Introduction
Cervical cancer was reported as the most common
gynecological tumor in developing countries in 2012 (1). It was also estimated that 527,600 women
were first diagnosed with cervical cancer and 265,700 women
succumbed to this disease worldwide in the same year (1). In recent years, cervical cytology
screening and vaccination have been used to prevent cervical cancer
development due to human papillomavirus (HPV) infection (2). However, thousands of women are still
affected by cervical cancer, with detrimental effects on their
quality of life (3). Alongside the
increased amount of research that makes the molecular mechanism
underlying cervical cancer more clear, particularly with the
knowledge that HPV and E6 oncoproteins potentially affect the
progress of cervical cancer, an increasing number of genes have
been demonstrated to be implicated in cervical cancer (4,5).
Identifying new molecular targets for the prevention and treatment
of cervical cancer would further improve the quality of life of the
patients.
Runt-related transcription factor 3 (RUNX3),
along with RUNX1 and RUNX2, are members of the Runt
domain family (6). RUNX3 is
involved in the transforming growth factor β (TGF-β) signaling
pathway, which is a key downstream effector affecting the
progression of tumors (7). In a
number of previous studies on gastric, hepatocellular and breast
cancer, RUNX3 has been revealed to play a significant role
in tumor suppression (8–11). Recent research has demonstrated that
RUNX3 may also act as a tumor suppressor gene in cervical
cancer (12). RUNX3
expression may be suppressed by promoter hypermethylation, gene
deletions, inactivating mutations and protein mislocalization
(8,11,13–16). By
contrast, other researchers concluded that RUNX3 acts as an
oncogene in skin, ovarian and head and neck cancer, where its
expression level was observed as significantly increased (17–20). In
addition, Lotem et al (21)
hypothesized that this gene plays important roles in immunity and
inflammation, and may affect the development of epithelial
tumors.
In a previous study, it was observed that the
polymorphisms of RUNX3 may be associated with cervical
cancer, and the mRNA expression of RUNX3 was significantly
different between the cervical cancer group and the healthy female
subjects (22). However, the
specific molecular mechanism through which RUNX3 regulates
cancer-associated signaling pathways and affects tumorigenesis
remains elusive. The aim of the present study was to investigate
the effect of RUNX3 on cervical cancer cell lines, and to
identify the transcriptome changes in cervical cancer.
Materials and methods
Cell culture
SiHa, HeLa and C33A cells were obtained from the
Laboratory of Molecular Translational Medicine, West China
Institute of Women and Children's Health, Key Laboratory of
Obstetric & Gynecological and Pediatric Diseases and Birth
Defects of Ministry of Education, West China Second University
Hospital, Sichuan University (Sichuan, China). SiHa is an HPV-16
infected cervical cancer cell line, HeLa is an HPV-18 infected
cervical cell line, and C33A is an HPV-negative cervical cancer
cell line. The cells were cultured in DMEM high-glucose medium at
37°C in 5% CO2, supplemented with 10% fetal bovine serum
and penicillin/streptomycin (50 U/ml) (both Thermo Fisher
Scientific, Inc.). There was no contamination of SiHa and C33A
cells by HeLa cells.
Construction of plasmids, RNA
interference and transfection
Homo sapiens RUNX3 mRNA sequence (23) was used to design and identify the
RUNX3 overexpression model and empty plasmid (plasmid
EX-NEG-M98; Guangzhou FulenGen Co., Ltd.), was used as the control;
in addition, RUNX3 short-hairpin RNA (shRNA) was designed
and identified (Guangzhou FulenGen Co., Ltd.). The following base
pairs of shRNA (shRNA31, shRNA32, shRNA33 and shRNA34) and the
non-targeting sequence control shRNA (shRNA001) were used for
RUNX3 gene interference (Table
I). After the single clones were obtained, the extracted
plasmids were identified and sequenced via digestion, following the
instructions of endotoxin-free plasmid extraction kit (Tiangen
Biotech Co., Ltd.). Briefly, 8 ml buffer P1 with RNase A was added
to the collected bacteria and vortexed. Subsequently, 8 ml buffer
P2 was added, and the samples were shaken gently and left to stand
at 25°C for 5 min. This was repeated with buffer P4, after which
the samples were centrifuged at 8,228 × g for 10 min at 25°C.
Following which, 0.3 of the volume of isopropanol and 2.5 ml buffer
BL was added, and the samples were transferred to CP6 columns and
centrifuged at 8,228 × g for 2 min at 25°C, twice. A total of 10 ml
buffer PW with absolute ethanol was added and the samples were
centrifuged at 8,228 g for 2 min at 25°C, twice. Subsequently,
cervical cancer cells were transfected with RUNX3
overexpression plasmid or shRNA (0.5 µg plasmid) by X-tremeGene HP
DNA Transfection Reagent (Roche Diagnostics). In the present study,
cells with RUNX3 overexpression were referred to as ‘ORF’
RUNX3 cells, the empty plasmid was the control of ORF
RUNX3 cells as ‘NEG’ control cells, and those with
RUNX3 shRNA as ‘shRNA’ RUNX3 cells. In addition, the
control groups were treated only with transfection reagent in SiHa,
HeLa and C33A cells, respectively which were referred to as
‘Control’, and without any intervention as ‘cell line name’
(baseline controls). At 24, 48 and 72 h after transfection, the
cells were harvested and prepared for subsequent experiments.
 | Table I.shRNAs used in the present study. |
Table I.
shRNAs used in the present study.
| Clone name | Symbol | Chromosome
location | Length | 5′-3′ |
|---|
|
HSH021393-31-LVRH1GP(OS503663) | RUNX3 | 815 | 21 |
GGCAATGACGAGAACTACTCC |
|
HSH021393-32-LVRH1GP(OS545091) | RUNX3 | 159 | 21 |
GGAATCCAAATTCTTGGGTAC |
|
HSH021393-33-LVRH1GP(OS503664) | RUNX3 | 2895 | 21 |
GGTCTCTTACAGGTATAGTTC |
|
HSH021393-34-LVRH1GP(OS545092) | RUNX3 | 3858 | 21 |
GGGATAGTAAATAAATTGCTC |
|
CSHCTR001-1-LVRH1GP(OSNEG20) |
|
| 19 |
GCTTCGCGCCGTAGTCTTA |
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR) analysis
Total RNA was extracted from cervical cancer cells
and purified using TRIzol® reagent according to the
manufacturer's protocol (Thermo Fisher Scientific, Inc.). RT-PCR
was performed using a One-Step RT-PCR kit (Bioneer), according to
the manufacturer's protocol. RT-qPCR was performed using the SYBR
Green PCR Master Mix (Roche Diagnostics). The samples of all genes
were amplified in a thermocycler as follows: 95°C for 10 min (1
cycle), 95°C for 15 sec and 60°C for 1 min (48 cycles). The primer
sequences are presented in Table
II. Data were normalized against β-actin expression with the
comparative quantification cycle method. Triplicate Cq values were
averaged and the relative expression levels were determined as
2−ΔΔCq (24).
 | Table II.Primer sequences used in the present
study. |
Table II.
Primer sequences used in the present
study.
| Gene | Gene ID | Forward
(5′-3′) | Reverse
(5′-3′) | Product size,
bp |
|---|
| TNC | NM_002160.3 |
TCGCTACAAGCTGAAGGTGG |
GTTAACGCCCTGACTGTGGT | 214 |
| PTGS2 | NM_000963.3 |
CAAATTGCTGGCAGGGTTGC |
AGGGCTTCAGCATAAAGCGT | 139 |
| ICAM1 | NM_000201.2 |
ATGGCAACGACTCCTTCTCG |
GCCGGAAAGCTGTAGATGGT | 142 |
| TNF
SF10 | NM_001190942.1 |
TGCGTGCTGATCGTGATCTT |
TCTTGGAGTCTTTCTAACGAGC | 234 |
| IL6 | NM_000600.3 |
TTCAATGAGGAGACTTGCCTGG |
CTGGCATTTGTGGTTGGGTC | 206 |
| IL7R | NM_002185.3 |
TGAAATATGTGGGGCCCTCG |
GTCATTGGCTCCTTCCCGAT | 223 |
| FOSL1 | NM_005438.4 |
AGCCCAGCAGAAGTTCCAC |
CCTCTTCCTCCGGGCTGAT | 227 |
| IL32 | NM_001308078 |
AGACAGTGGCGGCTTATTATGAGG |
GCCTCGGCACCGTAATCC | 86 |
| TGF-β | NM_000660.4 |
TATCGACATGGAGCTGGTGAAG |
CAGCTTGGACAGGATCTGGC | 67 |
| β-actin | NM_001101.3 |
TGACGTGGACATCCGCAAAG |
CTGGAAGGTGGACAGCGAGG | 205 |
WST-1 measurement and flow
cytometry
WST-1 measurement was used to detect cell viability
using a WST-1 cell proliferation and cytotoxicity assay kit (Boster
Biological Technology Co., Ltd.) according to the manufacturer's
protocol. Briefly, the cervical cancer cells were seeded at a
density of 104 cells/ml into 96-well plates and
incubated overnight at 37°C. Subsequently, the plasmids were
transfected into cancer cells for 24/48/72 h 3 times, as
aforementioned. WST-1 (10 µl) was added, followed by incubation for
a further 2 h at 37°C. In order to exclude the effect of the WST-1
reagent, the same concentrations of transfection reagent were added
to the cells directly when the WST-1 measurement was performed. The
absorbance of cells was monitored at 450 nm.
