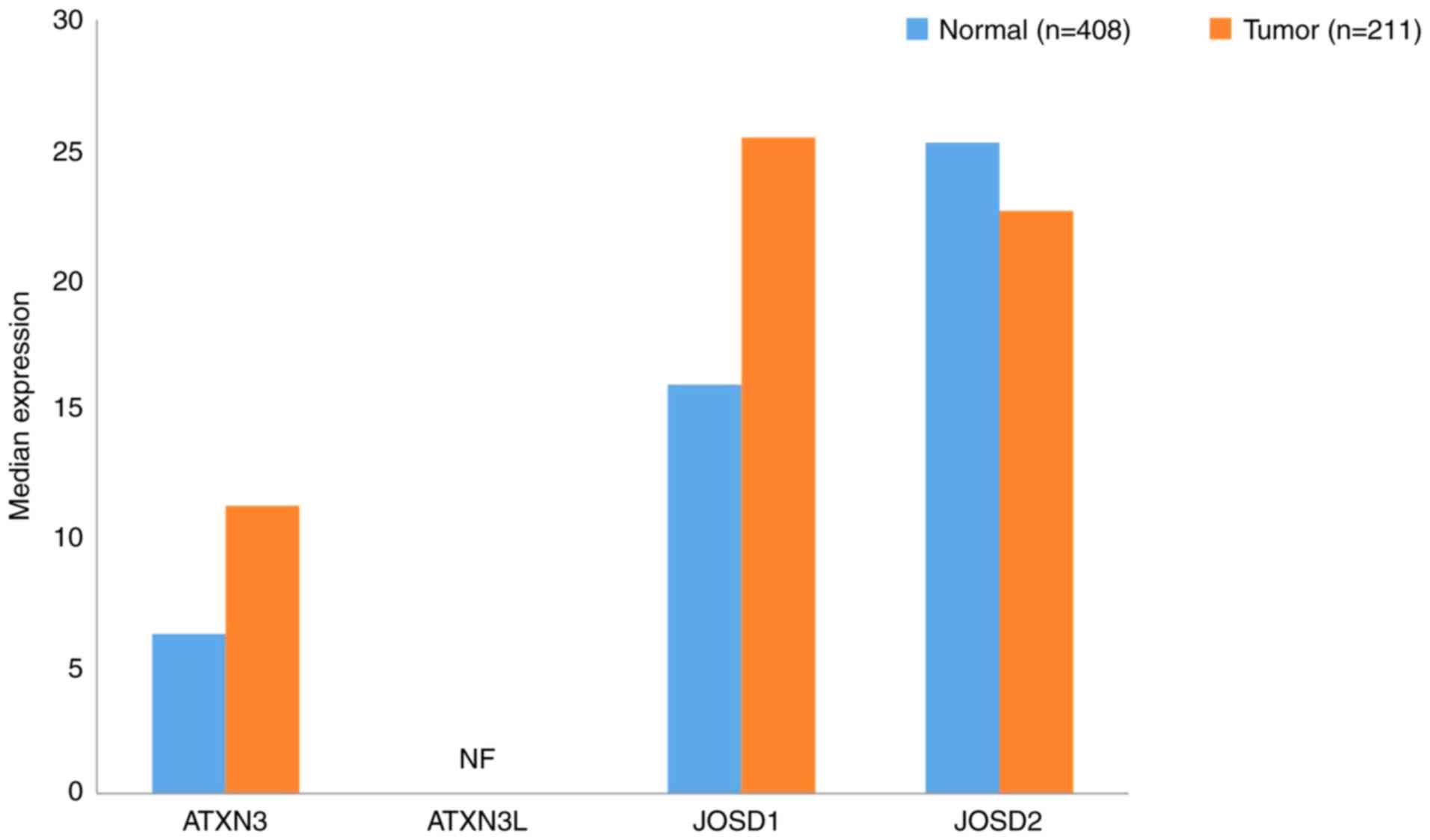
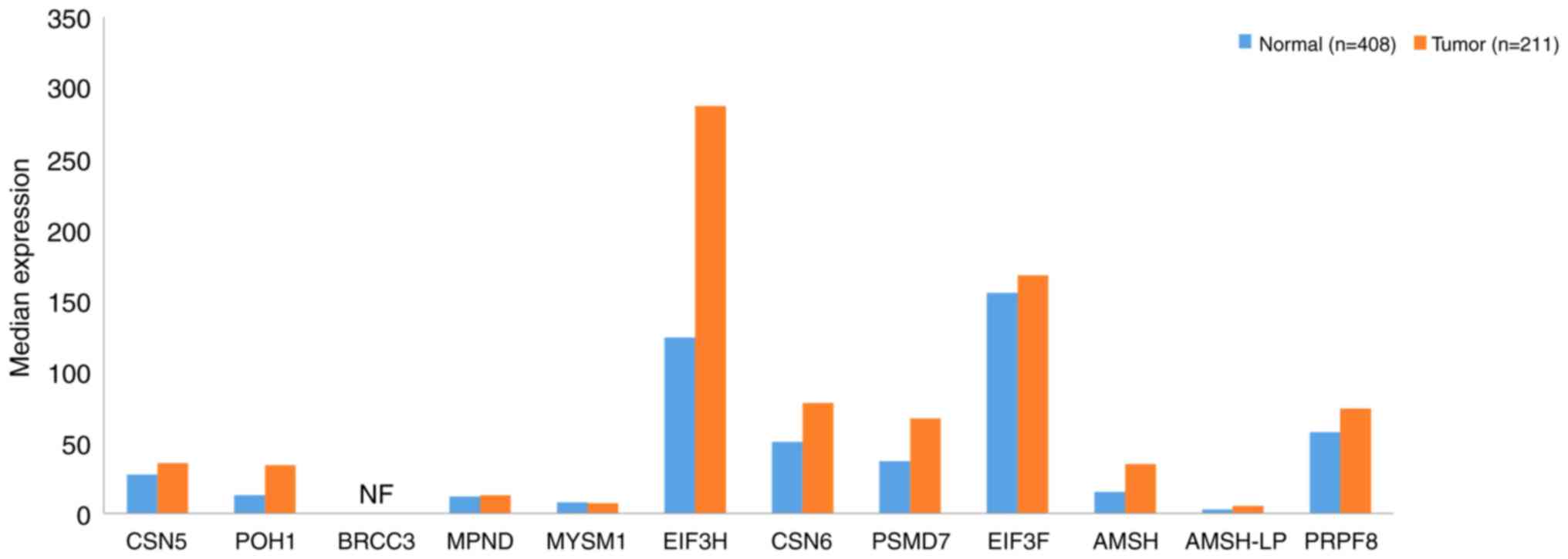
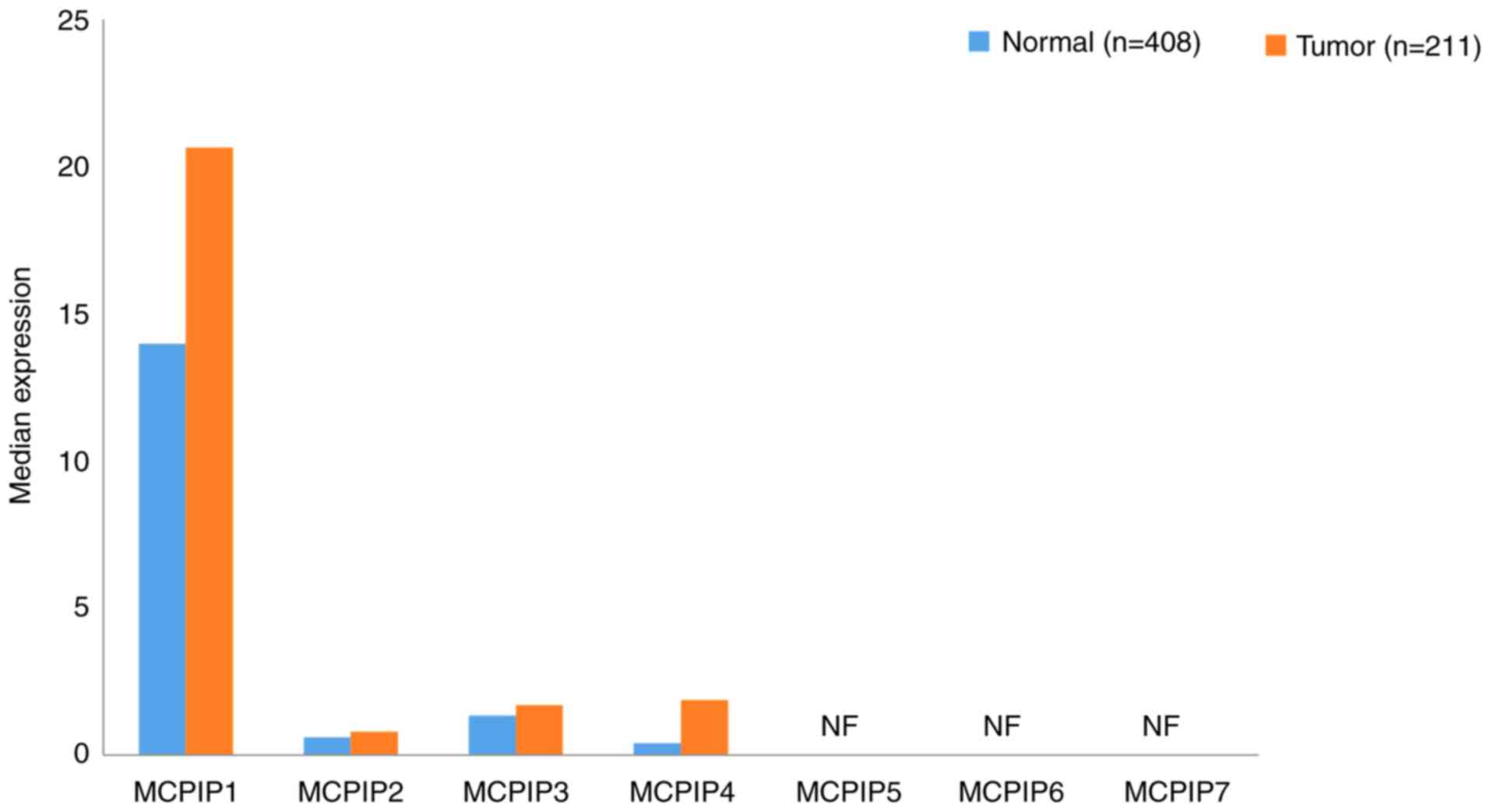
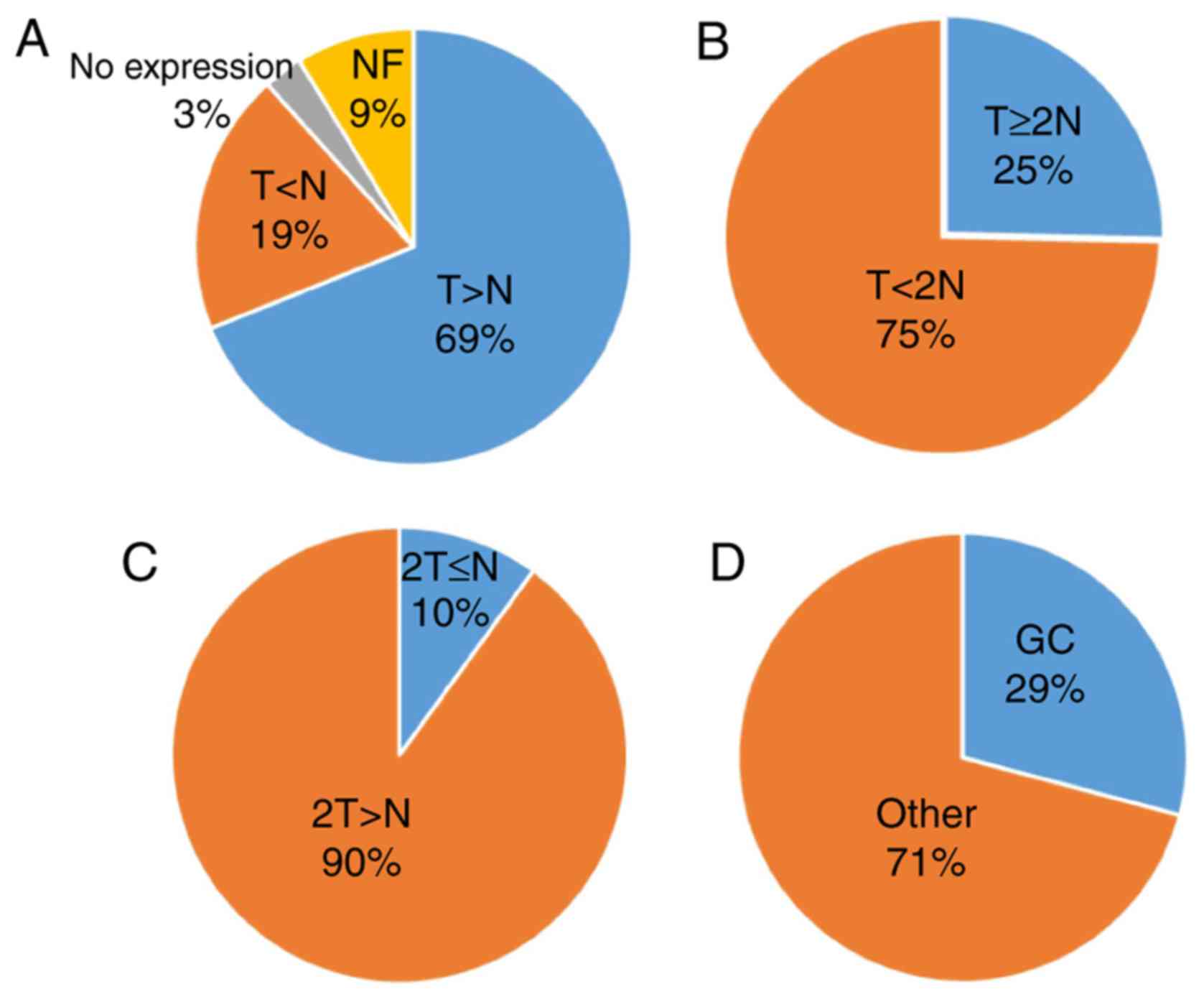
|
1
|
Pidsley R, Lawrence MG, Zotenko E,
Niranjan B, Statham A, Song J, Chabanon RM, Qu W, Wang H, Richards
M, et al: Enduring epigenetic landmarks define the cancer
microenvironment. Genome Res. 28:625–638. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zentner GE and Henikoff S: High-resolution
digital profiling of the epigenome. Nat Rev Genet. 15:814–827.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Onder O, Sidoli S, Carroll M and Garcia
BA: Progress in epigenetic histone modification analysis by mass
spectrometry for clinical investigations. Expert Rev Proteomics.
12:499–517. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Swatek KN and Komander D: Ubiquitin
modifications. Cell Res. 26:399–422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rogov V, Dotsch V, Johansen T and Kirkin
V: Interactions between autophagy receptors and ubiquitin-like
proteins form the molecular basis for selective autophagy. Mol
Cell. 53:167–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hershko A: Ubiquitin: Roles in protein
modification and breakdown. Cell. 34:11–12. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pickart CM: Mechanisms underlying
ubiquitination. Annu Rev Biochem. 70:503–533. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hershko A and Ciechanover A: The ubiquitin
