Introduction
Hepatocellular carcinoma (HCC) is the most common
primary malignancy of the liver (1)
and the fourth leading cause of cancer-related deaths in China
(2). HCC incidence rates are highest
in Eastern Asia and sub-Saharan Africa, but its prevalence is
rapidly increasing in Western countries; for instance, in the
United States, the incidence rate of HCC has increased from ~3% in
1980 to ~17% in 2012 (3). For
early-stage HCC, surgical resection resulted in improved overall
survival (OS) rates compared with local ablation therapy (LAT) or
locoregional therapy (LRT) (4,5).
However, recurrence rates after surgery are 40–80% within 5 years,
making recurrence the leading cause of postoperative death among
patients with HCC (6–8). Although many studies have reported
prognostic markers for HCC, such as Capn4 (9), PEBP1 (10), CD24 (11) and EZH2 (12), the accuracy and clinical application
of these markers are still limited. Therefore, developing novel
effective biomarkers to classify patients with high risk of death
or recurrence will assist clinicians to select the best therapeutic
strategies for patients with HCC and provide personalized therapy
according to the predicted risk of survival or recurrence (13).
Lipopolysaccharide binding protein (LBP) is a serum
protein that is synthesized in the liver and involved in the
recognition, binding, and transport of the bacterial cell wall
compound lipopolysaccharide (LPS)/endotoxin (14,15),
which is a cell wall component of Gram-negative bacteria that plays
a crucial role in aggravating HCC (16,17). LBP
is commonly elevated in the liver and systemic circulation in
patients with chronic liver diseases due to the increased
intestinal permeability and bacterial translocation (18,19). LBP
has also been reported to be involved in the pathogenesis of sepsis
(20). The increased research into
LBPs has led to an appreciation of the diagnostic value of
infection in patients with cancer with febrile neutropenia
(21) and its prognostic value in
serum and tissue levels in colorectal carcinoma (22) and renal cell carcinoma (23). However, the expression pattern and
prognostic value of LBP in HCC remains unclear. To address this
question, western blot analysis and immunohistochemistry (IHC) were
used to evaluate LBP expression in patients with HCC following
surgery, and then retrospectively explore its prognostic value in
this patient population.
Materials and methods
Patients, specimens, and
follow-up
For the present study, 346 formalin-fixed
paraffin-embedded (FFPE) HCC specimens (10% neutral formaldehyde
solution at room temperature for 12–24 h) were retrospectively
obtained from patients (age, 22–77 years; male, n=302 and female,
n=43) who underwent surgical resection between December 2005 and
December 2008 at the Eastern Hepatobiliary Surgery Hospital (EHBH).
Among them, 77 pairs of tumors and adjacent non-tumor liver tissues
(peritumor liver tissues, distance between tumors and non-tumor
tissue at least 2 cm) were used to explore LBP expression.
Additionally, LBP expression was explored in low-grade dysplastic
nodules (LGDN, n=15), high-grade dysplastic nodules (HGDN, n=12)
and well-differentiated HCC (well-HCC, n=18) (Table S1) in specimens obtained from
patients (age, 7–79 years; male, n=31 and female, n=14) who
underwent curative resection between January 2005 and December 2011
at the EHBH. For western blot analyses, five paired HCC and
peritumor liver tissues (age, 28–84; male, n=3 and female, n=2)
were obtained from EHBH in July 2017 and frozen at −80°C. For
diagnosis, hematoxylin and eosin (H&E)-stained sections were
reviewed by two experienced hepatopathologists. H&E staining
was performed at room temperature and observed under a Leica DM
IRE2 microscope (Leica Microsystems Imaging Solutions Ltd).
Diagnoses of HCC was based on the criteria proposed by the World
Health Organization (WHO) (24). The
inclusion criteria of the patients for the present study were: i) A
diagnosis of HCC consistent with histological diagnostic criteria
of the World Health Organization; ii) no pre-operative anticancer
treatment; and iii) No evidence of extrahepatic metastases
(25). LGDN, HGDN and well-HCC was
diagnosed based on previously described criteria (26). Briefly, hepatocytes in LGDN appear
normal or exhibit minimal nuclear atypia and a slightly increased
nucleus to cytoplasm (N:C) ratio, but mitotic figures are absent.
HGDN is characterized by cytologic and/or structural atypia, but
insufficient for a diagnosis of well-HCC. Well-HCC was diagnosed
based on the following criteria: i) Increased cell density (more
than twice compared with that of the surrounding liver) with an
increased N:C ratio; ii) irregular thin trabecular pattern of
growth; iii) pseudoglandular structures; iv) fat distribution
change; v) unpaired arteries; vi) intratumoral portal tracts; and
vii) stromal invasion.
Each patient provided written informed consent, and
the institutional review board of EHBH approved the present study.
Overall survival (OS) time was defined as the interval between
surgery and death or the last follow-up. Time to recurrence (TTR)
was measured from the date of tumor resection to the date of
detecting a tumor recurrence or the last follow-up (27). Patients were followed-up at the
clinic every 3 months during the first year after surgery and every
6 months thereafter until December 2013. Follow-up observations
were performed by two physicians who were blinded to the study.
Abdomen ultrasonography, chest X-ray and a test for serum α
fetoprotein (AFP, AFP (+), serum AFP>20 ng/ml; AFP (−), serum
AFP ≤20 ng/ml) concentration were used to monitor the patients
every 3 months during the first year after surgery and every 3–6
months thereafter. Serum AFP were determined on Roche Modular E170
immunology analyzer (Roche Diagnostics GmbH) with serum AFP test
kits (cat. no. 11731327; Roche Diagnostics GmbH). Magnetic
resonance imaging or computed tomography scanning of the abdomen
were performed every 6 months or immediately after a recurrence was
suspected (28).
Protein extraction and western blot
analysis
Western blot analysis was performed according to a
previous study (29). Briefly,
tissue samples were homogenized in a RIPA buffer (Qiagen, Inc.)
supplemented with a cocktail of proteinase inhibitors and with a
cocktail of phosphatase inhibitors (both Roche Diagnostics).
