Introduction
Colorectal cancer (CRC) is the third most common
cancer in males, and the second most common in females, with an
incidence of ~1.1 million new cases and 551,269 mortalities per
year worldwide in 2018 (1,2). Although there has been significant
progress in cancer screening programs, the survival rate of
patients with CRC remains unsatisfactory (3). In the past 20 years, the 5-year
survival rate of patients with early-stage CRC was 69%, while that
of patients with advanced-stage disease was 12% (4). The high mortality of CRC is in part due
to limitations in the currently available therapies, which are the
result of a limited understanding of the molecular mechanisms
underlying CRC (5). Several factors
are associated with the development of CRC, including smoking,
obesity and alcohol consumption (6).
In addition, it is estimated that genetic factors account for ~30%
of CRC cases (7). However, the
precise molecular mechanism remains unclear. It has been reported
that early- and late-onset CRC may evolve in distinct ways, and
there may be different molecular mechanisms according to the age at
onset (8). Compared with late-onset
CRC in patients who were diagnosed after the age of 50 years,
early-onset CRC, before the age of 50 years, is more frequently
associated with aggressive histology and distant metastasis
(9). Although numerous efforts have
been made to elucidate the genetic mechanism underlying CRC, the
treatment of early-onset CRC remains challenging (10,11).
Therefore, there is an urgent need to uncover the mechanisms of
this disease.
High-throughput platforms have been widely used to
search for genetic biomarkers for CRC. Microarrays have the
capacity to analyze genes implicated in the development of CRC
(12–14). Sun et al (15) used integrated bioinformatics analysis
to identify seven genes that may serve as novel biomarkers for CRC.
He et al (16) identified
four long non-coding RNAs associated with the progression of CRC by
analyzing gene data cohorts and constructing a competing endogenous
RNA network. Zhang et al (17) used a bioinformatics approach to
demonstrate that the hypermethylation of CpG islands in
transforming growth factor β induced was associated with poor
disease-free survival rates in patients with CRC. Furthermore, Yu
et al (18) constructed a
weighted gene co-expression network analysis (WGCNA) and revealed
that ribosomal proteins play a key role in the development of CRC.
However, to the best of the authors' knowledge, no previous studies
have uncovered the mechanism of early-onset CRC.
In the present study, the GSE39582 dataset (11) was downloaded from the Gene Expression
Omnibus (GEO) database and the gene expression data and clinical
information of patients who were diagnosed with CRC before the age
of 50 years were selected for further investigation. WGCNA was
subsequently used to identify the most relevant modules in
early-onset CRC. Gene Ontology (GO) and Kyoto Encyclopedia of Genes
and Genomes (KEGG) analyses were performed on the hub genes and a
protein-protein interaction (PPI) network was constructed. The
diagnostic values of the hub genes that had a high degree score of
protein-protein connection in the PPI network were analyzed. The
genes identified in the present study may shed new light on the
molecular mechanisms underlying the progression of early-onset CRC,
and may serve as novel prognostic biomarkers.
Materials and methods
Data collection and preprocessing
The normalized gene expression dataset GSE39582
(19) and the corresponding clinical
data were retrieved from the GEO database (www.ncbi.nlm.nih.gov/geo) using a GPL570 platform
(Affymetrix Human Genome U133 Plus 2.0 Array). The GSE39582 dataset
contained the gene expression information of 566 CRC tissues and 19
adjacent non-tumorous colorectal mucosal samples from patients with
CRC. In order to explore the key genes associated with the
progression of early-onset CRC, 53 CRC samples from patients in the
GSE39582 dataset who were diagnosed before the age of 50 years were
