Introduction
At present, colorectal cancer (CRC) is the third
most common malignancy in males and the second in females worldwide
(1,2). Due to the high mortality rate,
primarily as a result of occult or clinically overt metastases
present at the time of diagnosis, and increased understanding of
the molecular mechanisms underlying the malignant transformation of
normal colorectal epithelial cells in the progression of adenoma to
adenocarcinoma is required (3).
Colorectal tumorigenesis is a complex process mediated by a number
of genes and various signaling pathways, which ultimately results
in the robust growth of transformed cells (4,5). Despite
notable progress in the current understanding of the mechanisms
underlying this process, the aggressive nature and increased degree
of metastasis of certain types of CRC requires further
investigation. From a clinical perspective, improved understanding
of these differences may aid the identification of prognostic
biomarkers for CRC, consequently facilitating the personalized
management of CRC to improve the prognosis of patients with this
disease.
Bone morphogenetic proteins (BMPs) are members of
the transforming growth factor-β (TGF-β) family, which are
multi-functional cytokines (6). BMPs
are divided into several subgroups, including the BMP-2/4,
BMP-5/6/7/8 and BMP-9/10 groups, and the growth differentiation
factor (GDF)-5/6/7 group, depending on their structural
similarities and ability to bind certain type I receptors (7). Of note, 20 BMPs have been reported to
possess a number of functions that regulate different physiological
processes, including cellular proliferation, differentiation,
migration and apoptosis (8,9). BMP-9, also known as GDF-2, is a potent
BMP member of the TGF-β family (10). Previous studies have demonstrated
that BMP-9 acts as a multifunctional mediator in numerous
biological processes, including the regulation of cell
proliferation, differentiation, adhesion, migration and apoptosis
(11–13). These processes are involved in bone
morphogenesis, hepatic reticuloendothelial system function,
neuronal differentiation, hematopoiesis, angiogenesis, and iron and
glucose homeostasis (10,14–19).
Accumulating evidence has demonstrated that BMP-9 is
involved in tumorigenesis, and its expression varies according to
tumor types. For example, compared with normal thyroid tissues,
BMP-9 expression in thyroid tumors is increased (20). In addition, ~25% of ovarian cancers
exhibit upregulated BMP-9, which has been associated with the
tumorigenesis and progression of the disease (21). BMP-9 promotes the growth of
hepatocellular carcinoma cells, but not that of immortalized human
hepatocytes (22). Furthermore,
BMP-9 enhances the invasiveness of hepatocellular carcinoma cells
by inducing the epithelial-to-mesenchymal transition (23). Conversely, BMP-9 was demonstrated to
act as an apoptotic regulator, suppressing tumor growth in prostate
cancer and myeloma (24,25). BMP-9 inhibits the proliferation,
invasion and metastasis of breast cancer cells (26–28).
Due to its complex function, the ambiguous roles of
BMP-9 as a tumor-promoting and tumor-suppressive factor require
further investigation. Furthermore, the role of BMP-9 in colorectal
tumorigenesis remains to be elucidated. The present study aimed to
evaluate the prognostic value of BMP-9 expression in colorectal
carcinoma, and to determine the association between the
clinicopathological parameters of CRC and the prognostic potential
of BMP-9 in patients with this disease.
Materials and methods
Ethics statement
The present study was performed in accordance with
the Declaration of Helsinki and approved by the Ethics Committee of
The First Affiliated Hospital of Jinzhou Medical University. The
Ethics Committee waived the need for written informed consent from
the patients due to the retrospective nature of the present study.
Each tissue sample and respective clinical data was anonymized and
no additional patient intervention was performed.
Clinical samples
A total of 65 patients with pathologically confirmed
colorectal adenocarcinoma and a history of adenoma were enrolled in
the present study; patients were surgically treated in The First
Affiliated Hospital of Jinzhou Medical University (Jinzhou, China)
between April 2012 and December 2014. The mean age of the 56
patients was 65 years (range, 47–84 years), and there were 47 men
and 18 women. The inclusion criteria were as follows: i)
Pathologically diagnosed with colorectal adenocarcinoma and a
history of adenoma; ii) no previous neoadjuvant radiotherapy or
chemotherapy treatment; and iii) no surgery for colorectal
diseases. The exclusion criteria were as follows: i) Patients with
other malignant tumors; ii) patients with severe organ dysfunction;
and iii) patients with other types of colorectal diseases. The
tissues obtained were fixed in 10% formaldehyde in PBS for 24 h at
room temperature, and embedded in paraffin. For each patient, three
paraffin-embedded tissue blocks were analyzed, comprising normal
mucosa, adenoma and adenocarcinoma. All 195 tissue blocks were
obtained from the Department of Pathology at The First Affiliated
Hospital of Jinzhou Medical University. Pathological reports and
the clinical history of patients were obtained from medical
records. Follow-up information was available for 48 patients
(73.85%). The patient demographics and the clinicopathological
features of the specimens are summarized in Table I.
 | Table I.Clinical and demographic
characteristics of patients enrolled in the present study. |
Table I.
Clinical and demographic
characteristics of patients enrolled in the present study.
|
Characteristics | n (%) |
|---|
| Sex |
|
|
Male | 47/65 (72.3) |
|
Female | 18/65 (27.7) |
| Age, years |
|
|
≤55 | 11/65 (16.9) |
|
>55 | 54/65 (83.1) |
| Tumor site |
|
|
Rectum | 39/65 (60.0) |
|
Left-side colon | 17/65(26.2) |
|
Right-side colon | 9/65 (13.8) |
| Gross tumor
type |
|
|
Ulcerative | 49/65 (75.4) |
|
Elevated | 16/65 (24.6) |
| Tumor
differentiation |
|
|
Poor | 6/65 (9.2) |
|
Moderate | 54/65 (83.1) |
|
High | 5/65 (7.7) |
| Tumor histological
type |
|
| Tubular
papillary | 16/65 (24.6) |
|
Tubular | 44/65 (67.7) |
|
Mucinous | 5/65 (7.7) |
| Tumor WHO
classification |
|
|
Low | 53/65 (81.5) |
|
High | 12/65 (18.5) |
| Blood vessel tumor
embolus |
|
|
Positive | 7/65 (10.8) |
|
Negative | 58/65 (89.2) |
| TNM stage |
|
|
I–II | 46/65 (70.8) |
|
III–IV | 19/65 (29.2) |
| Serum CEA
levels |
|
|
High | 22/52 (42.3) |
|
Normal | 30/52 (57.7) |
| Serum CA19-9
levels |
|
|
High | 9/44 (20.5) |
|
Normal | 35/44 (79.5) |
| Adenomas site |
|
|
Left-side colon | 14/65 (21.5) |
|
Right-side colon | 11/65 (16.9) |
|
Rectum | 40/65 (61.6) |
| Number of
adenomas |
|
|
Single | 56/65 (86.2) |
|
Multiple | 9/65 (13.8) |
| Adenomas
histological type |
|
|
Tubular | 41/65 (63.1) |
|
Tubulovillous | 24/65 (36.9) |
| Adenoma
dysplasia |
|
|
Mild | 29/65 (44.6) |
|
Moderate | 27/65 (41.5) |
|
Severe | 9/65 (13.9) |
Pathological examination and
laboratory analysis
The Tumor-Node-Metastasis (TNM) staging and
histological type of patient samples were primarily determined
according to the 7th edition of the American Joint Committee's
Cancer Staging Manual (29,30). Dukes stages (A, B, C and D) were
classified according to cancer invasion, lymph node metastasis and
distant metastasis, referring to the Dukes staging of Colorectal
Cancer (1984) (31).
