Introduction
Breast cancer is the most commonly diagnosed
malignancy and one of the leading causes of cancer-associated death
in women worldwide (1–5). Breast tumors are heterogeneous
neoplasms, composed of multiple subtypes, which exhibit distinct
morphologies and clinical features (6,7).
Clinically, although great advances have been made in the treatment
of breast cancer over the last decade, recurrence and metastasis
remain the principal causes of mortality in patients with this
disease (2,8–11).
Therefore, elucidating the pathogenic mechanisms underlying breast
cancer development and progression to identify prognostic
biomarkers may provide potentially novel therapeutic targets.
Long noncoding RNAs (lncRNAs) are a class of
noncoding RNAs >200 nucleotides in length which are transcribed
from across the genome and participate in a variety of
physiological and pathological processes as posttranscriptional
regulators of gene expression (12–16).
Long intervening noncoding RNAs (lincRNAs) are a type of lncRNA
that have been demonstrated to be transcript units between protein
coding genes, and display distinct tissue- and cell-specific
expression (12,13). Accumulating evidence indicates that
the abnormal expression of specific lincRNAs is closely associated
with tumor initiation, progression, and metastasis (17,18).
Linc-OIP5, a novel cancer-associated lincRNA, is
dysregulated in various types of cancer (12,19); for
example, Deng et al (12)
demonstrated that Linc-OIP5 functions as an oncogene in lung
adenocarcinoma. Additionally, Linc-OIP5 contributes to
carcinogenic potential by controlling multiple myeloma cell
proliferation and apoptosis (19).
Therefore, Linc-OIP5 is considered to be an oncogene
involved in tumorigenesis and progression. Although a number of
reports have functionally characterized Linc-OIP5 in several
tumors, the functional significance of Linc-OIP5 in breast
cancer is largely unknown.
Yes-associated protein 1 (YAP1) and Jagged 1 (JAG1)
are key components of the Hippo and Notch pathways, respectively,
which participate in several biological processes, including
maintenance of tissue homeostasis, regulation of stem cells in
adults and progression of various tumors (6,7,20–25). YAP
is a transcriptional coactivator which controls the activity of
Hippo signaling through its phosphorylation and dephosphorylation,
and binds with the TEA domain (TEAD1) transcription factor to
activate target genes downstream of Hippo signaling (25–27).
JAG1 is a key ligand of the Notch pathway, implicated in
tumorigenesis and vascularization (28–30).
YAP1 has been demonstrated to regulate oncogenic phenotypes of
breast cancer cells (31–34), and JAG1 is associated with recurrence
and poor prognosis in patients with breast cancer (35–37).
Interestingly, it has previously been shown that YAP1 acts upstream
of the Notch pathway and upregulates JAG1 expression (20,25,38).
Based on unpublished data from our laboratory, it has been
demonstrated that Linc-OIP5 knockdown influences the
proliferation, migration, and tube formation of endothelial cells
when cocultured with breast cancer cells. Furthermore, the
expression of YAP1 and JAG1 in breast cancer cells with high-grade
malignancy is significantly higher compared with breast cancer
cells with moderate-grade malignancy, highlighting their potential
as breast tumor markers, which warrant further investigation
(unpublished data). In the present study, it was demonstrated that
YAP1 and JAG1 can synergistically regulate the tumorigenesis and
progression of breast cancer cells and the effects of
Linc-OIP5 on this regulation was determined.
Linc-OIP5 may regulate the proliferation,
apoptosis, migration and invasion of breast cancer cells, at least
partly, by modulating the signaling pathways involving YAP1/JAG1,
highlighting the therapeutic potential of targeting
Linc-OIP5 for treating patients with breast cancer.
Materials and methods
Cell lines and culture conditions
The human breast cancer cell lines (MDA-MB-231 and
MCF-7) were purchased from The Cell Bank of Type Culture Collection
of the Chinese Academy of Sciences and cultured in DMEM (Thermo
Fisher Scientific Inc.) supplemented with 10% FBS (Thermo Fisher
Scientific Inc.) and 1% penicillin-streptomycin (Hyclone; GE
Healthcare Life Sciences) in a humidified atmosphere at 37°C with
5% CO2.
Reverse transcription-quantitative
(RT-q)PCR and RT-PCR
Total RNA from cultured cells was isolated using a
miRNA kit (Omega Bio-Tek Inc.), according to the manufacturer's
protocol. Total RNA concentration was evaluated by measuring the
absorbance at 260/280 nm using a NanoDrop-2000 (Thermo Fisher
Scientific Inc.). RNA samples were reverse transcribed using a
Reverse Transcription kit (Roche Diagnostics GmbH) to synthesize
cDNA, according to the manufacturer's protocol. The reverse
transcription temperature protocol was: 65°C for 10 min, 25°C for
10 min, 50°C for 1 h and 85°C for 5 min.
RT-PCR was performed on a thermal cycler (Bio-Rad
Laboratories Inc.) with 2× Taq PCR Master mix (KT201-01; Tiangen
Biotech Co., Ltd.), according to the manufacturer's protocols. The
thermocycling conditions for Linc-OIP5 and YAP1 were:
Pre-denaturation at 95°C for 5 min; followed by 30 cycles of 95°C
for 30 sec, 60°C for 30 sec and 72°C for 2 min; with a final
extension step at 72°C for 5 min. The thermocycling conditions for
JAG1 mRNA was: Pre-denaturation at 95°C for 5 min; followed
by 30 cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C for 30
sec; with a final extension at 72°C for 7 min. The thermocycling
conditions for GAPDH was: Pre-denaturation at 94°C for 3
min; followed by 30 cycles of 94°C for 30 sec, 55°C for 30 sec and
72°C for 1 min; with a final extension step at 72°C for 5 min. The
following primer pairs were used to detect the mRNA levels of the
indicated genes by RT-PCR: Linc-OIP5 forward,
5′-GCTGCGAAGATGGCGGAGTAAG-3′ and reverse,
5′-GCACGGACGCGCCTAACAC-3′; YAP1 forward,
5′-ACTCGGCTTCAGGTCCTCTTCC-3′ and reverse,
5′-TGGCTACGCAGGGCTAACTCC-3′; JAG1 forward,
5′-CAGTGCTACAACCGTGCCAGTG-3′ and reverse,
5′-CCCTCCCAGCCGTCACTACAG-3′; and GAPDH forward,
5′-CAGGAGGCATTGCTGATGAT-3′ and reverse, 5′-GAAGGCTGGGGCTCATTT-3′.
The RT-PCR products were detected using agarose gel
electrophoresis, and the images were sequentially scanned using a
gel imaging system (Bio-Rad Laboratories Inc.).