selected and used as the discovery dataset. Therefore, the gene
expression information and clinical traits of the aforementioned 53
CRC samples were used in the present study. Ten clinical traits
were studied in the present study, and included sex, age,
tumor-node-metastasis (TNM) stage, T stage, M stage, N stage,
recurrence-free survival (RFS) event (time from surgery to the
first recurrence that was capped at 5 years), RFS days, overall
survival (OS) event (time from surgery to death) and OS days. Prior
to WGCNA, the probes without known gene symbols were filtered, and
the collapse Rows function (version 1.17.0) (20) was used to merge the retained probes
and gene symbols in expression profiles in the dataset. In the
present study, the sample quality of the discovery dataset was
assessed by sample clustering, according to the inter-array
correlation (IAC) (21); an IAC
>0.2 in the sample clustering tree was considered as the
criterion for screening outlier samples. Based on this criterion,
no samples were eliminated from the present study.
Co-expression module detection
The R package WGCNA (version 1.66) (22) was used to generate the gene
co-expression network for the selected genes in the GSE39582
dataset. According to the variation of median absolute deviation
(23), the expression profile of the
top 10,000 genes and information about their clinical traits were
used to construct the WGCNA. The threshold for identifying outlier
samples was set as 0.2. All genes were then analyzed with each
other using the Pearson's correlation test, and a matrix of
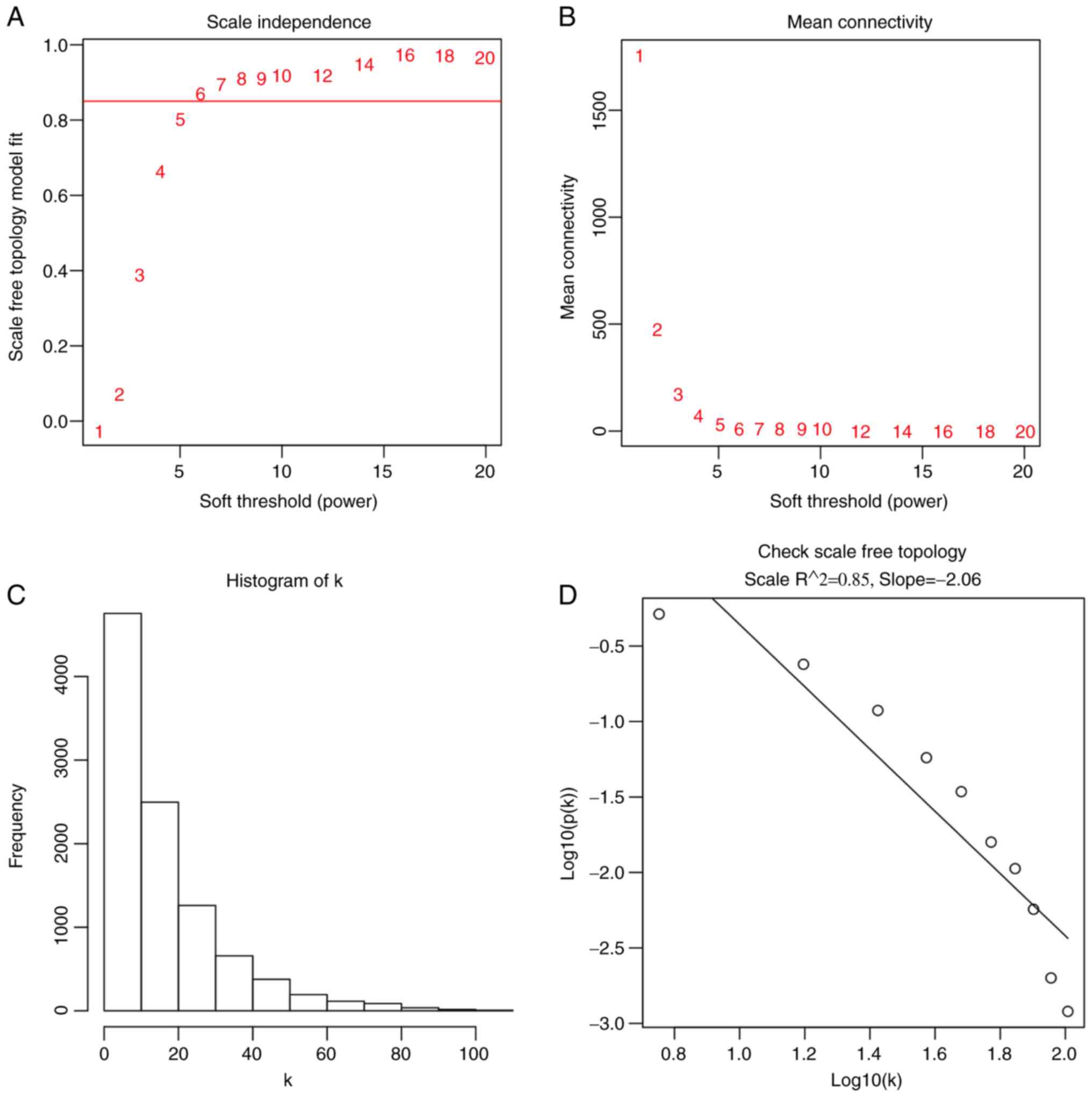
similarity was constructed based on this analysis. A soft power of
β=6 was selected based on the scale-free topology criterion of
>0.85 (22). The adjacency matrix
of gene expression data from all patients with early-onset CRC was
then clustered using topological overlap matrix analysis. Finally,
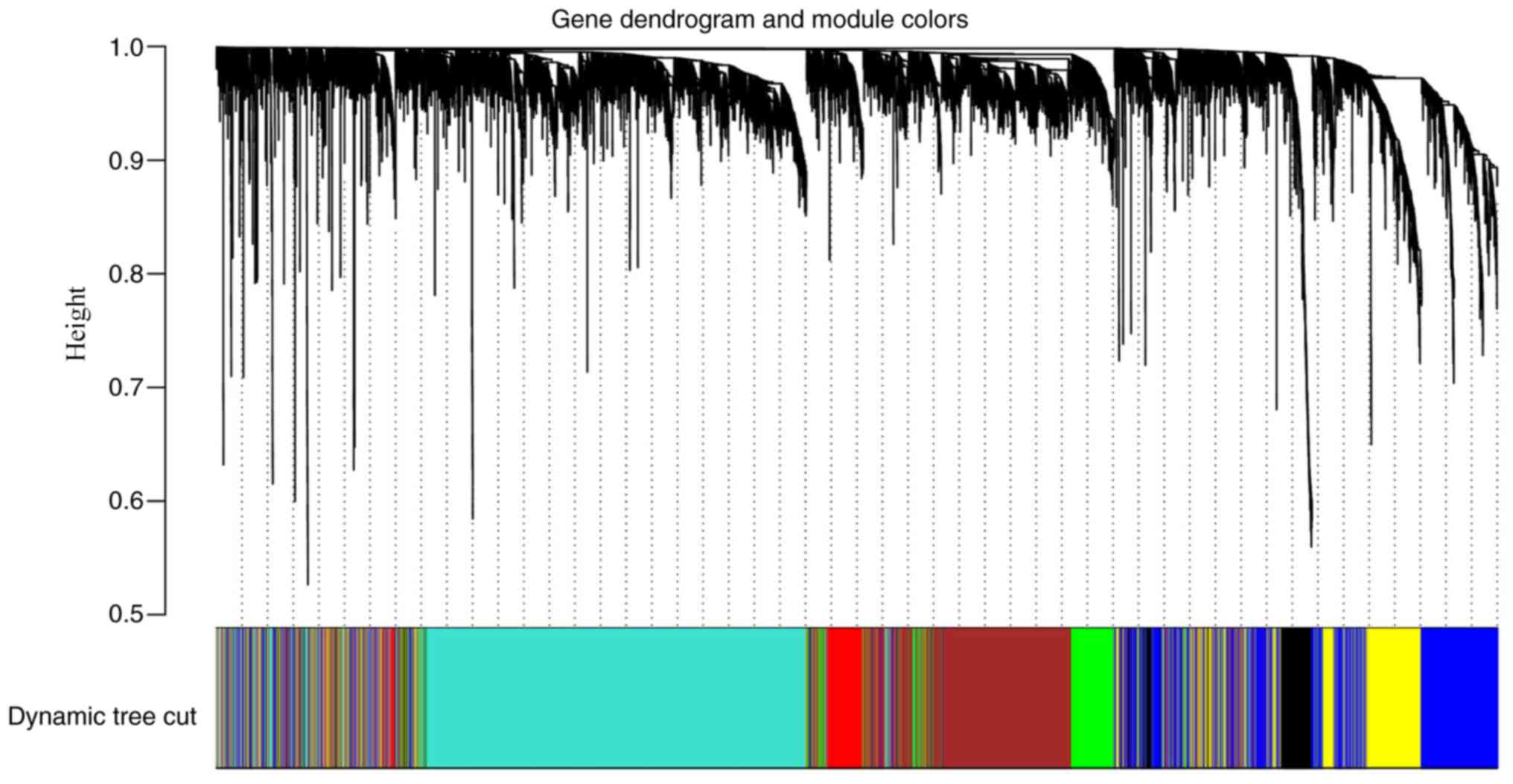
the dynamic tree cut algorithm in the R package WGCNA (version
1.66) (22), was applied to the
dendrogram for module identification with the mini-size of module
gene numbers set as 200.
Identification of clinically
significant modules
In the present study, the module eigengene (ME) was
used to represent each module, and was determined by the first
principal component obtained from the principal component analysis
of the expression matrix of each gene. In addition, the Pearson's
correlation between the ME of each module and clinical information
was defined as module significance (MS). The gene modules with an
ME >0.3 and an MS <0.05 were considered significant in
relation to clinical traits. All genes in these significant modules
were selected for further analysis.
Screening and functional annotation of
module hub genes
Hub genes are usually considered to be important in
the majority of biological processes in the gene module (24). In the present study, all genes in the
significant modules were screened to identify the hub genes, based
on a gene significance (GS) >0.2 and module membership (MM)
>0.8. The corresponding module hub gene information was
subsequently uploaded onto the Database for Annotation,
Visualization, and Integrated Discovery (DAVID; david.abcc.ncifcrf.gov) (version 6.8) to perform GO
(http://geneontology.org/) and KEGG (https://www.genome.jp/kegg/) analyses. P<0.05 was
used as the screening threshold.
PPI network construction and key gene
screening
The Search Tool for the Retrieval of Interacting
Genes (STRING; version 11.0; www.string-db.org) is a database that provides
information for known and predicted PPIs (25). In the present study, STRING was used
to analyze the PPIs among the module hub genes associated with
tumor TNM stage in patients with early-onset CRC. A confidence
score >0.4 was chosen to construct the PPI network, which was
subsequently visualized using Cytoscape software (version 3.7.0)
(26). Genes with ≥5 degrees were
considered to be the key genes in the PPI network (27).