system. Annu Rev Biochem. 67:425–479. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Finley D, Ciechanover A and Varshavsky A:
Ubiquitin as a central cellular regulator. Cell. 116 (Suppl
2):S29–S32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou MJ, Chen FZ and Chen HC:
Ubiquitination involved enzymes and cancer. Med Oncol. 31:932014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johnston SC, Riddle SM, Cohen RE and Hill
CP: Structural basis for the specificity of ubiquitin C-terminal
hydrolases. EMBO J. 18:3877–3887. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fang Y and Shen X: Ubiquitin
carboxyl-terminal hydrolases: Involvement in cancer progression and
clinical implications. Cancer Metastasis Rev. 36:669–682. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McDonough M, Sangan P and Gonda DK:
Characterization of novel yeast RAD6 (UBC2) ubiquitin-conjugating
enzyme mutants constructed by charge-to-alanine scanning
mutagenesis. J Bacteriol. 177:580–585. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu JC, Dawson VL and Dawson TM: Usp16: Key
controller of stem cells in Down syndrome. EMBO J. 32:2788–2789.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Avanzato D, Pupo E, Ducano N, Isella C,
Bertalot G, Luise C, Pece S, Bruna A, Rueda OM, Caldas C, et al:
High USP6NL levels in breast cancer sustain chronic AKT
phosphorylation and GLUT1 stability fueling aerobic glycolysis.
