Introduction
The prevalence of obesity has rapidly increased
globally in recent years with changing lifestyles and diets. An
epidemiological study in 2017, on ~130 million people aged >5
years, demonstrated that obesity rates increased from <1%
(equivalent to 5 million girls and 6 million boys) to ~6% in girls
(50 million) and ~8% in boys (74 million) between 1975 and 2016;
combined, the number rose by more than tenfold globally (1). Obesity has been associated with a
number of malignancies in epidemiological studies on oesophageal,
liver, gallbladder, pancreatic, kidney and colorectal cancer (CRC)
(2–4), and is considered an important risk
factor for cancer (5).
CRC is a common malignant tumour of the digestive
system. The incidence rate of CRC has increased year by year, and
the cause of the disease has been thoroughly studied; according to
data from GLOBOCAN in 2012, there are 1.6 million new cases of CRC
and 694,000 people that die from CRC worldwide every year (6). Obesity is associated with CRC, but the
pathogenesis of obesity-induced CRC remains unclear (7). Previous studies have suggested that
adipokines secreted by the adipose tissue serve an important role
in the interaction between obesity (especially abdominal obesity),
insulin resistance and CRC (8–10);
therefore, adipokines have received increasing attention.
Adipose tissue not only stores excess energy, but
also secretes adipokines, which are produced by adipocytes (e.g.
resistin, visfatin, leptin and adiponectin) and the stromovascular
fraction of adipose tissue cells, such as tumour necrosis factor α
(TNF-α), interleukin-6 (IL-6) and plasminogen activator
inhibitor-1. These adipokines affect numerous physiological
processes, including thermogenesis, neuroendocrine function,
glucose and lipid metabolism, inflammation, appetite, energy
balance, reproduction, angiogenesis, cell proliferation and
atherosclerosis (11–13). In addition, an association between
adipokines and several obesity-related disorders, including cancer,
has been demonstrated (14,15).
Omentin-1 is a 34-kDa protein; its gene, also termed
intelectin, was initially cloned in intestinal paneth cells, and
was considered to participate in intestinal microbial surveillance
(16). Later, omentin-1 was
demonstrated to be secreted from human visceral adipose tissue, and
was reported as an adipokine that increases adipocyte glucose
intake and insulin sensitivity (17). Omentin-1 is associated with metabolic
syndromes (such as obesity and insulin resistance) (18), as well as with cardiac function
(19), immune response (20) and reproductive diseases (21). Studies have identified a close
association between omentin-1 and the development of CRC. Fazeli
et al (22) demonstrated that
circulating omentin-1 levels were significantly increased in
patients with CRC compared with those in healthy subjects
independent of obesity. Aleksandrova et al (23) reported that omentin-1 concentration
was an independent predictor of CRC risk during a mean follow-up
time of 10.4 years. These data suggested that omentin-1 may be
implicated in the risk of CRC. However, the cause of the high
circulating omentin-1 level in patients with CRC, and whether CRC
cells express this adipokine remained to be determined. Our
previous study demonstrated that omentin-1 induced the
proliferation of CRC cells (24).
Thus, the present study aimed to clarify whether CRC cells
endogenously secreted and expressed omentin-1, which may act on CRC
cells in an autocrine manner.
Materials and methods
Patient samples and tissue
collection
This study was conducted at The First Affiliated
Hospital of Anhui Medical University (Hefei, China) between April
and December 2014. The study was approved by the Ethics Committee
of The First Affiliated Hospital of Anhui Medical University and
all procedures performed in studies involving human participants
were in accordance with the ethical standards of the institutional
and/or national research committee and with the 1964 Helsinki
declaration and its later amendments or comparable ethical
standards. Informed consent was obtained from all participants. A
total of 24 patients (13 males and 11 females; mean age, 55.13±8.67
years; age range, 30–72 years) with a first diagnosis of
histologically confirmed CRC. None of whom had undergone
radiotherapy or chemotherapy prior to surgery, were selected for
the study. The inclusion criteria were age ≥35 years and body mass
index (BMI) between 20 and 25 kg/m2. The exclusion
criteria were previous gastrointestinal tract surgery, familial
adenomatous polyposis, previous polypectomy, use of medications
that impair glucose tolerance, pregnancy, previous diagnosis of
CRC/recurrent patients, previous diagnosis of diabetes,
inflammatory bowel disease, serious liver and renal dysfunction,
and acute or chronic infectious disease. In addition,
intraoperative colon carcinoma tissues and para-carcinoma tissues
(>5 cm from cancer tissue) were collected from 24 patients with
CRC, in duplicate. One of the duplicate tissues (approximately
2×1×1 cm), were stored in a liquid nitrogen tank (−180°C) and the
other was stored in a liquid nitrogen tank fixed in 10%
formalin.
Cell culture and preparation
The human colon epithelial cancer cell lines SW480
and HCT116 were obtained from the Cell Bank of the Chinese Academy
of Sciences and were cultured in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% foetal bovine serum at 37°C with 5%
CO2. The European Collection of Authenticated Cell
Cultures PCR technology was used to confirm that the cells were not
contaminated with mycoplasma, and cell line authentication was
performed by short tandem repeat profiling to exclude misidentified
or cross-contaminated cell culture. After the cells reached the
logarithmic growth phase (the cell counts in the two cell lines
were essentially equal), the total medium was replaced with
serum-free medium for 6, 12, 24 or 48 h to avoid the toxic effect
of serum on cells and serum-derived contamination and allowed the
cells to secrete fresh proteins. To exclude the effect of
confluence in cell culture on the expression of omentin-1, the
supernatant and lysate of CRC cells were obtained by selecting
equal numbers of cells from serum-free cell flasks at 6, 12, 24 and
48 h. The expression level of omentin-1 in SW480 and HCT116 cell
lines was detected by reverse transcription-quantitative PCR
(RT-qPCR). Cells that expressed higher mRNA levels of omentin-1,
detected by RT-qPCR, were selected for further experiments
(25).
