Introduction
Cervical cancer is the fourth most frequently
diagnosed tumor and the fourth leading cause of cancer-associated
mortalities in women worldwide. In 2018, ~570,000 females were
diagnosed with cervical cancer and ~311,000 deaths were reported
(1). The burden of this type of
cancer remains heavy, particularly in low-to-middle-income
countries. In fact, the number of cervical cancer deaths in
developing countries accounted for ~90% of all cervical cancer
deaths worldwide in 2015 (2).
Cervical cancer ranks second in incidence and mortality in low- and
middle-income countries (1). In
high-income countries, the incidence and mortality rates of
cervical cancer have decreased dramatically due to screening
programs being made available in the mid-20th century (3). From 2006 to 2014 in the United States
of America (USA), delay-adjusted cervical cancer incidence rates
decreased at an average annual percentage rate of 0.3% (4). Mortality rates have also declined at an
average annual rate of 0.8% between 2003 and 2014 (4). However, in 2018, ~13,240 women were
diagnosed with invasive cervical cancer and 4,170 patients
succumbed to the disease in the USA (5). Human papilloma virus infection is a
risk factor for cervical cancer, but infection alone does not
necessarily lead to the development of the disease (6). Thus, the identification of novel
biomarkers with prognostic value is urgently required.
Additionally, this may clarify the mechanism underlying
tumorigenesis and aid the identification of novel therapeutic
targets.
Fibronectin type III domain containing 3B (FNDC3B),
also termed factor for adipocyte differentiation 104 (FAD104), was
initially determined to be a regulator of adipocyte differentiation
(7). A previous study used gene
targeting to demonstrate that FNDC3B was involved in cell
proliferation, adhesion, spreading and migration in
FNDC3B-deficient mice (8). FNDC3B
has been previously identified as an oncogene that promotes cell
migration in hepatocellular carcinoma (3,9).
However, to the best of the authors' knowledge, the prognostic
value and function of FNDC3B in cervical cancer has not yet been
elucidated.
Systematic biology comprehensively determines the
underlying mechanism and allows the identification of new
biomarkers in human disease on a global scale. Networks are
practical graphical representations of complex interactions
(10). The combination of systematic
biology and networks is therefore useful to visualize complex
biological activities and to annotate protein functions and
predictions (11). Thus, the present
study utilized systematic biology and network methods to predict
the effect of FNDC3B expression on the prognosis of patients with
cervical carcinoma and to annotate protein function.
The present study assessed the expression of FNDC3B
mRNA in patients with cervical cancer using the ONCOMINE database.
Subsequently, the association between FNDC3B expression and
prognosis was investigated, and the biological function and
mechanism of action of FNDC3B in patients with cervical cancer was
explored using publicly accessible databases.
Materials and methods
Expression analysis of FNDC3B in
cervical cancer
The expression value of FNDC3B mRNA in cervical
cancer was analyzed using the ONCOMINE database (version 4.5;
www.oncomine.org/resource/login.html) (12). Cancerous tissues and normal tissues
obtained from healthy volunteers were subsequently compared
according to the default settings of P<1×10−4,
fold-change >2 and gene ranking in the top 10% (13).
Survival analysis
FNDC3B gene expression data and the clinical
characteristics of patients with cervical cancer were downloaded
from The Cancer Genome Atlas (www.cbioportal.org) (14,15). The
association between FNDC3B expression and patient overall survival
(OS) was analyzed using the R package survival (version 2.43–3,
http://cran.r-project.org/web/views/Survival.html)
(16,17). Samples were then divided into high-
and low-expression groups using the median expression level of
FNDC3B mRNA as the cut-off point. The difference in OS between the
two groups was assessed using Kaplan-Meier curves followed by a
log-rank test.
Co-expression gene identification and
protein-protein interaction network visualization
The cBioportal database (cbioportal.org) was used to assess and visualize
cancer co-expression data (14),
which was subsequently downloaded. FNDC3B co-expression genes with
an absolute correlation coefficient of >0.4 and P<0.05 were
obtained from cBioPortal. The Search Tool for the Retrieval of
Interacting Genes/Proteins (STRING version 10.5, string-db.org) was used to perform protein-protein
interaction (PPI) analysis (18).
Data was subsequently downloaded and the PPI network was
constructed using Cytoscape software (version 3.7.1) (19).
Gene ontology (GO) and Kyoto
encyclopedia of genes and genomes (KEGG) enrichment analysis
The clusterProfiler package (version 3.8.1,
http://bioconductor.org/packages/release/bioc/html/clusterProfiler.html)
(20) in R was used to identify and
visualize the GO terms (geneontology.org) and KEGG pathways (www.genome.jp/kegg) associated with the FNDC3B
co-expression genes. The P-value was adjusted using the
Benjamini-Hochberg method. P<0.05 and q<0.05 were set as the
cut-off criteria for significant enrichment.
Localization of FNDC3B in cells
The cellular localization of FNDC3B was determined
using The Human Protein Atlas (version 18.1, www.proteinatlas.org) (21). The key word used for searching was
‘FNDC3B’. The location of FNDC3B in cells was determined using the
immunofluorescence with anti-FNDC3B antibodies (cat. no. HPA007859;
Atlas Antibodies AB). Images were obtained from www.proteinatlas.org/ENSG00000075420-FNDC3B/cell#img.
