Introduction
Thyroid cancer is the most common endocrine
malignancy, and its incidence has rapidly increased globally over
the past few decades. The incidence rate of thyroid cancer tripled
from 4.9 to 14.3 per 100,000 individuals between 1975 and 2009
(1). Papillary thyroid carcinoma
(PTC) is the most frequent type of thyroid cancer, accounting for
80–90% of all thyroid malignancies (2), and conventional PTC is the main
histological variant (3).
The BRAF-V600E mutation gene is the most
frequent oncogenic genetic alteration in PTC, accounting for
approximately 45% of PTC cases, and has been shown to be associated
with a poor prognosis of PTC (4).
This mutation is associated with aggressive tumor behavior, disease
recurrence and disease-specific mortality in PTC (5–7).
Numerous studies have documented the oncogenic molecular mechanisms
of BRAF-V600E driving the aggressiveness of PTC
(8,9). The American Joint Committee on Cancer
(AJCC)/Union for International Cancer Control reconvened in 2016 to
update the tumor node metastasis (TNM) staging system and published
its 8th edition, in which significant changes were introduced
regarding thyroid cancer (10). The
age factor is a major change in the revised 8th edition of AJCC, as
the age of 55 years has been introduced as the cut-off point
demarcating the age-related prognostic risk of thyroid cancer, and
patients aged >55 years appear to have a worse prognosis
compared with patients younger than 55 years (10). A large multicenter study by Xing
et al (5) demonstrated that
the BRAF-V600E mutation was significantly associated
with the risk of recurrence in PTC. Based on these data, it appears
that the BRAF-V600E status in isolation is not
sufficient to substantially contribute to risk stratification in
the majority of the patients. In addition, an incremental
improvement in risk stratification may be achieved if the
BRAF-V600E mutational status is considered in the
context of other standard clinicopathological risk factors
(11).
Therefore, synergistic interaction between
BRAF-V600E mutation and age stratification may better
predict the prognosis of PTC. However, it was previously reported
that the BRAF-V600E mutational status was not an
independent prognostic factor when patients were grouped by age
into two categories (<45 and ≥45 years) and into three
categories (<35, 35–60 and ≥60 years) (12). The aim of the present study was to
investigate whether the synergistic interaction between
BRAF-V600E mutational status and the new age
stratification with a cut-off age of 55 years is more efficient in
predicting the prognosis of PTC.
Materials and methods
Data source
The Cancer Genome Atlas (TCGA) Data Portal
(https://tcga-data.nci.nih.gov) is the
result of a collaboration between the National Cancer Institute and
the National Human Genome Research Institute (13). A comprehensive multi-dimensional map
of the key genomic changes in 33 types of cancer has been
generated. High-quality tumor and matched normal samples from 507
patients with thyroid cancer were collected and identified via the
TCGA database, of which 475 patients had complete
clinicopathological data.
Patient grouping and definition
A total of 475 patients were divided into two groups
according to the new cut-off age, namely the <55 years and the
≥55 years age groups. Lymph node positivity was defined as a count
of positive lymph nodes >5. Tumors were staged according to the
TNM staging system recommended by the 8th edition of AJCC (10). Based on the 2015 American Thyroid
Association (ATA) Management Guidelines (11), patients with gross extrathyroidal
extension (ETE), incomplete tumor resection, distant metastases or
lymph nodes >3 cm were classified into the high risk of
recurrence group; those with aggressive histological
characteristics, minor ETE, vascular invasion, or >5 involved
lymph nodes (0.2–3 cm) were classified into the intermediate risk
of recurrence group; and those with intrathyroidal differentiated
thyroid cancer (DTC) (11) and ≤5
lymph node micrometastases (<0.2 cm) were considered as
the low risk of recurrence group.
General information
In the present study, gene-level gene expression
data from mRNA-seq, BRAF-V600E mutation data and
clinicopathological information of 475 patients with PTC were
extracted from TCGA up until September 26, 2018. A total of 475
patients diagnosed with PTC, including 352 women (74.1%) and 123
men (25.9%), were investigated. The median age of the patients was
46 years [interquartile range (IQR), 35–58 years]. Based on the
cut-off age of 55 years, the patients were divided into the <55
years and the ≥55 years age groups. Patient age, sex, ethnicity,
tumor size, multifocality, lymphocytic thyroiditis, histology,
lymph node positivity, ETE, residual tumor, radioactive iodine
(RAI) therapy, RAI dose, recurrence follow-up time, mortality
follow-up time, TNM stage and AJCC stage (Tables I and II) were compared between the two groups.
The overall BRFA-V600E mutation prevalence was 50.3% and
the overall PTC recurrence rate was 6.4%.
 | Table I.Univariate analyses of association
between BRAF-V600E mutation status and
clinicopathological parameters in <55-age group. |
Table I.
Univariate analyses of association
between BRAF-V600E mutation status and
clinicopathological parameters in <55-age group.