RT-qPCR was performed on a StepOne™ PCR system
(Thermo Fisher Scientific Inc.) with a SYBR® Premix Ex
Taq™ (Roche Diagnostics GmbH), according to the manufacture's
protocols. The RT-qPCR thermocycling conditions were: Prevention of
cross-contamination at 50°C for 2 min; 95°C pre-denaturation for 10
min; followed by 40 cycles of 95°C denaturation for 15 sec, 60°C
annealing for 1 min and 95°C extension for 15 sec; with a final
extension step at 72°C for 5 min. Fluorescent signals were
collected at 72°C. The following primer pairs were used to detect
the mRNA levels of the indicated genes by RT-qPCR: Linc-OIP5
forward, 5′-GCTGCGAAGATGGCGGAGTAAG-3′ and reverse,
5′-CACGGTCCAACAGATGCACTCG-3′; YAP1 forward,
5′-CCTGCGTAGCCAGTTACCAACAC-3′ and reverse,
5′-GCTGCTCATGCTTAGTCCACTGTC-3′; JAG1 forward,
5′-TGTGGCTTGGATCTGTTGCTTGG-3′ and reverse,
5′-ACGTTGTTGGTGGTGTTGTCCTC-3′; and GAPDH forward,
5′-CAGGAGGCATTGCTGATGAT-3′ and reverse, 5′-GAAGGCTGGGGCTCATTT-3′.
The relative expression of the genes was calculated using the
2−∆∆Cq method and the experiments were performed three
times (39). The relative abundance
of specific mRNA molecules was calculated using GAPDH mRNA
for normalization. All the primers for RT-PCR and RT-qPCR were
purchased from Sangon Biotech (Shanghai Sangon).
Immunofluorescence
A total of 7×104 cells were seeded into
24-well plates with a coverslip on the bottom and incubated at 37°C
in 5% CO2. After 8 h, the cells on the coverslips were
fixed with 4% paraformaldehyde (Wuhan Boster Biological Technology,
Ltd.) for 10 min at room temperature and permeabilized for 20 min
in 0.1% Triton X-100 (Beijing Solarbio Science & Technology
Co., Ltd.). After blocking in goat serum (Wuhan Boster Biological
Technology, Ltd.) for 30 min, slides were incubated with a primary
antibody overnight at 4°C. Subsequently, slides were incubated with
a goat anti-rabbit immunoglobulin G (IgG) FITC-conjugated secondary
antibody (1:50 dilution; cat. no. SA00003-2; ProteinTech Group,
Inc.) for 1 h at room temperature. Slides were subsequently
counterstained with DAPI (cat. no. C1002; Beyotime Institute of
Biotechnology) in the dark for 5 min at room temperature. The
following primary antibodies were used for immunofluorescence
staining: YAP1 (1:200 dilution; cat. no. GTX129151; GeneTex, Inc.)
and JAG1 (1:100 dilution; cat. no. GTX48691; GeneTex, Inc.).
Immunofluorescence images were acquired using an inverted
fluorescence microscope (Ti-SR; Nikon Corporation; magnification,
×100).
Western blotting
Cells were lysed in RIPA lysis buffer supplemented
with protease inhibitors (Applygen Technologies, Inc.). Protein
concentrations were quantified using a bicinchoninic acid Protein
assay kit (Applygen Technologies, Inc.) and equivalent quantities
of protein (20 µg) from each sample were loaded on a 10% SDS gel
and resolved using SDS-PAGE and transferred to PVDF membranes (EMD
Millipore). Membranes were blocked with 5% non-fat milk in
TBS-Tween (TBST; OriGene Technologies, Inc.) for 1 h at room
temperature, and subsequently incubated overnight at 4°C with the
indicated primary antibodies. The following day, PVDF membranes
were washed three times with TBST and incubated with the
appropriate horseradish peroxidase-conjugated Affinipure Goat
anti-mouse/rabbit IgG secondary antibodies (1:10,000 dilution; cat.
no. SA00001-1/SA00001-2, respectively; ProteinTech Group, Inc.) for
1 h at room temperature. Immunoreactive bands were visualized using
an enhanced chemiluminescence kit (Biosharp Life Sciences). β-actin
was used as the loading control. The following primary antibodies
were used: YAP1 (1:200 dilution; cat. no. GTX129151; GeneTex,
Inc.), JAG1 (1:100 dilution; cat. no. GTX48691; GeneTex, Inc.) and
β-actin (1:1,800 dilution; cat. no. RPB340Mi01; Beyotime Institute
of Biotechnology). An Amersham Imager 600 was used to image the
blots (GE Healthcare), Image-pro plus (version 7; Media
Cybernetics, Inc.) was used for densitometric analyses of
immunoblots and quantification results were normalized to those of
the loading control.
Small interfering RNAs (siRNAs)
Small interfering RNA duplexes targeting
Linc-OIP5 and negative control siRNA duplexes were
synthesized and purchased from Shanghai GenePharma Co., Ltd. The
siRNA sequences were as follows: Negative control (NC) siRNA
duplexes sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′; siLinc-OIP5 Duplex 1 sense,
5′-CCUACUGCCUUGUAAGAAUTT-3′ and antisense,
5′-AUUCUUACAAGGCAGUAGGTT-3′; siLinc-OIP5 Duplex 2 sense,
5′-CCAGCUGUCUUUGUGUCUUTT-3′ and antisense,
5′-AAGACACAAAGACAGCUGGTT-3′; siLinc-OIP5 Duplex 3 sense,
5′-CCAGUUAUCCUGCUAACAUTT-3′ and antisense,
5′-AUGUUAGCAGGAUAACUGGTT-3′.
A mixture of the three siRNAs targeting
Linc-OIP5 were used, in a 1:1:1 ratio. MDA-MB-231 cells when
they had reached 70–80% confluence using Lipofectamine®
3000 Transfection Reagent (Thermo Fisher Scientific Inc.) according
to the manufacturer's protocol. Cells were seeded at a
concentration density of 2.7×105 cells per well in
6-well plates. At 48 h post-transfection, the effectiveness of
siRNA knockdown was assessed by RT-qPCR.
Cell proliferation assays
MDA-MB-231 cells were collected 48 h after
transfection with Linc-OIP5 siRNA or NC siRNA, and analyzed
using a Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies,
Inc.), according to the manufacturer's protocols. Cells were seeded
in 96-well plates at a density of 3×103 cells/well and
CCK-8 reagent (10 µl/well) was added into medium without serum (90
µl/well) and incubated for 3 h at 37°C. The amount of formazan dye
generated by cellular dehydrogenase redox was measured by
absorbance at 450 nm using a microplate reader (Molecular Devices,
LLC), with the amount produced being proportional to the number of
living cells. Cell proliferation was measured every 24 h for 3
days, with the optical density values of each well representing the
survival/proliferation of cells. These experiments were repeated at
least three times independently.