Immunohistochemical staining
The tissues (obtained from patients with CRC) were
embedded in paraffin, sliced into 4-µm sections with a microtome
(Leica Instrument Co., Ltd.), dewaxed in the oven (60°C overnight)
in three incubations with xylene. Subsequently, the sections were
placed in 100, 95, 80 and 70% ethanol for 3–5 min, and placed in
distilled water for 3 min to hydrate (at room temperature). A
pressure cooker was used to hold 2.5 l double distilled water and
50 ml ethylenediaminetetraacetic acid (EDTA) repair solution (pH
8.0) was added and heated to boiling on the induction cooker.
Subsequently, the slices were put together with the dyeing rack
into the repair solution, fixed (10% neutral formaldehyde) for ~1
min and left at room temperature to cool down. The sections were
subsequently blocked for endogenous peroxidase activity by 3%
H2O2 (cat. no. ZLI-9311D; Zsbio) for 20 min
at 37°C and washed three times with PBS. An appropriate amount
(~100 µl) anti-omentin-1 antibody (cat. no. 11770-1-AP ProteinTech
Group, Inc.) was diluted 1:500 and incubated with the specimen for
30 min at 37°C, followed by rinsing (3 times with PBS) and drying
the slices. Horseradish peroxidase-labelled goat anti-rabbit
secondary antibody (1:10,000; cat. no. TA130003; OriGene
Technologies, Inc.) was added to the specimen for 30 min at 37°C.
3,3′-Diaminobenzidine colouring solution was added for ~1 min and
rinsed. The colour-developed sections were placed in haematoxylin
staining solution for ~3 min at 37°C and washed again. Omentin-1
expression and distribution were observed under an optical
microscope (CX43, Olympus; magnification, ×400). According to
manufacturer's instructions, omentin-1 was represented by brown
particles in the cytoplasm. The average optical density value (four
fields analyzed per section) was calculated by ImagePro Plus 6.0
(Wuhan Gene Beauty Ltd.) to quantitatively compare the difference
between the protein expressed by the positive cells in cancerous
and adjacent tissues.
Reverse transcription-quantitative PCR
(RT-qPCR)
The mRNA expression of omentin-1 was measured by
RT-qPCR. Total RNA was extracted from ground tissue material (24
patients with CRC) using 1 ml TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc.) per 100 mg tissue. In addition,
total RNA was extracted from SW480 and HCT116 cells with
TRIzol®, and cell lysates of SW480 cells and HCT-116
cells were obtained after 6, 12, 24 and 48 h of culture. Next, 0.2
ml chloroform was added, followed by centrifugation at 12,000 × g
for 15 min at 4°C. Total RNA was precipitated from the mixture in a
sterile RNase-free tube by adding 0.5 ml isopropyl alcohol and
incubating for 10 min at 24°C. The pellet, containing total RNA,
was washed with 75% ethanol and centrifuged at 12,000 × g for 10
min at 4°C. Complementary DNA (cDNA) was synthesised using 2 µg
total RNA and a cDNA synthesis kit (HiScript® II Reverse
Transcriptase; cat. no. R233-01; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The resulting cDNA
samples were subjected to PCR analysis using specific primer sets.
PCR was performed in tubes containing 1 µg cDNA, 5 µl
SYBR® Green Master Mix, 1 µl each of forward and reverse
primers (10 µM) and 2 µl deionized water to obtain a final volume
of 10 µl. Primers (Table I) were
designed by AlleleID 6.0 software (Wuhan Gene Beauty Ltd.) based on
the omentin-1 gene sequences indicated in the GenBank online
resource (https://www.ncbi.nlm.nih.gov/nucleotide/NM_017625.2?report=genbank&log$=nuclalign&blast_rank=1&RID=UAJGH89U014).
PCR amplification was performed using a PCR thermocycler (Thermo
Fisher Scientific, Inc.) under the following conditions: Initial
denaturation at 95°C for 5 min, followed by 40 cycles of 95°C for 5
sec, 60°C for 10 sec and 72°C for 20 sec.
 | Table I.Target genes and their primer
sequences. |
Table I.
Target genes and their primer
sequences.
| Gene | Primer sequence
(5′→3′) | Product size
(bp) |
|---|
| Human
omentin-1 | F:
AGGAAAGTGCAGCTGAGACT | 138 |
|
| R:
GGAGACGAAGAACAGGTCCA |
|
| Human β-actin | F:
GGGAAATCGTGCGTGACATTAAGG | 180 |
|
| R:
CAGGAAGGAAGGCTGGAAGAGTG |
|
Relative expression of the studied genes was
determined using the 2−∆∆Cq method (26), and the data were normalized to
β-actin. All the experiments were conducted in duplicate, and the
relative expression of genes was determined in comparison with the
expression of the β-actin gene as a control.
