Introduction
Treatment with demethylating agents, such as
5-azacytidine (5-azaC), not only prolongs the survival of patients
with intermediate 2 and high-risk myelodysplastic syndrome (MDS)
(1,2), but also leads to improvements in acute
myeloid leukemia (AML) and multiple myeloma (MM) (3–5).
However, the mechanisms behind the therapeutic effects of 5-azaC
are not yet clear. No definite evidence exists that the clinical
response is related to the induction and magnitude of DNA
hypomethylation (6).
A number of studies have pointed out the
immunoregulatory effects of 5-azaC, which are proven to be
important in the pathogenesis of MDS (7), though the mechanisms that underlie
these changes are not fully understood. 5-azaC treatment has been
shown to augment regulatory T cells (Tregs) after bone marrow
transplants, and thereby, to alleviate the graft-versus-host
disease (GvHD) (8). 5-azaC may
expand Tregs and contribute to the management of GvHD after
allogeneic bone marrow transplantation (8). However, 5-azaC or decitabine have been
shown to possess an intact graft-versus-leukemia (GVL) effect in
transplanted mice, with an increase in the number of
FoxP3+ T cells (9). In
clinical practice, high-risk MDS patients benefit from 5-azaC
treatment with significant prolongation of survival. For these
reasons our study focused on 5-azaC in the development of
CD4+ T cells, particularly Tregs and T-helper 17
(Th17).
Previous studies have shown that the number of Tregs
is significantly increased in high-risk and intermediate-2 MDS,
whereas in ‘low-risk’ MDS interleukin (IL)-17-producing
CD4+ T cells (Th17 cells) is increased, suggesting a
correlation between the number of Tregs and Th17 with the severity
of the disease (10,11). The promoter of FoxP3, a key gene for
the function of Tregs, is methylated in conventional
CD4+CD25− T cells (12). Treatment with DNA methyltransferase
(DNMT) inhibitors induces conversion of conventional
CD4+CD25− T cells into
CD25+FoxP3+ Tregs. This suggests that the
expansion of Tregs is associated with increased FoxP3 expression
due to FoxP3 promoter demethylation.
In order to understand the effects of 5-azaC on
CD4+ T cells, the serial peripheral blood and bone
marrow samples from patients with intermediate-2/high-risk MDS were
analyzed, including the number profile of T cells prior to and
following 5-azaC treatment. The effect of 5-azaC on the expression
and methylation status of FoxP3 in vitro was also
investigated.
Patients and methods
Patient samples
Thirty patients with intermediate-2 and high risk
MDS were risk-classified according to the International Prognostic
Scoring System (IPSS). The study was approved by the Ethics
Committee of Shanghai East Hospital, Tongji University School of
Medicine (Shanghai, China; research no. 136, 2018). Patients who
participated in this research signed an informed consent and had
complete clinical data. MDS patients with a median age of 62 years
were treated with 5-azaC subcutaneously at a dose of 75
mg/m2/day on the first 7 days of a 28-day cycle. The
median time of treatment with 5-azaC was 3 months. After informed
consent, all 30 patients provided peripheral blood and bone marrow
samples for analysis, prior to treatment, and at 1, 2 and 3 months
while on 5-azaC treatment (Table
I).
 | Table I.Clinical data of MDS patients before
5-azaC treatment. |
Table I.
Clinical data of MDS patients before
5-azaC treatment.
| Variables | Cases | Normal chromosome
karyotype n (%) | Complex abnormal
karyotype n (%) | P-value |
|---|
| Age (years) |
|
|
| 0.484 |
|
<65 | 16 | 6 (37.5) | 10 (62.5) |
|
|
≥65 | 14 | 8 (57.1) | 6
(42.9) |
|
| Sex |
|
|
| 0.569 |
|
Male | 17 | 5 (29.4) | 12 (70.6) |
|
|
Female | 13 | 6 (46.1) | 7
(53.8) |
|
| WHO
classification |
|
|
| 0.732 |
| RA | 2 | 1 (50.0) | 1
(50.0) |
|
|
RARS | 3 | 1 (33.3) | 2
(66.7) |
|
|
RCMD | 7 | 2 (28.5) | 5
(71.5) |
|
|
RAEB-1 | 8 | 4 (50.0) | 4
(50.0) |
|
|
RAEB-2 | 5 | 3 (60) | 2
(40) |
|
|
MDS-U | 3 | 2 (66.7) | 1
(33.3) |
|
|
CMML | 2 | 1 (50.0) | 1
(50.0) |
|
| IPSS grouping |
|
|
| 0.594 |
|
Intermediate-2 | 12 | 6 (50.0) | 6
(50.0) |
|
| High
risk | 18 | 7 (38.9) | 11
(61.1) |
|
Mononuclear cell separation
Mononuclear cells were separated from peripheral
blood (PBMC) by density gradient sedimentation. For the in
vitro assays, CD4+ T cells were subsequently
isolated by magnetic-activated cell sorting (MACS) using the
CD4+ isolation kit from Miltenyi Biotec, GmbH. To obtain
CD3+CD4+CD25+FoxP3+
Tregs and CD3+CD4+IL-17+ Th17
cells, PBMC were first enriched for CD4+ T cells using a
negative isolation kit (Miltenyi Biotec, Inc.) and were stained
with anti-human CD4, CD25 and FoxP3. Purified Tregs and Th17 cells,
defined as
CD3+CD4+CD25+FoxP3+ and
CD3+CD4+IL-17+, were sorted using
a FACSAria sorter (BD Biosciences).