Wound healing assays
Wound healing assays were used to analyze the
migratory ability of MDA-MB-231 cells transfected with
Linc-OIP5 siRNA. A total of 3×105 cells per well
were seeded into 24-well plates and cultured in DMEM with 1% FBS at
37°C in 5% CO2 for 48 h to allow the cells to adhere and
form a confluent monolayer. Subsequently, the monolayers were
scratched using the tip of a 10 µl pipette tip. The scratched wound
was rinsed three times with PBS to remove debris. Cells were
incubated at 37°C in 5% CO2 and monitored for 24 h.
Wound healing was monitored by taking digital images from three
different fields of view and from three independent samples at 0,
12, and 24 h after scratching. Scratch-wound images were captured
using an inverted light microscope (TE2000-S; Nikon Corporation;
magnification, ×40).
Transwell migration and invasion
assays
Transwell assays were performed using 24-well
Transwell plates (Corning Inc.). A total of 5×104 cells
in serum-free DMEM (200 µl) were added to the upper chamber of each
insert, while the medium in the lower chambers (600 µl) was
supplemented with 10% FBS. After a 24 h incubation at 37°C, cells
on the upper surface of the membrane were removed with a cotton
tip, while those on the lower surface were stained for 20 min at
room temperature with 0.1% crystal violet (Beijing Solarbio Science
& Technology Co., Ltd.). For Transwell invasion assays, cells
(1×105) were seeded in the upper chamber of inserts
precoated with 40 µl Matrigel (BD Biosciences) prior to adding the
200 µl serum-free DMEM to the upper chamber. In the lower chamber,
600 µl DMEM supplemented with 10% FBS was added. Cells were
incubated overnight at 37°C, after which the cells on the upper
surface of the upper chamber were removed, whereas the invasive
cells on the lower surface were fixed with methanol and stained
with 0.1% crystal violet for 20 min at room temperature. Images
were captured of three different fields of view, from three
independent samples, using an inverted light microscope (TE2000-S;
Nikon Corporation; magnification, ×100). Quantitative analysis of
migratory and invasive cells was performed using ImageJ software
(version 1.48 v; NIH Inc., Bethesda, Md, USA).
Flow cytometry
Apoptotic cells were determined using an Annexin
V-FITC apoptosis detection kit (cat. no. 556547; BD Biosciences,
Franklin Lakes, NJ, USA), according to the manufacturer's
protocols. The assay was performed with two-color analysis of FITC
(green)-labeled Annexin V binding and PI (red) uptake. Cells
(5×105) were harvested and resuspended in 100 µl 1×
binding buffer and stained with 5 µl FITC-Annexin V and 5 µl PI for
15 min in the dark at room temperature. Cell apoptosis was
quantified using Cell Quest version 0.9.13 alpha (BD Biosciences)
and data were analyzed using FlowJo version 10.1 (FlowJo LLC.).
Results were calculated from three independent experiments.
Confocal laser-scanning
microscopy
The annexin V-FITC apoptosis detection kit uses
double staining to identify apoptotic cells using a confocal
laser-scanning microscope (Olympus Corporation). The staining
procedure was the same as described above for flow cytometry, and a
drop of the cell suspension was placed on a glass slide and
observed under a confocal microscope. Confocal images were obtained
from three different fields of view from three independent samples
(magnification, ×200).
Statistics and repeatability of
experiments
Data are presented as the mean ± standard deviation
(s.d.) of at least three repeats, and all error bars indicate s.d.
SPSS version 21.0 (IBM Corp.) and GraphPad Prism version 7.0
(GraphPad Software Inc.) were used to evaluate statistical
significance. A one-way ANOVA was used to compare the mean values
between three or more data sets, with a post-hoc
Student-Newman-Keuls test or the non-parametric Tamhane T2 test to
compare the mean of each data set with that of every other data
set. Statistical comparisons of the means of two data sets were
performed using an unpaired Student's two-tailed t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
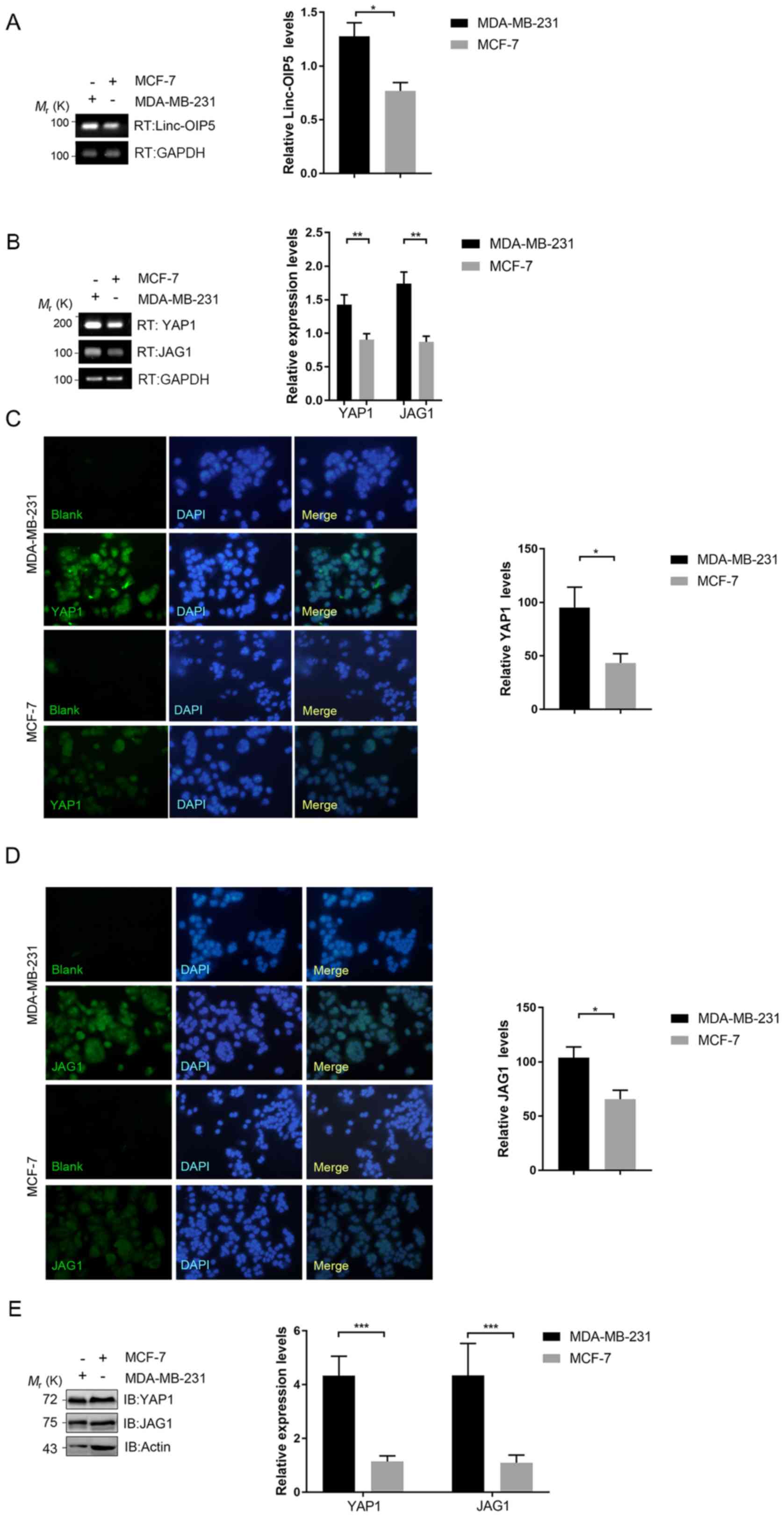
Expression of Linc-OIP5, YAP1 and JAG1
is upregulated in MDA-MB-231 cells
To understand the associations between Linc-OIP5,
YAP1 and JAG1 in human breast cancer, their expression
in breast cancer cells with different degrees of malignancy was
determined using RT-qPCR. Linc-OIP5, YAP1 and JAG1
were all expressed in all breast cancer cells assessed at the mRNA
level (Fig. 1A and B). The
expression levels of Linc-OIP5 (P<0.05), YAP1
(P<0.01) and JAG1 (P<0.01) in MDA-MB-231 cells, which
exhibit the highest degree of malignancy, were significantly higher
compared with MCF-7 cells which are typically less malignant
(Fig. 1A and B). Immunofluorescence
and western blotting analyses further demonstrated the differential
expression of YAP1 and JAG1 in the breast cancer cell lines at the
protein level (Fig. 1C-E). The
expression of YAP1 and JAG1 in MDA-MB-231 cells at the protein
level was significantly higher compared with MCF-7 cells (P<0.05
and P<0.001, respectively) (Fig.