Western blotting
Total protein was isolated from ground tissue
samples using radioimmunoprecipitation assay (RIPA; Biyuntian
Institute of Biotechnology) buffer. Briefly, 100 mg each sample was
mechanically pulverised and resuspended (100 mg tissue/ml) in 1 ml
RIPA buffer. Resuspended samples were sonicated for 3 sec, repeated
3 times with 10-sec intervals, on ice, and the insoluble material
was removed by centrifugation at 12,000 × g for 10 min at 4°C,
while the supernatant was preserved. The concentration of the
protein samples was determined by BCA assay and were separated by
SDS-PAGE on a 10% gel (10 µg per lane) and transferred to
nitrocellulose membranes. Following blocking in 5% non-fat milk in
Tris-buffered saline containing 0.05% Tween-20 (TBS-T) for 2 h at
37°C, membranes were incubated with a primary antibody against
omentin-1 (1:500; cat. no. 11770-1-AP; ProteinTech Group, Inc.) for
1 h at room temperature and subsequently with an anti-mouse
secondary antibody conjugated to horseradish peroxidase (1:10,000;
cat. no. TA130003; OriGene Technologies, Inc.) for 0.5 h at room
temperature. Antibody detection was performed by ECL (Thermo Fisher
Scientific, Inc.).
ELISA
The expression of omentin-1 protein was measured by
the Omentin-1 ELISA kit (Yuanye). Based on a quantitative sandwich
enzyme immunoassay technique with a sensitivity of 0.1 ng/ml, the
inter-assay and intra-assay coefficient of variation was <15%.
The supernatant and lysate of SW480 cells were obtained following
6, 12, 24 and 48 h of culture. The optical density of the
standards, controls and samples was assessed at a wavelength of 450
nm by a microplate reader (Bio Tek Instruments, Inc.). Triplicate
measurements were performed in a single experiment, and the results
were determined by comparing the optical density of the samples to
that of the standard curve.
Statistical analysis
The SPSS statistical software package for Windows
version 17.0 (SPSS, Inc.) was used for all statistical analyses.
Data are expressed as the mean ± SD. The paired t-test was used to
compare the protein and mRNA expression levels of omentin-1 between
carcinoma and para-carcinoma tissues. The independent Student's
t-test was used to compare the mRNA expression of omentin-1 between
SW480 and HCT116 cells. The independent Student's t-test and the
one-way analysis of variance (ANOVA) was used to compare the
protein and mRNA expression levels of omentin-1 in the supernatant
and lysate of SW480 cells at different times, and the
Student-Newman-Keuls (SNK-q) test was used for post hoc analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of omentin-1 protein in
human CRC and para-carcinoma tissues
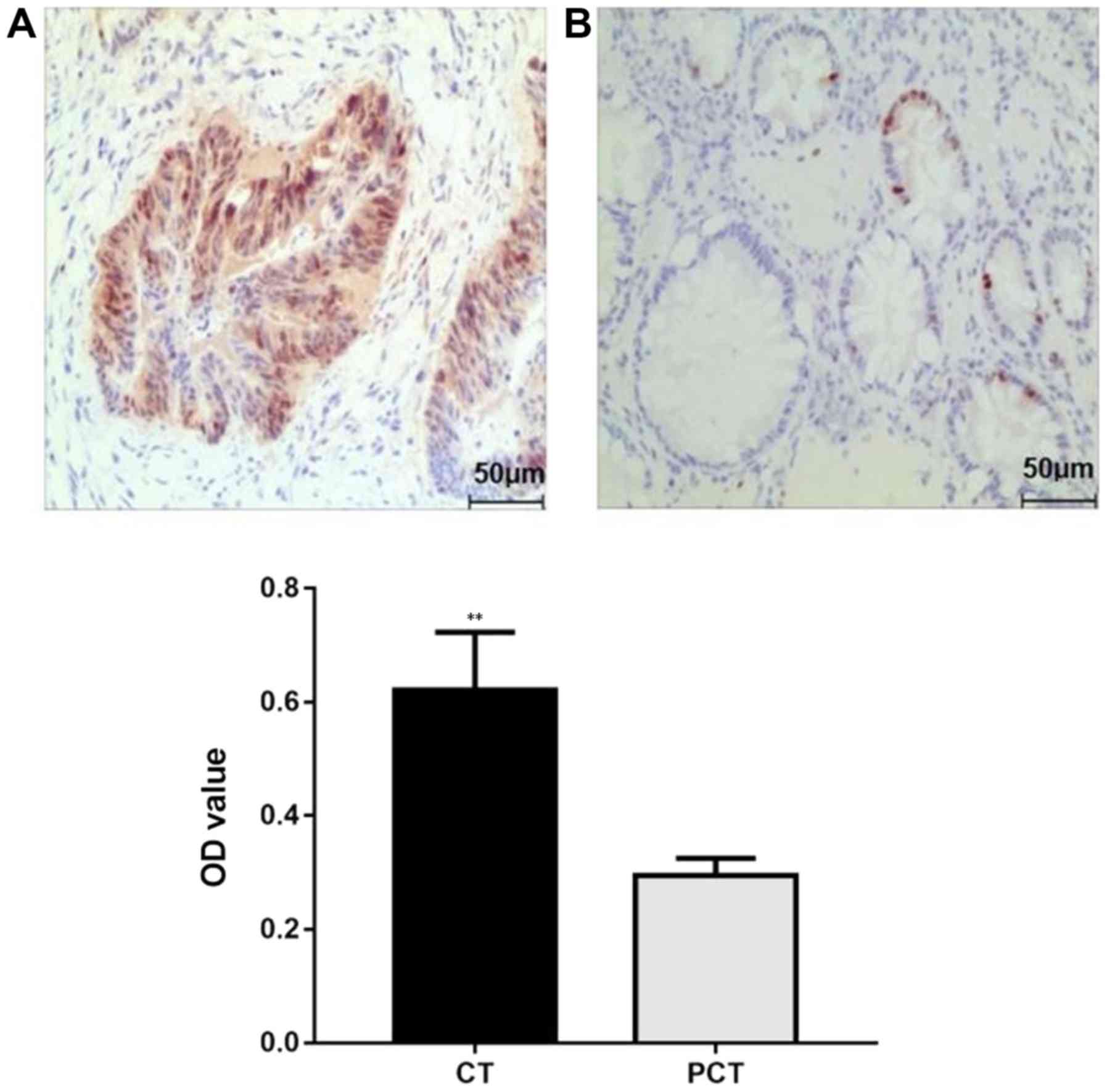
Immunohistochemical staining of cancer tissue
sections under optical microscopy revealed that the omentin-1
protein exhibited brownish-yellow staining in the cytoplasm of
cancer cells and the intercellular substance. Weak expression was
also observed in normal colonic epithelial cells in para-carcinoma
tissues (Fig. 1). The mean optical
density of the selected field of view of each slice was further
calculated by immunohistochemical image analysis software; the
results demonstrated that the expression of omentin-1 protein in
human CRC tissues was higher compared with that of para-carcinoma
tissues (0.621±0.102 vs. 0.295±0.030; P<0.01).
