Introduction
Pancreatic cancer (PC) is considered as a fatal
disease and is the third leading cause of cancer-associated
mortality in United States in 2019 (1). The overall 5-year survival rate was
9.3% between 2009 and 2015 in United States (1). Compared with other types of cancer, the
early diagnostic rate of PC is low, which remains to be one of the
critical factors contributing to its dismal prognosis (2). However, there has been no feasible
biomarker for the prediction or treatment of PC. Thus, the
investigation of the underlying molecular mechanism of PC
progression and development, and the identification of novel
biomarkers, is likely to be of benefit.
The microRNAs (miRNAs/miRs) are a group of small
non-coding class of RNAs containing >1,500 shortened non-coding
RNA molecules. miRNAs are single-stranded and ~22 nucleotides in
length (3–5). By controlling and targeting downstream
genes, miRNAs are likely to facilitate tumorigenesis or suppress
the growth of tumors (3,6).
A receptor for transforming growth factor-β is the
transmembrane serine/threonine kinase, TGF-β receptor type II
(TGFBR2). There are seven exons that encode 567 amino acids to
constitute a gene, which can be detected at chromosome 3p22,
forming a transmembrane region (referred to as a
NH2-terminal of ligand binding domain) and an active
COOH-terminal serine/threonine kinase domain (7). As a member of the TGF-β signal pathway,
TGFBR2 is vital for several biological processes, such as cell
proliferation, migration, apoptosis and differentiation (8,9). The
expression levels of TGFBR2 have been demonstrated as commonly
altered in various types of cancer (10).
The present study reported that the TGFBR2 may be
targeted by miR-23a-3p. Several different methods were adopted to
demonstrate the upregulation of miR-23a-3p expression in PC tissues
and cells. Furthermore, the promotion of proliferation, invasion
and migration of PC cells was likely to be associated with the
inhibition of TGFBR2 expression, mediated by the overexpression of
miR-23a-3p.
Materials and methods
Patients and microRNA arrays
PC and adjacent tissues obtained from regions
outside the tumor margin (>1 cm) were collected simultaneously
from 32 patients who underwent surgical resection in the Department
of General Surgery, The Affiliated Changzhou No. 2 People's
Hospital of Nanjing Medical University (Changzhou, China). Tumors
and adjacent tissues were stored in liquid nitrogen at −80°C. The
tissues included in the present study were collected between
February 2017 and May 2018. Of the samples, three were used for
microarrays, and the remainder were used for reverse
transcription-quantitative (RT-q)PCR. The demographics, clinical
information and procedural data were collected from patients'
medical records. The staging system and version used for
Tumor-Node-Metastasis staging of PC was based on the American Joint
Committee on Cancer and the Union for International Cancer Control
(11,12).
The high-throughput genome analysis of miRNAs was
conducted by Guangzhou RiboBio Co., Ltd. The results were analyzed
using the Partek Genomics suite software (version 6.6; Partek,
Inc.) for multi-dimensional scaling, clustering and heatmap
drawing. Upregulated miRNAs were screened based on the fold change
>2.
RT-qPCR analysis
Total RNA was extracted from tissues and cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA concentration was evaluated at an absorbance of
260 nm using the Thermo NanoDrop2000 spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). The Prime-Script™ RT
Reagent kit (Takara Biotechnology Co., Ltd.) was used to synthesize
cDNA in the following conditions: 37°C for 15 min, 85°C for 5 sec,
and finally maintained at 4°C. qPCR was conducted using the
SYBR® Premix Ex Taq® kit (Takara
Biotechnology Co., Ltd.) using a Real Time PCR system (Bio-Rad
Laboratories, Inc.). The housekeeping gene U6 was used as a control
to normalize the expression levels of the miRs. The primers for
qPCR were designed by Guangzhou RiboBio Co., Ltd., and the
sequences were as follows: miR-23a-3p: Forward,
5′-GCGATCACATTGCCAGGG-3′; and reverse, 5′-CAGTGCGTGTCGTGGAGT-3′;
U6: Forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′; and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The thermocycling conditions were as
follows: 95°C for 20 sec, followed by 40 cycles of denaturation at
60°C for 30 sec and dissociation at 95°C for 1 min and 55°C for 1
min. The miR-23a-3p level was calculated using the
2−ΔΔCq method (13).
Cell culture
Human PC cell lines (AsPC-1, MIA-Paca-2, BxPC-3,
Sw1990 and PANC-1) and human pancreatic ductal cell line (HPDE6-C7)
were purchased from the The Cell Bank of Type Culture Collection of
the Chinese Academy of Sciences. All cell lines were cultured in
DMEM or RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.), 1% penicillin and streptomycin (Sigma-Aldrich; Merck KGaA)
at 37°C in a 5% CO2 atmosphere.
Transfection
miR-23a-3p mimics, inhibitors and relative controls
were purchased from Guangzhou RiboBio Co., Ltd. The sequences of
the negative controls for the mimic and inhibitor are
non-targeting. The sequences were as follows: Mimics,
5′-AUCACAUUGCCAGGGAUUUCC-3′; 3′-UAGUGUAACGGUCCCUAAAGG-5′;
inhibitors, 5′-GGAAAUCCCUGGCAAUGUGAU-3′; mimics negative control,
5′-UUUGUACUACACAAAAGUACUG-3′; 3′-AAACAUGAUGUGUUUUCAUGAC-5′;
inhibitor negative control, 5′-CAGUACUUUUGUGUAGUACAAA-3′. The
mimics were transfected at a concentration of 100 nM/well and the
inhibitors at 150 nM/well into PANC-1 and SW-1990 cells using the
riboFECT™CP reagent (Guangzhou RiboBio Co., Ltd.) at 37°C,
according to the manufacturer's protocol. The cells were cultured
for 72 h following transfection prior to further experiments.
