Introduction
Ovarian cancer (OC) has the highest mortality rate
of all gynecological malignancies, with ~14,240 OC-associated
mortalities reported in the USA in 2016 (1). Due to a lack of methods for early
detection, almost 70% of patients present with an advanced stage of
OC at diagnosis, resulting in a poor prognosis (2). Therefore, the identification of
potential markers and improved understanding regarding the
molecular mechanisms underlying OC may facilitate the development
of early detection approaches and novel therapeutic strategies for
patients with OC.
The 26S proteasome non-ATPase regulatory subunit 10
(p28GANK), which is localized on human chromosome Xq22.3, is a
small protein of 226 amino acids containing 7 ankyrin repeats. This
protein is a subunit of the 26S proteasome that specifically
interacts with the S6b ATPase of the 19S regulator (3–5). p28GANK
promotes the hyperphosphorylation and degradation of retinoblastoma
(Rb), which releases the transcription factor E2F-1 from the Rb
repressor complex. Furthermore, p28GANK binds to cyclin-dependent
kinase 4 (CDK4) and prevents CDK4 inhibition by p16NK4a, which
accelerates cell cycle progression (5). p28GANK binds to the E3 ubiquitin
protein ligase MDM2, which enhances the ubiquitination and
degradation of cellular tumor antigen p53 (6). In addition, it has also been
demonstrated to regulate the nuclear factor-κB (7), signal transducer and activator of
transcription 3 (STAT3) (8) and
protein kinase B (9) pathways.
p28GANK serves an important role in cell metastasis, proliferation
and autophagy in cancer (8,10,11).
However, to the best of our knowledge, there is limited
understanding regarding its expression and clinical significance in
OC, which the present study aimed to investigate.
Materials and methods
Patients
OC tissue samples were obtained from who were
surgically treated at the Third Affiliated Hospital of Harbin
Medical University (Harbin, China) between January 1999 and 2006.
The exclusion criteria were as follows: i) Multifocal carcinoma;
ii) loss to follow-up [no overall survival (OS) follow-up and/or
progression data]; and iii) previous history of cancer. Inclusion
criteria were as follows: i) Presence of ovarian serous carcinoma
confirmed by pathological examination; ii) complete basic clinical
data; iii) absence of any prior treatment for cancer; iv) no
serious complications or any other malignant disease; v) informed
consent was provided by the patients and family members prior to
treatment; and vi) complete cytoreductive surgery with no peri- or
postoperative (within 45 days after surgery) mortality. The cohort
for analysis that met the inclusion criteria consisted of 114
patients. The mean age of the patients was 49 years (range, 28–76
years). Additionally, normal ovarian tissue samples were acquired
by endoscopy from non-tumor areas from 30 of the patients with OC
enrolled in the study. All tissue specimens used in the current
study were obtained with written informed consent from the
patients. The study protocol was approved by the Ethics Committee
of Harbin Medical University.
Immunohistochemistry (IHC)
IHC was performed following standard procedures as
described previously (12). All
samples were confirmed to contain >80% tumor cells by three
pathologists. Formalin-fixed and paraffin-embedded tissue sections
(4-µm thick) were dried out at 65°C for 2 h. Samples were
deparaffinized in xylene, and hydrated using a graded alcohol
series (70%, overnight; 80, 90 and 95% for 1 h each; and two
separate 10 min treatments in 100%. Subsequently, the tissue blocks
were placed in xylene for 10 min until the specimen were
transparent, after which the tissue blocks were placed in xylene +
paraffin and at 60°C for 2 h, to ensure the paraffin completely
penetrated the tissue. The dissolved paraffin was poured into a
metal frame, and the waxed tissue was placed in the center of the
metal frame and left to cool. The tissue was sectioned into 5 µM
thick slices and placed in a dish with warm water. The unfolded
tissue slices were placed on slides and dried at 60°C 4 h. The
sections were then treated with 3% H2O2 at
room temperature for 10 min and antigen retrieval was performed in
citrate buffer (pH 6.0) at 98°C for 10 min. The tissue sections
were incubated with anti-p28GANK mouse polyclonal antibodies (cat.
no. ab238999; 1:200 dilution; Abcam, Cambridge, UK) at 4°C
overnight. Following washing in PBS, the samples were incubated
with biotinylated secondary antibody (cat. no. 14709; 1:1,500
dilution; Cell Signaling Technology, Inc., Danvers, MA, USA) at
room temperature for 30 min and developed with diaminobenzadine at
room temperature for 5 min. As a negative staining control, the
primary antibody was replaced with PBS.
Each section was incubated in a 1:50 dilution of
anti-p28GANK antibody. An optical microscope (magnification, ×400)
was used for assessment of IHC. The IHC reaction was quantified by
multiplying the staining intensity by the percentage of positive
tumor cells. In the cytoplasm, staining intensity was graded as 0
(no staining), 1 (weak staining), 2 (moderate staining) or 3
(strong staining). The percentage of the extent of reactivity was
scored as follows: 0 (no positive tumor cells), 1 (<10%), 2
(10–50%) and 3 (>50%). Each case was scored independently and in
a blinded manner by two investigators. Final scores ≤4 were
regarded as low expression and the remainder were classified as
high expression.
