Introduction
To date, surgical resection is the preferred
treatment method for non-small cell lung cancer (NSCLC), especially
among patients at early stages (I, II and certain cases of IIIA)
(1). Although surgical resection is
frequently used for treatment, the recurrence rate of NSCLC remains
high (~30% within 5 years from surgery), which has emerged as a
major contributor to the poor prognosis of patients with NSCLC
(2–4). However, with the identification of
molecular markers in NSCLC, targeting pathogenic genes has been
used as second-line therapy as an alternative approach (5).
Activation of the Wnt/β-catenin pathway is crucial
for NSCLC progression (6). Without
stimulation, β-catenin mainly present in the cell membrane is
maintained at a low level by the ubiquitin proteasome system (UPS)
(7). Upon activation of Wnt, which
is the main regulator of β-catenin, β-catenin accumulates in the
cytoplasm and enters the nucleus to bind with the T-cell
factor/lymphoid enhancement factor to activate the downstream
oncogenes, such as cyclin D1 and matrix metalloproteinases (MMPs),
leading to the proliferation and sustained aggressiveness of
various cancer cells (7). β-catenin
is aberrantly enhanced in NSCLC, and its downregulation has been
suggested to inhibit disease development (8,9). The
activity of β-catenin can be modified by several pathways,
affecting its stability, cellular localization and transcriptional
activity. However, the specific mechanisms that modulate β-catenin
activity in NSCLC are not fully understood.
MicroRNAs (miRNAs), identified as a type of
endogenous non-coding RNA molecules with sequences of 19–25
nucleotides, are known for their gene regulation and have become
increasingly appreciated due to their regulatory roles in the
tumourigenesis of various types of cancer. For example, miR-212 in
lung cancer (10) and the miR-17-92
cluster in malignant lymphoma (11)
serve as oncogenes, whereas let-7 in lung cancer (12) and miR-551a/miR-483 in colorectal
cancer (13) act as tumor
suppressors. miRNAs function as tumour suppressors or promoters
most likely by regulating the transcription of tumour-related genes
by base pairing with the 3′-untranslated region (3′-UTR) of mRNAs,
resulting in the suppression of mRNAs and the subsequent
degradation of gene expression (14). To date, miR-512-5p has been
implicated in the tumourigenesis of a number of human malignancies,
including NSCLC (15–17). β-catenin is a target of miR-3619-5p
in NSCLC cells (18). However, the
exact mechanism of miR-512-5p inhibition of β-catenin in the
progression of NSCLC remains largely unknown.
The present study aimed to confirm the
downregulation of miR-512-5p and the upregulation of β-catenin in
human NSCLC. However, to the best of our knowledge, there are no
reports regarding the anticancer effect of miR-512-5p in patients
with NSCLC. In addition, the role of miR-512-5p in NSCLC remain
elusive; therefore, the present study aimed to investigate the
expression and tumour-suppressive behaviour of miR-512-5p in
patients with NSCLC.
Materials and methods
Human tissue samples
Tumourous lung tissues (n=30) and adjacent
non-tumourous lung tissues (n=30) were collected from patients with
NSCLC at the Department of Thoracic Surgery of the Shanghai Chest
Hospital (Shanghai, China) between January 2017 and December 2018.
Staging for each patient was performed according to the AJCC Cancer
Staging Manual, 7th edition (19),
and was based on findings from physical examination and surgical
resection. A total of 11 cases were stage II and 19 cases were
stage III. The following information was collected at the time of
diagnosis: Sex, age, pathological type and TNM stage (Table SI). All patients provided written
informed consent, and the use of patient tissues and data was
approved by the Ethics Committee of Shanghai Chest Hospital
[approval no. KS(P)1733].
Cell culture and treatment
A549, H460, HCC827, H1975 and H157 cells were
obtained from ATCC and cultured in DMEM (HyClone; GE Healthcare
Life Sciences) containing 100 U/ml penicillin and 100 µg/ml
streptomycin (Beijing Solarbio Science & Technology Co., Ltd.)
and 10% (v/v) foetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.). Cells in this complete culture medium were
cultured to form a monolayer at 37°C with 5% CO2.
Cell transfection
To overexpress miR-512-5p in NSCLC cells, a mimic of
miR-512-5p was synthesized using the following primers: Forward,
5′-CATCACTGCCACCCAGAAGACTG-3′ and reverse,
5′-TGCCAGTGAGCTTCCCGTTCAG-3′ with a 2 nt-30 overhang and a 2 nt-50
trim (Suzhou GenePharma Co., Ltd.). To downregulate miR-512-5p in
NSCLC cells, an inhibitor of miR-512-5p was synthesized using the
following single-stranded RNA: 5′-TAACTCGAGAACCCACTGCTTACT-3′. A
control mimic of a random miR-512-5p sequence (forward,
5′-TCGAGTCCCTCACTGTTACCCTTG-3′ and reverse,
5′-TAGATGACTTAAGCCTCAGCAGCA-3′) and a control inhibitor of a random
miR-512-5p sequence (5′-CTAGAAGGCACACTCGAGGCTGAT-3′) were used as
negative controls (NCs). To study the roles of miR-512-5p in the
progression of NSCLC in vitro, A549 and H1975 cells
(2×103 cells/ml; 100 µl) were seeded into a 96-well
plate and transfected with the miR-512-5p mimic inhibitor or the
corresponding control using Lipofectamine® 2000 (Thermo
Fisher Scientific, Inc.) for 48 h, according to the manufacturer's
instructions.
Flow cytometry for apoptosis
detection
Following transfection for 48 h, an apoptosis assay
of A549 and H1975 cells (1×106 cells) was performed
using an Annexin V-FITC Apoptosis Detection kit (Sangon Biotech
Co., Ltd.). A FACSCant II flow cytometry system (BD Biosciences)
was used to monitor apoptosis (FlowJo v10.0.7; FlowJo LLC). Early
apoptotic NSCLC cells were Annexin V (+)/propidium iodide (PI) (−)
and presented in the lower right quadrant, whereas late apoptotic
NSCLC cells were Annexin V (+)/PI (+) and presented in the upper
right quadrant.
Transwell invasion assay
The lower side of the membrane of the Transwell
inserts (Corning, Inc.) were pre-coated with a thin layer of
Matrigel. Following serum starvation for 24 h, A549 and H1975 cells
in 0.3 ml serum-free medium were seeded into the upper chamber
(4×105 cells/well), and 0.7 ml of complete medium was
added to the lower chamber. At 48 h, A549 and H1975 cells that
passed through the membrane were dyed using crystal violet (Beijing
Solarbio Science & Technology Co., Ltd.) and counted using a
Leica DM4P microscope (magnification, ×200) in three randomly
selected fields.