Statistical analysis
Statistical analyses were performed using R software
(version 3.5.1; R Foundation for Statistical Computing). The
relative expression of FNDC3B was presented as the mean ± standard
deviation. The differential expression of FNDC3B between cancerous
and non-cancerous samples was compared using an independent
Student's t-test. A total of 20 cancerous and eight non-cancerous
samples from the multi-cancer dataset published by Pyeon et
al (22) were selected for
analysis in the present study. An additional 32 cancerous and 21
non-cancerous samples were selected from a dataset published by
Scotto et al (23).
Kaplan-Meier survival analysis was performed to estimate the
survival distributions and the log-rank test was used to compare
the survival curves. The correlation of gene expression was
analyzed by Spearman's correlation test. P<0.05 was considered
to indicate a statistically significant difference.
Results
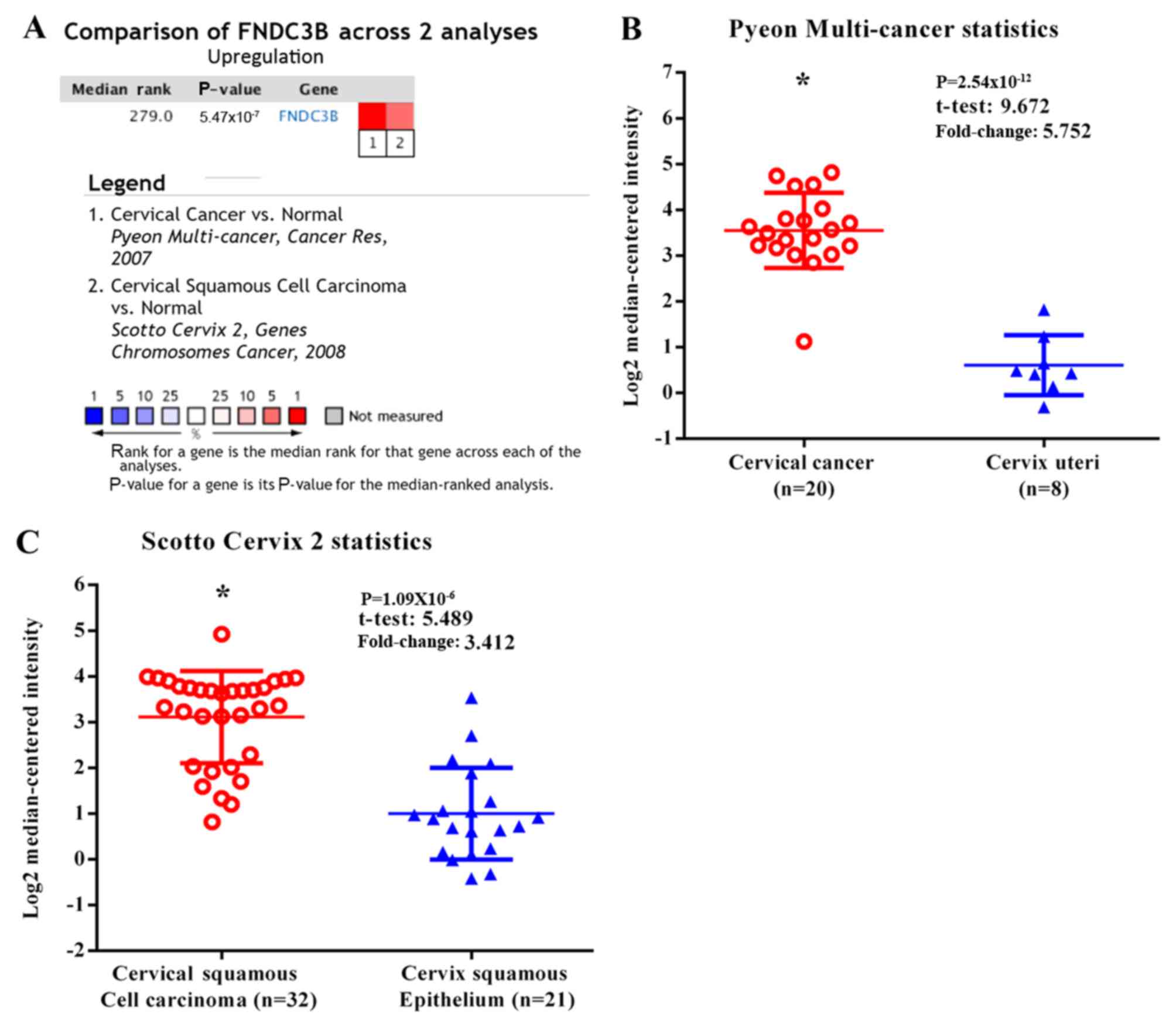
FNDC3B expression is upregulated in
cervical cancer
Analysis of the ONCOMINE database revealed that the
level of FNDC3B mRNA was significantly increased in cervical cancer
tissues compared with normal tissues. By contrast, no cervical
cancer tissues with downregulated FNDC3B expression were identified
(Fig. 1).