Follow up
All patients were followed until February 2015,
unless they succumbed prior to this date. The OS time was defined
as the interval from surgery to mortality or the date of the most
recent follow-up. Progression-free survival (PFS) time was
calculated from the date of surgery to the date of relapse,
including proven local recurrence or distant metastasis, or the
date of the most recent follow-up.
PrognoScan database analysis
The association between p28GANK expression and
survival in OC was analyzed using the PrognoScan database (13) PrognoScan searches for associations
between gene expression and patient prognosis, including OS and
disease-free survival (DFS), across a large collection of publicly
available cancer microarray datasets, were performed. The threshold
was adjusted to a Cox P-value <0.05.
GEPIA database analysis
Gene Expression Profiling Interactive Analysis
(GEPIA) (14) is a newly developed
interactive web server for analyzing RNA sequencing expression data
from The Cancer Genome Atlas (TCGA) database (15). The expression and survival analysis
of p28GANK was performed using GEPIA online software version 1.0.
OC and matched normal TCGA data and Genotype-Tissue Expression
(GTEx) data were used for the expression analysis (16), and log2 (TPM+1) was used
for the log-scale.
Statistical analysis
Statistical analysis was performed using SPSS
(version 18.0; SPSS Inc., Chicago, IL, USA). A Mann Whitney-U test
was used to compare two independent non-parametric samples.
χ2 tests were used to assess associations between
p28GANK expression level and clinicopathological features.
Kaplan-Meier survival curves and log-rank tests were used for
survival analysis. Prognostic factors were evaluated by univariate
and multivariate analyses using Cox proportional hazard regression
models. P<0.05 was considered to indicate a statistically
significant difference. Kaplan-Meier survival analysis was used on
the Gene Expression Omnibus dataset (GSE26712) using PrognoScan
(12). Histological grading was
based on the World Health Organization Histological grading system
(17).
Results
p28GANK expression in OC and normal
tissues
The expression level of p28GANK was higher in the
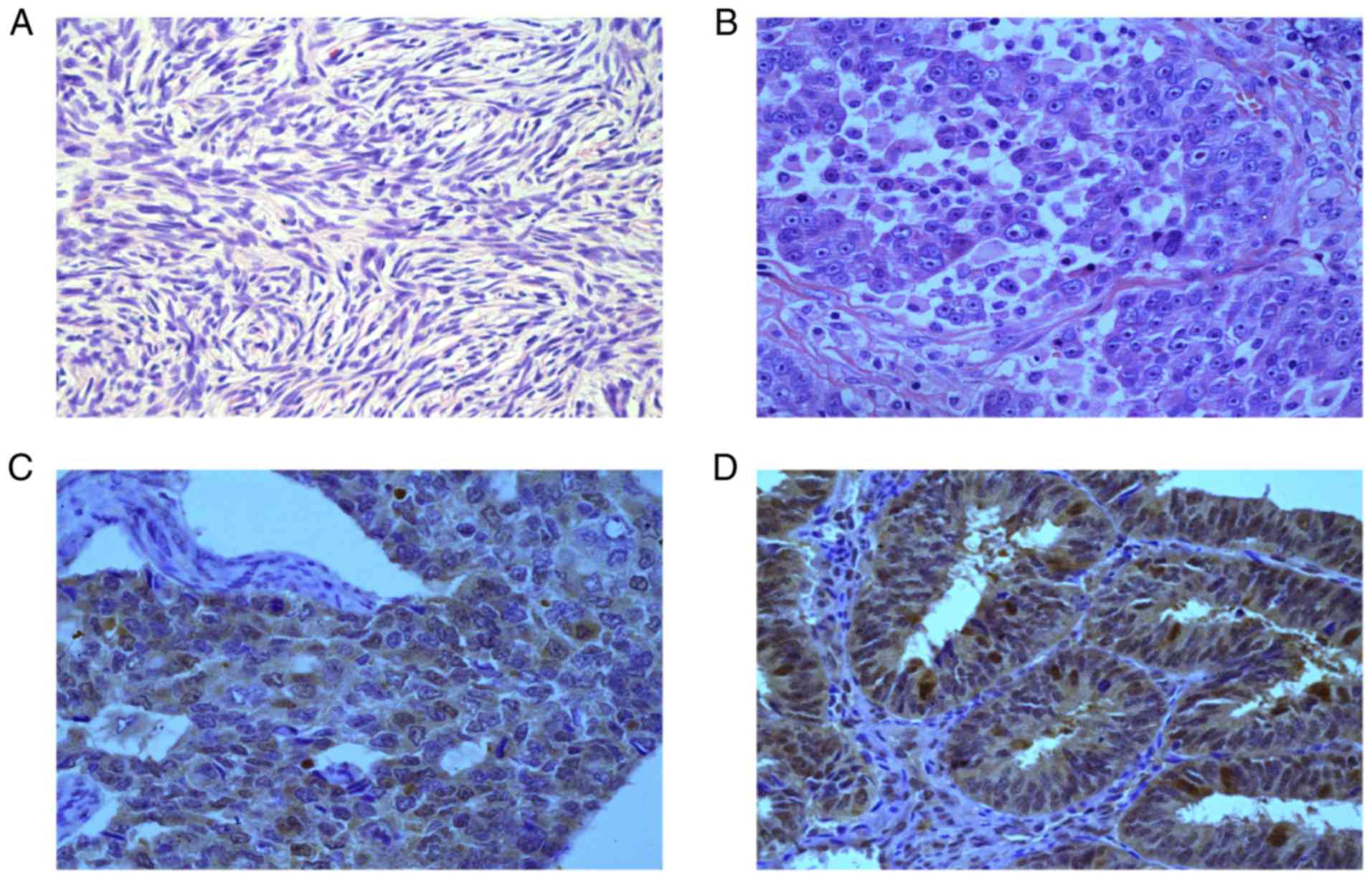
cytoplasm of cancer cells compared with normal tissues (Fig. 1). In the 30 normal controls, only 10
samples (33.3%) exhibited a positive p28GANK cytoplasmic staining.
