Introduction
The bone is a popular site of metastases within the
general population (1), along with
the liver and the lung. In bone metastases, the spine is most
commonly affected. Studies have suggested that spinal metastasis
(SM) accounts for up to 40% of patients suffering from metastasis
during the course of their disease (2–4). The
most common symptoms of SM include, radicular and back pain and
sensory disorder that leads to degradation of the patients' quality
of life (5–8). The treatment strategy for spinal
metastases depends on a number of factors, including: Histology,
the site of disease, the extent of metastases and the neurologic
status (9,10). Open surgery is one of the traditional
treatment options used for spinal metastases (11); however, this approach often results
in considerable trauma and severe side effects. Furthermore,
prolonged hospitalization may delay the treatment of the primary
disease (12,13). Given the complications associated
with surgery, external beam radiotherapy (EBRT) has become an
alternative option for the treatment and management of spinal
metastases (14). Nevertheless, the
conventional EBRT technique has a limited capability for dose
escalation when treating spinal cord bone metastases, due to the
dose limit of the organ-at-risk (OAR). For example, in order to
avoid the risk of radiation-induced myelitis, the OAR dose of the
spinal cord is kept below 45 Gy (3,15).
Furthermore, both surgery or EBRT may not be appropriate for
patients with medical problems or those unwilling to accept the
complication risks of surgery (16).
Radioactive iodine-125 (I-125) seed implantation
emits a low energy γ-ray and transfers steep dose gradients between
target volumes and the adjacent OAR (17). Satisfactory clinical outcomes have
been reported in the treatment of primary and secondary malignant
tumors with I-125 brachytherapy (18–21). The
steep dose gradients are particularly desirable for osteosarcoma or
vertebral column metastases where tumors abut sensitive critical
normal tissues, such as the spinal cord, and poor dose control can
result in myelitis and vertebral body fracture, which would be
catastrophic (3). Pre-implant
treatment planning is crucial to brachytherapy; however, it has
been suggested that the post-implant dosimetry may deviate from the
pre-implant treatment planning (22). To the best of our knowledge, there
have been limited studies that investigate this discrepancy. The
present study retrospectively examined patients with metastatic
spinal tumors who were treated with I-125 permanent interstitial
implantation. The dosimetric differences between the pre- and
post-implant treatment plans, with I-125 spinal metastases
brachytherapy, were compared.
Materials and methods
Patients
The retrospective analysis in the present study was
approved by the Ethics Committee of Shandong Provincial Hospital
(Jinan, China). A total of 11 patients with metastatic spinal
tumors, who were treated with I-125 permanent interstitial
implantation from September 2014 to January 2016, were included in
the present study. The following inclusion criteria were met by all
patients: i) Pathological or cytological confirmation of primary
malignant tumor; ii) Karnofsky performance score ≥60 (KPS; for
functional impairment); iii) adequate general health and functions
(hematological, hepatic, renal and cardiac); iv) ability to
maintain the prone position for at least 1 h; v) vertebral
destruction dominated by osteolytic lesions; vi) expected survival
time ≥3 months; vii) the patient underwent seed implantation, while
no surgery nor EBRT were conducted; viii) the patient was not in a
period of ulceration; ix) no other distant metastases besides bone
were observed and x) tumor identification via CT was performed
prior to I-125 seed implantation. The exclusion criteria were as
follows: i) Poor coagulation function or implantation could not be
performed; ii) no proper needle path and iii) rejection of
brachytherapy.
A total of six men and five women (median age, 52
years; range, 41–69 years) were enrolled in the present study,
including six cases of lung cancer, three cases of breast cancer
and two cases of kidney cancer. Metastases involving the vertebral
arch and the vertebral body accounted for 63.6% of all patients,
while 36.4% of the patients presented with metastasis in the
vertebral body alone. The degree of pre-implant pain in each
patient was assessed using the numerical rating scale (NRS) graded
from 1 to 10. One case (9.1%) had no pain (NRS=0), six patients
(54.5%) had moderate pain (NRS=4-6) and four patients (36.4%) had
severe pain (NRS=7-10). None of these patients received spinal
treatment prior to the I-125 interstitial brachytherapy. The
patients' characteristics are presented in Table I.
 | Table I.Patient characteristics (n=11). |
Table I.
Patient characteristics (n=11).
| Characteristic | No. of patients | Percentage, % |
|---|
| Sex |
|
|
| Male | 6 | 54.5 |
|
Female | 5 | 45.5 |
| Primary tumor |
|
|
| Lung | 6 | 54.5 |
|
Breast | 3 | 27.3 |
|
Kidney | 2 | 18.2 |
| Location of spine
metastasis |
|
|
|
Thoracic | 7 | 63.6 |
|
Lumbar | 4 | 36.4 |
| NRS score |
|
|
| 0 | 1 | 9.1 |
|
1–3 | 0 | 0.0 |
|
4–6 | 6 | 54.5 |
|
7–10 | 4 | 36.4 |
| KPS median
(range) | 60 (70–80) |
|
Radioactive source and
instruments
Radioactive I-125 seeds (HAT Co., Ltd.) were shaped
as a cylindrical titanium package body with a length of 4.5 mm, a
diameter of 0.8 mm and an activity range from 0.30–0.80 mCi. I-125
produces γ-rays (5% of 35 keV; 95% of 28 keV) with a half-life of
59.4 days, a half-value thickness of 0.025 mm lead and an incipient
rate of 7 cGy/h, at a distance of 1.7 cm (23).