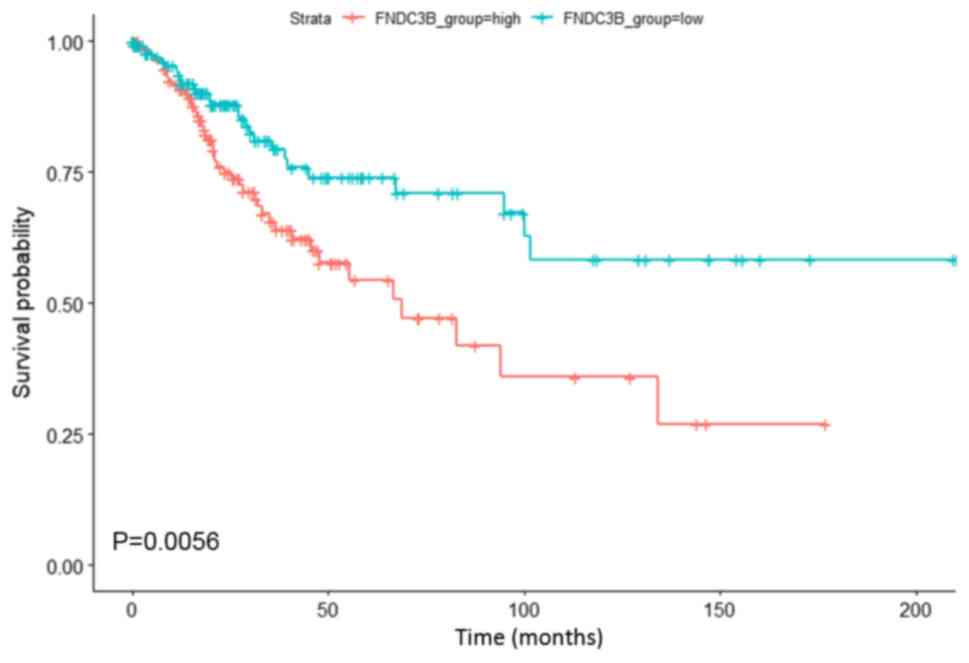
Survival prediction of FNDC3B in
cervical cancer
Survival analysis was performed to investigate the
association between upregulated FNDC3B expression and the clinical
outcome of patients with cervical cancer. As presented in Fig. 2, upregulated FNDC3B expression was
significantly associated with a lower OS in patients with cervical
cancer. The results indicated that upregulated FNDC3B expression
may serve as a biomarker of poor prognosis in patients with
cervical cancer.
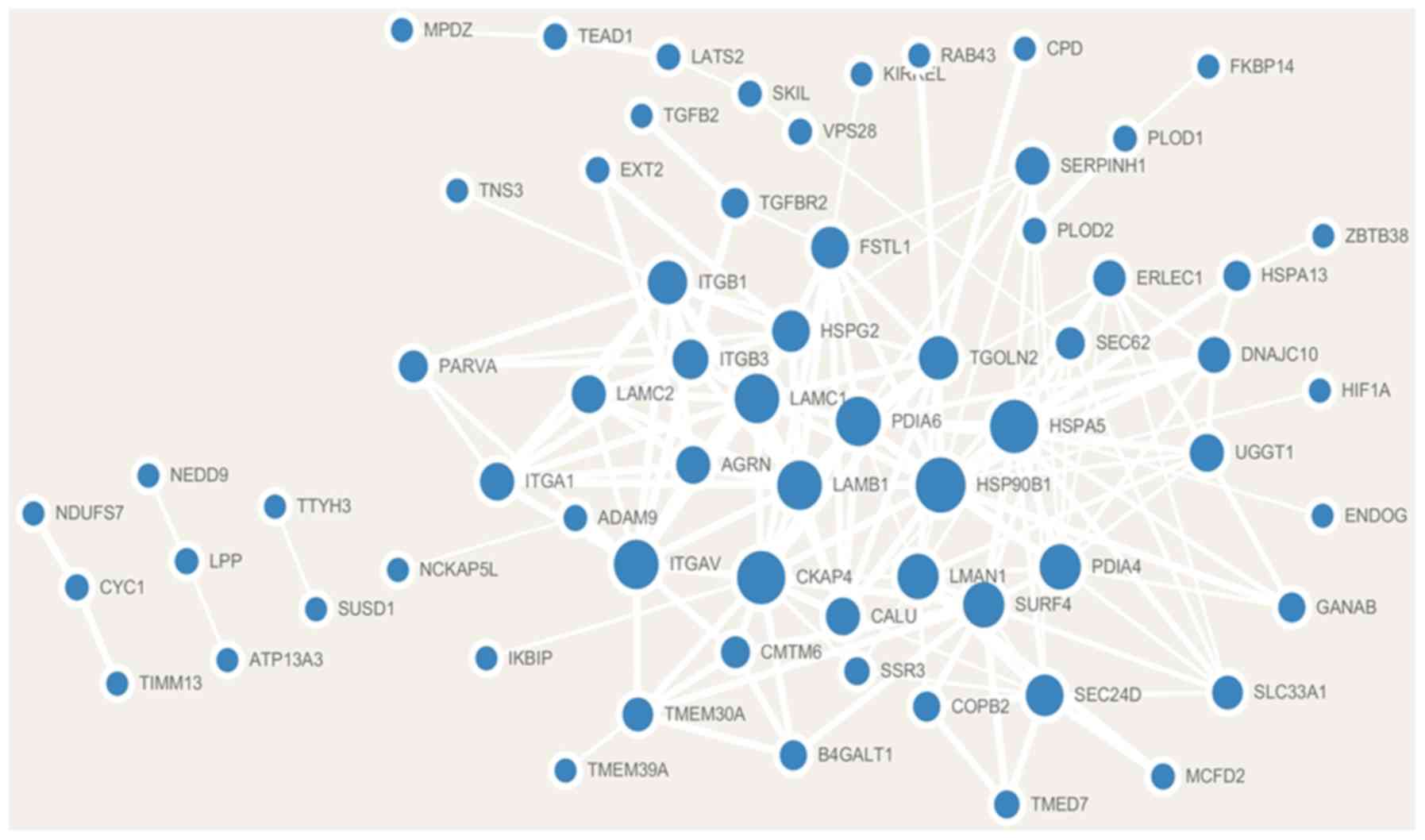
Co-expression gene identification and
PPI network visualization
Analysis of the cBioPortal database revealed that a
total of 88 genes were significantly co-expressed with FNDC3B.