Caspase activity assay
A549 cells were resuspended in hypotonic cell lysis
buffer at a density of 1×106 cells/ml and subjected to
three cycles of freezing and thawing. The cell lysates were
centrifuged at 16,000 × g for 20 min at 4°C, and the supernatant
fraction was collected and assayed for caspase-3 activity. Caspase
activity was measured using the Apo-ONE Homogeneous Caspase-3/7
Assay kit (Promega Corporation) according to the manufacturer's
instructions. Samples were analysed using a Synergy HT Plate Reader
(BioTek Instruments, Inc.) at a wavelength of 594 nm.
Dual-luciferase reporter gene
assay
The 3′-UTR of β-catenin targeting miR-512-5p
predicted through TargetScan Release 6.2 software (http://www.targetscan.org/vert_61/) was inserted
into the pGL3-Control vector (Promega Corporation). The sequences
of the wild-type 3′-UTR of β-catenin were as follows: Sense,
5′-GCGGAGCTCAACCAGAAGGCCAAGTC-3′ and antisense,
5′-GCGTCTAGAAAATGGACAAAGTGGGTGTGG-3′. The sequences of the mutant
3′-UTR of β-catenin were as follows: Sense,
5′-TCCCTCACTGTTACCCTT-3′ and antisense, 5′-ATGACTTAAGCCTCAGCAGC-3′.
A549 cells were co-transfected with 80 ng β-catenin 3′-UTR, 50 pM
miR-512-5p and 5 ng pRL-TK Renilla plasmid (Promega
Corporation) in a 96-well plate for 72 h using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) and
dissolved in LBL lysis buffer (Promega Corporation). The
Renilla/firefly luciferase activity ratio of A549 cells was
assessed using a Dual-Luciferase Reporter assay system (Promega
Corporation).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from lung tissues and NSCLC cell lines
(A549, H460, HCC827, H1975 and H157) was extracted with
TRIzol® Reagent (Sigma-Aldrich; Merck KGaA) and
reverse-transcribed using a cDNA Synthesis kit (Takara Bio, Inc.).
The primers used were as follows: β-catenin forward,
5′-TAACTCGAGAACCCACTGCTTACT-3′ and reverse,
5′-CTAGAAGGCACACTCGAGGCTGAT-3′; miR-512-5p forward,
5′-TCGAGTCCCTCACTGTTACCCTTG-3′ and reverse,
5′-TAGATGACTTAAGCCTCAGCAGCA-3′; let-7a forward,
5′-GCCGCTGAGGTAGTAGGTTGTA-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′;
APC forward, 5′-CTAGTACTGCTTCAACTAAGTC-3′ and reverse,
5′-TACCTGGAGATGTATATGACAT-3′; cyclin D1 forward,
5′-GCCTCACACGCTTCCTCTCCAGA-3′ and reverse,
5′-TGGCGCAGGCTTGACTCCAGCA-3′; MMP7 forward,
5′-ACAGGCTCAGGACTATCTCAAG-3′ and reverse,
5′-ATTTCTATGACGCGGGAGTTTAA-3′; GADPH forward,
5′-CATCACTGCCACCCAGAAGACTG-3′ and reverse,
5′-ATGCCAGTGAGCTTCCCGTTCAG-3′. GAPDH was used as a loading control
for analysing the expression of β-catenin, adenomatosis polyposis
coli (APC), cyclin D1 and MMP7 correspondingly. A SYBR Green PCR
kit (Thermo Fisher Scientific, Inc.) with an ABI 7300 system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) was used for
analysis. The amplification conditions were as follows: 95°C for 10
min, followed by 40 cycles of 95°C for 20 sec and 60°C for 45 sec.
The levels of miR-512-5p were normalized to let-7a, whereas the
levels of β-catenin, APC, cyclin D1 and MMP7 were normalized to
that of GADPH and quantified using the 2−∆∆Cq method
(20).
Western blot analysis
A549 cells were lysed in RIPA buffer (Beyotime
Institute of Biotechnology), and the extracted intracellular
proteins were evaluated using a BCA protein assay kit (Sangon
Biotech Co., Ltd.). Total protein (25 µg) was separated by 10%
SDS-PAGE. Electrophoretically pure β-catenin, APC, cyclin D1 and
MMP7 were transferred to a polyvinylidene difluoride membrane (EMD
Millipore) and incubated with primary antibodies (all purchased
from Abcam) against β-catenin (cat. no. ab2365; dilution, 1:1,000),
APC (cat. no. ab15270; dilution, 1:500), cyclin D1 (cat. no.
ab40754; dilution, 1:1,000), MMP7 (cat. no. ab5706; dilution,
1:1,000) and GAPDH (cat. no. ab16891; dilution, 1:10,000) at 4°C
overnight followed by incubation with the goat-anti-rabbit
secondary antibodies (cat. no. ab205718; dilution, 1:10,000) for 1
h at 25°C. The protein expression levels of β-catenin, miR-512-5p,
APC, cyclin D1 and MMP7 were quantified using an enhanced
chemiluminescence (ECL) system (EMD Millipore) with Bio-Rad Image
Lab software version 5.1 (Bio-Rad Laboratories, Inc.) and
normalized to that of GAPDH.
Immunohistochemistry
Paraffin-embedded tissue blocks were cut into 4-µm
sections, dried overnight at 60°C. Sections were subsequently
dewaxed in xylol, rehydrated in descending alcohol series (100, 95,
70 and 40%, 5 min each) and washed in distilled water. The sections
were immersed in distilled water containing 3% hydrogen peroxidase
twice to block endogenous oxidase activity. The sections were
incubated with an anti-β-catenin antibody (cat. no. ab2365;
dilution, 1:200) for 2 h at room temperature, followed by
incubation with a goat-anti-rabbit secondary antibody (cat. no.
ab205718; dilution, 1:1,000) at room temperature for 40 min. The
staining was developed by diaminobenzidine (DAB) chromogen (Bio-Rad
Laboratories, Inc.). Subsequently, the tissues were rinsed in
distilled water for 15 min, dehydrated in graded alcohol series and
then washed in xylene. The slides were sealed by neutral gum. Five
random fields of view were analysed under a light microscope with a
camera (Olympus, Japan) with ×100 magnification.