Cancer Res. 78:3432–3444. 2018.PubMed/NCBI
|
|
16
|
Weber A, Elliott PR, Pinto-Fernandez A,
Bonham S, Kessler BM, Komander D, El Oualid F and Krappmann D: A
linear diubiquitin-based probe for efficient and selective
detection of the deubiquitinating enzyme OTULIN. Cell Chem Biol.
24:1299–1313.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taneera J, Fadista J, Ahlqvist E, Atac D,
Ottosson-Laakso E, Wollheim CB and Groop L: Identification of novel
genes for glucose metabolism based upon expression pattern in human
islets and effect on insulin secretion and glycemia. Hum Mol Genet.
24:1945–1955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coombs N, Sompallae R, Olbermann P,
Gastaldello S, Goppel D, Masucci MG and Josenhans C: Helicobacter
pylori affects the cellular deubiquitinase USP7 and
ubiquitin-regulated components TRAF6 and the tumour suppressor p53.
Int J Med Microbiol. 301:213–224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saldana M, VanderVorst K, Berg AL, Lee H
and Carraway KL: Otubain 1: A non-canonical deubiquitinase with an
emerging role in cancer. Endocr Relat Cancer. 26:R1–R14. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rahman R, Asombang AW and Ibdah JA:
Characteristics of gastric cancer in Asia. World J Gastroenterol.
20:4483–5890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Todi SV and Paulson HL: Balancing act:
Deubiquitinating enzymes in the nervous system. Trends Neurosci.