|
|
|
BRAF-V600E |
|
|
|---|
|
|
|
|
|
|
|---|
| Patients'
parameters | Total | Mutation | Wild-type | Odds ratio (95%
CI) | P-value |
|---|
| Age, years (mean ±
standard deviation) | 38±10 | 39±10 | 37±10 | NA | 0.083 |
| Sex, n |
|
Female | 249 | 128 | 121 | 1 | 0.910 |
|
Male | 69 | 36 | 33 | 1.031
(0.605–1.759) |
|
| Ethnicity category,
n |
|
Caucasian | 207 | 116 | 91 | 1 | 0.742 |
|
Asian | 38 | 20 | 18 | 0.872
(0.436–1.744) |
|
|
Black | 15 | 7 | 8 | 0.686
(0.240–1.963) |
|
| Tumor size, cm
(mean ± standard deviation) | 1.7±0.8 | 1.6±0.9 | 1.7±0.8 | NA | <0.001a |
| Tumor foci, n |
|
Unifocality | 285 | 36 | 249 | 1 | 0.036a |
|
Multifocality | 33 | 0 | 33 | 1.133
(1.085–1.182) |
|
| Lymphocytic
thyroiditis, n |
| No | 240 | 119 | 120 | 1 | 0.099 |
|
Yes | 45 | 29 | 17 | 1.720
(0.898–3.296) |
|
| Histology, n |
|
CPTC | 237 | 142 | 95 | 1 | <0.001a |
|
FVPTC | 63 | 9 | 54 | 0.112
(0.053–0.237) |
|
|
TCPTC | 18 | 13 | 5 | 1.739
(0.600–5.039) |
|
| Lymph nodes
positivity, n (>5) |
| No | 190 | 102 | 88 | 1 | 0.618 |
|
Yes | 60 | 30 | 30 | 0.863
(0.483–1.542) |
|
| ETE, n (gross) |
| No | 307 | 160 | 147 | 1 | 1.000b |
|
Yes | 2 | 1 | 1 | 0.919
(0.057–14.822) |
|
| Residual tumor,
n |
| No | 253 | 137 | 116 | 1 | 0.098 |
|
Yes | 29 | 11 | 18 | 0.517
(0.235–1.140) |
|
| RAI therapy, n |
| No | 123 | 62 | 61 | 1 | 0.771 |
|
Yes | 163 | 85 | 78 | 1.072
(0.671–1.713) |
|
| RAI dose, mCi (mean
± standard deviation) | 121±59 | 120±50 | 122±68 | NA | <0.001a |
| Recurrence
follow-up, months | 21 (14–44) | 26 (16–50) | 18 (12–39) | NA | 0.001a |
| Recurrence, n |
| No | 278 | 143 | 135 | 1 | 0.865 |
|
Yes | 13 | 7 | 6 | 1.101
(0.361–3.360) |
|
| Mortality
follow-up, months | 21 (13–44) | 25 (15-51.25) | 18 (12-39.5) | NA | 0.001a |
| Mortality, n |
| No | 318 | 164 | 154 | 1 | NA |
|
Yes | 0 | 0 | 0 | NA |
|
| Recurrence risk
stage, n |
|
Low | 108 | 26 | 82 | 1 | <0.001a |
|
Intermediate | 163 | 124 | 39 | 10.028
(5.675–17.719) |
|
|
High | 33 | 13 | 20 | 2.050
(0.898–4.682) |
|
| T stage, nc |
| 1 | 221 | 114 | 107 | 1 | 0.981 |
| 2 | 69 | 36 | 33 | 1.024
(0.596–1.759) |
|
| 3 | 28 | 14 | 14 | 0.939
(0.428–2.061) |
|
| 4 | 0 | NA | NA | NA |
|
| N stage nc |
| 0 | 133 | 62 | 71 | 1 | 0.105 |
| 1a | 68 | 42 | 26 | 1.850
(1.019–3.357) |
|
| 1b | 45 | 21 | 24 | 1.002
(0.509–1.973) |
|
| M stage, nc |
| 0 | 178 | 96 | 82 | 1 | 1.000b |
| 1 | 4 | 2 | 2 | 0.854
(0.118–6.199) |
|
| AJCC stage, nc |
| I | 314 | 162 | 152 | 1 | 1.000b |
| II | 4 | 2 | 2 | 0.938
(0.131–6.744) |
|
 | Table II.Univariate analyses of association
between BRAF-V600E mutation status and
clinicopathological parameters in ≥55-age group. |
Table II.
Univariate analyses of association
between BRAF-V600E mutation status and
clinicopathological parameters in ≥55-age group.
|
|
|
BRAF-V600E |
|
|
|---|
|
|
|
|
|
|
|---|
| Patients'
parameters | Total | Mutation | Wild-type | Odds ratio (95%
CI) | P-value |
|---|
| Age, years (mean ±
standard deviation) | 65±9 | 65±8 | 65±9 | NA | 0.976 |
| Sex, n |
|
Female | 103 | 51 | 52 | 1 | 0.546 |
|
Male | 54 | 24 | 30 | 0.816
(0.421–1.580) |
|
| Ethnicity category,
n |
|
Caucasian | 107 | 57 | 50 | 1 | 0.428b |
|
Asian | 9 | 5 | 4 | 1.096
(0.279–4.309) |
|
|
Black | 11 | 3 | 8 | 0.329
(0.083–1.308) |
|
| Tumor size, cm
(mean ± standard deviation) | 1.9±1.0 | 1.7±0.9 | 2.1±1.1 | NA | 0.036a |
| Tumor foci, n |
|
Unifocality | 85 | 46 | 39 | 1 | 0.136 |
|
Multifocality | 69 | 29 | 40 | 0.615
(0.324–1.167) |
|
| Lymphocytic
thyroiditis, n |
| No | 116 | 59 | 57 | 1 | 0.784 |
|
Yes | 21 | 10 | 11 | 0.878
(0.346–2.227) |
|
| Histology, n |
|
CPTC | 106 | 53 | 53 | 1 | <0.001a |
|
FVPTC | 33 | 7 | 26 | 0.269
(0.108–0.674) |
|
|
TCPTC | 18 | 15 | 3 | 5.000
(1.367–18.287) |
|
| Lymph nodes
positivity, n (>5) |
| No | 93 | 46 | 47 | 1 | 0.231 |
|
Yes | 22 | 14 | 8 | 1.788
(0.685–4.665) |
|
| ETE, n (gross) |
| No | 134 | 63 | 71 | 1 | 0.169 |
|
Yes | 17 | 11 | 6 | 2.066
(0.722–5.910) |
|
| Residual tumor,
n |
| No | 110 | 51 | 59 | 1 | 0.111 |
|
Yes | 25 | 16 | 9 | 2.057
(0.837–5.051) |