In order to measure the levels of CEA and CA19-9,
blood samples from 52 patients (80%) and 44 patients (67.69%),
respectively, were obtained 2–3 days prior to surgery. Peripheral
blood was centrifuged at 1,800 × g for 20 min at 4°C to obtain
serum. The levels of these markers in the serum were measured using
an i2000 automatic immunoluminescence analyzer (Abbott
Laboratories). Serum levels of carcinoembryonic antigen (CEA)
>3.4 ng/ml and CA 19-9 >27 U/ml were considered abnormal.
Immunohistochemistry (IHC)
Formalin-fixed paraffin- embedded tissue blocks from
the 65 patients, including colorectal mucosa, adenoma and
adenocarcinoma specimens, were cut into 4 µm-thick sections and
affixed to glass slides. Sections were deparaffinized in xylene,
rehydrated via a descending alcohol series in double distilled
water (including 100, 80, 70, 50 and 30% alcohol) for 2 min per
concentration, and then rinsed in distilled water at room
temperature. The antigen retrieval process performed was specific
to each marker. For BMP-9, the slides were immersed in citrate
buffer (10 mmol/l citric acid and sodium citrate; pH 6.0) and
heated in a pressure cooker for 10 min at 100°C. In the case of
Ki-67, the slides were boiled in citrate buffer (10 mmol/l citric
acid and sodium citrate; pH of 6.0) at 100°C in a microwave oven
for 20 min. The slides were allowed to cool at room temperature for
60 min and were then blocked with 1% bovine serum albumin in TBS
(Sigma-Aldrich; Merck KGaA) for 60 min at room temperature.
Staining was performed in a humidified chamber overnight at 4°C
using the following antibodies (both from Abcam): Anti-BMP-9
(1:100; cat. no. ab35088) and anti-Ki-67 (1:250; cat. no. ab15580).
Following incubation with primary antibodies, endogenous peroxidase
activity was blocked by incubating the slides with 3% hydrogen
peroxide for 10 min at room temperature. The sections were
subsequently stained using the two-step plus® Poly-HRP
Anti-Mouse/Rabbit IgG Detection System (cat. no. PV-9000; OriGene
Technologies, Inc) for 20 min at room temperature. BMP-9 and Ki-67
expression was visualized via staining with 3,3′-diaminobenzidine
(Sigma-Aldrich; Merck KGaA) for 5 and 2 min at room temperature,
respectively. The nuclei were counterstained with hematoxylin for 8
min (Sigma-Aldrich; Merck KGaA) at room temperature. The slides
were dehydrated via an alcohol gradient, including 100, 70, 50 and
30% alcohol, in double distilled water for 1 min each time and then
immersed in xylene. Subsequently, the slides were fixed with
Histomount mounting medium (cat. no. HS-103-100ML; AGTC Bioproducts
Ltd.)
Evaluation of staining
All immunostained sections were evaluated
semi-quantitatively under an Olympus BX40 light microscope (Olympus
Corporation; magnification, ×400) by two investigators from the The
First Affiliated Hospital of Jinzhou Medical University (Jinzhou,
Liaoning) who were blinded to the clinicopathological data. In the
case of discrepancies between the evaluators, a consensus was
reached after obtaining a third and final opinion from the director
of the Department of Pathology at The First Affiliated Hospital of
Jinzhou Medical University. BMP-9 expression was calculated as the
product of the relative score, reflecting the percentage of
positively stained cells (0, <5%; 1, 5–25%; 2, 25–50%; 3,
50–75%; and 4, >75%), and the intensity of the staining (0,
negative; 1, weak; 2, moderate; and 3, strong). The final BMP-9
expression score ranged from 0–12, and was subsequently classified
into 4 levels: ‘−’ for negative (0), ‘+’ for mild (1–3), ‘++’
for moderate (4–7) and ‘+++’ for strong (8–12). For
the assessment of Ki-67 expression, cells with positive nuclear
staining were counted and the percentage of positively stained
cells was calculated as aforementioned. The staining was assessed
as ‘−’ (negative, <5%), ‘+’ (moderately positive, 5–25%), ‘++’
(positive, 25–50%) and ‘+++’ (strongly positive, >50%).
Bioinformatics analysis
To further analyze the role of BMP-9 in CRC, the
expression of BMP-9 and the clinicopathological data of patients
were extracted from The Cancer Genome Atlas (TCGA) (https://cancergenome.nih.gov/). Additionally, the
Kurashina (GSE11417) (32) and
Gaedcke (GSE20842) (33) dataset
from the Oncomine database (https://www.oncomine.org/), which is a cancer
microarray database and web-based data-mining platform for
genome-wide expression analyses, was downloaded. The data employed
were used to compare differences in BMP-9 expression at the mRNA
level between healthy colorectal tissue and tumor tissues. In
addition, the correlation between BMP-9 expression, and the
clinicopathological and prognostic data of patients with CRC was
determined. All data were log-transformed, median-centered per
array and standard deviation-normalized to one per array.
Statistical analysis
The expression of BMP-9 in colorectal tumorigenesis
and the associations between the clinicopathological features and
the status of BMP-9 expression were assessed via Spearman's rank
correlation analysis. A Kaplan-Meier analysis was performed in
order to estimate the 5-year overall survival (OS) rate for
patients with CRC with low- or high- BMP-9 expression levels. These
expression groups were defined according to the mean IHC score
(2.17); IHC scores 0–2 were considered to indicate low-BMP-9
expression, while a score of ≥3 was recorded as high BMP-9
expression. Differences between groups were analyzed with a
log-rank test. Significant factors from univariate analyses were
included in the multivariate models. A Cox proportional hazards
model was used to perform multivariate survival analyses in order
to identify independent prognostic factors. For the bioinformatics
analysis, the BMP-9 expression levels in different colorectal
tissues were compared using a repeated measures ANOVA. The
comparison of data from multiple groups against a single control
group was performed using a Dunnett's post hoc test. The comparison
of data from multiple groups against every other group was
performed using a Tukey's multiple comparisons test. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were conducted using SPSS software (version
20; IBM Corp.) and GraphPad Prism software (version 7.0; GraphPad
Software Inc.).