By contrast, of the 114 OC specimens, positive cytoplasmic
expression of p28GANK was identified in 72 samples (63.2%;
P<0.001; Table I).
 | Table I.p28GANK expression in normal and
ovarian cancer tissue samples. |
Table I.
p28GANK expression in normal and
ovarian cancer tissue samples.
|
| p28GANK expression, n
(%) |
|
|---|
|
|
|
|
|---|
| Category | Low | High | P-value |
|---|
| Tissue type |
|
| <0.001 |
|
Normal | 20 (66.7) | 10 (33.3) |
|
|
Tumor | 42 (36.8) | 72 (63.2) |
|
p28GANK overexpression is associated
with clinicopathological features
The association between the protein expression of
p28GANK and clinicopathological variables of OC are presented in
Table II. p28GANK expression was
significantly associated with the International Federation of
Gynecology and Obstetrics (FIGO) stage (18) (P=0.042), residual tumor size
(P=0.005) and response to chemotherapy (P<0.001). No significant
associations were identified between p28GANK expression and the
other clinicopathological factors.
 | Table II.Association between p28GANK expression
and clinicopathological factors and of samples from patients with
ovarian cancer (n=114). |
Table II.
Association between p28GANK expression
and clinicopathological factors and of samples from patients with
ovarian cancer (n=114).
|
|
| p28GANK expression, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Variable | n | Low | High | P-value |
|---|
| Age, years |
|
|
| 0.598 |
|
<50 | 36 | 12 (33.3) | 24 (66.7) |
|
| ≥50 | 78 | 30 (38.5) | 48 (61.5) |
|
| Omental
metastasis |
|
|
| 0.064 |
| No | 27 | 14 (51.9) | 13 (48.1) |
|
|
Yes | 87 | 28 (32.2) | 59 (67. 8) |
|
| Lymph node
metastasis |
|
|
| 0.765 |
| No | 101 | 38 (37.6) | 63 (62.4) |
|
|
Yes | 13 | 4 (30.8) | 9 (69.2) |
|
| FIGO stage |
|
|
| 0.042a |
|