Antibodies, reagents, and flow
cytometry
Peripheral blood CD4+ T cells
(1×106/ml) of patients were stimulated with 500 ng/ml
phorbol 12-myristate 13-acetate (PMA) and ionomycin in complete
medium for 4 h, After further 4 h, CD4+ T cells were
harvested and washed with PBS. To analyze the proportion of Th17
cells, CD4+ T cells were first stained with
FITC-conjugated anti-human CD4 antibody at 4°C for 30 min. Then,
they were fixed and permeabilized with fixation/permeabilization
buffer and were intracellularly stained with APC-conjugated
anti-human IL-17A antibody at room temperature in the dark for 30
min. To analyze the proportion of Tregs, CD4+ T cells
were simultaneously stained with FITC-conjugated anti-human CD4
antibody and PC7-conjugated anti-human CD25 antibody, then they
were fixed and permeabilized, and intracellularly stained with
PE-conjugated anti-human FoxP3 antibody at room temperature in the
dark for 30 min. Isotype-matched control antibodies were used in
all staining processes. Flow cytometry was performed on a FACSCanto
II system using FACSDiva software (BD Biosciences). Data were
analyzed on FlowJo software (Tree Star, Inc.). Antibodies: CD3-ECD
(mouse, monoclonal, dilution: 5 µl/test, cat. no. A07748, Beckman
Coulter, Inc.), CD4-PE (mouse, monoclonal, dilution: 5 µl/test,
cat. no. 347327; Becton, Dickinson and Company), CD4-FITC (mouse,
monoclonal, dilution: 5 µl/test, cat. no. A07750; Beckman Coulter,
Inc.), CD25-PC7 (mouse, monoclonal, dilution: 5 µl/test, cat. no.
A52882; Beckman Coulter, Inc.), FoxP3-PE (mouse, monoclonal,
dilution: 10 µl/test, cat. no. B46031; Beckman Coulter, Inc.),
IL-17-488A (rabbit, monoclonal, dilution: 10 µl/test, cat. no.
ab217359; Abcam).
Stimulation of isolated
CD4+ T-cell subset
5-azaC (Sigma- Aldrich: Merck KGaA) was dissolved in
acetic acid to a concentration of 20 mM and was used at 1 µM.
CD4+ T cells (20×106/ml) were treated by
freshly dissolved and diluted 5-azaC at a concentration of 1 µM or
an equal volume of vehicle (every 24 h for 96 h).
Carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE or CFSE)
dilution was determined by flow cytometry and the proliferation
index was calculated by ModFit software (Verity Software House,
Inc.).
Immunohistochemistry of bone
marrow
Bone marrow was collected from patients with MDS
after treatment with 5-azaC or vehicle for immunohistochemical
staining and the samples were fixed with formaldehyde. FoxP3, RORγt
and Tbet staining was carried out to determine the expression of
transcription factors in bone marrow. The results of
immunohistochemistry were obtained by a double-blind method. Five
high-power fields were selected, and the results were converted
into mm−2. The average value was selected as the final
result. Fixative (4%) was used at room temperature for 12 h, and
the samples were embedded in paraffin. The thickness of the
paraffin sections was approximately 6 µm. Bovine serum albumin
(BSA; 5%) was used for blocking at 37°C for 30 min. The sections
were incubated with rabbit anti-FoxP3 monoclonal antibody [SP97]
(cat. no. ab99963; Abcam), rabbit anti-RORγt monoclonal antibody
(cat. no. ab219496; Abcam), and goat anti-rabbit IgG H&L (HRP)
(cat. no. ab205718; Abcam) at 37°C for 30 min. Olympus CX41 (×400,
×200; Olympus, Corp.) was used.
Methylation assay
Genomic DNA was extracted with a blood and cell
culture DNA mini kit (Qiagen, Inc.) and was digested with an
EpiTect Methyl DNA Restriction kit (SABiosciences: Qiagen, Inc.).
The digested product was used to quantify the remaining DNA using
quantitative SYBR Green PCR. Primer sets used for amplification of
human FoxP3, RORγT and Tbet promoter CpG islands were purchased
from SABiosciences (Qiagen, Inc.). Quantitative PCR was performed
on an ABI Fast 7500 sequence detection system (Applied Biosystems:
Thermo Fisher Scientific, Inc.). Anti-mouse FoxP3 monoclonal
antibody (PE) (cat. no. E-AB-F1208I; Elabscience Biotechnology,
Inc.) was used. FoxP3 forward, 5′-TAGCCTCGATGTACGT-3′ and reverse,
5′-AGCCTGACGACCTAGCTCG-3′. β-actin was the reference gene; forward,
5′-TCACAGACACTGTGCTCATCTACGA-3′ and reverse,
5′-TAGCGTAACCGCTCGTTGCCAATGG-3′. Thermocycling conditions: 94°C
(hot start) for 10 min, followed by 30 cycles at 94°C for 30 sec,
at 55°C for 30 sec, and at 72°C for 30 sec, with a final extension
at 72°C for 10 min. 2−ΔΔCq was the quantification method
used in this study (13).