Results
Expression of BMP-9 in CRC
In order to investigate the role of BMP-9 in
colorectal tumorigenesis, the present study assessed BMP-9
expression in normal mucosa, adenoma and colorectal carcinoma
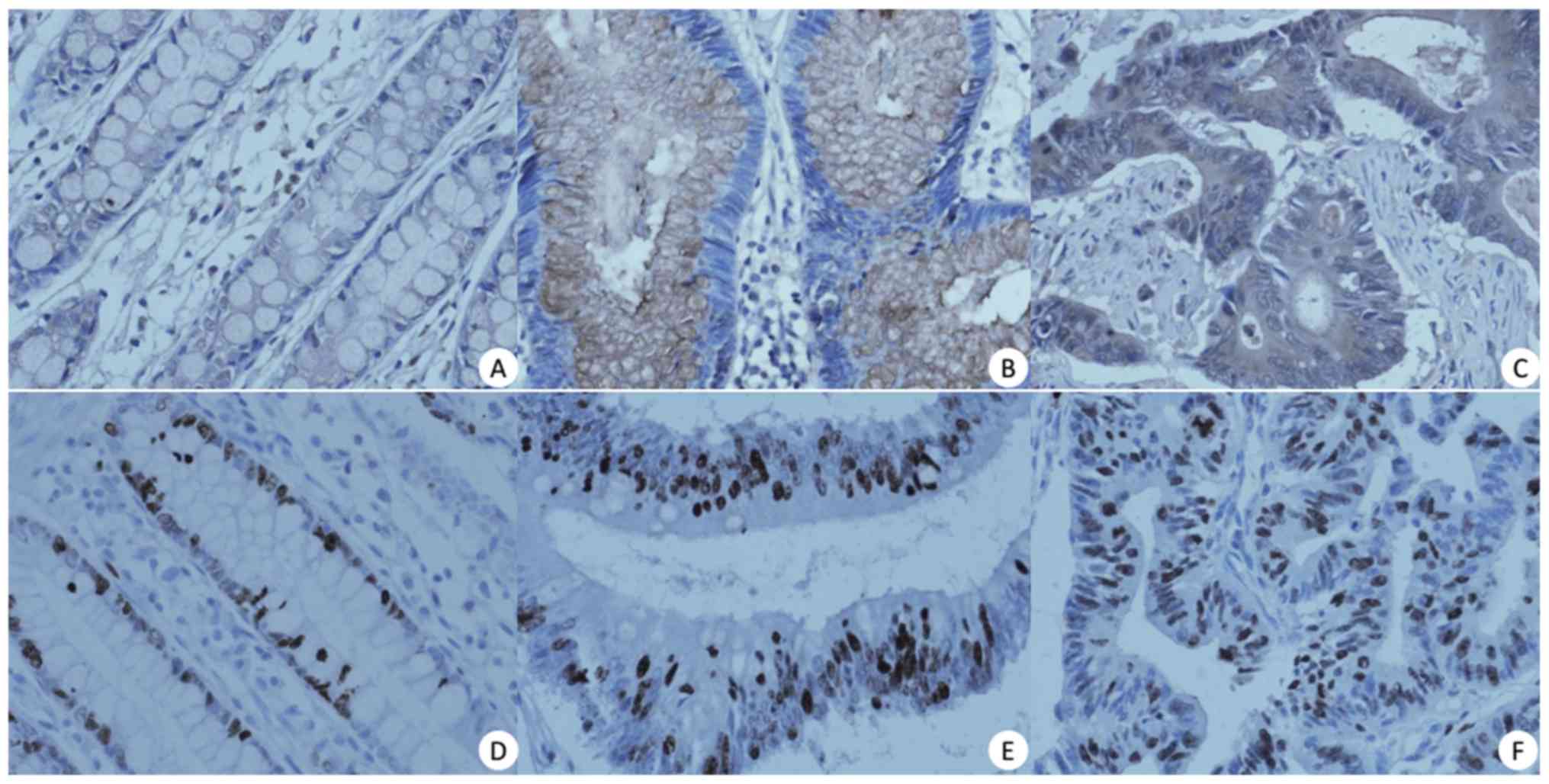
samples using IHC staining. In normal colorectal mucosa, decreased
BMP-9 expression was detected in the cell membrane and cytoplasm of
colorectal mucosal cells; in the majority of cases (60/65, 92.31%),
the protein was not present in the nucleus (Fig. 1A). In colorectal adenoma tissues,
BMP-9 was moderately expressed in the membrane and/or cytoplasm at
a level similar to that in normal tissues. In addition, BMP-9
expression was undetectable in the nucleus in the majority of cases
(62/65, 95.38%) (Fig. 1B). On the
contrary, in carcinoma tissues, BMP-9 was strongly expressed and
predominantly observed in the membrane and/or cytoplasm of cancer
cells, but was weakly expressed or undetectable in the nucleus
(Fig. 1C; Table II). The positive rates of BMP-9
expression in normal colorectal mucosa, adenoma and carcinoma
tissues were 68, 91 and 95%, respectively (Table II). The expression levels of BMP-9
presented an upward trend in the aforementioned tissues, with mean
values of 0.7, 1.8 and 2.2, respectively. The elevated BMP-9
expression levels of the three types of tissue were statistically
significant (r2=0.615; P<0.05). Furthermore, BMP-9
expression was significantly upregulated in carcinoma samples when
compared with adenoma samples (r2=0.221; P<0.05)
(Table II). Similarly,
significantly increased BMP-9 expression was observed in adenoma
tissues compared with normal mucosa (r2=0.572;
P<0.05). In the TCGA and Gaedcke and Kurashina datasets, BMP-9
mRNA expression was significantly increased in CRC tissues compared
with healthy tissues (P<0.05; Fig.
2A-C).
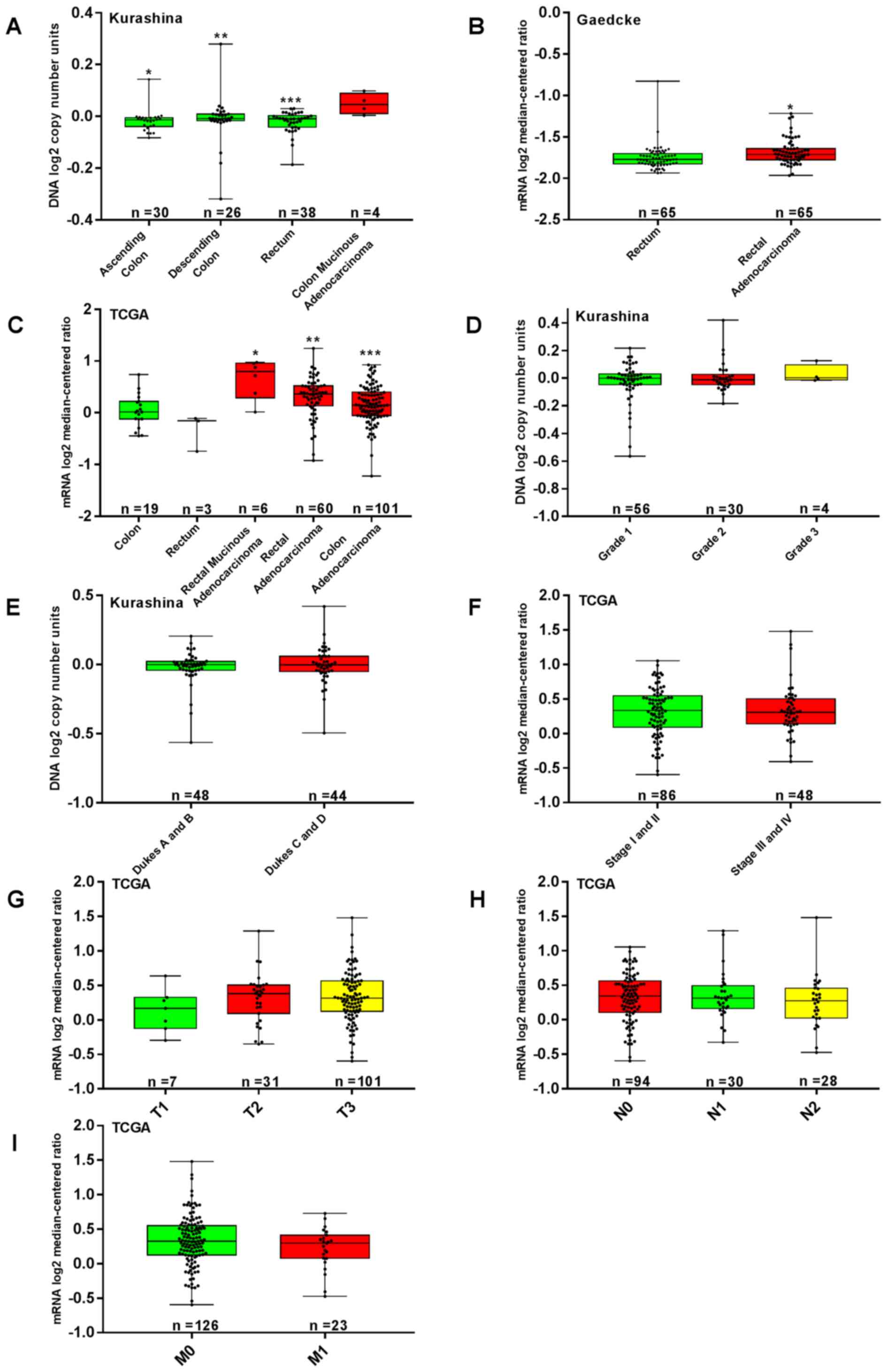 | Figure 2.Association between the
clinicopathological parameters of CRC and BMP-9 expression. TCGA
(mRNA), Kurashina (DNA) and Gaedcke (mRNA) datasets were utilized
for the bioinformatics analysis to investigate BMP-9 expression in
colorectal carcinogenesis. (A) Increased BMP-9 expression was
detected in CRC tissue compared with normal colorectal mucosa in
the Kurashina dataset. *P<0.001, **P<0.05, ***P<0.01 vs.
colon mucinous adenocarcinoma. (B) Analysis of the Gaedcke dataset
indicated that BMP-9 expression in rectal adenocarcinoma was
increased compared with normal rectal mucosa (*P<0.001). (C)
BMP-9 expression was significantly upregulated in CRC tissue
compared with normal colorectal mucosa in the TCGA dataset.