Protein concentrations were determined using a bicinchoninic acid
kit (Pierce; Thermo Fisher Scientific, Inc.). Proteins (20 µg/lane)
were separated using 10% SDS-PAGE and transferred to nitrocellulose
membranes (Bio-Rad Laboratories, Inc.). After blocking with 5%
bovine serum albumin/PBS at room temperature for 1 h, the membranes
were incubated with primary LBP antibody (anti-LBP antibody;
1:1,000 dilution; cat. no. ab169776; Abcam) and β-actin (1:1,000
dilution; cat. no. 4970; Cell Signaling Technology, Inc.) overnight
at 4°C. After washing with Tween-20 (0.05%) in PBS, the membranes
were incubated with horseradish peroxidase-conjugated goat
anti-rabbit IgG antibody (1:2,000 dilution; cat. no. 7074; Cell
Signaling Technology, Inc.) for 1 h at room temperature. LBP and
β-actin were detected using ECL development solution (Pierce;
Thermo Fisher Scientific, Inc.). LBP and β-actin expression levels
were determined using Quantity One v4.6.2 software (Bio-Rad
Laboratories, Inc.). β-actin was used as a loading control.
Tissue microarray (TMA), IHC and
scoring
For tissue microarray construction, H&E-stained
samples were reviewed by two experienced pathologists and the
representative cores were pre-marked in the paraffin blocks. A
tissue cylinder with a diameter of 1.5 mm was punched using a
Manual Tissue Microarrayer (Unitma Co., Ltd.) from the marked area
of each block and incorporated into a recipient paraffin block.
Sections (4-µm) were then placed on slides pre-coated with
3-aminopropyltriethoxysilane. Paraffin sections were deparaffinized
with xylene and rehydrated through decreasing concentrations of
ethanol (100, 95, and 85% for 5 min each) at room temperature.
Antigens were retrieved using microwave irradiation for 5 min in
citric acid buffer (pH 6.0) at 100°C, and slides were then cooled
at room temperature for 120 min, according to the protocol reported
by Jin et al (30) with minor
modifications. The slides were incubated with 3%
H2O2/phosphate-buffered saline to block
endogenous peroxidase activity, and then non-specific binding sites
were blocked using 100% goat serum (Beyotime Institute of
Biotechnology) for 1 h at room temperature. Primary anti-LBP
antibody (1:200 dilution; cat. no. HPA001508; Sigma-Aldrich; Merck
KGaA) was used for IHC. Tissue antigens were detected with an
EnVision detection kit (cat. no. GK500705; Gene Tech Biotechnology,
Co., Ltd.). Counterstaining with Hematoxylin was performed for 5
min at room temperature and observed using a Leica DM IRE2 light
microscope. Negative control slides were created for all assays and
consisted of omitting primary antibodies.
For semi-quantitative evaluation of LBP from IHC,
scoring was performed as follows: Staining intensity was first
scored as 0, negative; 1 weak; 2, moderate; 3, high, and then the
percentage of positive cells was scored as 0, 0% positive; 1, 1–10%
positive; 2, 11–50% positive; and 3, >50% positive. A final
score for each sample was obtained by multiplying the scores for
staining intensity and percentage of positive cells scores to
obtain LBP scores in HCC tissues.
Statistical analysis
Optimal LBP cut-off point for survival analysis was
obtained by X-tile (version 3.6.1; Rimm Lab; Yale School of
Medicine) as previously reported by Camp et al (31). LBP expression level in HCC and paired
peritumor tissues were analyzed using paired t-test. LBP expression
level between more than two groups of TNM, tumor differentiation
and tumor grade (based on the pathological diagnosis report) were
analyzed using one-way ANOVA and least significant difference
post-hoc test for multiple comparisons. χ2 tests were
used to compare qualitative variables. A cut-off point derived from
346 cases using The Mantel Cox log-rank test on X-tile was used to
determine the statistical significance of the association between
LBP expression and patient survival. SPSS standard version 13.0
(SPSS Inc.) was used to determine associations between variables,
univariate survival analysis, and multiple Cox proportional hazards
regression (forward, conditional likelihood ratio). P<0.05 was
considered to indicate a statistically significant difference.