I/II | 17 | 10 (58.8) | 7 (41.2) |
|
|
III/IV | 97 | 32 (33.0) | 65 (67.0) |
|
| Histological
grade |
|
|
| 0.553 |
| G1 | 28 | 9 (32.1) | 19 (67.9) |
|
|
G2/G3 | 86 | 33 (38.4) | 53 (61.6) |
|
| Residual disease,
cm |
|
|
| 0.005a |
| ≤2 | 98 | 41 (41.8) | 57 (50.0) |
|
|
>2 | 16 | 1 (6.3) | 15 (93.8) |
|
| Ascites |
|
|
| 0.841 |
| No | 34 | 13 (38.2) | 21 (61.8) |
|
|
Yes | 80 | 29 (36.3) | 51 (63.8) |
|
| CA-125, U/ml |
|
|
| 1.000 |
|
≤35 | 12 | 4 (33.3) | 8 (66.7) |
|
|
>35 | 102 | 38 (37.3) | 64 (62.7) |
|
| Response to
chemotherapy |
|
|
|
<0.001a |
|
Resistant | 39 | 5 (12.8) | 34 (87.2) |
|
|
Sensitive | 75 | 37 (49.3) | 38 (50.7) |
|
| Histological
classification |
|
|
| 0.893 |
|
Serous | 71 | 30 (42.3) | 41 (57.7) |
|
|
Mucinous | 18 | 6 (33.3) | 12 (66.7) |
|
|
Endometrioid | 20 | 9 (45.0) | 11 (55.0) |
|
| Clear
cell | 5 | 2 (40.0) | 3 (60.0) |
|
Association between p28GANK expression
and survival time of patients with OC
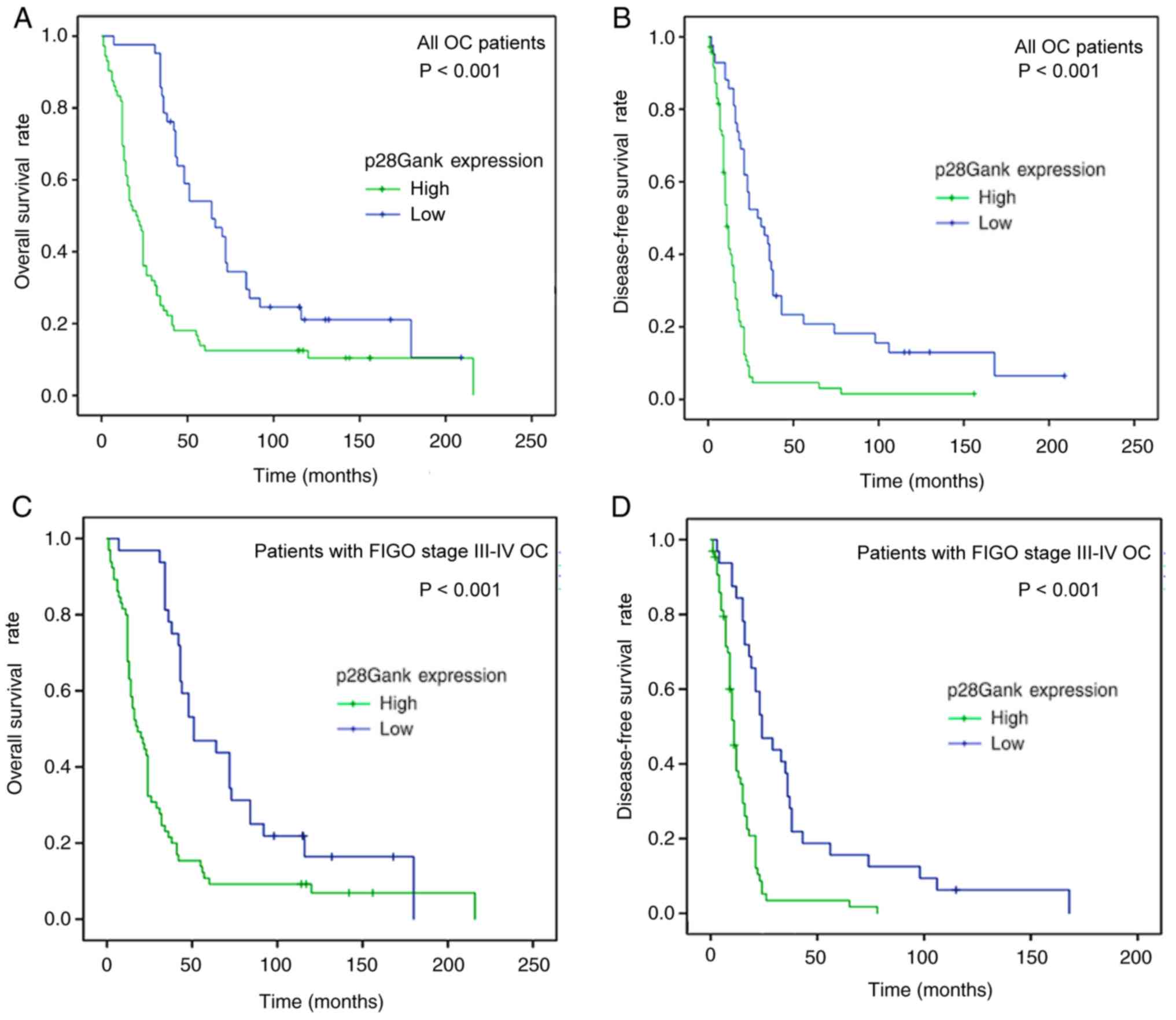
The 5-year survival curves stratified by p28GANK
expression are presented in Fig. 2.
p28GANK expression was revealed to be significantly associated with
OS (P<0.001) and DFS (P<0.001). A high expression level of
p28GANK was associated with worse OS and DFS times for patients
with OC. In addition, a FIGO-stratified analysis of all patients
was performed according to the level of p28GANK expression. The
results revealed that high expression of p28GANK was associated
with DFS and OS in patients with FIGO III and IV OC (both
P<0.001).
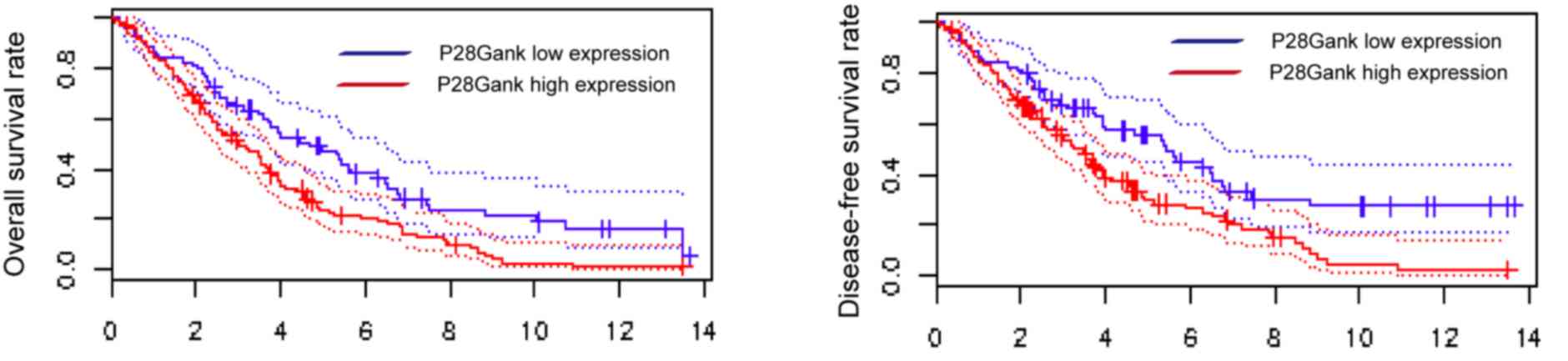
The Kaplan-Meier survival analysis demonstrated that
patients with high expression of p28GANK had significantly shorter
DFS (P=0.038) and OS (P=0.037) times using the Gene Expression
Omnibus dataset (GSE26712) from PrognoScan (12) (Fig.