*P<0.001, **P<0.01, ***P<0.05 vs. colon and rectum. From
the Kurashina dataset, BMP-9 expression was determined to not be
associated with (D) grade classifications and (E) Dukes staging of
CRC (P>0.05). (F-I) Analysis of the dataset of TCGA revealed
that BMP-9 mRNA expression were not associated with the TNM stages
of CRC (P>0.05). BMP-9, bone morphogenetic protein-9; CRC,
colorectal cancer; TCGA, The Cancer Genome Atlas; T, Tumor; N,
Node; M, metastasis. |
 | Table II.BMP-9 expression in colorectal
carcinoma, adenoma and normal colorectal mucosa. |
Table II.
BMP-9 expression in colorectal
carcinoma, adenoma and normal colorectal mucosa.
|
| BMP-9 expression, n
(%) |
|
|---|
|
|
|
|
|---|
| Tissue type | − | + | ++ | +++ | PR (%) |
|---|
| Normal colorectal
mucosa | 21 (32) | 42 (65) | 2 (3) | 0 (0) | 68 |
| Colorectal
adenoma | 6 (9) | 20 (31) | 22 (34) | 17 (26) | 91 |
| Colorectal
carcinoma | 3 (5) | 8 (12) | 29 (45) | 25 (38) | 95 |
Correlation between BMP-9 expression
and tumor cell proliferation in human CRC
In order to examine the correlation between BMP-9
expression and cell proliferation, the expression of Ki-67 in the
same sample set was evaluated by IHC. In normal mucosa, cells
positive for nuclear Ki-67 were localized primarily at the bottom
of the glands, whereas, in adenoma and carcinoma samples, such
cells were distributed randomly (Fig.
1D-F). The mean Ki-67 indices were significantly higher in
adenoma and carcinoma compared with normal tissues (2.4, 2.9 and
1.4, respectively; r2=0.711; P<0.001). Interestingly,
the Ki-67 index increased with more advanced cancer stages
(r2=0.50; P<0.001 adenoma vs. carcinoma; Table III). In addition, no correlation
between BMP-9 and Ki-67 expression in CRC was observed
(r2=−0.024; P>0.05; Table
IV).
 | Table III.Ki-67 expression in colorectal
carcinoma, adenoma and normal colorectal mucosa. |
Table III.
Ki-67 expression in colorectal
carcinoma, adenoma and normal colorectal mucosa.
|
| Ki-67 expression, n
(%) |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Tissue type | − | + | ++ | +++ | PR (%) |
r2-value | P-value |
|---|
| Normal colorectal
mucosa | 12 (18) | 24 (37) | 22 (34) | 7 (11) | 82 | 0.711 | P<0.001 |
| Colorectal
adenoma | 0 (0) | 6 (9) | 27 (42) | 32 (49) | 100 |
|
|
| Colorectal
carcinoma | 1 (2) | 0 (0) | 2 (3) | 62 (95) | 98 |
|
|
 | Table IV.Correlation between BMP-9 and Ki-67
expression in colorectal cancer. |
Table IV.
Correlation between BMP-9 and Ki-67
expression in colorectal cancer.
|
|
|
| BMP-9 expression,
n |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Intensity of Ki-67
expression |
| Number of patients,
n | − | + | ++ | +++ |
r2-value | P-value |
|---|
| Ki-67 expression,
n | – | 1 | 0 | 0 | 1 | 0 | −0.024 | 0.852 |
|
| + | 0 | 0 | 0 | 0 | 0 |
|
|
|
| ++ | 2 | 0 | 0 | 1 | 1 |
|
|
|
| +++ | 62 | 3 | 8 | 27 | 24 |
|
|
Correlation between BMP-9 expression
and the clinicopathological parameters of CRC
The expression of BMP-9 was significantly correlated
with age (r2=−0.329; P=0.007), but not with other
features, including sex, tumor location, gross tumor type,
differentiation, histological type, WHO classification, blood
vessel tumor emboli, TNM stage, and serum CEA and CA19-9 levels
(Table V). In the Oncomine dataset,
no significant association between BMP-9 mRNA and
clinicopathological parameters was observed (P>0.05). In TCGA
dataset, BMP-9 mRNA expression was not associated with the TNM
stage (P>0.05; Fig. 2F-I). In the
Kurashina dataset, BMP-9 expression was not associated with the
grade classifications and Dukes stages (P>0.05; Fig. 2D and E).
 | Table V.Association between BMP-9 expression
and the clinicopathological characteristics of patients with
colorectal cancer. |
Table V.
Association between BMP-9 expression
and the clinicopathological characteristics of patients with
colorectal cancer.
|
|
| BMP-9 expression,
n |
|
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | n | − | + | ++ | +++ |
r2-value | P-value |
|---|
| Sex |
|
|
|
|
| −0.146 | 0.246 |
|
Male | 47 | 3 | 5 | 18 | 21 |
|
|
|
Female | 18 | 0 | 3 | 11 | 4 |
|
|
| Age, years |
|
|
|
|
| −0.329 | 0.007 |
|
≤55 | 11 | 0 | 0 | 3 | 8 |
|
|
|
>55 | 54 | 3 | 8 | 26 | 17 |
|
|
| Site |
|
|
|
|
| 0.048 | 0.703 |
|
Rectum | 39 | 2 | 6 | 18 | 13 |
|
|
|
Left-side colon | 17 | 1 | 1 | 7 | 8 |
|
|
|
Right-side colon | 9 | 0 | 1 | 4 | 4 |
|
|
| Tumor gross
type |
|
|
|
|
| −0.067 | 0.596 |
|
Ulcerative type | 49 | 3 | 5 | 21 | 20 |
|
|
|
Elevated type | 16 | 0 | 3 | 8 | 5 |
|
|
|
Differentiation |
|
|
|
|
| −0.175 | 0.164 |
|
Poor | 6 | 0 | 0 | 3 | 3 |
|
|
|
Moderate | 54 | 2 | 6 | 26 | 20 |
|
|
|
High | 5 | 1 | 2 | 0 | 2 |
|
|
| Histological
type |
|
|
|
|
| −0.058 | 0.649 |
| Tubular
papillary | 16 | 1 | 0 | 10 | 5 |
|
|
|
Tubular | 44 | 1 | 6 | 19 | 18 |
|
|
|
Mucinous | 5 | 1 | 2 | 0 | 2 |
|
|
| WHO
classification |
|
|
|
|
| −0.023 | 0.856 |
|
Low | 53 | 2 | 6 | 25 | 20 |
|
|
|
High | 12 | 1 | 2 | 4 | 5 |
|
|
| Blood vessel tumor
emboli |
|
|
|
|
| −0.039 | 0.760 |
|
Positive | 7 | 0 | 1 | 3 | 3 |
|
|
|
Negative | 58 | 3 | 7 | 26 | 22 |
|
|
| TNM stage |
|
|
|
|
| −0.010 | 0.938 |
|
I–II | 46 | 2 | 6 | 20 | 18 |
|
|
|
III–IV | 19 | 1 | 2 | 9 | 7 |
|
|
| Serum CEA
levels |
|
|
|
|
| −0.035 | 0.805 |
|
High | 22 | 2 | 3 | 7 | 10 |
|
|
|
Normal | 30 | 1 | 3 | 16 | 10 |
|
|
| Serum CA19-9
levels |
|
|
|
|
| −0.110 | 0.476 |
|
High | 9 | 0 | 1 | 4 | 4 |
|
|
|
Normal | 35 | 2 | 5 | 16 | 12 |
|
|
Survival analysis
In the follow-up period of ≥3 years, the OS was 71%;
14 patients had succumbed, including 2 patients who underwent a
secondary operation. Of these 2 patients, one had undergone a
secondary operation for an intestinal fistula observed 1 month
after the initial procedure. The patient subsequently succumbed due
to infectious peritonitis and septic shock. For the other patient,
a secondary operation was conducted due to tumor relapse, which was
identified 3 months after the initial procedure. The patient
succumbed due to tumor progression and multiple organ metastasis 16
months after the primary surgery. Distant metastases were observed
in 3 other patients; in the liver (after 11 months), the brain
(after 25 months) and the bones (after 38 months), respectively. In
addition, locoregional relapse was observed in 2 patients after 14
and 26 months, respectively.