CT (Light Speed 16, GE Healthcare Sciences) of the
spine was performed using the following settings: 120 kV, 275 mA
and a 5 mm width. Prior to the I-125 seed implantation, dose
distribution was calculated using Beihang Treatment Planning System
(TPS; standard version; Beijing ASTRO Technology Development Co.,
Ltd.) based on the American Association of Physicists in Medicine
TG43 brachytherapy formalism.
Pre-treatment planning
Pre-treatment planning was performed 1–2 weeks
before the seed implantation. Axial images (at 5 mm intervals) of
the abdomen were obtained for all patients prior to the seed
implantation and were transferred to the TPS. Contouring was
performed in every CT slice. The prescription dose for the planned
target volume (PTV) was 90–110 Gy. The PTV was a 0.5–1.0 cm
expansion of the gross tumor volume (GTV). Needle locations were
drawn based on the lesion size and its association with the
surrounding tissues. There was a 0.5–1.0 cm spacing between
adjacent needles. The seeds were distributed in the needle passage
by the TPS, followed by modification according to the isodose curve
and dose-volume histogram (DVH). Pre-planning dosimetry aimed for
the majority of the target volume (>90%) to receive 100% of the
prescription dose (V100>90%) and <50% of the target volume to
receive 200% of the prescription dose (V200<50%).
Implant procedure
CT guided transperineal insertion of the permanent
seed implantation was performed according to the treatment plan,
under local anesthesia. The seeds were implanted and positioned
against its deepest margin using an 18-gauge needle with a
turntable gun (Beijing Atom High Tech). The I-125 seeds were spaced
0.5–1.0 cm from each other. Dose-sparing was ensured by implanting
the seeds 1.0 cm away from the spinal cord.
Post-implant dosimetry
CT scans were performed immediately following the
implantation. Images were captured at 5 mm intervals, without a
gap. Seeds were located on the CT images. Contouring was performed
by the same physician who performed the pre-implant contouring.
DVHs of the target and surrounding normal tissue structures were
generated from the pre- and post-implant scans. Parameters
including V100, V150, V200, the doses delivered to 90% of the
target volume (D90) and Dmax of the spinal cord were evaluated.
Follow-up schedule
