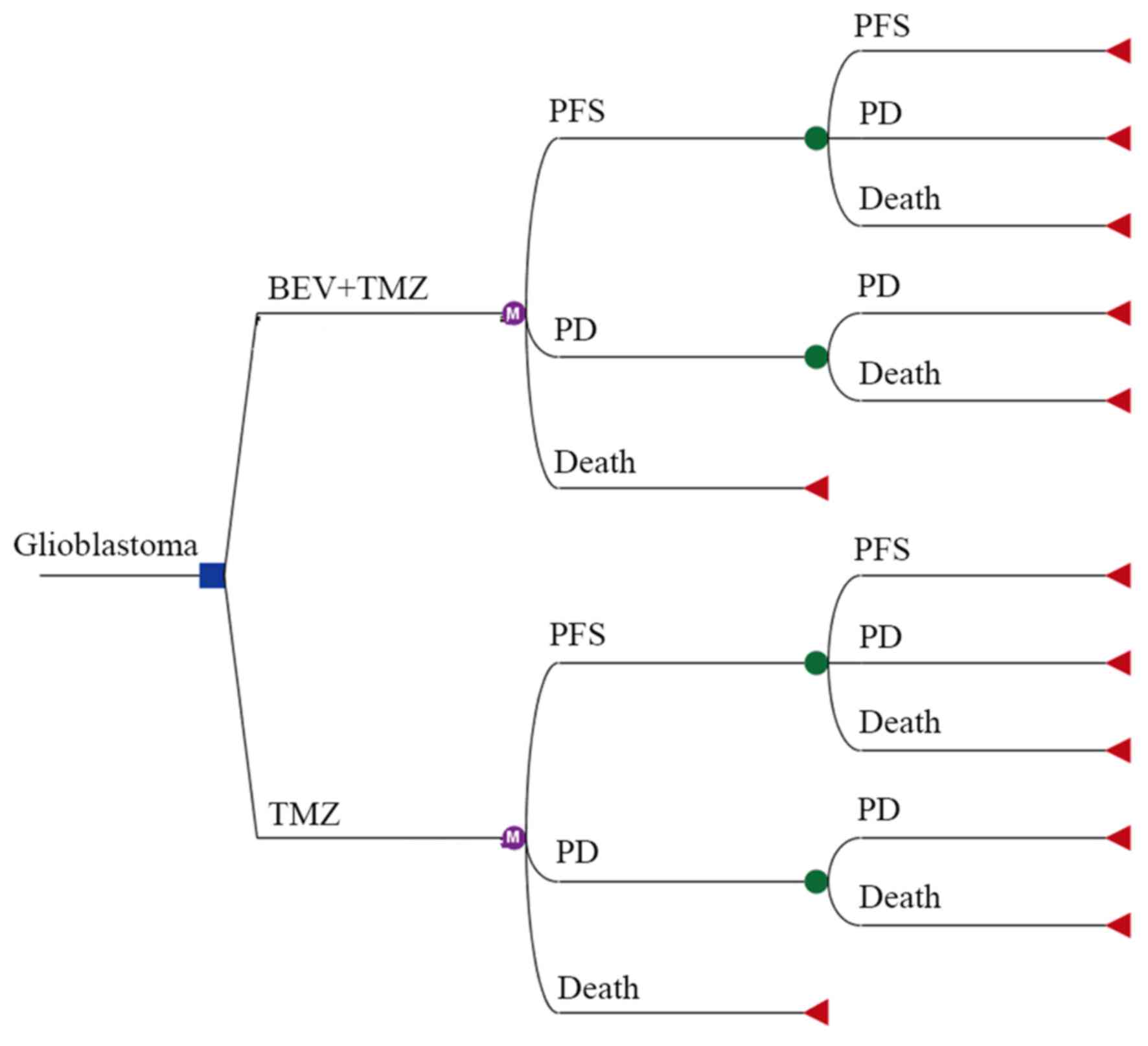
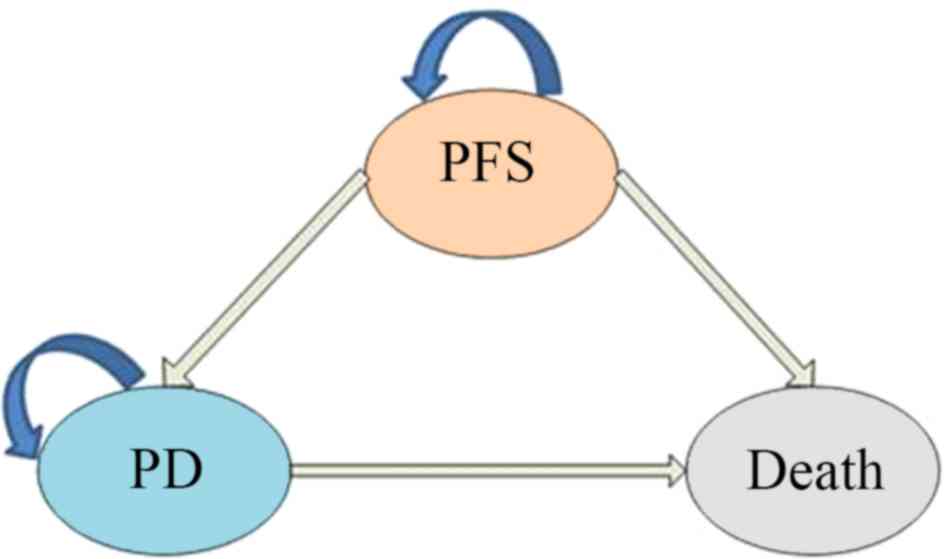
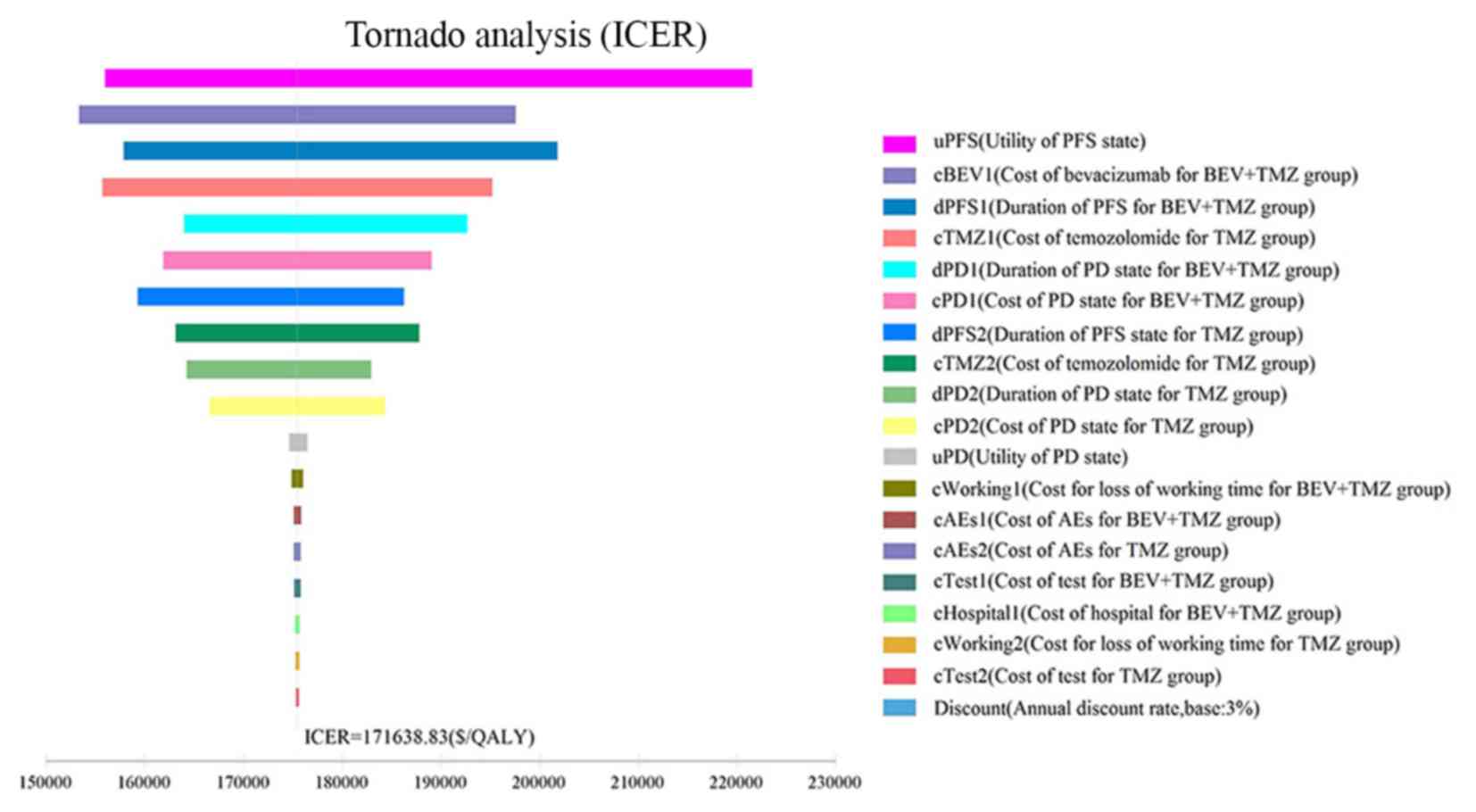
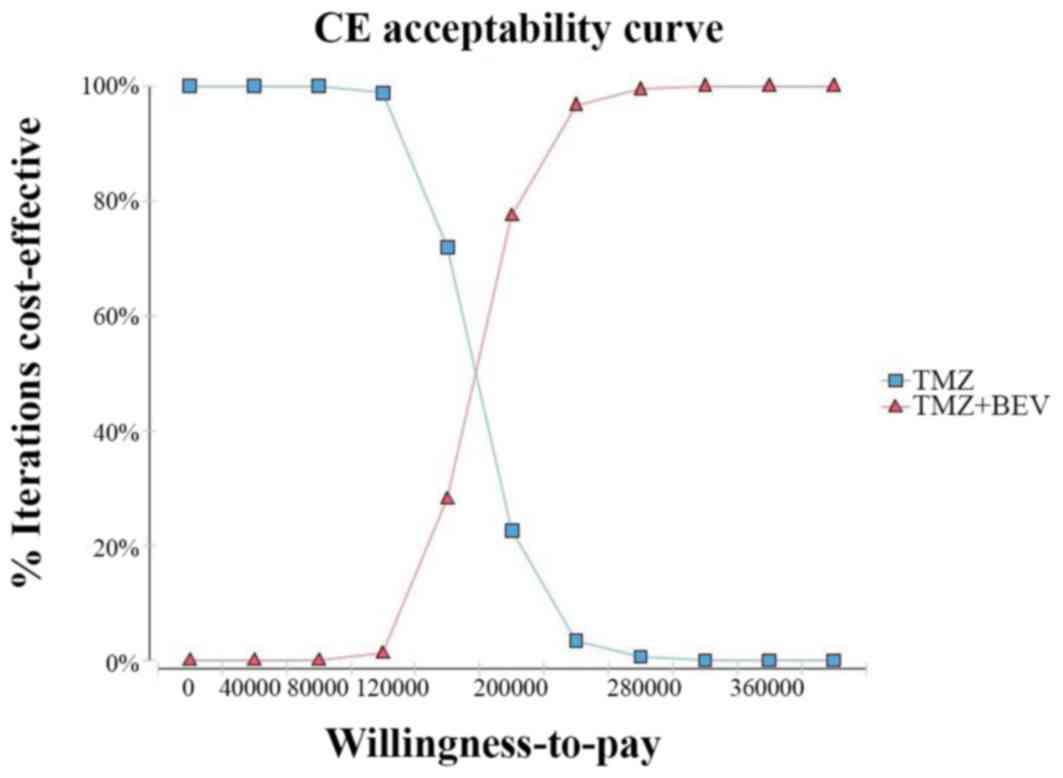
|
1
|
Therese A, Dolece k, Jennifer M, Prop p,
Nancy E, Strou p and Carol Kruchko: CBTRUS statistical report:
Primary brain and central nervous system tumors diagnosed in the
united states in 2005–2009. Neuro Oncol. 14 (Suppl):v1–v49. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aldape KD, Okcu MF, Bondy ML and Wrensch
M: Molecular epidemiology of glioblastoma. Cancer J. 9:99–106.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balana C, De Las Penas R, Sepúlveda JM,
Gil-Gil MJ, Luque R, Gallego O, Carrato C, Sanz C, Reynes G,
Herrero A, et al: Bevacizumab and temozolomide versus temozolomide
alone as neoadjuvant treatment in unresected glioblastoma: The
GENOM 009 randomized phase II trial. J Neurooncol. 127:569–579.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomized phase III study: 5-Year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Purmonen T, Martikainen JA, Soini EJ,