Statistical analysis
Data were analysed using GraphPad Prism 7.0 software
(GraphPad Software, Inc.) and are presented as the mean ± standard
deviation. The statistical analysis of two unpaired groups was
evaluated using an unpaired Student's t-test. Statistical analysis
of miR-512-5p and β-catenin expression between the tumourous lung
tissues and paired non-tumourous lung tissues was evaluated using a
paired Student's t-test. Any statistically significant differences
between multiple groups were performed using a one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
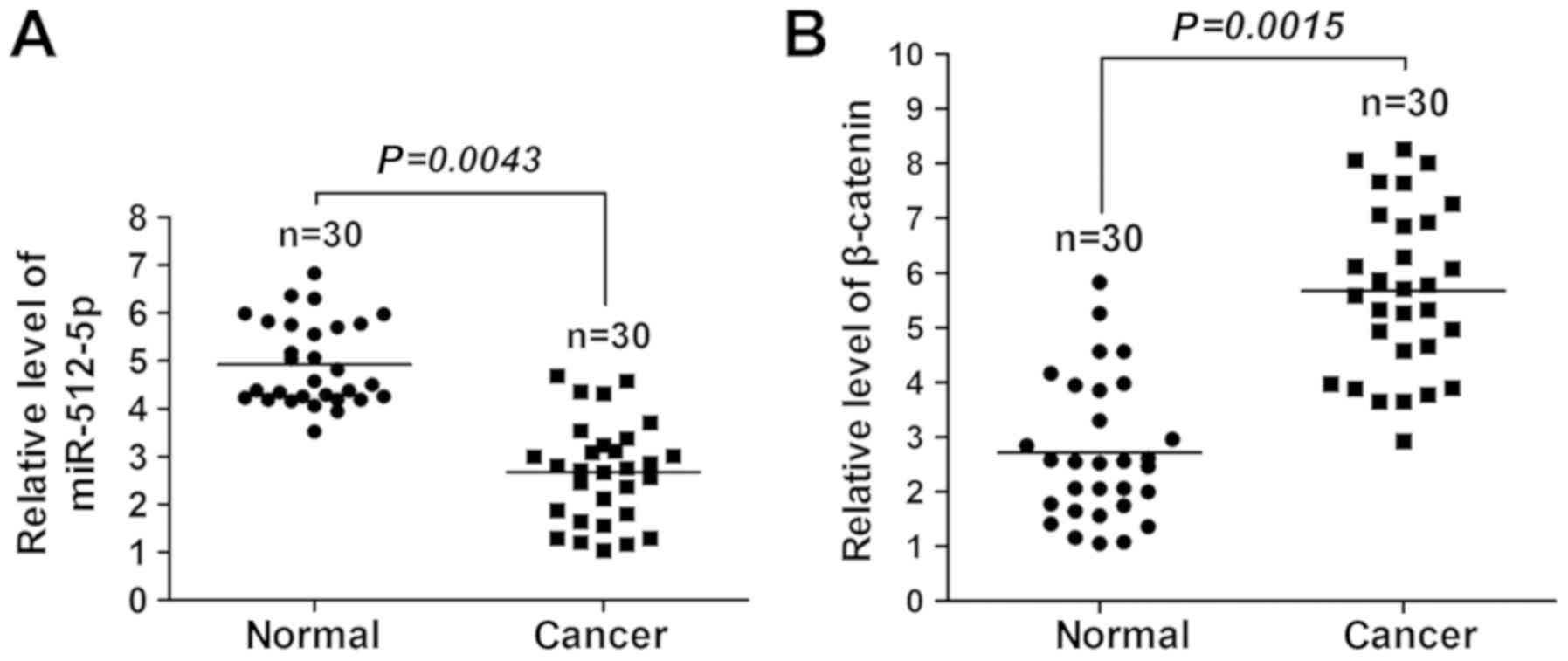
miR-512-5p is downregulated, whereas
β-catenin is upregulated in NSCLC
The miRNA/mRNA levels of miR-512-5p and β-catenin in
lung tissues of patients with NSCLC were detected. A significant
reduction in miR-512-5p (Fig. 1A)
and an increase in β-catenin (Fig.
1B) expression levels were observed in the tumourous lung
tissues compared with the non-tumourous lung tissues, which
suggested that miR-512-5p and β-catenin were involved in human
NSCLC.
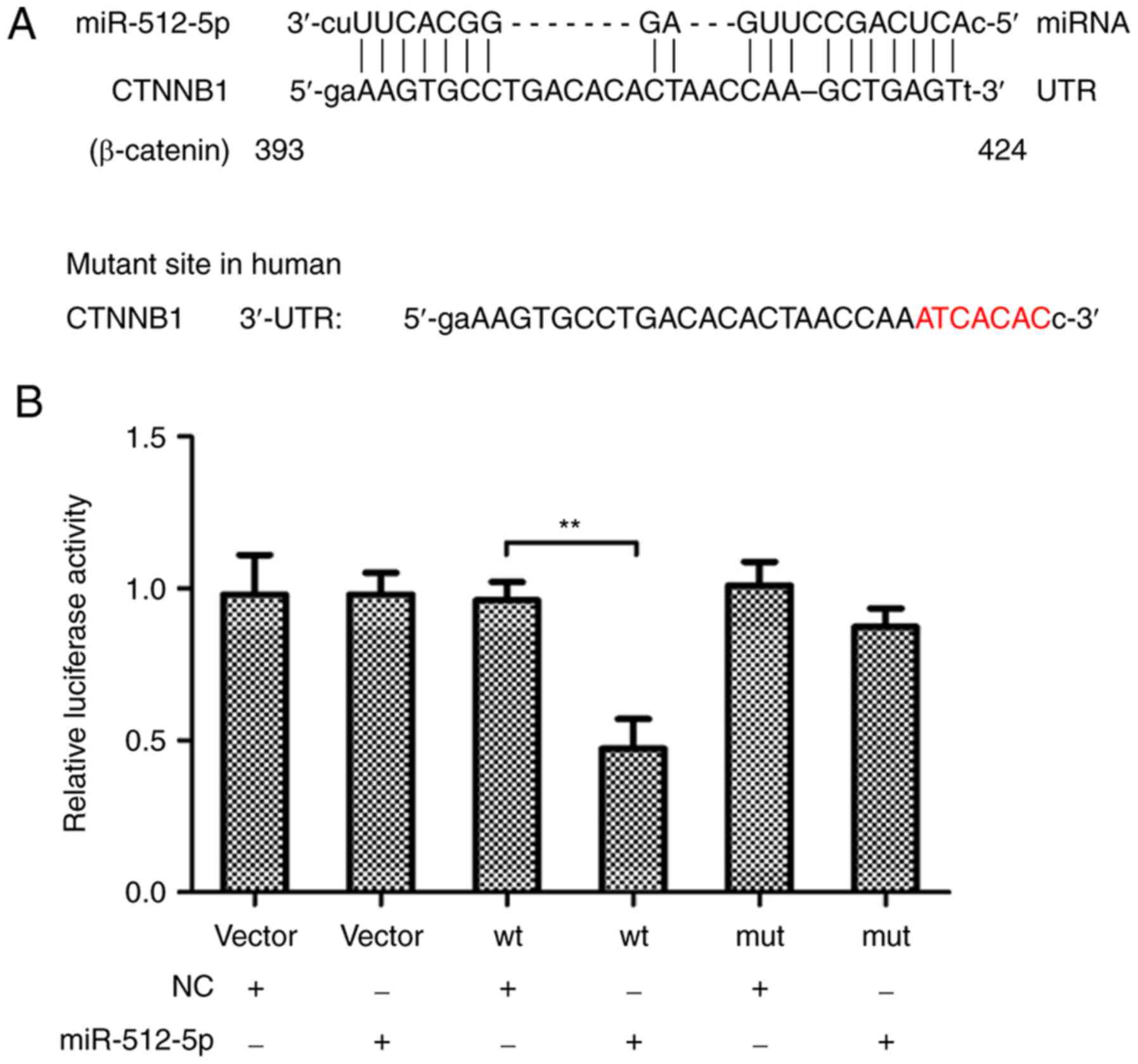
miR-512-5p targets the 3′-UTR of
β-catenin
The present explored whether β-catenin was a target
of miR-512-5p in human NSCLC. Two putative miR-512-5p target sites
were identified in β-catenin 3′-UTR using TargetScan Release 6.2
software. The results demonstrated that the 3′-UTR of β-catenin
directly bound to miR-512-5p (Fig.