Cell proliferation assay
Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) was used to measure cell proliferation. PANC-1
and SW-1990 cells were seeded at a density of 3,000 cells/well on
96-well plates. Cell viability was assayed at 0, 24, 48 and 72 h
post-transfection using CCK-8 reagent. The absorbance values at 450
nm were measured using the Quant Micro-plate spectrophotometer
(BioTek Instruments, Inc.).
Migration and invasion assays
For the migration assay, PANC-1 or SW-1990 cells
(3×104) were transfected for 72 h prior to seeding onto
the upper chamber of Transwell inserts (24-well insert, 8-µm pore
size; BD Biosciences). For the invasion assay, the membranes were
coated with Matrigel for 4 h at 37°C to form a matrix barrier prior
to seeding the PC cells. The upper chamber was filled with 200 µl
serum-free DMEM, and 500 µl DMEM supplemented with 10% fetal bovine
serum was used in the lower chamber. The cells were incubated at
37°C for 36 h for both migration and invasion assays. The membranes
of the Transwell inserts were fixed with absolute methanol for 20
min and stained with 0.1% crystal violet for 30 min at room
temperature. Cells were photographed at ×10 magnification using an
inverted microscope and counted.
Target genes prediction
The target genes of miR-23a-3p were predicted using
TargetScan (http://www.targetscan.org/), miRDB (http://www.mirdb.org/miRDB/), starBase (http://starbase.sysu.edu.cn/) and miRtarBase
(http://mirtarbase.mbc.nctu.edu.tw/php/index.php)
online analysis tools. The overlapping genes between above target
genes were screened for further study. The seed match sites of
miR-23a-3p on TGFBR2 were obtained from TargetScan.
Western blot analysis
Following the transfection of PANC-1 and SW-1990
cells with miR-23a-3p mimics, inhibitors and mimic controls, total
protein was extracted from these cells using
radioimmunoprecipitation assay lysis buffer containing protease
inhibitor (Beyotime Institute of Biotechnology). Protein
concentration was determined using a Nanodrop 2000
spectrophotometer (NanoDrop; Thermo Fisher Scientific, Inc.) by
measuring the optical density at 280 nm. Protein (20 µg/lane) was
loaded and separated via SDS-PAGE (12.5% gel). The proteins were
transferred to 0.22 µg polyvinylidene fluoride membranes. Following
blocking with 5% non-fat milk at room temperature for 1 h, the
membranes were incubated with antibodies targeting TGFBR2 (1:1,000;
cat. no. 79424s) and β-actin (1:1,000; cat. no. 8457s) at 4°C
overnight. Subsequently, the membranes were incubated with
anti-rabbit IgG (H+L) biotinylated antibody (1:3,000; cat. no.
14708s) diluted in TBS-Tween-20 (0.1% Tween-20) for 15 h at room
temperature. All antibodies were obtained from Cell Signaling