Validation of key genes in The Cancer
Genome Atlas (TCGA) samples
The prognostic value of the key genes identified
from the analysis of the PPI network was tested in the publically
available TCGA-COAD (colon adenocarcinoma) dataset (https://portal.gdc.cancer.gov/). The TCGA-COAD
dataset contains 480 patients with CRC, after screening out
patients without information of diagnostic age, 419 patients were
included as the validation dataset, which contained 53 patients
with early-onset CRC and 366 patients with late-onset CRC. The
associations between the key genes and the prognosis of patients
with early-onset CRC were evaluated using Kaplan-Meier survival
analysis in Prism (version 6.01; GraphPad Software, Inc.). All the
patients were divided into high-expression and moderate-low
expression groups according to the quartile method. Kaplan-Meier
curves were constructed to determine the OS time and the log-rank
test was used to compare the survival distribution. In addition, to
further verify whether the key genes were uniquely associated with
early-but not late-onset CRC, survival analysis validation of the
key genes was performed using TCGA late-onset CRC samples.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Construction of the co-expression
network and identification of key modules
Following the exclusion of late-onset patients, 53
early-onset CRC samples and the corresponding clinical information
were used for WGCNA (Fig. 1) using a
soft power of β=6 as the soft threshold (Fig. 2). A total of seven co-expressed
modules were obtained, and genes, which were not assigned to a
specific module were distributed in the grey module (Fig. 3). The seven co-expressed modules were
used for further analysis.
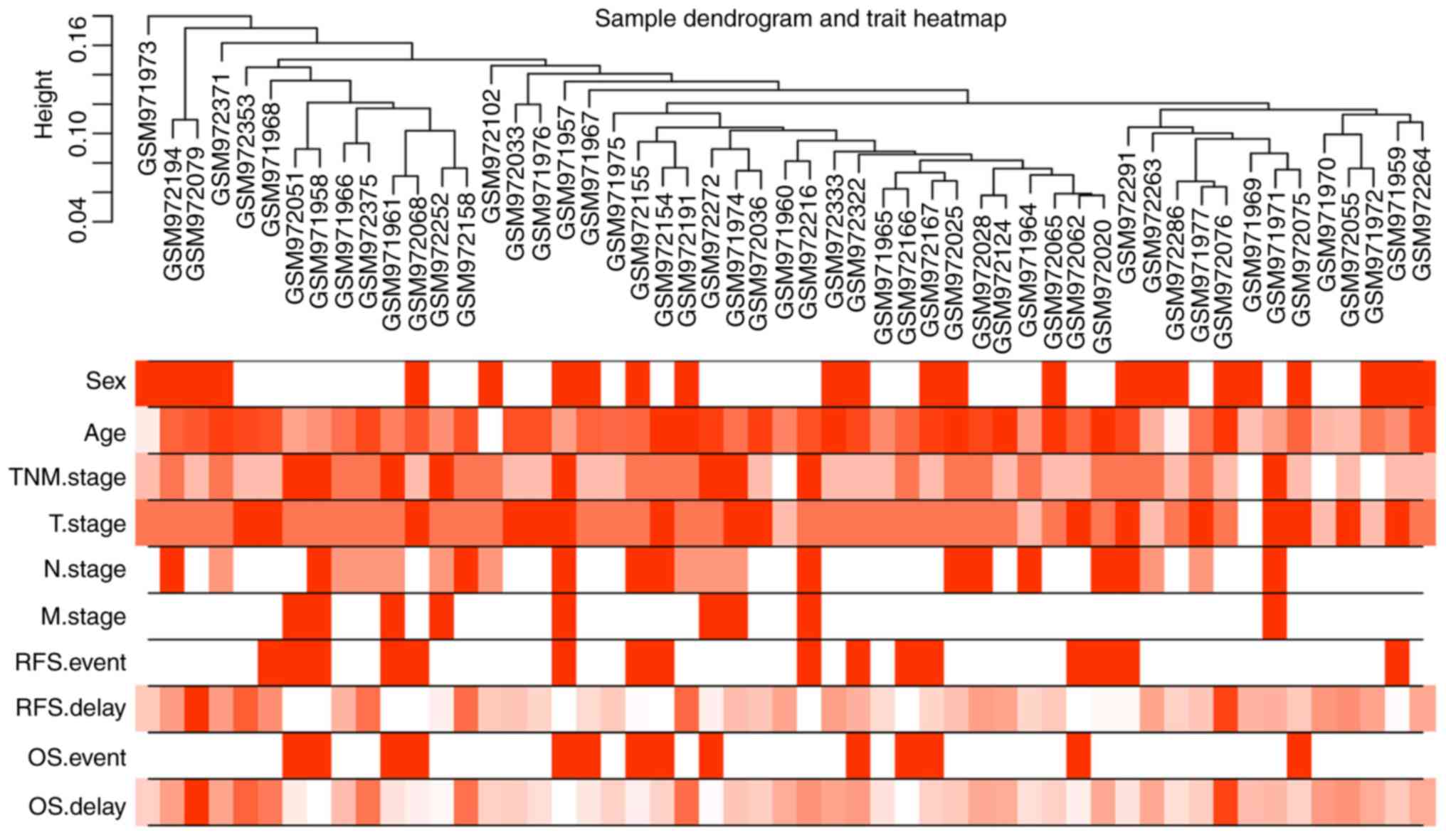 | Figure 1.Sample clustering tree and clinical
trait heat map of tumor samples. The cut height was set as 0.2 and
there was no deviated sample. The ten traits studied in the present
study included sex, age, TNM, TNM-T, TNM-N and TNM-M stage, RFS
survival event, RFS delay time, OS event and OS delay time. In the
heatmap of clinical traits, red color represents male and white
color represents female in sex; the red color depth of age, RFS
delay time and OS delay time is proportional to time; the red color
depth of TNM and TNM-T stage are divided into 4 scales according to
stage 1–4; the red color depth of TNM-N stage is divided into 3
scales according to stage 0–2; the red color depth of TNM-M stage
is divided into 2 scales according to stage 0–1; the red color