Statistical analysis
GraphPad Prism 5.0 (GraphPad Software, Inc.) and
SPSS 19.0 (IBM Corp.) software programs were used for statistical
analysis. Data are presented as n (%) or the mean ± standard
deviation (SD). The measurement data were compared using the
independent sample t-test, the enumeration data were compared by
χ2 test, and the paired t-test was used for comparisons
between two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
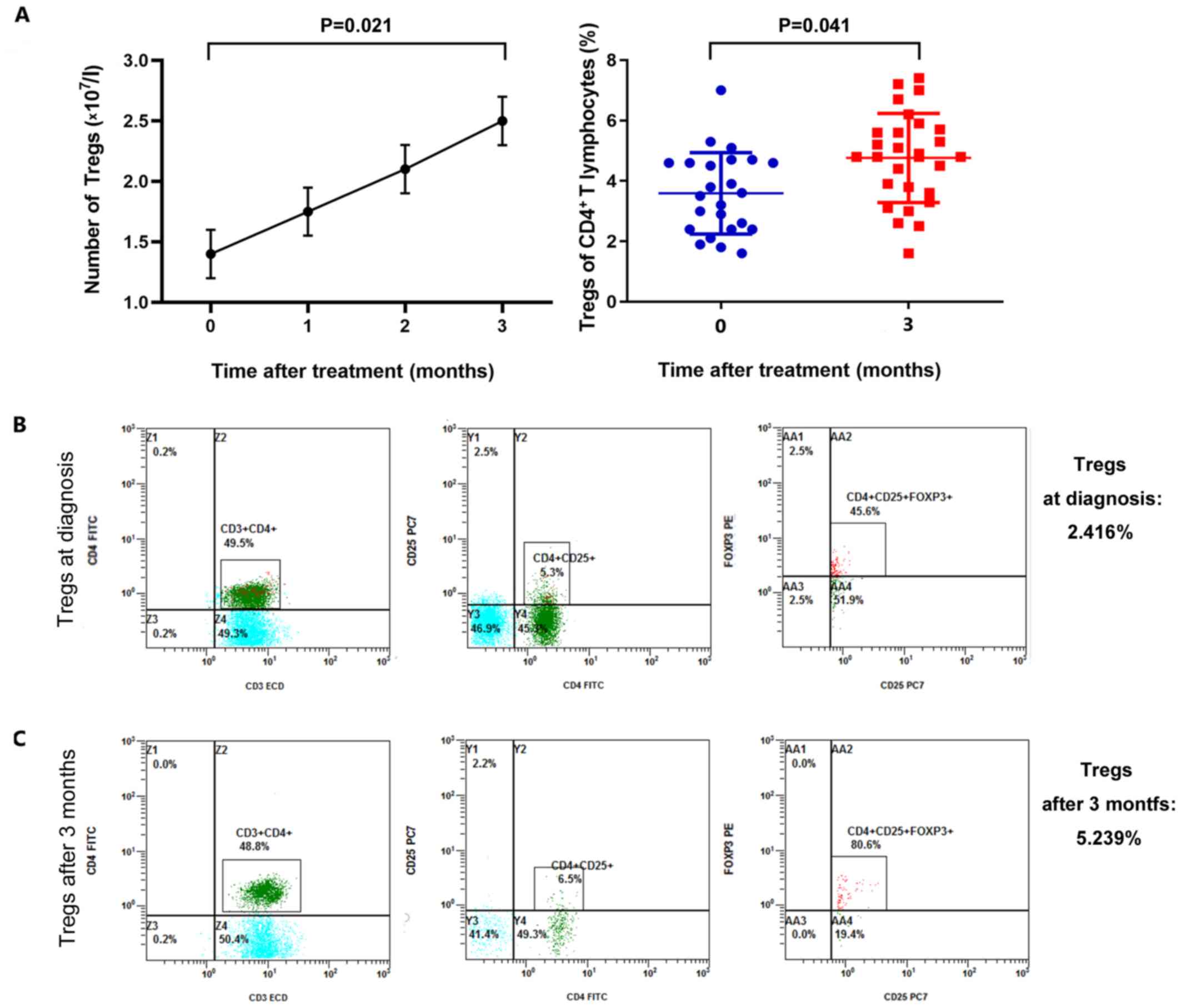
In vivo effect of 5-azaC on Tregs
Tregs isolated in vivo showed 98.2% cell
viability with 90.8% cell purity. Administration of 5-azaC
increased the absolute number of peripheral blood Tregs within 3
months [1.405±0.213 vs. 2.521±0.187 (×107/l)] (mean ±
SD, n=30) (P=0.021) (Fig. 1A). A
modest temporary increase in the number of Tregs arose following
5-azaC treatment in the first month, although this difference was
not statistically significant. The absolute numbers of peripheral
blood Tregs from an MDS patient are presented in Fig. 1B and C as a representative example.
An increase was observed from 2.416% of CD4+ T
lymphocytes (Fig. 1B) to 5.239% of
CD4+ T lymphocytes (Fig.
1C) after 3 months of therapy.