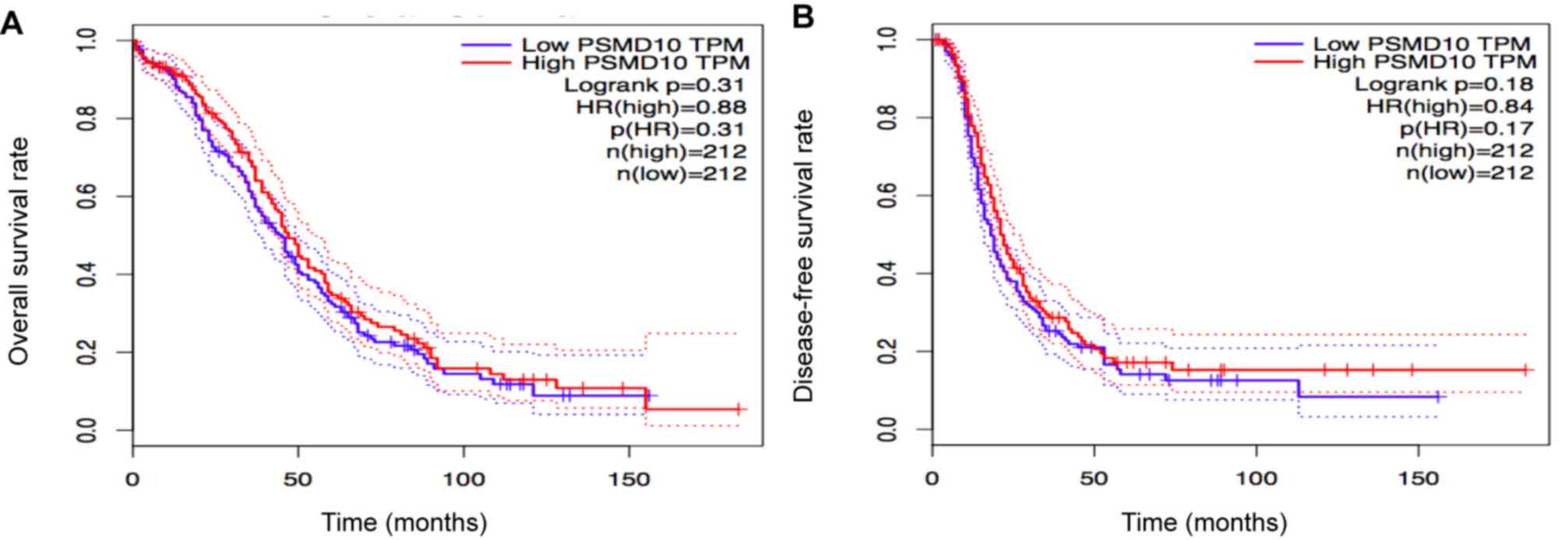
3). It was identified that p28GANK had no significant impact on
and DFS (P=0.18) and OS time (P=0.31; Fig. 4).
Univariate and multivariate analyses
of prognostic variables in patients with OC
Using univariate analysis, OS and DFS times were
revealed to be significantly associated with high p28GANK
expression (both P<0.001), omental metastasis (P=0.011 and
P=0.001, respectively), FIGO stage (P=0.007 and P=0.005,
respectively), residual tumor size (both P<0.001) and response
to chemotherapy (both P<0.001) (Table III).
 | Table III.Univariate and multivariate analyses
for overall survival. |
Table III.
Univariate and multivariate analyses
for overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years (<50
vs. ≥50) | 1.353 | 0.867–2.113 | 0.183 | 1.052 | 0.660–1.676 | 0.832 |
| Omental metastasis
(no vs. yes) | 1.926 | 1.163–3.190 | 0.011a | 1.271 | 0.603–2.682 | 0.528 |
| Lymph node
metastasis (absent vs. present) | 1.130 | 0.617–2.071 | 0.692 | 1.399 | 0.700–2.797 | 0.342 |
| FIGO stage (I/II
vs. III/IV) | 2.480 | 1.285–4.787 | 0.007a | 1.434 | 0.558–3.687 | 0.454 |
| Histological grade
(G1 vs. G2/G3) | 1.156 | 0.718–1.862 | 0.550 | 1.622 | 0.972–2.706 | 0.064 |
| Residual disease,
cm (≤2 vs. >2) | 6.715 | 3.748–12.032 |
<0.001a | 2.408 | 1.237–4.687 | 0.010a |
| Ascites (no vs.
yes) | 1.464 | 0.938–2.285 | 0.093 | 1.184 | 0.729–1.922 | 0.495 |
| CA-125, U/ml (≤35
vs. >35) | 1.125 | 0.564–2.243 | 0.739 | 0.794 | 0.379–1.665 | 0.542 |
| Response to
chemotherapy (resistant vs. sensitive) | 0.202 | 0.130–0.315 |
<0.001a | 0.276 | 0.161–0.472 |
<0.001a |
| p28GANK (low vs.
high) | 2.535 | 1.650–3.895 |
<0.001a | 1.818 | 1.137–2.908 | 0.013a |
The multivariate analysis revealed that high
expression of p28GANK (P=0.013), residual tumor size (P<0.010)
and response to chemotherapy (P=0.015) were independent prognostic
factors for OS in patients with OC (Table IV). Furthermore, it indicated that
high expression of p28GANK (P=0.001), histological grade (P=0.010),
residual tumor size (P=0.009) and response to chemotherapy
(P<0.001) were independent prognostic factors for DFS time in
these patients (Table IV).
 | Table IV.Univariate and multivariate analyses
for disease-free survival. |
Table IV.