2A). In addition, the luciferase reporter activity of β-catenin
3′-UTR was significantly reduced by miR-512-5p compared with the NC
(Fig. 2B), which suggested that
miR-512-5p was a novel β-catenin-targeting miRNA.
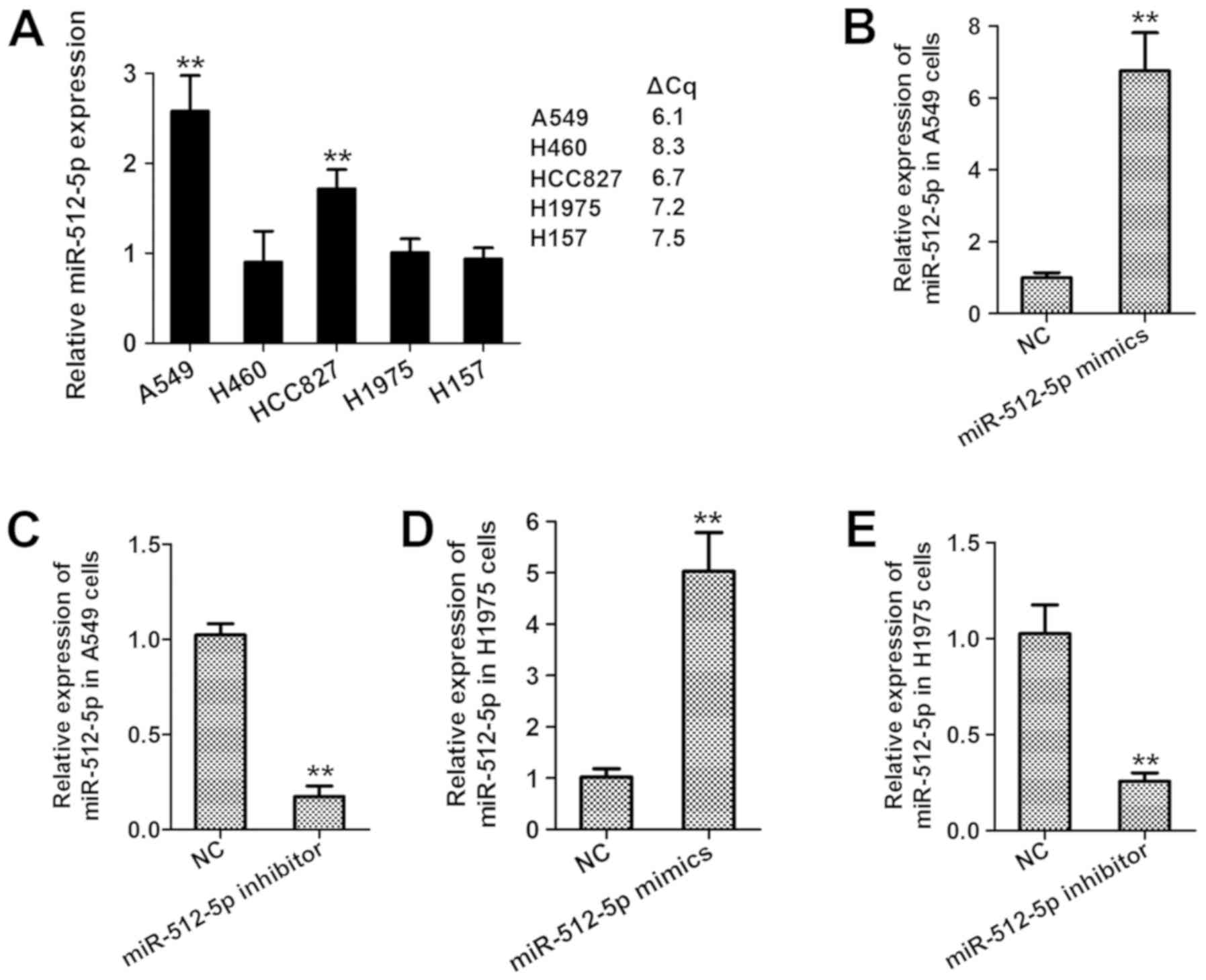
miR-512-5p contributes to the
progression of NSCLC in vitro
The expression levels of miR-512-5p were assessed in
several NSCLC cell lines. As presented in Fig. 3A, miR-512-5p was highly expressed in
A549 cells, but expressed at low levels in H460, H1975 and H157
cells. Thus, A549 and H1975 were selected for the transfection with
miR-512-5p mimics or inhibitors. The mRNA expression of miR-512-5p
was significantly enhanced by miR-512-5p mimics (Fig. 3B) and suppressed by the miR-512-5p
inhibitor compared with the NC (Fig.
3C), revealing the successful establishment of miR-512-5p
overexpression by the miR-512-5p mimics and miR-512-5p silencing by
the miR-512-5p inhibitor in the two NSCLC cell lines.