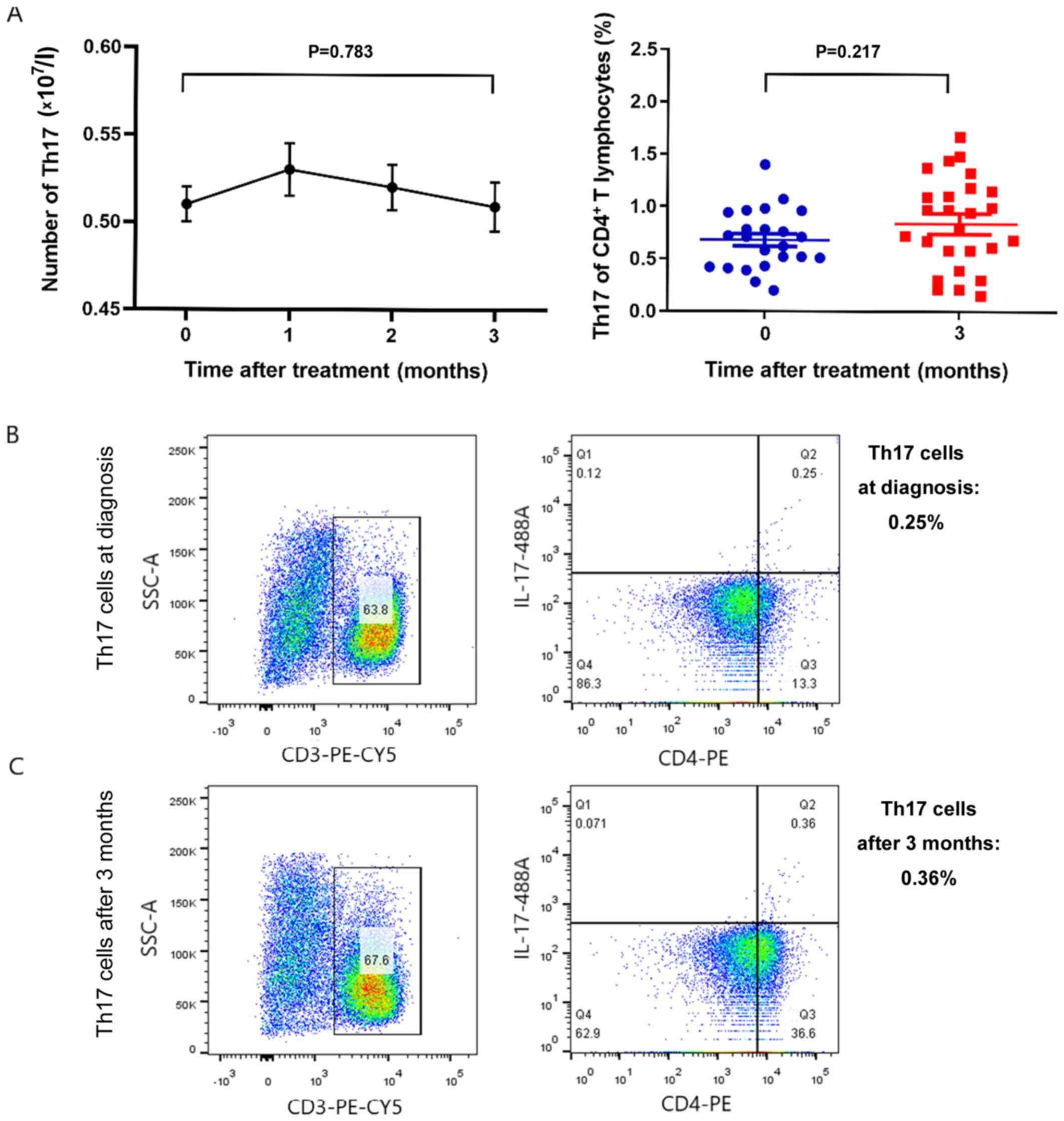
Following treatment with 5-azaC, the absolute number
of Th17 cells after 3 months of treatment showed no statistically
significant difference [0.518±0.012 vs. 0.509±0.014
(×107/l)] (mean ± SD, n=30) (P=0.783) (Fig. 2A). A representive example of an MDS
patient is presented in Fig. 2B and
C. The number of Th17 cells at diagnosis was 0.25% of
CD4+ T lymphocytes (Fig.
2B). After 3 months of therapy with 5-azaC, the number of Th17
cells was increased to 0.36% of CD4+ T lymphocytes
(Fig. 2C).