Additionally, 79 co-expressed genes were positively correlated with
FNDC3B and 9 co-expressed genes were negatively correlated with
FNDC3B (Table I). A PPI network
consisting of FNDC3B co-expression genes based on the STRING
database was constructed using Cytoscape software. The
co-expression network contained 66 nodes and 179 edges (Fig. 3).
 | Table I.Co-expressed genes associated with
fibronectin type III domain containing 3B. |
Table I.
Co-expressed genes associated with
fibronectin type III domain containing 3B.
| Correlated
gene | Cytoband | Spearman's
correlation coefficient | P-value |
|---|
| NCEH1 | 3q26.31 | 0.56 |
3.51×10−26 |
| B4GALT1 | 9p21.1 | 0.54 |
5.53×10−25 |
| CALU | 7q32.1 | 0.54 |
7.49×10−25 |
| LAMC1 | 1q25.3 | 0.54 |
1.87×10−24 |
| ITGB1 | 10p11.22 | 0.52 |
4.80×10−23 |
| MCFD2 | 2p21 | 0.52 |
2.19×10−22 |
| TMED7 | 5q22.3 | 0.51 |
6.07×10−22 |
| COPB2 | 3q23 | 0.51 |
8.75×10−22 |
| SKIL | 3q26.2 | 0.51 |
1.72×10−21 |
| UGGT1 | 2q14.3 | 0.50 |
3.66×10−21 |
| TMEM263 | 12q23.3 | 0.50 |
5.60×10−21 |
| HSPA5 | 9q33.3 | 0.50 |
2.20×10−20 |
| SEC62 | 3q26.2 | 0.49 |
4.63×10−20 |
| SUSD1 | 9q31.3-q32 | 0.49 |
1.62×10−19 |
| PLOD2 | 3q24 | 0.48 |
2.13×10−19 |
| TEAD1 | 11p15.3 | 0.47 |
2.76×10−18 |
| LMAN1 | 18q21.32 | 0.47 |
2.81×10−18 |
| HSP90B1 | 12q23.3 | 0.47 |
3.08×10−18 |
| FKBP14 | 7p14.3 | 0.47 |
6.05×10−18 |
| ITGB3 | 17q21.32 | 0.47 |
6.39×10−18 |
| CCDC50 | 3q28 | 0.47 |
6.82×10−18 |
| KIRREL1 | 1q23.1 | 0.47 |
6.89×10−18 |
| LPP | 3q27.3-q28 | 0.47 |
7.66×10−18 |
| SLC39A14 | 8p21.3 | 0.46 |
1.04×10−17 |
| NCKAP5L | 12q13.12 | 0.46 |
1.12×10−17 |
| ATP13A3 | 3q29 | 0.46 |
1.29×10−17 |
| EXT2 | 11p11.2 | 0.46 |
1.76×10−17 |
| LAMB1 | 7q31.1 | 0.46 |
2.85×10−17 |
| SLC33A1 | 3q25.31 | 0.46 |
2.99×10−17 |
| TTYH3 | 7p22.3 | 0.46 |
3.22×10−17 |
| FSTL1 | 3q13.33 | 0.46 |
4.40×10−17 |
| SSR3 | 3q25.31 | 0.46 |
4.76×10−17 |
| IKBIP | 12q23.1 | 0.45 |
6.75×10−17 |
| SERPINH1 | 11q13.5 | 0.45 |
2.10×10−16 |
| PDIA6 | 2p25.1 | 0.44 |
4.18×10−16 |
| TMEM30A | 6q14.1 | 0.44 |
4.84×10−16 |
| PLOD1 | 1p36.22 | 0.43 |
1.57×10−15 |
| PLBD2 | 12q24.13 | 0.43 |
1.63×10−15 |