Apoptosis was also analyzed via flow cytometry.
First, the transfected tumor cells in each group were lysed with
trypsin without EDTA (HyClone; GE Healthcare Life Sciences), and
the cells were stained using the FITC Annexin V Apoptosis Detection
kit with propidium iodide (PI; both BestBio, http://bestbio.bioon.com.cn/). Finally, for the cell
cycle analysis, cells in each group were stained with PI and then
analyzed by flow cytometry (Guava® easyCyte™), using
nCyte v2.7 software (both EMD Millipore; Merck KGaA).
Transcriptome sequencing
A total of 3 µg RNA from each sample was used, which
was prepared for input material of the RNA samples. Sequencing
libraries were generated, using the NEBNext® Ultra™ RNA
Library Prep kit for Illumina® (New England Biolabs)
according to the manufacturer's protocol. The index codes were
added to assign sequences to each sample. Briefly, mRNA was
purified from total RNA using poly-T oligo-attached magnetic beads
NEBNext® Ultra™ Directional RNA Library Prep kit for
Illumina® (New England Biolabs, Inc.) according to the
manufacturer's protocol. Fragmentation was then performed using
divalent cations at increasing temperatures of 25°C for 10 min,
42°C for 15 min, and 70°C for 15 min after which the samples were
held at 4°C, in NEBNext First-Strand Synthesis Reaction Buffer (5X)
(New England Biolabs, Inc.). Finally, the PCR products were
purified (AMPure XP system), and the library quality was assessed
on the Agilent Bioanalyzer 2100 system. According to the
manufacturer's instructions, clustering of the index-coded samples
was performed on a cBot Cluster Generation System, which used a
TruSeq PE Cluster kit v3-cBot-HS (Illumina, Inc.). The library
preparations were then sequenced on an Illumina Hiseq 2000/2500
platform, and 100/50 bp single-end reads were generated. Gene
Ontology (GO; http://geneontology.org) enrichment
analysis of differentially expressed genes was implemented by the
GOseq R package (clusterProfiler v3.4.4; http://bioconductor.org/), in which gene length bias
was corrected. GO terms with corrected P<0.05 were considered
significantly enriched by differentially expressed genes. The Kyoto
Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg) is a database resource for
understanding high-level functions and utilities of the biological
system, such as the cell, the organism and the ecosystem, from
molecular-level information, particularly from large-scale
molecular datasets generated by genome sequencing and other
high-throughput experimental technologies. The present study used
clusterProfiler v3.4.4 software (http://bioconductor.org/) in order to test the
statistical enrichment of differentially expressed genes in the
KEGG pathways. Through this analysis, different genes were
identified as potential regulators of cervical cancer, which may be
downstream or upstream of RUNX3.
Statistical analysis
GraphPad Prism software (version 5.01; GraphPad
Software, Inc.) was used for the data analysis and to assess the
normal distribution and equal variance of all data. The baseline
characteristics of the participants were assessed by Student's
t-test and single-factor Pearson's χ2 test. Only the
difference between two groups were evaluated by Student's t test.