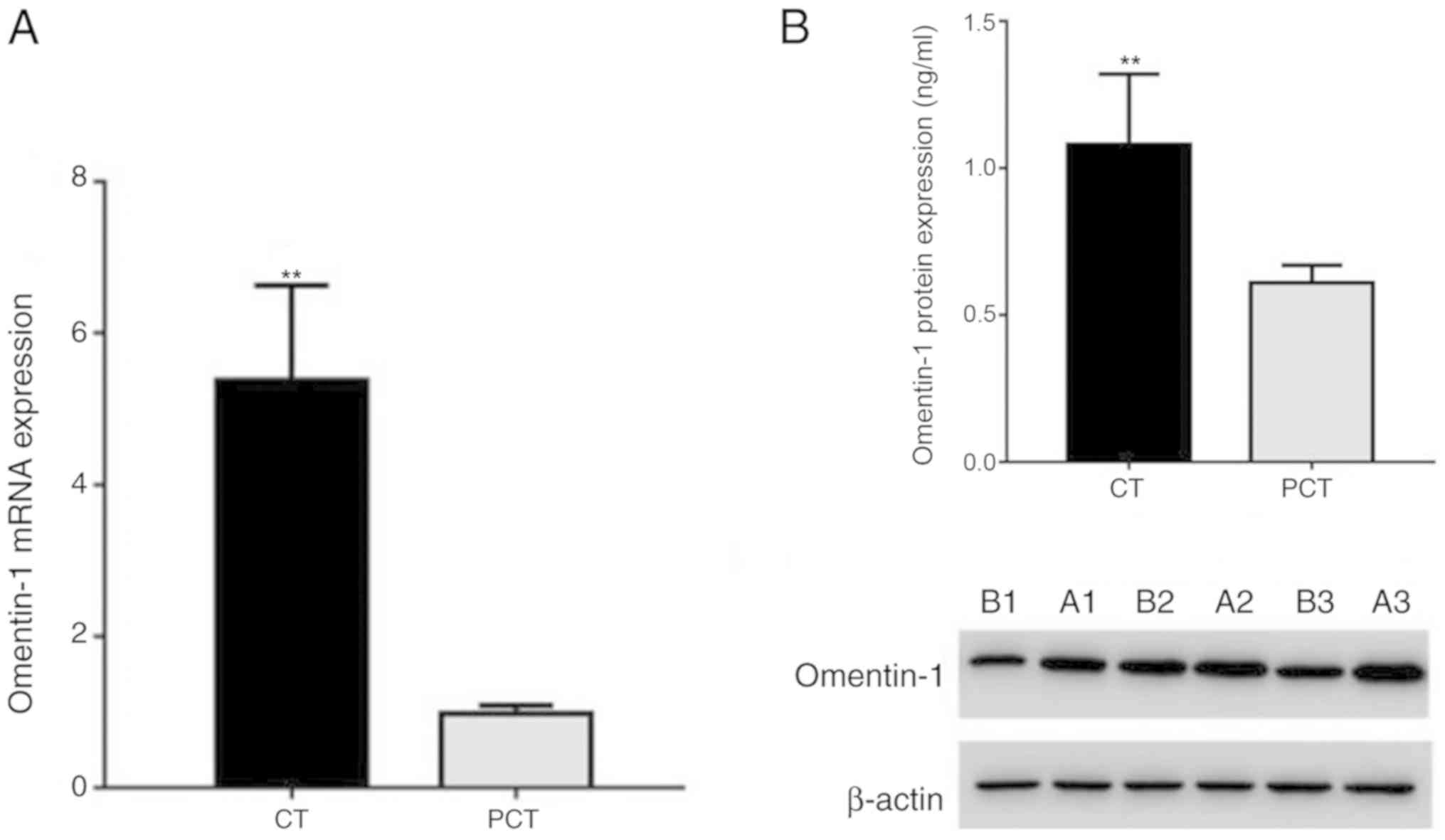
Omentin-1 mRNA and protein expression
levels in human CRC and para-carcinoma tissues
The mRNA (Fig. 2A)
and protein (Fig. 2B) levels of
omentin-1 determined by RT-qPCR and western blotting, respectively,
were significantly higher in human colon carcinoma tissues compared
with those in para-carcinoma tissues, and the difference was
statistically significant (mRNA, 5.38±1.25 vs. 0.98±0.11;
P<0.01; protein, 1.08±0.24 vs. 0.61±0.06; P<0.01).