| AGRN | 1p36.33 | 0.43 |
1.75×10−15 |
| GNS | 12q14.3 | 0.43 |
1.96×10−15 |
| ZNF281 | 1q32.1 | 0.43 |
2.00×10−15 |
| SLC41A2 | 12q23.3 | 0.43 |
3.07×10−15 |
| ADAM9 | 8p11.22 | 0.43 |
3.18×10−15 |
| TGFBR2 | 3p24.1 | 0.43 |
3.54×10−15 |
| HIF1A | 14q23.2 | 0.43 |
3.80×10−15 |
| STC1 | 8p21.2 | 0.43 |
4.46×10−15 |
| DNAJC10 | 2q32.1 | 0.43 |
5.28×10−15 |
| ITGAV | 2q32.1 | 0.43 |
7.41×10−15 |
| GANAB | 11q12.3 | 0.42 |
9.71×10−15 |
| PAPSS2 | 10q23.2-q23.31 | 0.42 |
1.02×10−14 |
| RAB43 | 3q21.3 | 0.42 |
1.05×10−14 |
| TGOLN2 | 2p11.2 | 0.42 |
1.63×10−14 |
| TMEM39A | 3q13.33 | 0.42 |
1.71×10−14 |
| ITFG1 | 16q12.1 | 0.42 |
2.29×10−14 |
| BICC1 | 10q21.1 | 0.42 |
2.66×10−14 |
| ZBTB38 | 3q23 | 0.41 |
3.83×10−14 |
| ERLEC1 | 2p16.2 | 0.41 |
5.09×10−14 |
| SEC24D | 4q26 | 0.41 |
5.18×10−14 |
| HSPA13 | 21q11.2 | 0.41 |
5.49×10−14 |
| LATS2 | 13q12.11 | 0.41 |
5.53×10−14 |
| MPDZ | 9p23 | 0.41 |
5.81×10−14 |
| LAMC2 | 1q25.3 | 0.41 |
5.85×10−14 |
| OSMR | 5p13.1 | 0.41 |
6.70×10−14 |
| RAI14 | 5p13.2 | 0.41 |
7.52×10−14 |
| HSPG2 | 1p36.12 | 0.41 |
7.53×10−14 |
| PDIA4 | 7q36.1 | 0.41 |
7.92×10−14 |
| ITGA1 | 5q11.2 | 0.41 |
9.88×10−14 |
| CMTM6 | 3p22.3 | 0.41 |
1.02×10−13 |
| SURF4 | 9q34.2 | 0.41 |
1.25×10−13 |
| TNS3 | 7p12.3 | 0.41 |
1.29×10−13 |
| CPD | 17q11.2 | 0.41 |
1.40×10−13 |
| OSBPL10 | 3p23 | 0.41 |
1.42×10−13 |
| CD276 | 15q24.1 | 0.40 |
2.47×10−13 |
| GALNT1 | 18q12.2 | 0.40 |
2.72×10−13 |
| CKAP4 | 12q23.3 | 0.40 |
2.91×10−13 |
| GPX8 | 5q11.2 | 0.40 |
2.97×10−13 |
| NEDD9 | 6p24.2 | 0.40 |
3.25×10−13 |
| TGFB2 | 1q41 | 0.40 |
3.30×10−13 |
| PARVA | 11p15.3 | 0.40 |
3.37×10−13 |
| NUDT8 | 11q13.2 | −0.40 |
3.28×10−13 |
| NOL12 | 22q13.1 | −0.40 |
3.28×10−13 |
| TIMM13 | 19p13.3 | −0.40 |
3.08×10−13 |
| ENDOG | 9q34.11 | −0.40 |
2.91×10−13 |
| COQ4 | 9q34.11 | −0.41 |
1.41×10−13 |
| VPS28 | 8q24.3 | −0.41 |
6.90×10−14 |
| CYC1 | 8q24.3 | −0.43 |
4.54×10−15 |
| COMTD1 | 10q22.2 | −0.43 |
3.48×10−15 |
| NDUFS7 | 19p13.3 | −0.43 |
1.82×10−15 |
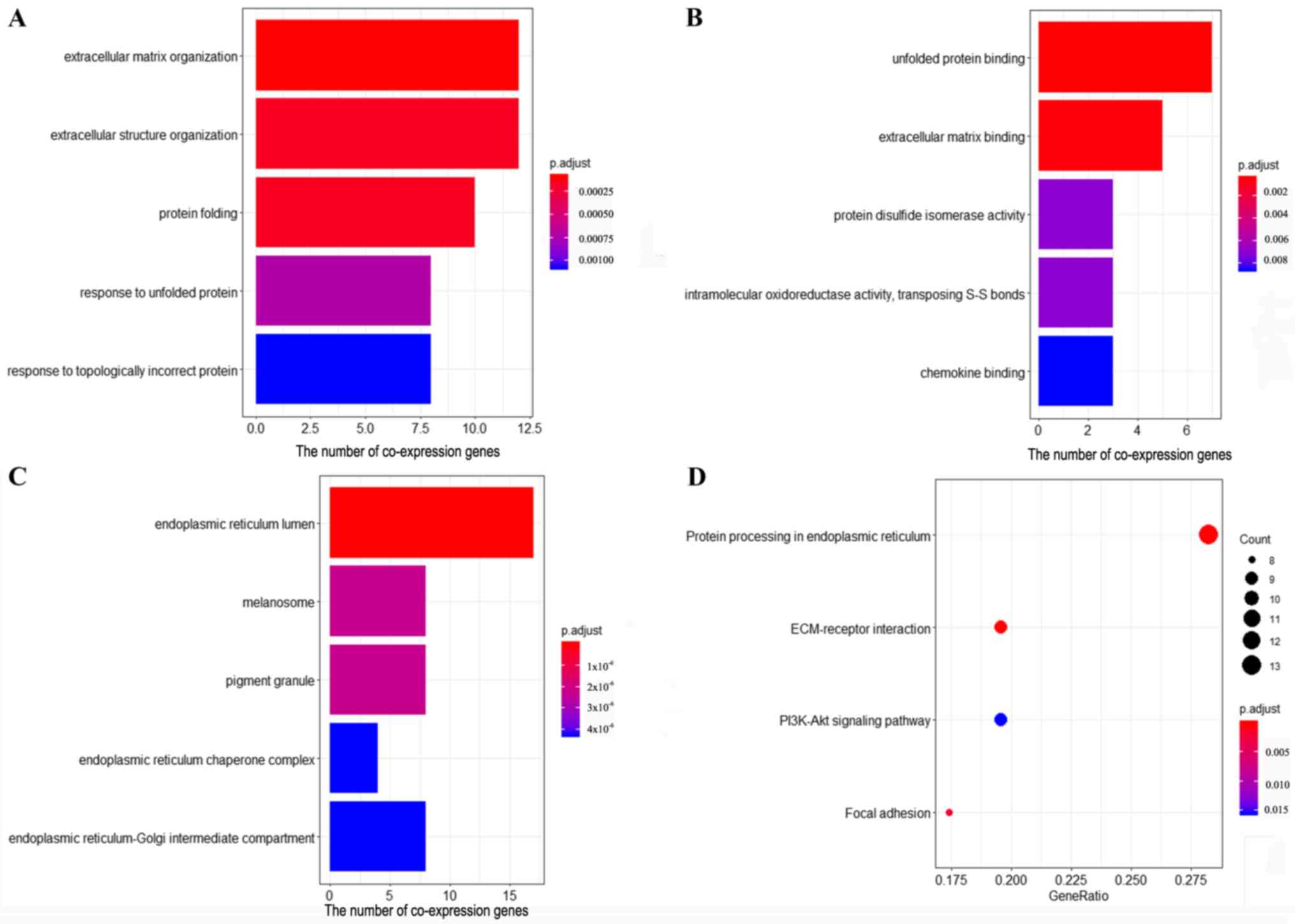
Gene co-expression network analysis is
associated with FNDC3B in cervical cancer