Results
LBP is expressed in tumor tissues
To investigate LBP expression in patients with HCC,
346 paraffin blocks from patients with HCC were used to determine
LBP expression as scores. IHC results demonstrated that LBP was
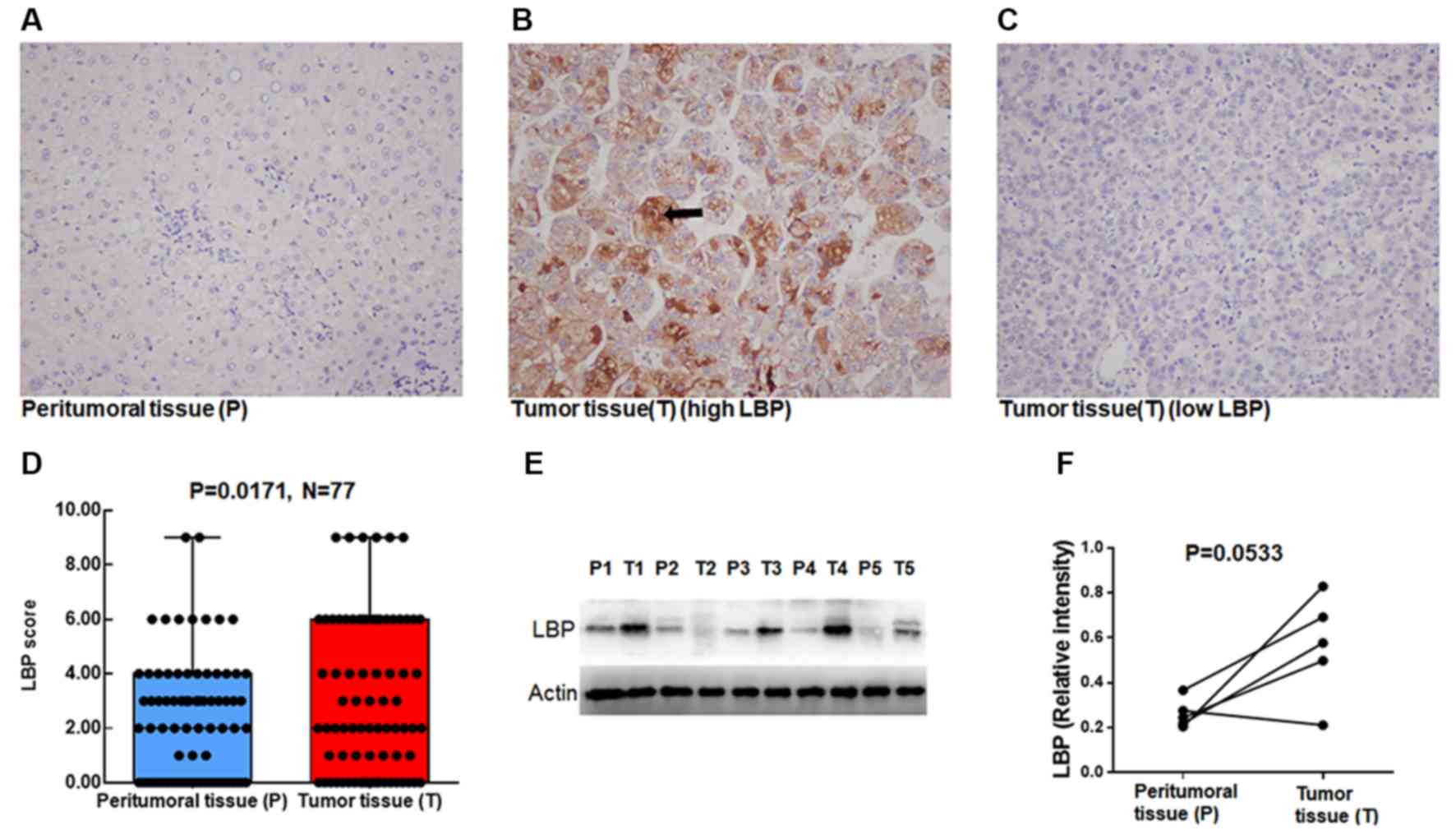
primarily cytosolic. As shown in Fig.
1, LBP was rarely expressed in peritumor liver tissues
(Fig. 1A), but frequently
overexpressed in HCC tissues (Fig.
1B); some HCC tissues were negative for LBP (Fig. 1C). IHC results of 77 paired tumor
tissues and peritumor liver tissues showed that LBP was highly
expressed in tumor tissues (Fig. 1D;
P=0.0171).
LBP scores in peritumor tissues were 0, 27 cases; 1,
3 cases; 2, 10 cases; 3, 16 cases; 4, 12 cases; 6, 7 cases, and 9,
2 cases. In tumor tissues the scores were 0, 20 cases; 1, 7 cases;
2, 13 cases; 3, 5 cases; 4, 8 cases; 6, 18 cases, and 9, 6 cases.
For further confirmation, LBP expression was analyzed in five
paired tumor and peritumor tissues using western blot analysis. As
presented in Fig. 1E and F, due to
the very low number of samples (n=5 pairs), no significant
differences were observed between tumor tissues and peritumor liver
tissues, although there was a trend for high LBP in tumor tissues
(P=0.0533). Taken together, these results indicate that LBP is
highly expressed in tumor tissues compared with that in peritumor
liver tissues.
Clinicopathological features of
patients with HCC
Subsequently, univariate and multivariate Cox
analyses were used to determine the prognostic significance of LBP
and the clinicopathological parameters in the 346 HCC cases
(Table I). Univariate analysis
showed that serum AFP (P=0.001), liver cirrhosis (P=0.006),
tumor-node-metastasis (TNM) stage (P<0.0001), tumor size
(P<0.0001), tumor number (P<0.0001), tumor differentiation
(P=0.012), vascular invasion (P=0.004), LBP (P=0.008), and the
LBP/serum AFP combination (P<0.0001) were significant prognostic
factors for OS time. Similarly, serum AFP (P=0.003), liver
cirrhosis (P<0.0001), TNM stage (P<0.0001), tumor size
(P<0.0001), tumor number (P<0.0001), tumor differentiation
(P=0.014), vascular invasion (P=0.019), LBP (P=0.002), and the
LBP/serum AFP combination (P<0.0001) were significant prognostic
factors for TTR using univariate analysis. Furthermore,
multivariate Cox analyses showed that, liver cirrhosis (HR,1.550;
95% Cl, 1.060–2.268; P=0.024), TNM (HR, 1.514; 95% Cl 1.188–1.929;
P=0.001), tumor size (HR, 1.671; 95% Cl, 1.192–2.343; P=0.003), and
the LBP/serum AFP combination (HR, 1.458; 95% Cl, 1.158–1.837,
P=0.001) were independent prognostic factors for OS time. Liver
cirrhosis (HR, 1.680; 95% Cl, 1.191–2.369; P=0.003), tumor size
(HR, 1.564; 95% Cl, 1.181–2.071; P=0.002), tumor number (HR, 1.950;
95% Cl, 1.437–2.646; P<0.0001), and the LBP/serum AFP
combination (HR, 1.382; 95% Cl, 1.124–1.700; P=0.002) were also
independent prognostic factors for TTR. While there are many
parameters associated with outcomes in patients with HCC, including
LBP, our results showed that the combination of LBP and serum AFP
is a novel valuable prognostic factor for OS time and TTR.
 | Table I.Univariate and multivariate analyses
of factors associated with OS and TTR in patients with
hepatocellular carcinoma. |
Table I.