|
| RAI therapy, n |
| No | 61 | 35 | 26 | 1 | 0.051 |
|
Yes | 83 | 34 | 49 | 0.515
(0.264–1.007) |
|
| RAI dose, mCi (mean
± standard deviation) | 122±52 | 125±20 | 102±62 | NA | 0.032a |
| Recurrence
follow-up, months | 19 (12–34) | 21 (12–47) | 18 (12-28.3) | NA | 0.018a |
| Recurrence, n |
| No | 132 | 58 | 74 | 1 | 0.031a |
|
Yes | 15 | 11 | 4 | 3.509
(1.062–11.590) |
|
| Mortality
follow-up, months | 20 (13–35) | 25 (14–48) | 18 (12.5–27.5) | NA | 0.006a |
| Mortality, n |
| No | 143 | 66 | 77 | 1 | 0.236 |
|
Yes | 14 | 9 | 5 | 1.968
(0.631–6.136) |
|
| Recurrence risk
stage, n |
|
Low | 54 | 9 | 45 | 1 | <0.001a |
|
Intermediate | 58 | 42 | 16 | 13.125
(5.238–32.887) |
|
|
High | 39 | 24 | 15 | 8.000
(3.052–20.967) |
|
| T stage, nc |
| 1 | 85 | 32 | 53 | 1 | 0.036a,b |
| 2 | 37 | 22 | 15 | 2.429
(1.103–5.349) |
|
| 3 | 27 | 15 | 12 | 2.070
(0.861–4.975) |
|
| 4 | 8 | 6 | 2 | 4.969
(0.945–26.116) |
|
| N stage, nc |
| 0 | 84 | 36 | 48 | 1 | 0.036a |
| 1a | 18 | 13 | 5 | 3.467
(1.133–10.606) |
|
| 1b | 26 | 16 | 10 | 2.133
(0.867–5.250) |
|
| M stage, nc |
| 0 | 88 | 44 | 44 | 1 | 1.000b |
| 1 | 5 | 3 | 2 | 1.500
(0.239–9.420) |
|
| AJCC stage, nc |
|
I/II | 143 | 64 | 79 | 1 | 0.016a |
|
III/IV | 14 | 11 | 3 | 0.938
(0.131–6.744) |
|
Clustering analysis
Genes that were differentially expressed between
positive and negative for BRAF-V600E mutation status in
the two age groups were assessed using RStudio software program
(http://www.r-project.org). The heat map was
generated using ‘pheatmap’ package in ‘R’ software (RStudio version
1.1.463) (14) to visualize the gene
expression pattern, with red and green color representing highly
expressed and lowly expressed genes, respectively.
Statistical analysis
Age, tumor size, and RAI dose are presented as mean
± SD while recurrence and mortality follow-up times are presented
as median and quartile ranges Sex, ethnicity, tumor foci,
lymphocytic thyroiditis, histology, lymph nodes positivity (>5),
ETE, residual tumor, RAI therapy, recurrence, recurrence risk
stage, T stage, N stage, M stage and AJCC stage are presented as
the frequency. The statistical analysis was performed using SPSS,
version 19 (IBM Corp.). The association of BRAF-V600E
mutation status and each clinicopathological variable was assessed
using the Pearson's χ2 test and Fisher's exact test when
the number of patient cases was <5. The Kaplan-Meier
analysis and log-rank test were used to analyze recurrence-free
survival (RFS) distribution and to compare the differences between
Kaplan-Meier curves for BRAF-V600E status. The odds
ratio (OR) was determined by univariate analysis, and ORs with 95%
confidence intervals (CIs) were calculated. Multivariate analyses
were conducted with the Cox regression analysis method on disease
recurrence, and hazard ratios (HRs) with 95% CIs were calculated.
All P-values were two-sided, and P<0.05 was considered to
indicate statistically significant differences.
Results
Patient demographics
The overall median follow-up time for all patients
was 20 months (IQR, 14–38.3 months). The median follow-up time was
21 months (IQR, 14–44 months) in the <55 years group and 19
months (IQR, 12–34 months) in the ≥55 years group.
Association between
BRAF-V600E mutation status and clinicopathological
parameters in the <55 years age group
In this group, of the 318 patients who were
diagnosed with PTC [including 249 women (78.3%) and 69 men (21.7%),
with a mean age at diagnosis of 38±10 years (range, 15–54 years)
and a mean tumor size of 1.7±0.8 cm], 232 patients (51.6%) were
positive for BRAF-V600E mutation.
The univariate analyses demonstrated a significant
association between the presence of BRAF-V600E and tumor
size (P<0.001), multifocality (P=0.036), histology (P<0.001),
RAI dose (P<0.001), follow-up time (P=0.001) and recurrence risk
stage (P<0.001), whereas there was no significant association
with age, sex, ethnicity, lymphocytic thyroiditis, lymph node
positivity, ETE, residual tumor, RAI treatment, recurrence, TNM
stage and AJCC stage (Table I).