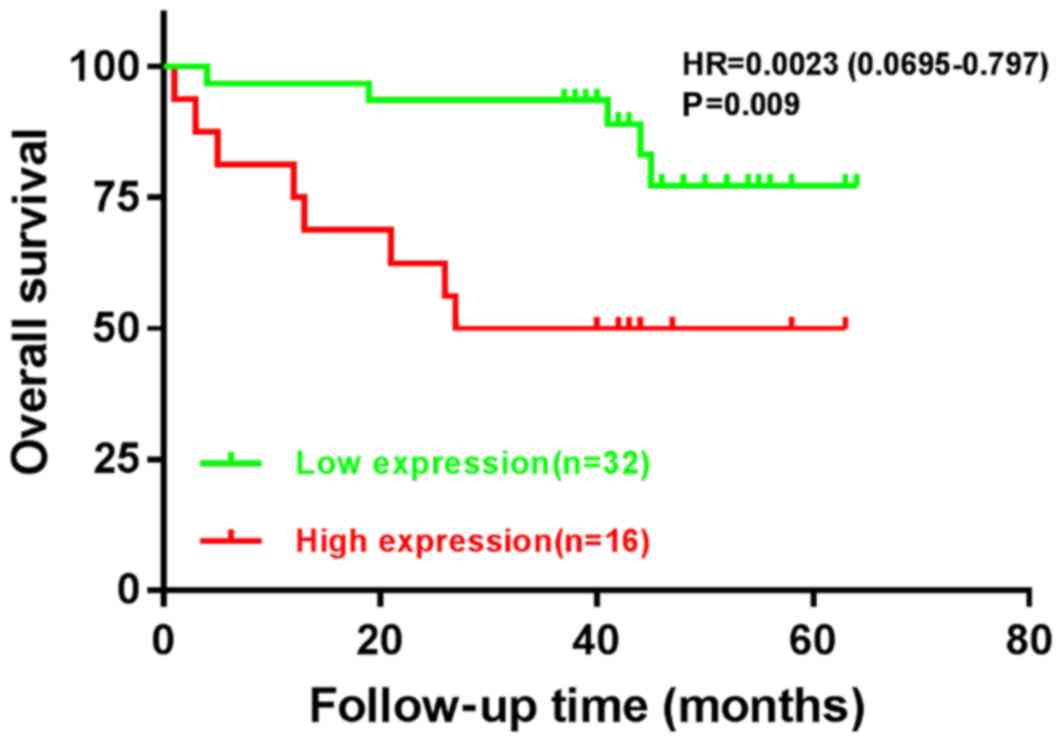
In order to assess the association between the
expression levels of BMP-9 and OS in colorectal carcinoma, BMP-9
expression was categorized into low- and high-expression groups
according to the mean of their IHC scores. A total of 32 patients
(67%) had low BMP-9 expression levels, and 16 patients (33%)
exhibited high BMP-9 expression. Low BMP-9 expression was
significantly associated with improved OS; the mean survival time
for such patients was 56.4 months with a 95% confidence interval
(CI) of 50.8–62.0. On the contrary, high BMP-9 expression was
associated with a mean survival time of 38.3 months with a 95% CI
of 25.7–50.8, whjch was significantly lower than the low-expression
group (P=0.009; Table VI; Fig. 3).
 | Table VI.Univariate analysis for BMP-9
expression and clinicopathological variables in patients with
colorectal cancer. |
Table VI.
Univariate analysis for BMP-9
expression and clinicopathological variables in patients with
colorectal cancer.
|
Characteristics | Number of patients,
n | Mean survival time,
months | 95% CI | P-value |
|---|
| BMP-9
expression |
| 50.485 | 44.330–56.640 | 0.009 |
|
Low | 32 | 56.420 | 50.838–62.001 |
|
|
High | 16 | 38.250 | 25.675–50.825 |
|
| Sex |
| 50.485 | 44.330–56.640 | 0.682 |
|
Male | 33 | 48.821 | 40.753–56.888 |
|
|
Female | 15 | 50.023 | 43.343–56.721 |
|
| Age, years |
| 50.485 | 44.330–56.640 | 0.404 |
|
≤55 | 7 | 43.143 | 25.195–61.091 |
|
|
>55 | 41 | 50.929 | 44.598–57.260 |
|
| Tumor site |
| 50.485 | 44.330–56.640 | 0.066 |
|
Rectum | 28 | 53.415 | 46.560–60.270 |
|
|
Left-side colon | 14 | 47.071 | 36.059–58.083 |
|
|
Right-side colon | 6 | 31.833 | 13.128–50.539 |
|
| Gross tumor
type |
| 50.485 | 44.330–56.640 | 0.083 |
|
Ulcerative type | 37 | 46.533 | 39.125–53.941 |
|
|
Elevated type | 11 | 61.909 | 58.002–65.817 |
|
|
Differentiation |
| 50.485 | 44.330–56.640 | 0.035 |
|
Poor | 5 | 30.000 | 11.555–48.445 |
|
|
Moderate | 38 | 53.690 | 47.209–60.171 |
|
|
High | 5 | 41.400 | 26.588–56.212 |
|
| Histological
type |
| 50.485 | 44.330–56.640 | 0.353 |
| Tubular
papillary | 11 | 52.176 | 42.042–62.291 |
|
|
Tubular | 32 | 51.031 | 43.061–59.001 |
|
|
Mucinous | 5 | 41.400 | 26.588–56.212 |
|
| WHO
classification |
| 50.485 | 44.330–56.640 | 0.035 |
|
Low | 37 | 53.346 | 46.691–60.002 |
|
|
High | 11 | 39.818 | 26.680–52.956 |
|
| Blood vessel tumor
embolus |
| 50.485 | 44.330–56.640 | <0.001 |
|
Positive | 43 | 54.003 | 48.395–59.611 |
|
|
Negative | 5 | 16.000 | 4.046–27.954 |
|
| TNM stage |
| 50.485 | 44.330–56.640 | 0.022 |
|
I–II | 32 | 54.088 | 47.489–60.686 |
|
|
III–IV | 16 | 41.193 | 29.813–52.572 |
|
| Serum CEA
levels |
| 49.350 | 42.681–56.018 | 0.185 |
|
High | 21 | 54.420 | 44.365–56.475 |
|
|
Normal | 18 | 44.222 | 32.885–55.559 |
|
| Serum CA19-9
levels |
| 45.344 | 38.817–51.871 | 0.073 |
|
High | 26 | 48.793 | 42.597–54.990 |
|
|
Normal | 7 | 32.429 | 15.235–49.622 |
|
In the univariate analyses, certain
clinicopathological parameters, including tumor differentiation,
WHO classification, blood vessel cancer embolus and TNM staging
were significant predictors of a poor prognosis (P=0.035, 0.035,
<0.001 and 0.022, respectively; Table VI). Sex, age, tumor location, gross
tumor type, histological type, serum CEA and CA 19-9 levels were
not associated with the prognosis of patients (all P>0.05;
Table VI).
