Introduction
According to the reports of National Cancer
Institutes (USA), pancreatic cancer was the tenth and eleventh most
prevalent case of cancer in men and women from 2010 to 2014 and the
fourth leading cause of death from cancer in men and women from
2011 to 2015. The case and death rates show an interesting tendency
(1): Pancreatic cancer has a higher
death rate than incidence rate. This is because early diagnosis is
difficult, and the cancer is mostly found after it has already
progressed to an advanced stage in many cases (2). Most pancreatic cancers are pancreatic
ductal adenocarcinoma (PDAC) (3).
Pancreatic cancer begins in minimally dysplastic epithelium and
progresses to invasive carcinoma by accumulation of mutations;
activation of KRAS oncogene, inactivation of tumor-suppressor genes
such as CDKN2A and TP53, and deletion of SMAD4 (4).
Tetraspanins form protein complexes with other
tetraspanins, integrins, and other membrane proteins, and the
protein complexes form tetraspanin-enriched microdomains (TEM) by
binding to membrane cholesterols and anchorage to the actin
cytoskeleton (5). The tetraspanins
are involved in regulation of cell differentiation, migration,
proliferation, and tumor progression (6,7).
Transmembrane 4 superfamily member 5 (TM4SF5), one of the
tetraspanins, was first reported in 1998, and the expression of
TM4SF5 was reported in human cancer such as hepatocellular
carcinoma (HCC), colon cancer, and pancreatic cancer (8,9). The
molecular function of TM4SF5 was intensively investigated in HCC
(9–13). TM4SF5 induces the
epithelial-mesenchymal transition (EMT), enhances translocation of
p27kip1 into cytosol, and reduces RhoA activity
(9–11). The cytosolic p27kip1
inhibits RhoA activity, and this leads to uncontrolled cell growth
and tumorigenesis by loss of contact inhibition (9,11). In
addition, TM4SF5 accelerates G1/S phase by controlling cytosolic
p27kip1 and RhoA activity (12). TM4SF5 induces vascular endothelial
growth factor (VEGF) expression and secretion, leading to an
increase of angiogenic activity (13). On the other hand, treatment of the
TM4SF5-targeted monoclonal antibody reverses cellular events
induced by TM4SF5 expression; anti-TM4SF5 antibody induces
translocation of p27kip1 into nucleus, increased RhoA
activity, reduced EMT, and suppression of tumor growth and
metastasis (10,14,15).
Since 1975, when the procedure of effectively
producing monoclonal antibodies (mAbs) was developed (16), antibodies were used for imaging and
therapy. In therapy, murine mAb triggered the stimulation of the
patient's immune system. In the early 1990s, a technique for
cloning IgG genes was developed and, as a result, IgG genes could
be expressed in eukaryotic cells (17). This development of antibody
technology has resulted in the production of antibodies such as
chimeric antibodies and humanized antibodies (18). The chimeric antibodies include 70% of
human sequences and the Fc portion is fully human. In order to
humanize more of the parts of the murine mAb, all parts except the
complementarity-determining region (CDR) portion are replaced with
human sequences. As a result, humanized antibodies include 85–90%
of human sequences and have lower immune responses than chimeric
antibodies (18). Anti-cancer
therapy using mAb targeting surface antigens expressed on tumor
cells is an efficacious strategy to treat cancer (19).
Previously, we found that TM4SF5-targeted monoclonal
antibody and peptide vaccination has preventive and therapeutic
effects on hepatocellular carcinoma and colon cancer models
(10,14,15,20,21).
Regarding the implication of TM4SF5 as a target of anticancer
strategy in pancreatic cancer, we revealed immunization with TM4SF5
peptide vaccine prevented tumor growth derived from
TM4SF5-expressing mouse pancreatic cancer cells in an allograft
mouse model (22). In this study, we
confirmed the implication of TM4SF5 expression and
anti-growth/motility effect of antibody targeting TM4SF5 in human
pancreatic cancer cells.