depth of RFS event and OS event are divided into 2 scales according
to whether the event occurs or not. TNM, tumor-node-metastasis;
RFS, recurrence-free survival; OS, overall survival. |
Identification of significant modules
and module hub genes
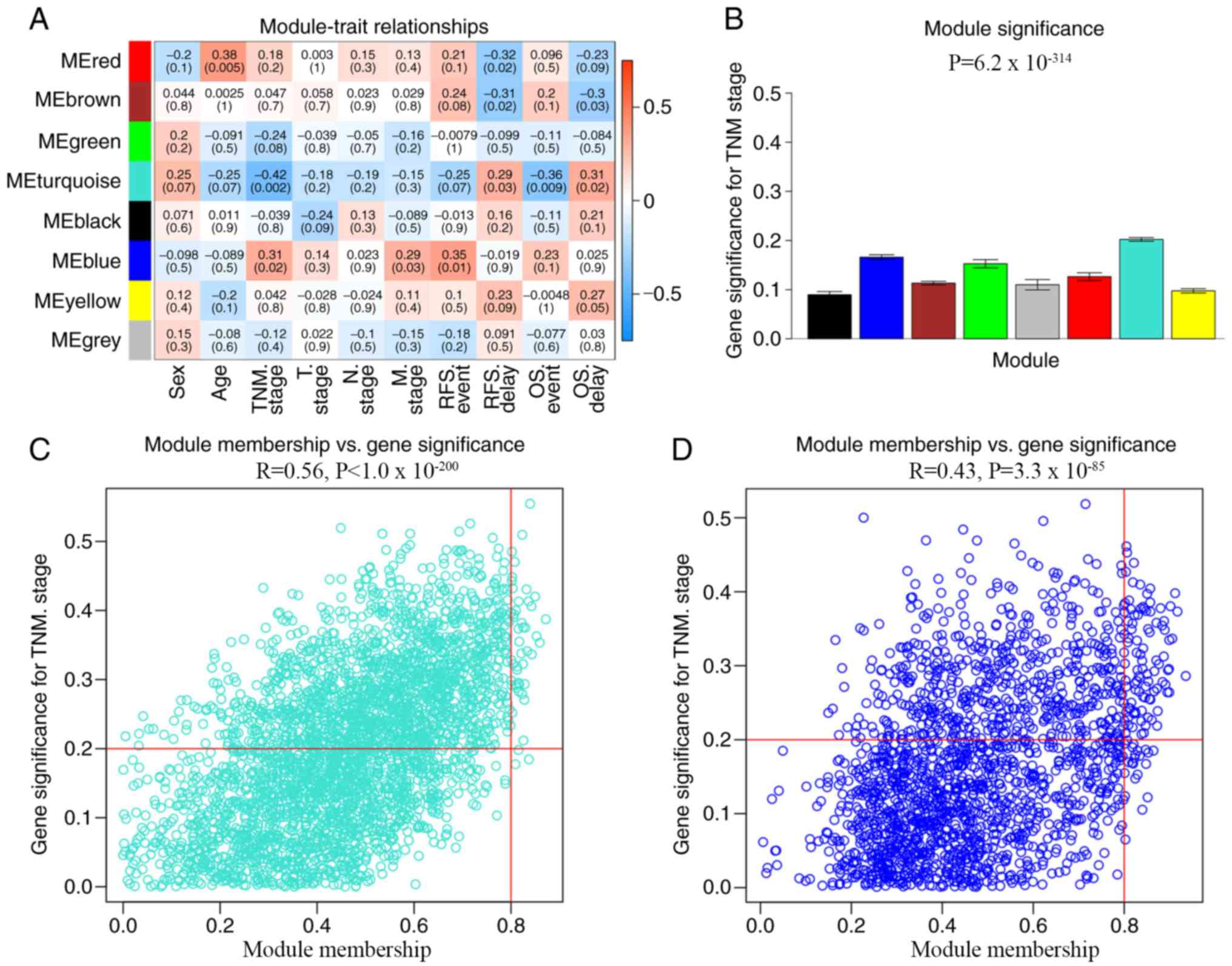
In the WGCNA, the association between modules and
clinical data was investigated using Pearson's correlation
analysis, and four modules were found to be significantly
associated with clinical traits. The red module was significantly
associated with age (R=0.38; P=0.005) and RFS delay time (R=−0.32;
P=0.02), while the brown module was significantly associated with
RFS delay time (R=−0.31; P=0.02) and OS delay time (R=−0.3;
P=0.03). The turquoise module was significantly associated with TNM
stage (R=−0.42; P=0.002), OS event (R=−0.31; P=0.009) and OS delay
time (R=0.31; P=0.02) and the blue module was significantly
associated with TNM stage (R=0.31; P=0.02) and RFS event (R=0.35;
P=0.01). Considering that TNM stage plays an important role in
evaluating the biological behavior and prognosis of CRC (28), the modules associated with TNM stage
were considered to be the most important modules in the present
study. The modules associated with TNM stage were the blue (R=0.43;
P<0.001) and turquoise (R=0.56; P<0.001) modules. The blue
and turquoise modules contained 1,874 and 3,331 genes,
respectively. All genes in the blue and turquoise modules were
screened based on a GS>0.2 and an MM>0.8 (Fig. 4). Finally, 95 blue and 45 turquoise
hub genes were screened for further functional analysis.
GO function and KEGG pathway
annotation of module hub genes
In order to better understand the biological
function of the hub genes in the two modules, the 140 hub genes
were uploaded onto the DAVID database for GO functional annotation
and KEGG pathway analysis. The genes were mainly enriched in four
GO terms: ‘mitochondrial large ribosomal subunit’, ‘structural
constituent of ribosome’, ‘poly(A) RNA binding’ and ‘collagen
binding and protein ubiquitination’. The KEGG pathway enrichment
analysis revealed that the module hub genes were significantly
involved in pathways associated with ribosomes.
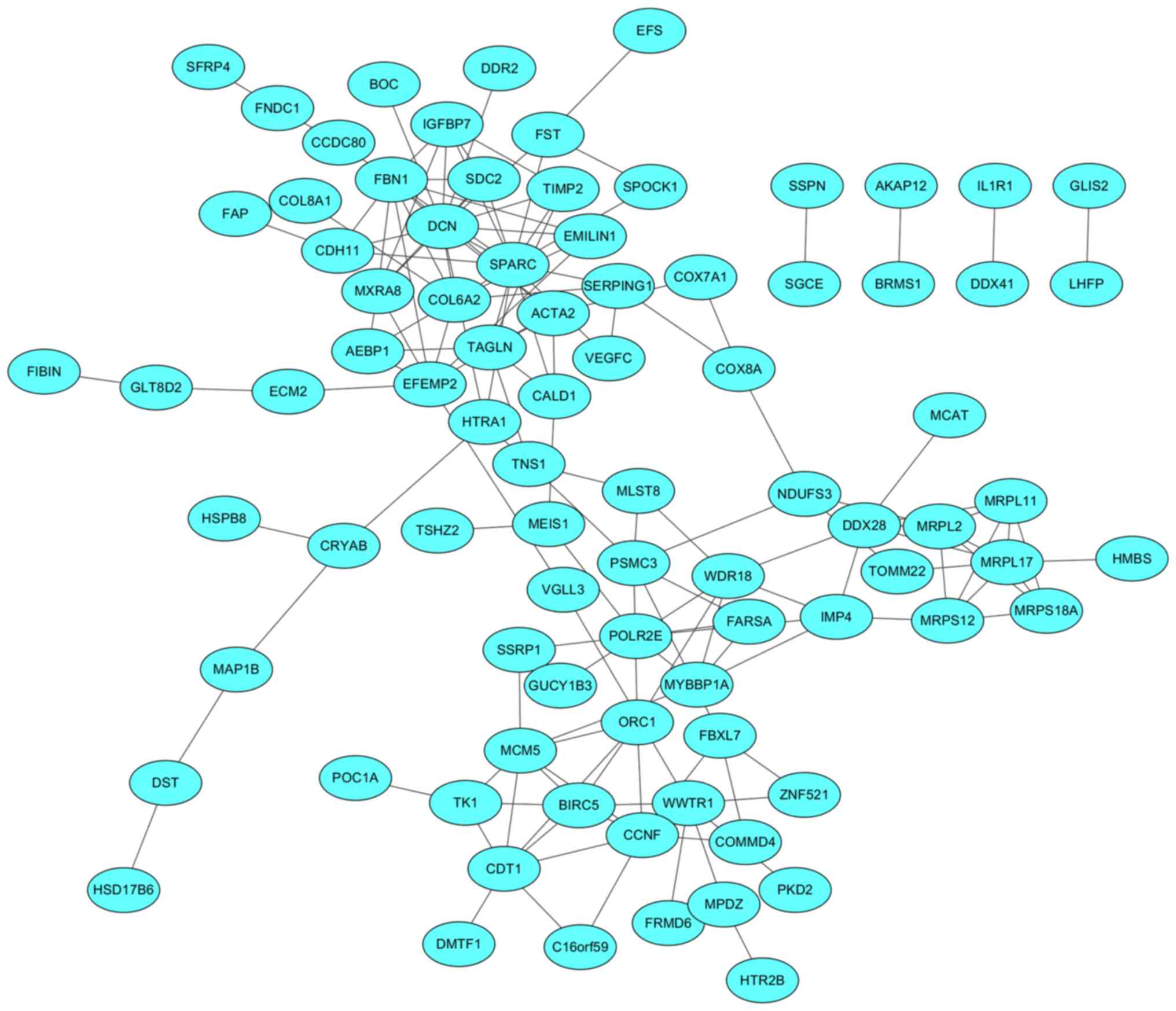
PPI network construction and module
analysis
The PPI network was composed of 85 nodes and 152
edges and was exported from the STRING database and visualized
using Cytoscape software (Fig. 5). A
total of 26 genes with ≥5 degrees were identified in the PPI
network and were selected for further validation using TCGA
samples.