Clinical and radiographical evaluation of the tumor
response was performed 1 month after implantation. Follow-ups were
scheduled every 2 months for the first year post-implantation and
every 3–6 months thereafter. The therapeutic outcome was assessed
according to the response evaluation criteria in solid tumors
(RECIST) standard (24), which
includes: Complete response (CR), partial response (PR), stable
disease (SD) and progressive disease (PD). Local tumor control
referred to the absence of tumor progression on CT (SD + PR +
CR).
Statistical analysis
Dosimetry parameters were reported as the means ±
standard deviation. Statistical analysis was performed using SPSS
20.0 software (IBM Corp.). Paired t-tests were performed to compare
the difference in dosimetric parameters between the pre- and
post-implant conditions. The data in the text are consistent with a
normal distribution. P<0.05 was considered to indicate a
statistically significant difference.
Results
Pre-implant dosimetric
characteristics
The V100 was between 96.50–99.80% (mean; 97.80%) and
the V200 was between 35.20–49.50% (mean; 43.97%). The Dmax was
between 18.17 and 74.32 Gy (mean, 63.54 Gy). The D90 was between
113.20 and 128.86 Gy (mean; 119.07 Gy) and the number of seeds per
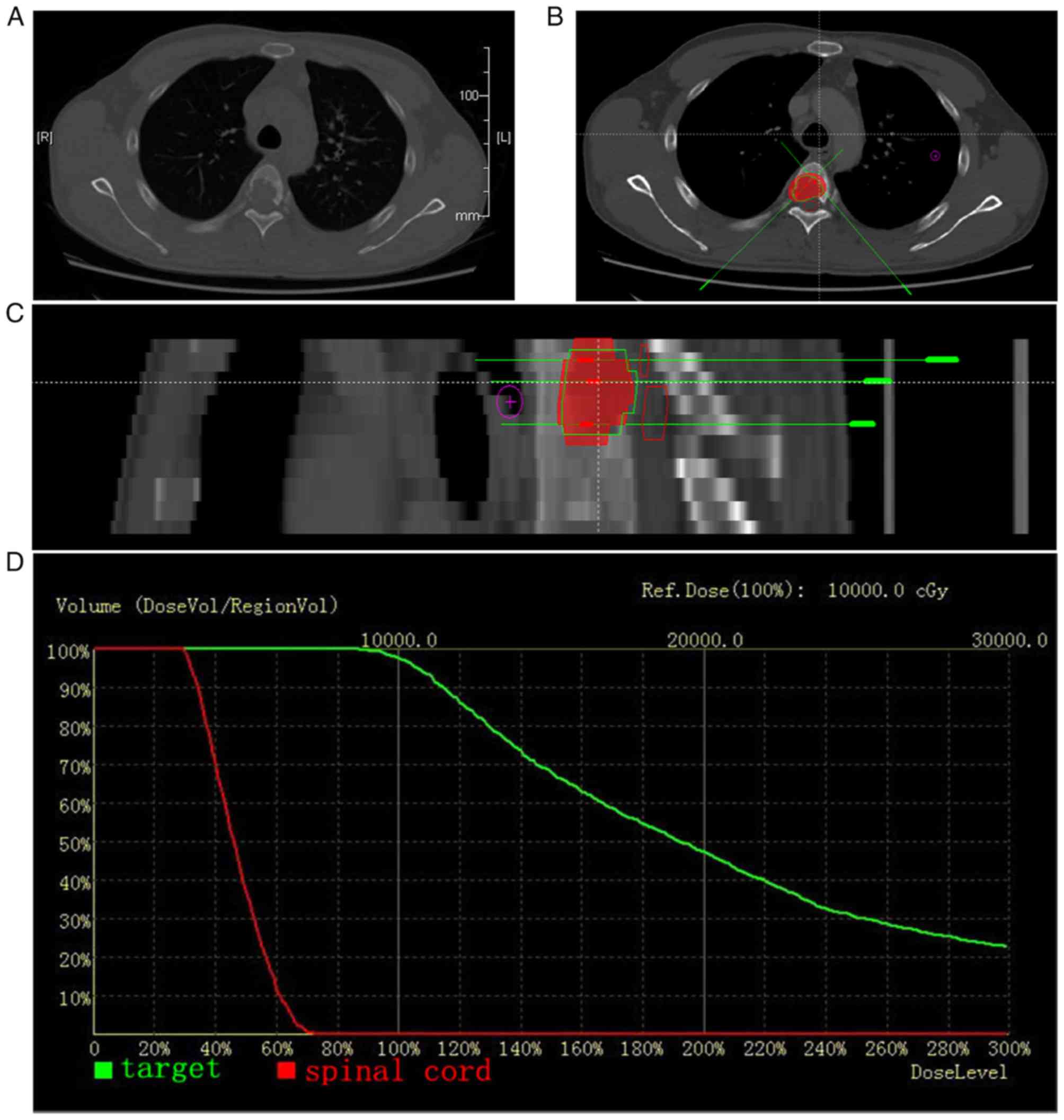
patient ranged from 8–44 (median, 30; Fig. 1A-D). The pre-implant dosimetric
characteristics are presented in Table
II.
 | Table II.Pre-implant treatment planning
characteristics. |
Table II.
Pre-implant treatment planning
characteristics.
| Patient no. | Location of spine
metastasis | GTV, cc | Seed activity,
mCi | No. of needles,
n | No. of seeds,
n | D90, Gy | GTV: V100, % | GTV: V150,% | GTV: V200, % | Dmax of spinal
cord, Gy |
|---|
| 1 | T10 | 30.5 | 0.8 | 10 | 30 | 117.08 | 97.00 | 72.40 | 49.20 | 74.17 |
| 2 | L2 | 8.2 | 0.8 | 7 | 11 | 115.95 | 98.00 | 64.60 | 38.20 | 69.38 |
| 3 | T2 | 4.9 | 0.8 | 5 | 8 | 128.86 | 99.80 | 75.00 | 49.50 | 60.33 |
| 4 | L4 | 51.2 | 0.8 | 15 | 44 | 114.00 | 96.50 | 71.40 | 48.70 | 74.11 |
| 5 | T5 | 8.3 | 0.6 | 9 | 15 | 114.54 | 97.60 | 68.10 | 47.30 | 72.62 |
| 6 | T7 | 14.4 | 0.6 | 12 | 23 | 116.92 | 97.20 | 69.00 | 40.20 | 73.26 |
| 7 | T12 | 5.0 | 0.6 | 4 | 11 | 127.64 | 99.00 | 78.00 | 45.60 | 18.17 |
| 8 | T11 | 34.5 | 0.6 | 8 | 39 | 118.40 | 97.00 | 73.30 | 49.30 | 74.32 |
| 9 | L2 | 32.3 | 0.6 | 11 | 37 | 123.95 | 98.70 | 70.30 | 37.60 | 46.16 |