Materials and methods
Cell culture
Mia-PaCa-2 and PANC-1 cells were maintained in
Dulbecco's modified Eagle's media (DMEM; Hyclone) with 10% fetal
bovine serum (FBS; Hyclone), 100 U/ml penicillin, and 100 µg/ml
streptomycin at 37°C under a humidified atmosphere of 5%
CO2. ASPC-1, Capan-1, and Capan-2 cells were maintained
in Roswell Park Memorial Institute medium (RPMI-1640; Hyclone) with
10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin. CFPAC-1
cells were maintained in Iscove's modified Dulbecco's medium (IMDM;
Hyclone) with 10% FBS, 100 U/ml penicillin, and 100 µg/ml
streptomycin. H6c7 cells were maintained in Keratinocyte-SFM
(Invitrogen; Thermo Fisher Scientific, Inc.) with 100 U/ml
penicillin, and 100 µg/ml streptomycin.
Reverse transcription (RT) PCR
Total RNA was isolated with the TRI
Reagent® according to the manufacturer's instructions
(MRC). Then, 2 µg of total RNA was reverse-transcribed in the
first-strand synthesis buffer containing 6 µg/ml oligo(dT) primer,
50 U M-MLV reverse transcriptase, 2 mM dNTP, 10 mM DTT, and 40 U
RNaseOUT™ recombinant ribonuclease inhibitor (Invitrogen; Thermo
Fisher Scientific, Inc.). The reaction was carried out at 37°C for
50 min and heat inactivated at 70°C for 15 min. One microliter of
the synthesized cDNA solution was subjected to a semi-quantitative
PCR of 25 (for GAPDH) or 30 (for TM4SF5) cycles consisting of
denaturation for 40 sec at 95°C, annealing for 40 sec at 58°C, and
extension for 40 sec at 72°C. The primer sequences used were as
follows: GAPDH, 5′-TCCACCACCCTGTTGCTGTA-3′ (sense) and
5′-ACCACAGTCCATGCCATCAC-3′ (anti-sense) (product size 452 bp);
human TM4SF5, 5′-AGCTTGCAAGTCTGGCTCAT-3′ (sense) and
5′-GCTGGATCCCACACAGTACT-3′ (anti-sense) (product size 401 bp).
Packaging and transduction of control
and TM4SF5-encoding retroviruses
The human TM4SF5 cDNA was amplified from
pcDNA3.1-hTM4SF5 (14) by PCR using
the following primer set: hTM4SF5 5′ primer,
5′-GAATTCGCCACCATGGAACAAAAACTCATCTCAGAAGAGGATCTGGGTGCAATGTGTACGGGAAAA-3′
and hTM4SF5 3′ primer, 5′-CTCGAGTCAGTGAGGTGTGTCCTG-3′. The cDNA
fragments were cloned into the expression vector pLXSN (Clontech
Laboratories, Inc.) using the XhoI and EcoRI sites.
GP2-293, a cell line derived from 293 cells, was obtained from
Clontech and used as a packaging cell line for preparation of the
retroviruses. GP2-293 cells were maintained in DMEM containing 10%
FBS in a 5% CO2 incubator at 37°C. Retroviral vectors
pLXSN or pLXSN-hTM4SF5 along with pVSV-G (Clontech Laboratories,
Inc.) encoding the pseudo-envelope protein gene were transfected
into the cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Twelve hours later, the medium was exchanged
with fresh culture medium supplemented with 10 mM sodium butyrate
(Sigma-Aldrich; Merck KGaA). After 48 h, the supernatant of the
culture medium was taken and filtrated through a filter with a 0.45
µm pore size. The retrovirus supernatants were concentrated using
Centricon centrifugal filters (Millipore) and stored at −80°C. The
viral supernatant was applied to Mia-PaCa-2 cells along with 8
µg/ml of polybrene (Sigma-Aldrich; Merck KGaA). Twenty-four hours
later, G418 (Sigma-Aldrich; Merck KGaA) was added at a
concentration of 1 mg/ml, and the G418-resistant transfected
Mia-PaCa-2 cells were selected by culture of the cells in the
presence of G418 for 2 weeks.