Furthermore, BMP-9 expression was independently
associated with poor prognosis in CRC (P=0.044) as determined by
multivariate analyses, which indicated the prognostic significance
of BMP-9 expression in CRC [hazard ratio (HR), 3.14; 95% CI,
1.03–9.57; Table VII].
 | Table VII.Multivariate Cox regression analysis
for overall survival in patients with colorectal cancer. |
Table VII.
Multivariate Cox regression analysis
for overall survival in patients with colorectal cancer.
|
Characteristics | HR | 95% CI | P-value |
|---|
| BMP-9
expression | 3.143 | 1.032–9.571 | 0.044 |
|
Differentiation | 1.021 | 0.435–2.395 | 0.962 |
| WHO
classification | 1.044 | 0.232–4.705 | 0.955 |
| Blood vessel tumor
embolus | 5.142 | 0.866–30.315 | 0.072 |
| TNM stage | 1.896 | 0.506–7.103 | 0.343 |
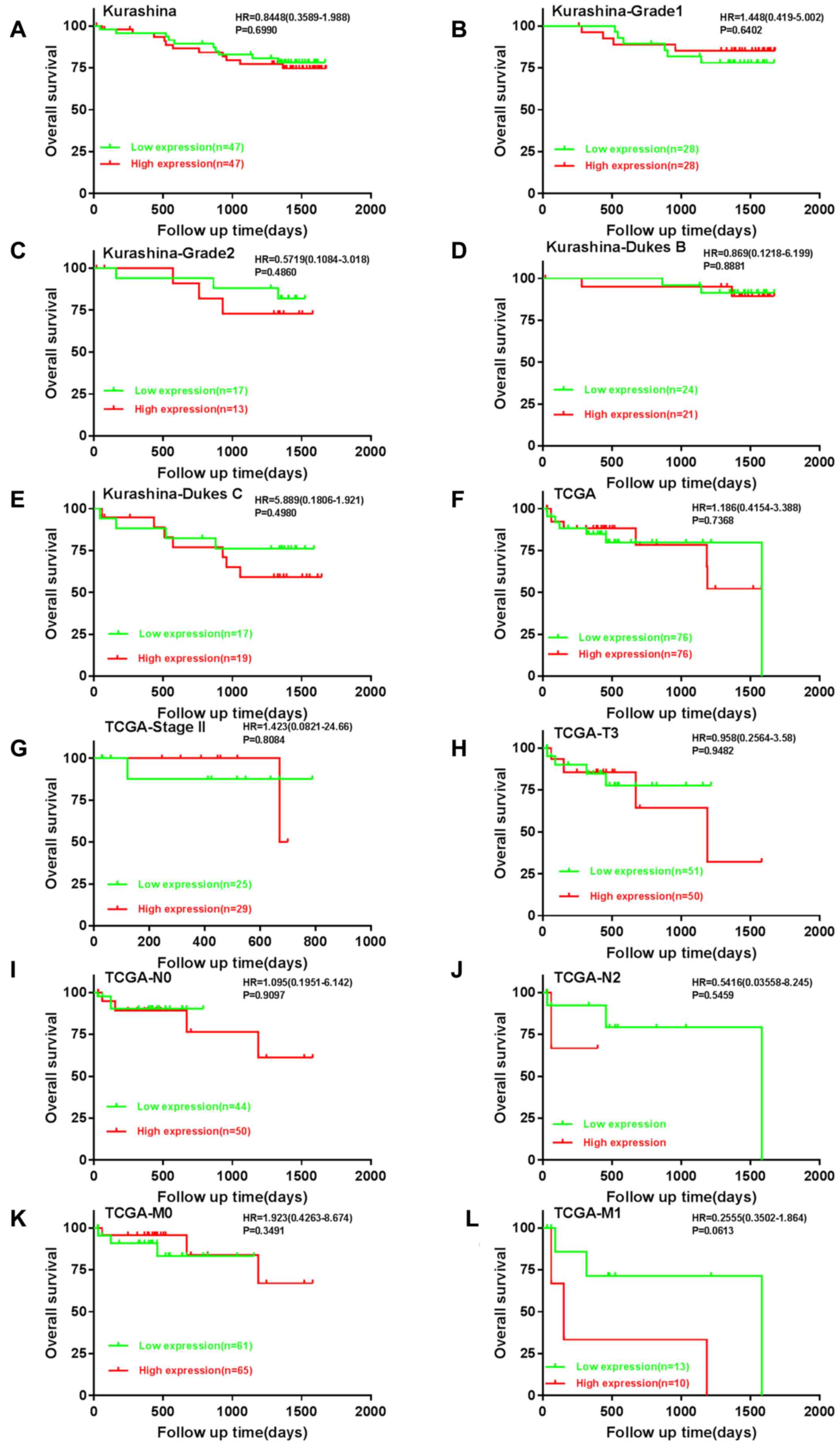
Bioinformatics analysis using the available datasets
confirmed the observations that patients with CRC and high BMP-9
mRNA expression exhibited decreased OS time compared with those
with low expression (Fig. 4A-L).
Discussion
BMP-9 belongs to the TGF-β family and serves as a
multifunctional mediator in numerous biological processes under
physiological and pathophysiological conditions, particularly in
tumorigenesis (10–19). The expression of BMP-9 and its
tumorigenic and antitumorigenic roles notably varies according to
the type of tumor (22,23,25,28). To
the best of our knowledge, the present study is the first to
investigate the role of BMP-9 in the various stages of colorectal
tumorigenesis, a process observed in the majority of types of
colorectal tumor (3,34,35), by
analyzing matched normal mucosa, adenoma and carcinoma samples. The
present study demonstrated that the expression of BMP-9
sequentially increased in normal mucosa, adenoma and carcinoma.
These results suggested that BMP-9 may be an important
protumorigenic factor in the development of CRC. In addition, it
was revealed that BMP-9 may serve as an independent prognostic
factor for patients with CRC, and that high BMP-9 expression was
significantly associated with poor prognosis and decreased survival
rate.
BMP-9 was first detected in fetal mouse liver, and
it can bind to activin receptor-like kinase 1, a TGF family type 1
receptor, to serve an important role in angiogenesis (10,19). It
is well known that angiogenesis is a critical process in
tumorigenesis (36). Recently, Na
et al (20) reported that
BMP-9 was upregulated in papillary thyroid carcinoma compared with
normal follicular cells (P<0.001), regardless of bone formation.
Similarly, Herrera et al (21) detected BMP-9 expression in epithelial
ovarian cancer cells, but not in normal human ovarian surface
epithelial cells. The results of the present study suggested an
increase in BMP-9 expression with advancing stages of colorectal
tumorigenesis. Conversely, Ren et al (27) revealed that BMP-9 may function as a
tumor suppressor in breast cancer cells in vitro and in
vivo. A similar role was also reported for BMP-9 in prostate
cancer (24,37), osteosarcoma (38) and myeloma (25). These discrepancies require further
investigation; however, they indicate that the role of BMP-9 may be
tissue- and tumor-specific.
BMP signaling has been investigated in the
development of colorectal carcinogenesis (39–41).
Although research has been performed following the detection of a
germline mutation of bone morphogenetic protein receptor type 1A in
patients with juvenile polyposis syndrome, the precise role of BMP
signaling in CRC remains unclear (42,43).