34:370–382. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fang Y, Fu D and Shen XZ: The potential
role of ubiquitin c-terminal hydrolases in oncogenesis. Biochim
Biophys Acta. 1806:1–6. 2010.PubMed/NCBI
|
|
25
|
Kim HJ, Kim YM, Lim S, Nam YK, Jeong J,
Kim HJ and Lee KJ: Ubiquitin C-terminal hydrolase-L1 is a key
regulator of tumor cell invasion and metastasis. Oncogene.
28:117–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dang LC, Melandri FD and Stein RL: Kinetic
and mechanistic studies on the hydrolysis of ubiquitin C-terminal
7-amido-4-methylcoumarin by deubiquitinating enzymes. Biochemistry.
37:1868–1879. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Case A and Stein RL: Mechanistic studies
of ubiquitin C-terminal hydrolase L1. Biochemistry. 45:2443–2452.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arpalahti L, Laitinen A, Hagström J,
Mustonen H, Kokkola A, Böckelman C, Haglund C and Holmberg CI:
Positive cytoplasmic UCHL5 tumor expression in gastric cancer is
linked to improved prognosis. PLoS One. 13:e01931252018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gu YY, Yang M, Zhao M, Luo Q, Yang L, Peng
H, Wang J, Huang SK, Zheng ZX, Yuan XH, et al: The de-ubiquitinase
UCHL1 promotes gastric cancer metastasis via the Akt and Erk1/2
pathways. Tumour Biol. 36:8379–8387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan S, He F, Luo R, Wu H, Huang M, Huang
C, Li Y and Zhou Z: Decreased expression of BRCA1-associated
protein 1 predicts unfavorable survival in gastric adenocarcinoma.
Tumour Biol. 37:6125–6133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nijman SM, Luna-Vargas MP, Velds A,
Brummelkamp TR, Dirac AM, Sixma TK and Bernards R: A genomic and
functional inventory of deubiquitinating enzymes. Cell.
123:773–786. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Das DS, Das A, Ray A, Song Y, Samur MK,
Munshi NC, Chauhan D and Anderson KC: Blockade of deubiquitylating
enzyme USP1 inhibits DNA repair and triggers apoptosis in multiple
myeloma cells. Clin Cancer Res. 23:4280–4289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kedersha N, Panas MD, Achorn CA, Lyons S,
Tisdale S, Hickman T, Thomas M, Lieberman J, McInerney GM, Ivanov P
and Anderson P: G3BP-Caprin1-USP10 complexes mediate stress granule
condensation and associate with 40S subunits. J Cell Biol.
212:845–860. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kapadia B, Nanaji NM, Bhalla K, Bhandary
B, Lapidus R, Beheshti A, Evens AM and Gartenhaus RB: Fatty Acid
Synthase induced S6Kinase facilitates USP11-eIF4B complex formation
for sustained oncogenic translation in DLBCL. Nat Commun.
9:8292018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aron R, Pellegrini P, Green EW, Maddison
DC, Opoku-Nsiah K, Wong JS, Daub AC, Giorgini F and Finkbeiner S:
Publisher correction: Deubiquitinase Usp12 functions
noncatalytically to induce autophagy and confer neuroprotection in
models of Huntington's disease. Nat Commun. 9:43332018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang S, Zhang M, Jing Y, Yin X, Ma P,
Zhang Z, Wang X, Di W and Zhuang G: Deubiquitinase USP13 dictates
MCL1 stability and sensitivity to BH3 mimetic inhibitors. Nat
Commun. 9:2152018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee BH, Lee MJ, Park S, Oh DC, Elsasser S,
Chen PC, Gartner C, Dimova N, Hanna J, Gygi SP, et al: Enhancement
of proteasome activity by a small-molecule inhibitor of USP14.
Nature. 467:179–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eichhorn PJ, Rodon L, Gonzalez-Junca A,
Dirac A, Gili M, Martinez-Saez E, Aura C, Barba I, Peg V, Prat A,
et al: USP15 stabilizes TGF-β receptor I and promotes oncogenesis
through the activation of TGF-β signaling in glioblastoma. Nat Med.
18:429–435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Adorno M, Sikandar S, Mitra SS, Kuo A,
Nicolis Di Robilant B, Haro-Acosta V, Ouadah Y, Quarta M, Rodriguez
J, Qian D, et al: Usp16 contributes to somatic stem-cell defects in
Down's syndrome. Nature. 501:380–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shaw JA, Page K, Blighe K, Hava N, Guttery
D, Ward B, Brown J, Ruangpratheep C, Stebbing J, Payne R, et al:
Genomic analysis of circulating cell-free DNA infers breast cancer
dormancy. Genome Res. 22:220–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Malakhov MP, Malakhova OA, Kim KI, Ritchie
KJ and Zhang DE: UBP43 (USP18) specifically removes ISG15 from
conjugated proteins. J Biol Chem. 277:9976–9981. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Combaret L, Adegoke OA, Bedard N, Baracos
V, Attaix D and Wing SS: USP19 is a ubiquitin-specific protease
regulated in rat skeletal muscle during catabolic states. Am J
Physiol Endocrinol Metab. 288:E693–E700. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xie L, Wei J, Qian X, Chen G, Yu L, Ding Y
and Liu B: CXCR4, a potential predictive marker for docetaxel
sensitivity in gastric cancer. Anticancer Res. 30:2209–2216.
2010.PubMed/NCBI
|
|
44
|
Fu Y, Ma G, Liu G, Li B, Li H, Hao X and
Liu L: USP14 as a novel prognostic marker promotes cisplatin
resistance via Akt/ERK signaling pathways in gastric cancer. Cancer
Med. 7:5577–5588. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zeng Z, Wu HX, Zhan N, Huang YB, Wang ZS,
Yang GF, Wang P and Fu GH: Prognostic significance of USP10 as a
tumor-associated marker in gastric carcinoma. Tumour Biol.
35:3845–3853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu Y, Zhang Y, Sui Z, Zhang Y, Liu M and
Tang H: USP14 de-ubiquitinates vimentin and miR-320a modulates
USP14 and vimentin to contribute to malignancy in gastric cancer
cells. Oncotarget. 8:48725–48736. 2017.PubMed/NCBI
|
|
47
|
Renatus M, Parrado SG, D'Arcy A, Eidhoff
U, Gerhartz B, Hassiepen U, Pierrat B, Riedl R, Vinzenz D,
Worpenberg S and Kroemer M: Structural basis of ubiquitin
recognition by the deubiquitinating protease USP2. Structure.