Technology, Inc. The signals were exposed to film (FujiFilm), using
the enhanced chemiluminescence reagent (ECL plus; Beyotime
Institute of Biotechnology), in accordance with the manufacturer's
protocol. The ChemDoc imaging system (Abcam) (Pierce; Thermo Fisher
Scientific, Inc.) was used for visualizing the bound antibodies.
The ImageJ software (version 1.46r; National Institutes of Health)
was used for the semi-quantification of densitometry, which was
normalized to β-actin.
Statistical analysis
All data were obtained from at least three
independent experiments, and are presented as the mean ± standard
deviation. Paired student's t-test was used to compare differences
between two groups and one-way analysis of variance followed by the
least significant difference post hoc test was used to compare
differences among three or more groups. Clinical features of the
patients, alongside miR-23a-3p expression, were assessed using
Fisher's exact probability test. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using SPSS software (version 22.0; IBM
Corp.).
Results
Association between miR-23a-3p and
clinical pathology of PC
The age, sex and lymph node metastasis of patients
were not associated with the expression of miR-23a-3p (Table I). However, high miR-23a-3p
expression was associated with low Tumor-Node-Metastasis (TNM)
stage and large tumor size.
 | Table I.Association between miR-23a-3p
expression and clinical features. |
Table I.
Association between miR-23a-3p
expression and clinical features.
|
|
| miR-23a-3p
expression, n |
|
|---|
|
|
|
|
|
|---|
| Variables | Total, n | Low | High | P-value |
|---|
| Age, years |
|
|
| >0.500 |
|
<60 | 8 | 0 | 8 |
|
|
≥60 | 21 | 3 | 18 |
|
| Sex |
|
|
| >0.500 |
|
Male | 17 | 2 | 15 |
|
|
Female | 12 | 1 | 11 |
|
| Tumor
sizea, cm |
|
|
| 0.003 |
|
<3 | 5 | 3 | 2 |
|
| ≥3 | 24 | 0 | 24 |
|
| TNM
stagea |
|
|
| 0.005 |
|
I–II | 23 | 0 | 23 |
|
|
III–IV | 6 | 3 | 3 |
|
| Lymph node
metastasis |
|
|
| >0.500 |
|
Positive | 13 | 1 | 12 |
|
|
Negative | 16 | 2 | 14 |
|
miRNA expression profile of PC
In order to identify the dysregulated miRNAs that
may participate in the tumorigenesis of PC, miRNA microarrays were
performed on three individual pairs of tissues from PC and adjacent
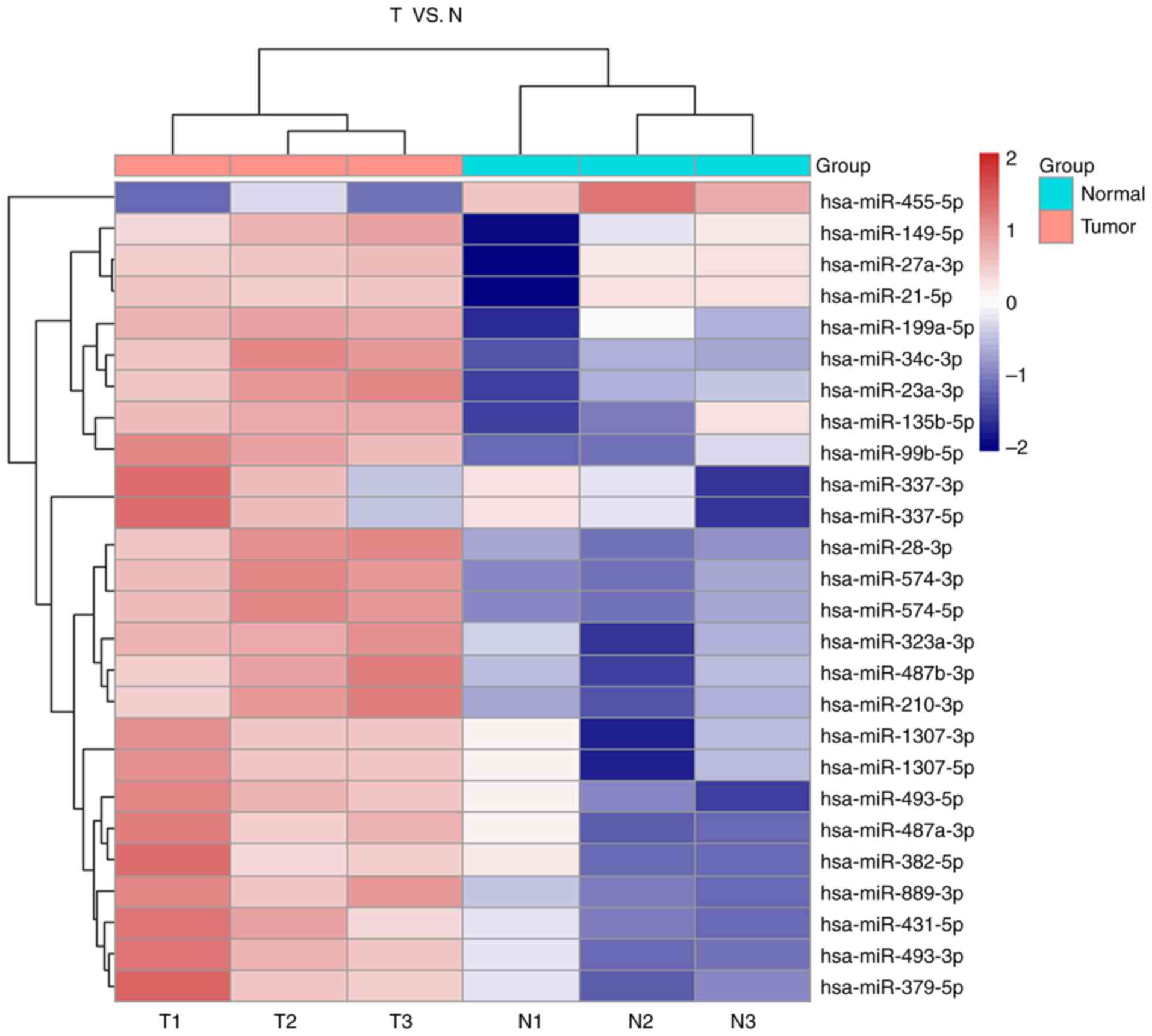
non-cancerous pancreatic tissues (Fig.
1). Paired student's t-test was used for the analysis and the
dysregulated miRNAs were demonstrated to have ≥2-fold higher number
of changes in their expression levels. The P-value threshold was
set at 0.01 to screen for a total of 100 differential miRNAs
compared with the paired tissues (Fig.
2A).
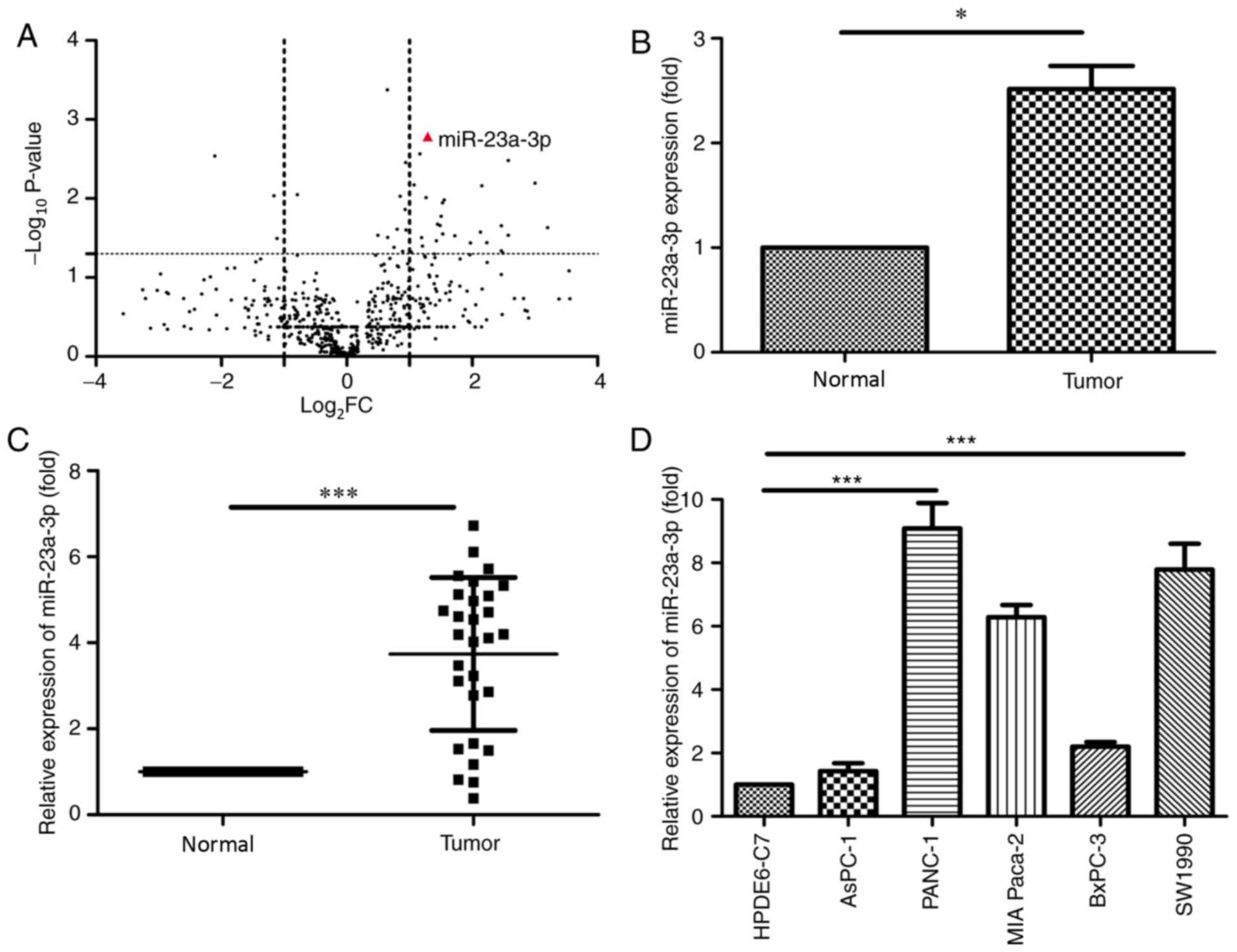
miR-23a-3p was highly expressed in PC tumors
compared with the adjacent normal tissues (Fig. 2B).
