evaluate the expression levels and subcellular
localization of HJURP protein in PCa tissues. The specimens were
fixed in 10% neutral buffered formalin and were subsequently
processed by gradient dehydration in ethanol, embedded in paraffin
and sectioned into 4-µm sections for hematoxylin and eosin or
immunohistochemistry staining with the DAKO EnVision System (Dako
Diagnostics). Following antigen retrieval with citric acid buffer
at 95°C for 8 min, the sections were blocked with peroxidase at
room temperature for 15 min and 10% goat serum (cat. no. BA1056;
Wuhan Boster Biological Technology, Ltd.) at room temperature for
30 min. Subsequently, the sections were incubated overnight with a
rabbit anti-HJRUP primary antibody (cat. no. PAB20427; dilution,
1:50; Abnova Corporation) at 4°C. Following three washes (5 min
each) in phosphate buffer saline, the sections were incubated with
an avidin-conjugated goat anti-rabbit secondary antibody
(undiluted; cat. no. BA1056; Wuhan Boster Biological Technology
Ltd.) at room temperature for 30 min.
Streptavidin-peroxidase-labeled polymer (50 µl for 15 min at room
temperature) and substrate-chromogen (100 µl for 2 min at room
temperature) were used to observe the staining of the target
protein. Negative controls were obtained by omitting the primary