The median follow-up time was 21 months (IQR, 14–44
months), during which time 13 patients (4.5%) developed recurrence;
there were no reported fatalities. The univariate analyses revealed
no significant association between the BRAF-V600E
mutation and PTC recurrence (P=0.865). Furthermore, the
Kaplan-Meier plot for RFS did not reveal a statistically
significant difference between the presence and absence of
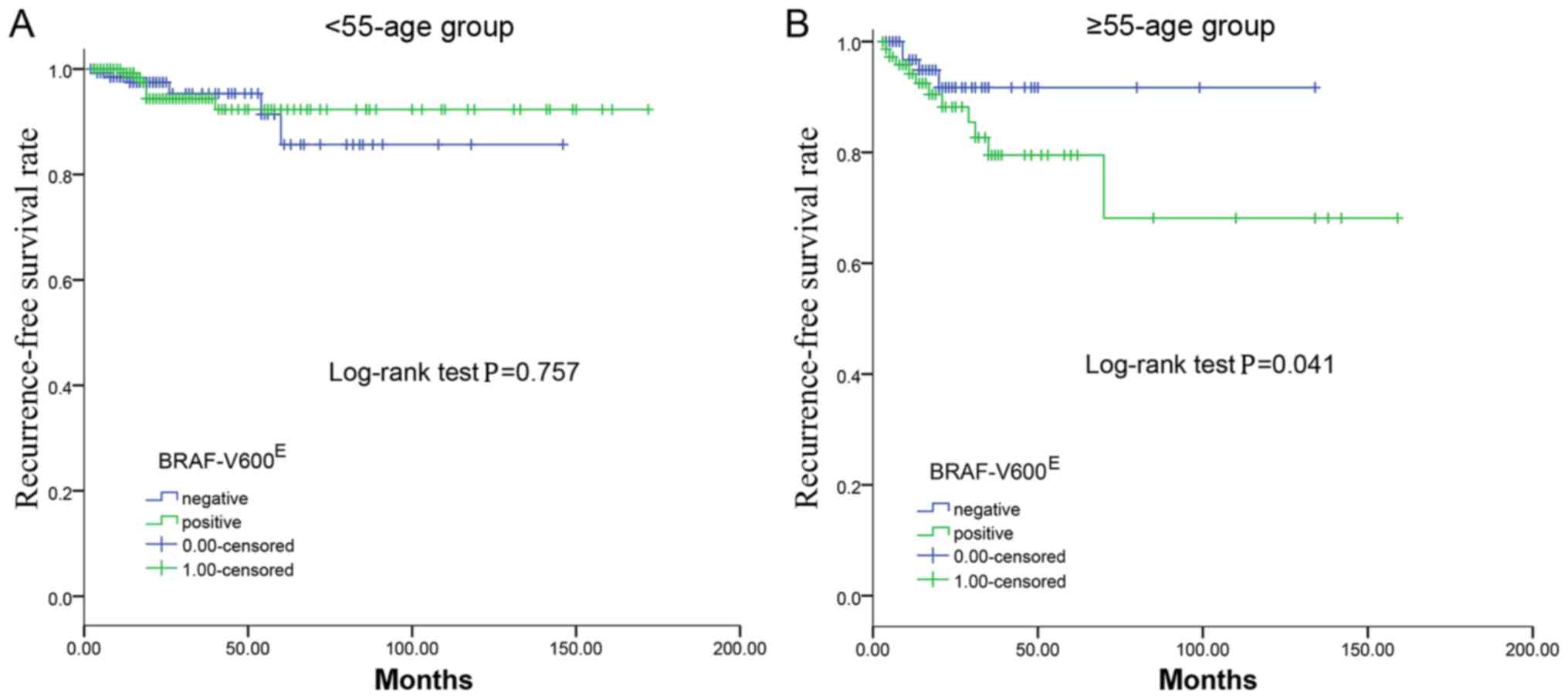
BRAF-V600E (P=0.757; Fig.
1A).
Association between
BRAF-V600E mutation status and clinicopathological
parameters in the ≥55 years age group
The group included a total of 157 patients diagnosed
with PTC, including 103 women (65.6%) and 54 men (34.4%). The mean
age of the patients was 65±9 years and the mean tumor size was
1.9±1.0 cm. The prevalence rate of the BRAF-V600E
mutation was 47.8% (75 cases).
In the ≥55 years age group, the results on
univariate analysis demonstrated a significant association between
BRAF-V600E mutation and tumor size (P=0.036), histology
(P<0.001), RAI therapy dose (P=0.032), recurrence follow-up time
(P=0.018), recurrence (P=0.031), mortality follow-up time
(P=0.006), recurrence risk stage (P<0.001), advanced T stage
(P=0.036), advanced N stage (P=0.036) and advanced AJCC stage
(P=0.016) (Table II). There was no
significant association between BRAF-V600E and age, sex,
ethnicity, multifocality, lymphocytic thyroiditis, lymph node
positivity, ETE, residual tumor, RAI therapy, mortality or M stage
(all P≥0.05).
During a mean follow-up of 28 months (range, 3–157
months), 15 cases (10.2%) of recurrence were recorded. The presence
of BRAF-V600E mutation was associated with lower RFS in
the ≥55 year age group (P=0.041; Fig.
1B).