miR-23a-3p expression in PC cell lines
and tissues
In order to detect the expression of miR-23a-3p in
PC cell lines and tissues, RT-qPCR was performed. The expression of
miR-23a-3p was upregulated in PC tissues compared with adjacent
tissues (Fig. 2C). Subsequently, the
expression level of miR-23a-3p was determined in five PC cell lines
(AsPC-1, PANC-1, MIA Paca-2, BxPC-3 and SW1990) and a pancreatic
cell line (HPDE6-C7). The expression of miR-23a-3p was increased in
PC cell lines, notably in PANC-1 and the SW1990 cells, compared
with HPDE6-C7 cells (Fig. 2D).
Cell proliferation of PC cells is
promoted by miR-23a-3p
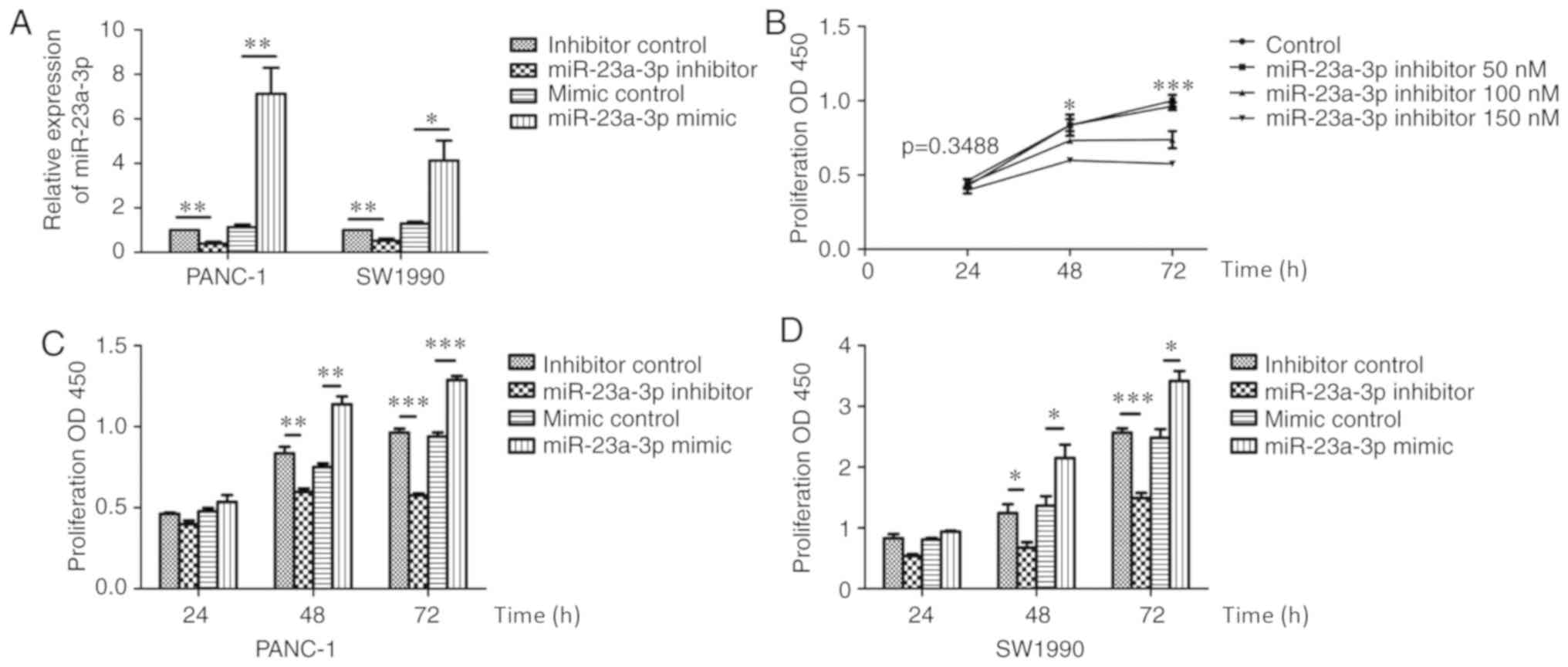
The effect of miR-23a-3p on the proliferation of PC
was investigated in the present study. The expression of miR-23a-3p
was affected by mimics and inhibitors in PANC-1 and SW1990
(Fig. 3A). PANC-1 cells were
transfected with the miR-23a-3p inhibitor at different
concentrations. The CCK-8 assay was performed at 24, 48 and 72 h
after transfection (Fig. 3B), which
revealed that the amount of proliferation decreased as the dose of
inhibitor increased. Subsequently, the effects of miR-23a-3p
inhibitor and mimic on the proliferation of PANC-1 and SW1990 cells
were investigated using the CCK-8 assay. The proliferation was
decreased at 48 and 72 h in PANC-1 and SW1990 cells transfected
with the miR-23a-3p inhibitor 150 nM, whereas those transfected
with the miR-23a-3p mimic 100 nM demonstrated enhanced
proliferation (Fig. 3C and D).