antibody. Light microscopy at ×400 magnification was used to
examine the sections.
Immunostaining was scored by two experienced
independent pathologists who were blinded to the
clinicopathological data and clinical outcomes of the patients. The
scores of the two pathologists were compared, and any discrepancy
between the scores were dealt with by re-examination of the
staining by both pathologists to achieve a consensus score. The
immunolabeling of cancer cells and stromal cells was evaluated
separately. The number of positive-staining cells in ten
representative microscopic fields was counted, and the percentage
of positive cells was calculated. Given the homogeneity of the
staining of the target proteins, tumor specimens were scored in a
semi-quantitative manner. The percentage scoring of immunoreactive
tumor cells was as follows: 0, 0–5%; 1, 6–25%; 2, 26–50%; 3,
51–75%; and 4, >75%. The staining intensity was visually scored
and stratified as follows: 0, negative; 1, weak; 2, moderate; and
3, strong. An IRS value for HJURP staining was obtained for each
case by adding the percentage and the intensity score.
Statistical analysis
Statistical analyses were performed using SPSS 19.0
(IBM Corp.). Unpaired two-tailed Student's t test was used to
analyze the results and the data were expressed as the mean ± SD.
The survival analysis was performed by Kaplan-Meier curves and the
groups were compared by log-rank (Mantel-Cox) test. P<0.05 was
considered to indicate a statistically significant difference.
Results
HJURP is overexpressed in PCa
tissues
RT-qPCR and immunohistochemistry results revealed
that HJURP was upregulated in PCa tissues. As indicated by the
RT-qPCR results in Fig. 1, the mRNA
expression levels of HJURP were higher in PCa compared with benign
prostate tissues (P<0.001). Furthermore, the protein expression
levels of HJURP in PCa were investigated using immunohistochemical
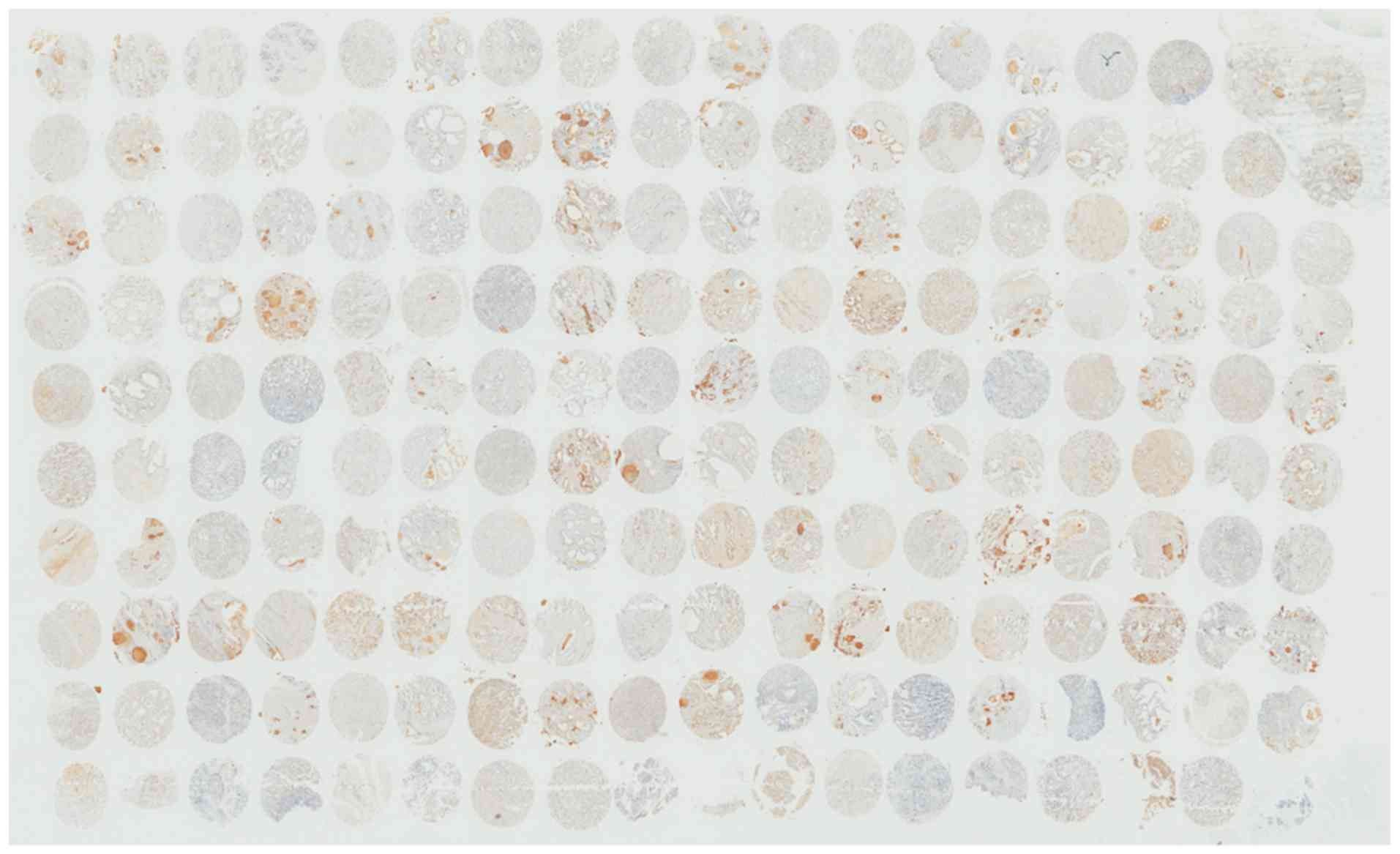
analysis. The panoramic view examined with immunohistochemical
analysis of TMA is presented in Fig.
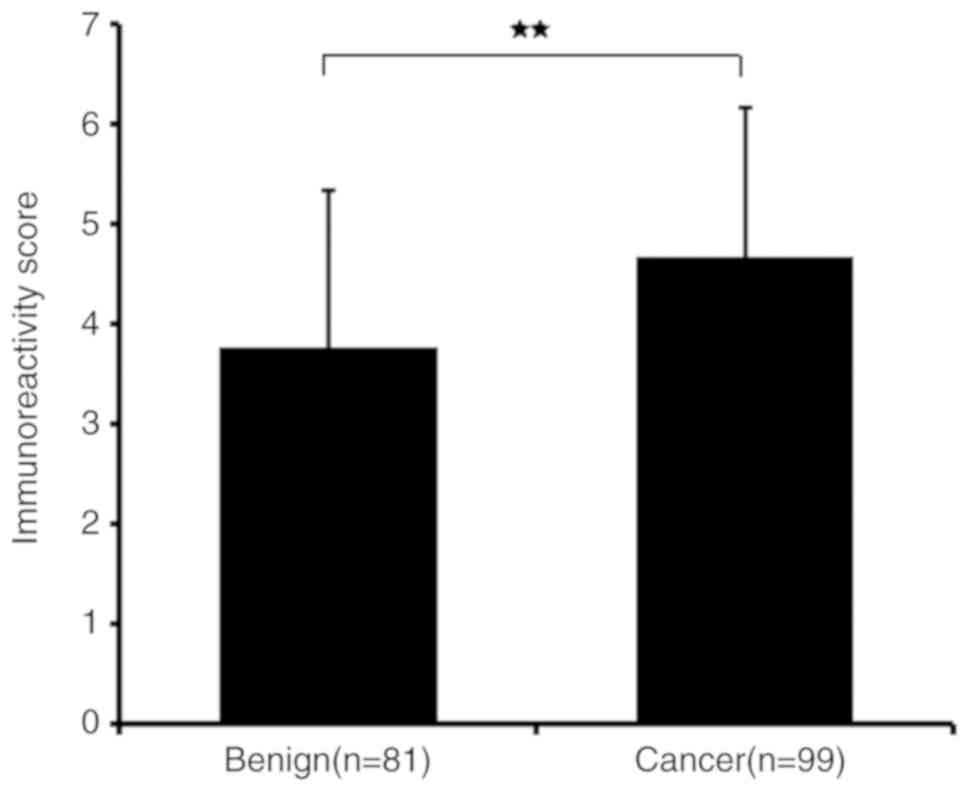
2. As demonstrated in Fig. 3,
IRS values revealed that the protein expression levels of HJURP
were higher in PCa compared with benign prostate tissues
(P<0.001), which was in accordance with the results of RT-qPCR
and with statistical analysis of the Taylor dataset (P<0.001;
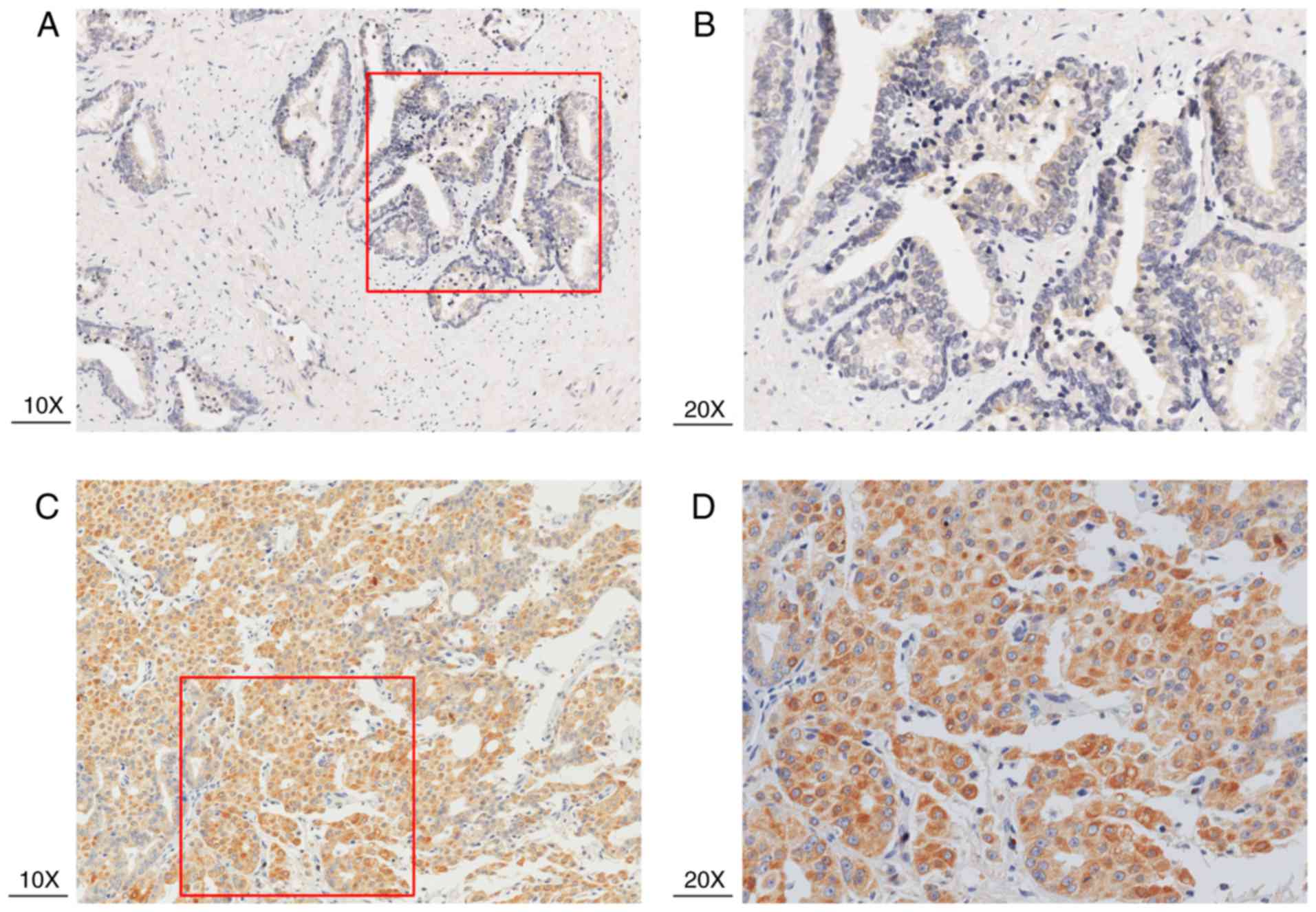
Table II). As shown in Fig. 4A-D, immunohistochemical staining of
HJURP protein revealed that it was predominantly expressed in the
cytoplasm. Notably, weak or negative staining was found in the
benign prostate tissues (Fig. 4A and
B).