Univariate and multivariate analyses
for disease-free survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years (<50
vs. ≥50) | 1.184 | 0.773–1.813 | 0.438 | 0.982 | 0.632–1.523 | 0.934 |
| Omental metastasis
(no vs. yes) | 2.286 | 1.389–3.761 | 0.001a | 1.735 | 0.806–3.738 | 0.159 |
| Lymph node
metastasis (absent vs. present) | 0.960 | 0.535–1.721 | 0.890 | 0.865 | 0.444–1.683 | 0.699 |
| FIGO stage (I/II
vs. III/IV) | 2.395 | 1.301–4.409 | 0.005a | 1.036 | 0.418–2.564 | 0.940 |
| Histological grade
(G1 vs. G2/G3) | 1.305 | 0.810–2.101 | 0.274 | 1.962 | 1.174–3.278 | 0.010a |
| Residual disease,
cm (≤2 vs. >2) | 7.351 | 3.980–13.577 |
<0.001a | 2.709 | 1.277–5.747 | 0.009a |
| Ascites (no vs.
yes) | 1.412 | 0.921–2.165 | 0.114 | 1.077 | 0.676–1.718 | 0.754 |
| CA-125, U/ml (≤35
vs. >35) | 1.579 | 0.792–3.147 | 0.194 | 1.443 | 0.676–3.080 | 0.343 |
| Response to
chemotherapy (resistant vs. sensitive) | 0.013 | 0.003–0.053 |
<0.001a | 0.014 | 0.003–0.061 |
<0.001a |
| p28GANK (low vs.
high) | 3.126 | 2.027–4.821 |
<0.001a | 2.390 | 1.459–3.914 | 0.001a |
Discussion
The present study first analyzed the expression
levels of p28GANK by IHC, revealing that it was higher in OC
tissues compared with normal tissues. This finding is consistent
with the results of previous studies (19).
A high p28GANK expression level has also been
reported in several other types of cancer, including lung cancer,
pancreatic cancer, colorectal cancer, esophageal squamous cell
carcinoma, mammary carcinoma, endometrial carcinoma and
hepatocellular carcinoma (10,12,20–24)
To further characterize the clinical prognostic
implications of p28GANK, the present study analyzed the
associations between p28GANK expression and the clinicopathological
features and prognosis of patients with OC. The IHC results
indicated significant associations between the expression of
p28GANK and FIGO stage, residual disease and response to
cisplatin-based chemotherapy. In addition, a significant
association was revealed between the expression of p28GANK and poor
prognosis for OC.
Chen et al (19) identified that a high expression level
of p28GANK was positively associated with clinical stage and serum
cancer antigen 125 levels, and negatively associated with tumor
grade. Furthermore, high levels of p28GANK expression were
associated with a poor prognosis and early relapse. In addition, a
high expression level of p28GANK has been demonstrated to be
associated with primary tumor, lymph node metastasis, clinical
stage and poor prognosis in esophageal squamous cell carcinoma
(22). In breast cancer, p28GANK
overexpression has been associated with lymph node metastasis.
Knockdown of p28GANK has been reported to inhibit tumor metastases
to the lungs in animal models (23).
Furthermore, p28GANK expression was revealed to be significantly
higher in cases of hepatocellular carcinoma with increased tumor
size and distant metastases (8). A
similar observation has also been reported for colorectal cancer
(21). In summary, these findings
indicate a significant role of p28GANK in tumor metastasis and
progression.
Higashitsuji et al (4) first identified p28GANK as an oncogenic
protein that is overexpressed in hepatocellular carcinoma. This
protein controls the phosphorylation of Rb by CDK4 and promotes the
ubiquitination of p53 by MDM2 (3,6). Man
et al (10) revealed that
p28GANK serves an essential role in Ras-initiated tumorigenesis in
human lung cancer. It may decrease cancer cell focal adhesions by
regulating the activity of Ras-related C3 botulinum toxin substrate
1, resulting in metastasis (23).
p28GANK promotes tumor growth and metastasis in
hepatocarcinogenesis via the phosphoinositide 3-kinase (9), STAT3 (8)
and β-catenin (11) signaling
pathways. Further investigation has confirmed that overexpression
of p28GANK enhances the epithelial-mesenchymal transition, which is
defined as the switching of polarized epithelial cells to a
migratory fibroblastoid phenotype, strengthening matrix
metalloproteinase 2 activity, and increasing vascular endothelial
growth factor expression (8,9). Therefore, p28GANK may promote cancer
metastasis in numerous ways.
Patients with OC have been treated with carboplatin
since 1989 (25). To the best of our
knowledge, the present study is the first to report that p28GANK
overexpression was associated with the response to platinum-based
chemotherapy and the OS time of patients with OC. Accumulating data
suggest that cancer stem cells (CSCs) exhibit a higher capacity for
self-renewal and chemoresistance. Sun et al (26) identified that p28GANK mediates the
dedifferentiation of hepatocytes via a hepatocyte nuclear factor 4
α-dependent mechanism. The decrease in p28GANK levels leads to a
significant decrease in the proportion of CSCs and the degradation
of octamer-binding transcription factor 4 in hepatoma cells
(27). p28GANK has also been
reported to be significantly associated with prominin-1 and Nanog
in colorectal cancer (28). These
data suggest that p28GANK may stimulate cancer cell stemness,
resulting in resistance to cisplatin chemotherapy.