miR-23a-3p expression enhances cell
invasion and migration
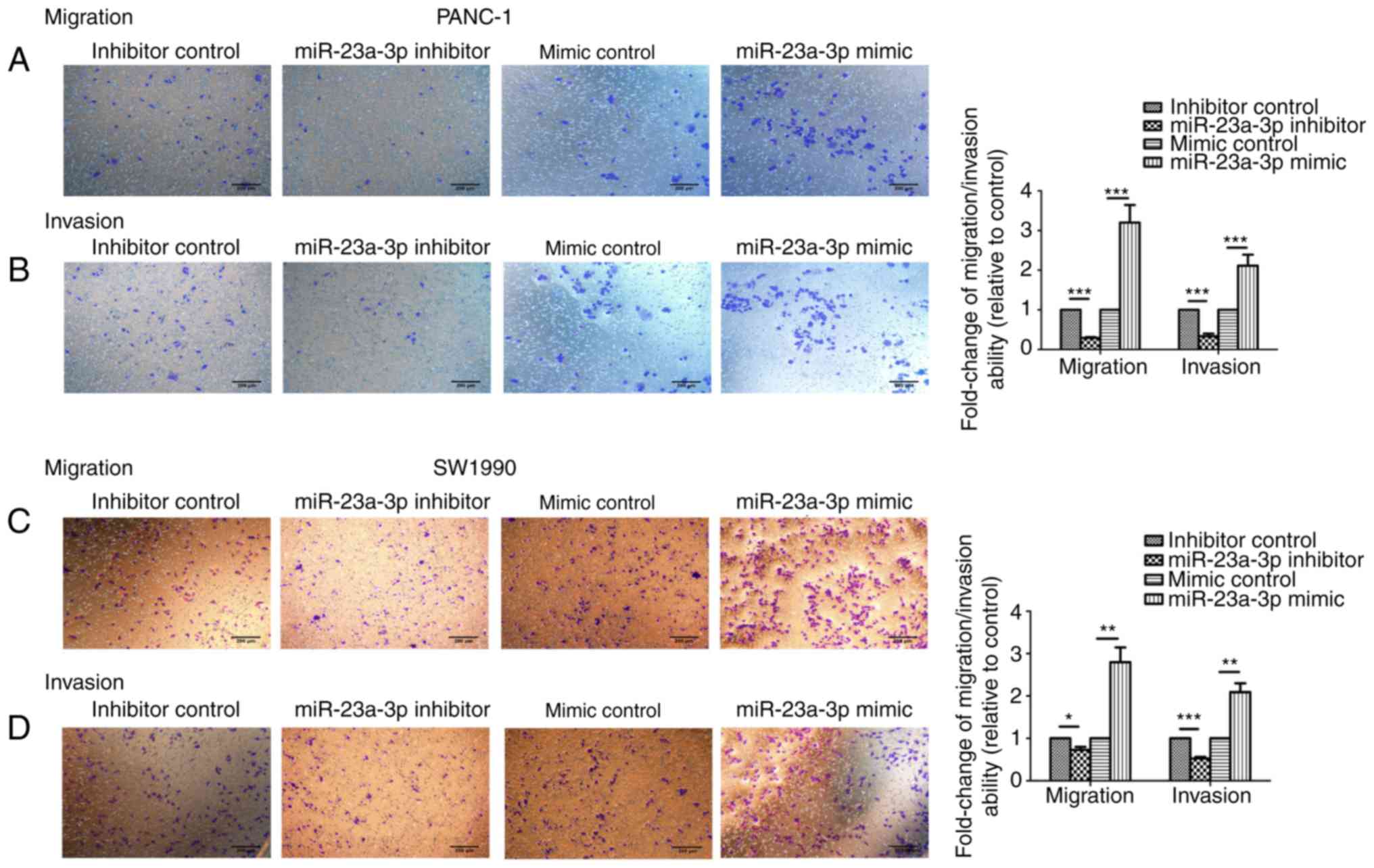
The invasion and migration capabilities of PANC-1
and SW1990 cells transfected with miR-23a-3p mimics and inhibitors
were assessed via Transwell migration and matrigel invasion assays.
Following a 24-h incubation period the number of migrated/invaded
cells were counted. The invasion and migration capability of PANC-1
and SW1990 were significantly increased following the
overexpression of the miR-23a-3p compared with the control cells;
the opposing effect was observed in cells transfected with the
miR-23a-3p inhibitor (Fig. 4).