 | Table II.Association of Holliday junction
recognition protein mRNA expression with clinicopathological
characteristics in the Taylor dataset. |
Table II.
Association of Holliday junction
recognition protein mRNA expression with clinicopathological
characteristics in the Taylor dataset.
|
|
| HJURP |
|---|
|
|
|
|
|---|
| Clinical
features | Cases, n | Mean ± SD | P-value |
|---|
| Tissues |
|
| <0.001 |
|
Benign | 29 | 7.10±0.130 |
|
|
Cancer | 150 | 7.31±0.245 |
|
| Age, years |
|
| 0.805 |
|
<60 | 93 | 7.31±0.261 |
|
|
≥60 | 57 | 7.30±0.219 |
|
| Serum
prostate-specific antigen, ng/ml |
|
| 0.004 |
|
<10 | 105 | 7.25±0.201 |
|
|
≥10 | 42 | 7.40±0.279 |
|
| Gleason score |
|
| 0.005 |
|
<8 | 117 | 7.25±0.184 |
|
| ≥8 | 22 | 7.44±0.288 |
|
| Clinical stage |
|
| 0.731 |
|
<T2a | 80 | 7.29±0.199 |
|
|
≥T2a | 65 | 7.30±0.287 |
|
| Pathological
stage |
|
| 0.007 |
|
T2a-T2c | 86 | 7.24±0.162 |
|
|
T3a-T4 | 55 | 7.35±0.266 |
|
| Metastasis |
|
| <0.001 |
| No | 122 | 7.24±0.165 |
|
|
Yes | 28 | 7.60±0.311 |
|
| Prostate-specific
antigen failure |
|
| <0.001 |
|
Negative | 104 | 7.23±0.168 |
|
|
Positive | 36 | 7.42±0.260 |
|
Association of HJURP mRNA expression
with the clinicopathological characteristics of PCa
The association of HJURP mRNA expression levels with
clinicopathological features was determined (Table II). It was also demonstrated that
PSA levels ≥10, a Gleason score ≥8, pathological stage T3a-T4
(16–18), metastasis and PSA failure, including
biochemical relapse (BCR) of prostate cancer, which is an important
endpoint that can indicate recurrent prostate cancer (19), were associated with higher mRNA
expression levels of HJURP compared with PSA <10 (P=0.004), a
Gleason score <8 (P=0.005), pathological stage T2a-T2c
(P=0.007), no metastasis (P<0.001) and no PSA failure
(P<0.001; Table II).