Differences among multiple groups were evaluated by the one-way
ANOVA, followed by Bonferroni's multiple comparisons test. The
RUNX3 mRNA expression levels were compared between
transfected cells, or between groups of cervical cancer cells and
controls using Bonferroni's multiple comparisons test. P<0.05
was considered to indicate a statistically significant difference.
According to transcriptome sequencing, differential expression
analysis of two groups was performed using the DESequencing (DESeq)
R package (v1.10.1; http://www.bioconductor.org/). DESeq provides
statistical analysis for determining differential expression in
digital gene expression data using a model based on the negative
binomial distribution. The resulting P-values were adjusted using
the Benjamini and Hochberg's approach for controlling the false
discovery rate. Genes with an adjusted P-value (q value) found by
DESeq were classified as differentially expressed. q<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of RUNX3 in cervical cancer
lines
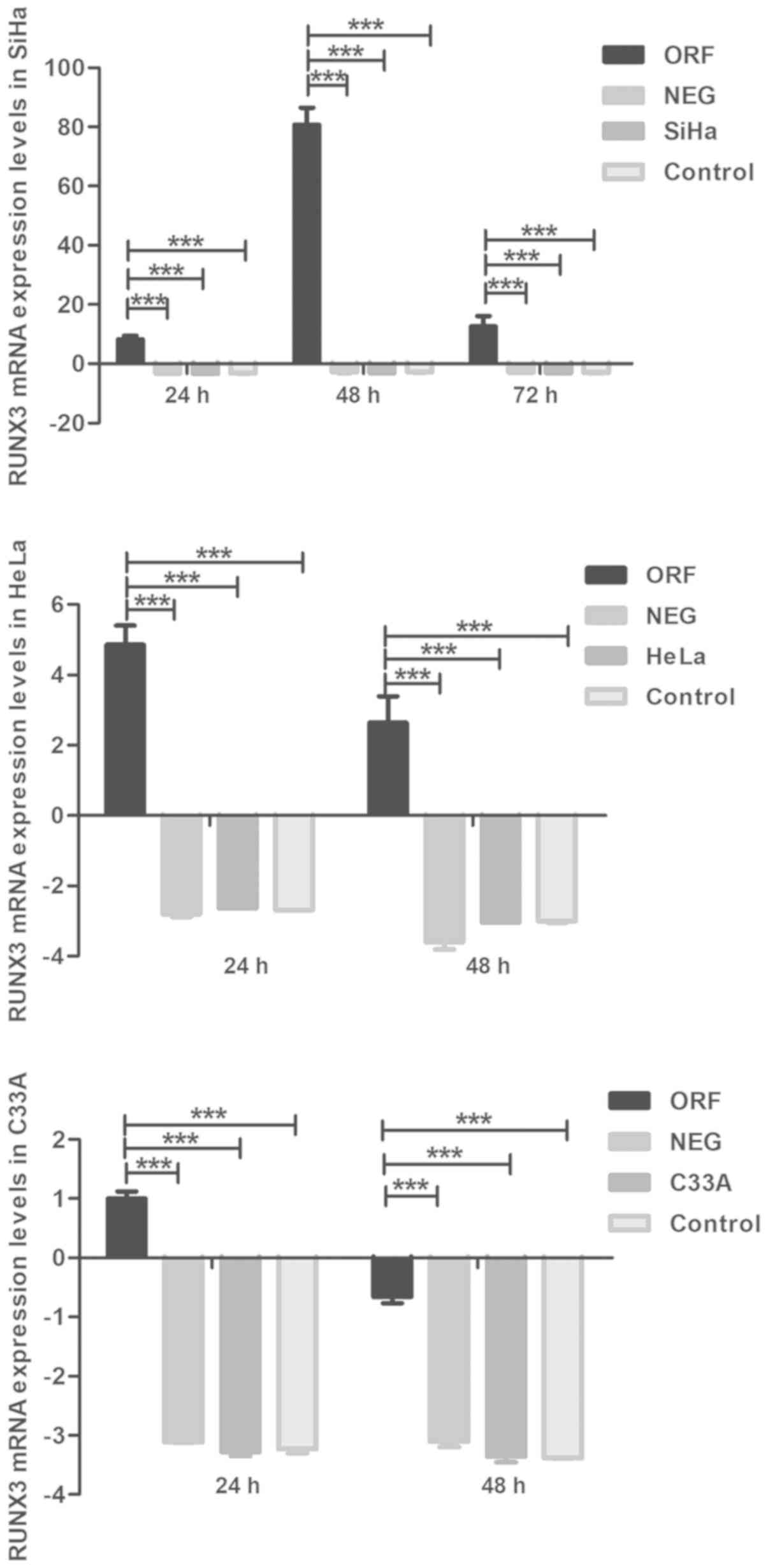
The efficiency of exogenous RUNX3 expression
and RUNX3 shRNA in cervical cancer cells was verified via
RT-qPCR analysis. The mRNA levels of RUNX3 were markedly
higher in ORF RUNX3 cells compared with the NEG control
group of the three cervical cancer cell lines (P<0.001; Fig. 1), which were also extremely
significantly higher than the ‘Control’ and ‘cell name’ groups
(P<0.001; Fig. 1). It also
demonstrated the low expression levels of RUNX3 in all the
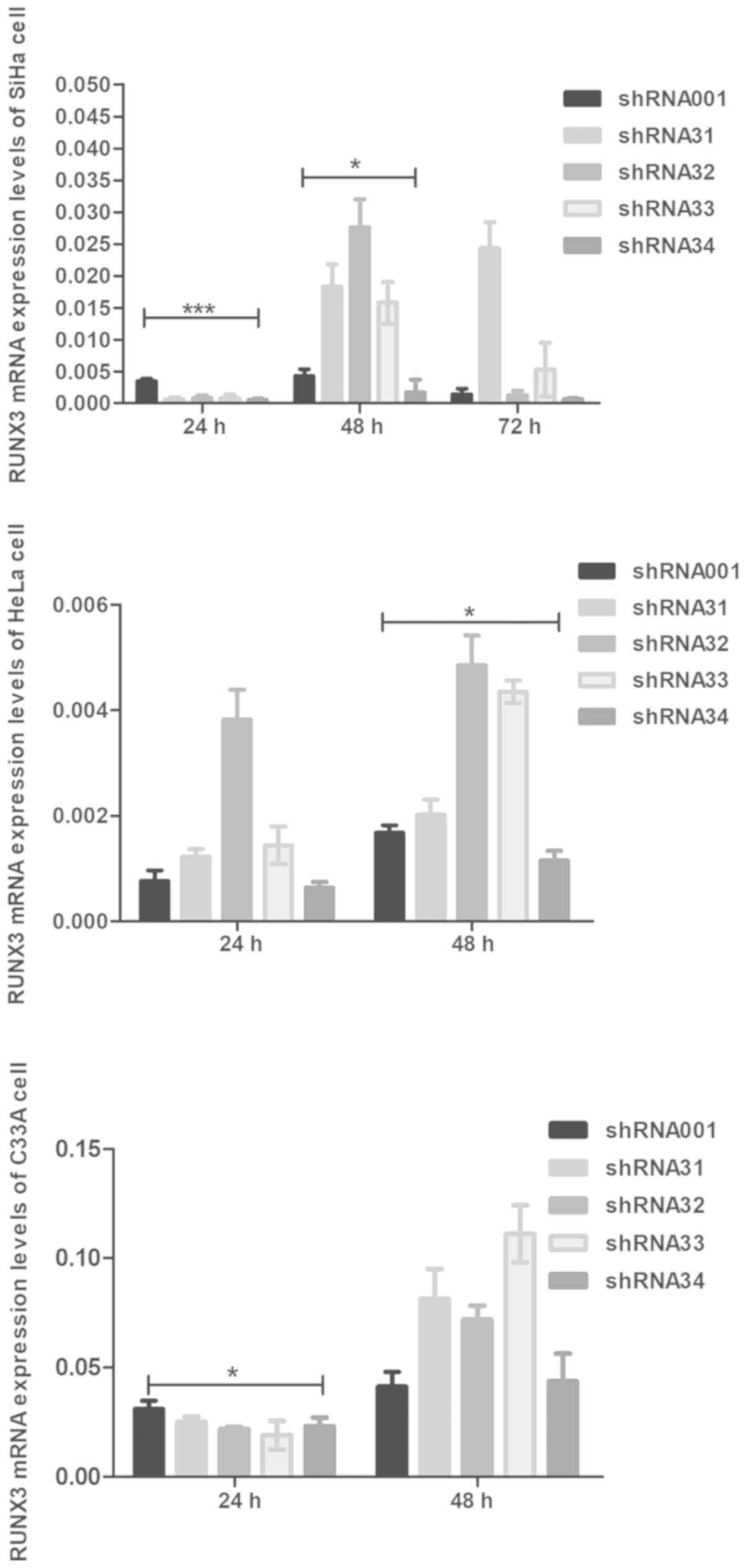
control groups, with or without intervention. In addition, the
shRNA RUNX3 and control groups (shRNA001) were significantly
different in the three cell lines, particularly in SiHa cells
(P<0.001 and P<0.05, respectively; Fig. 2). As the RUNX3 gene was
successfully suppressed by shRNA34 in SiHa, HeLa and C33A cells,
particularly the SiHa cell line at 24 (P<0.001) and 48 h
(P<0.05), shRNA34 was selected as the interference plasmid for
subsequent experiments (Fig. 2).
Role of RUNX3 in proliferation and
apoptosis of cervical cancer cells
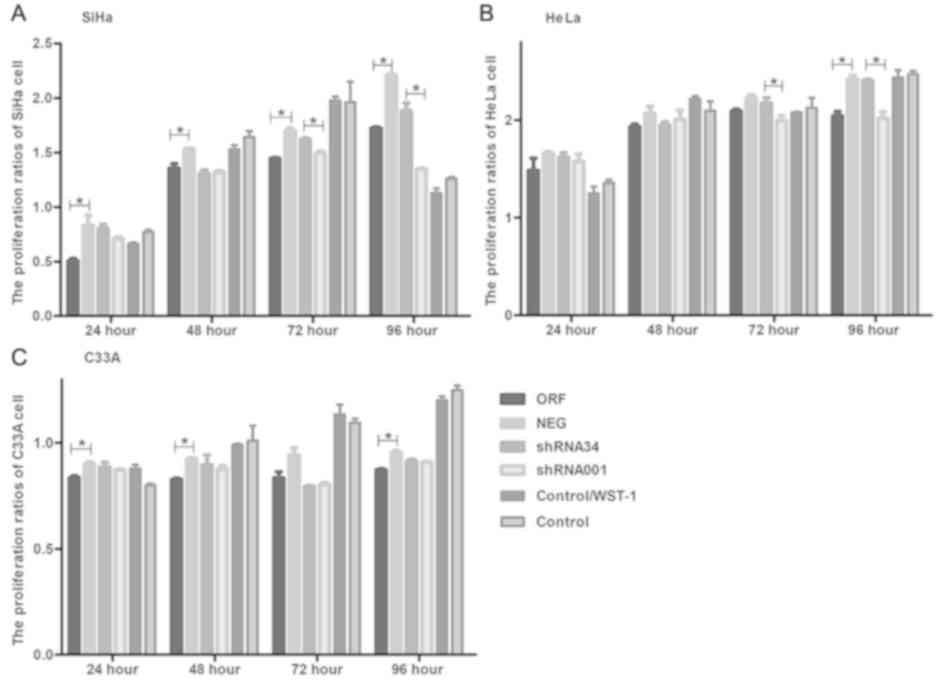
The WST-1 results suggested that there were
significant differences among ORF and NEG plasmid transfection
groups and shRNA34 and shRNA001 groups in the SiHa cell line
(P<0.05; Fig. 3A), However, the
shRNA34 and shRNA001, and ORF and NEG groups exhibited less
prominent differences in HeLa and C33A cell lines, respectively,
although the results were still significant; (Fig. 3B and C). There was no significant
difference between the groups of cervical cancer cells with WST-1
or without WST-1 (Fig. 3). In
addition, the results revealed that there were significant
differences at different transfection times; the ORF and NEG groups
were significantly different at 24, 48, 72 and 96 h in the SiHa
cell line, the shRNA34 and shRNA001 groups were significantly
different at 72 and 96 h in the HeLa cell line and the ORF and NEG
groups were significantly different at 24, 48 and 96 h in the C33A
cell line (P<0.05). The results also demonstrated that the
effects on different cervical cancer cells varied and indicated
that RUNX3 may inhibit the proliferation of cervical cancer
cells.