14:1293–1302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Berthouze M, Venkataramanan V, Li Y and
Shenoy SK: The deubiquitinases USP33 and USP20 coordinate beta2
adrenergic receptor recycling and resensitization. EMBO J.
28:1684–1796. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ye Y, Akutsu M, Reyes-Turcu F, Enchev RI,
Wilkinson KD and Komander D: Polyubiquitin binding and
cross-reactivity in the USP domain deubiquitinase USP21. EMBO Rep.
12:350–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang XY, Varthi M, Sykes SM, Phillips C,
Warzecha C, Zhu W, Wyce A, Thorne AW, Berger SL and McMahon SB: The
putative cancer stem cell marker USP22 is a subunit of the human
SAGA complex required for activated transcription and cell-cycle
progression. Mol Cell. 29:102–111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang L, Lubin A, Chen H, Sun Z and Gong
F: The deubiquitinating protein USP24 interacts with DDB2 and
regulates DDB2 stability. Cell Cycle. 11:4378–4384. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Stouffs K, Lissens W, Tournaye H, Van
Steirteghem A and Liebaers I: Possible role of USP26 in patients
with severely impaired spermatogenesis. Eur J Hum Genet.
13:336–340. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Weber A, Heinlein M, Dengjel J, Alber C,
Singh PK and Häcker G: The deubiquitinase Usp27× stabilizes the
BH3-only protein Bim and enhances apoptosis. EMBO Rep. 17:724–738.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Popov N, Wanzel M, Madiredjo M, Zhang D,
Beijersbergen R, Bernards R, Moll R, Elledge SJ and Eilers M: The
ubiquitin-specific protease USP28 is required for MYC stability.
Nat Cell Biol. 9:765–774. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu J, Chung HJ, Vogt M, Jin Y, Malide D,
He L, Dundr M and Levens D: JTV1 co-activates FBP to induce USP29
transcription and stabilize p53 in response to oxidative stress.
EMBO J. 30:846–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhao LJ, Zhang T, Feng XJ, Chang J, Suo
FZ, Ma JL, Liu YJ, Liu Y, Zheng YC and Liu HM: USP28 contributes to
the proliferation and metastasis of gastric cancer. J Cell Biochem.
Nov 28–2018.(Epub ahead of print). doi: 10.1002/jcb.28040.
|
|
57
|
Wang C, Yang C, Ji J, Jiang J, Shi M, Cai
Q, Yu Y, Zhu Z and Zhang J: Deubiquitinating enzyme USP20 is a
positive regulator of Claspin and suppresses the malignant
characteristics of gastric cancer cells. Int J Oncol. Mar
8–2017.(Epub ahead of print). doi: 10.3892/ijo.2017.3904.
|
|
58
|
Ma Y, Fu HL, Wang Z, Huang H, Ni J, Song
J, Xia Y, Jin WL and Cui DX: USP22 maintains gastric cancer stem
cell stemness and promotes gastric cancer progression by
stabilizing BMI1 protein. Oncotarget. 8:33329–33342.
2017.PubMed/NCBI
|
|
59
|
He Y, Jin YJ, Zhang YH, Meng HX, Zhao BS,
Jiang Y, Zhu JW, Liang GY, Kong D and Jin XM: Ubiquitin-specific
peptidase 22 overexpression may promote cancer progression and poor
prognosis in human gastric carcinoma. Transl Res. 16:407–416. 2015.
View Article : Google Scholar
|
|
60
|
Yang DD, Cui BB, Sun LY, Zheng HQ, Huang
Q, Tong JX and Zhang QF: The co-expression of USP22 and BMI-1 may
promote cancer progression and predict therapy failure in gastric
carcinoma. Cell Biochem Biophys. 61:703–710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nicassio F, Corrado N, Vissers JH, Areces
LB, Bergink S, Marteijn JA, Geverts B, Houtsmuller AB, Vermeulen W,
Di Fiore PP and Citterio E: Human USP3 is a chromatin modifier
required for S phase progression and genome stability. Curr Biol.
17:1972–1977. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bingol B, Tea JS, Phu L, Reichelt M,
Bakalarski CE, Song Q, Foreman O, Kirkpatrick DS and Sheng M: The
mitochondrial deubiquitinase USP30 opposes parkin-mediated
mitophagy. Nature. 510:370–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tzimas C, Michailidou G, Arsenakis M,
Kieff E, Mosialos G and Hatzivassiliou EG: Human ubiquitin specific
protease 31 is a deubiquitinating enzyme implicated in activation
of nuclear factor-kappaB. Cell Signal. 18:83–92. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Akhavantabasi S, Akman HB, Sapmaz A,
Keller J, Petty EM and Erson AE: USP32 is an active, membrane-bound
ubiquitin protease overexpressed in breast cancers. Mamm Genome.
21:388–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sy SM, Jiang J, O WS, Deng Y and Huen MS:
The ubiquitin specific protease USP34 promotes ubiquitin signaling
at DNA double-strand breaks. Nucleic Acids Res. 41:8572–8580. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang Y, Serricchio M, Jauregui M, Shanbhag
R, Stoltz T, Di Paolo CT, Kim PK and McQuibban GA: Deubiquitinating
enzymes regulate PARK2-mediated mitophagy. Autophagy. 11:595–606.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Endo A, Matsumoto M, Inada T, Yamamoto A,
Nakayama KI, Kitamura N and Komada M: Nucleolar structure and
function are regulated by the deubiquitylating enzyme USP36. J Cell
Sci. 122:678–686. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Huang X, Summers MK, Pham V, Lill JR, Liu
J, Lee G, Kirkpatrick DS, Jackson PK, Fang G and Dixit VM:
Deubiquitinase USP37 is activated by CDK2 to antagonize APC(CDH1)
and promote S phase entry. Mol Cell. 42:511–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lin M, Zhao Z, Yang Z, Meng Q, Tan P, Xie
W, Qin Y, Wang RF and Cui J: USP38 Inhibits type I interferon
signaling by editing TBK1 Ubiquitination through NLRP4 Signalosome.