| 10 | L4 | 42.0 | 0.8 | 12 | 37 | 113.20 | 97.30 | 69.10 | 42.90 | 72.94 |
| 11 | T7 | 8.8 | 0.3 | 9 | 30 | 119.25 | 97.70 | 64.40 | 35.20 | 63.48 |
Post-implant dosimetric
characteristics
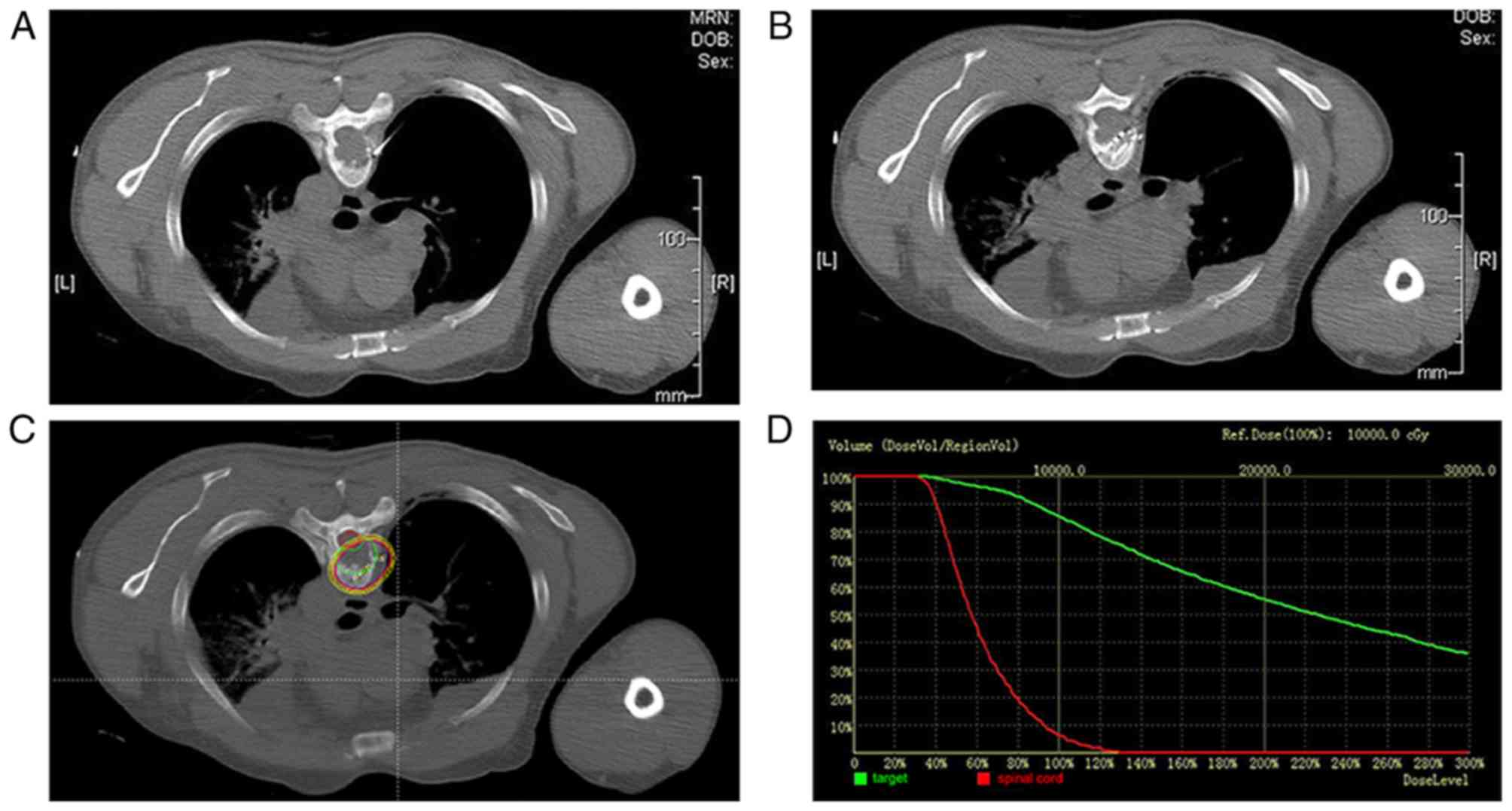
The number of I-125 seeds that were implanted ranged
from 10–58 (median, 30; Fig. 2A-D).
The specific activity of the I-125 seeds ranged from 0.3–0.8 mCi
per seed (median; 0.6 mCi). The V100 was between 66.40 and 96.70%
(mean, 84.46%) and the V200 was between 21.10 and 67.90%) (mean,
45.73%). The D90 ranged from 62.31–128.39 Gy (mean, 94.15 Gy). The
Dmax ranged from 16.07–274.30 Gy (mean, 112.78 Gy), for the cauda
equina. The post-implant dosimetirc characteristics are presented
in Table III.
 | Table III.Post-implant dosimetric
characteristics. |
Table III.
Post-implant dosimetric
characteristics.
| Patient no. | Location of spine
metastasis | GTV, cc | Seed activity,
mCi | No. of needles,
n | No. of seeds,
n | D90, Gy | GTV: V100, % | GTV: V150, % | GTV: V200,% | Dmax of spinal
cord, Gy |
|---|
| 1 | T10 | 30.6 | 0.8 | 5 | 25 | 64.72 | 73.20 | 54.40 | 40.60 | 123.32 |
| 2 | L2 | 8.3 | 0.8 | 4 | 12 | 79.18 | 75.00 | 58.00 | 40.70 | 92.61 |
| 3 | T2 | 4.9 | 0.8 | 3 | 11 | 102.53 | 90.70 | 71.90 | 54.60 | 38.31 |
| 4 | L4 | 51.2 | 0.8 | 5 | 42 | 103.72 | 91.70 | 66.00 | 47.60 | 119.87 |
| 5 | T5 | 8.3 | 0.6 | 3 | 22 | 88.13 | 85.80 | 68.50 | 55.60 | 129.73 |
| 6 | T7 | 14.4 | 0.6 | 6 | 37 | 128.39 | 96.70 | 83.30 | 65.30 | 274.30 |
| 7 | T12 | 5.1 | 0.6 | 2 | 10 | 101.01 | 90.30 | 65.10 | 29.70 | 16.07 |
| 8 | T11 | 34.6 | 0.6 | 2 | 34 | 62.31 | 66.40 | 38.90 | 21.10 | 159.30 |
| 9 | L2 | 32.4 | 0.6 | 9 | 58 | 113.17 | 93.40 | 79.90 | 67.90 | 77.64 |
| 10 | L4 | 41.9 | 0.8 | 9 | 52 | 107.88 | 92.10 | 74.90 | 56.40 | 110.85 |
| 11 | T7 | 8.8 | 0.3 | 3 | 22 | 84.61 | 73.80 | 33.00 | 23.50 | 98.54 |
Pre- and post-implant dosimetric
comparisons
The pre- and post-implanting plan-associated
parameters are presented in Table
IV. A greater number of needles were used in the pre-implant
treatment planning (mean, 9) compared with the implantation (mean,
4). However, the mean GTV, number of seeds and the activity per
seed were revealed to be similar between the two groups.
 | Table IV.Plan-associated parameters for
pre-planning and post implantation. |
Table IV.