1C-E). Based on these data, MDA-MB-231 cells were used for
further experimental analyses.
Knockdown of Linc-OIP5 inhibits cell
proliferation and increases apoptosis in MDA-MB-231 cells
To determine the biological effects of
Linc-OIP5 during the initiation and progression of breast
cancer, its expression was knocked down in MDA-MB-231 cells using
siRNA and the effects of knockdown on proliferation and apoptosis
were investigated. RT-qPCR analysis showed that the mRNA expression
levels of Linc-OIP5 was significantly decreased following
transfection of Linc-OIP5 siRNA compared with NC-siRNA or
mock-treated controls (P<0.01; Fig.
2A). The results of the cell proliferation (CCK-8) assays
showed that Linc-OIP5 knockdown significantly decreased
proliferation of MDA-MB-231 cells compared with the NC siRNA group
after 24 h (P<0.01; Fig. 2B).
Additionally, the rate of apoptosis in MDA-MB-231 cells following
knockdown of Linc-OIP5 was detected using Annexin V-FITC/PI
double staining combined with flow cytometry (Fig. 2C) and confocal laser-scanning
microscopy (Fig. 2D). The results
showed a significant increase in the rate of apoptosis and Annexin
V staining when Linc-OIP5 was knocked down in MDA-MB-231
cells compared with the NC siRNA group (flow cytometry, P<0.01;
confocal microscopy, P<0.001). These results suggest that
Linc-OIP5 knockdown significantly inhibited the
proliferation and promoted apoptosis of MDA-MB-231 cells.
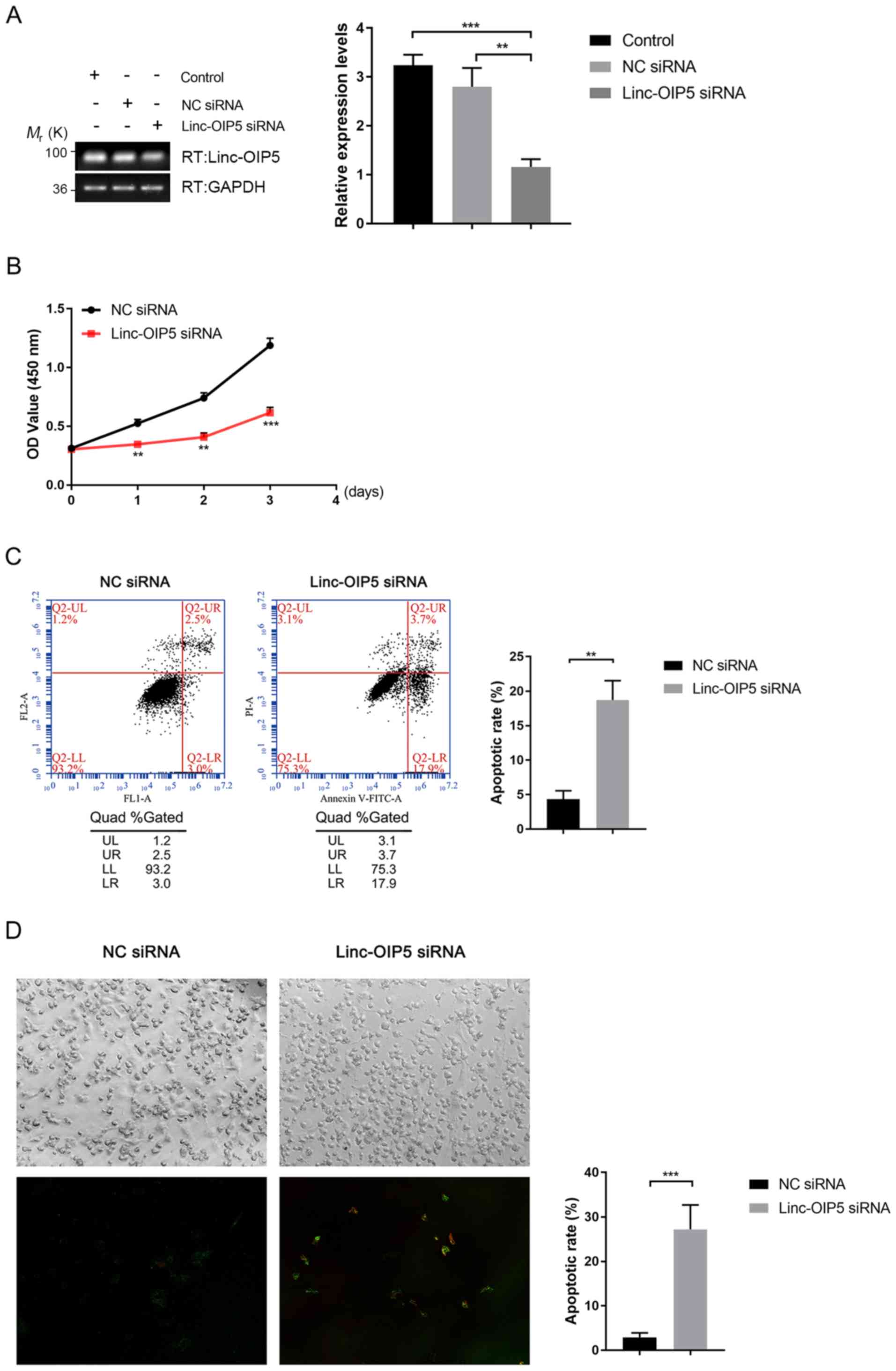 | Figure 2.Knockdown of Linc-OIP5 decreases
proliferation and promotes apoptosis in MDA-MB-231 cells. (A)
Relative expression levels of Linc-OIP5 in cells transfected
with Linc-OIP5 siRNA were significantly decreased compared
with cells transfected with the NC siRNA. **P<0.01,
***P<0.001. (B) Knockdown of Linc-OIP5 significantly
decreased the proliferative capacity of MDA-MB-231 cells compared
with the NC siRNA transfected cells. **P<0.01, ***P<0.001.