Recurrence risk factors
Multivariate regression analysis controlling for
BRAF-V600E, male sex, multifocality, histology, ETE,
residual tumor, T stage, N stage, M stage and AJCC stage found that
only advanced N stage (P=0.038) and advanced M stage (P=0.028) were
independent predictors of recurrence in the <55 age group
(Table III). Notably, multivariate
analysis demonstrated that male sex (P=0.026), multifocality
(P=0.034), N stage (P=0.001) and M stage (P=0.005) were independent
predictors of recurrence in the ≥55 age group (Table III). However, the Cox multivariate
regression demonstrated that BRAF-V600E was not an
independent predictor of recurrence in either of the two groups
(P=0.758 and 0.993, respectively; Table III). In addition, the Kaplan-Meier
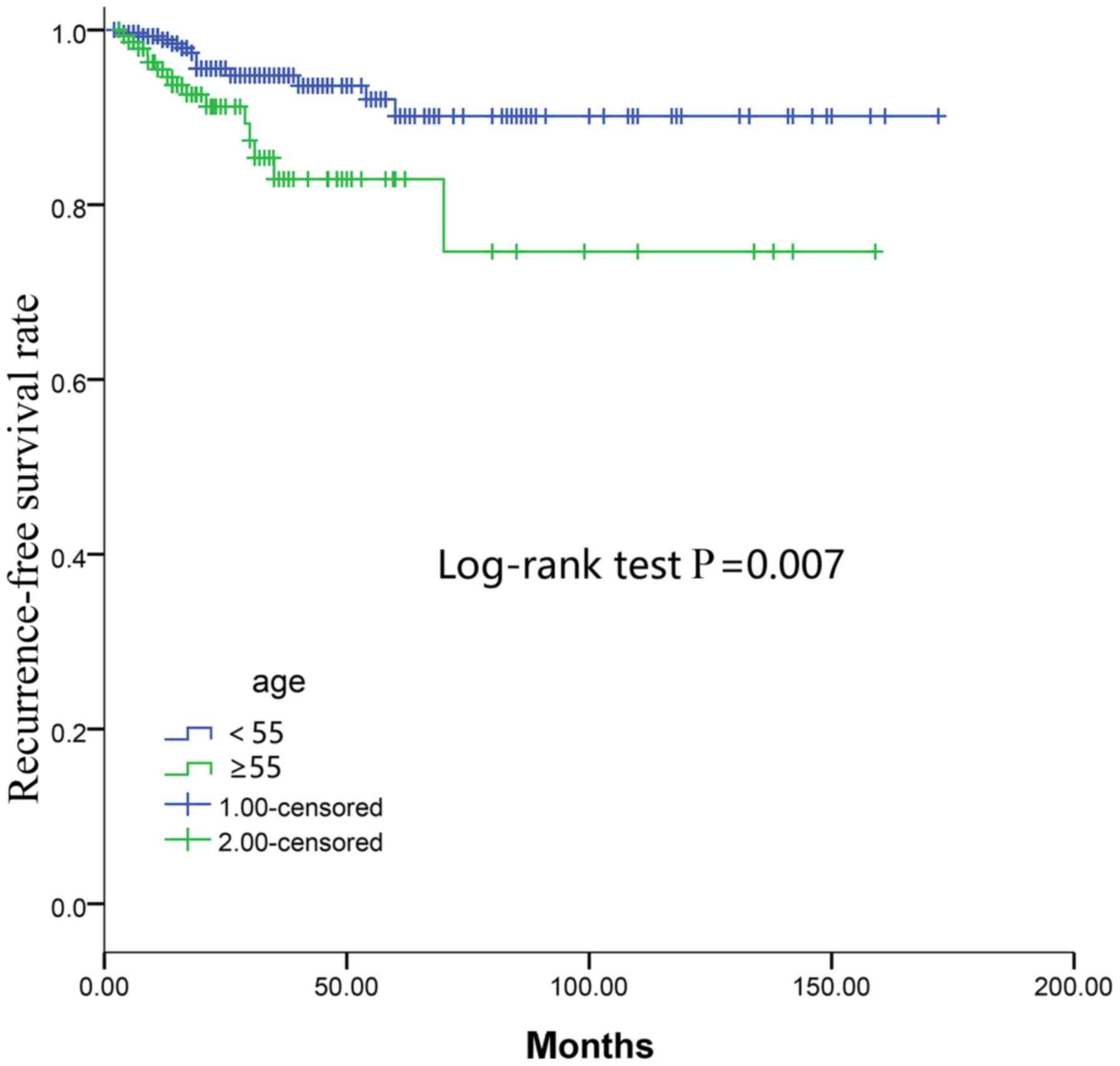
curves of RFS revealed that the ≥55 age group had a lower RFS rate
compared with the <55 age group as determined by the log-rank
test (P=0.007; Fig. 2).
 | Table III.Cox multivariate regression analyses
of factors associated with recurrence. |
Table III.
Cox multivariate regression analyses
of factors associated with recurrence.
|
| <55-age
group | ≥55-age group |
|---|
|
|
|
|
|---|
| Clinicopathologic
features | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| BRAF | 0.842
(0.282–2.513) | 0.758 | 0.995
(0.356–2.781) | 0.993 |
| Male sex | 1.423
(0.315–6.422) | 0.647 | 2.345
(1.108–4.963) | 0.026a |
| Multifocality | 1.753
(0.583–5.272) | 0.318 | 3.180
(2.932–5.850) | 0.034a |
| Histology | 0.716
(0.190–2.693) | 0.621 | 1.333
(0.677–2.623) | 0.405 |
| ETE | 0.716
(0.190–2.693) | 0.621 | 0.422
(0.055–3.256) | 0.408 |
| Residual tumor | 2.484
(0.682–9.050) | 0.168 | 0.999
(0.219–4.559) | 0.999 |
| T stage | 1.363
(0.641–2.902) | 0.421 | 1.268
(0.779–2.065) | 0.340 |
| N stage | 2.453
(1.051–5.722) | 0.038a | 7.873
(5.121–14.781) | 0.001a |
| M stage | 16.010
(1.358–188.707) | 0.028a | 9.043
(1.966–41.602) | 0.005a |
| AJCC stage | 4.215
(0.541–32.855) | 0.170 | 1.093
(0.240–4.968) | 0.908 |
Heat map result by clustering
analysis
Gene variability was then computed using the median
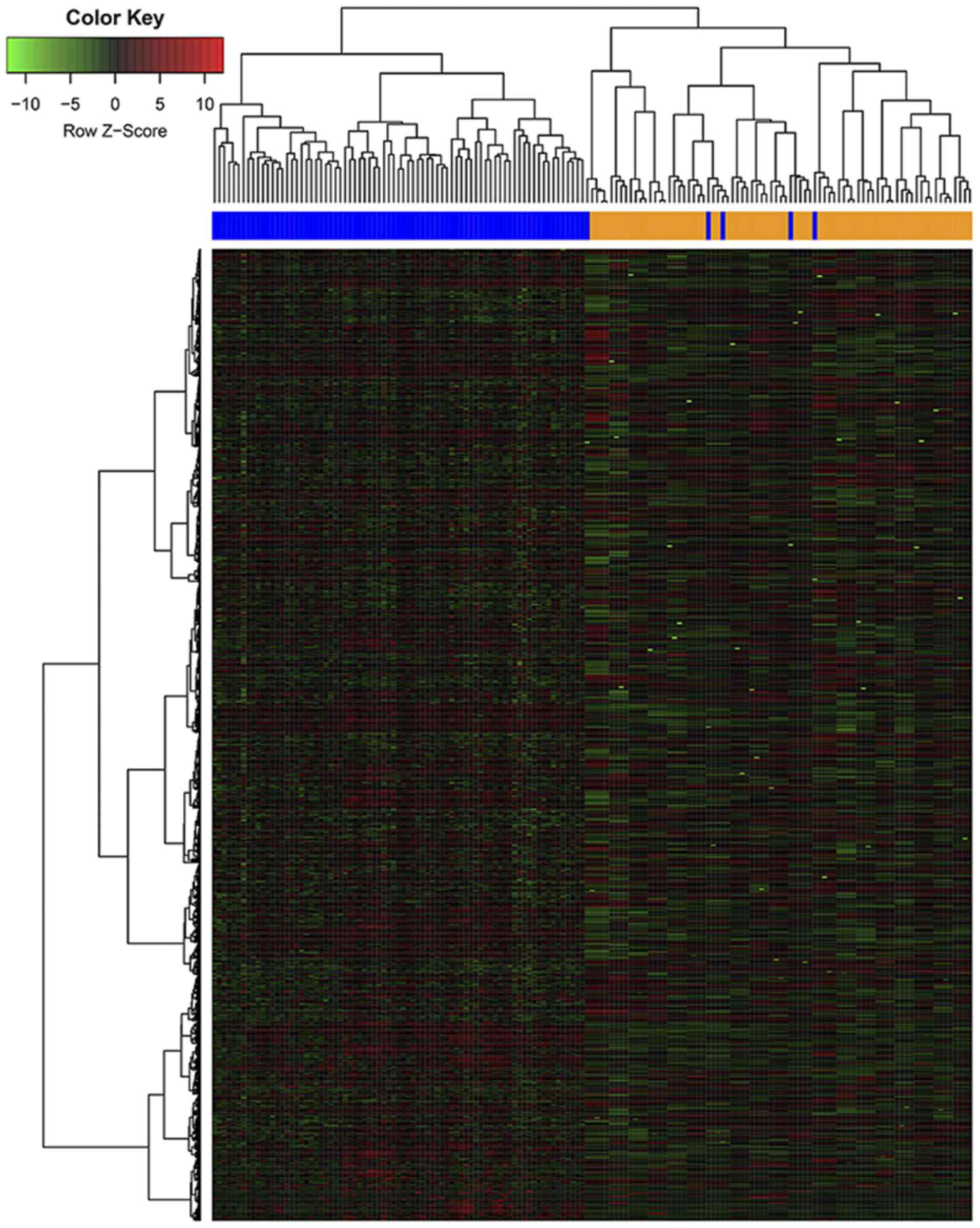
absolute deviation. The 3,120 most variable genes were selected.
The heat map generated from the gene expression data revealed that
there was a significant clustering effect between the positivity
and negativity for BRAF-V600E mutation in the ≥55 year
age group (Fig. 3). By contrast, the
gene expression data demonstrated that there was no significant
difference between the two groups in the <55 year age group.
Discussion
PTC is a well-differentiated papillary carcinoma
with a relatively low mortality rate among thyroid cancers
(14). However, the rate of disease
recurrence or persistence is high, up to 30% (15,16). The
BRAF-V600E mutation has been reported as a prognostic