Plan-associated parameters for
pre-planning and post implantation.
| Parameter | Pre-planning |
Post-implanting | P-value |
|---|
| GTV, mean (range)
cc | 21.83
(4.9–51.2) | 21.87
(4.9–51.2) | 0.104 |
| Number of needles,
median (range) | 9 (4–15) | 4 (2–9) | <0.001 |
| Number of seeds,
median (range) | 30 (8–44) | 30 (10–58) | 0.231 |
| Activity per seed,
median (range) | 0.6 (0.3–0.8) | 0.6 (0.3–0.8) | 1 |
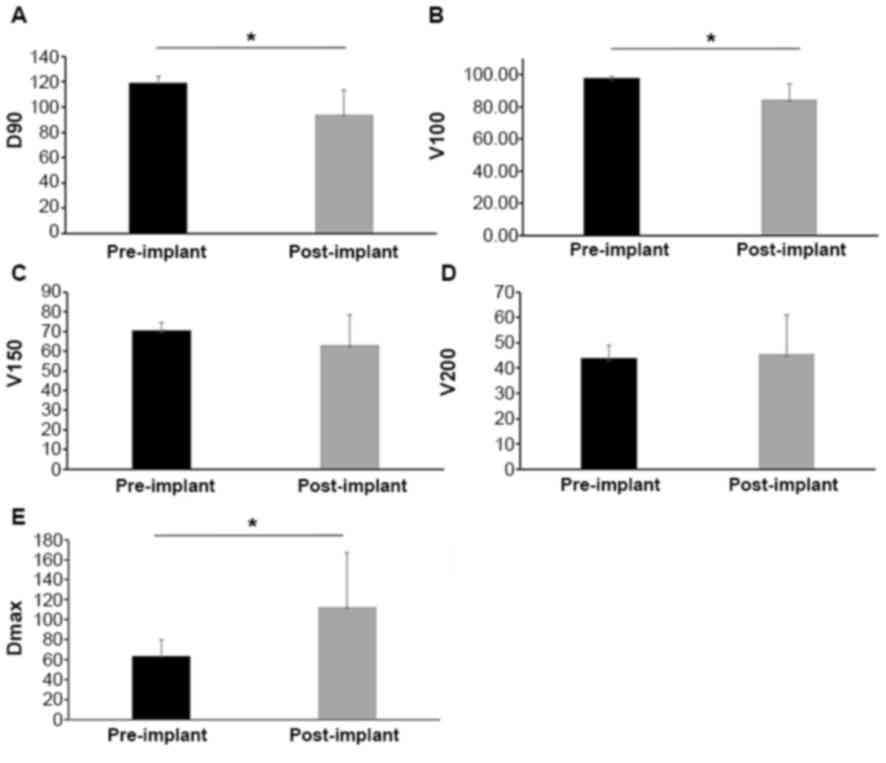
The pre- and post-implant dosimetric comparisons are
presented in Table V and Fig. 3. The mean D90 value in the
pre-implant planning images of the spine was greater than the
post-implant dosimetry (119.07 vs. 94.15 Gy; P<0.05). Similarly,
the mean pre-implant V100 was greater than the mean post-implant
V100 (97.80 vs. 84.46%; P<0.05), of the prescribed doses. These
differences may be due to variations in the shape, puncture path,
position of critical organs, such as the spinal cord, and bone
obstruction. Therefore, it is difficult to precisely implant the
seeds according to the pre-implant plan. The mean Dmax of the
spinal cord in post-implant dosimetry was higher compared with
pre-implant planning (112.78 vs. 63.54; P<0.05). However, the
V150 and V200 were revealed to be similar between the two
groups.
 | Table V.Pre- and post-implant dosimetric
comparisons. |
Table V.
Pre- and post-implant dosimetric
comparisons.
| Parameter | Pre-implant | Post-implant | P-value |
|---|
| D90, mean (range),
Gy | 19.07
(113.20–128.86) | 94.15
(62.31–128.39) | 0.002 |
| V100, mean (range),
% | 97.80
(96.50–99.80) | 84.46
(66.40–96.70) | 0.001 |
| V150, mean (range),
% | 70.51
(64.40–78.00) | 63.08
(33.00–83.30) | 0.148 |
| V200, mean (range),
% | 43.97
(35.20–49.50) | 45.73
(21.10–67.90) | 0.746 |
| Dmax of cord, mean
(range), Gy | 63.54
(18.17–74.32) | 112.78
(16.78–274.30) | 0.018 |
Local control and survival
The mean time between the implantation and the
follow-up was 5.45 months (range, 2–17 months). All patients
survived until the end of the follow-up. No cases of CR were
observed in the combined-treatment group, while one case of PR
(9.1%), 10 cases of SD (90.9%) and no cases of PD (0.0%) were
observed, with a local control rate of 100.0%.