Western blot analysis
Harvested cells were lysed in a lysis buffer (pH
8.0, 20 mM Tris-HCl, 137 mM NaCl, 10% glycerol, 10 mM EDTA, 0.5%
sodium deoxycholate, 0.1% SDS, 1% NP-40, protease inhibitor
cocktail, and phosphatase inhibitor). Proteins were resolved by
SDS-polyacrylamide gel electrophoresis and electro-transferred to
polyvinylidene fluoride (PVDF) membranes (Millipore). The membranes
were blocked with 5% dry milk in phosphate buffered saline-Tween-20
(PBS-T; 140 mM NaCl, 2.7 mM KCl, 10 mM
Na2HPO4, 2 mM KH2PO4,
and 0.05% Tween-20) and probed with an appropriate primary
antibody. The monoclonal anti-GAPDH antibody was purchased from
Santa Cruz Biotechnology, Inc. The anti-E-cadherin and
anti-Vimentin polyclonal antibodies were purchased from Cell
Signaling Technology, Inc. Immunoreactive proteins were visualized
by horseradish peroxidase-conjugated anti-rabbit or anti-mouse
secondary antibodies (Santa Cruz Biotechnology, Inc.) and an ECL
solution (ATTO).
Humanized anti-hTM4SF5 monoclonal
antibody
The humanized anti-hTM4SF5 monoclonal antibody
(anti-hTM4SF5 antibody), hEC2-C-2, was established based on the
mouse monoclonal antibody mEC2-C obtained by immunization with the
cyclic peptide hTM4SF5EC2-C as an antigen (14). The anti-hTM4SF5 antibody recognizes
structural epitope of the second extracellular loop region of
TM4SF5. To obtain humanized antibody with intact IgG format, VH and
Vk encoding genes originated from mEC2-C were synthesized
(Bioneer). The synthesized genes were then inserted into the
modified pcDNA 3.4 expression vector (Invitrogen; Thermo Fisher
Scientific, Inc.) carrying the human IgG1 constant regions
(CH1-hinge-CH2-CH3) or human kappa chain constant region (CL) for
mammalian cell expression in HEK 293F cells. The antibodies were
purified using Protein A affinity chromatography following the
manufacturer's protocol after 5–7 days of cell culture.
Immunostaining and confocal
microscopy
The cells were fixed with 4% paraformaldehyde and
blocked with 3% BSA containing 0.1% Triton X-100. Cells were
treated with anti-hTM4SF5 antibody (3 µg/ml) for 3 h. After
extensive washing with PBS, the samples were incubated with Alexa
Flour 488-conjugated goat anti-human IgG (Invitrogen; Thermo Fisher
Scientific, Inc.) for 1 h. The nuclei were stained with Hoechst
33258 (Sigma-Aldrich).
Immunoprecipitation
Whole cell lysates were lysed in a lysis buffer.
After anti-hTM4SF5 antibody was conjugated to protein A-agarose
beads (Roche Diagnostics), the whole cell lysates were
immunoprecipitated with the antibody-conjugated agarose beads.
Immunoprecipitated proteins were washed with PBS and processed for
a standard western blot analysis using the mouse anti-hTM4SF5
monoclonal antibody (mEC2-CF, 1 µg/ml), which we previously
reported (23).
Cell proliferation assay
The cell proliferation ELISA, BrdU colorimetric kit
(Roche Diagnostics), was used to measure the cell proliferation
according to the manufacturer's instructions. Cells were treated
with normal IgG or anti-hTM4SF5 antibody (10 µg/ml) for 3 days. The
BrdU solution was added to each well, and then the plates were
incubated for 4 h at 37°C. After fixation of the cells, anti-BrdU
antibody conjugated with peroxidase was added to each well for 90
min at room temperature. A colorimetric assay was developed with a
substrate solution, and the absorbance at 370 nm with a reference
wavelength of 492 nm was measured using a microplate reader
(Bio-Rad Laboratories, Inc.).
