Introduction
Renal cell carcinoma (RCC) is a malignant tumor that
originates in the renal pituitary epithelial system and has the
second highest incidence rate of genitourinary tumors (1). In the United States of America in 2018,
RCC had the eighth highest incidence rate among adult tumors, with
65,340 new cases diagnosed (1).
According to the American Cancer Society, RCC had the highest
mortality rate with 14,240 mortalities in 2018 (1). Approximately one-third of patients with
RCC had metastases prior to their first hospital visit, and
exhibited a high recurrence rate and poor prognosis in the later
stage of disease (2). Even with
early detection and early radical resection, 20–40% of patients
experience distant metastasis or recurrence (3). The 5-year overall survival (OS) time of
patients with metastatic RCC (mRCC) is <10% (4). RCC is not sensitive to traditional
radiotherapy and chemotherapy, including cytotoxic chemotherapy,
5-fluorouracil- and floxuridin (FUDR)-based chemotherapy (5), and radical or partial nephrectomy is
currently the primary method of treatment for RCC (6). Targeted therapy is the most effective
treatment for mRCC (7).
Retrospective analysis revealed that, prior to 2005,
high-dose interleukin 2 (IL-2) or interferon α (IFN-α) were the
first-line drugs for the clinical management of RCC (8). Randomized phase III clinical trials
demonstrated that temsirolimus, sunitinib and bevacizumab had
clinical benefit compared with IFN-α for patients with RCC in terms
of OS and progression free survival (PFS). Tesirolimus is a
selective inhibitor of mammalian target of rapamycin (mTOR), a key
component of the intracellular signaling transduction cascade that
mediates cell proliferation and tumor angiogenesis. Tesirolimus
synergistically binds to 12-kDa FK506-binding protein (FKBP-12) to
form a complex that inhibits mTOR kinase activity. This inhibition
impairs translation of key regulatory proteins in the cell cycle,
ultimately leading to G1/S arrest (9). Sunitinib is an oral tyrosine kinase
inhibitor (TKI), targeting vascular endothelial growth factor
(VEGF) (10). This receptor tyrosine
kinase plays a key role in the pathogenesis of clear-cell
carcinoma, the predominant type of renal-cell carcinoma, through
involvement of the von Hippel-Lindau (VHL) gene. VHL is inactivated
by deletion, mutation or methylation in up to 80% of sporadic cases
of clear-cell carcinoma (11). VHL
is a tumor-suppressor gene that encodes a protein involved in the
regulation of the production of VEGF and a number of other
hypoxia-inducible proteins. Inactivation of the VHL gene
upregulates the VEGF receptor (VEGFR), and the resulting persistent
stimulation of which may promote tumor angiogenesis and growth and
metastasis (12). Bevacizumab and
sunitinib bind to circulating VEGF, which produces a significant
prolongation of time to disease progression in patients with
metastatic RCC (13). In addition to
temsirolimus, sunitinib and bevacizumab, sorafenib, pazopanib,
everolimus, axitinib, cabozantinib, lenvantinib, dovitinib and
nivolumab have been used for clinical trials for the treatment of
RCC over the past two decades (14–44).
Sunitinib, sorafenib, pazopanib, axitinib, cabozantinib,
lenvantinib and dovitinib are TKIs that inhibit a number of
receptors, including platelet derived growth factor receptor,
Fms-like tyrosine kinase-3, fibroblast growth factor receptor,
stem-cell growth factor and VEGFR (10,21,23,24,32,45,46).
Nivolumab is a humanized immunoglobulin monoclonal antibody that
binds to programmed cell death 1 (PD-1) on activated immune cells
and blocks binding of this receptor to its ligands PD-L1 and 2,
thereby eliminating the inhibition of immunosuppressive signal and
enhancing the host's anti-tumor response (25). The development of targeted molecular
therapies against VEGFR, PD-1 and mTOR has made the selection of
the optimal treatment for patients challenging. However,
mechanistically, agents that target PD-L1 or PD-1 are forms of
immunotherapy and are distinct from targeted therapies.
There is currently a lack of direct comparison of
the drugs used to treat RCC in clinical trials. Network
meta-analysis is a novel technique that combines direct and
indirect evidence, and provides useful comparisons between the
different therapies (47).
Furthermore, the results of different interventions for the
treatment of similar diseases may be aggregated and quantitatively
analyzed synthesized. The outcomes of the interventions are
subsequently ranked, allowing the selection of the optimal
treatment (48). The aim of the
present study was to evaluate and compare the effectiveness and
safety of various targeted therapies for RCC using a network
meta-analysis.
Materials and methods
Inclusion and exclusion criteria
The present study followed the Preferred Reporting
Items for Systematic Reviews and Meta-Analyses (PRISMA) extension
statement for network meta-analyses (49). Prior to performing the network
meta-analysis, a protocol was published on PROSPERO (www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018086692)
(50). Randomized control trials
investigating temsirolimus, sunitinib, bevacizumab, sorafenib,
pazopanib, everolimus, axitinib, cabozantinib, lenvantinib,
dovitinib and nivolumab as monotherapy or combination therapy for
the treatment of patients with RCC were included in the present
study, irrespective of whether the patients had received any
systemic therapy, including first-line sunitinib and
cytokine-containing regimen. Studies for which full text was
unavailable and participants in crossover populations were
excluded, and parallel group trials allocate each participant to a
single intervention for comparison with one or more alternative
interventions. By contrast, crossover trials allocate each
participant to a sequence of interventions. It is difficult to
extract suitable data from a trial allowing crossover, and the
carry-over (a type of period-by-intervention interaction) effects
the assessment (51). Additionally,
studies with treatment strategies that were not compared with other
drugs or which lacked data on the primary outcomes investigated in
the current study were excluded.
Search strategy
The Web of Science (apps.webofknowledge.com), PubMed (https://www.ncbi.nlm.nih.gov/pubmed), EMBASE
(https://www.embase.com), Cochrane Library of
Controlled Trials (https://www.cochranelibrary.com) and Clinical
trials.gov (https://clinicaltrials.gov) were all searched for
relevant publications published in the English language from
inception to February 2019. Conference or seminal articles were
also searched. A comprehensive search strategy was utilized,
included the following terms: ‘Renal cell carcinoma’,
‘temsirolimus’, ‘sunitinib’, ‘bevacizumab’, ‘sorafenib’,
‘pazopanib’, ‘everolimus’, ‘axitinib’, ‘cabozantinib’,
‘lenvantinib’, ‘dovitinib’, ‘nivolumab’ and ‘randomized controlled
trial’. The full search strategy is available in the aforementioned
published protocol (50).
Study selection and data
collection
The reference management software EndNote (version
X7) (52) was used to identify and
remove duplicate records. The abstract and title of each
publication were scanned independently by two reviewers to exclude
studies that did not meet the inclusion criteria, and if required,
full articles were assessed according to the PRISMA statement
(53). If discrepancies arose,
another reviewer participated in this process. The following data
were extracted from the selected articles: Date of publication,
first author, trial ID, trial phase, number of participants, drug
used and patient outcome data. In the data extracting stage, the
present study aimed to contact authors in order to obtain more
information, but they were unavailable. Stata (version 13)
(54) was used to draw a network
plot to present the cumulative number of trials for each comparison
and the number of enrolled participants, and to decide which trials
to exclude according to the aforementioned exclusion criteria.
Outcome measures
The primary outcome of the present study was PFS,
defined as the time from randomization to the first documentation
of objective disease progression, or to mortality from any cause.
Objective disease progression indicated a minimum 20% increase in
the sum of diameters of target lesions, taking as reference the
smallest value in the study, including the baseline, and an
absolute increase of at least 5 mm, appearance of one or more new
target or non-target lesions, or unequivocal progression of
existing non-target lesions. Secondary outcomes included OS, which
was defined as the time from randomization to the date of death;
objective response rate (ORR; assessed according to the Response
Evaluation Criteria in Solid Tumors) (55) and safety (graded according to the
Common Terminology Criteria for Adverse Events) (56).
Statistical analysis
The data analysis combined direct and indirect
meta-analyses. A traditional pair-wise meta-analysis was performed
to compare the same interventions. The fixed effects model was used
to analyze data if the χ2 test and I2 index
for testing heterogeneity among the study revealed that there was
no statistically significant heterogeneity (P>0.1;
I2≤25%). Otherwise, a random effects model was used.