Discussion
I-125 brachytherapy has served as a tumor treatment
strategy for a number of years. Several studies have demonstrated
that I-125 brachytherapy provides satisfactory local control of
solid tumors, including prostate carcinoma, lung cancer, lung
metastasis and pancreatic cancer (25–27).
I-125 seeds are permanently implanted into the tumor and low energy
γ-rays are continuously emitted. Due to the low penetration of low
energy γ-rays, dose deposition to tissue decreases rapidly with
distance from the radioactive source (23). The radiobiological advantages of
interstitial I-125 seed implants include decreased treatment time,
a high radiation dose conformity to the tumor and the sparing of
surrounding normal tissues (17).
These traits are particularly desirable for the treatment of spinal
tumors, because poor dose control can result in myelitis and
vertebral body fracture, which would be catastrophic.
Pre-implant treatment planning assesses the dose
distribution and seed arrangement based on volumes recorded in CT
images, which are acquired several days or weeks prior to
implantation. Accurate dose distribution increases the efficacy of
I-125 brachytherapy (28). Although
seeds are implanted into patients according to a predetermined
arrangement, studies have suggested that the post-implant dosimetry
is usually different from the pre-implant planning in prostate
brachytherapy (29–31). The potential reasons for such
discrepancies between the pre- and post-implant dosimetry include,
post-operative prostatic inflammation and edema (32,33),
difficulty to precisely implant the seeds according to the
pre-implant plan (34,35), measures taken by the implanting
physician to spare the surrounding normal tissues (36,37) and
post-operative seed displacement (36–38).
However, to the best of our knowledge, very few studies have
directly investigated this issue in spinal metastases
brachytherapy.
The present study compared the pre- and post-implant
dosimetry in I-125 spinal metastases brachytherapy. The results
revealed a difference in the mean V100 and D90 between the two
groups. In all 11 cases, the post-implant V100 was lower compared
with the pre-implant treatment plans, whereas there was only one
case in which the post-implant D90 was greater than that of the
pre-implant. In this case, osteolytic destruction was serious and
both needle puncture and seed implantation were easy. It has been
suggested that the stiffness and shape of the bone are vital to the
implantation procedure, enabling the correct implantation of the
seeds and protection of the OAR, particularly the spinal cord
(39). Similarly, the average number
of needles used in the implantation was lower than the pre-implant
treatment planning. Despite a difference in the V100 and D90
between the two groups, the local control rate remained at 100%.
This may indicate that V100 <90% is effective in controlling
bone metastatic diseases.
The prostate volume changes that were observed
during and after the seed implantation were primarily due to
prostatic inflammation and edema (40). In the spinal cases, the present data
revealed little difference in the GTV both before and after
implantation. This may be due to the fact that the spine is not
prone to edema upon surgery. The Dmax of the spinal cord in
post-implant dosimetry was generally greater than in the
pre-implant treatment plans. In two of the cases (no. 3 and no. 7),
the Dmax in the spinal cord was lower following implantation, and
the lesion was located away from the spinal cord. In one case, the
Dmax of the spinal cord increased to 274.3 Gy without the
occurrence of myelitis. Rogers et al (41) reported that radiation myelitis was
not recorded despite the delivery of 167.3 Gy. Similarly, Harrison
et al (42) demonstrated that
brachytherapy, using permanent or temporary implants, revealed no
myelitis following 60 Gy in paraspinal tumors, pancoast carcinoma
or other sarcoma treatment. Although the recommended clinical dose
limit for the spinal cord is 45 Gy (41), no myelitis was observed. This may
indicate that: i) CT used for these two plans had spinal cord
coverage larger than the true spinal cord; ii) dose distribution
was calculated using a TPS brachytherapy planning system based on
the AAPM TG43 formalism that does not account for the complex
internal environment in humans or iii) the 45 Gy for the spinal
cord was obtained from previous data in the study of conventional
radiotherapy and EBRT (3,43), and currently, there is no equivalent
conversion. Future studies should continue to investigate the
recommended clinical dose limit of spinal cord in brachytherapy.
Furthermore, a longer follow-up period should be implemented for
the evaluation of a suitable spinal cord dosage and the assessment
of the clinical significance of suboptimal PTV dose coverage in
patients who attain good dosimetry.
A high rate of tumor control and rapid pain relief
was achieved with interstitial I-125 seed brachytherapy. The
present study demonstrated that CT guided I-125 seed brachytherapy
in the treatment of spinal metastases tumors is both safe and
effective. However, the seed number and position in the
post-implant dosimetry was observed to deviate from the pre-implant
treatment planning. Thus, strict adherence to the pre-implant
treatment plan remains crucial.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81272351) and the Natural
Science Foundation of Shandong Province (grant nos. 2012G0021826
and ZR2012HM020).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GC analyzed and interpreted the data, and wrote the
manuscript. MH designed the present study. All authors approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shandong Provincial Hospital (approval no. 2015-011;
Jinan, China). Written informed consent for publication was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors have declared that they have no
competing interests.