Validation survival analysis of key
genes in TCGA samples
All 26 key genes [acetyl-CoA acetyltransferase 2,
baculoviral IAP repeat containing 5, cyclin F, chromatin licensing
and DNA replication factor 1, collagen type VI α 2 chain, cold
shock domain containing C2 (CSDC2), decorin (DCN), DEAD-box
helicase 28 (DDX28), EGF containing fibulin extracellular matrix
protein 2, fibrilin 1 (FBN1), insulin like growth factor binding
protein 7, IMP U3 small nucleolar ribonucleoprotein 4,
minichromosome maintenance complex component 5, mitochondrial
ribosomal protein L2, mitochondrial ribosomal protein L11,
mitochondrial ribosomal protein L17, mitochondrial ribosomal
protein S12, matrix remodeling associated 8, MYB binding protein
1a, origin recognition complex subunit 1, RNA polymerase II subunit
E, proteasome 26S subunit-ATPase 3, secreted protein acidic and
cysteine rich (SPARC), transgelin (TAGLN), WD repeat domain 1, WW
domain containing transcription regulator 1 (WWTR1)] identified
from the PPI network were analyzed using TCGA-COAD dataset. All
patients in TCGA-COAD dataset who were diagnosed before the age of
50 (n=53) were selected for survival analysis. Patients were
divided into high-expression (n=14) and moderate-low expression
(n=39) groups according to the quartile method. Kaplan-Meier curves
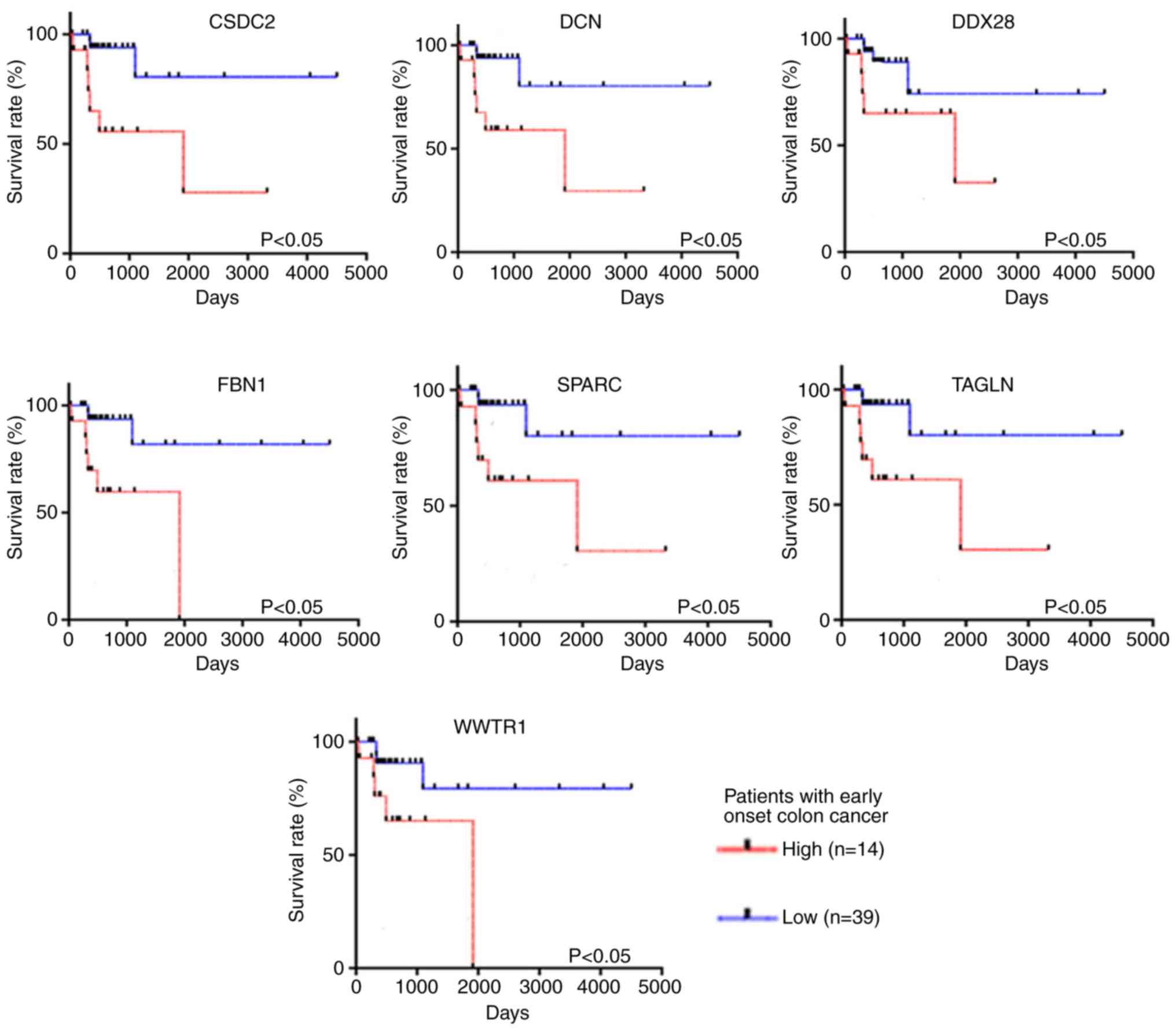
were constructed to determine the OS time. The results revealed
that seven genes, including SPARC, DCN, FBN1, WWTR1, TAGLN, DDX28
and CSDC2, remained statistically significant prognostic factors
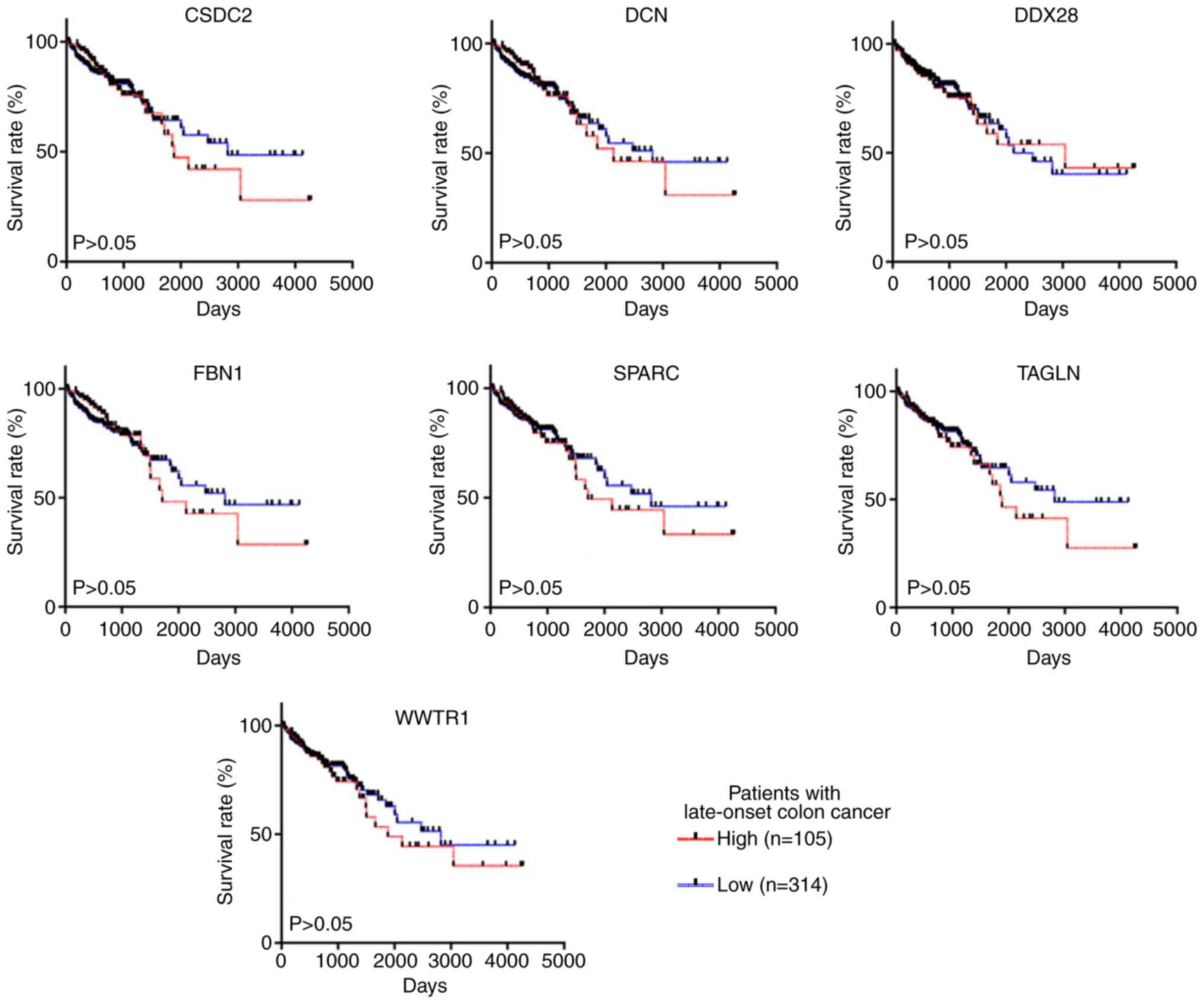
for patients with early-onset CRC (P<0.05; Fig. 6). On the other hand, the expression
level of these seven key genes was not significantly associated
with OS time in patients with late-onset CRC (P>0.05; Fig. 7).
Discussion
In contrast to the decreasing incidence of
late-onset CRC, the incidence and mortality of early-onset CRCs has
been increasing (29). According to
the latest data, patients with early-onset CRC account for 2–8% of
all CRC cases (30,31). Various studies have shown that
polygenic changes play a key role in the development of early-onset
CRC (10,32). However, there is a limited
understanding of the latent molecular mechanism in the development
and progression of CRC.
Gene expression data of patients with early-onset
CRC extracted from the GEO dataset GSE39582 were investigated in
the present study. WGCNA revealed that the functions of the genes
associated with TNM stage in early-onset CRC were enriched in
‘mitochondrial large ribosomal subunit’, ‘structural constituent of
ribosome’ and ‘poly (A) RNA binding’. PPI network analysis and
TCGA-COAD dataset revealed that there were seven genes with an
important role in the progression of early-onset CRC, including
SPARC, DCN, FBN1, WWTR1, TAGLN, DDX28 and CSDC2.