Univariate and multivariate analyses
of factors associated with OS and TTR in patients with
hepatocellular carcinoma.
|
| OS | TTR |
|---|
|
|
|
|
|---|
|
| Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
factors | P-value | HR | 95% Cl | P-value | P-value | HR | 95% Cl | P-value |
|---|
| Sex (male vs.
female) | 0.665 |
|
|
| 0.611 |
|
|
|
| Age, years (≤50 vs.
>50) | 0.988 |
|
|
| 0.947 |
|
|
|
| HBsAg (positive vs.
negative) | 0.148 |
|
|
| 0.107 |
|
|
|
| Serum AFP, ng/ml
(≤20 vs. >20) | 0.001a |
|
|
| 0.003a |
|
|
|
| Liver cirrhosis
(yes vs. no) | 0.006a | 1.550 | 1.060–2.268 | 0.024a |
<0.001a | 1.680 | 1.191–2.369 | 0.003a |
| TNM (I vs. II vs.
III–IV) |
<0.001a | 1.514 | 1.188–1.929 | 0.001a |
<0.001a |
|
|
|
| Child-Pugh (A vs.
B) | 0.251 |
|
|
| 0.266 |
|
|
|
| Tumor size, cm (≤5
vs. >5) |
<0.001a | 1.671 | 1.192–2.343 | 0.003a |
<0.001a | 1.564 | 1.181–2.071 | 0.002a |
| Tumor number
(single vs. multiple) |
<0.001a |
|
|
|
<0.000a | 1.950 | 1.437–2.646 |
<0.001a |
| Tumor
differentiation (well vs. moderate vs. poor) | 0.012a |
|
|
| 0.014a |
|
|
|
| Vascular invasion
(no vs. yes) | 0.004a |
|
|
| 0.019a |
|
|
|
| LBP (low vs.
high) | 0.008a |
|
|
| 0.002a |
|
|
|
| LBP/AFP [LBP
low/AFP (−) vs. LBP high/AFP (−) or LBP low/AFP (+) vs. AFP (+) and
LBP high] |
<0.001a | 1.458 | 1.158–1.837 | 0.001a |
<0.001a | 1.382 | 1.124–1.700 | 0.002a |
Prognostic value of LBP expression in
patients with HCC
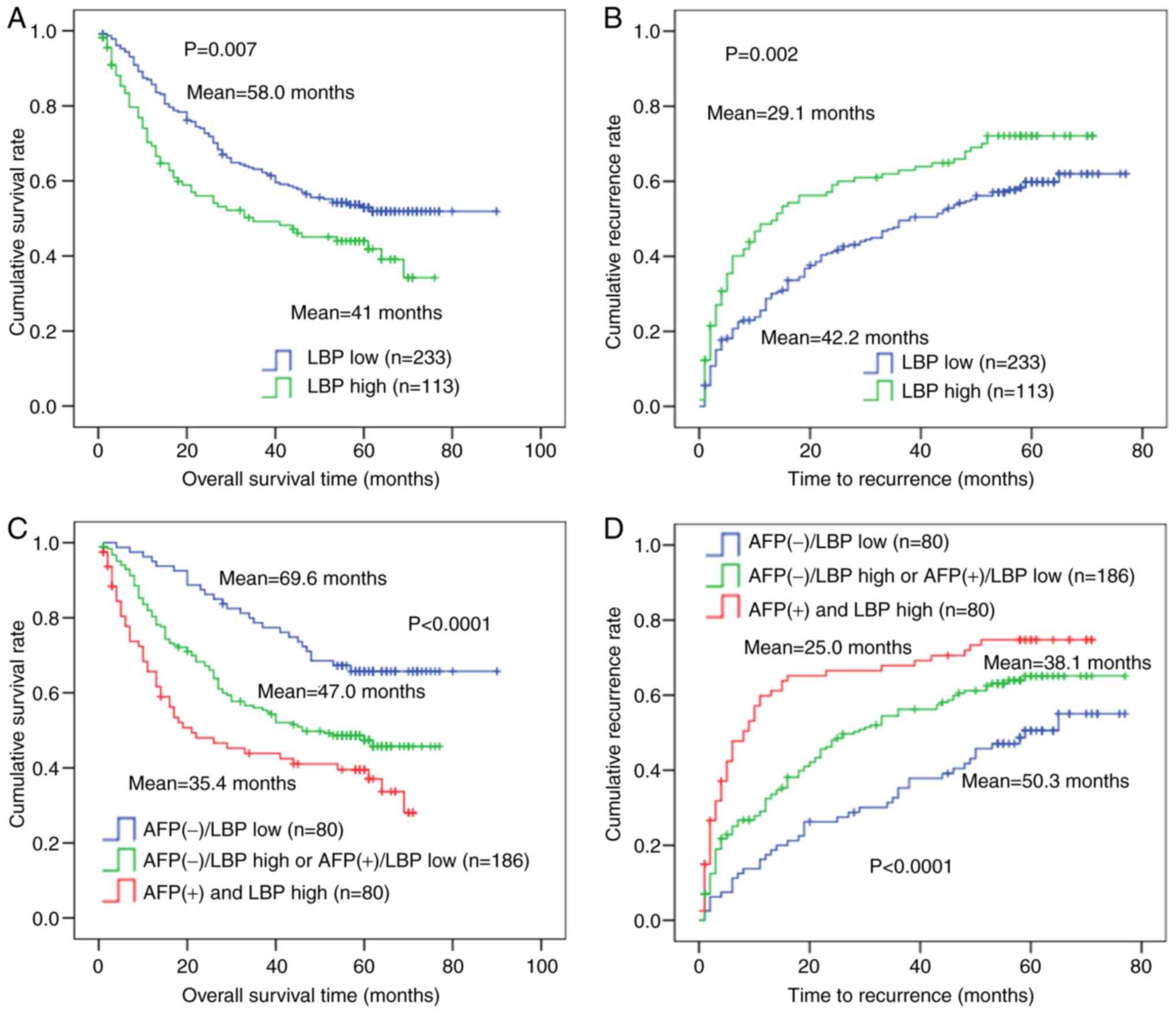
Kaplan-Meier curves were used to further determine
the association between LBP score and prognosis. LBP scores in HCC
tissues ranged from 0 to 9, 40.7% (141/346) were 0, 59.3% (205/346)
were 1–9. X-tile analysis of 346 cases showed that the best cut-off
point for survival analysis was 2. Thus, if an IHC score of HCC
tissue was ≤2, the patient was classified as low-LBP; if the score
was >2, the patients was classified as LBP-high. As shown in
Fig. 2, OS time and TTR were
significantly worse (P=0.007 and P=0.002, respectively) in the high
LBP expression group (n=113) compared with that in the LBP-low
expression group (n=233). Probability of post-operative survival
demonstrated that the mean OS time for patients with HCC with high
LBP levels was 41 months compared with 58 months for those with low
LBP expression (Fig. 2A). The mean
TTR for patients with HCC with high LBP levels was 29.1 months; for
those with high LBP levels, it was 42.2 months (Fig. 2B). The representative LBP expression
for shortest the OS time and TTR, and longest OS time and TTR is
shown in Fig. S1. Furthermore, the
346 patients with HCC were classified into three groups according
to LBP expression and serum AFP status: i) Group I, AFP (+) and LBP
high (n=80); ii) group II, AFP (+)/LBP-low or AFP (−)/LBP-high
(n=186); and iii) group III, AFP (−)/LBP-low (n=80). Kaplan-Meier
analysis demonstrated that patients in group I (AFP (+)/LBP-high)
had the shortest OS time (mean, 35.4 months) and TTR (mean, 25.0
months), whereas patients in group III had the longest OS time
(mean, 69.6 months) and TTR (mean, 50.3 months) (Fig. 2C and D).