miR-23a-3p decreases the expression of
the target TGFBR2
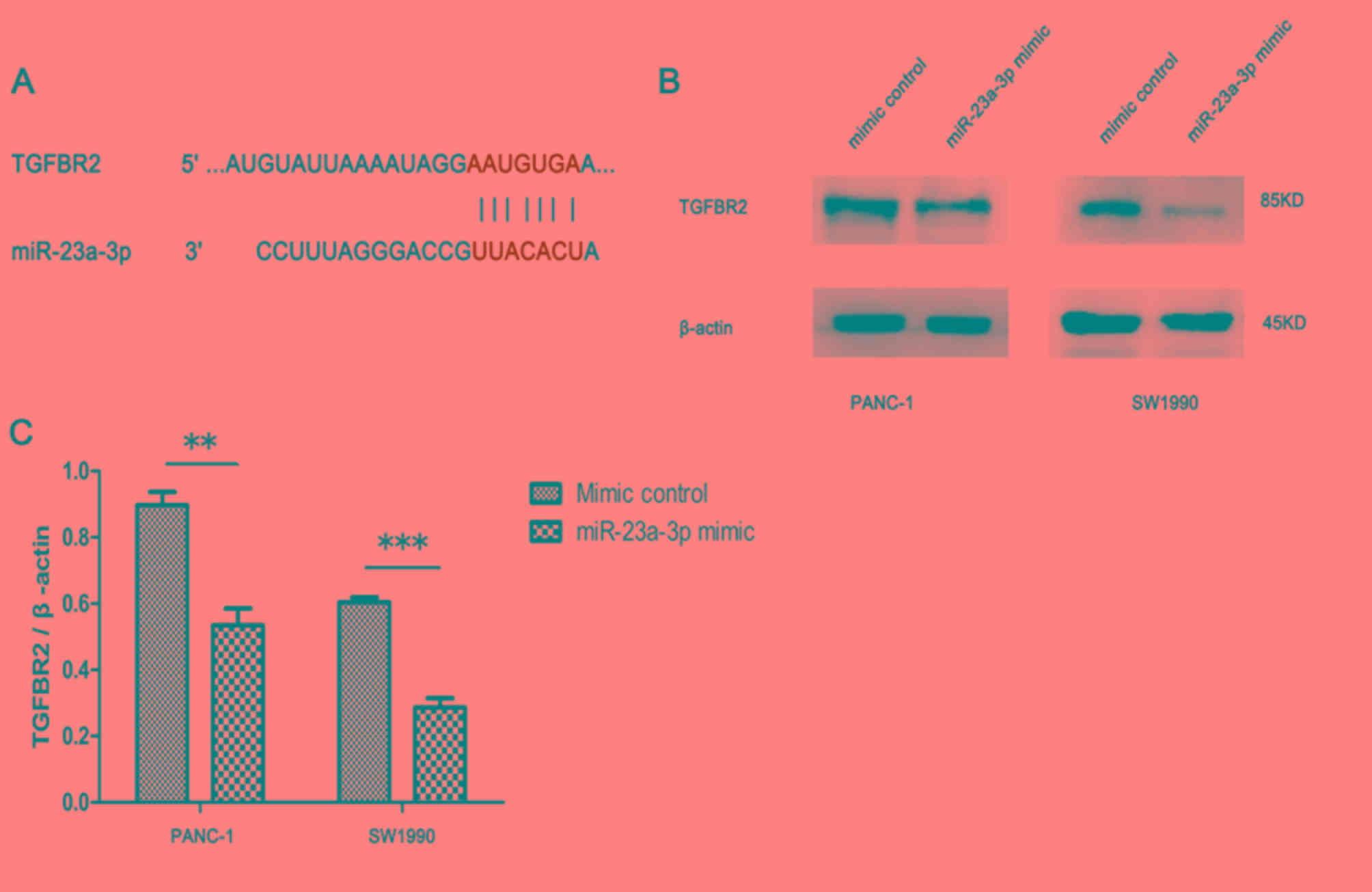
Several candidate targets of miR-23a-3p were
predicted by online analysis tools, of which TGFBR2 was further
investigated in the present study (Fig.
5A). Western blotting revealed decreased ratio of TGFBR2 to
β-actin in PANC-1 and SW1990 cells transfected with miR-23a-3p
mimic compared with those transfected with mimic control (Fig. 5B and C). Thus, the expression of
miR-23a-3p was negatively associated with TGFBR2 protein
expression.
Discussion
miRNAs act as both oncogenes and tumor suppressor
genes in various types of cancer (14,15) and
are critical to the pathological and the physiological processes
(such as proliferation, metabolism, differentiation and apoptosis)
(16).
Several recent studies demonstrated that miR-23a-3p
was associated with the development of several cancer types, such
as melanoma cancer, liver cancer and renal cell carcinoma (17–20).
Thus, miRNAs are potential biomarkers and therapeutic targets
(17,21). It has been reported that there are
higher levels of miR-23a-3p expression in the serum of patients
with colon cancer compared with that of healthy donors (22,23). The
higher expression level of miR-23a-3p could be vital for the early
processes of carcinogenesis. The metastasis suppressor 1 (MTSS1)
was demonstrated to be a direct target of miR-23a-3p, which
potentially participates in the invasion of cancer (24). Zhu et al (25) demonstrated higher expression levels
of miR-23a-3p in esophageal squamous cell cancer due to its close
association with tumor differentiation, and could play a
significant role in the microenvironment of esophageal carcinoma.
Furthermore, high expression levels of miR-23a-3p were detected in
lung adenocarcinoma, as well as in cervical cancer (26,27).
Since the finding by Calatayud et al
(28) that miR-23a-3p was
upregulated in PC, few studies have investigated the detailed roles
and other molecular mechanisms of miR-23a-3p in PC; thus far the
conclusions remain unclear and contradictory.
In the present study, the ≥2-fold change in
expression level was defined as differentially expressed, and the
P-value threshold was set as 0.01. Subsequently, miR-23a-3p
expression in PC was detected using three pairs of PC samples via
high-throughput genome analysis. The microarray results revealed 19
differentially expressed genes, of which miR-23a-3p expression was
upregulated in PC. In order to verify the feasibility of the
microarray, the remaining tissue specimens and five PC cell lines
were employed to perform RT-qPCR, which demonstrated increased
expression levels of miR-23a-3p in PC tissues and cells compared
with non-neoplastic controls. Furthermore, clinical information
indicated the association of high miR-23a-3p expression with larger
tumor size. Thus, it was speculated that miR-23a-3p may exhibit
oncogenic activities in PC. Furthermore, miR-23a-3p expression
promoted cell proliferation and facilitated cell invasion and
migration. However, lymph node metastasis was not associated with
miR-23a-3p expression, whereas miR-23a-3p expression was negatively
associated with TNM stage. There were two explanations considered
regarding the findings of the present study: i) miR-23a-3p
primarily affected PC by enhancing the ability of invasion, rather
than lymph node metastasis; and ii) insufficient organized
specimens limited the study, and more samples are required in order
to enhance feasibility.