(C) Flow cytometry of Annexin V-FITC/PI double stained cells to
determine the effect of Linc-OIP5 knockdown on apoptosis.
**P<0.01. (D) Confocal laser-scanning microscopy showed a
significant increase in the apoptotic rates of MDA-MB-231 cells
transfected with Linc-OIP5 siRNA compared with NC siRNA,
determined by FITC-labeled Annexin V (green) and PI (red) staining.
Magnification, ×200. Values were normalized against the NC siRNA
group. ***P<0.001. FL, fluorescence parameter; FITC, fluorescein
isothiocyanate; PI, propidium iodide; NC, negative control; si,
small interfering; UL, upper left, UR, upper right; LL, lower left;
lower right. |
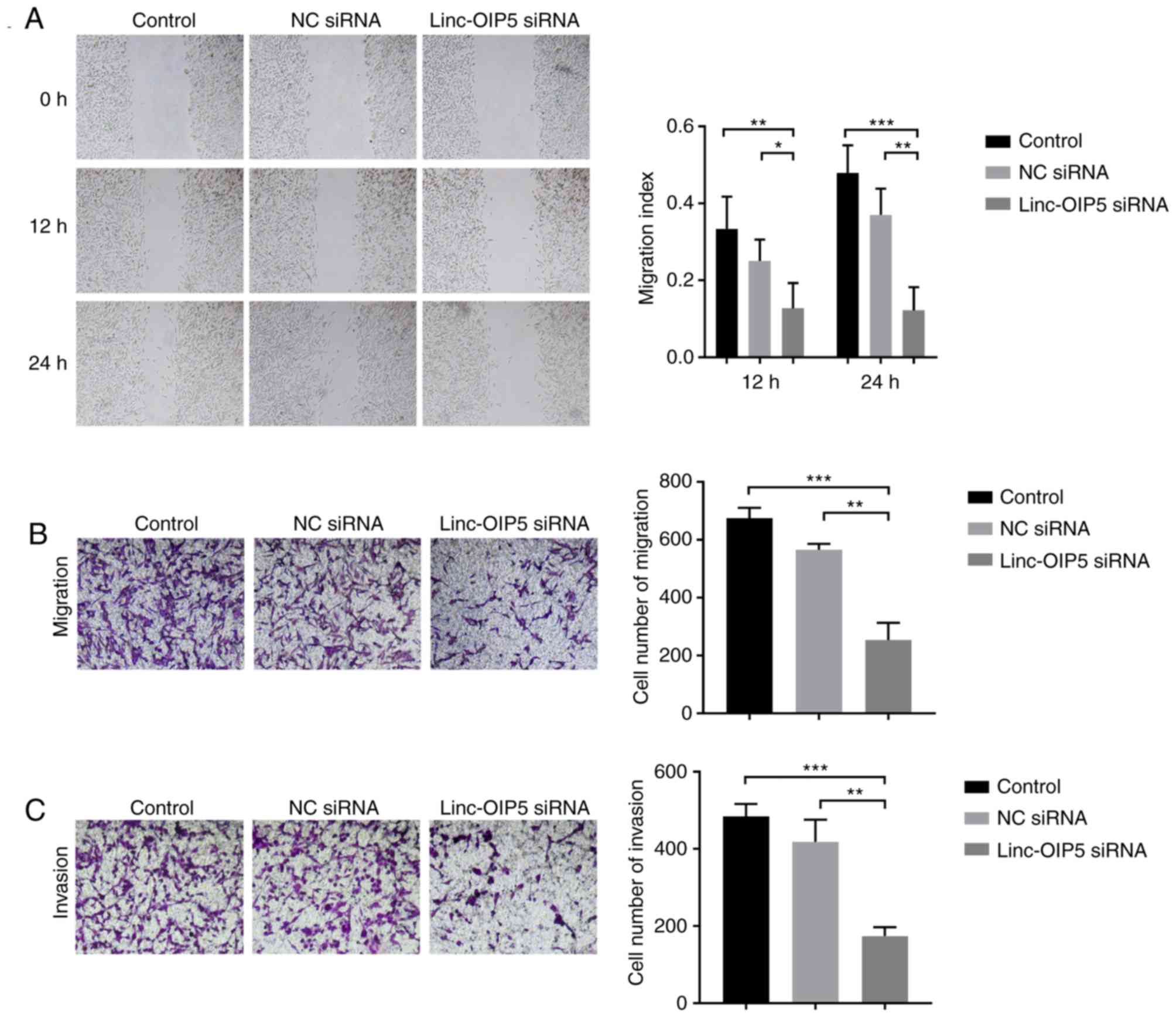
Knockdown of Linc-OIP5 decreases cell
migration and invasion in MDA-MB-231 cells
To determine the role of Linc-OIP5 in
regulating the malignancy of MDA-MB-231 cells, cell migration and
invasion were measured following Linc-OIP5 knockdown in
vitro. Wound healing and transwell migration assays
demonstrated that the migratory ability of MDA-MB-231 cells was
reduced significantly following knockdown of Linc-OIP5.
MDA-MB-231 cells transfected with Linc-OIP5 siRNA exhibited
a decreased migratory capacity in the wound healing assay compared
with the control group after 12 and 24 h (P<0.05 and P<0.01,
respectively; Fig. 3A). Similarly,
the results of the transwell migration assays showed that the
number of cells which had migrated through the membrane was
significantly decreased in cells transfected with Linc-OIP5
siRNA compared with the control group (P<0.05, P<0.01,
respectively; Fig. 3B).
Additionally, a transwell invasion assay was used to elucidate the
effects of Linc-OIP5 knockdown on the invasive ability of
MDA-MB-231 cells. The results showed that the number of cells which
had invaded through the Matrigel was significantly decreased in the
MDA-MB-231 cells transfected with Linc-OIP5 siRNA
(P<0.01; Fig. 3C). These data
demonstrate that Linc-OIP5 enhances the metastatic capacity
of MDA-MB-231 breast cancer cells.