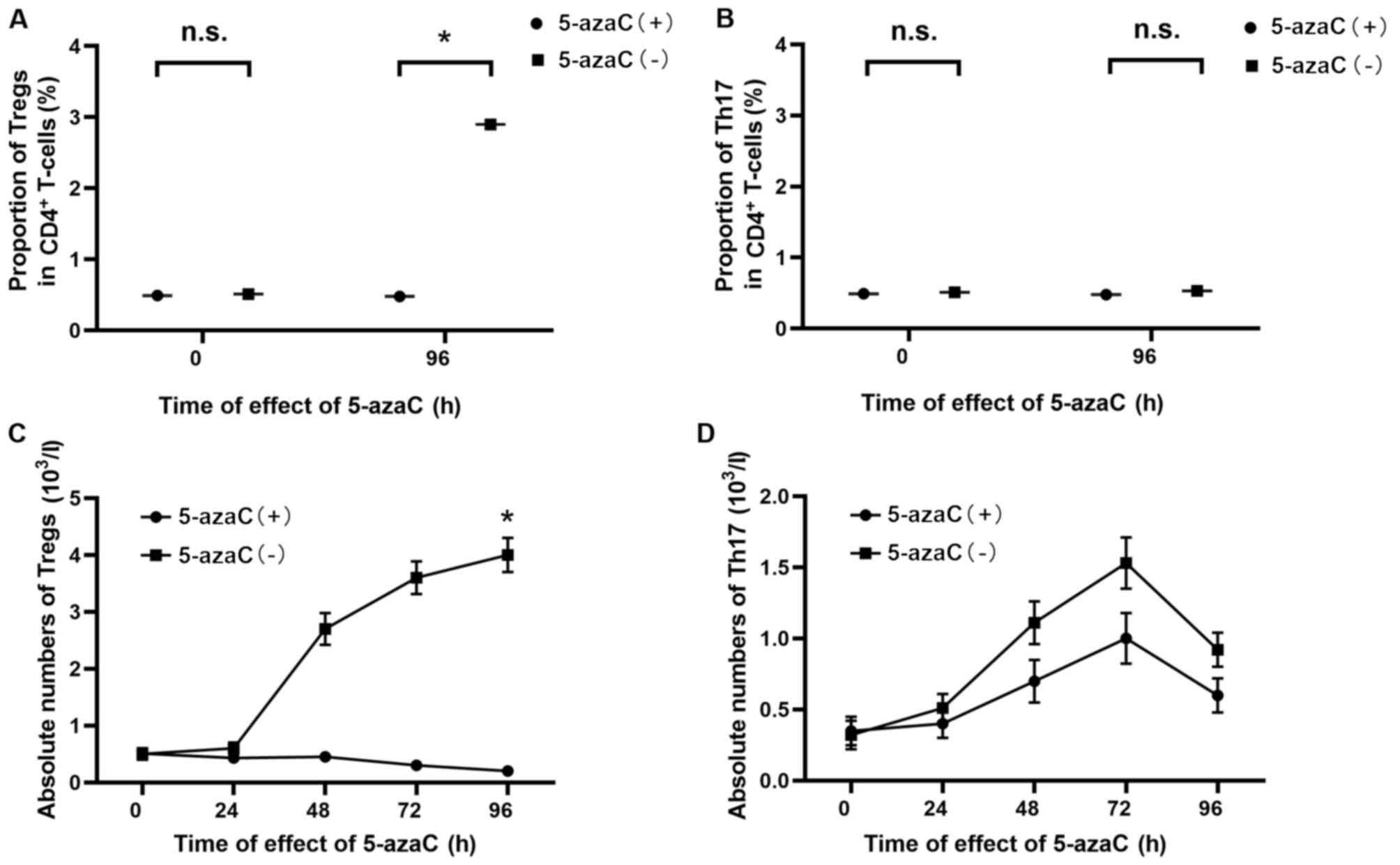
In vitro effect of 5-azaC on the
development of Tregs
According to a dose-response experiment, examining
T-cell proliferation (8,9), a dose previously shown to be non-toxic
was used. The overall proportion of Tregs in CD4+ T
cells from thirty patients was increased by 5-azaC at a
concentration of 1 µM (every 24 h for 96 h) (0.491±0.157 vs.
2.912±0.403%) (P=0.014) (Fig. 3A),
while the overall proportion of Th17 cells remained unchanged
(0.480±0.169 vs. 0.583±0.238%) (P=0.308) (Fig. 3B). These findings raise the question
as to whether the increase in Tregs among CD4+ T cells
is due to conversion of conventional CD25− cells or
proliferation of CD25+FoxP3+ cells. To
address this question, 5-azaC (1 µM) was added to Tregs and Th17
cells separately (every 24 h for 96 h). Addition of 5-azaC caused a
significant reduction in the absolute numbers of Tregs (0.417±0.193
vs. 4.064±0.345%) (P=0.018) (Fig.
3C). The proliferation of Th17 cells was reduced following
5-azaC addition (Fig. 3D). However,
this difference was not statistically significant (0.614±0.127 vs.
0.924±0.142%) (P=0.351). Thus, Tregs ceased to proliferate when
cultured in the presence of 5-azaC, indicating that the increase in
Tregs among proliferating CD4+ T cells in the presence
of 5-azaC is due to conversion of conventional CD25−
cells, rather than proliferation of
CD25+FoxP3+ cells.
Effect of 5-azaC on the expression of
transcription factors
To study whether the conversion was due to a change
in the expression of transcription factors, immunohistochemistry
was performed on CD4+ T cells in bone marrow. No obvious
changes in Tbet and RORγT mRNA transcription were
observed, while FoxP3 mRNA transcription was enhanced
significantly (80.631±10.489 vs. 30.541±5.815%) (P=0.028) (Fig. 4A). Previous studies have reported
that methylation status of the FoxP3 gene promoter plays an
important role in the regulation of FoxP3 expression, demonstrating
an upregulation of FoxP3 expression in CD4+ T cells upon
stimulation by 5-azaC (14,15). Tregs from MDS patients were
stimulated by 5-azaC or vehicle for 96 h. The methylation status of
CpG islands in the promoter of the FoxP3 gene was assessed.