Mol Cell. 64:267–281. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
van Leuken RJ, Luna-Vargas MP, Sixma TK,
Wolthuis RM and Medema RH: Usp39 is essential for mitotic spindle
checkpoint integrity and controls mRNA-levels of aurora B. Cell
Cycle. 7:2710–2719. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Fang CL, Lin CC, Chen HK, Hseu YC, Hung
ST, Sun DP, Uen YH and Lin KY: Ubiquitin-specific protease 3
overexpression promotes gastric carcinogenesis and is predictive of
poor patient prognosis. Cancer Sci. 109:3438–3449. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chen Y, Pang X, Ji L, Sun Y and Ji Y:
Reduced expression of deubiquitinase USP33 is associated with tumor
progression and poor prognosis of gastric adenocarcinoma. Med Sci
Monit. 24:3496–505. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang X, Yu Q, Huang L and Yu P:
Lentivirus-mediated inhibition of USP39 suppresses the growth of
gastric cancer cells via PARP activation. Mol Med Rep. 14:301–306.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Dong X, Su H, Jiang F, Li H, Shi G and Fan
L: miR-133a, directly targeted USP39, suppresses cell proliferation
and predicts prognosis of gastric cancer. Oncol Lett. 15:8311–3818.
2018.PubMed/NCBI
|
|
75
|
Zhang L, Zhou F, Drabsch Y, Gao R,
Snaar-Jagalska BE, Mickanin C, Huang H, Sheppard KA, Porter JA, Lu
CX and ten Dijke P: USP4 is regulated by AKT phosphorylation and
directly deubiquitylates TGF-β type I receptor. Nat Cell Biol.
14:717–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li Y, Schrodi S, Rowland C, Tacey K,
Catanese J and Grupe A: Genetic evidence for ubiquitin-specific
proteases USP24 and USP40 as candidate genes for late-onset
Parkinson disease. Hum Mutat. 27:1017–1023. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Pinilla-Vera M, Xiong Z, Zhao Y, Zhao J,
Donahoe MP, Barge S, Horne WT, Kolls JK, McVerry BJ, Birukova A, et
al: Full spectrum of LPS activation in alveolar macrophages of
healthy volunteers by whole transcriptomic profiling. PLoS One.
11:e01593292016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hock AK, Vigneron AM, Carter S, Ludwig RL
and Vousden KH: Regulation of p53 stability and function by the
deubiquitinating enzyme USP42. EMBO J. 30:4921–4930. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
He L, Liu X, Yang J, Li W, Liu S, Liu X,
Yang Z, Ren J, Wang Y, Shan L, et al: Imbalance of the reciprocally
inhibitory loop between the ubiquitin-specific protease USP43 and
EGFR/PI3K/AKT drives breast carcinogenesis. Cell Res. 28:934–951.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Borrero J, Jimenez JJ, Gutiez L, Herranz
C, Cintas LM and Hernandez PE: Use of the usp45 lactococcal
secretion signal sequence to drive the secretion and functional
expression of enterococcal bacteriocins in Lactococcus lactis. Appl
Microbiol Biotechnol. 89:131–143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Schweitzer K and Naumann M: CSN-associated
USP48 confers stability to nuclear NF-kappaB/RelA by trimming
K48-linked Ub-chains. Biochim Biophys Acta. 1853:453–469. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Weinstock J, Wu J, Cao P, Kingsbury WD,
McDermott JL, Kodrasov MP, McKelvey DM, Suresh Kumar KG, Goldenberg
SJ, Mattern MR and Nicholson B: Selective dual inhibitors of the
cancer-related deubiquitylating proteases USP7 and USP47. ACS Med
Chem Lett. 3:789–792. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhang B, Yin Y, Hu Y, Zhang J, Bian Z,
Song M, Hua D and Huang Z: MicroRNA-204-5p inhibits gastric cancer
cell proliferation by downregulating USP47 and RAB22A. Med Oncol.
32:3312015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Naghavi L, Schwalbe M, Ghanem A and
Naumann M: Deubiquitinylase USP47 promotes RelA phosphorylation and
survival in gastric cancer cells. Biomedicines. 6(pii): E622018.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hou K, Zhu Z, Wang Y, Zhang C, Yu S, Zhu Q
and Yan B: Overexpression and biological function of
ubiquitin-specific protease 42 in gastric cancer. PLoS One.
11:e01529972016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Nishimura S, Oki E, Ando K, Iimori M,
Nakaji Y, Nakashima Y, Saeki H, Oda Y and Maehara Y: High
ubiquitin-specific protease 44 expression induces DNA aneuploidy
and provides independent prognostic information in gastric cancer.
Cancer Med. 6:1453–1464. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Dayal S, Sparks A, Jacob J, Allende-Vega
N, Lane DP and Saville MK: Suppression of the deubiquitinating
enzyme USP5 causes the accumulation of unanchored polyubiquitin and
the activation of p53. J Biol Chem. 284:5030–5041. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Aressy B, Jullien D, Cazales M, Marcellin
M, Bugler B, Burlet-Schiltz O and Ducommun B: A screen for
deubiquitinating enzymes involved in the G2/M checkpoint
identifies USP50 as a regulator of HSP90-dependent Wee1 stability.
Cell Cycle. 9:3815–3822. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang Z, Zhang H, Liu J, Cheruiyot A, Lee
JH, Ordog T, Lou Z, You Z and Zhang Z: USP51 deubiquitylates
H2AK13,15ub and regulates DNA damage response. Genes Dev.
30:946–959. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yang S, Liu L, Cao C, Song N, Wang Y, Ma
S, Zhang Q, Yu N, Ding X, Yang F, et al: USP52 acts as a
deubiquitinase and promotes histone chaperone ASF1A stabilization.
Nat Commun. 9:12852018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kazmierczak M, Harris SL, Kazmierczak P,
Shah P, Starovoytov V, Ohlemiller KK and Schwander M: Progressive
hearing loss in mice carrying a mutation in Usp53. J Neurosci.
35:15582–15598. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Fraile JM, Campos-Iglesias D, Rodriguez F,
Espanol Y and Freije JM: The deubiquitinase USP54 is overexpressed
in colorectal cancer stem cells and promotes intestinal
tumorigenesis. Oncotarget. 7:74427–74434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liu Y, Wang WM, Zou LY, Li L, Feng L, Pan
MZ, Lv MY, Cao Y, Wang H, Kung HF, et al: Ubiquitin specific
peptidase 5 mediates Histidine-rich protein Hpn induced cell
apoptosis in hepatocellular carcinoma through P14-P53 signaling.