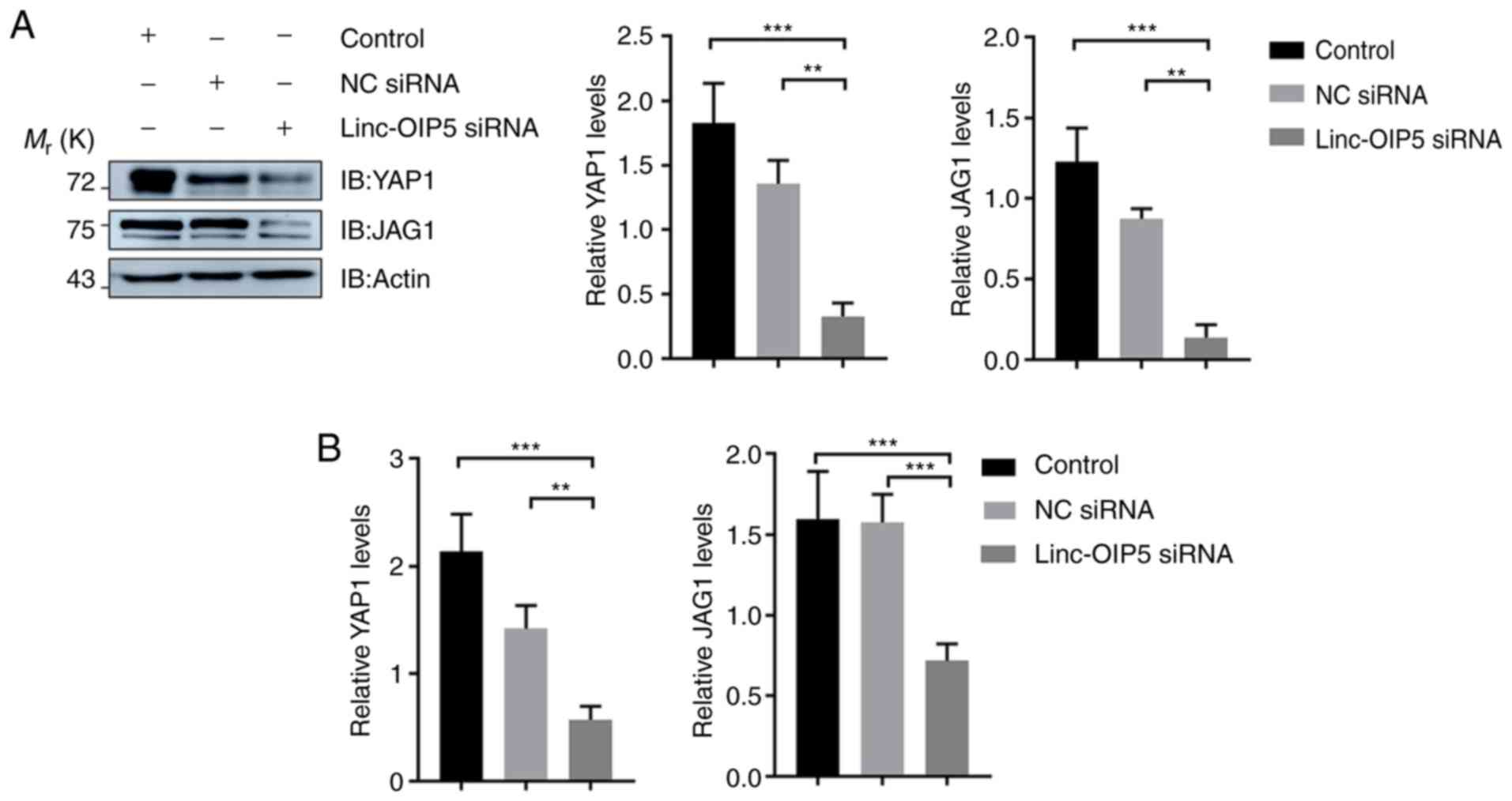
Linc-OIP5 knockdown downregulates the
expression of YAP1 and JAG1 in MDA-MB-231 cells
To determine the mechanisms underlying the effects
of Linc-OIP5 in increasing the malignant potential of
MDA-MB-231 breast cancer cells, the expression levels of YAP1 and
JAG1 were measured in MDA-MB-231 cells transfected with
Linc-OIP5 siRNA, relative to NC-siRNA- or mock-treated
controls. The results of western blotting showed that
Linc-OIP5 knock down significantly reduced the protein
expression levels of YAP1 and JAG1 in MDA-MB-231 cells compared
with the control group (P<0.01; Fig.
4A). Consistent with these findings, RT-qPCR analysis
demonstrated that the YAP1 (P<0.01) and JAG1
(P<0.001) mRNA expression levels were also significantly reduced
by knocking down Linc-OIP5 in MDA-MB-231 cells (Fig. 4B). Therefore Linc-OIP5
knockdown decreased YAP1 and JAG1 expression levels in MDA-MB-231
cells.
Discussion
Several lncRNAs have emerged as important regulators
of gene expression in mammary tumors. For example, loss of lncRNA
Malat1 reduces the differentiation and metastatic capacity
of mammary tumors (14), and lncRNA
HOTAIR contributes to the development and tumorigenesis of
breast cancer (3). Furthermore,
Linc-OIP5 is closely associated with tumor initiation,
progression and metastasis (17,18).
Previous studies have shown that Linc-OIP5 contributes to
the carcinogenic potential of lung adenocarcinoma and multiple
myeloma (12,19). Based on unpublished data from our
lab, Linc-OIP5 knockdown regulates the proliferation, migration and
tube formation of endothelial cells when cocultured with breast
cancer cells, suggesting that this molecule may be a promising
marker for breast tumors. Additionally, Zeng et al (40) demonstrated that Linc-OIP5 was
aberrantly expressed in breast cancer.
In the present study, to elucidate the functional
effects of Linc-OIP5 on the development and progression of
breast cancer, and the link between Linc-OIP5, YAP1 and
JAG1, the expression levels of Linc-OIP5, YAP1, and JAG1 in
breast cancer cell lines with different degrees of malignancy were
determined. Expression of Linc-OIP5, YAP1, and JAG1 was
shown to be highest in MDA-MB-231 cells, and thus, they were used
for all subsequent experiments. Given that dysregulation of cell
proliferation and apoptosis are hallmarks of cancer progression,
the effects of Linc-OIP5 knockdown on cell proliferation and
apoptosis in MDA-MB-231 cells were evaluated. It was demonstrated
that Linc-OIP5 knockdown significantly increased
proliferation and promoted apoptosis. Therefore, Linc-OIP5
may serve as an oncogene in breast cancer and its effects on cell
proliferation may be associated with regulation of apoptosis. It
should be noted however, that the green staining observed in the
immunofluorescence experiments was not typical of Annexin-V
staining. A possible explanation for this may be the uneven
distribution of apoptotic protein (Annexin V) in MDA-MB-231 breast
cancer cells. Furthermore, there is a misalignment between the
bright-field and fluorescence images. This misalignment was the
result of using two separate microscopes, as the confocal
laser-scanning microscope used does not possess bright-field
optics. Migration and invasion are considered to mediate the
malignancy and metastatic capacity of tumors (2). To determine the role of
Linc-OIP5 in metastasis of MDA-MB-231 breast cancer cells,
the migratory and invasive capacities of the cells following
knockdown of Linc-OIP5 were examined. The results showed
that Linc-OIP5 knockdown resulted in a significant reduction
of cell migration and invasion, suggesting that Linc-OIP5 may serve
as an oncogenic regulator which increases the malignant behaviors
of MDA-MB-231 breast cancer cells, and that inhibiting
Linc-OIP5 function may suppress the progression and
metastasis of breast cancer.