In Tregs treated by 5-azaC, the percentage of moderately methylated
promoter decreased from 56.782±6.021% to 28.541±10.815% (P=0.026)
(Fig. 4B), whereas the percentage of
unmethylated promoter increased from 25.127±10.315% to
53.619±12.614% (P=0.032) (Fig.
4B).
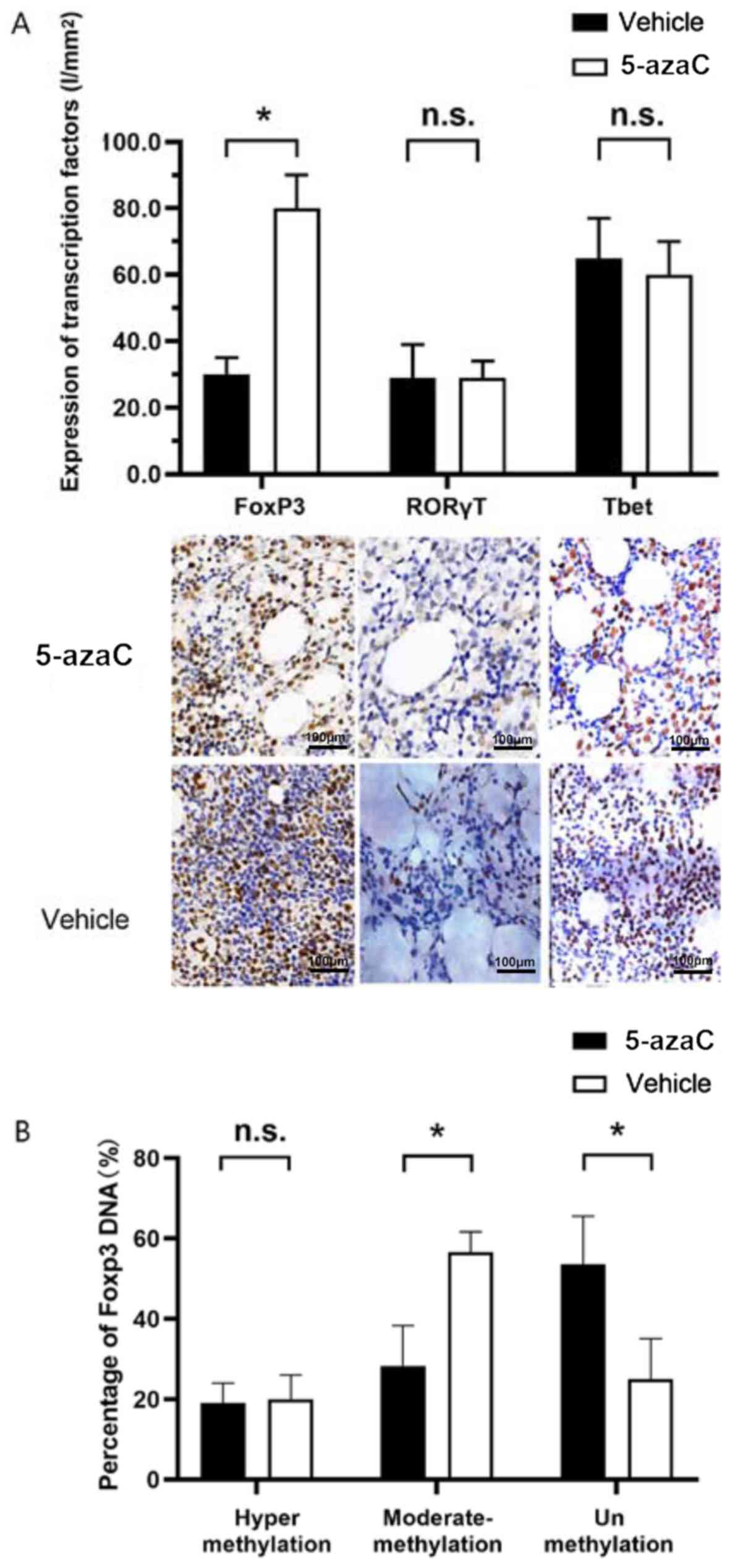 | Figure 4.(A) Transcription factor expression
of FoxP3 with 5-azaC was significantly reduced
(80.631±10.489 vs. 30.541±5.815%) (*P<0.05, P=0.028), in
CD4+ T cells in bone marrow immunohistochemistry, while
the expression of RORγT and Tbet was not
significantly changed (n.s., P>0.05; P=0.631 and 0.319,
respectively). (B) In Tregs treated with 5-azaC, the percentage of
moderately methylated promoter decreased from 56.782±6.021% to
28.541±10.815% (*P<0.05, P=0.026). The percentage of
unmethylated promoter increased from 25.127±10.315% to
53.619±12.614% (*P<0.05, P=0.032). 5-azaC, 5-azacytidine; Tregs,
regulatory T cells. |
Discussion
5-azaC prolongs overall survival, in comparison to
the conventional care regimens, inducing hematologic responses in
up to 56.5% of MDS patients and a prolongation of patients'
survival of ~10 months (2). The
advantage of 5-azaC therapy has also been verified in numerous
other studies. Goodyear et al (5) have shown that 5-azaC treatment induces
CD4+ T-cell responses with an increase in the number of
Tregs. Choi et al (9) have
shown an intact GVL effect in transplanted mice following treatment
with 5-azaC or decitabine, also with an increase in the number of
FoxP3+ T cells. Costantini et al (16) have shown that Treg numbers increase
in responders after 9 months of treatment. All these findings
suggest that post-treatment Tregs do actually exert a GVL effect,
rather than function as ‘suppressors’.