SPARC, a multifunctional calcium-binding
glycoprotein, belongs to a group of matricellular proteins
(33,34). Previous studies have shown that SPARC
is usually secreted into the extracellular matrix and plays a key
role in cellular processes, including proliferation, migration,
adhesion and differentiation (33,34).
SPARC is highly expressed in oral squamous cell carcinoma and has
the potential to promote oral squamous cell carcinoma cell
proliferation and metastasis (35).
Furthermore, the high expression level of SPARC indicated a poor
outcome in patients with esophageal squamous cell carcinoma
(36). Additionally, the expression
level of SPARC is associated with lymph node metastasis in
pancreatic cancer (37). However, a
study by Chew et al (38)
concluded that high SPARC expresion is associated with improved
disease outcome in stage II CRC and may be a prognostic indicator
of cancer-specific survival. DCN belongs to the small leucine-rich
proteoglycans family (39,40). Accumulating evidence has shown that
the DCN expression is dysregulated in several types of cancer,
including pancreatic and breast cancer (39,40). The
expression of DCN is decreased in renal cell carcinoma, and the
ectopic expression of DCN can decrease cell proliferation and
metastasis in renal cell carcinoma (41). Overexpression of DCN also has the
capacity to decrease CRC cell proliferation and migration by
increasing the expression of cyclin dependent kinase inhibitor 1A
and E-cadherin (42). However, the
present study revealed that DCN was associated with TNM stage, and
a high expression of DCN predicted poor prognosis in patients with
early-onset CRC. Therefore, further experimental studies are
required to investigate the role of DCN in early-onset CRC.
FBN1 encodes fibrillin, which is the primary
component of microfibrils in the extracellular matrix (43). A previous study reported that
downregulated FBN1 expression plays a key role in the development
of germ cell tumors (44). FBN1 is
also a target gene of microRNA (miR)-133b and promotes gastric
cancer cell proliferation and invasion (45). Moreover, hypermethylated FBN1 was
detected in patients with CRC but not in healthy controls, which
suggested that hypermethylated FBN1 is a sensitive biomarker for
CRC (46). WWTR1 is a
transcriptional coactivator with the capacity to combine with
various transcription factors and promote their effect (47). WWTR1 is highly expressed in renal
cancer and is associated with TNM stage (48). Furthermore, the nuclear localization
of WWTR1 is correlated with worse clinical outcomes in lung
squamous cell carcinomas compared to adjacent normal lung tissues
(49). Furthermore, another study
suggested that WWTR1 expression may serve as a prognostic indicator
and therapeutic target in CRC (50).