LBP expression in aggressive tumor
tissues
The χ2 test was used to identify
associations with LBP expression with different variables. First,
the associations between clinicopathological variables and LBP
expression in patients with HCC (n=346) were investigated. The
results showed that LBP expression in HCC tissues was associated
with TNM stage (P=0.035) and tumor differentiation (P=0.011), but
was not associated with sex, age, Hepatitis B surface antigen
(HBsAg), serum AFP, liver cirrhosis, Child-Pugh class, tumor size,
tumor number and vascular invasion (all P>0.05; Table II). Based on these χ2
test results, LBP expression was further analyzed in subgroups of
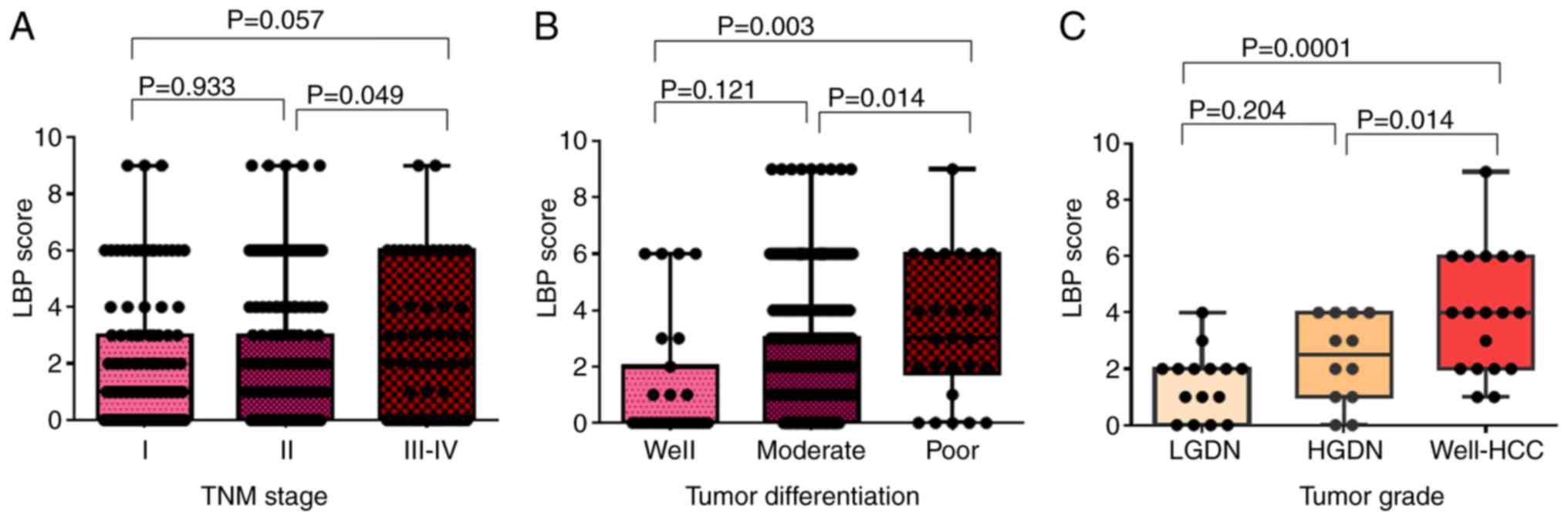
TNM stage and tumor grade. Results showed that LBP expression in
tumors with TNM stage III–IV were higher compared with that in
stage I and II tumors; however, there were no statistically
significant differences between LBP expression in TNM stages I and
II and between I and III–IV (Fig.
3A). LBP score was significantly higher in poorly
differentiated tumors compared with that in well-differentiated
(P=0.003) and moderately differentiated (P=0.014) tumors (Fig. 3B). LBP expression was gradually
increased in LGDN, HGDN and well-HCC, respectively (HGDN vs.
well-HCC, P=0.014; LGDN vs. well-HCC, P=0.0001; Fig. 3C). The representative LBP expression
for each stage of TNM, tumor differentiation, and tumor grade is
shown in Fig. S2.
 | Table II.Relationship between LBP expression
and clinicopathological features in hepatocellular carcinoma. |
Table II.