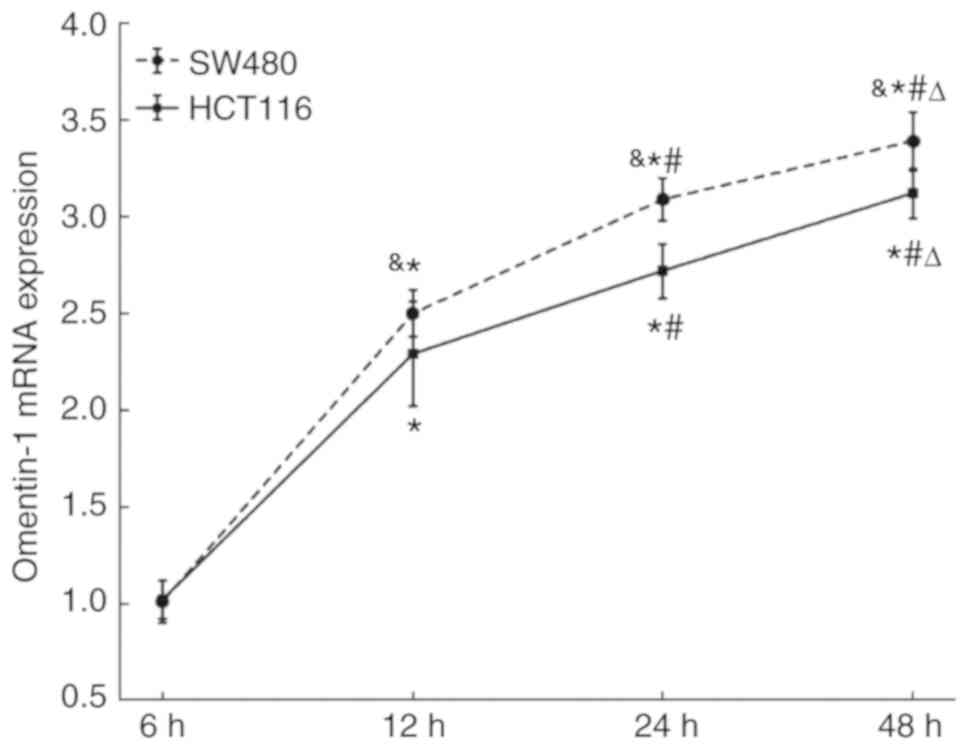
Comparison of the omentin-1 mRNA
expression between SW480 cells and HCT116 cells at different
times
SW480 and HCT116 CRC cells secreted omentin-1 mRNA
in a time-dependent manner (P<0.05). In addition, the expression
of omentin-1 mRNA in was higher in SW480 cells compared with that
in HCT116 cells at 12, 24 and 48 h, however, the difference was not
statistically significant (Fig. 3).
Therefore, SW480 cells were selected for the following
experiment.
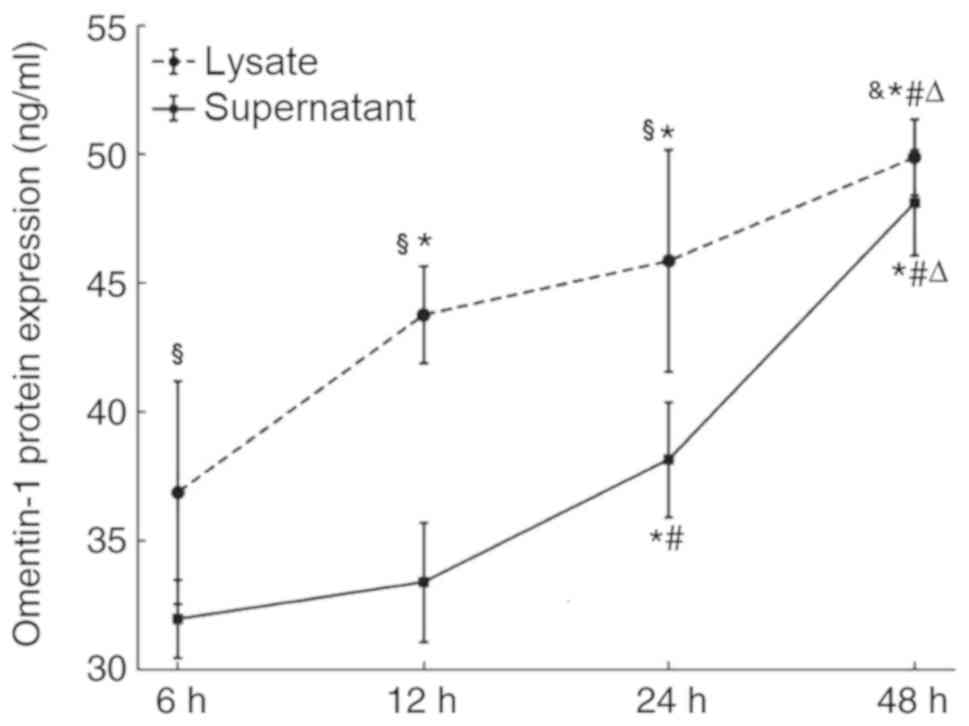
Omentin-1 protein expression in the
supernatant and lysate of SW480 cells
The ELISA results demonstrated that the supernatant
of SW480 cells contained 31.99, 33.40, 38.16 and 48.10 ng/ml
omentin-1 protein at 6, 12, 24 and 48 h, respectively. The lysate
of SW480 cells contained 36.88, 43.76, 45.86 and 49.87 ng/ml
omentin-1 protein at 6, 12, 24 and 48 h, respectively. The
concentration of omentin-1 was higher in the cell lysates compared
with that in the supernatants and the difference is statistically
significant at 6, 12 and 24 h. Moreover, the secretion of omentin-1
in the cell lysates and the supernatants increased over time
(Fig. 4).