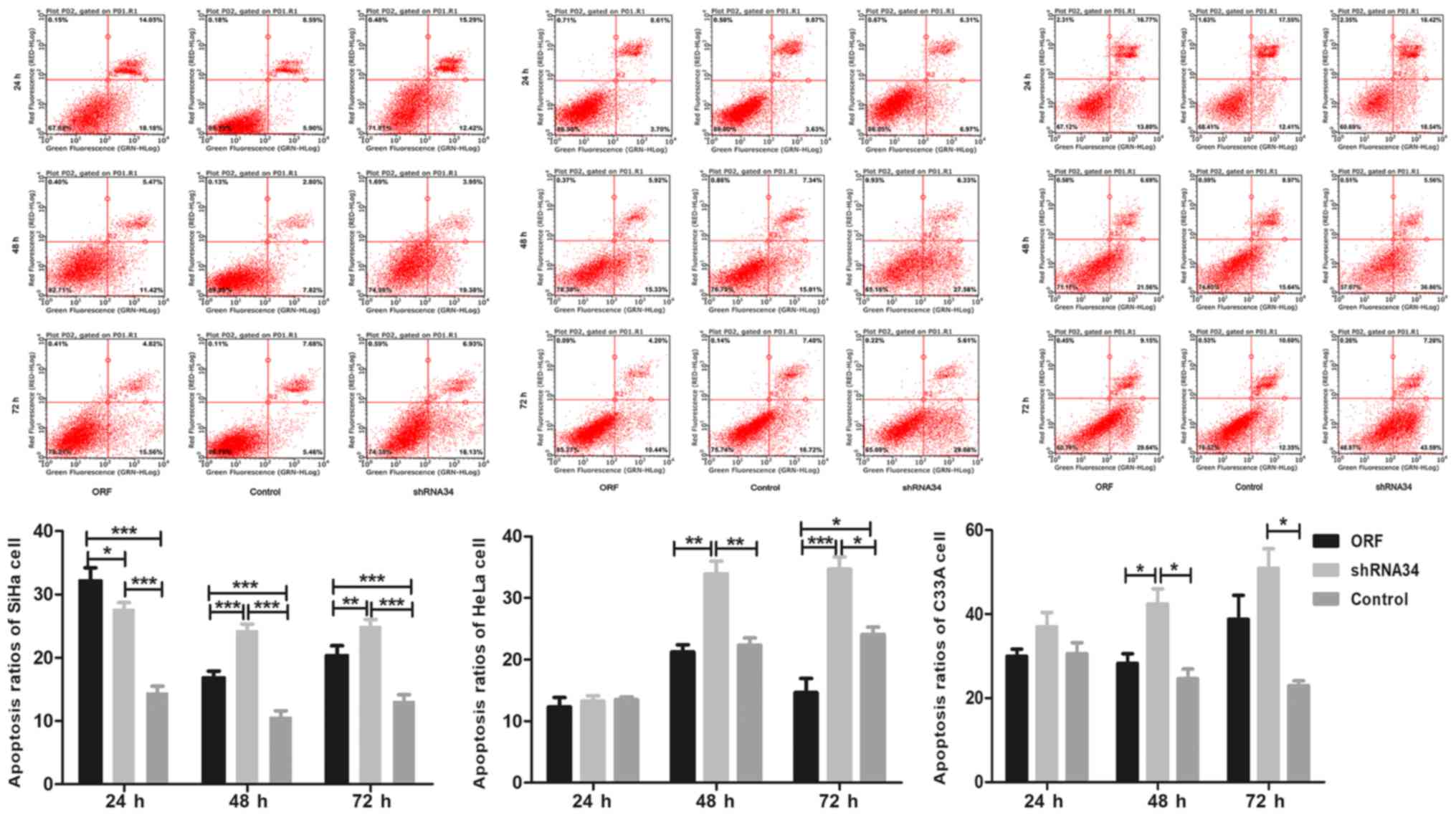
PI/Annexin V flow cytometry analysis was performed
in the present study in order to detect the apoptosis rates in
RUNX3-transfected cell lines. The apoptosis rates of
different cervical cancer cell groups were significantly different,
especially in SiHa cells (Fig. 4).
The apoptosis rate of the ORF group was significantly higher
compared with that in the control group in SiHa cell at 24, 48 and
72 h (P<0.001), indicating an apoptosis-promoting role of
RUNX3 in cervical cancer. Furthermore the apoptosis rate of
the shRNA34 group was significantly higher compared with that in
the control group at 24, 48 and 72 h, which were inconsistent as
expected (P<0.001, Fig. 4). In
addition, the apoptosis ratios of HeLa and C33A cells were not
significantly higher in the ORF group compared with that in the
control group at 24 and 48 h, which were not consistent with those
of the SiHa cells in ORF and control group, but in the 48 and 72 h
time periods, shRNA34 group was significantly higher compared with
that in the control group, in both the HeLa and C33A cells, which
is consistent with the SiHa cells (P<0.05; Fig. 4). Finally the apoptosis ratios of ORF
group were significantly lower compared with that in the shRNA34
group at 48 h in SiHa, HeLa and C33A cell lines (P<0.05;
Fig. 4), and at 72 h in SiHa and
HeLa cell lines (P<0.05; Fig. 4).
The results may suggest the low expression of RUNX3 in
cervical cancer is complexity to interfere and further research
should be preceded.
Effects of RUNX3 on cervical cancer
cells by transcriptome sequencing
The molecular mechanisms through which RUNX3
may affect cervical cancer were investigated in SiHa cells in
vitro. Relative transcript levels were tested by transcriptome
sequencing in order to determine the difference in transcripts
mediated by RUNX3 in SiHa cells. The present study
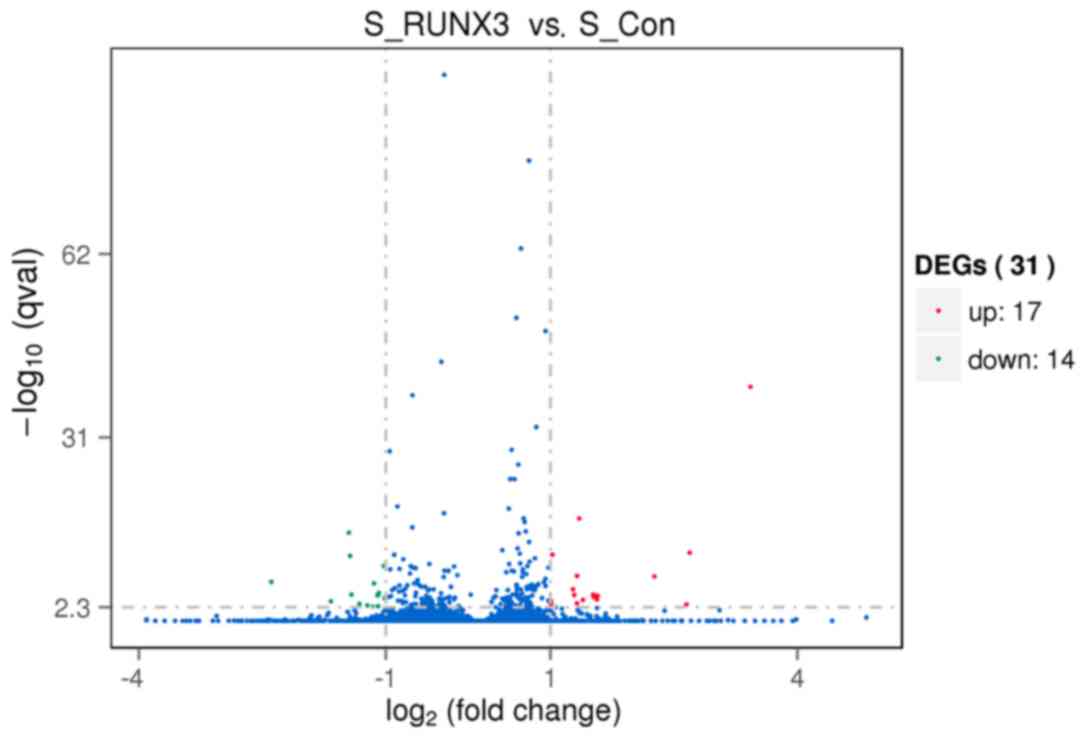
identified 31 genes that were differentially expressed in
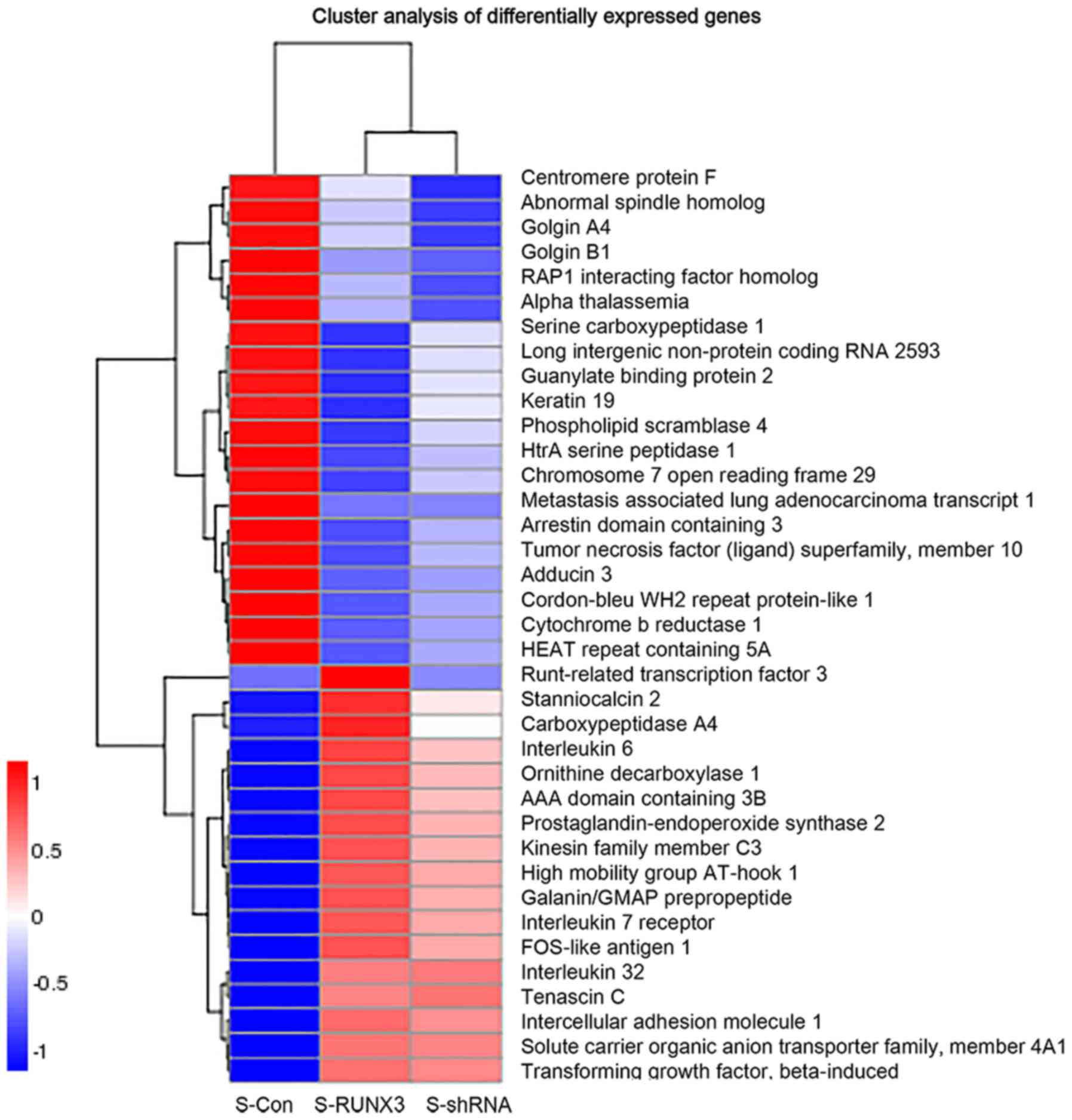
RUNX3-overexpressing SiHa cells. A total of 9 genes showed
differences in the three groups simultaneously, including IL-6,
PTGS2, FOSL1, TNC, ICAM1, IL-7R, IL-32, TGF-β and
TNFSF10 (Figs. 5 and 6), which may be potential regulators of
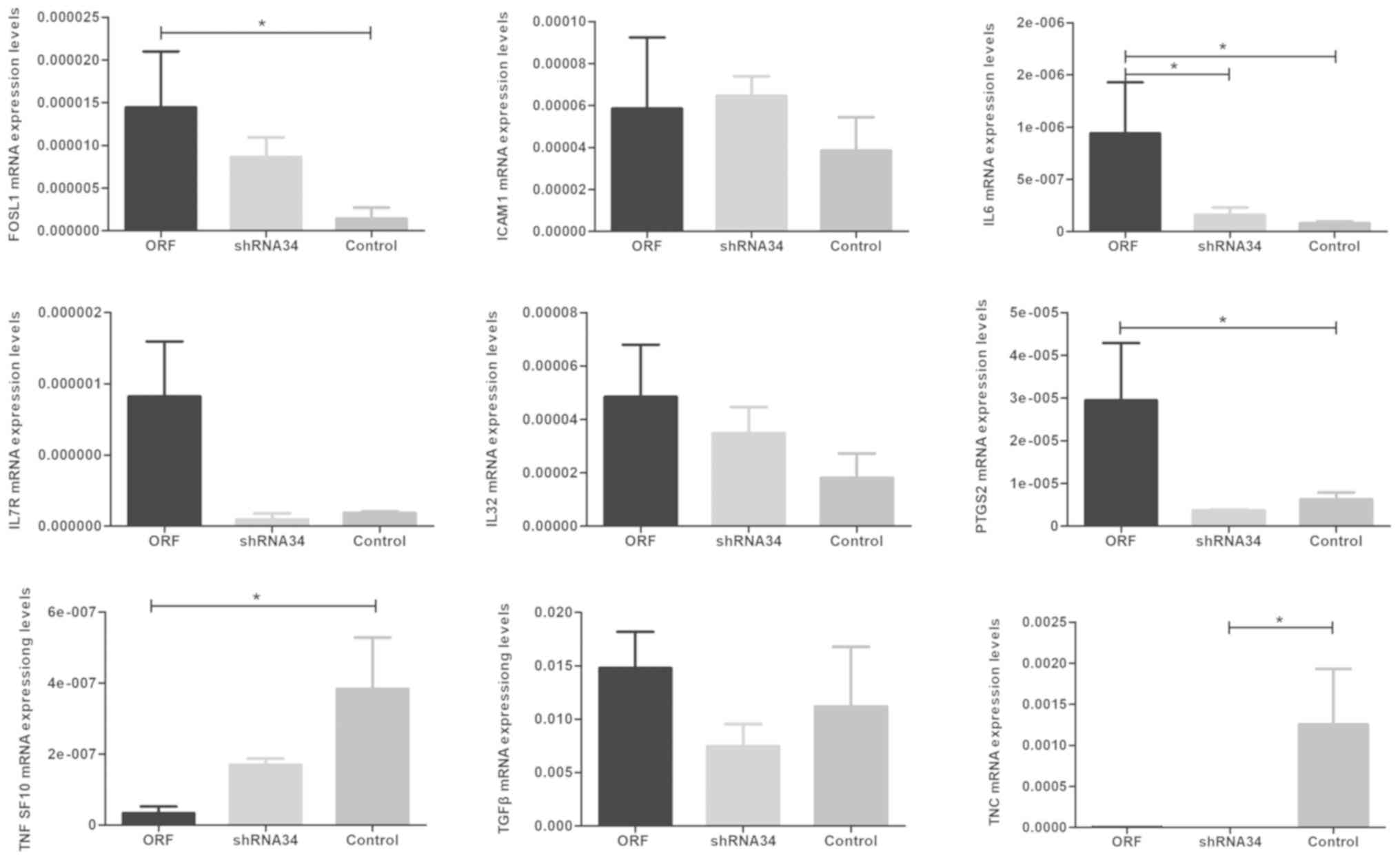
cervical cancer combined with RUNX3. The mRNA expressions of
the 9 genes confirmed the results of the transcriptome sequencing
(Fig. 7), although further research
is required to clarify the connection between RUNX3 and
these genes. However, in the shRNA34 groups, except with the
TNC and IL-6 gene, the expression levels of these
genes were not changed, which may be due to the low expression of
RUNX3 mRNA in cervical cancer and limited interference of
shRNA34. Additional research is required in order to develop an
improved understanding. Furthermore, following verification of the
mRNA expression levels, there was no significant difference between