Proteomics. 172017.doi: 10.1002/pmic.201600350. PubMed/NCBI
|
|
94
|
Oliveira AM, Perez-Atayde AR, Inwards CY,
Medeiros F, Derr V, Hsi BL, Gebhardt MC, Rosenberg AE and Fletcher
JA: USP6 and CDH11 oncogenes identify the neoplastic cell in
primary aneurysmal bone cysts and are absent in so-called secondary
aneurysmal bone cysts. Am J Pathol. 165:1773–1780. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kang A, Kumar JB, Thomas A and Bourke AG:
A spontaneously resolving breast lesion: Imaging and cytological
findings of nodular fasciitis of the breast with FISH showing USP6
gene rearrangement. BMJ Case Rep. 2015(pii): bcr20152130762015.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Jian F, Cao Y, Bian L and Sun Q: USP8: A
novel therapeutic target for Cushing's disease. Endocrine.
50:292–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Fu X, Xie W, Song X, Wu K, Xiao L, Liu Y
and Zhang L: Aberrant expression of deubiquitylating enzyme USP9X
predicts poor prognosis in gastric cancer. Clin Res Hepatol
Gastroenterol. 41:687–692. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Deng S, Zhou H, Xiong R, Lu Y, Yan D, Xing
T, Dong L, Tang E and Yang H: Over-expression of genes and proteins
of ubiquitin specific peptidases (USPs) and proteasome subunits
(PSs) in breast cancer tissue observed by the methods of RFDD-PCR
and proteomics. Breast Cancer Res Treat. 104:21–30. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Xia JT, Chen LZ, Jian WH, Wang KB, Yang
YZ, He WL, Chen D and Li W: MicroRNA-362 induces cell proliferation
and apoptosis resistance in gastric cancer by activation of NF-B
signaling. J Transl Med. 12:332014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Sun B, Li L, Ma W, Wang S and Huang C:
MiR-130b inhibits proliferation and induces apoptosis of gastric
cancer cells via CYLD. Tumour Biol. 37:7981–9787. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Bermejo JL, Kabisch M, Dunnebier T,
Schnaidt S, Melchior F, Fischer HP, Harth V, Rabstein S, Pesch B,
Brüning T, et al: Exploring the association between genetic
variation in the SUMO isopeptidase gene USPL1 and breast cancer
through integration of data from the population-based GENICA study
and external genetic databases. Int J Cancer. 133:362–372. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Mevissen TE, Hospenthal MK, Geurink PP,
Elliott PR, Akutsu M, Arnaudo N, Ekkebus R, Kulathu Y, Wauer T, El
Oualid F, et al: OTU deubiquitinases reveal mechanisms of linkage
specificity and enable ubiquitin chain restriction analysis. Cell.
154:169–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wiener R, Zhang X, Wang T and Wolberger C:
The mechanism of OTUB1-mediated inhibition of ubiquitination.
Nature. 483:618–622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kato K, Nakajima K, Ui A, Muto-Terao Y,
Ogiwara H and Nakada S: Fine-tuning of DNA damage-dependent
ubiquitination by OTUB2 supports the DNA repair pathway choice. Mol
Cell. 53:617–630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wang YQ, Zhang QY, Weng WW, Wu Y, Yang YS,
Shen C, Chen XC, Wang L, Liu KJ, Xu MD and Sheng WQ: Upregulation
of the Non-coding RNA OTUB1-isoform 2 contributes to gastric cancer
cell proliferation and invasion and predicts poor gastric cancer
prognosis. Int J Biol Sci. 12:545–557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Carneiro AP, Reis CF, Morari EC, Maia YC,
Nascimento R, Bonatto JM, de Souza MA, Goulart LR and Ward LS: A
putative OTU domain-containing protein 1 deubiquitinating enzyme is
differentially expressed in thyroid cancer and identifies
less-aggressive tumours. Br J Cancer. 111:551–558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Flierman D, van der Heden van Noort GJ,
Ekkebus R, Geurink PP, Mevissen TE, Hospenthal MK, Komander D and
Ovaa H: Non-hydrolyzable diubiquitin probes reveal linkage-specific
reactivity of deubiquitylating enzymes mediated by S2 pockets. Cell
Chem Biol. 23:472–482. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Yuan L, Lv Y, Li H, Gao H, Song S, Zhang
Y, Xing G, Kong X, Wang L, Li Y, et al: Deubiquitylase OTUD3
regulates PTEN stability and suppresses tumorigenesis. Nat Cell
Biol. 17:1169–1181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhao Y, Majid MC, Soll JM, Brickner JR,
Dango S and Mosammaparast N: Noncanonical regulation of alkylation
damage resistance by the OTUD4 deubiquitinase. EMBO J.
34:1687–1703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Luo J, Lu Z, Lu X, Chen L, Cao J, Zhang S,
Ling Y and Zhou X: OTUD5 regulates p53 stability by
deubiquitinating p53. PLoS One. 8:e776822013. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kim SY, Kwon SK, Lee SY and Baek KH:
Ubiquitin-specific peptidase 5 and ovarian tumor deubiquitinase 6A
are differentially expressed in p53+/+ and
p53−/− HCT116 cells. Int J Oncol. Mar 5–2018.(Epub ahead
of print). doi: 10.3892/ijo.2018.4302. View Article : Google Scholar
|
|
112
|
Santiago-Sim T, Burrage LC, Ebstein F,
Tokita MJ, Miller M, Bi W, Braxton AA, Rosenfeld JA, Shahrour M,
Lehmann A, et al: Biallelic variants in OTUD6B cause an
intellectual disability syndrome associated with seizures and
dysmorphic features. Am J Hum Genet. 100:676–688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Evans PC, Smith TS, Lai MJ, Williams MG,
Burke DF, Heyninck K, Kreike MM, Beyaert R, Blundell TL and Kilshaw
PJ: A novel type of deubiquitinating enzyme. J Biol Chem.