References
|
1
|
Ibrahim T, Mercatali L, Casadei R and
Sabbatini R: Clinical manifestation. Osteoncology textbook. Amadori
D, Cascinu S, Conte P and Ibrahim T: Poletto editore; Milan: pp.
258–276. 2010, PubMed/NCBI
|
|
2
|
Ortiz Gómez JA: The incidence of vertebral
body metastases. Int Orthop. 19:309–311. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sciubba DM, Petteys RJ, Dekutoski MB,
Fisher CG, Fehlings MG, Ondra SL, Rhines LD and Gokaslan ZL:
Diagnosis and management of metastatic spine disease. A review. J
Neurosurg Spine. 13:94–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nielson OS, Munro AJ and Tannock IF: Bone
metastasis: Pathophysiology and management policy. J Clin Oncol.
9:509–524. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
García-Picazo A, Capilla Ramírez P, Pulido
Rivas P and Garcia de Sola R: Utility of surgery in the treatment
of epidural vertebral metastases. Acta Neurochir (Wien).
103:131–138. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bach F, Larsen BH, Rohde K, Børgesen SE,
Gjerris F, Bøge-Rasmussen T, Agerlin N, Rasmusson B, Stjernholm P
and Sørensen PS: Metastatic spinal cord compression. Occurrence,
symptoms, clinical presentations and prognosis in 398 patients with
spinal cord compression. Acta Neurochir (Wien). 107:37–43. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gilbert RW, Kim JH and Posner JB: Epidural
spinal cord compression from metastatic tumor: Diagnosis and
treatment. Ann Neurol. 3:40–51. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wild WO and Porter RW: Metastatic epidural
tumor of the spine. A study of 45 cases. Arch Surg. 87:825–830.
1963. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aoude A, Fortin M, Aldebeyan S, Ouellet J,
Amiot LP, Weber MH and Jarzem P: The revised Tokuhashi score;
analysis of parameters and assessment of its accuracy in
determining survival in patients afflicted with spinal metastasis.
Eur Spine J. 27:835–840. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ibrahim T, Mercatali L and Amadori D: A
new emergency in oncology: Bone metastases in breast cancer
patients (Review). Oncol Lett. 6:306–310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Patchell RA, Tibbs PA, Regine WF, Payne R,
Saris S, Kryscio RJ, Mohiuddin M and Young B: Direct decompressive
surgical resection in the treatment of spinal cord compression
caused by metastatic cancer: A randomised trial. Lancet.
366:643–648. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Delank KS, Wendtner C, Eich HT and Eysel
P: The treatment of spinal metastases. Dtsch Arztebl Int.
108:71–79. 2011.PubMed/NCBI
|
|
13
|
Jacobs WB and Perrin RG: Evaluation and
treatment of spinal metastases: An overview. Neurosurg Focus.
11:e102001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Markoe AM and Schwade JG: The role of
radiation therapy in the management of spine and spinal cord
tumors. In Spine Tumors. Rea GL: American Association of
Neurological Surgeons; Park Ridge: pp. ILpp23–35. 1994
|
|
15
|
Ryu SI, Chang SD, Kim DH, Murphy MJ, Le
QT, Martin DP and Adler JR Jr: Image-guided hypo-fractionated
stereotactic radiosurgery to spinal lesions. Neurosurgery.
49:838–846. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang SD, Adler J Jr and Hancock S:
Clinical uses of radiosurgery. Oncology (Williston Park).
12:1181-1188–1191-1192. 1998.
|
|
17
|
Shi S, Yang J and Sun D: CT-guided 125I
brachytherapy on pulmonary metastases after resection of colorectal
cancer: A report of six cases. Oncol Lett. 9:375–380. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Lu J, Liu L, Liu T, Chen K, Liu F
and Huang G: Clinical application of CT-guided 125I seed
interstitial implantation for local recurrent rectal carcinoma.
Radiat Oncol. 6:1382011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhongmin W, Yu L, Fenju L, Kemin C and
Gang H: Clinical efficacy of CT-guided iodine-125 seed implantation
therapy in patients with advanced pancreatic cancer. Eur Radiol.
20:1786–1791. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Z, Lu J, Liu T, Chen K, Huang G and
Liu F: CT-guided interstitial brachytherapy of inoperable non-small
cell lung cancer. Lung Cancer. 74:253–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martínez-Monge R, Pagola M, Vivas I and
López-Picazo JM: CT-guided permanent brachytherapy for patients
with medically inoperable early-stage non-small cell lung cancer
(NSCLC). Lung Cancer. 61:209–213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nasser NJ, Sappiatzer J, Wang Y, Borg J
and Saibishkumar EP: Dosimetric evaluation of clinical target
volume in the postimplant analysis of low-dose-rate brachytherapy
for prostate cancer. Brachytherapy. 14:189–196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Z, Tan J, Zhao R, Wang J, Sun H, Wang
X, Xu L, Jiang H and Zhang J: Clinical investigations on the spinal
osteoblastic metastasis treated by combination of percutaneous
vertebroplasty and (125)I seeds implantation versus radiotherapy.
Cancer Biother Radiopharm. 28:58–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cosset JM, Flam T, Thiounn N, Rosenwald
JC, Pontvert D, Timbert M, Solignac S and Chauveinc L:
Brachytherapy for prostate cancer: Old concept, new techniques.