Relationship between LBP expression
and clinicopathological features in hepatocellular carcinoma.
|
| LBP |
|
|---|
|
|
|
|
|---|
| Variable | Low (n=233) | High (n=113) | P-value |
|---|
| Sex |
|
| 0.160 |
|
Male | 200 | 103 |
|
|
Female | 33 | 10 |
|
| Age, years |
|
| 0.389 |
|
≤50 | 104 | 56 |
|
|
>50 | 129 | 57 |
|
| HBsAg |
|
| 0.640 |
|
Negative | 44 | 19 |
|
|
Positive | 189 | 94 |
|
| Serum AFP,
ng/ml |
|
| 0.117 |
|
≤20 | 88 | 33 |
|
|
>20 | 145 | 80 |
|
| Liver
cirrhosis |
|
| 0.068 |
| No | 69 | 23 |
|
|
Yes | 164 | 90 |
|
| TNM |
|
| 0.035a |
| I | 79 | 34 |
|
| II | 124 | 52 |
|
|
III–IV | 30 | 27 |
|
| Child-Pugh
class |
|
| 0.452 |
| A | 214 | 101 |
|
| B | 19 | 12 |
|
| Tumor size, cm |
|
| 0.113 |
| ≤5 | 116 | 46 |
|
|
>5 | 117 | 67 |
|
| Tumor number |
|
| 0.073 |
|
Single | 187 | 81 |
|
|
Multiple | 46 | 32 |
|
| Tumor
differentiation |
|
| 0.011a |
|
Well | 21 | 6 |
|
|
Moderate | 201 | 92 |
|
|
Poor | 11 | 15 |
|
| Vascular
invasion |
|
| 0.254 |
| No | 91 | 37 |
|
|
Yes | 142 | 76 |
|
In the present study, both LBP expression level and
the combination of LBP expression level and serum AFP level were
significant prognostic factors for both OS time and TTR (Fig. 2). The prognostic value of LBP in the
different status of serum HBsAg and liver cirrhosis was further
explored (Fig. S3). The results
demonstrated that a high level of LBP might indicate poor prognosis
for OS time and TTR in patients who are HBsAg-positive but not for
patients who are HBsAg-negative. Similar results were observed in
patients with liver cirrhosis (Fig.
S4). In addition, in the HBsAg positive group, the OS rate and
TTR rate was worse in AFP (+)/LBP high patients compared with AFP
(−)/LBP low patients, whereas no significant differences were
observed in the HBsAg negative group (Fig. S5). In the liver cirrhosis and
non-cirrhosis group, the OS and TTR rates were worse in AFP (+)/LBP
high patients compared with AFP (−)/LBP low patients (Fig. S6). Thus, measurement of both LBP and
serum AFP may provide useful prognostic information for patients
with HCC.
Discussion
The primary function of LBP is to act in the
recognition, binding, and transport of the LPS (14,15). LBP
has previously been associated with the pathogenesis of sepsis
(20), and with the prognosis of
colorectal carcinoma and renal cell carcinoma (22,23).
However, the prognostic role of LBP in HCC and its expression in
HCC tumor tissues and peritumor liver tissues has not been
reported, to the best of our knowledge. In the present study, LBP
expression in peritumor liver tissues was weak overall only nine
cases (11.6%; 9/77) strongly expressed LBP in non-tumor liver
tissues (scores of 6 or 9), whereas LBP was strongly expressed in
tumor tissues in 24 cases (31.1%, 24/77, scores of 6 or 9).
Quantified analysis of LBP expression by western blot analysis
further reveals that LBP was overexpressed in tumor tissues
compared with peritumor liver tissues (Fig. 1E and F). Furthermore, as shown in
Fig. 3B, LBP was highly expressed in
poorly differentiated tumors compared with that in well- or
moderately differentiated tumors, suggesting high LBP expression
might reflect poor differentiation status of HCC.
Hepatocarcinogenesis is a complex and multistep
process from preneoplastic lesions, including cirrhosis, LGDNs and
HGDN to early- and well-differentiated HCC (32). Therefore, exploring molecular
pathogenesis during this multistep process, may assist in
understanding how the critical transition happens during HCC
initiation at a molecular level. As shown in Fig. 3C, LBP expression levels gradually
increased in order of LGDN, HGDN and well-HCC; thus, LBP expression
may partially reflect HCC initiation and progression.
Serum AFP is a common serum biomarker for HCC
diagnosis (33). However, due to its
low sensitivity and specificity (33–35),
Golgi protein 73 (36), vitamin K or
antagonist-II (PIVKA-II) (37) were
proposed as new serological biomarkers for diagnosing of HCC. Since
then, many studies have focused on using AFP and PIVKA-II to
predict prognosis (38,39). The prognosis of patients with HCC
might also be predicted by molecular classification.
Biomarker-based classes of HCC would be useful to predict prognosis
and design clinical trials for targeted therapy (40). Although progress in clinical
predictive biomarkers has been made (11,25,41), the
clinical utility of these biomarkers for predicting the prognosis
of HCC patients is still unclear.
The present study has some limitations. First,
because of its retrospective nature and mono-center design, a
multicenter and prospective study should be performed to further
evaluate the prognostic value of LBP in HCC. Second, as LBP has
been proposed as a diagnostic serum biomarker for ovarian cancer
(42), Kawasaki disease (43), and rheumatoid arthritis (44), the diagnostic and prognostic role of
serum LBP in patients with HCC should be explored by further
research. Finally, the mechanism of LBP overexpression in patients
with HCC, and whether LBP could be a therapeutic biomarker for
patients with HCC should be evaluated in the future.
In conclusion, the present study is the first to
report that LBP is overexpressed in HCC tissues, and that LBP
status may indicate the aggressiveness of HCC, and the prognosis of
HCC patients after surgery. Significant differences in the
prognosis of patients with HCC stratified by the combination of LBP
expression and serum AFP was also found.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Cong Wen-Ming and
Dr Dong Hui from the Department of Pathology, EHBH for reviewing
hematoxylin and eosin staining.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81671739 and
81472769).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
NYJ, GZJ and QYC designed and prepared the study and
the manuscript. JHJ proposed the study QYC, JHJ, RMJ and GZJ
collected and analyzed the data and wrote the first draft of the
manuscript. QYC and NYJ performed data analysis. All authors
reviewed and approved the manuscript.