In vitro wound-healing assay
Cells were placed in a 6-well plate, cultured
overnight to 80 ~90% confluence in a medium containing serum, and
the monolayer was wounded with a pipette tip (3 wounds/well).
Normal IgG or anti-hTM4SF5 antibody (10 µg/ml) was added to the
medium for the indicated periods. The cells were fixed with 4%
paraformaldehyde (Biosesang) for 20 min and stained with 0.01%
crystal violet (Sigma-Aldrich; Merck KGaA) for 20 min. The
wound-healing activity of cells was calculated by the following
formula: The cells migrating into wound (%)=[(the wounded area at 0
day-cell-free space in the wounded area)/wounded area at 0 day
×100]. The percent ratio of migrated area to wounded area was
measured at 3 points per each wound (9 points/well in total) under
a microscope (Nikon).
In vitro cell migration and invasion
assays
Trans-well chambers with 8 µm porosity (Corning
Incorporated) were used for these assays. For the migration assays,
the lower side of the trans-well chamber membranes was coated with
gelatin (10 µg/well; Sigma-Aldrich; Merck KGaA). For the invasion
assays, a Matrigel invasion chamber (Corning Incorporated) was
used. Cells were suspended in serum-free medium with normal IgG or
anti-hTM4SF5 antibody (10 µg/ml) and placed on the top of the
trans-well chamber. DMEM medium containing 10% FBS was placed in
the lower chamber. After incubation for 48 h, the cells that
invaded the lower surface of the filters were fixed, stained with
crystal violet, and counted under a microscope (Nikon). The
migrated and invaded cells were calculated by the following
formula: The migrated or invaded cells (%)=(the total
area-cell-free space in the total area)/the total area ×100.
Statistics
The results are shown as the mean ± standard error
of the mean (SEM) from at least three independent experiments.