Bull Cancer. 93:761–766. 2006.(In French). PubMed/NCBI
|
|
26
|
Peretz T, Nori D, Hilaris B, Manolatos S,
Linares L, Harrison L, Anderson LL, Fuks Z and Brennan MF:
Treatment of primary unresectable carcinoma of the pancreas with
I-125 implantation. Int J Radiat Oncol Biol Phys. 17:931–935. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lewis JW Jr, Ajlouni M, Kvale PA, Groux N,
Bae Y, Horowitz BS and Magilligan DJ Jr: Role of brachytherapy in
the management of pulmonary and mediastinal malignancies. Ann
Thorac Surg. 49:728–323. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao F, Li C, Gu Y, Huang J and Wu P:
CT-guided 125I brachytherapy for mediastinal metastatic lymph nodes
recurrence from esophageal carcinoma: Effectiveness and safety in
16 patients. Eur J Radiol. 82:e70–e75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Potters L, Roach M III, Davis BJ, Stock
RG, Ciezki JP, Zelefsky MJ, Stone NN, Fearn PA, Yu C, Shinohara K
and Kattan MW: Postoperative nomogram predicting the 9-year
probability of prostate cancer recurrence after permanent prostate
brachytherapy using radiation dose as a prognostic variable. Int J
Radiat Oncol Biol Phys. 76:1061–1065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stock RG, Stone NN, Cesaretti JA and
Rosenstein BS: Biologically effective dose values for prostate
brachytherapy: Effects on PSA failure and posttreatment biopsy
results. Int J Radiat Oncol Biol Phys. 64:527–533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee WR, Bae K, Lawton CA, Gillin MT,
Morton G, Firat S, Baikadi M, Kuettel M, Greven K and Sandler H: A
descriptive analysis of postimplant dosimetric parameters from
Radiation Therapy Oncology Group P0019. Brachytherapy. 5:239–243.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marshall RA, Buckstein M, Stone NN and
Stock R: Treatment outcomes and morbidity following definitive
brachytherapy with or without external beam radiation for the
treatment of localized prostate cancer: 20-year experience at Mount
Sinai medical center. Urol Oncol. 32:38.e1–e7. 2014. View Article : Google Scholar
|
|
33
|
Kovtun KA, Wolfsberger L, Niedermayr T,
Sugar EN, Graham PL, Murciano-Goroff Y, Beard C, D'Amico AV, Martin
NE, Orio PF and Nguyen PL: Dosimetric quality and evolution of
edema after low-dose-rate brachytherapy for small prostates:
Implications for the use of newer isotopes. Brachytherapy.
13:152–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saibishkumar EP, Borg J, Yeung I,
Cummins-Holder C, Landon A and Crook J: Sequential comparison of
seed loss and prostate dosimetry of stranded seeds with loose seeds
in 125I permanent implant for low-risk prostate cancer. Int J
Radiat Oncol Biol Phys. 73:61–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saibishkumar EP, Borg J, Yeung I,
Cummins-Holder C, Landon A and Crook JM: Loose seeds vs. stranded
seeds: A comparison of critical organ dosimetry and acute toxicity
in (125)I permanent implant for low-risk prostate cancer.
Brachytherapy. 7:200–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Davis BJ, Horwitz EM, Lee WR, Crook JM,
Stock RG, Merrick GS, Butler WM, Grimm PD, Stone NN, Potters L, et
al: American brachytherapy society consensus guidelines for
transrectal ultrasound-guided permanent prostate brachytherapy.
Brachytherapy. 11:6–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Price JG, Stone NN and Stock RG:
Predictive factors and management of rectal bleeding side effects
following prostate cancer brachytherapy. Int J Radiat Oncol Biol
Phys. 86:842–847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Sappiatzer J, Borg J, Rink A and
Saibishkumar EP: Analysis of seed loss and seed displacement and
its dosimetry impact in prostate cancer patients treated with low
dose rate brachytherapy. Med Phys Int. 1 (1 Suppl):S5362013.
|
|
39
|
Cao Q, Wang H, Meng N, Jiang Y, Jiang P,
Gao Y, Tian S, Liu C, Yang R, Wang J and Zhang K: CT-guidance
interstitial (125)Iodine seed brachytherapy as a salvage therapy
for recurrent spinal primary tumors. Radiat Oncol. 9:3012014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sarkar A, Donavanik V, Zhang I, Chen H,
Koprowski C, Hanlon A, Mourtada F, Strasser J and Raben A: Prostate
implant dosimetric outcomes and migration patterns between
bio-absorbable coated and uncoated brachytherapy seeds.
Brachytherapy. 12:356–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rogers CL, Theodore N, Dickman CA, Sonntag
VK, Thomas T, Lam S and Speiser BL: Surgery and permanent 125I seed
paraspinal brachytherapy for malignant tumors with spinal cord
compression. Int J Radiat Oncol Biol Phys. 54:505–513. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Harrison LB, Zelefsky MJ, Armstrong JG,
Schupak KD and Brennan MF: Brachytherapy and function preservation
in the localized management of soft tissue sarcomas of the
extremity. Semin Radiat Oncol. 3:260–269. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
St Clair WH, Arnold SM, Sloan AE and
Regine WF: Spinal cord and peripheral nerve injury: Current
management and investigations. Semin Radiat Oncol. 13:322–332.
2003. View Article : Google Scholar : PubMed/NCBI
|