In addition, bioinformatics analysis predicted
TGFBR2 as a potential target gene of miR-23a-3p. Despite little
emphasis on TGFBR2 in the literature, its mutation and
downregulation was detected in various types of cancer such as
colorectal cancer, lung cancer and breast cancer (20,29–32).
Furthermore, Shima et al (33) reported that mutations in TGFBR2 were
associated with 5-year survival rates in colorectal cancer. Zhou
et al (34) reported that the
linc00462/miR-665/TGFBR1-TGFBR2/smad2/3 axis was vital for cell
migration, invasion, proliferation and tumor metastasis in PC.
Furthermore, Yang et al (35)
demonstrated that lower TGFBR2 expression levels in patients were
associated with poor prognosis in cervical cancer. In the present
study, western blotting revealed a negative association between the
expression levels of miR-23a-3p and TGFBR2 protein levels. Thus,
the dysregulation of miR-23a-3-p by targeting TGFBR2 could impact
the pathological process of PC.
The limitations of the present study included
insufficient number of specimens, lack of rescue experiments and
in vivo experiments.
Overall, the present study indicated that the
expression of miR-23a-3p may be associated with the expression of
TGFBR2, and partially facilitate the progression of PC.
Furthermore, the findings of the present study could provide novel
approaches for PC diagnosis and treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Social
Development Foundation of Science and Technology of Jiangsu (grant.
no. BE2016658), The Changzhou Sci & Tech Program (grant. no.
CE20165020), The High-Level Medical Talents Training Project of
Changzhou (grant. no. 2016CZLJ007) and The Role of ENO-1 on
Proliferation of Cholangiocarcinoma and Underlying Mechanism
(grant. no. 2018K005).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request. The datasets are available from TargetScan (http://www.targetscan.org/), miRDB (http://www.mirdb.org/miRDB/), starBase (http://starbase.sysu.edu.cn/) and miRtarBase
(http://mirtarbase.mbc.nctu.edu.tw/php/index.php).
Authors' contributions
CZ, JC, LJ, XZ, DD, SW and XQ contributed to the
conception and design of the study. CZ, JC, LJ, LM, BZ, SS, XY, PG
and JL provided the study materials and patients. JC, LM, BZ, SS,
XY, PG, JL, CZ and XQ contributed to the collection and assembly of
data. All authors contributed to data analysis and interpretation.
JC wrote the manuscript. All authors approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Study
Committee of Changzhou No. 2 People's Hospital [approval no.
(2018)KY024-01]. Written informed consent was obtained from each
participating patient (or guardian) prior to entry into the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
US Preventive Services Task Force, ; Owens
DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, Curry
SJ, Doubeni CA, Epling JW Jr, et al: Screening for pancreatic
cancer: US preventive services task force reaffirmation
recommendation statement. JAMA. 322:438–444. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGuigan A, Kelly P, Turkington RC, Jones
C, Coleman HG and McCain RS: Pancreatic cancer: A review of
clinical diagnosis, epidemiology, treatment and outcomes. World J
Gastroenterol. 24:4846–4861. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Yang P and Wang XF:
Microenvironmental regulation of cancer metastasis by miRNAs.
Trends Cell Biol. 24:153–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tassinari V, Cesarini V, Silvestris DA and
Gallo A: The adaptive potential of RNA editing-mediated
miRNA-retargeting in cancer. Biochim Biophys Acta Gene Regul Mech.
1862:291–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwok GT, Zhao JT, Weiss J, Mugridge N,
Brahmbhatt H, MacDiarmid JA, Robinson BG and Sidhu SB:
Translational applications of microRNAs in cancer, and therapeutic
implications. Noncoding RNA Res. 2:143–150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frampton AE, Castellano L, Colombo T,
Giovannetti E, Krell J, Jacob J, Pellegrino L, Roca-Alonso L, Funel
N, Gall TM, et al: MicroRNAs cooperatively inhibit a network of
tumor suppressor genes to promote pancreatic tumor growth and
progression. Gastroenterology. 146:268–277.e18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L, Gao LG, Zhang M and Zhou XL:
Genotype-phenotype analysis of F-helix mutations at the kinase
domain of TGFBR2, including a type 2 Marfan syndrome familial
study. Mol Vis. 18:55–63. 2012.PubMed/NCBI
|
|
8
|
Luo J, Chen XQ and Li P: The Role of TGF-β
and its receptors in gastrointestinal cancers. Transl Oncol.
12:475–484. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pu XH, Li F, Miao XL, Ye JL and Lu LG:
Transforming growth factor beta regulates hepatic progenitor cells
migration via PI3K/AKT/mTOR/p70S6K pathway. Zhonghua Gan Zang Bing
Za Zhi. 26:680–685. 2018.(In Chinese). PubMed/NCBI
|
|
10
|
Zhou H, Wu G, Ma X, Xiao J, Yu G, Yang C,
Xu N, Zhang B, Zhou J, Ye Z and Wang Z: Attenuation of TGFBR2
expression and tumour progression in prostate cancer involve
diverse hypoxia-regulated pathways. J Exp Clin Cancer Res.
37:892018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC Cancer Staging
Manual and the Future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Greene FL and Sobin LH: A worldwide
approach to the TNM staging system: Collaborative efforts of the
AJCC and UICC. J Surg Oncol. 99:269–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KG and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Olive V, Bennett MJ, Walker JC, Ma C,
Jiang I, Cordon-Cardo C, Li QJ, Lowe SW, Hannon GJ and He L: miR-19
is a key oncogenic component of mir-17-92. Genes Dev. 23:2839–2849.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang D, Liu G and Wang K: miR-203 Acts as
a tumor suppressor gene in osteosarcoma by regulating RAB22A. PLoS
One. 10:e01322252015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou J, Lu S, Yang S, Chen H, Shi H, Miao
M and Jiao B: MicroRNA-127 post-transcriptionally downregulates
Sept7 and suppresses cell growth in hepatocellular carcinoma cells.