High HJURP expression is associated
with reduced BCR-free survival time
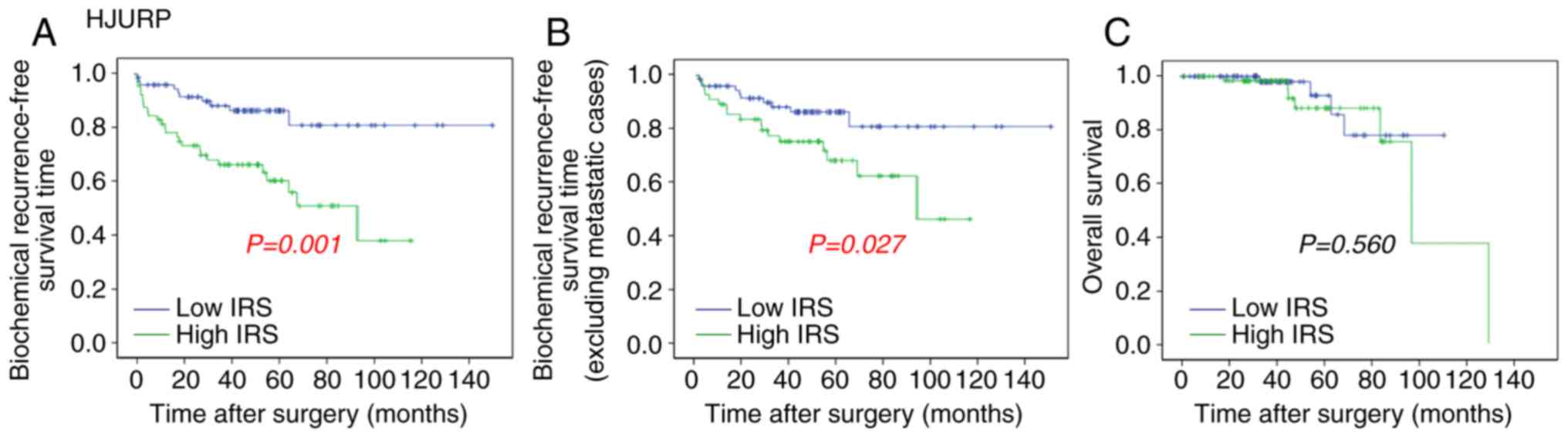
The association of HJURP expression with overall
survival and BCR-free survival of patients with PCa was analyzed by
the Kaplan-Meier method using the Taylor dataset (Fig. 5). The median value (HJURP median
expression =7.22) of HJURP expression level in PCa was used as the
cut-off point to separate the patients into high (n=75) and low
(n=65) HJURP expression level groups. Comparison between PCa
patients with high HJURP expression and low HJURP expression
indicated that there was a significant statistical difference in
the BCR-free survival time between these groups (P=0.001; Fig. 5A). A statistically significant
difference was also observed in the BCR-free survival when patients
with metastasis were excluded (P=0.027; Fig. 5B). However, there was no significant
difference found between HJURP expression and overall survival
(P=0.560; Fig. 5C).
Discussion
Although many patients with PCa achieve long-term
survival after RP, the prognosis of late stage PCa is currently
unsatisfactory. In general, BCR and metastasis lead to
cancer-associated mortality. Despite close follow-up, the low
sensitivity of tumor indices and biomarkers impedes the evaluation
of the progression of PCa (2,3).
Therefore, there is a need for the identification of novel and
effective prognostic biomarkers to improve the clinical management
of PCa which may help to lower mortality and establish personalized
treatment for patients with PCa (3).
Accumulating evidence shows that HJURP may be an
oncogene that has a crucial role in PCa (10–12). To
the best of our knowledge, the current study is the first to report
the role of HJURP expression in PCa. The findings demonstrated that
HJURP was upregulated in PCa tissues compared with benign prostate
tissues, which was in accordance with the Taylor dataset.
Upregulation of HJURP has also been identified in other types of
cancer, including brain, breast, ovarian and lung cancer,
suggesting that HJURP may be involved in tumor progression
(10–13). According to the analysis of the
Taylor dataset in the present study, upregulation of HJURP was
associated with aggressive tumor progression in patients with PCa.
In addition, Kaplan-Meier results indicated that high HJURP mRNA
expression was associated with shorter BCR-free survival time, and
hence, HJURP expression may be useful to determine BCR-free
survival in patients with PCa. It has been previously reported that
upregulation of HJURP mRNA is associated with the progesterone and
estrogen negative status, which has relatively poor prognosis and
aggressive behavior in breast cancer (11). Similar results have also been
identified in lung cancer and glioma reports (12,13).
Studies suggest that members of the CENP family can
become functionally disordered during cell division giving rise to
chromosome instability, segregation defects and cancer development
(20–22). It has previously been reported that
abnormal expression of CENP can lead to cancer progression and
prognosis (20–22). The CENP-A chaperone, HJURP plays a
significant role in localizing CENP-A on the centromere
facilitating accurate chromosome segregation (9). Previous studies have demonstrated that
knockdown or inhibition of HJURP in cancer cells strongly affects
CENP-A deposition, centrosomes and chromosome stability and
contributes to cell cycle arrest and cell death (11–13).