the control groups. In addition, the GO enrichment analysis of
biological processes revealed that certain genes were enriched,
such as those involved in the regulation of carboxypeptidase,
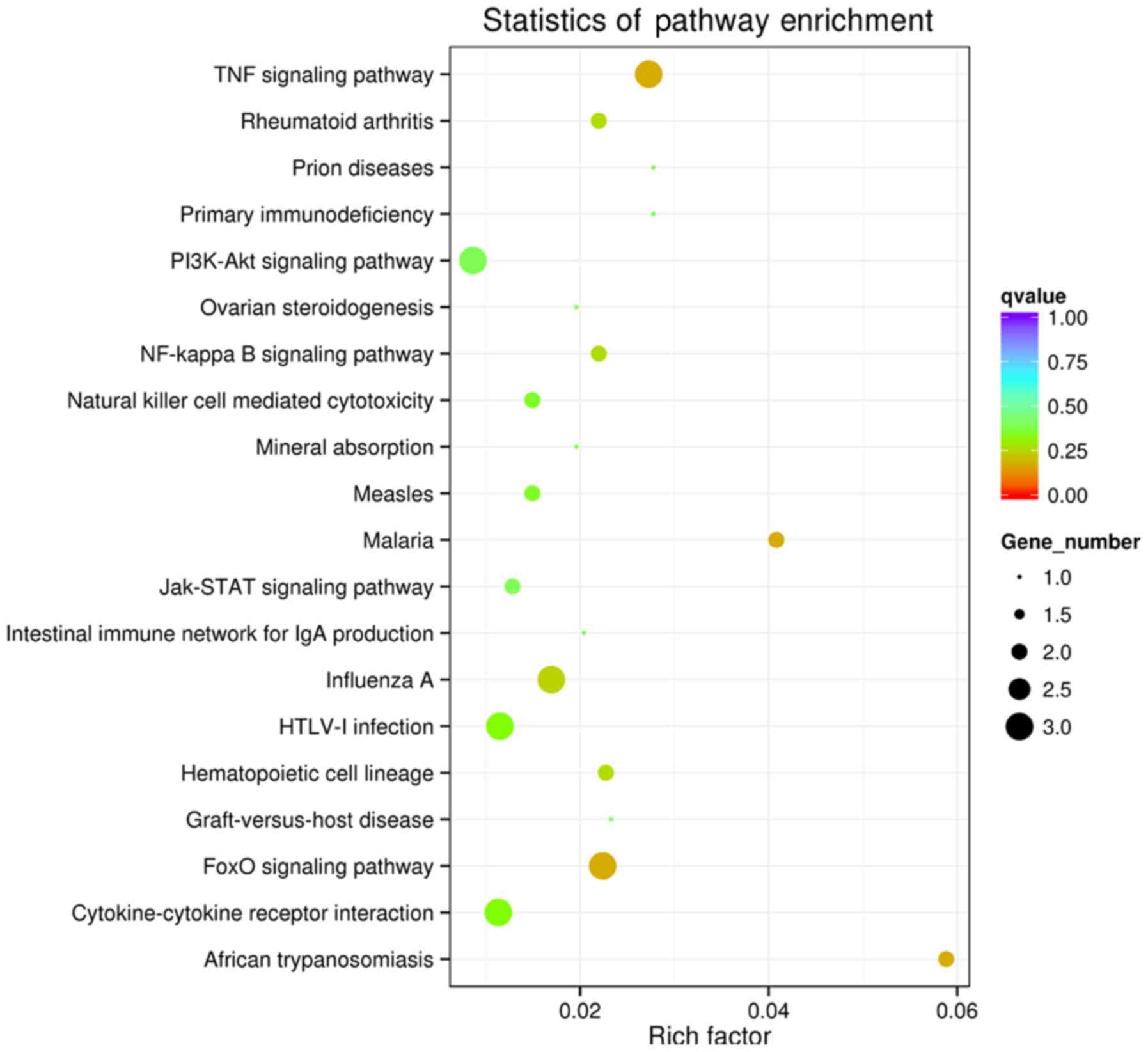
exopeptidase and hydrolase activity. Furthermore, the GO enrichment
analyses of the KEGG pathways concluded that these genes were
enriched in signaling pathways such as the tumor necrosis factor
(TNF) pathway, Forkhead box O (FoxO) pathway, African
trypanosomiasis and malaria (Fig.
8). Transcription factors and proto-oncogenes, such as
HMGA1 and FOSL1, IL7R and MALAT1, were
demonstrated as likely to be associated with RUNX3 in
cervical cancer (Fig. 6) and may be
associated with microfollicular thyroid adenoma, various benign
mesenchymal tumors and renal cell carcinoma (25,26).
Finally, the transcriptome sequencing analysis results indicated
that two long non-coding RNAs, RP11-54O7.3 and
MALAT1, were significantly different between groups,
suggesting that RUNX3 may affect their expression level
(Fig. 6). These findings indicate
that the expression of RUNX3 may affect the expression of
other genes, which may also be associated with cervical cancer
in vitro.
Discussion
Cervical cancer has been confirmed to be associated
with HPV infection; furthermore 0.7% of women initially found to be
infected with high-risk HPV will develop invasive carcinomas within
3 years (2,3). In recent years, immunological
mechanisms and genetic factors have been demonstrated to play
critical roles in cervical cancer (4,27–30). A
previous study demonstrated that RUNX3 is likely a potential
gene involved in cervical cancer susceptibility, and was associated
with the type of HPV infection and cervical intraepithelial
neoplasia progression (22). In the
present study, three types of cervical cancer cell lines were
selected: SiHa, HeLa and C33A, infected by HPV-16, HPV-18 or not
infected, respectively. It was recently reported that RUNX3
may play a key role in the development and progression of cervical
cancer (12). It was also observed
in the present study that RUNX3 may inhibit cervical cancer
cell proliferation, particularly in the SiHa cell lines, indicating
its potential function as a tumor suppressor; inconsistencies in
these results may be due to the different cell lines. In addition,
the expression levels of RUNX3 were lower in the three cell
lines, which were almost the same level in the control groups with
or without any intervention. The molecular mechanisms and signaling
pathways underlying the role of RUNX3 in cervical cancer
remain elusive. The aim of the present study was to elucidate the
potential effects of RUNX3 on cervical cancer by
transcriptome sequencing, which may help improve the current
therapy options and prognosis of patients with cervical cancer.
It was previously demonstrated that the
tumorigenicity of human gastric cancer cell lines was significantly
associated with the level of RUNX3 in nude mice, which