molecular marker in PTC (8,17,18).
However, the prevalence of the BRAF-V600E mutation in
PTC ranges between 30 and 80% (19).
Therefore, this molecular marker is of limited value for clinical
decision-making in the majority of PTC cases. For thyroid cancer,
the recent 8th edition of the AJCC staging system strongly
emphasizes the overall age-related risk, with a cut-off age at 55
years (10). In addition, several
studies have demonstrated an association of BRAF-V600E
with older age and poor clinical outcomes, including PTC recurrence
and PTC-specific mortality (5,7). The
present study investigated the hypothesis that
BRAF-V600E may better predict PTC aggressiveness and
recurrence based on this age stratification.
This study demonstrated no significant difference
between the presence of BRAF-V600E mutation and
ethnicity, in the ≥55-age group and the <55-age group.
Therefore, we believe that the BRAF-V600E mutation is
not significantly heterogeneous in different ethnicities. By
categorizing patients in the aforementioned two age groups the
effectiveness of BRAF-V600E in predicting aggressiveness
and recurrence of PTC in terms of the cut-off age at 55 years
improved. Furthermore, the heat map revealed significant clustering
between the positive and negative BRAF-V600E cases in
the ≥55 age group. Similar to the results reported by Xing et
al (5), in the ≥55 group, the
presence of the BRAF-V600E mutation was significantly
associated with tumor size, histology, RAI dose, lymph node
metastasis (LNM), advanced AJCC stage (III/IV) and tumor
recurrence, which are the major factors associated with a worse
prognosis of PTC (11). By contrast,
in the <55 age group, the prognostic implications of the
BRAF-V600E mutation in PTC were limited.
In the present study, the RFS distribution suggested
that the ≥55 group exhibited a lower survival rate compared with
the <55 group, and the latter group had a better prognosis. In
addition, it was demonstrated that BRAF-V600E is useful
for predicting prognosis based on age stratification with the
cut-off at 55 years. The molecular mechanism underlying the
age-dependent effect of the BRAF-V600E mutation on the
prognosis of patients with PTC remains to be defined (20). It is possible that certain
age-related genes, such as immune response-associated genes
(21), may cooperate with
BRAF-V600E in conferring poor prognosis, as
BRAF-V600E was shown to be associated with abnormal
immune response in human cancers, including PTC (22,23). The
present study further demonstrated that the presence of the
BRAF-V600E mutation was associated with a lower survival
rate on RFS analysis in the ≥55 age group. However, in the <55
age group, there was no significant difference in survival between
the presence and absence of the BRAF-V600E mutation.