278:23180–23186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Xu Z, Pei L, Wang L, Zhang F, Hu X and Gui
Y: Snail1-dependent transcriptional repression of Cezanne2 in
hepatocellular carcinoma. Oncogene. 33:2836–2845. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Virdee S, Ye Y, Nguyen DP, Komander D and
Chin JW: Engineered diubiquitin synthesis reveals Lys29-isopeptide
specificity of an OTU deubiquitinase. Nat Chem Biol. 6:750–757.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Guo T, Zhang Y, Qu X, Che X, Li C, Fan Y,
Wan X, Ma R, Hou K, Zhou H, et al: miR-200a enhances TRAIL-induced
apoptosis in gastric cancer cells by targeting A20. Cell Biol Int.
42:506–514. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Lork M, Verhelst K and Beyaert R: CYLD,
A20 and OTULIN deubiquitinases in NF-B signaling and cell death: So
similar, yet so different. Cell Death Differ. 24:1172–1183. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Weng W, Zhang Q, Xu M, Wu Y, Zhang M, Shen
C, Chen X, Wang Y and Sheng W: OTUB1 promotes tumor invasion and
predicts a poor prognosis in gastric adenocarcinoma. Am J Transl
Res. 8:2234–2244. 2016.PubMed/NCBI
|
|
119
|
Wang X, Zhang L, Zhang Y, Zhao P, Qian L,
Yuan Y, Liu J, Cheng Q, Xu W, Zuo Y, et al: JOSD1 negatively
regulates type-I interferon antiviral activity by deubiquitinating
and stabilizing SOCS1. Viral Immunol. 30:342–349. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhang B, Zheng A, Hydbring P, Ambroise G,
Ouchida AT, Goiny M, Vakifahmetoglu-Norberg H and Norberg E: PHGDH
defines a metabolic subtype in lung adenocarcinomas with poor
prognosis. Cell Rep. 19:2289–2303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zhang J, Huang JY, Chen YN, Yuan F, Zhang
H, Yan FH, Wang MJ, Wang G, Su M, Lu G, et al: Whole genome and
transcriptome sequencing of matched primary and peritoneal
metastatic gastric carcinoma. Sci Rep. 5:137502015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Butler LR, Densham RM, Jia J, Garvin AJ,
Stone HR, Shah V, Weekes D, Festy F, Beesley J and Morris JR: The
proteasomal de-ubiquitinating enzyme POH1 promotes the
double-strand DNA break response. EMBO J. 31:3918–3934. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Py BF, Kim MS, Vakifahmetoglu-Norberg H
and Yuan J: Deubiquitination of NLRP3 by BRCC3 critically regulates
inflammasome activity. Mol Cell. 49:331–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Sun H, Guo D, Su Y, Yu D, Wang Q, Wang T,
Zhou Q, Ran X and Zou Z: Hyperplasia of pericytes is one of the
main characteristics of microvascular architecture in malignant
glioma. PLoS One. 9:e1142462014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhou L, Shi L, Guo H and Yao X: MYSM-1
suppresses migration and invasion in renal carcinoma through
inhibiting epithelial-mesenchymal transition. Tumour Biol. Sep
27–2015.(Epub ahead of print).
|
|
126
|
Xiao D, Yang S, Huang L, He H, Pan H and
He J: COP9 signalosome subunit CSN5, but not CSN6, is upregulated
in lung adenocarcinoma and predicts poor prognosis. J Thorac Dis.
10:1596–1606. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Niu Z, Lei R, Shi J, Wang D, Shou W, Wang
Z, Wang Y, Wang Z and Huang W: A polymorphism rs17336700 in the
PSMD7 gene is associated with ankylosing spondylitis in Chinese
subjects. Ann Rheum Dis. 70:706–907. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
McCullough J, Clague MJ and Urbe S: AMSH
is an endosome-associated ubiquitin isopeptidase. J Cell Biol.
166:487–492. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Zhu W, Liu Y and Ling B: Quantum mechanics
and molecular mechanics study of the catalytic mechanism of human
AMSH-LP domain deubiquitinating enzymes. Biochemistry.
54:5225–5234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wickramasinghe VO, Gonzalez-Porta M,
Perera D, Bartolozzi AR, Sibley CR, Hallegger M, Ule J, Marioni JC
and Venkitaraman AR: Regulation of constitutive and alternative
mRNA splicing across the human transcriptome by PRPF8 is determined
by 5′ splice site strength. Genome Biol. 16:2012015. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Sang MM, Du WQ, Zhang RY, Zheng JN and Pei
DS: Suppression of CSN5 promotes the apoptosis of gastric cancer
cells through regulating p53-related apoptotic pathways. Bioorg Med
Chem Lett. 25:2897–2901. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Wang X, Wang H, Zhao S, Sun P, Wen D, Liu
T, Liu H, Yang Z and Ma Z: Eukaryotic translation initiation factor
EIF3H potentiates gastric carcinoma cell proliferation. Tissue
Cell. 53:23–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Cheng Y, Jia C, Li G and Li H: Expression
of eukaryotic initiation factor 3f is associated with prognosis in
gastric carcinomas. Oncol Res Treat. 37:198–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Tahara H, Kay MA, Yasui W and Tahara E:
MicroRNAs in cancer: The 22nd hiroshima cancer Seminar/the 4th
Japanese Association for RNA interference joint international
symposium, 30 August 2012, grand prince hotel Hiroshima. Jpn J Clin
Oncol. 43:579–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Huang S, Liu S, Fu JJ, Tony Wang T, Yao X,
Kumar A, Liu G and Fu M: Monocyte chemotactic protein-induced
protein 1 and 4 form a complex but act independently in regulation
of interleukin-6 mRNA degradation. J Biol Chem. 290:20782–20792.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Roy A and Kolattukudy PE: Monocyte
chemotactic protein-induced protein (MCPIP) promotes inflammatory
angiogenesis via sequential induction of oxidative stress,
endoplasmic reticulum stress and autophagy. Cell Signal.
24:2123–2131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Mansour MA: Ubiquitination: Friend and foe
in cancer. Int J Biochem Cell Biol. 101:80–93. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Suk FM, Chang CC, Lin RJ, Lin SY, Chen YT
and Liang YC: MCPIP3 as a potential metastasis suppressor gene in
human colorectal cancer. Int J Mol Sci. 19:E13502018. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|