Network meta-analyses were conducted using the
Bayesian Markov Chain Monte Carlo model with WinBUGS (version 14)
(57). Each model was run for 40,000
burn-in simulations and 200,000 runs, which were then thinned every
20th simulation to decrease autocorrelation. The mean log hazard
ratio (HR) and standard error for each treatment were extracted to
compare PFS and OS (58). The total
number of events was used to compare ORR and safety. By comparing
the deviance information criterion (DIC) value of fixed and random
effect model, the model with lowest DIC was selected, or
alternatively, the fixed effect model was selected when the
discrepancy was <10 (59). If
direct and indirect evidence was available, consistency was checked
via model extensions to estimate the validation of mixed treatment
comparison.
The probability of an intervention being the optimal
intervention was calculated using rank code in WinBUGS and the
surface under the cumulative ranking curve (SUCRA) values of all
treatments were compared for efficacy and safety. Node-splitting
analyses were used to assess inconsistencies in the network
meta-analysis, which assessed whether direct and indirect evidence
on a specific node (the split node) were in agreement.
Results
Study characteristics
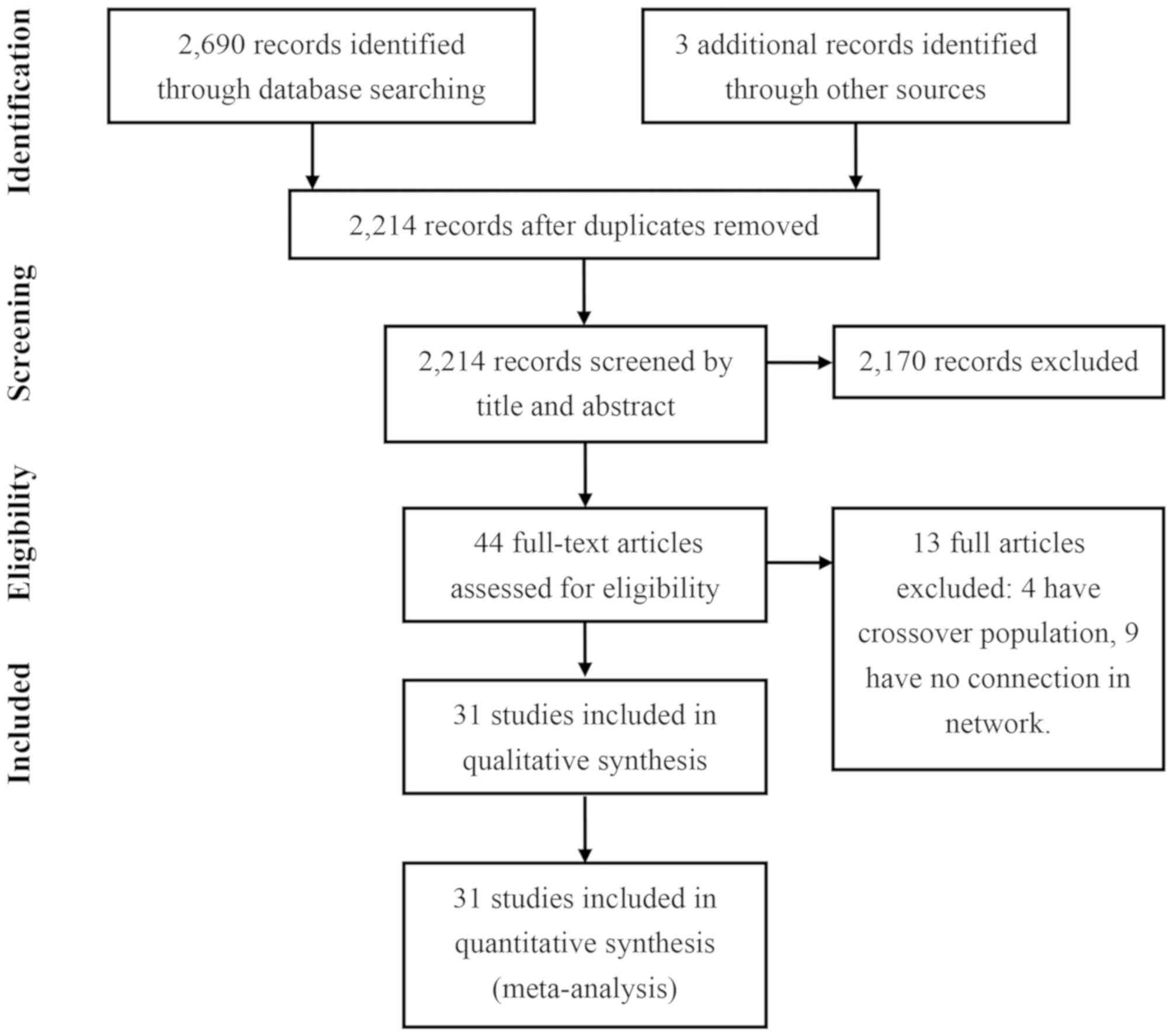
The present study identified 2,693 potentially
relevant publications. After excluding 479 duplications and 2,170
reports that did not meet the eligibility criteria (Fig. 1), 44 full articles were assessed for
eligibility. A further 13 studies were excluded due to the
following reasons: Having no connection to other treatments or were
not the focus of the present study (including studies on the
comparison of sorafenib, IFN-α2, combination of bevacizumab and
IFN-α2 and the combination of erlotinib and bevacizumab),
allocating each participant to a sequence of interventions, which
may result in low quality conclusion and influence the result of
the network meta-analysis. Overall, 31 publications covering 18
trials between 2007 and 2018 were included in the comparison
meta-analysis of the present study (Table SI) (14–44). The
11,498 participants were randomly assigned to 16 treatment groups
(including placebo, nivolumab, everolimus, lenvantinib plus
everolimus, lenvantinib, cabozantinib, sunitinib, IFNα, sorafenib,
axitinib, temsirolimus, pazopanib, dovitinib, bevacizumab plus
IFNα, temsirolimus plus IFN-α, or temsirolimus plus bevacizumab)
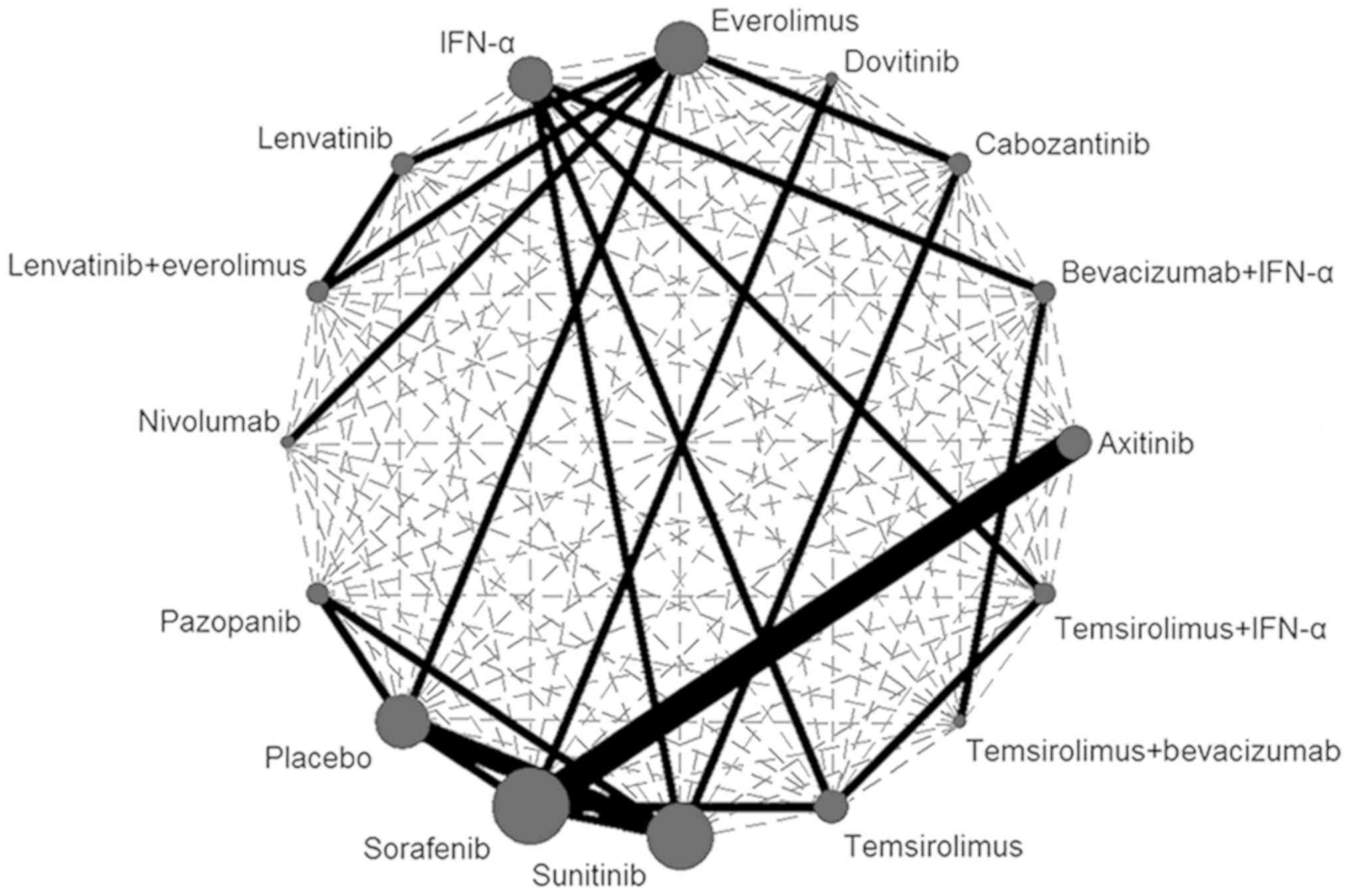
and were included in the multiple-treatments meta-analysis. The
dashed lines in the network plot indicated all possible direct
comparisons (Fig. 2). Due to the
limited number of published trials, data on a number of possible
direct comparisons were not available. The solid lines represented
existing evidence that was available.