TAGLN is an actin stress fiber binding protein that stabilizes the
cytoskeleton through actin binding and plays a key role in cancer
cell migration, invasion and proliferation (51). A previous study demonstrated that the
expression level of TAGLN was decreased in bladder cancer compared
to normal bladder mucosae tissues (52). Exogenous TAGLN decreases the
migration and proliferation of CRC cells in vitro (53). However, in the present study,
patients with early-onset CRC with high TAGLN expression were found
to have a lower OS time compared with patients with moderate-low
expression. Therefore, TAGLN may be an oncogene in early-onset CRC.
DDX28, a conserved mitochondrial matrix protein, is essential for
mitochondrial oxidative phosphorylation (54). A previous study revealed that high
DDX28 expression was associated with high risk of CRC (55). CSDC2 is an mRNA-binding protein and a
target gene of miR-373 (56). CSDC2
plays a key role in decidua development (57). However, its role in cancer
development remains unknown.
In conclusion, the present study used WGCNA and
other analysis methods, including GO, KEGG, PPI network and
survival analysis, to identify seven genes (SPARC, DCN, FBN1,
WWTR1, TAGLN, DDX28 and CSDC2) associated with the development and
prognosis of early-onset CRC. These genes may serve as novel
biomarkers for the diagnosis of early-onset CRC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and analyzed during the
present study are available in the GEO database (http://www.ncbi.nlm.nih.gov/geo/) and TCGA
database (https://portal.gdc.cancer.gov/).
Authors' contributions
XM, ZS and HQ conceived and designed the study. XM,
BY and ZZ collected the data and wrote the manuscript. BY and SL
performed the data analysis. HQ contributed to the language
editing. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
GEO
|
Gene Expression Omnibus
|
|
RFS
|
recurrence-free survival
|
|
OS
|
overall survival
|
|
WGCNA
|
weighted gene co-expression
network
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
PPI
|
protein-protein interaction
|
References
|
1
|
Dienstmann R, Vermeulen L, Guinney J,
Kopetz S, Tejpar S and Tabernero J: Consensus molecular subtypes
and the evolution of precision medicine in colorectal cancer. Nat
Rev Cancer. 17:79–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Veenstra CM and Krauss JC: Emerging
systemic therapies for colorectal cancer. Clin Colon Rectal Surg.
31:179–191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gonzalez N, Prieto I, Del Puerto-Nevado L,
Portal-Nuñez S, Ardura JA, Corton M, Fernández-Fernández B,
Aguilera O, Gomez-Guerrero C, Mas S, et al: 2017 update on the
relationship between diabetes and colorectal cancer: Epidemiology,
potential molecular mechanisms and therapeutic implications.
Oncotarget. 8:18456–18485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jayasekara H, English DR, Haydon A, Hodge
AM, Lynch BM, Rosty C, Williamson EJ, Clendenning M, Southey MC,
Jenkins MA, et al: Associations of alcohol intake, smoking,
physical activity and obesity with survival following colorectal
cancer diagnosis by stage, anatomic site and tumor molecular
subtype. Int J Cancer. 142:238–250. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lichtenstein P, Holm NV, Verkasalo PK,
Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A and Hemminki
K: Environmental and heritable factors in the causation of
cancer-analyses of cohorts of twins from Sweden, Denmark, and
Finland. N Engl J Med. 343:78–85. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ballester V, Rashtak S and Boardman L:
Clinical and molecular features of young-onset colorectal cancer.
World J Gastroenterol. 22:1736–1744. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yeo H, Betel D, Abelson JS, Zheng XE,
Yantiss R and Shah MA: Early-onset colorectal cancer is distinct
from traditional colorectal cancer. Clin Colorectal Cancer.
16:293–299.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cavestro GM, Mannucci A, Zuppardo RA, Di
Leo M, Stoffel E and Tonon G: Early onset sporadic colorectal
cancer: Worrisome trends and oncogenic features. Dig Liver Dis.
50:521–532. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burnett-Hartman AN, Powers JD, Chubak J,
Corley DA, Ghai NR, McMullen CK, Pawloski PA, Sterrett AT and
Feigelson HS: Treatment patterns and survival differ between
early-onset and late-onset colorectal cancer patients: The patient
outcomes to advance learning network. Cancer Causes Control.
30:747–755. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Williams SP, Barthorpe AS, Lightfoot H,
Garnett MJ and McDermott U: High-throughput RNAi screen for
essential genes and drug synergistic combinations in colorectal
cancer. Sci Data. 4:1701392017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao M, Zhong A, Patel N, Alur C and Vyas
D: High throughput RNA sequencing utility for diagnosis and
prognosis in colon diseases. World J Gastroenterol. 23:2819–2825.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiong W, Ai YQ and Li YF, Ye Q, Chen ZT,
Qin JY, Liu QY, Wang H, Ju YH, Li WH and Li YF: Microarray analysis
of circular RNA expression profile associated with
5-fluorouracil-based chemoradiation resistance in colorectal cancer
cells. Biomed Res Int. 2017:84216142017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun G, Li Y, Peng Y, Lu D, Zhang F, Cui X,
Zhang Q and Li Z: Identification of differentially expressed genes
and biological characteristics of colorectal cancer by integrated
bioinformatics analysis. J Cell Physiol. 2019.(Epub ahead of
print). View Article : Google Scholar
|
|
16
|
He M, Lin Y and Xu Y: Identification of
prognostic biomarkers in colorectal cancer using a long non-coding
RNA-mediated competitive endogenous RNA network. Oncol Lett.
17:2687–2694. 2019.PubMed/NCBI
|
|
17
|
Zhang H, Dong S and Feng J: Epigenetic
profiling and mRNA expression reveal candidate genes as biomarkers
for colorectal cancer. J Cell Biochem. 120:10767–10776. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu C, Hong H, Zhang S, Zong Y, Ma J, Lu A,
Sun J and Zheng M: Identification of key genes and pathways
involved in microsatellite instability in colorectal cancer. Mol
Med Rep. 19:2065–2076. 2019.PubMed/NCBI
|
|
19
|
Marisa L, de Reynies A, Duval A, Selves J,
Gaub MP, Vescovo L, Etienne-Grimaldi MC, Schiappa R, Guenot D,
Ayadi M, et al: Gene expression classification of colon cancer into
molecular subtypes: Characterization, validation, and prognostic
value. PLoS Med. 10:e10014532013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miller JA, Cai C, Langfelder P, Geschwind
DH, Kurian SM, Salomon DR and Horvath S: Strategies for aggregating
gene expression data: the collapseRows R function. BMC
Bioinformatics. 12:3222011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giorgi FM, Bolger AM, Lohse M and Usadel
B: Algorithm-driven artifacts in median polish summarization of
microarray data. BMC Bioinformatics. 11:5532010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Langfelder P and Horvath S: WGCNA: An R
package for weighted correlation network analysis. BMC
Bioinformatics. 9:5592008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chung N, Zhang XD, Kreamer A, Locco L,
Kuan PF, Bartz S, Linsley PS, Ferrer M and Strulovici B: Median
absolute deviation to improve hit selection for genome-scale RNAi
screens. J Biomol Screen. 13:149–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Horvath S, Zhang B, Carlson M, Lu KV, Zhu
S, Felciano RM, Laurance MF, Zhao W, Qi S, Chen Z, et al: Analysis
of oncogenic signaling networks in glioblastoma identifies ASPM as
a molecular target. Proc Natl Acad Sci USA. 103:17402–17407. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu C, Chen N, Huang K, Jiang M, Liang H,
Sun Z, Tian J and Wang D: Identifying hub genes and potential
mechanisms associated with senescence in human annulus cells by
gene expression profiling and bioinformatics analysis. Mol Med Rep.