Discussion
Adipose tissue is not only a depot of energy, but
also an active endocrine organ, which directly or indirectly
affects the pathogenesis of obesity-related disorders through
generating large quantities of bioactive proteins termed adipokines
or adipocytokines (27). Adipokines
are crucial to insulin resistance, regulation of local and systemic
inflammation, and associated metabolic complications in a paracrine
or endocrine manner. Previous studies have identified associations
between cancer biology and certain adipokines (5,11,28).
Omentin-1 may promote tumour proliferation or
apoptosis depending on the type of cancer cells. A previous study
has demonstrated that omentin-1 promotes human hepatocellular
carcinoma apoptosis in vitro via activation of the JNK
signalling pathway and p53 up-regulation (29). Another study has demonstrated that
the loss of omentin-1 may be involved in obesity-related
carcinogenesis (30). By contrast,
Uyeturk et al (31) reported
a moderate negative correlation between omentin-1 and BMI in
patients with benign prostatic hyperplasia; conversely, circulating
omentin-1 levels were elevated in patients with prostate cancer.
These results may reflect the pathological and ‘Janus-faced’
biological characteristics of omentin-1 in different types of
cancer (32). Therefore, the aim of
the present study was to explore positive or negative outcomes in
patients with CRC.
As we know, the expression of omentin-1 is inversely
correlated with obesity (33), and
obesity is a risk factor for CRC; therefore, it is speculated that
the expression of omentin-1 in patients with obesity and at risk of
CRC should be low. However, Aleksandrova et al (23) demonstrated in a prospective cohort
study that omentin-1 concentration was positively correlated with
the risk of CRC, similar to the results of Fazeli et al
(22). A previous study also
reported this contradiction: The expression of omentin-1 was
decreased in patients with obesity compared with that in healthy
subjects; the risk of CRC was increased in patients with obesity;
and omentin-1 promoted the proliferation of colon cancer cells
(24). This suggested that, in
addition to human visceral adipose tissue, omentin-1 may be
secreted by other cells, at least in patients with CRC. A previous
study suggested that the colon cancer cell line HCT116 did not
produce resistin in CRC and that other cells that secrete this
adipokine may be present in the cancer stroma; however, the
concentration of visfatin was greater in the cell lysate compared
with that in the supernatant, and the secretion of this adipokine
was increased over time (34). These
results demonstrated that HCT116 cells endogenously secreted and
expressed visfatin. Therefore, it is speculated that the colon
cancer cell lines SW480 and HCT116 can also secrete autocrine
omentin-1. The immunohistochemical experiments in the present study
demonstrated that the expression of omentin-1 protein was mainly
located in the cytoplasm and interstitial CRC cells. Further
analysis revealed that the protein and mRNA levels of omentin-1 in
human CRC tissues of 24 patients were significantly higher compared
with those of the para-carcinoma tissues. To test this hypothesis,
human SW480 and HCT116 CRC cells were cultured and the results
indicated that SW480 and HCT11 expressed omentin-1, and SW480 cells
exhibited higher expression levels of omentin-1 mRNA compared with
those of HCT116 cells, however, with no statistical significance.
Thus, SW480 was selected as the cell line for subsequent studies.
The study found that the concentration of omentin-1 was higher in
the cell lysates compared with that in the supernatants with
statistical significance at 6, 12 and 24 h. Moreover, the secretion
of omentin-1 in the cell lysates and supernatants increased over
time. These results revealed that SW480 cells could endogenously
secrete and express omentin-1. A previous study demonstrated that
omentin-1 induced the proliferation of colon cancer SW480 cells
(24); similarly, omentin-1 gene
silencing may inhibit the proliferation and promote the apoptosis
of human SW480 cells (35). The
present study further demonstrated that the CRC cell line SW480 may
secrete this adipokine. Therefore, based on the above results,
omentin-1 may act on CRC cells in an autocrine manner.
A previous study has demonstrated that omentin-1
increased glucose intake through stimulating insulin production in
subcutaneous and omental human adipocytes (17). Conversely, insulin and glucose
dose-dependently decreased omentin-1 mRNA and protein expression,
as well as secretion (36). In
clinical studies, omentin-1 levels were decreased in patients with
type 2 diabetes compared with those of normal subjects (37) and obesity compared with those of lean
subjects (38). Therefore, omentin-1
levels are considered to be an indicator of the metabolic
consequences or co-morbidities associated with obesity (39). It is unclear whether high omentin-1
expression is associated with positive or negative outcomes in
patients with different diseases. Shibata et al (40) reported that circulating omentin-1
levels exhibited a negative correlation with carotid intima-media
thickness in apparently healthy Japanese men. Greulich et al
(41) identified omentin-1 as a
cardioprotective adipokine, and demonstrated that cardiovascular
dysfunction in type 2 diabetes may be due to a decline in omentin-1
levels. In addition, omentin-1 inhibits TNF-α-induced vascular
inflammation in human endothelial cells (42). Similarly, in vascular smooth muscle
cells, omentin-1 inhibits TNF-α-induced vascular cell adhesion
molecule-1 expression (43).
Circulating omentin-1 concentration negatively correlates with
C-reactive protein in subjects with normal and impaired glucose
tolerance (44). These results
suggest an anti-inflammatory role of omentin-1, and provide a
different perspective on the role of adipokines in chronic
inflammation, as well as in the occurrence and progression of colon
cancer. To further understand the effects of different adipokines
in cancer progression, further studies are needed.
The present study had several limitations. Firstly,
unneglectable variations exist between different CRC cell lines,
presented by highly variable gene expression, proliferation and
metastatic potential, which results in clinically heterogeneous
cancer development. To simplify the analysis, only two cell lines
were examined in the present study. Furthermore, the present study
did not explore the role of inflammation or immune cells in
omentin-1 expression in cancerous tissues. To determine whether CRC
cells exhibit variable omentin-1 expression abilities, further
in vivo experiments focusing on CRC are required.