Efficacy
The result of standard pair-wise meta-analysis only
revealed a significant difference in PFS (HR, 0.69; 95% CI,
0.60–0.79; P<0.0001) and ORR (odds ratio, 2.51; 95% CI,
1.18–3.51) between axitinib and sorafenib. The HRs of PFS were
pooled by the fixed effect model, as the DIC values of the fixed
and random effect models were 3.868 and −4.528, respectively. The
results of the primary outcome are presented in Table I. In terms of PFS, except for IFN-α
(HR, 1.30; 95% CI, 0.97–1.90), bevacizumab plus IFN-α (HR, 0.96;
95% CI, 0.67–1.37) and bevacizumab plus temsirolimus (HR, 1.03; 95%
CI, 0.69–1.55), all treatments were significantly different from
the placebo. Multiple comparisons of the HR of OS and the
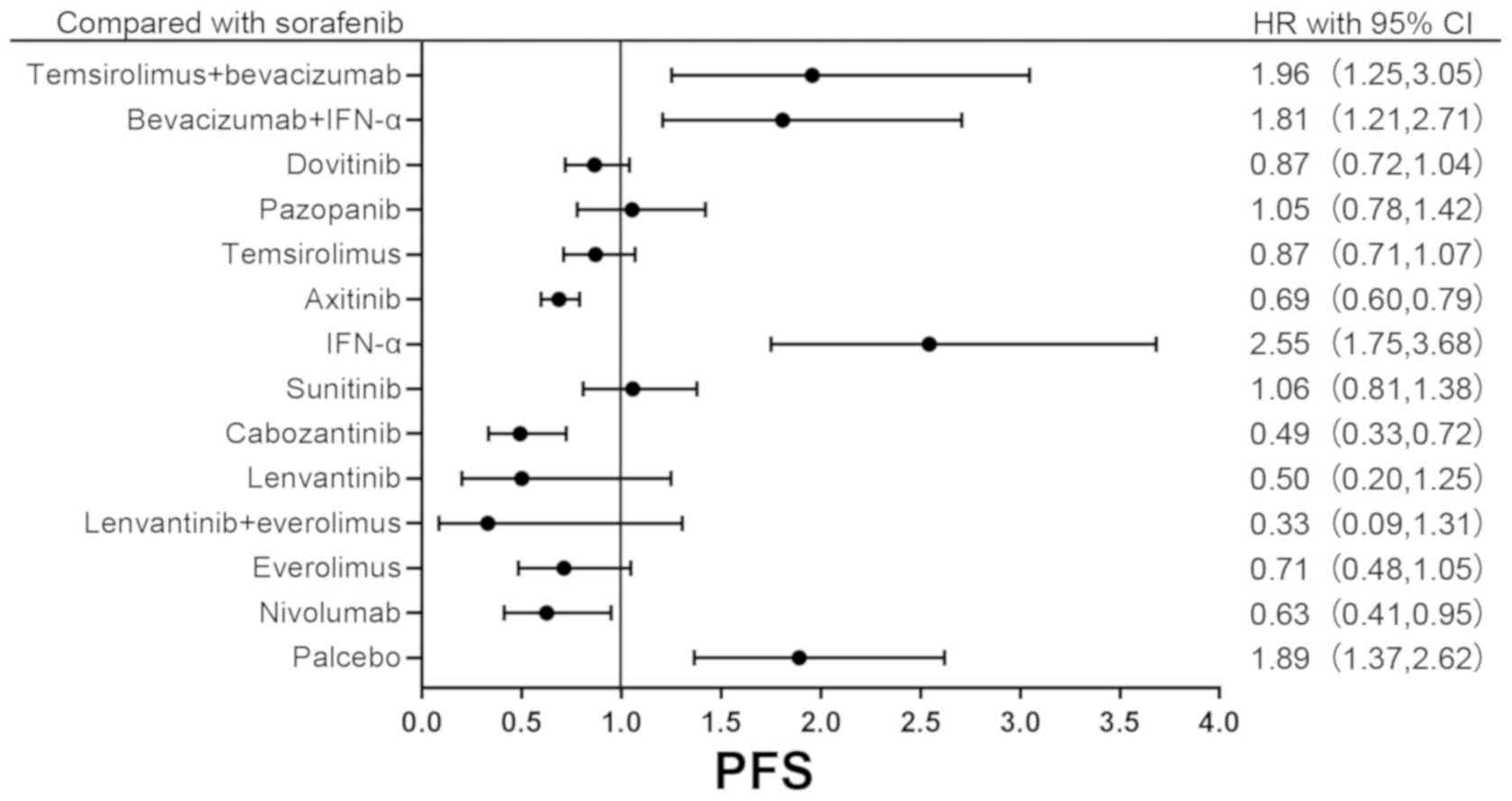
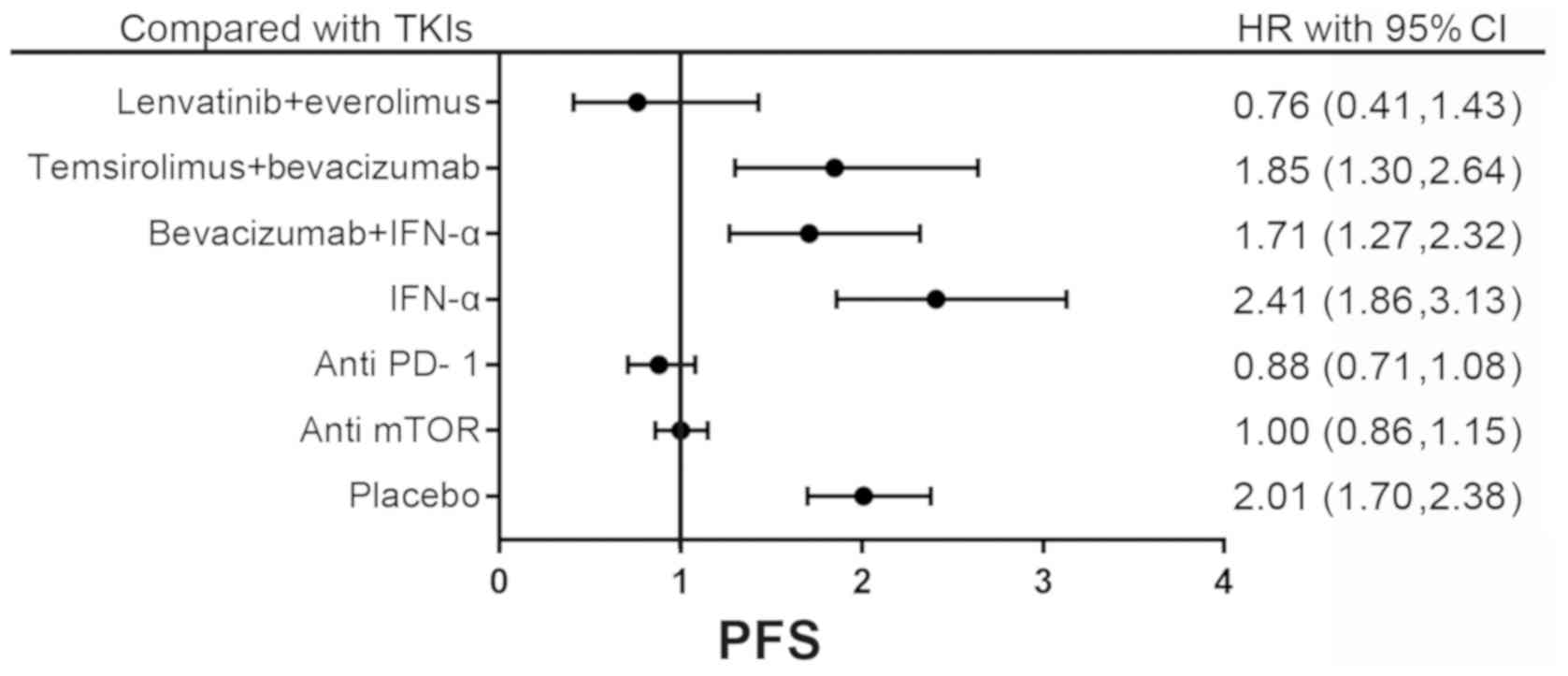
occurrence rate (OR) of ORR are presented in Tables SII and III. In addition, the forest plot comparing
the primary outcomes between sorafenib and other drugs is presented
in Fig. 3, as sorafenib was the
first drug used as a targeted therapy in the treatment of RCC
(60).