A previous study suggested that p28GANK inhibits
apoptosis in hepatocellular carcinoma cells by enhancing the
adaptive response and endoplasmic reticulum chaperone BiP
expression under endoplasmic reticulum stress conditions (29). Furthermore, it increases resistance
to apoptosis and enhances autophagy with sorafenib treatment
(30). In summary, these findings
indicate that p28GANK may be a therapeutic target for OC and a
p28GANK inhibitor is likely to enhance the effects of cisplatin
chemotherapy.
Chen et al (19) identified that p28GANK can promote OC
cell proliferation. Consistent with this study, the present
findings revealed that high expression of p28GANK was associated
with FIGO stage and drug resistance. Therefore, p28GANK may promote
tumor progression by enhancing resistance to treatment and may be a
valuable therapeutic target.
In conclusion, the current study demonstrated high
expression levels of p28GANK in OC. This expression was associated
with FIGO stage, residual tumor size, response to chemotherapy, and
poor OS and DFS. The present results highlight the importance of
p28GANK in the progression of OC, and suggest that it may be a
potential prognostic marker and therapeutic target for the
improvement of OC clinical management.
Acknowledgements
Not applicable.
Funding
The present study was part of the Program on the
Clinical Study of Chinese Medicine funded by the National Natural
Science Foundation of China (grant no. 81373673). The present study
was also supported by Heilongjiang Health and Family Planning
Commission Foundation (grant no. 2016-085), Heilongjiang
Postdoctoral Foundation, China (grant no. LBH-Z16246) and
Innovation Scientific Research Fund of Harbin Medical University
(grant no. 2017LCZX81).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XF supervised the entire study and participated in
study design and coordination. GY, NL and WW performed the
experiments and statistical analysis, and drafted the manuscript.
MN performed the statistical analysis and article revision. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Harbin Medical University Cancer Hospital
(Harbin, China). Written informed consent was obtained from all
patients involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis CE, Siegel RL, Sauer AG, Miller
KD, Fedewa SA, Alcaraz KI and Jemal A: Cancer statistics for
African Americans, 2016: Progress and opportunities in reducing
racial disparities. CA Cancer J Clin. 66:290–308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tørring ML, Falborg AZ, Jensen H, Neal RD,
Weller D, Reguilon I; ICBP Working Group, ; Menon U and Vedsted P:
Advanced-stage cancer and time to diagnosis: An international
cancer benchmarking partnership (ICBP) cross-sectional study. Eur J
Cancer Care (Engl). May 22–2019.(Epub ahead of print). View Article : Google Scholar
|
|
3
|
Dawson S, Apcher S, Mee M, Higashitsuji H,
Baker R, Uhle S, Dubiel W, Fujita J and Mayer RJ: Gankyrin is an
ankyrin-repeat oncoprotein that interacts with CDK4 kinase and the
S6 ATPase of the 26 S proteasome. J Biol Chem. 277:10893–10902.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Higashitsuji H, Itoh K, Nagao T, Dawson S,
Nonoguchi K, Kido T, Mayer RJ, Arii S and Fujita J: Reduced
stability of retinoblastoma protein by gankyrin, an
oncogenicankyrin-repeat protein overexpressed in hepatomas. Nat
Med. 6:96–99. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krzywda S, Brzozowski AM, Higashitsuji H,
Fujita J, Welchman R, Dawson S, Mayer RJ and Wilkinson AJ: The
crystal structure of gankyrin, an oncoprotein found in complexes
with cyclin-dependent kinase 4, a 19 S proteasomal ATPase
regulator, and the tumor suppressors Rb and p53. J Biol Chem.
279:1541–1545. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Higashitsuji H, Higashitsuji H, Itoh K,
Sakurai T, Nagao T, Sumitomo Y, Masuda T, Dawson S, Shimada Y,
Mayer RJ and Fujita J: The oncoprotein gankyrin binds to MDM2/HDM2,
enhancing ubiquitylation and degradation of p53. Cancer Cell.
8:75–87. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y, Li HH, Fu J, Wang XF, Ren YB, Dong
LW, Tang SH, Liu SQ, Wu MC and Wang HY: Oncoprotein p28 GANK binds
to RelA and retains NF-kappaB in the cytoplasm through nuclear
export. Cell Res. 17:1020–1029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng T, Hong X, Wang J, Pei T, Liang Y,
Yin D, Song R, Song X, Lu Z, Qi S, et al: Gankyrin promotes tumor
growth and metastasis through activation of IL-6/STAT3 signaling in
human cholangiocarcinoma. Hepatology. 59:935–946. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fu J, Chen Y, Cao J, Luo T, Qian YW, Yang
W, Ren YB, Su B, Cao GW, Yang Y, et al: p28GANK overexpression
accelerates hepatocellular carcinoma invasiveness and metastasis
via phosphoinositol 3-kinase/AKT/hypoxia-inducible factor-1α
pathways. Hepatology. 53:181–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Man JH, Liang B, Gu YX, Zhou T, Li AL, Li
T, Jin BF, Bai B, Zhang HY, Zhang WN, et al: Gankyrin plays an
essential role in Ras-induced tumorigenesis through regulation of
the RhoA/ROCK pathway in mammalian cells. J Clin Invest.