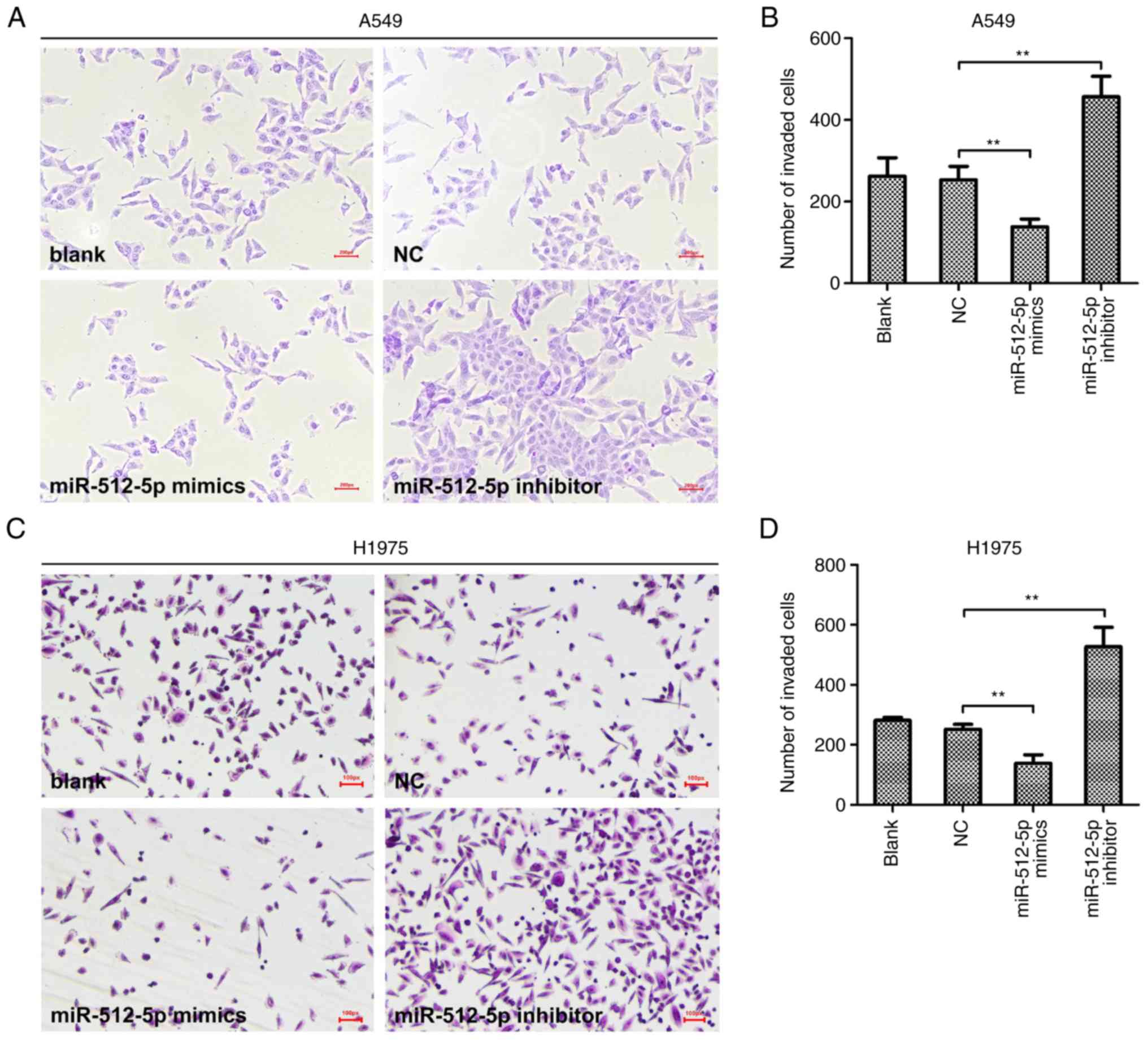
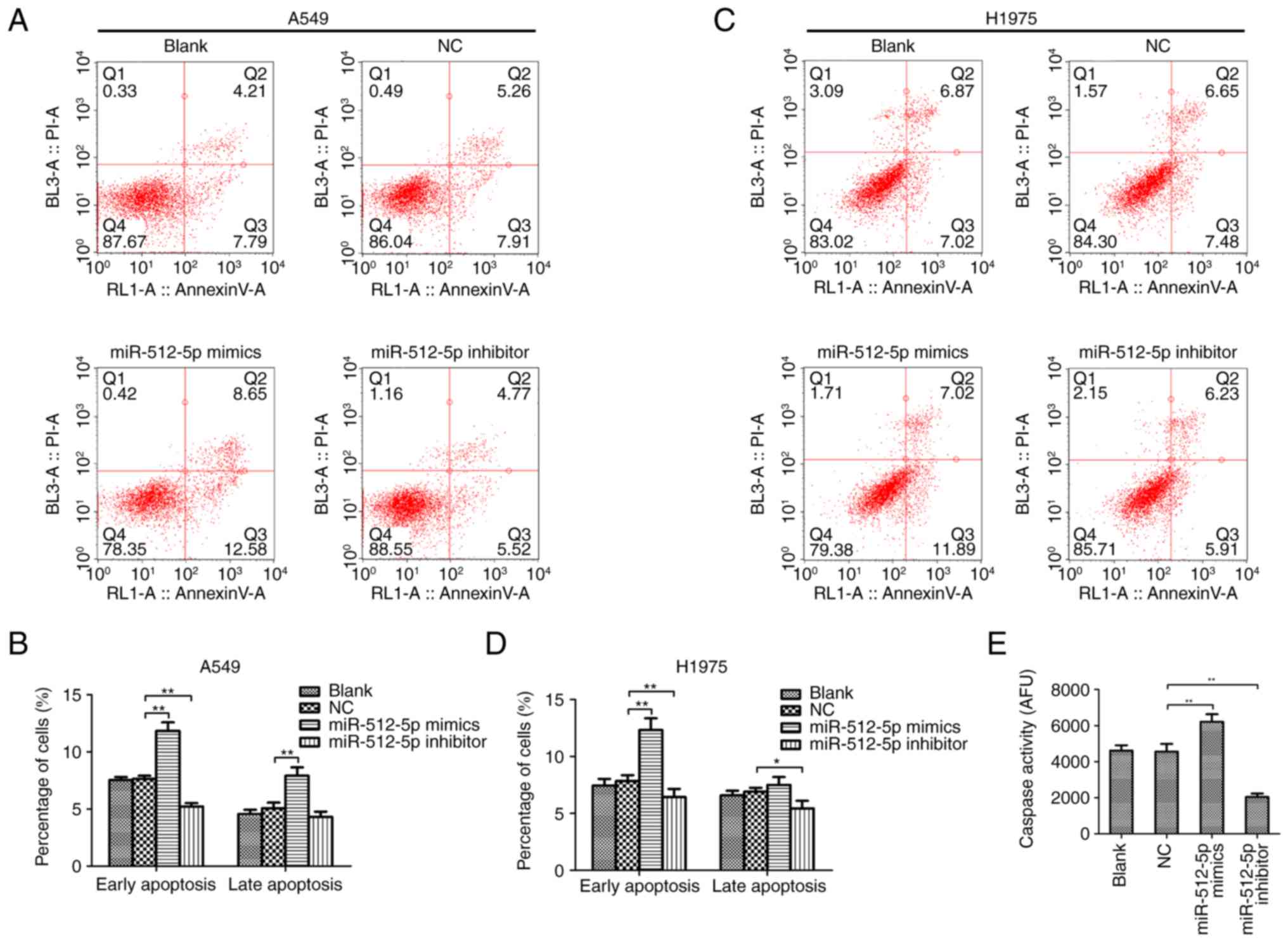
Following transfection, the malignant behaviours of
NSCLC cells were assessed. Transwell assays revealed that the
invasive abilities of A549 (Fig. 4A and
B) and H1975 (Fig. 4C and D)
cells were reduced by miR-512-5p overexpression and increased by
miR-512-5p silencing. In addition, a flow cytometry assay was
performed to further determine the effects of miR-512-5p on lung
cancer cell apoptosis. The results demonstrated that the group
transfected with miR-512-5p mimic exhibited a significantly higher
proportion of early apoptotic cells compared with the NC group in
A549 (Fig. 5A and B) and H1975
(Fig. 5C and D) cells. Consistent
with these findings, miR-512-5p mimic significantly increased
caspase activity in A549 cells, and the level of caspase activity
was decreased in miR-512-5p inhibitor-transfected A549 cells
(Fig. 5E). These results suggested
an anticancer effect of miR-512-5p in NSCLC in vitro. By
contrast, the miR-512-5p inhibitor resulted in the opposite
effects, promoting NSCLC cell invasion and reducing apoptosis
(P<0.05), indicating the promotive effect of the miR-512-5p
inhibitor in the progression of NSCLC in vitro.