Therefore, BRAF-V600E may better predict the prognosis
and recurrence of PTC based on the 55 year age stratification.
In addition, this study demonstrated that the
synergistic interaction of BRAF-V600E and age
stratification was of greater assistance for clinicians in terms of
optimal decision-making regarding surgical approach and the extent
of surgery. It has been reported that central LNM is the most
common cause of disease recurrence in PTC (24,25).
However, prophylactic central lymph node dissection is not
routinely recommended based on the currently published guideline
(11). The results of the present
study demonstrated that BRAF-V600E was significantly
associated with LNM in the ≥55 age group, and positive
BRAF-V600E mutation status was associated with a higher
risk of LNM compared with the negative mutation status. This study
confirmed the association between the BRAF-V600E
mutation and a higher risk of LNM in the ≥55 age group. By
contrast, in the <55 age group, there was no significant
association between BRAF-V600E and LNM. Whether the
presence of BRAF-V600E with regional LNM exert a
synergistic effect on increasing the rate of locoregional disease
recurrence remains elusive. Further RFS analysis demonstrated that
there was no significant difference between positive and negative
BRAF-V600E mutation status in the LNM subgroup in the
two age groups. Therefore, prophylactic central neck dissection may
be performed in positive BRAF-V600E mutation cases,
particularly in the ≥55 years age group. However, the synergistic
interaction between BRAF-V600E and LNM is unclear, and
patients with PTC who are BRAF-V600E-positive with LNM
may not require more aggressive treatment compared with patients
with PTC who are BRAF-V600E-negative with LNM.
Although RAI is routinely recommended by the 2015
ATA guidelines for patients with intermediate- to high-risk DTC,
there is currently no consensus regarding the dose required for
ablation (11). There may be an
association between the BRAF-V600E mutation and the
expression of certain genes, including sodium/iodide symporter,
thyroid-stimulating hormone receptor, thyroperoxidase,
thyroglobulin, and pendrin (26).
The sodium/iodide symporter gene is involved in RAI metabolism,
therefore mutations could result in impaired sodium/iodide
symporter expression, and also a decrease in iodide-metabolizing
gene expression of thyrotropin receptor, thyroglobulin and
thyroperoxidase (27). This may be a
possible explanation for RAI therapy failure and recurrence of PTC
(28). Although tumors with the
BRAF-V600E mutation tend to be RAI-resistant, that
knowledge, to the best of our knowledge, has yet to affect
decision-making regarding RAI therapy. The results of the present
study revealed no significant association between
BRAF-V600E and RAI therapy in the <55 and ≥55 age
groups. However, the presence of BRAF-V600E was
associated with higher RAI therapy dose in the ≥55 age group. A
large multicenter study reported that the association between
BRAF-V600E-positive mutation and high-dose RAI therapy
is controversial (5). Further
research conducted by the present study revealed no significant
difference between high- and low-dose RAI on RFS analysis in a low-
to intermediate-risk group (based on the 2015 ATA risk
stratification system). Therefore, it is uncertain whether
higher-dose RAI therapy is required for patients with PTC, who
harbor the BRAF-V600E mutation. However, larger samples
and cohort studies are required to confirm whether older patients
with the BRAF-V600E mutation require high-dose RAI
therapy to improve prognosis.
In addition, the presence of BRAF-V600E
was not found to be an independent prognostic factor for predicting
recurrent disease on univariate and multivariate analyses, when
patients were grouped into <45 and ≥45 years age groups
(12). Although
BRAF-V600E had no independent impact on RFS, the
BRAF-V600E mutation better predicted aggressive
clinicopathological characteristics and PTC recurrence based on age
stratification using 55 years as the cut-off.
There were several limitations to the present study.
First, the sample size was relatively small, with 475 cases
downloaded from TCGA. Second, not all of the clinicopathological
data was complete therefore some data is missing. Third, this study
was a retrospective analysis using TCGA data, and prospective
clinical trials are required to provide more reliable results.
Fourth, information associated with the extent of surgery and
thyrotropin suppression is not recorded by TCGA. Finally, the sex
ratio of women to men is 352 to 123, consistent with the ratio of
epidemiology (3.1:1) (1). However,
in the <55 age group, gender distribution was unequal at 249
women and 69 male, which may cause bias.
In summary, the BRAF-V600E mutation in
PTC better predicts aggressive and recurrent disease based on
stratification by 55 years of age. The synergistic interaction
between the BRAF-V600E mutation and age stratification
with a cut-off of 55 years may be more helpful for clinicians and
facilitate optimal decision-making regarding surgical approach and
the extent of surgery.
Acknowledgements
The authors would like to thank Ms. Yiming Tan
(Southwestern University of Finance and Economics, Chengdu) for
editing the language within the manuscript.
Funding
This study was supported by Guangzhou Medicine and
Health Care Technology projects (grant nos., 20161A011008 and
20171A011243), Guangdong Medical Research fund project (grant no.,
A2017415), Guangdong Traditional Chinese Medicine Scientific
research subject (grant no., 20152039).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in The Cancer Genome Atlas repository,
(https://tcga-data.nci.nih.gov).
Authors' contributions
XG and BX conceived the present study. XG wrote the
manuscript. XG, LP and JL designed and revised the manuscript. FS,
JF, MG, ZC and WW analyzed and interpreted the data. WC, PH, XD, WZ
assisted in data analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davies L and Welch HG: Current thyroid
cancer trends in the United States. JAMA Otolaryngol Head Neck
Surg. 140:317–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alzahrani AS and Xing M: Impact of Iymph
node metastases identified on central neck dissection (CND) on the
recurrence of papillary thyroid cancer: Potential role of BRAFV600E
mutation in defining CND. Endocr Relat Cancer. 20:13–22. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen X, Liu R and Xing M: A Six-genotype
genetic prognostic model for papillary thyroid cancer. Endocr Relat
Cancer. 24:41–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xing M: BRAF mutation in thyroid cancer.