The molecular mechanism underlying the effects of
Linc-OIP5 on breast cancer cells were investigated in this
study. It has been suggested that YAP1 and JAG1 are associated with
progression and recurrence in breast cancer (31–37).
YAP1 acts upstream of the Notch pathway, as well as upregulating
JAG1 expression (20,25,38).
Based on the above studies, it was hypothesized that
Linc-OIP5 may influence the proliferation, migration,
invasion and apoptosis of MDA-MB-231 breast cancer cells, and may
act upstream of YAP1/JAG1 signaling. The expression of YAP1 and
JAG1 were determined in MDA-MB-231 breast cancer cells treated with
or without Linc-OIP5 siRNA. The results suggested that both
the mRNA and protein expression levels of YAP1 and JAG1 were
significantly lower in MDA-MB-231 cells treated with
Linc-OIP5 siRNA compared with the control, indicating that
the effect of Linc-OIP5 on the malignant behaviors of MDA-MB-231
cells was partially associated with YAP1/JAG1 signaling. Together,
the results indicate that Linc-OIP5 may act upstream of
YAP1/JAG1 signaling to affect the proliferation, apoptosis,
invasion and migration of MDA-MB-231 breast cancer cells.
In order to demonstrate a causal link between
silencing of Linc-OIP5 and the lower expression levels of
YAP1 and JAG1, rescue experiments were performed (data not shown).
Unfortunately, the experimental results were not as expected. After
consulting with a Plasmid Construction Company, the unexpected
results may have been due to the use of a DNA construct >8,000
bp for Linc-OIP5 overexpression. This may have been too
long, and ideally a <1,000 bp should be used as this typically
leads to a higher success rate (Personal communication with the
manufacturer). Therefore, establishing a causal link between
Linc-OIP5 and YAP1/JAG1 will be the aim of future
experiments. Future experiments should also investigate the
function of Linc-OIP5 in EMT and in in vivo studies,
possibly using xenograft mice models. EMT refers to the
transformation of epithelial cells to mesenchymal cells under
certain conditions, providing these cells with increased invasive
and migratory capabilities (41,42). EMT
is generally viewed as one of the primary mechanisms by which
invasion and metastasis is increased (43). Therefore future research should
establish the association between Linc-OIP5 and EMT to
ascertain whether the effects of Linc-OIP5 observed in
vitro translates to in vivo.
In conclusion, the results of the present study
demonstrate that Linc-OIP5 is upregulated in MDA-MB-231
breast cancer cells. In addition, Linc-OIP5 knockdown
inhibits MDA-MB-231 cell proliferation, migration and invasion,
while inducing apoptosis, at least in part, through YAP1/JAG1
signaling. Linc-OIP5 may regulate JAG1 signaling through
YAP1 signaling. Regulation of YAP1 and JAG1 by Linc-OIP5 may
be a novel mechanism of oncogenic signal regulation in breast
cancer. Therefore, Linc-OIP5 may serve as a breast cancer
oncogene and may be a suitable therapeutic target for treatments
aimed at preventing tumor progression and metastasis in patients
with breast cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding from the
Project on Basic Scientific Research for Higher Education
Institutions affiliated to Heilongjiang Province (Heilongjiang,
China; grant no. 2018-KYYWFMY-0006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SG and QZ designed the study. QZ and YY performed
the experiments. QZ, XD, YL and HW analyzed the data. QZ wrote the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CCK-8
|
Cell Counting Kit-8
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
lincRNAs
|
long intervening noncoding RNAs
|
|
lncRNAs
|
long noncoding RNAs
|
|
NC
|
negative control
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
siRNA
|
small interfering RNA
|
|
s.d.
|
standard deviation
|
References
|
1
|
Lobba ARM, Carreira ACO, Cerqueira OLD,
Fujita A, DeOcesano-Pereira C, Osorio CAB, Soares FA, Rameshwar P
and Sogayar MC: High CD90 (THY-1) expression positively correlates
with cell transformation and worse prognosis in basal-like breast
cancer tumors. PLoS One. 13:e01992542018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan S, Fan C, Liu N, Huang K, Fang X and
Wang K: Downregulation of the long non-coding RNA ZFAS1 is
associated with cell proliferation, migration and invasion in
breast cancer. Mol Med Rep. 17:6405–6412. 2018.PubMed/NCBI
|
|
3
|
Zhao W, Geng D, Li S, Chen Z and Sun M:
LncRNA HOTAIR influences cell growth, migration, invasion, and
apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer. Cancer
Med. 7:842–855. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang P, Wang F, Wang L and Pan JH:
Proprotein convertase subtilisin/kexin type 6 activates the
extracellular signal-regulated kinase 1/2 and Wnt family member 3A
pathways and promotes in vitro proliferation, migration and
invasion of breast cancer MDA-MB-231 cells. Oncol Lett. 16:145–150.