 | Table I.Result of the network meta-analysis
comparing the effect [hazard ratio (95% confidence interval)] of
immunotherapy and targeted therapies on the progression-free
survival in renal cell carcinoma. |
Table I.
Result of the network meta-analysis
comparing the effect [hazard ratio (95% confidence interval)] of
immunotherapy and targeted therapies on the progression-free
survival in renal cell carcinoma.
| Treatment | Placebo | Nivolumab | Everolimus | Lenvantinib +
Everolimus | Lenvantinib | Cabozantinib | Sunitinib | IFN-α | Sorafenib | Axitinib | Temsi-rolimus | Pazopanib | Dovitinib |
Bevacizumab+IFN-α |
Temsi-rolimus+Bevacizumab |
|---|
| Placebo | – | 0.33 | 0.38 | 0.18 | 0.26 | 0.26 | 0.56 | 1.3 | 0.53 | 0.36 | 0.46 | 0.56 | 0.46 | 0.96 | 1.03 |
|
|
| (0.25,0.45) | (0.29,0.48) | (0.05,0.67) | (0.11,0.63) | (0.20,0.35) | (0.46,0.67) | (0.97,1.9) | (0.38,0.73) | (0.25,0.52) | (0.31,0.68) | (0.45,0.68) | (0.31,0.66) | (0.67,1.37) | (0.69,1.55) |
| Nivolumab |
| – | 1.14 | 0.53 | 0.80 | 0.79 | 1.70 | 4.06 | 1.60 | 1.10 | 1.39 | 1.68 | 1.38 | 2.89 | 3.13 |
|
|
|
| (0.97,1.33) | (0.14,2.00) | (0.34,1.86) | (0.60,1.04) | (1.23,2.33) | (2.68,6.15) | (1.05,2.42) | (0.71,1.70) | (0.87,2.21) | (1.20,2.35) | (0.88,2.17) | (1.86,4.50) | (1.94,5.05) |
| Everolimus |
|
| – | 0.46 | 0.70 | 0.59 | 0.92 | 2.20 | 0.87 | 0.59 | 0.75 | 0.83 | 0.75 | 1.57 | 1.70 |
|
|
|
|
| (0.124,1.72) | (0.30,1.61) | (0.47,0.73) | (0.73,1.14) | (1.56,3.10) | (0.61,1.22) | (0.41,0.86) | (0.51,1.13) | (0.66,1.03) | (0.51,1.11) | (1.08,2.28) | (1.11,2.58) |
| Lenvantinib |
|
|
| – | 1.51 | 1.48 | 3.18 | 7.66 | 3.00 | 2.06 | 2.62 | 3.16 | 2.60 | 5.44 | 5.89 |
| +Everolimus |
|
|
|
| (0.75,3.06) | (0.40,5.69) | (0.84,12.41) | (1.97,30.68) | (0.78,12.09) | (0.53,8.31) | (0.67,10.65) | (0.84,12.41) | (0.66,10.56) | (1.39,21.98) | (1.49,23.99) |
| Lenvantinib |
|
|
|
| – | 0.99 | 2.11 | 5.09 | 2 | 1.38 | 1.74 | 2.10 | 1.73 | 3.62 | 3.92 |
|
|
|
|
|
|
| (0.42,2.33) | (0.88,5.09) | (2.04,12.74) | (0.80,5.00) | (0.54,3.48) | (0.68,4.46) | (0.87,5.12) | (0.68,4.41) | (1.44,9.20) | (1.53,10.13) |
| Cabozantinib |
|
|
|
|
| – | 2.15 | 5.17 | 2.03 | 1.40 | 1.77 | 2.14 | 1.76 | 3.68 | 3.98 |
|
|
|
|
|
|
|
| (1.62,2.84) | (3.52,7.58) | (1.38,2.98) | (0.92,2.10) | (1.14,2.74) | (1.58,2.90) | (1.15,2.69) | (2.42,5.55) | (2.52,6.26) |
| Sunitinib |