The results of GO enrichment analysis revealed that
the co-expression genes were significantly enriched in 72
biological processes (BPs), 29 molecule functions (MFs) and 50
cellular components (CCs). The five top ranked BPs, MFs and CCs
were as follows: ‘Extracellular matrix organization’,
‘extracellular structure organization’, ‘protein folding’,
‘response to unfolded protein’, ‘response to topologically
incorrect protein’, ‘unfolded protein binding’, ‘extracellular
matrix binding’, ‘protein disulfide isomerase activity’,
‘intramolecular oxidoreductase activity, transposing S-S bonds’,
‘chemokine binding’, ‘endoplasmic reticulum (ER) lumen’,
‘melanosome’, ‘pigment granule’, ‘ER chaperone complex’ and
‘ER-Golgi intermediate compartment’ (Fig. 4A-C). The results of KEGG pathway
enrichment analysis demonstrated that the co-expression genes of
FNDC3B were significantly enriched in four pathways, including
‘protein processing in ER’, ‘extracellular matrix (ECM)-receptor
interaction’, ‘focal adhesion’ and ‘PI3K-Akt signaling pathway’
(Fig. 4D).
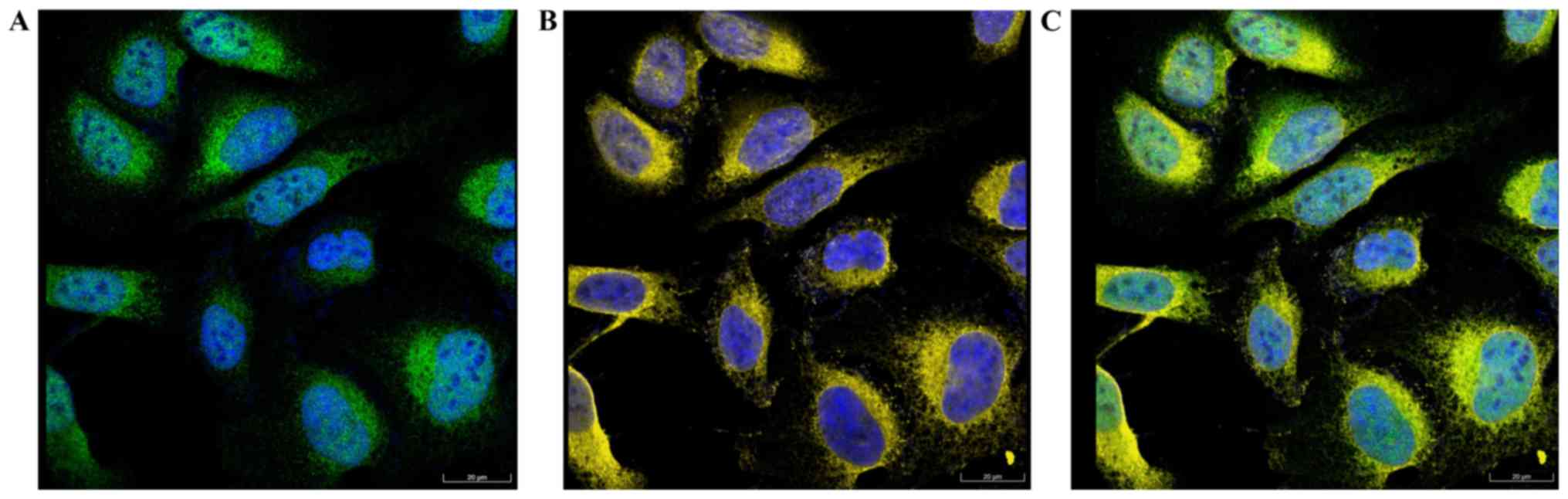
Cellular location of FNDC3B
The results of immunofluorescence analysis obtained
from The Human Protein Atlas database are presented in Fig. 5. Co-localization of FNDC3B (green)
and ER (yellow) was observed, indicating that FNDC3B was localized
to the ER.
Discussion
The present study assessed the prognostic effect of
FNDC3B and its potential underlying molecular mechanisms in
cervical cancer using bioinformatics tools. FNDC3B is an important
oncogenic driver gene that was identified in an oncogenomic screen
for oncogenes in hepatocellular carcinoma (3). Lin et al (9) identified FNDC3B as a biomarker and
therapeutic target for hepatocellular carcinoma metastasis. In the
present study, FNDC3B expression was upregulated in cervical cancer
tissues and was associated with a poor prognosis. As the function
of FNDC3B in cervical cancer is unknown, the present study
investigated its potential functions by constructing a
co-expression network. GO and KEGG enrichment analyses revealed
that FNDC3B was associated with ER stress and UPR signaling.