BMP-2 and BMP-3 were determined to have growth-suppressive
activities in colon cancer cells (8,44).
However, Yokoyama et al (6)
reported that the expression levels of BMP-4 in human CRC cells and
tissues were upregulated when compared with those in normal
epithelium or adenoma, while inhibition of BMP-4 promoted the
apoptosis of CRC cells in vitro and in vivo. These
findings were consistent with the reports of the present study.
Furthermore, Yuan et al (45)
demonstrated that BMP-9 was essential for the antiproliferative
effects of resveratrol on human colon cancer. Conversely,
Lorente-Trigos et al (46)
revealed that BMP signaling promoted the growth of primary human
colon carcinomas in vivo. Whether the inconsistency in these
reports is due to context differences or cross-talk involving other
signaling pathways in colorectal tumorigenesis at different stages
requires further investigation.
Notably, in the present study, the expression of
BMP-9 gradually increased within the cytoplasm or/and membrane in
tissues of advancing tumor stages, from normal colorectal mucosa to
adenoma and carcinoma. This suggested a potential role of high
cytoplasmic or membranous BMP-9 expression in colorectal
tumorigenesis. Similarly, an increase of galectin 3 in the
cytoplasm has been associated with the progression of CRC (47). Miyata et al (48) also observed a similar correlation
between the enhanced expression of ELAV like RNA binding protein 1
in the cytoplasm, and the aggressiveness and poor prognosis of
bladder cancer. However, due to the limitations of IHC staining in
the present study, the correlation between the cytoplasmic
expression of BMP-9 and poor prognosis in patients with CRC could
not be assessed.
In order to evaluate the prognostic value of BMP-9
expression in patients with CRC, a Kaplan-Meier univariate survival
analysis was performed in the present study, which revealed an
association between high BMP-9 expression levels and poor prognosis
in patients with this disease. When the Cox proportional hazards
model was generated, high BMP-9 expression levels were determined
to be an independent factor for predicting the unfavorable
prognosis of patients with CRC. These results suggested that BMP-9
may be a prognostic marker for patients with this disease.
Numerous proteins or genes identified thus far have
been used as prognostic biomarkers for various types of CRC
(49,50); however, improvements are required.
Considering the results from the present study, BMP-9 may be a
valuable addition to the clinical management of patients with CRC
and could contribute to the improvement of treatment outcomes.
An increasing number of studies have focused on
BMP-9 as a therapeutic target for the treatment of cancer,
particularly tumor angiogenesis (51,52). In
light of the results from the present study, the development of a
novel therapeutic strategy targeting BMP-9 in CRC is warranted.
The primary limitation of the present study was the
evaluation of BMP-9 expression using IHC staining. The
semi-quantitative nature of this analysis may have negatively
influenced its statistical power. Therefore, reverse
transcription-quantitative PCR should be performed in order to
confirm the results of the present study, along with a more
thorough correlation analysis. Furthermore, other limitations
included the small sample size employed, and the lack
recurrence-free and disease-free survival data.
The findings of the present study suggested that
increased BMP-9 expression may serve an important role in promoting
colorectal tumorigenesis by driving the transformation of
colorectal normal mucosa to adenoma, and subsequent carcinoma. In
addition, upregulated BMP-9 levels may be an independent predictor
of poor prognosis for patients with CRC. Thus, modulating BMP-9
activity may be considered as a novel therapeutic strategy in the
treatment of CRC; however, further investigation is warranted to
elucidate the mechanism underlying the protumorigenic effects of
BMP-9 in the disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the Oncomine and TCGA databases:
oncomine.org/;ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE11417;
ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE20842; and
cancergenome.nih.gov/.
Authors' contributions
HS and HZ designed the study. YF designed the study
and wrote the manuscript. YF and WW collected the data. YF, LG and
WW performed the experiments. YF and CJ analyzed the data. All
authors read and approved the final manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the First Affiliated Hospital of Jinzhou Medical University
(Jinzhou, China). The Ethics Committee waived the need for written
informed consent due to the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vincent MD: Cancer: Beyond speciation. Adv
Cancer Res. 112:283–350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Terzić J, Grivennikov S, Karin E and Karin
M: Inflammation and colon cancer. Gastroenterology. 138:2101–2114.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yokoyama Y, Watanabe T, Tamura Y,
Hashizume Y, Miyazono K and Ehata S: Autocrine BMP-4 signaling is a
therapeutic target in colorectal cancer. Cancer Res. 77:4026–4038.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyazono K, Kamiya Y and Morikawa M: Bone
morphogenetic protein receptors and signal transduction. J Biochem.
147:35–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Chen X, Qiao M, Zhang BQ, Wang N,
Zhang Z, Liao Z, Zeng L, Deng Y, Deng F, et al: Bone morphogenetic
protein 2 inhibits the proliferation and growth of human colorectal
cancer cells. Oncol Rep. 32:1013–1020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen D, Zhao M, Harris SE and Mi Z: Signal
transduction and biological functions of bone morphogenetic
proteins. Front Biosci. 9:349–358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Celeste AJ: Bone morphogenetic protein-9,
a new member of the TGF-β superfamily. J Bone Miner Res. 1(9):
S1361994.
|
|
11
|
Wagner DO, Sieber C, Bhushan R, Börgermann
JH, Graf D and Knaus P: BMPs: From bone to body morphogenetic
proteins. Sci Signal. 3:mr12010.PubMed/NCBI
|
|
12
|
Plouhinec JL, Zakin L and De Robertis EM:
Systems control of BMP morphogen flow in vertebrate embryos. Curr
Opin Genet Dev. 21:696–703. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reddi AH and Reddi A: Bone morphogenetic
proteins (BMPs): From morphogens to metabologens. Cytokine Growth
Factor Rev. 20:341–342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ploemacher RE, Engels LJ, Mayen AE, Thies
S and Neben S: Bone morphogenetic protein 9 is a potent synergistic
factor for murine hemopoietic progenitor cell generation and colony
formation in serum-free cultures. Leukemia. 13:428–437. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Helm GA, Alden TD, Beres EJ, Hudson SB,
Das S, Engh JA, Pittman DD, Kerns KM and Kallmes DF: Use of bone
morphogenetic protein-9 gene therapy to induce spinal arthrodesis
in the rodent. J Neurosurg. 92 (Suppl):S191–S196. 2000.
|
|
16
|
Lopez-Coviella I, Berse B, Krauss R, Thies
RS and Blusztajn JK: Induction and maintenance of the neuronal
cholinergic phenotype in the central nervous system by BMP-9.
Science. 28:313–316. 2000. View Article : Google Scholar
|
|
17
|
Chen C, Grzegorzewski KJ, Barash S, Zhao
Q, Schneider H, Wang Q, Singh M, Pukac L, Bell AC, Duan R, et al:
An integrated functionalgenomics screening program reveals a role
for BMP-9 in glucose homeostasis. Nat Biotechnol. 21:294–301. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Truksa J, Peng HF, Lee P and Beutler E:
Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1
expression independently of Hfe, transferrin receptor 2 (Tfr2), and
IL-6. Proc Natl Acad Sci USA. 103:10289–10293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
David L, Mallet C, Mazerbourg S, Feige JJ
and Bailly S: Identification of BMP9 and BMP10 as functional
activators of the orphan activin receptor-like kinase 1 (ALK1) in
endothelial cells. Blood. 109:1953–1961. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Na KY, Kim HS, Lee SK, Jung WW, Sung JY,
Kim YW and Park YK: Papillary thyroid carcinoma with bone
formation. Pathol Res Pract. 209:14–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Herrera B, van Dinther M, Ten Dijke P and
Inman GJ: Autocrine bone morphogenetic protein-9 signals through
activin receptor-like kinase-2/Smad1/Smad4 to promote ovarian
cancer cell proliferation. Cancer Res. 69:9254–9262. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herrera B, García-Álvaro M, Cruz S, Walsh
P, Fernández M, Roncero C, Fabregat I, Sánchez A and Inman GJ: BMP9
is a proliferative and survival factor for human hepatocellular
carcinoma cells. PLoS One. 8:e695352013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Q, Gu X, Weng H, Ghafoory S, Liu Y,
Feng T, Dzieran J, Li L, Ilkavets I and Kruithof-de Julio M: Bone
morphogenetic protein-9 induces epithelial to mesenchymal
transition in hepatocellular carcinoma cells. Cancer Sci.