|
|
|
|
|
| – | 2.40 | 0.95 | 0.65 | 0.83 | 1.00 | 0.82 | 1.71 | 1.85 |
|
|
|
|
|
|
|
|
| (1.85,3.13) | (0.73,1.23) | (0.48,0.88) | (0.59,1.15) | (0.87,1.15) | (0.59,1.13) | (1.26,2.32) | (1.30,2.64) |
| IFN-α |
|
|
|
|
|
|
| – | 0.39 | 0.27 | 0.34 | 0.41 | 0.34 | 0.71 | 0.77 |
|
|
|
|
|
|
|
|
|
| (0.27,0.57) | (0.18,0.40) | (0.22,0.52) | (0.31,0.56) | (0.22,0.52) | (0.61,0.83) | (0.61,0.98) |
| Sorafenib |
|
|
|
|
|
|
|
| – | 0.69 | 0.87 | 1.05 | 0.87 | 1.81 | 1.96 |
|
|
|
|
|
|
|
|
|
|
| (0.60,0.79) | (0.71,1.07) | (0.78,1.42) | (0.72,1.04) | (1.21,2.71) | (1.25,3.05) |
| Axitinib |
|
|
|
|
|
|
|
|
| – | 1.27 | 1.53 | 1.26 | 2.63 | 2.85 |
|
|
|
|
|
|
|
|
|
|
|
| (0.99,1.62) | (1.1,2.14) | (1,1.59) | (1.72,4.03) | (1.78,4.54) |
| Temsirolimus |
|
|
|
|
|
|
|
|
|
| – | 1.21 | 0.99 | 2.08 | 2.25 |
|
|
|
|
|
|
|
|
|
|
|
|
| (0.84,1.74) | (0.75,1.31) | (1.32,3.27) | (1.38,3.66) |
| Pazopanib |
|
|
|
|
|
|
|
|
|
|
| – | 0.82 | 1.72 | 1.86 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| (0.58,1.17) | (1.23,2.39) | (1.27,2.72) |
| Dovitinib |
|
|
|
|
|
|
|
|
|
|
|
| – | 2.09 | 2.26 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (1.34,3.26) | (1.40,3.65) |
| Bevacizumab + |
|
|
|
|
|
|
|
|
|
|
|
|
| – | 1.08 |
| IFN-α |
|
|
|
|
|
|
|
|
|
|
|
|
|
| (0.90,1.30) |
| Temsirolimus+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
| – |
| Bevacizumab |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
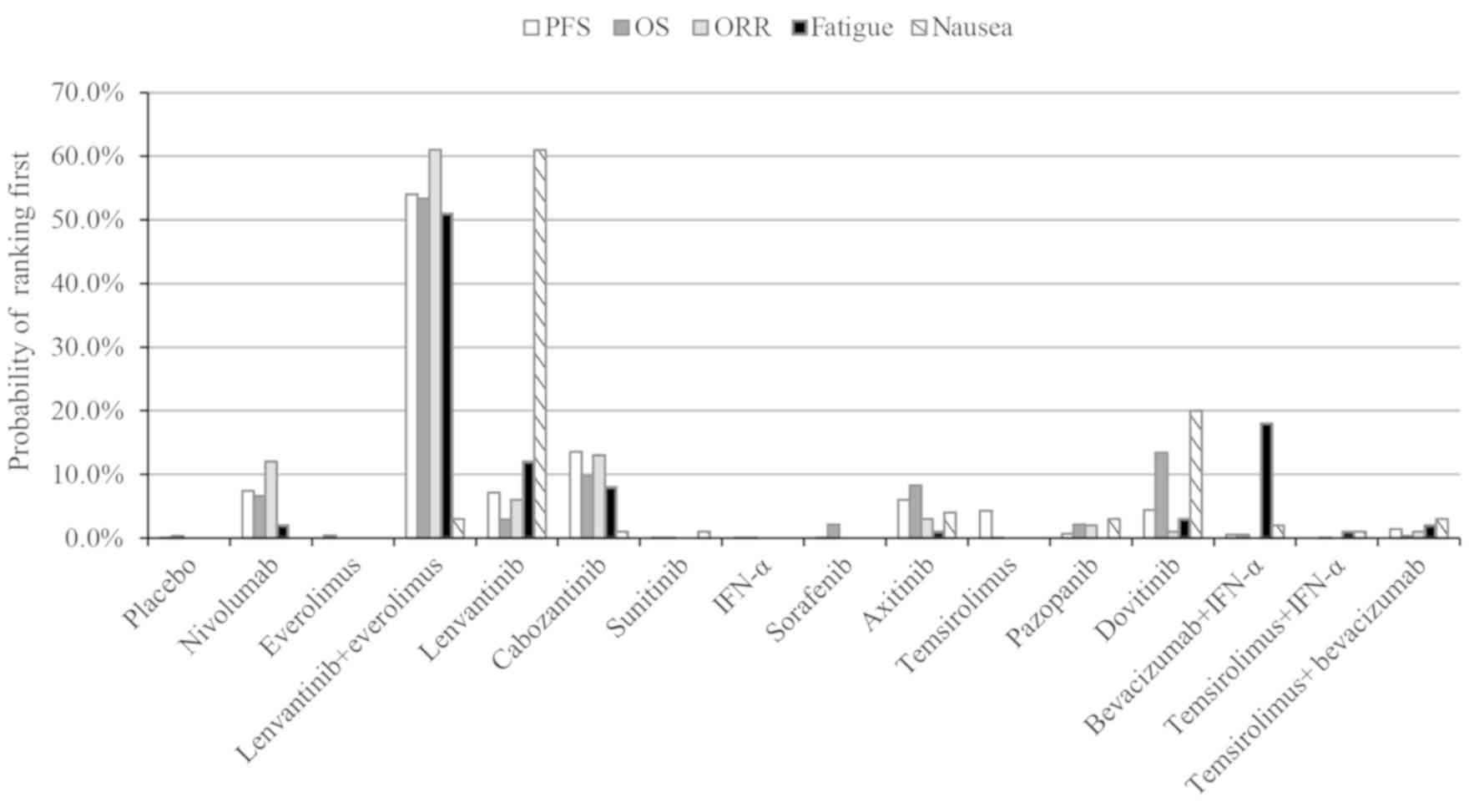
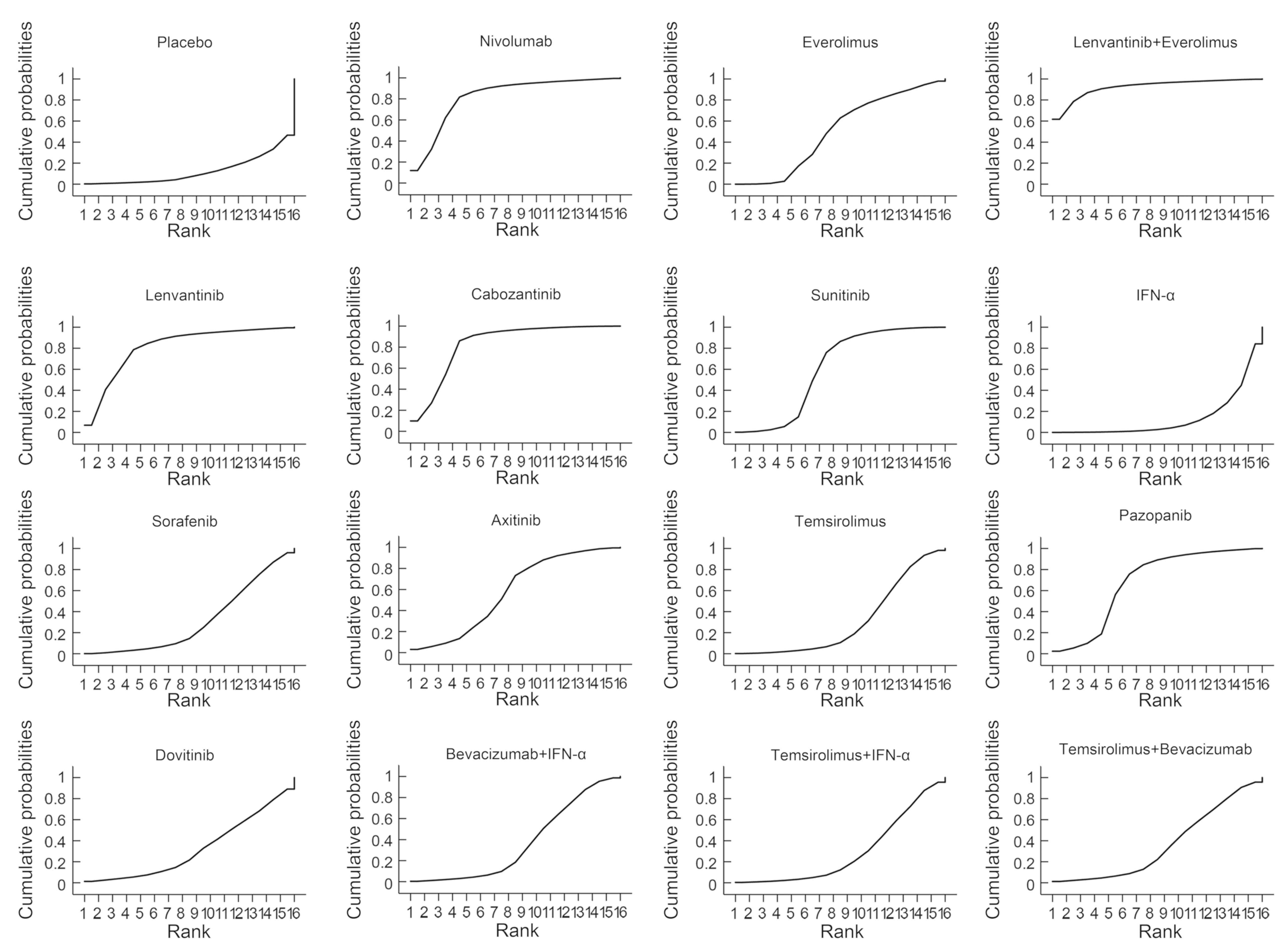
The probability of each treatment being ranked the
highest in terms of survival, response and safety is presented in
Fig. 4. The lenvantinib and
everolimus combination ranked first out of the 16 treatments in
terms of PFS, OS, ORR and OR of fatigue (probability of 54.0, 53.4,
61.0 and 51.0%, respectively). Lenvantinib monotherapy ranked first
for nausea and second in the fatigue OR (probability of 61 and 25%,
respectively). Lenvantinib plus everolimus had the highest SUCRA
value (92.3%; Fig. 5). Consistency
tests revealed there was no significant difference between the
indirect and direct evidence (P>0.35). The forest plot for
comparing PFS between TKIs and drugs targeting the same target is
presented in Fig. 6. The result of
network meta-analysis revealed no significant difference in PFS
between TKIs and other drugs.