suggested that suppression of RUNX3 function was directly
associated with the occurrence and progression of human gastric
cancer (9). In a previous study on
ovarian cancer, the researchers observed that the overexpression of
RUNX3 in A2780s cells rendered them more resistant to
carboplatin, whereas the sensitivity of A2780cp cells to
carboplatin increased significantly following inhibition of
RUNX3 (31). In the present
study, the upregulated expression of RUNX3 consistently
inhibited the proliferation and promoted the apoptosis of cervical
cancer cells, particularly in the SiHa cell line. The results were
similar to those of a recent study on cervical cancer, which
reported that upregulated expression of RUNX3 inhibited the
proliferation, migration and invasion of cervical cancer cells
(12). According to these results,
it may be concluded that RUNX3 acts as a tumor suppressor
gene in cervical cancer (12).
As the mechanisms of action of RUNX3 have not
yet been fully elucidated, the molecular mechanisms and signaling
pathways of RUNX3 have become a focus in cancer research. It
is generally recognized that the transcription factor RUNX3,
which is a key effector of the TGF-β signaling pathway, acts
on the TGF-β receptor, thereby promoting cell proliferation
and apoptosis through the TGF-β signal transduction pathway
(7,10,32–34).
This may explain its wide involvement in tumorigenesis (35). However, several other signaling
pathways are affected by RUNX3. The Wnt signaling
pathway was confirmed to be associated with RUNX3 in
intestinal tumorigenesis (36)
through the formation of a ternary complex with
β-catenin/TCF4 and attenuation of Wnt signaling
activity, whereas the inactivation of RUNX3 may promote
intestinal adenoma formation (36).
In addition, the mitochondria-mediated pathway has been
demonstrated to be associated with RUNX3, inducing apoptosis
in gastric cancer cells (37). In a
study on hepatocellular carcinoma (HCC), the researchers reported
that RUNX3 suppressed Notch signaling in HCC SMMC7721
cells (38). In the present study,
the TNF and FoxO signaling pathways were demonstrated
to be associated with RUNX3.
TNF is a member of the TNF superfamily
of cytokines, which mediates cell processes such as
differentiation, inflammation, proliferation and apoptosis
(39), and has double the effect in
cancer cells. More specifically, it has been reported that
TNF is associated with cervical cancer (40). In addition, the
TNF-α/TNFR1/NF-κB pathway is potentially implicated in
tongue cancer and lung metastasis (41). The NF-κB signaling axis
(defined by the interactions of NF-κB dimers, IκB
regulators and IKK complexes) is responsive to external
stimuli and signal received (42).
FoxO is a subfamily member of the forkhead transcription
factor family. FoxO has been revealed as a key determinant
of cell fate, and to play an important functional role as a tumor
suppressor in different types of cancer (43,44).