17:3465–3472. 2018.PubMed/NCBI
|
|
28
|
Kawakami H, Zaanan A and Sinicrope FA:
Microsatellite instability testing and its role in the management
of colorectal cancer. Curr Treat Options Oncol. 16:302015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Connell LC, Mota JM, Braghiroli MI and
Hoff PM: The rising incidence of younger patients with colorectal
cancer: Questions about screening, biology, and treatment. Curr
Treat Options Oncol. 18:232017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Siegel RL, Jemal A and Ward EM: Increase
in incidence of colorectal cancer among young men and women in the
United States. Cancer Epidemiol Biomarkers Prev. 18:1695–1698.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stigliano V, Sanchez-Mete L, Martayan A
and Anti M: Early-onset colorectal cancer: A sporadic or inherited
disease? World J Gastroenterol. 20:12420–12430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vaz J, Ansari D, Sasor A and Andersson R:
SPARC: A potential prognostic and therapeutic target in pancreatic
cancer. Pancreas. 44:1024–1035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng J and Tang L: SPARC in tumor
pathophysiology and as a potential therapeutic target. Curr Pharm
Des. 20:6182–6190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jing Y, Jin Y, Wang Y, Chen S, Zhang X,
Song Y, Wang Z, Pu Y, Ni Y and Hu Q: SPARC promotes the
proliferation and metastasis of oral squamous cell carcinoma by
PI3K/AKT/PDGFB/PDGFRβ axis. 2019.(Epub ahead of print). View Article : Google Scholar
|
|
36
|
Chen Y, Zhang Y, Tan Y and Liu Z: Clinical
significance of SPARC in esophageal squamous cell carcinoma.
Biochem Biophys Res Commun. 492:184–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu XZ, Guo ZY, Di Y, Yang F, Ouyang Q, Fu
DL and Jin C: The relationship between SPARC expression in primary
tumor and metastatic lymph node of resected pancreatic cancer
patients and patients' survival. Hepatobiliary Pancreat Dis Int.
16:104–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chew A, Salama P, Robbshaw A, Klopcic B,
Zeps N, Platell C and Lawrance IC: SPARC, FOXP3, CD8 and CD45
correlation with disease recurrence and long-term disease-free
survival in colorectal cancer. PLoS One. 6:e220472011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Neill T, Schaefer L and Iozzo RV: Decorin
as a multivalent therapeutic agent against cancer. Adv Drug Deliv
Rev. 97:174–185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang W, Ge Y, Cheng Q, Zhang Q, Fang L
and Zheng J: Decorin is a pivotal effector in the extracellular
matrix and tumour microenvironment. Oncotarget. 9:5480–5491.
2018.PubMed/NCBI
|
|
41
|
Ho TH, Serie DJ, Parasramka M, Cheville
JC, Bot BM, Tan W, Wang L, Joseph RW, Hilton T, Leibovich BC, et
al: Differential gene expression profiling of matched primary renal
cell carcinoma and metastases reveals upregulation of extracellular
matrix genes. Ann Oncol. 28:604–610. 2017.PubMed/NCBI
|
|
42
|
Bi X, Pohl NM, Qian Z, Yang GR, Gou Y,
Guzman G, Kajdacsy-Balla A, Iozzo RV and Yang W: Decorin-mediated
inhibition of colorectal cancer growth and migration is associated
with E-cadherin in vitro and in mice. Carcinogenesis. 33:326–330.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Milewicz DM, Guo DC, Tran-Fadulu V, Lafont
AL, Papke CL, Inamoto S, Kwartler CS and Pannu H: Genetic basis of
thoracic aortic aneurysms and dissections: Focus on smooth muscle
cell contractile dysfunction. Annu Rev Genomics Hum Genet.
9:283–302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cierna Z, Mego M, Jurisica I, Machalekova
K, Chovanec M, Miskovska V, Svetlovska D, Kalavska K, Rejlekova K,
Kajo K, et al: Fibrillin-1 (FBN-1) a new marker of germ cell
neoplasia in situ. BMC Cancer. 16:5972016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang D, Zhao D and Chen X: MiR-133b
inhibits proliferation and invasion of gastric cancer cells by
up-regulating FBN1 expression. Cancer Biomark. 19:425–436. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Guo Q, Song Y, Zhang H, Wu X, Xia P and
Dang C: Detection of hypermethylated fibrillin-1 in the stool
samples of colorectal cancer patients. Med Oncol. 30:6952013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wei J, Wang L, Zhu J, Sun A, Yu G, Chen M,
Huang P, Liu H, Shao G, Yang W and Lin Q: The Hippo signaling
effector WWTR1 is a metastatic biomarker of gastric cardia
adenocarcinoma. Cancer Cell Int. 19:742019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ruan H, Bao L, Song Z, Wang K, Cao Q, Tong
J, Cheng G, Xu T, Chen X, Liu D, et al: High expression of TAZ
serves as a novel prognostic biomarker and drives cancer
progression in renal cancer. Exp Cell Res. 376:181–191. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang Y, Han Y, Guo Z, Yang Y and Ren T:
Nuclear TAZ activity distinctly associates with subtypes of
non-small cell lung cancer. Biochem Biophys Res Commun.
509:828–832. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yuen HF, McCrudden CM, Huang YH, Tham JM,
Zhang X, Zeng Q, Zhang SD and Hong W: TAZ expression as a
prognostic indicator in colorectal cancer. PLoS One. 8:e542112013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dos Santos Hidalgo G, Meola J, Rosa E
Silva JC, Paro de Paz CC and Ferriani RA: TAGLN expression is
deregulated in endometriosis and may be involved in cell invasion,
migration, and differentiation. Fertil Steril. 96:700–703. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu Y, Wu X, Wang G, Hu S, Zhang Y and
Zhao S: CALD1, CNN1, and TAGLN identified as potential prognostic
molecular markers of bladder cancer by bioinformatics analysis.
Medicine (Baltimore). 98:e138472019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li Q, Shi R, Wang Y and Niu X: TAGLN
suppresses proliferation and invasion, and induces apoptosis of
colorectal carcinoma cells. Tumor Biol. 34:505–513. 2013.
View Article : Google Scholar
|
|
54
|
Tu YT and Barrientos A: The Human
mitochondrial DEAD-box protein DDX28 resides in RNA granules and
functions in mitoribosome assembly. Cell Rep. 10:854–864. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Loo LW, Cheng I, Tiirikainen M, Lum-Jones
A, Seifried A, Dunklee LM, Church JM, Gryfe R, Weisenberger DJ,
Haile RW, et al: cis-Expression QTL analysis of established
colorectal cancer risk variants in colon tumors and adjacent normal
tissue. PLoS One. 7:e304772012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Place RF, Li LC, Pookot D, Noonan EJ and
Dahiya R: MicroRNA-373 induces expression of genes with
complementary promoter sequences. Proc Natl Acad Sci USA.
105:1608–1613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Vallejo G, Mestre-Citrinovitz AC,
Winterhager E and Saragueta PE: CSDC2, a cold shock domain
RNA-binding protein in decidualization. J Cell Physiol.
234:740–748. 2018. View Article : Google Scholar : PubMed/NCBI
|