Safety
Fatigue and nausea were considered as two important
adverse events. Using a Bayesian meta-analysis it was observed that
lenvantinib plus everolimus, cabozantinib and sunitinib had higher
probability of fatigue (OR, 5.89; 95% CI, 1.07–33.90; and OR, 3.81,
95% CI, 1.24–12.92; and OR 2.06; 95% CI, 1.16–3.95, respectively)
compared with the placebo, whereas the other drugs demonstrated no
significant difference from the placebo and with each other. With
the exception of pazopanib and sunitinib (OR, 3.34; 95% CI,
1.12–11.02 and OR, 3.41; 95% CI, 1.08–11.04, respectively), the
drugs were not significantly different from placebo in the
probability of nausea (Tables SIV
and V). Notably, patients treated
with lenvantinib were more susceptible to nausea compared with
those treated with nivolumab or everolimus.
The results regarding drug safety from the
probability analysis are presented in Fig. 4. Lenvantinib plus everolimus caused
greater levels of fatigue than lenvantinib monotherapy or
everolimus monotherapy. Patients receiving levantinib and eovitinib
were more prone to experiencing nausea.
Due to insufficient data, other adverse events were
not analyzed by the Bayesian meta-analysis, and the results
regarding safety that were extracted from the included publications
are summarized in Table II.
Cabozantinib and lenvantinib plus everolimus had the highest rate
of any grade of adverse events, and grade 3 or 4 adverse events
most frequently occurred in the bevacizumab plus IFN-α treatment
group.
 | Table II.Most common adverse events in each
treatment group and their occurrence rate. |
Table II.
Most common adverse events in each
treatment group and their occurrence rate.
| Treatment | Most common adverse
event | Any grade (%) | Grades 3 or 4
(%) |
|---|
| Nivolumab | Fatigue | 33 | 2 |
| Everolimus | Fatigue | 20–46 | 2–7 |
|
| Stomatitis | 24–42 | 2–4 |
| Cabozantinib | Fatigue | 56-85.9 | 6.4–9 |
|
| Diarrhea | 71–74 | 10.3–11 |
|
Lenvatinib+Everolimus | Diarrhoea | 85 | 20 |
| Lenvatinib | Proteinuria | 31 | 19 |
|
| Diarrhoea | 72 | 12 |
| Sunitinib | Fatigue | 13.6–81.9 | 2–18 |
|
| Hypertension | 6.4–68.1 | 2–22 |
|
| Diarrhea | 14.1–56.9 | 1–11 |
| Sorafenib | Hand-foot
syndrome | 39-56.5 | 7.2–33 |
|
| Diarrhoea | 30.4–63 | 1.4–9 |
|
| Hypertension | 28-36.3 | 1–17 |
| Axitinib | Hypertension | 42-49.6 | 14-19.3 |
|
| Diarrhoea | 34.1–54 | 3–11 |
|
Bevacizumab+IFN-α | Fatigue | – | 37 |
|
| Proteinuria | 27 | 13–15 |
|
| Pyrexia | 39 | 3 |
| IFN-α | Fatigue | 32 | 13–30 |
| Temsirolimus | Fatigue | 24.5–40 | 5.3–6 |
|
| Anemia | 21.6–34 | 9-9.6 |
|
| Rash | 22.6–42 | 2–3 |
|
Temsirolimus+IFN-α | Fatigue | 29.80 | 13.50 |
|
| Anemia | 29.30 | 18.30 |
|
Temsirolimus+Bevacizumab | Proteinuria | 36 | 16 |
| Pazopanib | Fatigue | 19–55 | 2–10 |
|
| Diarrhea | 52 | 3 |
|
| Hypertension | 40 | 4 |
| Dovitinib |
Hypertriglyceridaemia | 20 | 14 |
|
| Fatigue | 41 | 10 |
|
| Diarrhea | 68 | 7 |
Discussion
To the best of the authors' knowledge, the present
network meta-analysis was the first to provide an indirect
comparison of the efficacy and safety of all existing targeted
therapies (temsirolimus, sunitinib, bevacizumab, sorafenib,
pazopanib, everolimus, axitinib, cabozantinib, lenvantinib,
dovitinib and nivolumab) for the treatment of RCC. The present
study was based on 31 publications, which included 11,498
individuals randomly assigned to 16 different targeted therapies.
As the primary analysis was reliant on the Bayesian Markov Chain
Monte Carlo model, the HR of PFS for targeted therapies and the
probability of each treatment being most effective was assessed,
and it was revealed that lenvantinib plus everolimus was ranked
first in terms of PFS, OS and ORR. Lenvantinib plus everolimus was
also ranked first in terms of the occurrence of fatigue. Although
not every comparison had a significant outcome, the treatments were
still ranked. The results of the present study revealed that the
use of lenvatinib plus everolimus, lenvatinib monotherapy,
cabozantinib, nivolumab and everolimus significantly extended PFS.
The use of lenvatinib plus everolimus, lenvatinib monotherapy,
cabozantinib, sorafenib and axitinib significantly increased OS.
The use of lenvatinib plus everolimus, lenvatinib monotherapy,
nivolumab, cabozantinib and pazopanib significantly improved the
ORR. Previous evidence had indicated that patients with a poor
prognosis benefited from the use of cabozantinib in terms of
survival, whereas the use of nivolumab exhibited a greater benefit
for patients with a better prognosis (61). The present study demonstrated that
there was no significant difference between nivolumab and
cabozantinib use in PFS, OS and ORR. Inconsistencies arose when the
two drugs were analyzed in one network, which was influenced by
numerous factors, including the extent of heterogeneity in indirect
comparison that was significantly associated with the inconsistency
(62). The present analysis
suggested that lenvantinib plus everolimus may be considered
superior to other drugs. Axitinib and sorafenib differed
significantly in both the network meta-analysis and pair-wise
meta-analysis. This was consistent with the results of the direct
comparison, in which axitinib was more beneficial than
sorafenib.
The strength of the analysis in the present study
rests on its transparent design. The analysis was conducted
according to a predesigned and published protocol (50), and all predefined research questions
were answered. A traditional standard meta-analysis is based on
evidence from direct comparisons, the results from which may help
to improve the health policy and reduce the risk of mortality,
however it could not analyze more than two interventions at once or
select the best option (47). In the
present study, the therapies were ranked based on the Bayesian
network meta-analysis. To the best of the authors' knowledge, there
are currently limited published data comparing all available
targeted drugs for RCC. A similar network meta-analysis published
by He et al (63) in 2017
ranked single-drug targeted therapies in terms of response while
Rousseau et al (64) assessed
the efficacy of antiangiogenic agents for metastatic RCC. The
present study focused on PFS and included an analysis of the
combination of targeted therapies, which makes the present study
relevant in the clinical setting and provides improved
transitivity.
Although IFN-α does not belong to the targeted
therapy or immunotherapy treatment types, it was still included in
the network meta-analysis, as it is indispensable as a link between
sunitinib, temsirolimus, temsirolimus plus IFN-α and bevacizumab
plus IFN-α. A total of 13 publications were excluded after reading
the full-text as they may have influenced the transitivity of the
results.