Furthermore, KEGG pathways analysis revealed that FNDC3B was
enriched in ‘protein processing in ER’. The ER is a subcellular
organelle that is associated with protein synthesis, folding and
quality control (24). Adequately
folded proteins are subsequently transported to their destined
sites, whereas terminally misfolded proteins are subjected to
degradation via ER-associated degradation pathways (25). Certain biological processes including
‘protein folding’, ‘response to unfolded protein’ and ‘response to
topologically incorrect protein’ were enriched in the present study
and were associated with ER stress and UPR activation. The highest
enrichment of MF and CC were ‘unfolded protein binding’ and ‘ER
lumen’, respectively. These indicated that the function of FNDC3B
may be associated with ER stress and UPR. The role of ER stress and
UPR activation in the development of cancer has been previously
revealed in various types of cancer, including cervical cancer
(26–28). Tumor growth can produce several
cell-intrinsic and extrinsic stresses (29). The effects induced by these stresses
disrupt the ER protein-folding environment, resulting in protein
misfolding and the accumulation of misfolded proteins, which is
referred to as ER stress (26).
Tolerable levels of ER stress promote tumor development by
bolstering viability under hypoxia and nutrient deprivation,
enhancing metastatic spread by supporting epithelial-mesenchymal
transition (EMT), tumor cell dormancy and tumor-initiating cell
function, thereby stimulating angiogenesis (29). ER stress can activate the UPR, which
is mediated by three ER membrane localized stress sensing proteins:
Inositol-requiring enzyme 1, activating transcription factor 6 and
protein kinase RNA-like ER kinase (30). Additionally, UPR activation may be
tumor-supportive or suppressive depending on the intensity and
duration of ER stress (31). The UPR
also acts to restore ER homeostasis for cancer cell survival
(32). When corrective efforts are
insufficient, the cell will undergo apoptosis (33). In the current study, FNDC3B was
localized to the ER. The results of the present study may therefore
indicate the function of FNDC3B in ER stress and UPR.
Although FNDC3B acts as an oncogenic gene, its
target genes have not been identified. However, certain studies
have indicated that FNDC3B may be involved in stress granule
formation-mediated ER stress (34–36).
Stress granules are dense aggregations that are composed of mRNAs
and proteins under conditions of stress. FNDC3B is primarily
composed of fibronectin type III domains (9). FNDC3B was identified as an RNA-binding
protein candidate via the interactome capture of proliferating
human HeLa cells (37). When cells
were challenged with ER stress, stress granules were formed
(35). FNDC3B has also been
identified in stress granule proteomes (34). Stress granules recruit various mRNAs
and signaling proteins, including receptor for activated C kinase
1/mitogen-activated protein kinase 14/JNK, integrated stress
response/phosphorylated-eukaryotic translation initiation factor
2A, rapamycin and Rho GTPase signaling pathways, which modulate
metabolism, growth and survival (36). However, the role of FNDC3B in stress
granule formation requires further elucidation.
In congruence with the current study, a previous
study has indicated that FNDC3B induces and activates the PI3K/Akt
signaling pathway (3). The FNDC3B
co-expression genes identified in the present study were enriched
in the PI3K/Akt signaling pathway, which serves a pivotal role in
tumor growth, proliferation, metabolism, motility, migration,
invasion, angiogenesis, survival and autophagy (38). Considering the additional pathway
enrichment of ‘ECM-receptor interaction’ and ‘focal adhesion’
determined in the current study, FNDC3B may be involved in
migration and invasion.
In conclusion, the present study revealed that
FNDC3B was upregulated in cervical cancer tissue compared with
normal tissue. Furthermore, elevated FNDC3B levels were associated
with poor OS. Therefore, it was determined that elevated FNDC3B may
be a biomarker for poor prognosis in patients with cervical cancer.
Coupled with the co-expression network analysis of the current
study, it was inferred that FNDC3B may serve an oncogenic role in
cancer development via ER stress, UPR, cell migration and invasion.
However, further studies are required to determine the exact
molecular mechanism of FNDC3B in the development of cervical cancer
and its potential as a novel therapeutic target.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Major New
Drugs Research & Development Special Project of the Ministry of
Science and Technology of P.R. China (grant no.
2018ZX09303015).
Availability of data and materials
All datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ, JT and HW designed the present study. BH and JZ
performed the experiments, analyzed the data and prepared the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics statement and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen PA, Jhingran A, Oaknin A and Denny
L: Cervical cancer. Lancet. 393:169–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cai C, Rajaram M, Zhou X, Liu Q, Marchica
J, Li J and Powers RS: Activation of multiple cancer pathways and
tumor maintenance function of the 3q amplified oncogene FNDC3B.
Cell Cycle. 11:1773–1781. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith RA, Andrews KS, Brooks D, Fedewa SA,
Manassaram-Baptiste D, Saslow D, Brawley OW and Wender RC: Cancer
screening in the United States, 2018: A review of current American
Cancer Society guidelines and current issues in cancer screening.
CA Cancer J Clin. 68:297–316. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi YJ and Park JS: Clinical significance
of human papillomavirus genotyping. J Gynecol Oncol. 27:e212016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tominaga K, Kondo C, Johmura Y, Nishizuka
M and Imagawa M: The novel gene fad104, containing a fibronectin
type III domain, has a significant role in adipogenesis. FEBS Lett.