These findings suggest that HJURP may serve a key role in cell
proliferation, and the abnormal HJURP expression may have an
influence on chromosome stability and cell oncogenesis.
During cell division, DNA damage or double-strand
breaks (DSB) can initiate the DNA damage response (DDR). Notably,
the DDR causes cell cycle arrest or induces cell death, which is
beneficial for DNA repair and for preventing genomic instability
(23,24). Recent studies have revealed cancer
cells might have some mechanisms to resist the DDR process
protecting cancer cells from chromosomal instability and
contributing to tumorigenesis (25,26).
Many DSB repair proteins play a role in inhibiting the DDR in
cancer cells and are significantly associated with poor prognosis
(27). During the DSB process, HJURP
is upregulated after DNA damage induction, interacts with proteins
mutS homolog 5 and/or NBS1, and mediates DNA repair via homologous
recombination (13). Therefore,
HJURP may play a critical role in DNA repair mechanisms and
abnormal HJURP expression may suppress the DDR process, which
maintains chromosome stability in cancer cells (13). Thus, the strict regulation of HJURP
during the cell cycle is a key factor associated with genomic
stability.
Further research into this subject is required,
including western blot analysis to confirm the findings that HJURP
expression is increased in PCa tissues. Cell line experiments to
investigate proliferation, migration, invasion and clonal formation
are required to unravel the biological mechanism of HJURP in
patients with PCa. Future work will further investigate the
interaction between HJURP expression and PCa development.
To conclude, the results of the present study
indicate that HJURP was upregulated in PCa tissues and may play a
crucial role in the prognosis of PCa. High expression of HJURP was
also associated, with poor clinicopathological features of PCa, and
may predict BCR-free survival time in patients. Although the
results presented in this study require further validation and the
biological mechanism of HJURP in PCa needs elucidation, the present
study extends our knowledge about the prognostic value of HJURP in
PCa.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81571427
and 81600620), the Science and Technology Project of Guangdong
Province (grant no. 201607010398 and 2016A020215018), the Science
and Technology Project of Huadu District (grant no. HD15CXY005) and
Projects of Guangdong Key Laboratory of Clinical Molecular Medicine
and Diagnostics.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Author's contributions
WZ, ZH, YW and YL participated in study design,
coordination, analysis and interpretation of data, obtained funding
and supervised the study. YC, YL and BW performed the majority of
the experiments and statistical analysis and drafted the
manuscript. JY, DY, JL and SZ performed certain experiments and
sample collection. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Research Ethics
Committee of Guangzhou First People's Hospital (Guangzhou, China).
All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Artibani W, Porcaro AB, De Marco V,
Cerruto MA and Siracusano S: Management of biochemical recurrence
after primary curative treatment for prostate cancer: A review.
Urol Int. 100:251–262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spahn M, Boxler S, Joniau S, Moschini M,
Tombal B and Karnes RJ: What is the need for prostatic biomarkers
in prostate cancer management? Curr Urol Rep. 16:702015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gaither TL, Merrett SL, Pun MJ and Scott
KC: Centromeric barrier disruption leads to mitotic defects in
schizosaccharomyces pombe. G3 (Bethesda). 4:633–642. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barnhart MC, Kuich PH, Stellfox ME, Ward
JA, Bassett EA, Black BE and Foltz DR: HJURP is a CENP-A chromatin
assembly factor sufficient to form a functional de novo
kinetochore. J Cell Biol. 194:229–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mishra PK, Au WC, Choy JS, Kuich PH, Baker
RE, Foltz DR and Basrai MA: Misregulation of Scm3p/HJURP causes
chromosome instability in saccharomyces cerevisiae and human cells.