The transcription factor FoxP3, produced by Tregs,
usually exists in the form of hypermethylation, and its expression
and methylation are dissimilar in different diseases. Thymic
regulatory T cell (nTreg) is the main Treg in immune tolerance of
healthy people or patients after haematopoietic stem cell
transplantation (HSCT). While in tumor diseases, such as MDS, it is
replaced by induced T cell (iTreg) (17). Because the degree of demethylation of
FoxP3 in iTreg cells is relatively weak and the stability is poor,
the number of Tregs in MDS which have an immune tolerance effect is
lower than that of HSCT. According to the results of this study,
some Tregs were transformed from T cells of CD25− and
transformed from naïve T cells in the presence of cytokines. The
mechanism of Tregs in MDS patients is different from that in HSCT
patients. So the occurrence of GVL is not only related to the
induction of CD4+ T cells, but also to the demethylation
of FoxP3 transcription factor promoter.
We recruited 30 patients with intermediate/high-risk
MDS for this study and serial peripheral blood samples were tested
for CD4+ T-cell subsets. Intriguingly, Treg numbers
increased steadily during the 3 months of treatmnet. Initially, we
compared the effect of 5-azaC on Tregs and Th17 cells from MDS
patients. While 5-azaC significantly inhibited the proliferation of
Tregs, the proportion of FoxP3+ Tregs was enhanced in
vitro. This suggests that the increase in the percentage of
Tregs is due to conversion of conventional
CD4+CD25− T cells, rather than proliferation
of CD25+FoxP3+ cells.
Tregs play an important role in maintaining the
balance of immune response between human health and disease. While
MDS patients benefit from demethylation, the number of Tregs
increases, inhibiting antitumor immune response and promoting tumor
cell escape. On the other hand, Tregs can downregulate excessive
inflammation by producing adenosine (Ado), and protect patients
from tissue injury and tumor development. Its plasticity is
controlled and evolved based on the microenvironment. Therefore, it
is very important to monitor the number and function of Tregs and
detect the effect of demethylation on Treg transcription factor
FoxP3 in MDS.
5-azaC sequesters and promotes degradation of DNMT,
inducing DNA hypomethylation, thereby causing re-expression of
genes, leading to differentiation and/or apoptosis of the myeloid
leukemia cells (18–21). Methylation of the FoxP3 promoter
plays an important role in the regulation of FoxP3 expression.
5-azaC sequesters and promotes degradation of DNMT, inducing DNA
hypomethylation, thereby causing upregulation of genes (8,14,15).
FoxP3 expression acts as the master switch for Tregs and expansion
of Tregs is associated with increased FoxP3 expression due to FoxP3
promoter demethylation (22,23). In the present study we confirmed
these results and demonstrate that during T-cell activation 5-azaC
increases the transcription of FoxP3. Moreover, the reduction in
methylation was specific to FoxP3 in the sorted Tregs from thirty
patients and then was stimulated for 96 h in the presence of
5-azaC. The reduction in FoxP3 promoter methylation was shown to be
associated with increased FoxP3 expression.
The effect of 5-azaC on the function Tregs needs
further investigation. Costantini et al (16) have shown that the 5-azaC-treated
Tregs have reduced suppressive function. The expanded
FoxP3+ cells do not have a regulatory function, unable
to suppress the secretion of pro-inflammatory cytokines. However,
co-culture of 5-azaC-treated Tregs and T-effectors lead to higher
levels of IL-17 secretion in comparison with the T effectors alone.
Thus, the expanded Tregs no longer function as ‘suppressors’.
Examining the effect of 5-azaC on the function of Tregs will be the
aim of our future research.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81570190 and
81529001).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XJ and WY conceived and designed the study. XZ
acquired the patients' data. LH analyzed and interpreted the data
regarding the numbers of CD4+ T-cell subsets in the 30
patients with intermediate-2/high-risk MDS. JS performed
quantitative PCR. XJ was a major contributor in writing the
manuscript. JS reviewed the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shanghai East Hospital, Tongji University School of Medicine
(Shanghai, China; research no. 136, 2018). Patients who
participated in this research signed an informed consent and had
complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fenaux P, Mufti GJ, Hellstrom-Lindberg E,
Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz
G, List A, et al International Vidaza High-Risk MDS Survival Study
Group, : Efficacy of azacitidine compared with that of conventional
care regimens in the treatment of higher-risk myelodysplastic
syndromes: A randomised, open-label, phase III study. Lancet Oncol.
10:223–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fenaux P, Mufti GJ, Hellström-Lindberg E,
Santini V, Gattermann N, Germing U, Sanz G, List AF, Gore S,
Seymour JF, et al: Azacitidine prolongs overall survival compared
with conventional care regimens in elderly patients with low bone
marrow blast count acute myeloid leukemia. J Clin Oncol.
28:562–569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pinto A and Zagonel V:
5-Aza-2-deoxycytidine (Decitabine) and 5-azacytidine in the
treatment of acute myeloid leukemias and myelodysplastic syndromes:
Past, present and future trends. Leukemia. 7 (Suppl 1):51–60.