In conclusion, omentin-1 protein was demonstrated to
be synthesized by colon epithelial cancer cells, and omentin-1
affected SW480 CRC cells in an autocrine and endocrine (produced by
adipocytes) manner. Therefore, the novel adipokine omentin-1 may
serve an important role in the therapeutic strategy for CRC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Anhui (grant. no. 1508085MH150).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and XZ designed the study, collected the samples
and materials, analysed the data and wrote the manuscript. MC
designed the study and analysed the data with YZ and XZ. All
authors approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Anhui Medical
University. Informed consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
NCD Risk Factor Collaboration (NCD-RisC),
. Worldwide trends in body-mass index, underweight, overweight, and
obesity from 1975 to 2016: A pooled analysis of 2416
population-based measurement studies in 128·9 million children,
adolescents, and adults. Lancet. 390:2627–2642. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iyengar NM, Morris PG, Hudis CA and
Dannenberg AJ: Obesity, inflammation, and breast cancer. Obesity,
Inflammation and Cancer. Dannenberg A and Berger N: Springer; New
York, NY: pp. 181–217. 2013, View Article : Google Scholar
|
|
3
|
Yoon HH, Lewis MA, Shi Q, Khan M, Cassivi
SD, Diasio RB and Sinicrope FA: Prognostic impact of body mass
index stratified by smoking status in patients with esophageal
adenocarcinoma. J Clin Oncol. 29:4561–4567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iyengar NM, Hudis CA and Dannenberg AJ:
Obesity and inflammation: New insights into breast cancer
development and progression. Am Soc Clin Oncol Educ Book. 33:46–51.
2013. View Article : Google Scholar
|
|
5
|
van Kruijsdijk RC, van der Wall E and
Visseren FL: Obesity and cancer: The role of dysfunctional adipose
tissue. Cancer Epidemiol Biomarkers Prev. 18:2569–2578. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Durko L and Malecka-Panas E: Lifestyle
modifications and colorectal cancer. Curr Colorectal Cancer Rep.
10:45–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamaji T, Iwasaki M, Sasazuki S and
Tsugane S: Interaction between adiponectin and leptin Influences
the risk of colorectal adenoma. Cancer Res. 70:5430–5437. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Otake S, Takeda H, Fujishima S, Fukui T,
Orii T, Sato T, Sasaki Y, Nishise S and Kawata S: Decreased levels
of plasma adiponectin associated with increased risk of colorectal
cancer. World J Gastroenterol. 16:1252–1257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumor A, Daniel P, Pietruczuk M and
Malecka-Panas E: Serum leptin, adiponectin, and resistin
concentration in colorectal adenoma and carcinoma (CC) patients.
Int J Colorectal Dis. 24:275–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nowakowska-Zajdel E, Wierzchowiec O, Kokot
T, Fatygaa E and Muc-Wierzgon M: Metabolic abnormalities in
colorectal cancer patients. J Endocrinol Metab. 2:135–138.
2012.
|
|
12
|
Halberg N, Wernstedt-Asterholm I and
Scherer PE: The adipocyte as an endocrine cell. Endocrinol Metab
Clin North Am. 37:753–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trujillo ME and Scherer PE: Adipose
tissue-derived factors: Impact on health and disease. Endocr Rev.
27:762–789. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paz-Filho G, Lim EL, Wong ML and Licinio
J: Associations between adipokines and obesity-related cancer.
Front Biosci (Landmark Ed). 16:1634–1650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Balistreri CR, Caruso C and Candore G: The
role of adipose tissue and adipokines in obesity-related
inflammatory diseases. Mediators Inflamm. 2010:8020782010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wrackmeyer U, Hansen GH, Seya T and
Danielsen EM: Intelectin: A novel lipid raft-associated protein in
the enterocyte brush border. Biochemistry. 45:9188–9197. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang RZ, Lee MJ, Hu H, Pray J, Wu HB,
Hansen BC, Shuldiner AR, Fried SK, McLenithan JC and Gong DW:
Identification of omentin as a novel depot-specific adipokine in
human adipose tissue: Possible role in modulating insulin action.
Am J Physiol Endocrinol Metab. 290:E1253–E1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sepandar F, Rashidbeygi E, Maghbooli Z,
Khorrami-Nezhad L, Hajizadehoghaz M and Mirzaei K: The association
between resting metabolic rate and metabolic syndrome May Be
mediated by adipokines in overweight and obese women. Diabetes
Metab Syndr. 13:530–534. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li F, Pang LZ, Zhang L, Zhang Y, Zhang YY,
Yu BY and Kou JP: YiQiFuMai powder injection ameliorates chronic
heart failure through cross-talk between adipose tissue and
cardiomyocytes via up-regulation of circulating adipokine omentin.
Biomed Pharmacother. 119:1094182019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zabetian-Targhi F, Mirzaei K, Keshavarz SA
and Hossein-Nezhad A: Modulatory Role of omentin-1 in inflammation:
Cytokines and dietary intake. J Am Coll Nutr. 35:670–678. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Briana DD and Malamitsi-Puchner A:
Intrauterine growth restriction: The controversial role of
perinatal adipocytokines in the prediction of metabolic adult
disease. J Matern Fetal Neonatal Med. 1–6. 2019:Sept 25–2019.(Epub
ahead of print). doi: 10.1080/14767058.2019.1669556.). View Article : Google Scholar
|
|
22
|
Fazeli MS, Dashti H, Akbarzadeh S, Assadi
M, Aminian A, Keramati MR and Nabipour I: Circulating levels of
novel adipocytokines in patients with colorectal cancer. Cytokine.