104:398–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ye L, Kynaston H and Jiang WG: Bone
morphogenetic protein-9 induces apoptosis in prostate cancer cells,
the role of prostate apoptosis response-4. Mol Cancer Res.
6:1594–1606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Olsen OE, Wader KF, Misund K, Våtsveen TK,
Rø TB, Mylin AK, Turesson I, Størdal BF, Moen SH, Standal T, et al:
Bone morphogenetic protein-9 suppresses growth of myeloma cells by
signaling through ALK2 but is inhibited by endoglin. Blood Cancer
J. 4:e1962014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wan S, Liu Y, Weng Y, Wang W, Ren W, Fei
C, Chen Y, Zhang Z, Wang T, Wang J, et al: BMP9 regulates
cross-talk between breast cancer cells and bone marrow-derived
mesenchymal stem cells. Cell Oncol (Dordr). 37:363–375. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ren W, Liu Y, Wan S, Fei C, Wang W, Chen
Y, Zhang Z, Wang T, Wang J, Zhou L, et al: BMP9 inhibits
proliferation and metastasis of HER2-positive SK-BR-3 breast cancer
cells through ERK1/2 and PI3K/AKT pathways. PLoS One. 9:e968162014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ren W, Sun X, Wang K, Feng H, Liu Y, Fei
C, Wan S, Wang W, Luo J, Shi Q, et al: BMP9 inhibits the bone
metastasis of breast cancer cells by downregulating CCN2
(connective tissue growth factor, CTGF) expression. Mol Biol Rep.
41:1373–1383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
America Joint Committee on Cancer, . The
7th edition of the AJCC cancer staging manual. 7th. New York:
Springer; 2010
|
|
30
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sarma DP: Dukes' classification of rectal
cancer. South Med J. 81:407–408. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kurashina K, Yamashita Y, Ueno T, Koinuma
K, Ohashi J, Horie H, Miyakura Y, Hamada T, Haruta H, Hatanaka H,
et al: Chromosome copy number analysis in screening for prognosis-
related genomic regions in colorectal carcinoma. Cancer Sci.
99:1835–1840. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gaedcke J, Grade M, Jung K, Camps J, Jo P,
Emons G, Gehoff A, Sax U, Schirmer M, Becker H, et al: Mutated KRAS
results in overexpression of DUSP4, a MAP-kinase phosphatase, and
SMYD3, a histone methyltransferase, in rectal carcinomas. Genes
Chromosomes Cancer. 49:1024–1034. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Muto T, Bussey HJ and Morson BC: The
evolution of cancer of the colon and rectum. Cancer. 36:2251–2270.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kinzler KW and Vogelstein B: Lessons from
hereditary colorectal cancer. Cell. 87:159–170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Herrera B, Dooley S and Breitkopf-Heinlein
K: Potential roles of bone morphogenetic protein (BMP)-9 in human
liver diseases. Int J Mol Sci. 15:5199–5220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Astrologo L, Zoni E, Karkampouna S, Gray
PC, Klima I, Grosjean J, Goumans MJ, Hawinkels LJAC, van der Pluijm
G, Spahn M, et al: ALK1Fc Suppresses the human prostate cancer
growth in in vitro and in vivo preclinical models. Front Cell Dev
Biol. 5:1042017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lv Z, Yang D, Li J, Hu M, Luo M, Zhan X,
Song P, Liu C, Bai H, Li B, et al: Bone morphogenetic protein 9
overexpression reduces osteosarcoma cell migration and invasion.
Mol Cells. 36:119–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hardwick JC, Kodach LL, Offerhaus GJ and
van den Brink GR: Bone morphogenetic protein signalling in
colorectal cancer. Nat Rev Cancer. 8:806–812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Allaire JM, Roy SA, Ouellet C, Lemieux É,
Jones C, Paquet M, Boudreau F and Perreault N: Bmp signaling in
colonic mesenchyme regulates stromal microenvironment and protects
from polyposis initiation. Int J Cancer. 138:2700–2712. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Voorneveld PW, Kodach LL, Jacobs RJ, van
Noesel CJ, Peppelenbosch MP, Korkmaz KS, Molendijk I, Dekker E,
Morreau H, van Pelt GW, et al: The BMP pathway either enhances or
inhibits the Wnt pathway depending on the SMAD4 and p53 status in
CRC. Br J Cancer. 112:122–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Howe JR, Bair JL, Sayed MG, Anderson ME,
Mitros FA, Petersen GM, Velculescu VE, Traverso G and Vogelstein B:
Germline mutations of the gene encoding bone morphogenetic protein
receptor 1A in juvenile polyposis. Nat Genet. 28:184–187. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Davis H, Raja E, Miyazono K, Tsubakihara Y
and Moustakas A: Mechanisms of action of bone morphogenetic
proteins in cancer. Cytokine Growth Factor Rev. 27:81–92. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Loh K, Chia JA, Greco S, Cozzi SJ,
Buttenshaw RL, Bond CE, Simms LA, Pike T, Young JP, Jass JR, et al:
Bone morphogenetic protein 3 inactivation is an early and frequent
event in colorectal cancer development. Genes Chromosomes Cancer.
47:449–460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yuan SX, Wang DX, Wu QX, Ren CM, Li Y,
Chen QZ, Zeng YH, Shao Y, Yang JQ, Bai Y, et al: BMP9/p38 MAPK is
essential for the antiproliferative effect of resveratrol on human
colon cancer. Oncol Rep. 35:939–947. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lorente-Trigos A, Varnat F, Melotti A,
Ruiz I and Altaba A: BMP signaling promotes the growth of primary
human colon carcinomas in vivo. J Mol Cell Biol. 2:318–332. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sanjuan X, Fernández PL, Castells A,
Castronovo V, van den Brule F, Liu FT, Cardesa A and Campo E:
Differential expression of galectin 3 and galectin 1 in colorectal
cancer progression. Gastroenterology. 113:1906–1915. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Miyata Y, Watanabe S, Sagara Y, Mitsunari
K, Matsuo T, Ohba K and Sakai H: High expression of HuR in
cytoplasm, but not nuclei, is associated with malignant
aggressiveness and prognosis in bladder cancer. PLoS One.
8:e590952013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Steinke-Lange V and Holinski-Feder E:
Genetic screening and personalized prevention in colorectal cancer.
Visc Med. 35:226–230. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
De Roock W, De Vriendt V, Normanno N,
Ciardiello F and Tejpar S: KRAS, BRAF, PIK3CA, and PTEN mutations:
Implications for targeted therapies in metastatic colorectal
cancer. Lancet Oncol. 12:594–603. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yoshimatsu Y, Lee YG, Akatsu Y, Taguchi L,
Suzuki HI, Cunha SI, Maruyama K, Suzuki Y, Yamazaki T, Katsura A,
et al: Bone morphogenetic protein-9 inhibits lymphatic vessel
formation via activin receptor-like kinase 1 during development and
cancer progression. Proc Natl Acad Sci USA. 110:18940–18945. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cunha SI and Pietras K: ALK1 as an
emerging target for antiangiogenic therapy of cancer. Blood.
117:6999–7006. 2011. View Article : Google Scholar : PubMed/NCBI
|