miR-512-5p prevents the activation of
the Wnt/β-catenin pathway
The roles of miR-512-5p in modulating the
Wnt/β-catenin pathway were investigated. In the present study, the
overexpression of miR-512-5p suppressed the mRNA expression of
β-catenin, APC, cyclin D1 and MMP7 compared with the NC; silencing
miR-512-5p resulted in the opposite effects (Fig. 6A and B). In addition, the protein
levels of β-catenin, cyclin D1 and MMP7 in A549 cells transfected
with the miR-512-5p mimics or inhibitor and the corresponding NC
were quantified by western blot analysis (Fig. 6C-G). The results of the
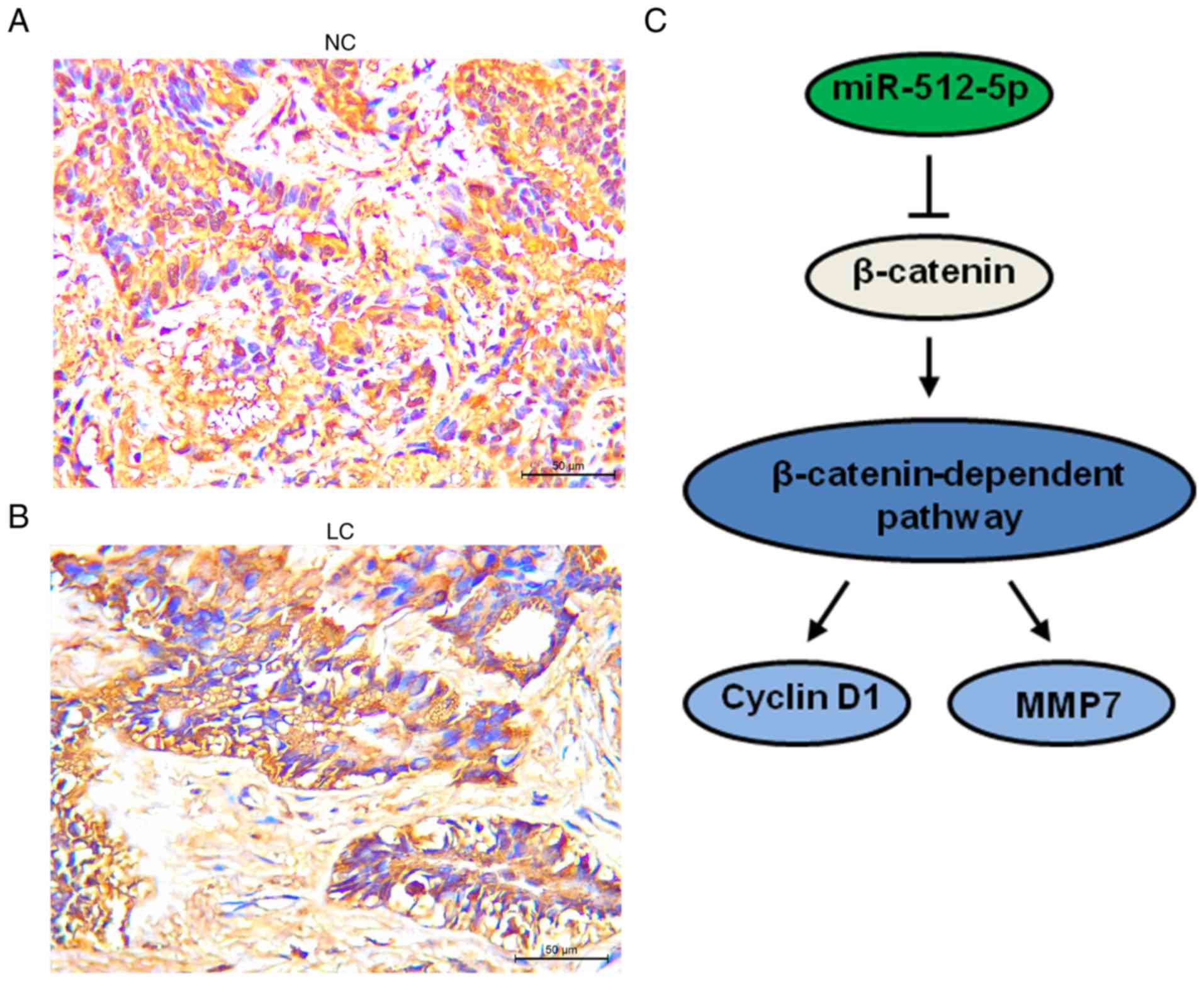
immunohistochemistry assay revealed that the protein expression of
β-catenin was higher in lung cancer tissues compared with adjacent
normal tissues, consistent with the RT-qPCR results (Fig. 7A and B). As β-catenin is a direct
target gene of miR-512-5p, the results of the present study
suggested that cyclin D1 and MMP7 were downstream effectors of
miR-512-5p and were at least partly induced by β-catenin signalling
(Fig. 7C).