Endocr Relat Cancer. 12:245–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xing M, Alzahrani AS, Carson KA, Shong YK,
Kim TY, Viola D, Elisei R, Bendlová B, Yip L, Mian C, et al:
Association between BRAF V600E mutation and recurrence of papillary
thyroid cancer. J Clin Oncol. 33:42–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li C, Lee KC, Schneider EB and Zeiger MA:
BRAF V600E mutation and its association with clinicopathological
features of papillary thyroid cancer: A meta-analysis. J Clin
Endocrinol Metab. 97:4559–4570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elisei R, Ugolini C, Viola D, Lupi C,
Biagini A, Giannini R, Romei C, Miccoli P, Pinchera A and Basolo F:
BRAF (V600E) mutation and outcome of patients with papillary
thyroid carcinoma: A 15-year median follow-up study. J Clin
Endocrinol Metab. 93:3943–3949. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim TH, Park YJ, Lim JA, Ahn HY, Lee EK,
Lee YJ, Kim KW, Hahn SK, Youn YK, Kim KH, et al: The association of
the BRAF(V600E) mutation with prognostic factors and poor clinical
outcome in papillary thyroid cancer: A meta-analysis. Cancer.
118:1764–1773. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amin MB: AJCC Cancer Staging Manual. 8th.
Springer; New York, NY: 2017, View Article : Google Scholar
|
|
11
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American Thyroid Association Management
Guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American Thyroid Association
Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid
Cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takacsova E, Kralik R, Waczulikova I,
Zavodna K and Kausitz J: A different prognostic value of BRAFV600E
mutation positivity in different age groups of patients with
papillary thyroid cancer. Neoplasma. 64:156–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tomczak K, Czerwińska P and Wiznerowicz M:
The Cancer Genome Atlas (TCGA): An immeasurable source of
knowledge. Contemp Oncol (Pozn). 19:A68–A77. 2015.PubMed/NCBI
|
|
14
|
Eisen MB, Spellman PT, Brown PO and
Botstein D: Cluster analysis and display of genome-wide expression
patterns. Proc Natl Acad Sci USA. 95:14863–14868. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Omry-Orbach G: Risk stratification in
differentiated thyroid cancer: An ongoing process. Rambam
Maimonides Med. 72016.
|
|
16
|
Luster M, Clarke SE, Dietlein M, Lassmann
M, Lind P, Oyen WJ, Tennvall J and Bombardieri E; European
Association of Nuclear Medicine (EANM), : Guidelines for
radioiodine therapy of differentiated thyroid cancer. Eur J Nucl
Med Mol Imaging. 35:1941–1959. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cohen Y, Xing M, Mambo E, Guo Z, Wu G,
Trink B, Beller U, Westra WH, Ladenson PW and Sidransky D: BRAF
mutation in papillary thyroid carcinoma. J Natl Cancer Inst.
95:625–627. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kimura ET, Nikiforova MN, Zhu Z, Knauf JA,
Nikiforov YE and Fagin JA: High prevalence of BRAF mutations in
thyroid cancer: Genetic evidence for constitutive activation of the
RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma.
Cancer Res. 63:1454–1457. 2003.PubMed/NCBI
|
|
19
|
Xing M: BRAF mutation in papillary thyroid
cancer: Pathogenic role, molecular bases, and clinical
implications. Endocr Rev. 28:742–762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen X, Zhu G, Liu R, Viola D, Elisei R,
Puxeddu E, Fugazzola L, Colombo C, Jarzab B, Czarniecka A, et al:
Patient age-associated mortality risk is differentiated by BRAF
V600E status in papillary thyroid cancer. J Clin Oncol. 36:438–445.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haymart MR: Understanding the relationship
between age and thyroid cancer. Oncologist. 14:216–221. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sumimoto H, Imabayashi F, Iwata T and
Kawakami Y: The BRAF-MAPK signaling pathway is essential for
cancer-immune evasion in human melanoma cells. J Exp Med.
203:1651–1656. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Angell TE, Lechner MG, Jang JK, Correa AJ,
LoPresti JS and Epstein AL: BRAF V600E in papillary thyroid
carcinoma is associated with increased programmed death ligand 1
expression and suppressive immune cell infiltration. Thyroid.
24:1385–1393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim H, Kim TH, Choe JH, Kim JH, Kim JS, Oh
YL, Hahn SY, Shin JH, Chi SA, Jung SH, et al: Patterns of initial
recurrence in completely resected papillary thyroid carcinoma.
Thyroid. 27:908–914. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suh YJ, Kwon H, Kim SJ, Choi JY, Lee KE,
Park YJ, Park DJ and Youn YK: Factors affecting the locoregional
recurrence of conventional papillary thyroid carcinoma after
surgery: A retrospective analysis of 3381 patients. Ann Surg Oncol.
22:3543–3549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xing M: Prognostic utility of BRAF
mutation in papillary thyroid cancer. Mol Cell Endocrinol.
321:86–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Durante C, Puxeddu E, Ferretti E, Morisi
R, Moretti S, Bruno R, Barbi F, Avenia N, Scipioni A, Verrienti A,
et al: BRAF mutations in papillary thyroid carcinomas inhibit genes
involved in iodine metabolism. J Clin Endocrinol Metab.
92:2840–2843. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zoghlami A, Roussel F, Sabourin JC, Kuhn
JM, Marie JP, Dehesdin D and Choussy O: BRAF mutation in papillary
thyroid carcinoma: Predictive value for long-term prognosis and
radioiodine sensitivity. Eur Ann Otorhinolaryngol Head Neck Dis.
131:7–13. 2014. View Article : Google Scholar : PubMed/NCBI
|