The present study revealed that a combination of
lenvatinib plus everolimus was ranked first in terms of PFS, OS and
ORR and that nivolumab may be used in patients with moderate
disease, as this group exhibited fewer adverse events. Therefore,
these results may aid in the selection of therapeutic drugs for
patients with RCC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
WW, RP, LK, CX, YC, LZ, XW, QQ, CZ, WL and SX
participated in the design of the study. WW wrote the article, RP
scanned and excluded studies, LK searched the database and
extracted data, CX, YC LZ, XW, QQ and CZ searched the database and
analyzed the data and WL checked the grammar and spelling. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brookman-May SD, May M, Shariat SF, Novara
G, Zigeuner R, Cindolo L, De Cobelli O, De Nunzio C, Pahernik S,
Wirth MP, et al: Time to recurrence is a significant predictor of
cancer-specific survival after recurrence in patients with
recurrent renal cell carcinoma-results from a comprehensive
multi-centre database (CORONA/SATURN-Project). BJU Int.
112:909–916. 2013.PubMed/NCBI
|
|
3
|
Tosco L, Van Poppel H, Frea B, Gregoraci G
and Joniau S: Survival and impact of clinical prognostic factors in
surgically treated metastatic renal cell carcinoma. Eur Urol.
63:646–652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garcia JA and Rini BI: Recent progress in
the management of advanced renal cell carcinoma. CA Cancer J Clin.
57:112–125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartmann JT and Bokemeyer C: Chemotherapy
for renal cell carcinoma. Anticancer Res. 19:1541–1543.
1999.PubMed/NCBI
|
|
6
|
Motzer RJ and Russo P: Systemic therapy
for renal cell carcinoma. J Urol. 163:408–417. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gore ME and Larkin JM: Challenges and
opportunities for converting renal cell carcinoma into a chronic
disease with targeted therapies. Br J Cancer. 104:399–406. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Motzer RJ, Bacik J, Murphy BA, Russo P and
Mazumdar M: Interferon-alfa as a comparative treatment for clinical
trials of new therapies against advanced renal cell carcinoma. J
Clin Oncol. 20:289–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harding MW: Immunophilins, mTOR and
Pharmacodynamic Strategies for a targeted cancer therapy. Clin
Cancer Res. 9:2882–2886. 2003.PubMed/NCBI
|
|
10
|
Mendel DB, Laird AD, Xin X, Louie SG,
Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, et
al: In vivo antitumor activity of SU11248, a novel tyrosine kinase
inhibitor targeting vascular endothelial growth factor and
platelet-derived growth factor receptors: Determination of a
pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res.
9:327–337. 2003.PubMed/NCBI
|
|
11
|
Gnarra JR, Tory K, Weng Y, Schmidt L, Wei
MH, Li H, Latif F, Liu S, Chen F, Duh FM, et al: Mutations of the
VHL tumour suppressor gene in renal carcinoma. Nat Genet. 7:85–90.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Na XI, Wu G, Ryan CK, Schoen SR,
di'Santagnese PA and Messing EM: Overproduction of vascular
endothelial growth factor related to von Hippel-Lindau tumor
suppressor gene mutations and hypoxia-inducible factor-1 alpha
expression in renal cell carcinomas. J Urol. 170:588–592. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang JC, Haworth L, Sherry RM, Hwu P,
Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX and
Rosenberg SA: A Randomized trial of bevacizumab, an anti-vascular
endothelial growth factor antibody, for metastatic renal cancer. N
Engl J Med. 349:427–434. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Motzer RJ, Ravaud A, Patard JJ, Pandha HS,
George DJ, Patel A, Chang YH, Escudier B, Donskov F, Magheli A, et
al: Adjuvant Sunitinib for High-risk renal cell carcinoma after
nephrectomy: Subgroup analyses and updated overall survival
results. Eur Urol. 73:62–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tomita Y, Fukasawa S, Shinohara N,
Kitamura H, Oya M, Eto M, Tanabe K, Kimura G, Yonese J, Yao M, et
al: Nivolumab versus everolimus in advanced renal cell carcinoma:
Japanese subgroup analysis from the CheckMate 025 study. Jpn J Clin
Oncol. 47:639–646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hutson TE, Al-Shukri S, Stus VP, Lipatov
ON, Shparyk Y, Bair AH, Rosbrook B, Andrews GI and Vogelzang NJ:
Axitinib Versus Sorafenib in First-line metastatic renal cell
carcinoma: Overall survival from a randomized phase III trial. Clin
Genitourin Cancer. 15:72–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choueiri TK, Halabi S, Sanford BL, Hahn O,
Michaelson MD, Walsh MK, Feldman DR, Olencki T, Picus J, Small EJ,
et al: Cabozantinib versus sunitinib as initial targeted therapy
for patients with metastatic renal cell carcinoma of poor or
intermediate risk: The alliance A031203 CABOSUN trial. J Clin
Oncol. 35:591–597. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ravaud A, Motzer RJ, Pandha HS, George DJ,
Pantuck AJ, Patel A, Chang YH, Escudier B, Donskov F, Magheli A, et
al: Adjuvant sunitinib in high-risk renal-cell carcinoma after
nephrectomy. N Engl J Med. 375:2246–2254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Motzer RJ, Hutson TE, Ren M, Dutcus C and
Larkin J: Independent assessment of lenvatinib plus everolimus in
patients with metastatic renal cell carcinoma. Lancet Oncol.
17:e4–e5. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haas NB, Manola J, Uzzo RG, Flaherty KT,
Wood CG, Kane C, Jewett M, Dutcher JP, Atkins MB, Pins M, et al:
Adjuvant sunitinib or sorafenib for high-risk, non-metastatic
renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind,
placebo-controlled, randomised, phase 3 trial. Lancet.
387:2008–2016. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choueiri TK, Escudier B, Powles T, Tannir
NM, Mainwaring PN, Rini BI, Hammers HJ, Donskov F, Roth BJ, Peltola
K, et al: Cabozantinib versus everolimus in advanced renal cell
carcinoma (METEOR): Final results from a randomised, open-label,
phase 3 trial. Lancet Oncol. 17:917–927. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beaumont JL, Salsman JM, Diaz J, Deen KC,
McCann L, Powles T, Hackshaw MD, Motzer RJ and Cella D:
Quality-adjusted time without symptoms or toxicity analysis of
pazopanib versus sunitinib in patients with renal cell carcinoma.
Cancer. 122:1108–1115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qin S, Bi F, Jin J, Cheng Y, Guo J, Ren X,
Huang Y, Tarazi J, Tang J, Chen C, et al: Axitinib versus sorafenib
as a second-line therapy in Asian patients with metastatic renal
cell carcinoma: Results from a randomized registrational study.
Onco Targets Ther. 8:1363–1373. 2015.PubMed/NCBI
|
|
24
|
Motzer RJ, Hutson TE, Glen H, Michaelson
MD, Molina A, Eisen T, Jassem J, Zolnierek J, Maroto JP, Mellado B,
et al: Lenvatinib, everolimus and the combination in patients with
metastatic renal cell carcinoma: A randomised, phase 2, open-label,
multicentre trial. Lancet Oncol. 16:1473–1482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choueiri TK, Escudier B, Powles T,
Mainwaring PN, Rini BI, Donskov F, Hammers H, Hutson TE, Lee JL,
Peltola K, et al: Cabozantinib versus Everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1814–1823. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rini BI, Bellmunt J, Clancy J, Wang K,
Niethammer AG, Hariharan S and Escudier B: Randomized phase III
trial of temsirolimus and bevacizumab versus interferon alfa and
bevacizumab in metastatic renal cell carcinoma: INTORACT trial. J
Clin Oncol. 32:752–759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Motzer RJ, Porta C, Vogelzang NJ,
Sternberg CN, Szczylik C, Zolnierek J, Kollmannsberger C, Rha SY,
Bjarnason GA, Melichar B, et al: Dovitinib versus sorafenib for
third-line targeted treatment of patients with metastatic renal
cell carcinoma: An open-label, randomised phase 3 trial. Lancet
Oncol. 15:286–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hutson TE, Escudier B, Esteban E,
Bjarnason GA, Lim HY, Pittman KB, Senico P, Niethammer A, Lu DR,
Hariharan S and Motzer RJ: Randomized phase III trial of
temsirolimus versus sorafenib as second-line therapy after
sunitinib in patients with metastatic renal cell carcinoma. J Clin
Oncol. 32:760–767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ueda T, Uemura H, Tomita Y, Tsukamoto T,
Kanayama H, Shinohara N, Tarazi J, Chen C, Kim S, Ozono S, et al:
Efficacy and safety of axitinib versus sorafenib in metastatic
renal cell carcinoma: Subgroup analysis of Japanese patients from
the global randomized Phase 3 AXIS trial. Jpn J Clin Oncol.