Kataja V, Vuorinen RL and Kellokumpu-Lehtinen PL: Economic
evaluation of sunitinib malate in second-line treatment of
metastatic renal cell carcinoma in Finland. Clin Ther. 30:382–392.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miller DK and Homan SM: Determining
transition probabilities: Confusion and suggestions. Med Decis
Making. 14:52–58. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu B, Ye M, Chen H and Shen JF: Costs of
trastuzumab in combination with chemotherapy for HER2-positive
advanced gastric or gastroesophageal junction cancer: An economic
evaluation in the Chinese context. Clin Ther. 34:468–479. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
http://www.sc.gov.cn/10462/12771/2018/1/31/10444117.shtmlMarch
15–2018
|
|
10
|
http://www.boc.cn/sourcedb/whpj/Jan 31–2018
|
|
11
|
Garside R, Pitt M, Anderson R, Rogers G,
Dyer M, Mealing S, Somerville M, Price A and Stein K: The
effectiveness and cost-effectiveness of carmustine implants and
temozolomide for the treatment of newly diagnosed high-grade
glioma: A systematic review and economic evaluation. Health Technol
Assess. 11:iii–iv, ix-221. 2007. View
Article : Google Scholar
|
|
12
|
https://book.douban.com/subject/26922525/Jan
31–2018
|
|
13
|
Wu B, Miao Y, Bai Y, Ye M, Xu Y, Chen H,
Shen J and Qiu Y: Subgroup economic analysis for glioblastoma in a
health resource-limited setting. PLoS One. 7:e345882012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
http://www.stats.gov.cn/english/PressRelease/201801/t20180118_1574943.htmlJan
31–2018
|
|
15
|
Murray CJ, Evans DB, Acharya A and
Baltussen RM: Development of WHO guidelines on generalized
cost-effectiveness analysis. Health Econ. 9:235–251. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cohen MH, Shen YL, Keegan P and Pazdur R:
FDA drug approval summary: Bevacizumab (Avastin) as treatment of
recurrent glioblastoma multiforme. Oncologist. 14:1131–1138. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu P, He YS, Huang Q, Ding T, Cen YC, Zhao
HY and Wei X: Bevacizumab treatment for newly diagnosed
glioblastoma: Systematic review and meta-analysis of clinical
trials. Mol Clin Oncol. 4:833–838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kovic B1 and Xie F: Economic evaluation of
bevacizumab for the first-line treatment of newly diagnosed
glioblastoma multiforme. J Clin Oncol. 33:2296–2302. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Minion LE, Bai J, Monk BJ, Robin Keller L,
Ramez EN, Forde GK, Chan JK and Tewari KS: A Markov model to
evaluate cost-effectiveness of antiangiogenesis therapy using
bevacizumab in advanced cervical cancer. Gynecol Oncol.
137:490–496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goulart B and Ramsey S: A trial-based
assessment of the cost-utility of bevacizumab and chemotherapy
versus chemotherapy alone for advanced non-small cell lung cancer.
Value Health. 14:836–845. 2011. View Article : Google Scholar : PubMed/NCBI
|