120:2829–2841. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong LW, Yang GZ, Pan YF, Chen Y, Tan YX,
Dai RY, Ren YB, Fu J and Wang HY: The oncoprotein p28GANK
establishes a positive feedback loop in β-catenin signaling. Cell
Res. 21:1248–1261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Yang Y, Zhang Z, He Y, Liu Z, Yu
Y, Wu S, Cai B and Feng Y: Gankyrin plays an essential role in
estrogen-driven and GPR30-mediated endometrial carcinoma cell
proliferation via the PTEN/PI3K/AKT signaling pathway. Cancer Lett.
339:279–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mizuno H, Kitada K, Nakai K and Sarai A:
PrognoScan: A new database for meta-analysis of the prognostic
value of genes. BMC Med Genomics. 2:182009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo Q, He Y, Sun L, Kong C, Cheng Y and
Zhang G: In silico detection of potential prognostic circRNAs
through a re-annotation strategy in ovarian cancer. Oncol Lett.
17:3677–3686. 2019.PubMed/NCBI
|
|
16
|
Keen JC and Moore HM: The genotype-tissue
expression (GTEx) project: Linking clinical data with molecular
analysis to advance personalized medicine. J Pers Med. 5:22–29.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Silverberg SG: Histopathologic grading of
ovarian carcinoma: A review and proposal. Int J Gynecol Pathol.
19:7–15. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pecorelli S, Benedet JL, Creasman WT and
Shepherd JH: FIGO staging of gynecologic cancer. 1994–1997 FIGO
committee on gynecologic oncology. international federation of
gynecology and obstetrics. Int J Gynaecol Obstet. 65:243–249. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen J, Bai M, Ning C, Xie B, Zhang J,
Liao H, Xiong J, Tao X, Yan D, Xi X, et al: Gankyrin facilitates
follicle-stimulating hormone-driven ovarian cancer cell
proliferation through the PI3K/AKT/HIF-1 α/cyclin D1 pathway.
Oncogene. 35:2506–2517. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng Y, He L, Guo X, Tang S, Zhao X, Du R,
Jin J, Bi Q, Li H, Nie Y, et al: Gankyrin promotes the
proliferation of human pancreatic cancer. Cancer Lett. 297:9–17.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bai Z, Tai Y, Li W, Zhen C, Gu W, Jian Z,
Wang Q, Lin JE, Zhao Q, Gong W, et al: Gankyrin activates IL-8 to
promote hepatic metastasis of colorectal cancer. Cancer Res.
73:4548–4558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ortiz CM, Ito T, Tanaka E, Tsunoda S,
Nagayama S, Sakai Y, Higashitsuji H, Fujita J and Shimada Y:
Gankyrin oncoprotein overexpression as a critical factor for tumor
growth in human esophageal squamous cell carcinoma and its clinical
significance. Int J Cancer. 122:325–332. 2010. View Article : Google Scholar
|
|
23
|
Zhen C, Chen L, Zhao Q, Liang B, Gu YX,
Bai ZF, Wang K, Xu X, Han QY, Fang DF, et al: Gankyrin promotes
breast cancer cell metastasis by regulating Rac1 activity.
Oncogene. 32:3452–3460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin X, Wang X, Liu F, Morris LE, Wang X,
Jiang B and Zhang Y: Gankyrin activates mTORC1 signaling by
accelerating TSC2 degradation in colorectal cancer. Cancer Lett.
376:83–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lloyd K: The resurgence of platinum-based
cancer chemotherapy. Nat Rev Cancer. 7:573–584. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun W, Ding J, Wu K, Ning BF, Wen W, Sun
HY, Han T, Huang L, Dong LW, Yang W, et al: Gankyrin-mediated
dedifferentiation facilitates the tumorigenicity of rat hepatocytes
and hepatoma cells. Hepatology. 54:1259–1272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qian YW, Chen Y, Yang W, Fu J, Cao J, Ren
YB, Zhu JJ, Su B, Luo T, Zhao XF, et al: p28 GANK prevents
degradation of oct4 and promotes expansion of tumor-initiating
cells in hepatocarcinogenesis. Gastroenterology. 142:1547–1558.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mine H, Sakurai T, Kashida H, Matsui S,
Nishida N, Nagai T, Hagiwara S, Watanabe T and Kudo M: Association
of Gankyrin and Stemness factor expression in human; Colorectal
cancer. Dig Dis Sci. 58:2337–2344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dai RY, Chen Y, Fu J, Dong LW, Ren YB,
Yang GZ, Qian YW, Cao J, Tang SH, Yang SL and Wang HY: p28GANK
inhibits endoplasmic reticulum stress-induced cell death via
enhancement of the endoplasmic reticulum adaptive capacity. Cell
Res. 19:1243–1257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo T, Fu J, Xu A, Su B, Ren Y, Li N, Zhu
J, Zhao X, Dai R, Cao J, et al: PSMD10/gankyrin induces autophagy
to promote tumor progression through cytoplasmic interaction with
ATG7 and nuclear transactivation of ATG7 expression. Autophagy.
12:1355–1371. 2016. View Article : Google Scholar : PubMed/NCBI
|