43:616–628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sternberg CN, Hawkins RE, Wagstaff J,
Salman P, Mardiak J, Barrios CH, Zarba JJ, Gladkov OA, Lee E,
Szczylik C, et al: A randomised, double-blind phase III study of
pazopanib in patients with advanced and/or metastatic renal cell
carcinoma: Final overall survival results and safety update. Eur J
Cancer. 49:1287–1296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Motzer RJ, Hutson TE, Cella D, Reeves J,
Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, et
al: Pazopanib versus sunitinib in metastatic renal-cell carcinoma.
N Engl J Med. 369:722–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Motzer RJ, Escudier B, Tomczak P, Hutson
TE, Michaelson MD, Negrier S, Oudard S, Gore ME, Tarazi J,
Hariharan S, et al: Axitinib versus sorafenib as second-line
treatment for advanced renal cell carcinoma: Overall survival
analysis and updated results from a randomised phase 3 trial.
Lancet Oncol. 14:552–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hutson TE, Lesovoy V, Al-Shukri S, Stus
VP, Lipatov ON, Bair AH, Rosbrook B, Chen C, Kim S and Vogelzang
NJ: Axitinib versus sorafenib as first-line therapy in patients
with metastatic renal-cell carcinoma: A randomised open-label phase
3 trial. Lancet Oncol. 14:1287–1294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rini BI, Escudier B, Tomczak P, Kaprin A,
Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME,
Rusakov IG, et al: Comparative effectiveness of axitinib versus
sorafenib in advanced renal cell carcinoma (AXIS): A randomised
phase 3 trial. Lancet. 378:1931–1939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sternberg CN, Davis ID, Mardiak J,
Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA,
Kavina A, et al: Pazopanib in locally advanced or metastatic renal
cell carcinoma: Results of a randomized phase III trial. J Clin
Oncol. 28:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rini BI, Halabi S, Rosenberg JE, Stadler
WM, Vaena DA, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J
and Small EJ: Phase III trial of bevacizumab plus interferon alfa
versus interferon alfa monotherapy in patients with metastatic
renal cell carcinoma: Final results of CALGB 90206. J Clin Oncol.
28:2137–2143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Motzer RJ, Escudier B, Oudard S, Hutson
TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA,
Hollaender N, et al: Phase 3 trial of everolimus for metastatic
renal cell carcinoma: Final results and analysis of prognostic
factors. Cancer. 116:4256–4265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili
R, Bjarnason GA, et al: Overall survival and updated results for
sunitinib compared with interferon alfa in patients with metastatic
renal cell carcinoma. J Clin Oncol. 27:3584–3590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dutcher JP, de Souza P, McDermott D,
Figlin RA, Berkenblit A, Thiele A, Krygowski M, Strahs A, Feingold
J and Hudes G: Effect of temsirolimus versus interferon-alpha on
outcome of patients with advanced renal cell carcinoma of different
tumor histologies. Med Oncol. 26:202–209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rini BI, Halabi S, Rosenberg JE, Stadler
WM, Vaena DA, Ou SS, Archer L, Atkins JN, Picus J, Czaykowski P, et
al: Bevacizumab plus interferon alfa compared with interferon alfa
monotherapy in patients with metastatic renal cell carcinoma: CALGB
90206. J Clin Oncol. 26:5422–5428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Motzer RJ, Escudier B, Oudard S, Hutson
TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA,
Hollaender N, et al: Efficacy of everolimus in advanced renal cell
carcinoma: A double-blind, randomised, placebo-controlled phase III
trial. Lancet. 372:449–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hudes G, Carducci M, Tomczak P, Dutcher J,
Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi
I, et al: Temsirolimus, interferon alfa, or both for advanced
renal-cell carcinoma. N Engl J Med. 356:2271–2281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee SH, Lopes de Menezes D, Vora J, Harris
A, Ye H, Nordahl L, Garrett E, Samara E, Aukerman SL, Gelb AB and
Heise C: In vivo target modulation and biological activity of
CHIR-258, a multitargeted growth factor receptor kinase inhibitor,
in colon cancer models. Clin Cancer Res. 11:3633–3641. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43-9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tonin FS, Rotta I, Mendes AM and Pontarolo
R: Network meta-analysis: A technique to gather evidence from
direct and indirect comparisons. Pharm Pract (Granada). 15:9432017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Salanti G, Ades AE and Ioannidis JP:
Graphical methods and numerical summaries for presenting results
from multiple-treatment meta-analysis: An overview and tutorial. J
Clin Epidemiol. 64:163–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hutton B, Salanti G, Caldwell DM, Chaimani
A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen
JP, et al: The PRISMA extension statement for reporting of
systematic reviews incorporating network meta-analyses of health
care interventions: Checklist and explanations. Ann Intern Med.
162:777–784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wei W, Peng R, Zeng L, Xu C, Cao Y, Chen W
and Xia S: Comparative efficacy and safety of targeted therapy in
the treatment of renal cell carcinoma: A Bayesian network analysis.
Value Health. 21 (Suppl 2):S1132018. View Article : Google Scholar
|
|
51
|
Higgins JPT and Green S: Cochrane handbook
for systematic reviews of interventions 4.2.6The Cochrane Library.
4:157–161. 2006.
|
|
52
|
Bramer WM, Giustini D, de Jonge GB,
Holland L and Bekhuis T: De-duplication of database search results
for systematic reviews in EndNote. J Med Libr Assoc. 104:240–243.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group, : Preferred reporting items for systematic
reviews and meta-analyses: The PRISMA statement. Int J Surg.
8:336–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chaimani A, Higgins JP, Mavridis D,
Spyridonos P and Salanti G: Graphical tools for network
meta-analysis in STATA. PLoS One. 8:e766542013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lunn DJ, Thomas A, Best N and
Spiegelhalter D: WinBUGS-A Bayesian modelling framework: Concepts,
structure and extensibility. Stat Computing. 10:325–337. 2000.
View Article : Google Scholar
|
|
58
|
Woods BS, Hawkins N and Scott DA: Network
meta-analysis on the log-hazard scale, combining count and hazard
ratio statistics accounting for multi-arm trials: A tutorial. BMC
Med Res Methodol. 10:542010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Spiegelhalter DJ, Best NG, Carlin BP and
Van Der Linde A: Bayesian measures of model complexity and fit. J
Royal Stat Soc Series B (Statistical Methodology). 64:583–639.
2002. View Article : Google Scholar
|
|
60
|
Kane RC, Farrell AT, Saber H, Tang S,
Williams G, Jee JM, Liang C, Booth B, Chidambaram N, Morse D, et
al: Sorafenib for the treatment of advanced renal cell carcinoma.
Clin Cancer Res. 12:7271–7278. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wiecek W and Karcher H: Nivolumab versus
cabozantinib: Comparing overall survival in metastatic renal cell
carcinoma. PLoS One. 11:e01553892016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Song F, Xiong T, Parekh-Bhurke S, Loke YK,
Sutton AJ, Eastwood AJ, Holland R, Chen YF, Glenny AM, Deeks JJ and
Altman DG: Inconsistency between direct and indirect comparisons of
competing interventions: Meta-epidemiological study. BMJ.
343:d49092011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
He HL and Yao WX: A network meta-analysis
of short-term efficacy of different single-drug targeted therapies
in the treatment of renal cell carcinoma. Biosci Rep. 37(pii):
BSR201708272017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Rousseau B, Kempf E, Desamericq G,
Boissier E, Chaubet-Houdu M, Joly C, Saldana C, Boussion H,
Neuzillet C, Macquin-Mavier I, et al: First-line antiangiogenics
for metastatic renal cell carcinoma: A systematic review and
network meta-analysis. Crit Rev Oncol Hematol. 107:44–53. 2016.
View Article : Google Scholar : PubMed/NCBI
|