During apoptosis, FoxO is involved in mitochondria-dependent
and -independent processes that trigger the expression of death
receptor ligands, such as Fas ligand, TNF apoptosis ligand,
Bcl XL, bNIP3 and Bim (43,44). The
most important pathway associated with FoxO is the
PI3K/AKT pathway. The PI3K/AKT pathway is also
dysregulated and activated in a wide variety of cancers, such as
breast, thyroid and cervical cancers (43). In addition, the Ras/MEK/ERK,
IKK and AMPK pathways have also been demonstrated to be
associated with FoxO, and they may play a role in
tumorigenesis (43–45). However, the role of FoxO in
cervical cancer has not been extensively investigated. An
association between FoxO and TGF-β in tumors has also
been reported (46,47). In an HCC study, the Thr32 residue of
FoxO3 was proven to be a critical factor for
TGF-β-induced apoptosis, which was mediated via Bim
(47). Due to the complexity and
uncertainty of the associations between RUNX3 and signaling
pathways, further research is required in order to confirm the
association between RUNX3 and the TNF/FoxO pathway in
cervical cancer.
Recently, the associations of lncRNAs with
RUNX3 in different cancers were investigated. A previous
study identified a potential competing endogenous RNA regulatory
network involving MT1JP and the regulation of RUNX3
expression and progression of gastric cancer (48). In a study of human colorectal cancer,
miR-532-5p mimic was revealed to markedly downregulate the
mRNA and protein levels of RUNX3, potentially acting as an
oncogenic miRNA (49). Finally, the
present study demonstrated that RUNX3 may affect the
expression levels of RP11-54O7.3 and MALAT1.
According to previous reports, MALAT1 may participate in
tumor formation, such as lung, prostate and ovarian cancer
(50–53). It was recently reported that
overexpression of MALAT1 could sponge miR-429 and
regulate cervical cancer pathogenesis in vivo and in
vitro (54), while the
associations between MALAT1 and RUNX3 remain unclear.
However, the role of RP11-54O7.3 has not yet been fully
elucidated, and the association between RP11-54O7.3 and
RUNX3 remains unknown (55,56).
These results indicate that RUNX3 may affect the expression
of lncRNAs, which may be associated with cervical cancer
in vitro. The specific mechanisms of action and role of
RUNX3 in cervical cancer will be further investigated in
future studies.
In conclusion, the present study demonstrated that
RUNX3 inhibited proliferation and promoted the apoptosis of
cervical cancer cells. In addition, the TNF and FoxO
pathways were demonstrated to be affected by RUNX3, and the
effects of MALAT1 and RP11-54O7.3 are likely mediated
by RUNX3 in cervical cancer. However, further research is
required in order to achieve an improved understanding of the
molecular complexities and functions of RUNX3 in cervical
cancer.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National
Natural Science Foundation of China (grant nos. 81572573 and
81172440), and the New Bud Research Foundation of West China Second
University Hospital (grant no. kx091), Sichuan University.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
XY, ZZ and YS contributed to analysis and
manuscript preparation. QG and MX performed the data analyses and
wrote the manuscript. BZ, YW, LZ and HL helped perform the analysis
with constructive discussions.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berkhof J, de Bruijne MC, Zielinski GD and
Meijer CJ: Natural history and screening model for high-risk human
papillomavirus infection, neoplasia and cervical cancer in the
Netherlands. Int J Cancer. 115:268–275. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McCredie MR, Sharples KJ, Paul C, Baranyai
J, Medley G, Jones RW and Skegg DC: Natural history of cervical
neoplasia and risk of invasive cancer in women with cervical
intraepithelial neoplasia 3: A retrospective cohort study. Lancet
Oncol. 9:425–434. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
González-Herrera L, Rodríguez-Morales P,
Gonza Lez-Losa Mdel R, Pérez-Mendoza G, Canul-Canché J,
Rosado-López I and Cetina TC: MTHFR/p53 polymorphisms as genetic
factors for cervical intraepithelial neoplasia and cervical cancer
in HPV-infected Mexican women. Int J Biol Markers. 29:e142–e149.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuglik P, Kasikova K, Smetana J, Vallova
V, Lastuvkova A, Moukova L, Cvanova M and Brozova L: Molecular
cytogenetic analyses of hTERC (3q26) and MYC (8q24) genes
amplifications in correlation with oncogenic human papillomavirus
infection in Czech patients with cervical intraepithelial neoplasia
and cervical carcinomas. Neoplasma. 62:130–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lund AH and van Lohuizen M: RUNX: A
trilogy of cancer genes. Cancer Cell. 1:213–215. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ito Y and Miyazono K: RUNX transcription
factors as key targets of TGF-beta superfamily signaling. Curr Opin
Genet Dev. 13:43–47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hwang KT, Han W, Bae JY, Hwang SE, Shin
HJ, Lee JE, Kim SW, Min HJ and Noh DY: Downregulation of the RUNX3
gene by promoter hypermethylation and hemizygous deletion in breast
cancer. J Korean Med Sci. 22 (Suppl):S24–S31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li QL, Ito K, Sakakura C, Fukamachi H,
Inoue K, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB, et al: Causal
relationship between the loss of RUNX3 expression and gastric
cancer. Cell. 109:113–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Subramaniam MM, Chan JY, Yeoh KG, Quek T,
Ito K and Salto-Tellez M: Molecular pathology of RUNX3 in human
carcinogenesis. Biochim Biophys Acta. 1796:315–331. 2009.PubMed/NCBI
|
|
11
|
Xiao WH and Liu WW: Hemizygous deletion
and hypermethylation of RUNX3 gene in hepatocellular carcinoma.
World J Gastroenterol. 10:376–380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Z, Fan P, Deng M and Zeng C: The roles
of RUNX3 in cervical cancer cells in vitro. Oncol Lett.
15:8729–8734. 2018.PubMed/NCBI
|
|
13
|
Gao F, Huang C, Lin M, Wang Z, Shen J,
Zhang H, Jiang L and Chen Q: Frequent inactivation of RUNX3 by
promoter hypermethylation and protein mislocalization in oral
squamous cell carcinomas. J Cancer Res Clin Oncol. 135:739–747.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ito K, Liu Q, Salto-Tellez M, Yano T, Tada
K, Ida H, Huang C, Shah N, Inoue M, Rajnakova A, et al: RUNX3, a
novel tumor suppressor, is frequently inactivated in gastric cancer
by protein mislocalization. Cancer Res. 65:7743–7750. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim WJ, Kim EJ, Jeong P, Quan C, Kim J, Li
QL, Yang JO, Ito Y and Bae SC: RUNX3 inactivation by point
mutations and aberrant DNA methylation in bladder tumors. Cancer
Res. 65:9347–9354. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lau QC, Raja E, Salto-Tellez M, Liu Q, Ito
K, Inoue M, Putti TC, Loh M, Ko TK, Huang C, et al: RUNX3 is
frequently inactivated by dual mechanisms of protein
mislocalization and promoter hypermethylation in breast cancer.
Cancer Res. 66:6512–6520. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kudo Y, Tsunematsu T and Takata T:
Oncogenic role of RUNX3 in head and neck cancer. J Cell Biochem.
112:387–393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsunematsu T, Kudo Y, Iizuka S, Ogawa I,
Fujita T, Kurihara H, Abiko Y and Takata T: RUNX3 has an oncogenic
role in head and neck cancer. PLoS One. 4:e58922009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JH, Pyon JK, Kim DW, Lee SH, Nam HS,
Kang SG, Kim CH, Lee YJ, Chun JS and Cho MK: Expression of RUNX3 in
skin cancers. Clin Exp Dermatol. 36:769–774. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Häfner N, Steinbach D, Jansen L, Diebolder
H, Dürst M and Runnebaum IB: RUNX3 and CAMK2N1 hypermethylation as
prognostic marker for epithelial ovarian cancer. INT J Cancer.
138:217–228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lotem J, Levanon D, Negreanu V, Bauer O,
Hantisteanu S, Dicken J and Groner Y: Runx3 at the interface of
immunity, inflammation and cancer. Biochim Biophys Acta.
1855:131–143. 2015.PubMed/NCBI
|
|
22
|
Gao QQ, Zhou B, Yu XZ, Zeng X, Zhang Z,
Quan Y, Wang YY, Pu Y, Cheng P, Song YP, et al: RUNX3 polymorphisms
and the susceptibility to cervical cancer and cervical
intraepithelial neoplasia in Western China. Int J Clin Exp Pathol.
9:10617–10626. 2016.
|
|
23
|
Lee JW, Kim DM, Jang JW, Park TG, Song SH,
Lee YS, Chi XZ, Park IY, Hyun JW, Ito Y and Bae SC: RUNX3 regulates
cell cycle-dependent chromatin dynamics by functioning as a pioneer
factor of the restriction-point. Nat Commun. 10:18972019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dal Cin P, Fusco A, Belge G, Chiappetta G,
Fedele M, Pauwels P, Bullerdiek J and Van den Berghe H: Involvement
of the HMGI(Y) gene in a microfollicular adenoma of the thyroid.