Ethics approval and consent to
participate
Each patient provided informed consent for use of
these samples and the institutional review board at Eastern
Hepatobiliary Surgery Hospital approved the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khemlina G, Ikeda S and Kurzrock R: The
biology of Hepatocellular carcinoma: Implications for genomic and
immune therapies. Mol Cancer. 16:1492017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang A, Hallouch O, Chernyak V, Kamaya A
and Sirlin CB: Epidemiology of hepatocellular carcinoma: Target
population for surveillance and diagnosis. Abdom Radiol (NY).
43:13–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ziogas DE, Kyrochristos ID, Glantzounis
GK, Christodoulou D, Felekouras E and Roukos DH: Primary liver
cancer genome sequencing: Translational implications and
challenges. Expert Rev Gastroenterol Hepatol. 11:875–883.
2017.PubMed/NCBI
|
|
5
|
Shin J, Yu JH, Jin YJ, Suh YJ, Kim DH,
Byun S and Lee JW: Effective therapeutic options for elderly
patients with hepatocellular carcinoma: A nationwide cohort study.
Medicine (Baltimore). 98:e161502019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lai EC, Lo CM, Fan ST, Liu CL and Wong J:
Postoperative adjuvant chemotherapy after curative resection of
hepatocellular carcinoma: A randomized controlled trial. Arch Surg.
133:183–188. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tung-Ping Poon R, Fan ST and Wong J: Risk
factors, prevention, and management of postoperative recurrence
after resection of hepatocellular carcinoma. Ann Surg. 232:10–24.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xia Y, Qiu Y, Li J, Wang K, Xi T, Shen F,
Yan Z and Wu M: Adjuvant therapy with capecitabine postpones
recurrence of hepatocellular carcinoma after curative resection: A
randomized controlled trial. Ann Surg Oncol. 17:3137–3144. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bai DS, Dai Z, Zhou J, Liu YK, Qiu SJ, Tan
CJ, Shi YH, Huang C, Wang Z, He YF and Fan J: Capn4 overexpression
underlies tumor invasion and metastasis after liver transplantation
for hepatocellular carcinoma. Hepatology. 49:460–470. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu YF, Yi Y, Qiu SJ, Gao Q, Li YW, Dai CX,
Cai MY, Ju MJ, Zhou J, Zhang BH and Fan J: PEBP1 downregulation is
associated to poor prognosis in HCC related to hepatitis B
infection. J Hepatol. 53:872–879. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang XR, Xu Y, Yu B, Zhou J, Li JC, Qiu
SJ, Shi YH, Wang XY, Dai Z, Shi GM, et al: CD24 is a novel
predictor for poor prognosis of hepatocellular carcinoma after
surgery. Clin Cancer Res. 15:5518–5527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cai MY, Tong ZT, Zheng F, Liao YJ, Wang Y,
Rao HL, Chen YC, Wu QL, Liu YH, Guan XY, et al: EZH2 protein: A
promising immunomarker for the detection of hepatocellular
carcinomas in liver needle biopsies. Gut. 60:967–976. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Llovet JM, Bru C and Bruix J: Prognosis of
hepatocellular carcinoma: The BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schumann RR, Kirschning CJ, Unbehaun A,
Aberle HP, Knope HP, Lamping N, Ulevitch RJ and Herrmann F: The
lipopolysaccharide-binding protein is a secretory class 1
acute-phase protein whose gene is transcriptionally activated by
APRF/STAT/3 and other cytokine-inducible nuclear proteins. Mol Cell
Biol. 16:3490–3503. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schumann RR, Leong SR, Flaggs GW, Gray PW,
Wright SD, Mathison JC, Tobias PS and Ulevitch RJ: Structure and
function of lipopolysaccharide binding protein. Science.
249:1429–1431. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong
W, Tang L, Lin Y, He YQ, Zou SS, et al: Endotoxin accumulation
prevents carcinogen-induced apoptosis and promotes liver
tumorigenesis in rodents. Hepatology. 52:1322–1333. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dapito DH, Mencin A, Gwak GY, Pradere JP,
Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A,
Bataller R, et al: Promotion of hepatocellular carcinoma by the
intestinal microbiota and TLR4. Cancer Cell. 21:504–516. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Darnaud M, Faivre J and Moniaux N:
Targeting gut flora to prevent progression of hepatocellular
carcinoma. J Hepatol. 58:385–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang HL, Yu LX, Yang W, Tang L, Lin Y, Wu
H, Zhai B, Tan YX, Shan L, Liu Q, et al: Profound impact of gut
homeostasis on chemically-induced pro-tumorigenic inflammation and
hepatocarcinogenesis in rats. J Hepatol. 57:803–812. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Malhotra R and Bird MI: L-selectin: A
novel receptor for lipopolysaccharide and its potential role in
bacterial sepsis. Bioessays. 19:919–923. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garcia de Guadiana-Romualdo L,
Espanol-Morales I, Cerezuela-Fuentes P, Consuegra-Sánchez L,
Hernando-Holgado A, Esteban-Torrella P, Jiménez-Santos E,
Viqueira-González M, de Béjar-Almira Á and Albaladejo-Otón MD:
Value of lipopolysaccharide binding protein as diagnostic marker of
infection in adult cancer patients with febrile neutropenia:
Comparison with C-reactive protein, procalcitonin, and interleukin
6. Support Care Cancer. 23:2175–2182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen R, Luo FK, Wang YL, Tang JL and Liu
YS: LBP and CD14 polymorphisms correlate with increased colorectal
carcinoma risk in Han Chinese. World J Gastroenterol. 17:2326–2331.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kovacs G, Peterfi L, Farkas N, Javorhazy
A, Pusztai C and Szanto A: Expression of inflammatory
lipopolysaccharide binding protein (LBP) predicts the progression
of conventional renal cell carcinoma-a short report. Cell Oncol
(Dordr). 40:651–656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO Classification of tumours of the digestive system.