PLoS Genet. 7:e10023032011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang W, Mao JH, Zhu W, Jain AK, Liu K,
Brown JB and Karpen GH: Centromere and kinetochore gene
misexpression predicts cancer patient survival and response to
radiotherapy and chemotherapy. Nat Commun. 7:126192016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Verdaasdonk JS and Bloom K: Centromeres:
Unique chromatin structures that drive chromosome segregation. Nat
Rev Mol Cell Biol. 12:320–332. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Foltz DR, Jansen LE, Bailey AO, Yates JR
III, Bassett EA, Wood S, Black BE and Cleveland DW:
Centromere-specific assembly of CENP-a nucleosomes is mediated by
HJURP. Cell. 137:472–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Valente V, Serafim RB, de Oliveira LC,
Adorni FS, Torrieri R, Tirapelli DP, Espreafico EM, Oba-Shinjo SM,
Marie SK, Paçó-Larson ML and Carlotti CG Jr: Modulation of HJURP
(Holliday Junction-Recognizing Protein) levels is correlated with
glioblastoma cells survival. PLoS One. 8:e622002013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu Z, Huang G, Sadanandam A, Gu S, Lenburg
ME, Pai M, Bayani N, Blakely EA, Gray JW and Mao JH: The expression
level of HJURP has an independent prognostic impact and predicts
the sensitivity to radiotherapy in breast cancer. Breast Cancer
Res. 12:R182010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rouam S, Moreau T and Broët P: Identifying
common prognostic factors in genomic cancer studies: A novel index
for censored outcomes. BMC Bioinformatics. 11:1502010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kato T, Sato N, Hayama S, Yamabuki T, Ito
T, Miyamoto M, Kondo S, Nakamura Y and Daigo Y: Activation of
holliday junction recognizing protein involved in the chromosomal
stability and immortality of cancer cells. Cancer Res.
67:8544–8553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taylor BS, Schultz N, Hieronymus H,
Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva
B, et al: Integrative genomic profiling of human prostate cancer.
Cancer Cell. 18:11–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brimo F, Montironi R, Egevad L,
Erbersdobler A, Lin DW, Nelson JB, Rubin MA, van der Kwast T, Amin
M and Epstein JI: Contemporary grading for prostate cancer:
Implications for patient care. Eur Urol. 63:892–901. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kweldam CF, van Leenders GJ and van der
Kwast T: Grading of prostate cancer: A work in progress.
Histopathology. 74:146–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soares R and Eden CG: Surgical treatment
of high-risk prostate cancer. Minerva Urol Nefrol. 67:33–46.
2015.PubMed/NCBI
|
|
19
|
Giacalone NJ, Wu J, Chen MH, Renshaw A,
Loffredo M, Kantoff PW and D'Amico AV: Prostate-specific antigen
failure and risk of death within comorbidity subgroups among men
with unfavorable-risk prostate cancer treated in a randomized
trial. J Clin Oncol. 34:3781–3786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao WT, Feng Y, Li ML, Liu GL, Li MZ,
Zeng MS and Song LB: Overexpression of centromere protein H is
significantly associated with breast cancer progression and overall
patient survival. Chin J Cancer. 30:627–637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McGovern SL, Qi Y, Pusztai L, Symmans WF
and Buchholz TA: Centromere protein-A, an essential centromere
protein, is a prognostic marker for relapse in estrogen
receptor-positive breast cancer. Breast Cancer Res. 14:R722012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dai Y, Liu L, Zeng T, Zhu YH, Li J, Chen
L, Li Y, Yuan YF, Ma S and Guan XY: Characterization of the
oncogenic function of centromere protein F in hepatocellular
carcinoma. Biochem Biophys Res Commun. 436:711–718. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Georgoulis A, Vorgias CE, Chrousos GP and
Rogakou EP: Genome instability and γH2AX. Int J Mol Sci.
18:E19792017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagaria P, Robert C and Rassool FV: DNA
double-strand break response in stem cells: Mechanisms to maintain
genomic integrity. Biochim Biophys Acta. 1830:2345–2353. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Majidinia M and Yousefi B: DNA damage
response regulation by microRNAs as a therapeutic target in cancer.
DNA Repair (Amst). 47:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Raschellà G, Melino G and Malewicz M: New
factors in mammalian DNA repair-the chromatin connection. Oncogene.
36:4673–4681. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhen Y, Xiao R, Chen X, Yuan C, Sun Y and
Li J: A non-synonymous polymorphism is associated with progression
from chronic hepatitis B virus infection to hepatocellular
carcinoma in a Chinese population. Onco Targets Ther. 11:563–569.
2018. View Article : Google Scholar : PubMed/NCBI
|