Genes Chromosomes Cancer. 24:286–289. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hirata H, Hinoda Y, Shahryari V, Deng G,
Nakajima K, Tabatabai ZL, Ishii N and Dahiya R: Long Noncoding RNA
MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and
interacts with miR-205. Cancer Res. 75:1322–1331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Quan Y, Zhou B, Wang Y, Duan R, Wang K,
Gao Q, Shi S, Song Y, Zhang L and Xi M: Association between IL17
polymorphisms and risk of cervical cancer in Chinese women. Clin
Dev Immunol. 2012:258–293. 2012. View Article : Google Scholar
|
|
28
|
Walch-Rückheim B, Mavrova R, Henning M,
Vicinus B, Kim YJ, Bohle RM, Juhasz-Böss I, Solomayer EF and Smola
S: Stromal fibroblasts induce CCL20 through IL6/C/EBPβ to support
the recruitment of Th17 cells during cervical cancer progression.
Cancer Res. 75:5248–5259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zidi S, Sghaier I, Gazouani E, Mezlini A
and Yacoubi-Loueslati B: Evaluation of Toll-like receptors 2/3/4/9
gene polymorphisms in cervical cancer evolution. Pathol Oncol Res.
22:323–330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zidi S, Stayoussef M, Gazouani E, Mezlini
A, Yacoubi-Loueslati B and Almawi WY: Relationship of common
vascular endothelial growth factor polymorphisms and haplotypes
with the risk of cervical cancer in Tunisians. Cytokine.
74:108–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barghout SH, Zepeda N, Vincent K, Azad AK,
Xu Z, Yang C, Steed H, Postovit LM and Fu Y: RUNX3 contributes to
carboplatin resistance in epithelial ovarian cancer cells. Gynecol
Oncol. 138:647–655. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yano T, Ito K, Fukamachi H, Chi XZ, Wee
HJ, Inoue K, Ida H, Bouillet P, Strasser A, Bae SC and Ito Y: The
RUNX3 tumor suppressor upregulates Bim in gastric epithelial cells
undergoing transforming growth factor beta-induced apoptosis. Mol
Cell Biol. 26:4474–4488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamamura Y, Lee WL, Inoue K, Ida H and Ito
Y: RUNX3 cooperates with FoxO3a to induce apoptosis in gastric
cancer cells. J Biol Chem. 281:5267–5276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chi XZ, Yang JO, Lee KY, Ito K, Sakakura
C, Li QL, Kim HR, Cha EJ, Lee YH, Kaneda A, et al: RUNX3 suppresses
gastric epithelial cell growth by inducing p21(WAF1/Cip1)
expression in cooperation with transforming growth factor
{beta}-activated SMAD. Mol Cell Biol. 25:8097–8107. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Farooqi AA, Khalid S and Ahmad A:
Regulation of cell signaling pathways and miRNAs by resveratrol in
different cancers. Int J Mol Sci. 19(pii): E6522018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ito K, Lim AC, Salto-Tellez M, Motoda L,
Osato M, Chuang LS, Lee CW, Voon DC, Koo JK, Wang H, et al: RUNX3
attenuates beta-catenin/T cell factors in intestinal tumorigenesis.
Cancer Cell. 14:226–237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nagahama Y, Ishimaru M, Osaki M, Inoue T,
Maeda A, Nakada C, Moriyama M, Sato K, Oshimura M and Ito H:
Apoptotic pathway induced by transduction of RUNX3 in the human
gastric carcinoma cell line MKN-1. Cancer Sci. 99:23–30.
2008.PubMed/NCBI
|
|
38
|
Gao J, Chen Y, Wu KC, Liu J, Zhao YQ, Pan
YL, Du R, Zheng GR, Xiong YM, Xu HL and Fan DM: RUNX3 directly
interacts with intracellular domain of Notch1 and suppresses Notch
signaling in hepatocellular carcinoma cells. Exp Cell Res.
316:149–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Holdbrooks AT, Britain CM and Bellis SL:
ST6Gal-I sialyltransferase promotes tumor necrosis factor
(TNF)-mediated cancer cell survival via sialylation of the TNF
receptor 1 (TNFR1) death receptor. J Biol Chem. 293:1610–1622.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zidi S, Stayoussef M, Zouidi F, Benali S,
Gazouani E, Mezlini A and Yacoubi-Loueslati B: Tumor necrosis
factor alpha (−238/-308) and TNFRII–VNTR (−322) polymorphisms as
genetic biomarkers of susceptibility to develop cervical cancer
among tunisians. Pathol Oncol Res. 21:339–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tanaka T, Imamura T, Yoneda M, Irie A, Ogi
H, Nagata M, Yoshida R, Fukuma D, Kawahara K, Shinohara M and
Nakayama H: Enhancement of active MMP release and invasive activity
of lymph node metastatic tongue cancer cells by elevated signaling
via the TNF-α-TNFR1-NF-κB pathway and a possible involvement of
angiopoietin-like 4 in lung metastasis. Int J Oncol. 49:1377–1384.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mitchell S, Vargas J and Hoffmann A:
Signaling via the NFκB system. Wiley Interdiscip Rev Syst Biol Med.
8:227–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Farhan M, Wang H, Gaur U, Little PJ, Xu J
and Zheng W: foxo signaling pathways as therapeutic targets in
cancer. Int J Biol Sci. 13:815–827. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Coomans de Brachène A and Demoulin JB:
FOXO transcription factors in cancer development and therapy. Cell
Mol Life Sci. 73:1159–1172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Link W and Fernandez-Marcos PJ: FOXO
transcription factors at the interface of metabolism and cancer.
Int J Cancer. 141:2379–2391. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yadav H, Devalaraja S, Chung ST and Rane
SG: TGF-β1/Smad3 pathway targets PP2A-AMPK-FoxO1 signaling to
regulate hepatic gluconeogenesis. J Biol Chem. 292:3420–3432. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao X, Liu Y, Du L, He L, Ni B, Hu J, Zhu
D and Chen Q: Threonine 32 (Thr32) of FoxO3 is critical for
TGF-β-induced apoptosis via Bim in hepatocarcinoma cells. Protein
Cell. 6:127–138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu Y, Zhang G, Zou C, Zhang H, Gong Z,
Wang W, Ma G, Jiang P and Zhang W: LncRNA MT1JP suppresses gastric
cancer cell proliferation and migration through
MT1JP/MiR-214-3p/RUNX3 axis. Cell Physiol Biochem. 46:2445–2459.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang J, Zhou W, Liu Y, Liu T, Li C and
Wang L: Oncogenic role of microRNA-532-5p in human colorectal
cancer via targeting of the 5′UTR of RUNX3. Oncol Lett.
15:7215–7220. 2018.PubMed/NCBI
|
|
50
|
Xue D, Lu H, Xu HY, Zhou CX and He XZ:
Long noncoding RNA MALAT1 enhances the docetaxel resistance of
prostate cancer cells via miR-145-5p-mediated regulation of AKAP12.
J Cell Mol Med. 22:3223–3237. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bai L, Wang A, Zhang Y, Xu X and Zhang X:
Knockdown of MALAT1 enhances chemosensitivity of ovarian cancer
cells to cisplatin through inhibiting the Notch1 signaling pathway.
Exp Cell Res. 366:161–171. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fang Z, Chen W, Yuan Z, Liu X and Jiang H:
LncRNA-MALAT1 contributes to the cisplatin-resistance of lung
cancer by upregulating MRP1 and MDR1 via STAT3 activation. Biomed
Pharmacother. 101:536–542. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xiping Z, Bo C, Shifeng Y, Feijiang Y,
Hongjian Y, Qihui C and Binbin T: Roles of MALAT1 in development
and migration of triple negative and Her-2 positive breast cancer.
Oncotarget. 9:2255–2267. 2017.PubMed/NCBI
|
|
54
|
Shen F, Zheng H, Zhou L, Li W and Xu X:
Overexpression of MALAT1 contributes to cervical cancer progression
by acting as a sponge of miR-429. J Cell Physiol. 234:11219–11226.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li J, Wang W, Xia P, Wan L, Zhang L, Yu L,
Wang L, Chen X, Xiao Y and Xu C: Identification of a five-lncRNA
signature for predicting the risk of tumor recurrence in patients
with breast cancer. Int J cancer. 143:2150–2160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jiang W, Zhan H, Jiao Y, Li S and Gao W: A
novel lncRNA-miRNA-mRNA network analysis identified the hub lncRNA
RP11-159F24.1 in the pathogenesis of papillary thyroid cancer.
Cancer Med. 7:6290–6298. 2018. View Article : Google Scholar : PubMed/NCBI
|