2018.PubMed/NCBI
|
|
5
|
Liang Y, Chen H, Ji L, Du J, Xie X, Li X
and Lou Y: Talin2 regulates breast cancer cell migration and
invasion by apoptosis. Oncol Lett. 16:285–293. 2018.PubMed/NCBI
|
|
6
|
Wen Y, Ji Y, Zhang Y, Jiang B, Tang C,
Wang Q, Chen X, Jia L, Gu W and Xu X: Knockdown of Yes-associated
protein induces the apoptosis while inhibits the proliferation of
human periodontal ligament stem cells through crosstalk between Erk
and Bcl-2 signaling pathways. Int J Med Sci. 14:1231–1240. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang N, Ke B, Hjortjensen K, Iglesiasgato
D, Wang Z, Chang PC, Zhao Y, Niu XD, Wu T, Peng B, et al: YAP1
regulates prostate cancer stem cell-like characteristics to promote
castration resistant growth. Oncotarget. 8:115054–115067. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu Y, Lin X, Xu J, Jing H, Qin Y and Li Y:
SULT1E1 inhibits cell proliferation and invasion by activating
PPARγ in breast cancer. J Cancer. 9:1078–1087. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:e359–e386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng J, Deng H, Liu C, Liang Y and Wang S:
Long non-coding RNA OIP5-AS1 functions as an oncogene in lung
adenocarcinoma through targeting miR-448/Bcl-2. Biomed
Pharmacother. 98:102–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mendell JT: Targeting a long noncoding RNA
in breast cancer. N Engl J Med. 374:2287–2289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arun G, Diermeier S, Akerman M, Chang KC,
Wilkinson JE, Hearn S, Kim Y, MacLeod AR, Krainer AR, Norton L, et
al: Differentiation of mammary tumors and reduction in metastasis
upon Malat1 lncRNA loss. Genes Dev. 30:34–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bergmann JH and Spector DL: Long
non-coding RNAs: Modulators of nuclear structure and function. Curr
Opin Cell Biol. 26:10–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rinn JL and Chang HY: Genome regulation by
long noncoding RNAs. Ann Rev Biochem. 81:145–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meseure D, Drak Alsibai K, Nicolas A,
Bieche I and Morillon A: Long noncoding RNAs as new architects in
cancer epigenetics, prognostic biomarkers, and potential
therapeutic targets. Biomed Res Int. 2015:3202142015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pandey GK and Kanduri C: Long noncoding
RNAs and neuroblastoma. Oncotarget. 6:18265–18275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang N, Chen J, Zhang H, Wang X, Yao H,
Peng Y and Zhang WG: LncRNA OIP5-AS1 loss-induced microRNA-410
accumulation regulates cell proliferation and apoptosis by
targeting KLF10 via activating PTEN/PI3K/AKT pathway in multiple
myeloma. Cell Death Dis. 8:e29752017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu N, Nguyen Q, Wan Y, Zhou T, Venter J,
Frampton GA, DeMorrow S, Pan D, Meng F, Glaser S, et al: The Hippo
signaling functions through the Notch signaling to regulate
intrahepatic bile duct development in mammals. Lab Invest.
97:843–853. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gonzalez-King H, García NA, Ontoria-Oviedo
I, Ciria M, Montero JA and Sepúlveda P: Hypoxia Inducible Factor-1α
potentiates Jagged1-mediated angiogenesis by mesenchymal stem
cell-derived exosomes. Stem Cells. 35:1747–1759. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sheldon H, Heikamp E, Turley H, Dragovic
R, Thomas P, Oon CE, Leek R, Edelmann M, Kessler B, Sainson RC, et
al: New mechanism for Notch signaling to endothelium at a distance
by Delta-like 4 incorporation into exosomes. Blood. 116:2385–2394.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pan D: Hippo signaling in organ size
control. Genes Dev. 21:886–897. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan D: The hippo signaling pathway in
development and cancer. Dev Cell. 19:491–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yimlamai D, Christodoulou C, Galli GG,
Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ
and Camargo FD: Hippo pathway activity influences liver cell fate.
Cell. 157:1324–1338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ramos A and Camargo FD: The Hippo
signaling pathway and stem cell biology. Trends Cell Biol.
22:339–346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mammoto A, Muyleart M, Kadlec A, Gutterman
D and Mammoto T: YAP1-TEAD1 signaling controls angiogenesis and
mitochondrial biogenesis through PGC1α. Microvas Res. 119:73–83.
2018. View Article : Google Scholar
|
|
28
|
Oon CE, Bridges E, Sheldon H, Sainson RCA,
Jubb A, Turley H, Leek R, Buffa F, Harris AL and Li JL: Role of
Delta-like 4 in Jagged1-induced tumour angiogenesis and tumour
growth. Oncotarget. 8:40115–40131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Benedito R, Roca C, Sörensen I, Adams S,
Gossler A, Fruttiger M and Adams RH: The notch ligands Dll4 and
Jagged1 have opposing effects on angiogenesis. Cell. 137:1124–1135.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aspalter IM, Gordon E, Dubrac A, Ragab A,
Narloch J, Vizán P, Geudens I, Collins RT, Franco CA, Abrahams CL,
et al: Alk1 and Alk5 inhibition by Nrp1 controls vascular sprouting
downstream of Notch. Nat Comm. 17:72642015. View Article : Google Scholar
|
|
31
|
Lin C and Xu X: YAP1-TEAD1-Glut1 axis
dictates the oncogenic phenotypes of breast cancer cells by
modulating glycolysis. Biomed Pharmacother. 95:789–794. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang T, Mao B, Cheng C, Zou Z, Gao J, Yang
Y, Lei T, Qi X, Yuan Z, Xu W and Lu Z: YAP promotes breast cancer
metastasis by repressing growth differentiation factor-15. Biochim
Biophys Acta Mol Basis Dis. 1864:1744–1753. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu Q, Li J and Sun S, Chen X, Zhang H, Li
B and Sun S: YAP/TAZ-mediated activation of serine metabolism and
methylation regulation is critical for LKB1-deficient breast cancer
progression. Biosci Rep. 37(pii): BSR201710722017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hou L, Chen L and Fang L: Scutellarin
inhibits proliferation, invasion, and tumorigenicity in human
breast cancer cells by regulating HIPPO-YAP signaling pathway. Med
Sci Monit. 23:5130–5138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Selcuklu SD, Donoghue MT, Kerin MJ and
Spillane C: Regulatory interplay between miR-21, JAG1 and
17beta-estradiol (E2) in breast cancer cells. Biochem Biophys Res
Commun. 423:234–239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dickson BC, Mulligan AM, Zhang H, Lockwood
G, O'Malley FP, Egan SE and Reedijk M: High-level JAG1 mRNA and
protein predict poor outcome in breast cancer. Mod Pathol.
20:685–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reedijk M, Pinnaduwage D, Dickson BC,
Mulligan AM, Zhang H, Bull SB, O'Malley FP, Egan SE and Andrulis
IL: JAG1 expression is associated with a basal phenotype and
recurrence in lymph node-negative breast cancer. Breast Cancer Res
Treat. 111:439–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Slemmons KK, Crose LES, Riedel S,
Sushnitha M, Belyea B and Linardic CM: A novel Notch-YAP circuit
drives stemness and tumorigenesis in embryonal rhabdomyosarcoma.
Mol Cancer Res. 15:1777–1791. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zeng H, Wang J, Chen T, Zhang K, Chen J,
Wang L, Li H, Tuluhong D, Li J and Wang S: Downregulation of long
non-coding RNA Opa interacting protein 5-antisense RNA 1 inhibits
breast cancer progression by targeting sex-determining region Y-box
2 by microRNA-129-5p upregulation. Cancer Sci. 110:289–302.
2019.PubMed/NCBI
|
|
41
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Klymkowsky MW and Savagner P:
Epithelial-mesenchymal transition: A cancer researcher's conceptual
friend and foe. Am J Pathol. 174:1588–1593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ansieau S, Courtois-Cox S, Morel AP and
Puisieux A: Failsafe program escape and EMT: A deleterious
partnership. Semin Cancer Biol. 21:392–396. 2011.PubMed/NCBI
|