Lyon: IARC Press; 3. pp. 205–216. 2010
|
|
25
|
Jin GZ, Yu WL, Dong H, Zhou WP, Gu YJ, Yu
H, Yu H, Lu XY, Xian ZH, Liu YK, et al: SUOX is a promising
diagnostic and prognostic biomarker for hepatocellular carcinoma. J
Hepatol. 59:510–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
International Consensus Group for
Hepatocellular NeoplasiaThe International Consensus Group for
Hepatocellular Neoplasia, . Pathologic diagnosis of early
hepatocellular carcinoma: A report of the international consensus
group for hepatocellular neoplasia. Hepatology. 49:658–664. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang GH, Fan J, Xu Y, Qiu SJ, Yang XR, Shi
GM, Wu B, Dai Z, Liu YK, Tang ZY and Zhou J: Osteopontin combined
with CD44, a novel prognostic biomarker for patients with
hepatocellular carcinoma undergoing curative resection. Oncologist.
13:1155–1165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan N, Liu Q, Liu X, Gong Z, Zeng Y, Pan
G, Xu Q and He S: Low expression of B-cell-associated protein 31 in
human primary hepatocellular carcinoma correlates with poor
prognosis. Histopathology. 68:221–229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin H, Wang C, Jin G, Ruan H, Gu D, Wei L,
Wang H, Wang N, Arunachalam E, Zhang Y, et al: Regulator of
calcineurin 1 gene isoform 4, down-regulated in hepatocellular
carcinoma, prevents proliferation, migration, and invasive activity
of cancer cells and metastasis of orthotopic tumors by inhibiting
nuclear translocation of NFAT1. Gastroenterology. 153:799–811.e733.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin GZ, Li Y, Cong WM, Yu H, Dong H, Shu
H, Liu XH, Yan GQ, Zhang L, Zhang Y, et al: iTRAQ-2DLC-ESI-MS/MS
based identification of a new set of immunohistochemical biomarkers
for classification of dysplastic nodules and small hepatocellular
carcinoma. J Proteome Res. 10:3418–3428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kudo M: Multistep human
hepatocarcinogenesis: Correlation of imaging with pathology. J
Gastroenterol. 44 (Suppl):(19): S112–S118. 2009. View Article : Google Scholar
|
|
33
|
Ertle JM, Heider D, Wichert M, Keller B,
Kueper R, Hilgard P, Gerken G and Schlaak JF: A combination of
α-fetoprotein and des-gamma-carboxy prothrombin is superior in
detection of hepatocellular carcinoma. Digestion. 87:121–131. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Morimoto M, Numata K, Nozaki A, Kondo M,
Moriya S, Taguri M, Morita S, Konno M, Sugo A, Miyajima E, et al:
Novel Lens culinaris agglutinin-reactive fraction of α-fetoprotein:
A biomarker of hepatocellular carcinoma recurrence in patients with
low alpha-fetoprotein concentrations. Int J Clin Oncol. 17:373–379.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamamoto K, Imamura H, Matsuyama Y,
Hasegawa K, Beck Y, Sugawara Y, Makuuchi M and Kokudo N:
Significance of alpha-fetoprotein and des-gamma-carboxy prothrombin
in patients with hepatocellular carcinoma undergoing hepatectomy.
Ann Surg Oncol. 16:2795–2804. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Marrero JA, Romano PR, Nikolaeva O, Steel
L, Mehta A, Fimmel CJ, Comunale MA, D'Amelio A, Lok AS and Block
TM: GP73, a resident Golgi glycoprotein, is a novel serum marker
for hepatocellular carcinoma. J Hepatol. 43:1007–1012. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fujiyama S, Morishita T, Sagara K, Sato T,
Motohara K and Matsuda I: Clinical evaluation of plasma abnormal
prothrombin (PIVKA-II) in patients with hepatocellular carcinoma.
Hepatogastroenterology. 33:201–205. 1986.PubMed/NCBI
|
|
38
|
Chon YE, Choi GH, Lee MH, Kim SU, Kim DY,
Ahn SH, Kim KS, Choi JS, Han KH, Chon CY and Park JY: Combined
measurement of preoperative alpha-fetoprotein and des-gamma-carboxy
prothrombin predicts recurrence after curative resection in
patients with hepatitis-B-related hepatocellular carcinoma. Int J
Cancer. 131:2332–2341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kang SH, Kim DY, Jeon SM, Ahn SH, Park JY,
Kim SU, Kim JK, Lee KS, Chon CY and Han KH: Clinical
characteristics and prognosis of hepatocellular carcinoma with
different sets of serum AFP and PIVKA-II levels. Eur J
Gastroenterol Hepatol. 24:849–856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin Z, Xu SH, Wang HQ, Cai YJ, Ying L,
Song M, Wang YQ, Du SJ, Shi KQ and Zhou MT: Prognostic value of DNA
repair based stratification of hepatocellular carcinoma. Sci Rep.
6:259992016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hwang HW, Ha SY, Bang H and Park CK: ATAD2
as a poor prognostic marker for hepatocellular carcinoma after
curative resection. Cancer Res Treat. 47:853–861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Boylan KL, Andersen JD, Anderson LB,
Higgins L and Skubitz AP: Quantitative proteomic analysis by
iTRAQ(R) for the identification of candidate biomarkers in ovarian
cancer serum. Proteome Sci. 8:312010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kimura Y, Yanagimachi M, Ino Y, Aketagawa
M, Matsuo M, Okayama A, Shimizu H, Oba K, Morioka I, Imagawa T, et
al: Identification of candidate diagnostic serum biomarkers for
Kawasaki disease using proteomic analysis. Sci Rep. 7:437322017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wen W, Li Y, Cheng Y, He J, Jia R, Li C,
Guo J, Sun X and Li Z: Lipopolysaccharide-binding protein is a
sensitive disease activity biomarker for rheumatoid arthritis. Clin
Exp Rheumatol. 36:233–240. 2018.PubMed/NCBI
|