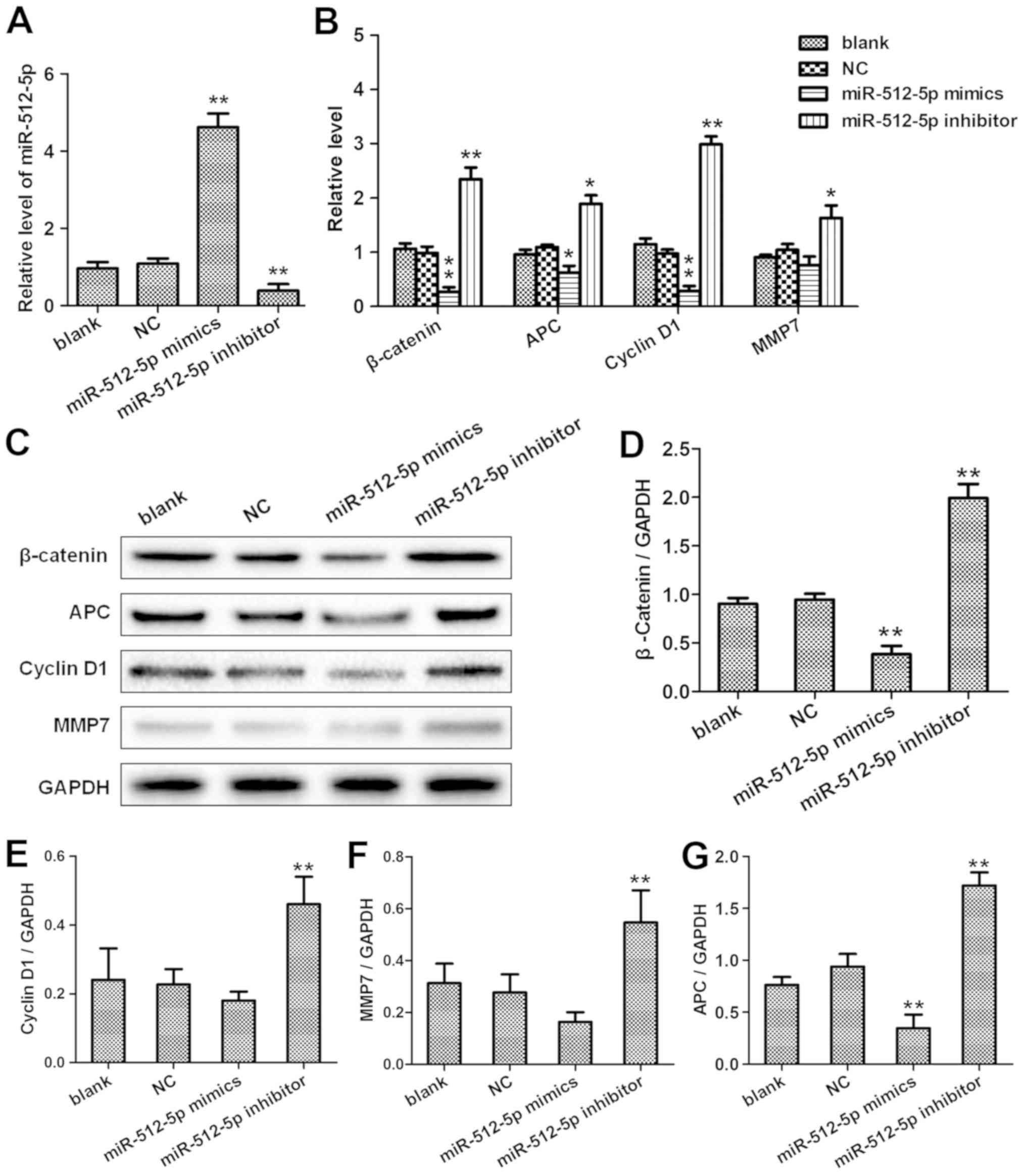 | Figure 6.miR-512-5p downregulates β-catenin,
APC, cyclin D1 and MMP7 expression in the A549 cell line. At 48 h
post-transfection with the NC, miR-512-5p mimic or the miR-512-5p
inhibitor, the expression levels of (A) miR-512-5p and (B)
β-catenin, APC, cyclin D1 and MMP7 in A549 cells was analysed by
reverse transcription-quantitative PCR. (C) Western blot analyses
were performed to evaluate the protein expression levels of
β-catenin, APC, cyclin D1 and MMP7 in A549 cells transfected with
the NC, miR-512-5p mimic or miR-512-5p inhibitor. Quantitative
analysis of protein levels of (D) β-catenin, (E) cyclin D1, (F)
MMP7 and (G) APC. *P<0.05, **P<0.01. miR, microRNA; NC,
negative control; APC, adenomatosis polyposis coli; MMP7, matrix
metallopeptidase 7. |
Discussion
The overexpression of β-catenin is a key contributor
to NSCLC development and is usually associated with the poor
prognosis of this disease (8,21). The
depletion of β-catenin by miRNAs impairs malignant behaviours in
the progression of various types of cancer, including NSCLC, and is
beneficial to the therapeutic outcome (18). The results of the present study
demonstrated an increase in β-catenin expression and a decrease in
miR-512-5p expression in NSCLC. Previous evidence has suggested
that miR-3619-5p targets β-catenin to inhibit β-catenin expression
and β-catenin-mediated malignancy of NSCLC in vitro
(18). However, whether and how
miR-512-5p regulates β-catenin in NSCLC carcinogenesis remains
largely unknown.
A previous report has revealed that miR-512-5p is
downregulated in NSCLC and restricts the progression of NSCLC in
vitro by promoting apoptosis and impairing migration; however,
it exerts almost no effect on cell proliferation (16). The present study demonstrated the
tumour-suppressive effect of miR-512-5p in NSCLC by suppressing
cell invasion and further suggested that miR-512-5p deficiency may
contribute to the malignant process of NSCLC in vitro. These
results suggested that miR-512-5p may be a promising target for
miRNA-based NSCLC treatment.
Previous research has demonstrated that the
translation of β-catenin is regulated by miRNAs that target the
3′-UTR of β-catenin mRNA in NSCLC cells, such as miR-3619-5p
(18). The present study first
demonstrated that β-catenin was also a target of miR-512-5p.
miR-512-5p inhibited the activity of β-catenin by base pairing with
the 3′-UTR of β-catenin, resulting in a reduction in β-catenin
expression and inactivation of the Wnt/β-catenin pathway, which
demonstrated the involvement of the Wnt/β-catenin pathway in the
anticancer effect of miR-512-5p in NSCLC. However, an
interventional study using a β-catenin activator needs to be
performed to further confirm whether miR-512-5p directly acts
through the Wnt/β-catenin pathway to regulate the progression of
NSCLC in vitro. In addition, cytoplasmic β-catenin is broken
down through the UPS pathway by a multiprotein destruction complex
containing APC and Axin, as well as kinases casein kinase 1α/ε and
glycogen synthase kinase 3 β (22).
APC, a tumour suppressor protein, is a core constitute of canonical
Wnt signalling (7). The
downregulation of APC triggers the activation of Wnt/β-catenin
signalling by accumulating β-catenin in the nucleus. Cyclin D1 and
MMP7 are two important targets of β-catenin and are associated with
NSCLC cell proliferation and invasion (23,24). The
results of the present study indicated an inhibitory effect of
miR-512-5p on APC and β-catenin, which may offset the enhanced
β-catenin expression caused by the downregulation of APC. In
addition, APC can be regulated by miRNA-129-5p and miR-582-5p in
cancer progression (25,26); however, whether and how miR-512-5p
influences APC expression need to be investigated in further
research.
The Wnt/β-catenin signalling pathway is an
evolutionarily highly conserved signalling pathway (7). When an external signal stimulates the
activation of the Wnt/β-catenin signalling pathway, it activates
the cytoplasmic dishevelled protein, and dephosphorylate β-catenin
(27). After accumulating to a
certain extent in the cytoplasm, β-catenin begins to translocate to
the nucleus and activates the downstream target genes c-myc, cyclin
Dl, survivin and MMP7, the promoters of which are exposed and
activated, resulting in abnormal cell proliferation (28). The results of the present study
indicated that overexpression of miR-512-5p negatively affected the
expression of β-catenin, cyclin D1 and MMP7 in A549 cells.
miR-512-5p does not contain a binding site for cyclin D1 or MMP7;
therefore, miR-512-5p may bind to and inhibit β-catenin to
downregulate its downstream target genes Cyclin D1 and MMP7.
In conclusion, miR-512-5p is a tumour-suppressive
regulator of tumour progression of NSCLC in vitro.
miR-512-5p may directly target β-catenin and inhibit the
Wnt/β-catenin pathway and the progression of NSCLC in vitro.