577:49–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishizuka M, Kishimoto K, Kato A, Ikawa M,
Okabe M, Sato R, Niida H, Nakanishi M, Osada S and Imagawa M:
Disruption of the novel gene fad104 causes rapid postnatal death
and attenuation of cell proliferation, adhesion, spreading and
migration. Exp Cell Res. 315:809–819. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin CH, Lin YW, Chen YC, Liao CC, Jou YS,
Hsu MT and Chen CF: FNDC3B promotes cell migration and tumor
metastasis in hepatocellular carcinoma. Oncotarget. 7:49498–49508.
2016.PubMed/NCBI
|
|
10
|
Noell G, Faner R and Agusti A: From
systems biology to P4 medicine: Applications in respiratory
medicine. Eur Respir Rev. 27(pii): 1701102018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin M, Ye M, Zhou J, Wang ZP and Zhu X:
Recent advances on the molecular mechanism of cervical
carcinogenesis based on systems biology technologies. Comput Struct
Biotechnol J. 17:241–250. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Cui S, Li W, Zhao Y, Yan X and Xu
J: PAX3 is a biomarker and prognostic factor in melanoma: Database
mining. Oncol Lett. 17:4985–4993. 2019.PubMed/NCBI
|
|
14
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Therneau T: A Package for survival
analysis in S. R package version 2.38. https://CRAN.R-project.org/package=survival2015
|
|
17
|
Terry MT and Grambsch PM: Modeling
survival data: Extending the cox model. Springer; New York:
2000
|
|
18
|
Szklarczyk D, Gable AL, Lyon D, Junge A,
Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork
P, et al: STRING v11: Protein-protein association networks with
increased coverage, supporting functional discovery in genome-wide
experimental datasets. Nucleic Acids Res. 47:D607–D613. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu G, Wang LG, Han Y and He QY:
ClusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uhlen M, Zhang C, Lee S, Sjöstedt E,
Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, et
al: A pathology atlas of the human cancer transcriptome. Science.
357(pii): eaan25072017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pyeon D, Newton MA, Lambert PF, den Boon
JA, Sengupta S, Marsit CJ, Woodworth CD, Connor JP, Haugen TH,
Smith EM, et al: Fundamental differences in cell cycle deregulation
in human papillomavirus-positive and human papillomavirus-negative
head/neck and cervical cancers. Cancer Res. 67:4605–4619. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scotto L, Narayan G, Nandula SV,
Arias-Pulido H, Subramaniyam S, Schneider A, Kaufmann AM, Wright
JD, Pothuri B, Mansukhani M and Murty VV: Identification of copy
number gain and overexpressed genes on chromosome arm 20q by an
integrative genomic approach in cervical cancer: Potential role in
progression. Genes Chromosomes Cancer. 47:755–765. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vitale A and Denecke J: The endoplasmic
reticulum-gateway of the secretory pathway. Plant Cell. 11:615–628.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jain BP: An overview of unfolded protein
response signaling and its role in cancer. Cancer Biother
Radiopharm. 32:275–281. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang M and Kaufman RJ: The impact of the
endoplasmic reticulum protein-folding environment on cancer
development. Nat Rev Cancer. 14:581–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang M, Law ME, Castellano RK and Law BK:
The unfolded protein response as a target for anticancer
therapeutics. Crit Rev Oncol Hematol. 127:66–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taguchi Y, Horiuchi Y, Kano F and Murata
M: Novel prosurvival function of Yip1A in human cervical cancer
cells: Constitutive activation of the IRE1 and PERK pathways of the
unfolded protein response. Cell Death Dis. 8:e27182017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cubillos-Ruiz JR, Bettigole SE and
Glimcher LH: Tumorigenic and immunosuppressive effects of
endoplasmic reticulum stress in cancer. Cell. 168:692–706. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostatic regulation. Science.
334:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang M and Kaufman RJ: Protein misfolding
in the endoplasmic reticulum as a conduit to human disease. Nature.
529:326–335. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tashiro E: Screening and identification of
inhibitors of endoplasmic reticulum stress-induced activation of
the IRE1a-XBP1 branch. J Antibiot (Tokyo). Aug 9–2019.(Epub ahead
of print). View Article : Google Scholar
|
|
33
|
Maurel M, McGrath EP, Mnich K, Healy S,
Chevet E and Samali A: Controlling the unfolded protein
response-mediated life and death decisions in cancer. Semin Cancer
Biol. 33:57–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jain S, Wheeler JR, Walters RW, Agrawal A,
Barsic A and Parker R: ATPase-modulated stress granules contain a
diverse proteome and substructure. Cell. 164:487–498. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Namkoong S, Ho A, Woo YM, Kwak H and Lee
JH: Systematic characterization of stress-induced RNA granulation.
Mol Cell. 70:175–187 e8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kedersha N, Ivanov P and Anderson P:
Stress granules and cell signaling: More than just a passing phase?
Trends Biochem Sci. 38:494–506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Castello A, Fischer B, Eichelbaum K, Horos
R, Beckmann BM, Strein C, Davey NE, Humphreys DT, Preiss T,
Steinmetz LM, et al: Insights into RNA biology from an atlas of
mammalian mRNA-binding proteins. Cell. 149:1393–1406. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McAuliffe PF, Meric-Bernstam F, Mills GB
and Gonzalez-Angulo AM: Deciphering the role of PI3K/Akt/mTOR
pathway in breast cancer biology and pathogenesis. Clin Breast
Cancer. 10 (Suppl 3):S59–S65. 2010. View Article : Google Scholar : PubMed/NCBI
|