1993.PubMed/NCBI
|
|
4
|
Garcia-Manero G and Fenaux P:
Hypomethylating agents and other novel strategies in
myelodysplastic syndromes. J Clin Oncol. 29:516–523. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goodyear O, Agathanggelou A,
Novitzky-Basso I, Siddique S, McSkeane T, Ryan G, Vyas P, Cavenagh
J, Stankovic T, Moss P, et al: Induction of a CD8+
T-cell response to the MAGE cancer testis antigen by combined
treatment with azacitidine and sodium valproate in patients with
acute myeloid leukemia and myelodysplasia. Blood. 116:1908–1918.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fandy TE, Herman JG, Kerns P, Jiemjit A,
Sugar EA, Choi SH, Yang AS, Aucott T, Dauses T, Odchimar-Reissig R,
et al: Early epigenetic changes and DNA damage do not predict
clinical response in an overlapping schedule of 5-azacytidine and
entinostat in patients with myeloid malignancies. Blood.
114:2764–2773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aggarwal S, van de Loosdrecht AA, Alhan C,
Ossenkoppele GJ, Westers TM and Bontkes HJ: Role of immune
responses in the pathogenesis of low-risk MDS and high-risk MDS:
Implications for immunotherapy. Br J Haematol. 153:568–581. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sánchez-Abarca LI, Gutierrez-Cosio S,
Santamaría C, Caballero-Velazquez T, Blanco B, Herrero-Sánchez C,
García JL, Carrancio S, Hernández-Campo P, González FJ, et al:
Immunomodulatory effect of 5-azacytidine (5-azaC): Potential role
in the transplantation setting. Blood. 115:107–121. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi J, Ritchey J, Prior JL, Holt M,
Shannon WD, Deych E, Piwnica-Worms DR and DiPersio JF: In vivo
administration of hypomethylating agents mitigate graft-versus-host
disease without sacrificing graft-versus-leukemia. Blood.
116:129–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kordasti SY, Afzali B, Lim Z, Ingram W,
Hayden J, Barber L, Matthews K, Chelliah R, Guinn B, Lombardi G, et
al: IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and
apoptosis are increased in low risk myelodysplastic syndrome. Br J
Haematol. 145:64–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kordasti SY, Ingram W, Hayden J, Darling
D, Barber L, Afzali B, Lombardi G, Wlodarski MW, Maciejewski JP,
Farzaneh F, et al: CD4+CD25high
Foxp3+ regulatory T cells in myelodysplastic syndrome
(MDS). Blood. 110:847–850. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Polansky JK, Kretschmer K, Freyer J,
Floess S, Garbe A, Baron U, Olek S, Hamann A, von Boehmer H and
Huehn J: DNA methylation controls Foxp3 gene expression. Eur J
Immunol. 38:1654–1663. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lal G, Zhang N, van der Touw W, Ding Y, Ju
W, Bottinger EP, Reid SP, Levy DE and Bromberg JS: Epigenetic
regulation of Foxp3 expression in regulatory T cells by DNA
methylation. J Immunol. 182:259–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagar M, Vernitsky H, Cohen Y, Dominissini
D, Berkun Y, Rechavi G, Amariglio N and Goldstein I: Epigenetic
inheritance of DNA methylation limits activation-induced expression
of FOXP3 in conventional human CD25−CD4+ T
cells. Int Immunol. 20:1041–1055. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Costantini B, Kordasti SY, Kulasekararaj
AG, Jiang J, Seidl T, Abellan PP, Mohamedali A, Thomas NS, Farzaneh
F and Mufti GJ: The effects of 5-azacytidine on the function and
number of regulatory T cells and T-effectors in myelodysplastic
syndrome. Haematologica. 98:1196–1205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Whiteside TL: Regulatory T cell subsets in
human cancer: Are they regulating for or against tumor progression?
Cancer Immunol Immunother. 63:67–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mund C, Brueckner B and Lyko F:
Reactivation of epigenetically silenced genes by DNA
methyltransferase inhibitors: Basic concepts and clinical
applications. Epigenetics. 1:7–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Raj K, John A, Ho A, Chronis C, Khan S,
Samuel J, Pomplun S, Thomas NS and Mufti GJ: CDKN2B methylation
status and isolated chromosome 7 abnormalities predict responses to
treatment with 5-azacytidine. Leukemia. 21:1937–1944. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pinto A, Attadia V, Fusco A, Ferrara F,
Spada OA and Di Fiore PP: 5-Aza-2-deoxycytidine induces terminal
differentiation of leukemic blasts from patients with acute myeloid
leukemias. Blood. 64:922–929. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang AS, Doshi KD, Choi SW, Mason JB,
Mannari RK, Gharybian V, Luna R, Rashid A, Shen L, Estecio MR, et
al: DNA methylation changes after 5-aza-2-deoxycytidine therapy in
patients with leukemia. Cancer Res. 66:5495–5503. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim HP and Leonard WJ: CREB/ATF-dependent
T cell receptor-induced FoxP3 gene expression: A role for DNA
methylation. J Exp Med. 204:1543–1551. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wilson CB, Rowell E and Sekimata M:
Epigenetic control of T-helper-cell differentiation. Nat Rev
Immunol. 9:91–105. 2009. View
Article : Google Scholar : PubMed/NCBI
|