Cell Physiol Biochem. 33:1537–1546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen F, Qi S, Zhang X, Wu J, Yang X and
Wang R: miR-23a-3p suppresses cell proliferation in oral squamous
cell carcinomas by targeting FGF2 and correlates with a better
prognosis: miR-23a-3p inhibits OSCC growth by targeting FGF2.
Pathol Res Pract. 215:660–667. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ding F, Lai J, Gao Y, Wang G, Shang J,
Zhang D and Zheng S: NEAT1/miR-23a-3p/KLF3: A novel regulatory axis
in melanoma cancer progression. Cancer Cell Int. 19:2172019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Fan L, Yu H, Zhang J, He Y, Feng D,
Wang F, Li X, Liu Q, Li Y, et al: Endoplasmic reticulum stress
causes liver cancer cells to release exosomal miR-23a-3p and
Up-regulate programmed death ligand 1 expression in macrophages.
Hepatology. 70:241–258. 2019.PubMed/NCBI
|
|
20
|
Quan J, Pan X, Li Y, Hu Y, Tao L, Li Z,
Zhao L, Wang J, Li H, Lai Y, et al: MiR-23a-3p acts as an oncogene
and potential prognostic biomarker by targeting PNRC2 in RCC.
Biomed Pharmacother. 110:656–666. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karmakar S, Kaushik G, Nimmakayala R,
Rachagani S, Ponnusamy MP and Batra SK: MicroRNA regulation of
K-Ras in pancreatic cancer and opportunities for therapeutic
intervention. Semin Cancer Biol. 54:63–71. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vychytilova-Faltejskova P, Radova L,
Sachlova M, Kosarova Z, Slaba K, Fabian P, Grolich T, Prochazka V,
Kala Z, Svoboda M, et al: Serum-based microRNA signatures in early
diagnosis and prognosis prediction of colon cancer. Carcinogenesis.
37:941–950. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ostenfeld MS, Jensen SG, Jeppesen DK,
Christensen LL, Thorsen SB, Stenvang J, Hvam ML, Thomsen A,
Mouritzen P, Rasmussen MH, et al: miRNA profiling of circulating
EpCAM(+) extracellular vesicles: Promising biomarkers of colorectal
cancer. J Extracell Vesicles. 5:314882016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jahid S, Sun J, Edwards RA, Dizon D,
Panarelli NC, Milsom JW, Sikandar SS, Gumus ZH and Lipkin SM:
miR-23a promotes the transition from indolent to invasive
colorectal cancer. Cancer Discov. 2:540–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu L, Jin L, Jiang R, Wang Q, Jiang J,
Mao C and Chen D: Correlations between miRNAs and TGF-β1 in tumor
microenvironment of esophageal squamous cell cancer. Xi Bao Yu Fen
Zi Mian Yi Xue Za Zhi. 29:524–528. 2013.(In Chinese). PubMed/NCBI
|
|
26
|
Ito S, Kamoto Y, Sakai A, Sasai K, Hayashi
T, Toyooka S and Katayama H: Unique circulating microRNAs in
relation to EGFR mutation status in Japanese smoker male with lung
adenocarcinoma. Oncotarget. 8:114685–114697. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Honegger A, Schilling D, Bastian S,
Sponagel J, Kuryshev V, Sultmann H, Scheffner M, Hoppe-Seyler K and
Hoppe-Seyler F: Dependence of intracellular and exosomal microRNAs
on viral E6/E7 oncogene expression in HPV-positive tumor cells.
PLoS Pathog. 11:e10047122015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Calatayud D, Dehlendorff C, Boisen MK,
Hasselby JP, Schultz NA, Werner J, Immervoll H, Molven A, Hansen CP
and Johansen JS: Tissue MicroRNA profiles as diagnostic and
prognostic biomarkers in patients with resectable pancreatic ductal
adenocarcinoma and periampullary cancers. Biomark Res. 5:82017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Drabsch Y and ten Dijke P: TGF-β
signalling and its role in cancer progression and metastasis.
Cancer Metastasis Rev. 31:553–568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He H, Zhao X, Zhu Z, Du L, Chen E, Liu S,
Li Q, Dong J, Yang J and Lei L: MicroRNA-3191 promotes migration
and invasion by downregulating TGFBR2 in colorectal cancer. J
Biochem Mol Toxicol. e22308:2019.
|
|
31
|
Tarfiei GA, Shadboorestan A, Montazeri H,
Rahmanian N, Tavosi G and Ghahremani MH: GDF15 induced apoptosis
and cytotoxicity in A549 cells depends on TGFBR2 expression. Cell
Biochem Funct. 37:320–330. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Tan X, Tang Y, Zhang C, Xu J, Zhou
J, Cheng X, Hou N, Liu W, Yang G, et al: Dysregulated
Tgfbr2/ERK-Smad4/SOX2 signaling promotes lung squamous cell
carcinoma formation. Cancer Res. 79:4466–4479. 2019.PubMed/NCBI
|
|
33
|
Shima K, Morikawa T, Yamauchi M, Kuchiba
A, Imamura Y, Liao X, Meyerhardt JA, Fuchs CS and Ogino S: TGFBR2
and BAX mononucleotide tract mutations, microsatellite instability,
and prognosis in 1072 colorectal cancers. PLoS One. 6:e250622011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou B, Guo W, Sun C, Zhang B and Zheng F:
Linc00462 promotes pancreatic cancer invasiveness through the
miR-665/TGFBR1-TGFBR2/SMAD2/3 pathway. Cell Death Dis. 9:7062018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang H, Zhang H, Zhong Y, Wang Q, Yang L,
Kang H, Gao X, Yu H, Xie C, Zhou F and Zhou Y: Concomitant
underexpression of TGFBR2 and overexpression of hTERT are
associated with poor prognosis in cervical cancer. Sci Rep.
7:416702017. View Article : Google Scholar : PubMed/NCBI
|