62:81–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aleksandrova K, di Giuseppe R, Isermann B,
Biemann R, Schulze M, Wittenbecher C, Fritsche A, Lehmann R, Menzel
J, Weikert C, et al: Circulating omentin as a novel biomarker for
colorectal cancer risk: Data from the EPIC-Potsdam cohort study.
Cancer Res. 76:3862–3871. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan C, Xiaotong Zhao and Mingwei C: A
study on relationship of adipokine omentin-1 involved in colorectal
cancer. Acta Univ Med Anhui. 1:75–78. 2015.
|
|
25
|
Qin Y, Huo ZB, Song X, Chen X, Tian X and
Wang X: mir-106a regulates cell proliferation and apoptosis of
colon cancer cells through targeting the PTEN/PI3K/AKT signaling
pathway. Oncol Lett. 15:3197–3201. 2018.PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Booth A, Magnuson A, Fouts J and Foster M:
Adipose tissue, obesity and adipokines: Role in cancer promotion.
Horm Mol Biol Clin Investig. 21:57–74. 2015.PubMed/NCBI
|
|
28
|
Yang G, Fan W, Luo B, Xu Z, Wang P, Tang
S, Xu P and Yu M: Circulating resistin levels and risk of
colorectal cancer: A Meta-analysis. Biomed Res Int.
2016:73674852016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang YY and Zhou LM: Omentin-1, a new
adipokine, promotes apoptosis through regulating Sirt1-dependent
p53 deacetylation in hepatocellular carcinoma cells. Eur J
Pharmacol. 698:137–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Washimi K, Yokose T, Yamashita M, Kageyama
T, Suzuki K, Yoshihara M, Miyagi Y, Hayashi H and Tsuji S: Specific
expression of human intelectin-1 in malignant pleural mesothelioma
and gastrointestinal goblet cells. PLoS One. 7:398892012.
View Article : Google Scholar
|
|
31
|
Uyeturk U, Sarıci H, Kın Tekce B, Eroglu
M, Kemahl E, Uyeturk U and Gucuk A: Serum omentin level in patients
with prostate cancer. Med Oncol. 31:9232014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kawashima K, Maeda K, Saigo C, Kito Y,
Yoshida K and Takeuchi T: Adiponectin and intelectin-1: Important
adipokine players in obesity-related colorectal Carcinogenesis. Int
J Mol Sci. 18(pii): E8662017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jaikanth C, Gurumurthy P, Cherian KM and
Indhumathi T: Emergence of omentin as a pleiotropic adipocytokine.
Exp Clin Endocrinol Diabetes. 121:377–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ghaemmaghami S, Mohaddes SM, Hedayati M,
Gorgian Mohammadi M and Dehbashi G: Resistin and visfatin
expression in HCT-116 colorectal cancer cell line. Int J Mol Cell
Med. 2:143–150. 2012.
|
|
35
|
Li XT, Wan LJ, Zhang QH, Chen MW, Xia TJ,
Xu M and Tang SG: Effects of omentin-1 gene silence on
proliferation and apoptosis of colon cancer cells. Acta Univ Med
Anhui. 54:60–63. 2019.
|
|
36
|
Tan BK, Adya R, Farhatullah S, Lewandowski
KC, O'Hare P, Lehnert H and Randeva HS: Omentin-1, a novel
adipokine, is decreased in overweight insulin-resistant women with
polycystic ovary syndrome: Ex vivo and in vivo regulation of
omentin-1 by insulin and glucose. Diabetes. 57:801–808. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pan HY, Guo L and Li Q: Changes of serum
omentin-1levels in normal subjects and in patients with impaired
glucose regulation and with newly diagnosed and untreated type 2
diabetes. Diabetes Res Clin Pract. 88:29–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Souza Batista CM, Yang RZ, Lee MJ,
Glynn NM, Yu DZ, Pray J, Ndubuizu K, Patil S, Schwartz A, Kligman
M, et al: Omentin plasma levels and gene expression are decreased
in obesity. Diabetes. 56:1655–1661. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tan BK, Adya R and Randeva HS: Omentin: A
novel link between inflammation, diabesity, and cardiovascular
disease. Trends Cardiovasc Med. 20:143–148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shibata R, Takahashi R, Kataoka Y, Ohashi
K, Ikeda N, Kihara S, Murohara T and Ouchi N: Association of
fat-derived plasma protein omentin with carotid artery intima-media
thickness in apparently healthy men. Hypertens Res. 34:1309–1312.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Greulich S, Chen WJ, Maxhera B, Rijzewijk
LJ, van der Meer RW, Jonker JT, Mueller H, de Wiza DH, Floerke RR,
Smiris K, et al: Cardioprotective properties of omentin-1 in type 2
diabetes: Evidence from clinical and in vitro studies. PLoS One.
8:e596792013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yamawaki H, Kuramoto J, Kameshima S, Usui
T, Okada M and Hara Y: Omentin, a novel adipocytokine inhibits
TNF-induced vascular inflammation in human endothelial cells.
Biochem Biophys Res Commun. 408:339–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kazama K, Usui T, Okada M, Hara Y and
Yamawaki H: Omentin plays an anti-inflammatory role through
inhibition of TNF-α-induced superoxide production in vascular
smooth muscle cells. Eur J Pharmacol. 686:116–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moreno-Navarrete JM, Ortega F, Castro A,
Sabater M, Ricart W and Fernández-Real JM: Circulating omentin as a
novel biomarker of endothelial dysfunction. Obesity (Silver
Spring). 19:1552–1559. 2011. View Article : Google Scholar : PubMed/NCBI
|