To confirm these results, a future in vivo study using a
xenograft model will be performed.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the first author on reasonable
request.
Authors' contributions
ZW and XZ performed all the experiments and wrote
the manuscript. TZ performed immunohistochemistry experiments and
relevant specimen collection. ZW collected and analyzed the data
and assisted with the writing of the manuscript. FY conceived the
design and supervised the entire project. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All patients gave their full consent to participate
in the present study, and a written consent form was obtained from
each patient. All animal experiments were approved by the Research
Ethics Committee of Shanghai Chest Hospital (Shanghai, China) and
carried out in conformity with the Chinese governing law on the use
of medical laboratory animal (authorization No. 55,1998, by
ministry of health).
Patient consent for publication
A written consent form was obtained from each
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang H, Liu C, Tan Z and Zhang T:
Segmentectomy versus Wedge resection for stage I non-small cell
lung cancer: A Meta-analysis. J Surg Res. 243:371–379. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu H, Yin Q, Yang G and Qie P: Including
3564 patients prognostic impact of tumor spread through air spaces
in non-small cell lung cancers: A meta-analysis. Pathol Oncol Res.
2019.(Epub ahead of print).
|
|
3
|
Im Y, Park HY, Shin S, Shin SH, Lee H, Ahn
JH, Sohn I, Cho JH, Kim HK, Zo JI, et al: Prevalence of and risk
factors for pulmonary complications after curative resection in
otherwise healthy elderly patients with early stage lung cancer.
Respir Res. 20:1362019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hung JJ, Jeng WJ, Hsu WH, Chou TY, Huang
BS and Wu YC: Predictors of death, local recurrence, and distant
metastasis in completely resected pathological stage-I
non-small-cell lung cancer. J Thorac Oncol. 7:1115–1123. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peng B, Zhang J, Woods JS and Peng W:
Molecular markers and pathogenically targeted therapy in non-small
cell lung cancer. Front Med China. 3:245–255. 2009. View Article : Google Scholar
|
|
6
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst. 106:djt3562014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shang S, Hua F and Hu ZW: The regulation
of β-catenin activity and function in cancer: Therapeutic
opportunities. Oncotarget. 8:33972–33989. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu X, Sun PL, Li JZ, Jheon S, Lee CT and
Chung JH: Aberrant Wnt1/β-catenin expression is an independent poor
prognostic marker of non-small cell lung cancer after surgery. J
Thorac Oncol. 6:716–724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paul S and Dey A: Wnt signaling and cancer
development: Therapeutic implication. Neoplasma. 55:165–176.
2008.PubMed/NCBI
|
|
10
|
Chen W, Huang Y, Zhang S, Zheng X, Xie S,
Mao J, Cai Y, Lu X, Hu L, Shen J, et al: MicroRNA-212 suppresses
nonsmall lung cancer invasion and migration by regulating
ubiquitin-specific protease-9. J Cell Biochem. 120:6482–6489. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang C, Bi C, Jiang X, Tian T, Huang X,
Wang C, Fernandez MR, Iqbal J, Chan WC, McKeithan TW, et al: The
miR-17~92 cluster activates mTORC1 in mantle cell lymphoma by
targeting multiple regulators in the STK11/AMPK/TSC/mTOR pathway.
Br J Haematol. 185:616–620. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li JP, Liao XH, Xiang Y, Yao A, Song RH,
Zhang ZJ, Huang F, Dai ZT and Zhang TC: Hyperoside and let-7a-5p
synergistically inhibits lung cancer cell proliferation via
inducing G1/S phase arrest. Gene. 679:232–240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Loo JM, Scherl A, Nguyen A, Man FY,
Weinberg E, Zeng Z, Saltz L, Paty PB and Tavazoie SF: Extracellular
metabolic energetics can promote cancer progression. Cell.
160:393–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
John K, Wu J, Lee BW and Farah CS:
MicroRNAs in head and neck cancer. Int J Dent. 2013:6502182013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dinami R, Buemi V, Sestito R, Zappone A,
Ciani Y, Mano M, Petti E, Sacconi A, Blandino G, Giacca M, et al:
Epigenetic silencing of miR-296 and miR-512 ensures hTERT dependent
apoptosis protection and telomere maintenance in basal-type breast
cancer cells. Oncotarget. 8:95674–95691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chu K, Gao G, Yang X, Ren S, Li Y, Wu H,
Huang Y and Zhou C: miR-512-5p induces apoptosis and inhibits
glycolysis by targeting p21 in non-small cell lung cancer cells.
Int J Oncol. 48:577–586. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Lei H, Xu Y and Tao ZZ: miR-512-5p
suppresses tumor growth by targeting hTERT in telomerase positive
head and neck squamous cell carcinoma in vitro and in vivo. PLoS
One. 10:e01352652015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Niu X, Liu S, Jia L and Chen J: Role of
miR-3619-5p in β-catenin-mediated non-small cell lung cancer growth
and invasion. Cell Physiol Biochem. 37:1527–1536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu H, Lin D, Wang L, Liu N and Wang E:
Expression and mutation of β-catenin in non-small cell lung cancer.
Zhongguo Fei Ai Za Zhi. 7:409–413. 2004.(In Chinese). PubMed/NCBI
|
|
22
|
Vargas DA, Sun M, Sadykov K, Kukuruzinska
MA and Zaman MH: The integrated role of Wnt/β-catenin,
N-glycosylation, and E-cadherin-mediated adhesion in network
dynamics. PLoS Comput Biol. 12:e10050072016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie C, Jiang G, Fan C, Zhang X, Zhang Y,
Miao Y, Lin X, Wu J, Wang L, Liu Y, et al: ARMC8α promotes
proliferation and invasion of non-small cell lung cancer cells by
activating the canonical Wnt signaling pathway. Tumour Biol.
35:8903–8911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schneikert J and Behrens J: The canonical
Wnt signalling pathway and its APC partner in colon cancer
development. Gut. 56:417–425. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shu Z, Chen L and Ding D: miR-582-5P
induces colorectal cancer cell proliferation by targeting
adenomatous polyposis coli. World J Surg Oncol. 14:2392016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li M, Tian L, Wang L, Yao H, Zhang J, Lu
J, Sun Y, Gao X, Xiao H and Liu M: Down-regulation of miR-129-5p
inhibits growth and induces apoptosis in laryngeal squamous cell
carcinoma by targeting APC. PLoS One. 8:e778292013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang S, Dong Y, Zhang Y, Wang X, Xu L,
Yang S, Li X, Dong H, Xu L, Su L, et al: DACT2 is a functional
tumor suppressor through inhibiting Wnt/β-catenin pathway and
associated with poor survival in colon cancer. Oncogene.
34:2575–2585. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Y, Yang JJ, Tao H and Jin WS: New
perspectives on β-catenin control of cell fate and proliferation in
colon cancer. Food Chem Toxicol. 740:14–19. 2014. View Article : Google Scholar
|