Statistical significance of the differences between two samples was
evaluated using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Validation of the TM4SF5 expression in
human pancreatic cancer cell lines
Previously, we checked TM4SF5 expression in human
pancreatic cancer tissues by immunohistochemistry (10,22). To
validate the function of TM4SF5 in human pancreatic cancer cells,
we first checked the mRNA levels of TM4SF5 in non-cancerous human
pancreatic duct epithelial cell line H6c7 and several human
pancreatic cancer cell lines such as ASPC-1, Capan-1, Capan-2,
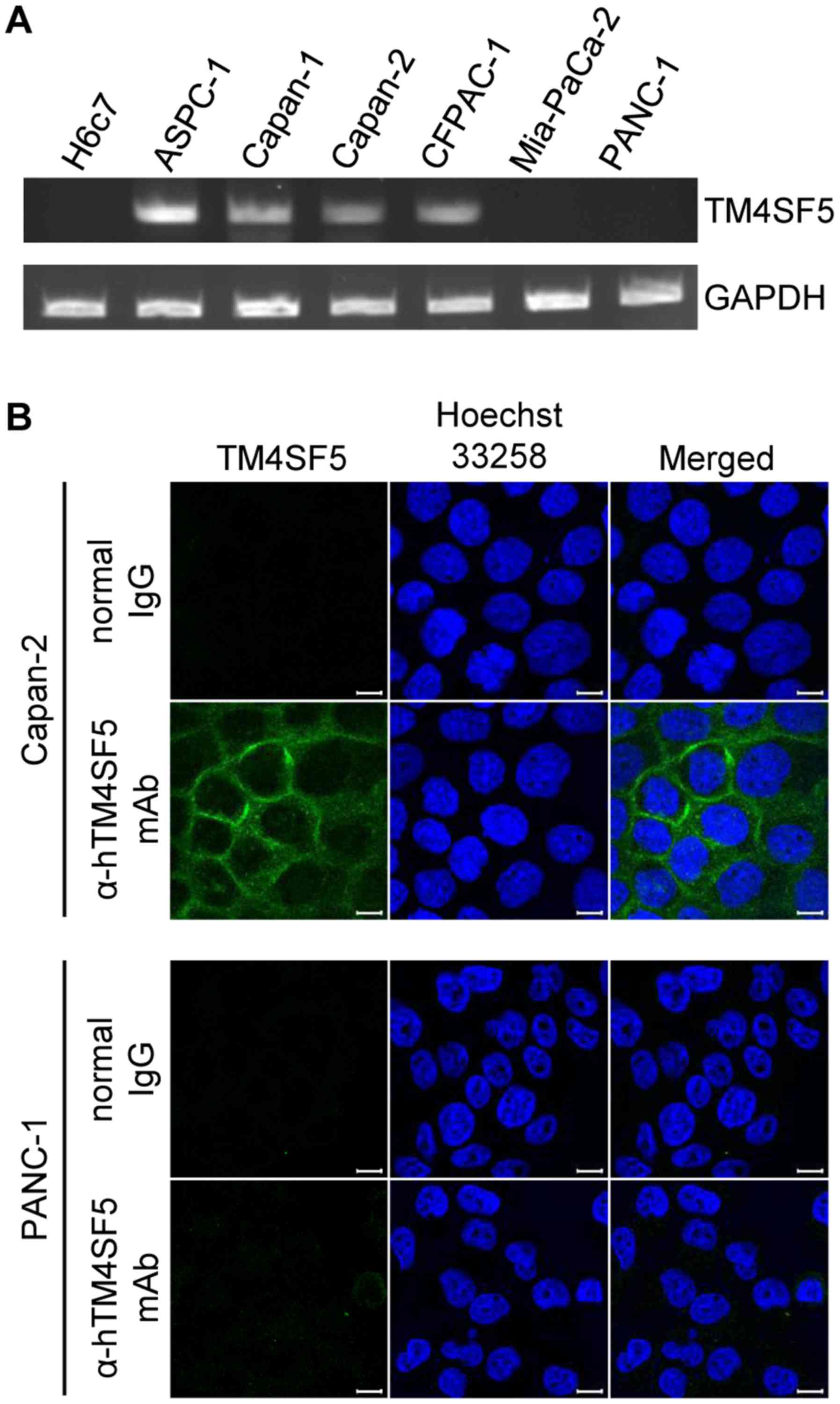
CFPAC-1, Mia-PaCa-2, and PANC-1 by RT-PCR. As shown in Fig. 1A, TM4SF5 mRNA was expressed in
ASPC-1, Capan-1, Capan-2, and CFPAC-1 cells but barely expressed in
Mia-PaCa-2, PANC-1, and H6c7 cells. Next, we investigated
expression of TM4SF5 protein using immunostaining and confocal
microscopy in Capan-2 and PANC-1 cells as a representative
TM4SF5-positive and TM4SF5-negative cell line, respectively. In
accordance with the mRNA expression, TM4SF5 protein was detected in
Capan-2 cells but not in PANC-1 cells (Fig. 1B). Expression of TM4SF5 protein in
the cells couldn't be verified by western blot analysis because the
anti-TM4SF5 antibody has very low sensitivity when used for western
blot analysis.
Suppression of human pancreatic cancer
cell growth by treatment with the anti-hTM4SF5 antibody
The tetraspanins, including TM4SF5, regulates tumor
growth and metastasis by interaction with integrins in HCC
(11,24–26).
Previously, we confirmed that immunization with the TM4SF5 peptide
vaccine suppressed growth of TM4SF5-expressing mouse pancreatic
cancer cells in vivo (22).
Therefore, we first checked the growth of TM4SF5-expressing human
pancreatic cancer cells after treatment with anti-hTM4SF5 antibody
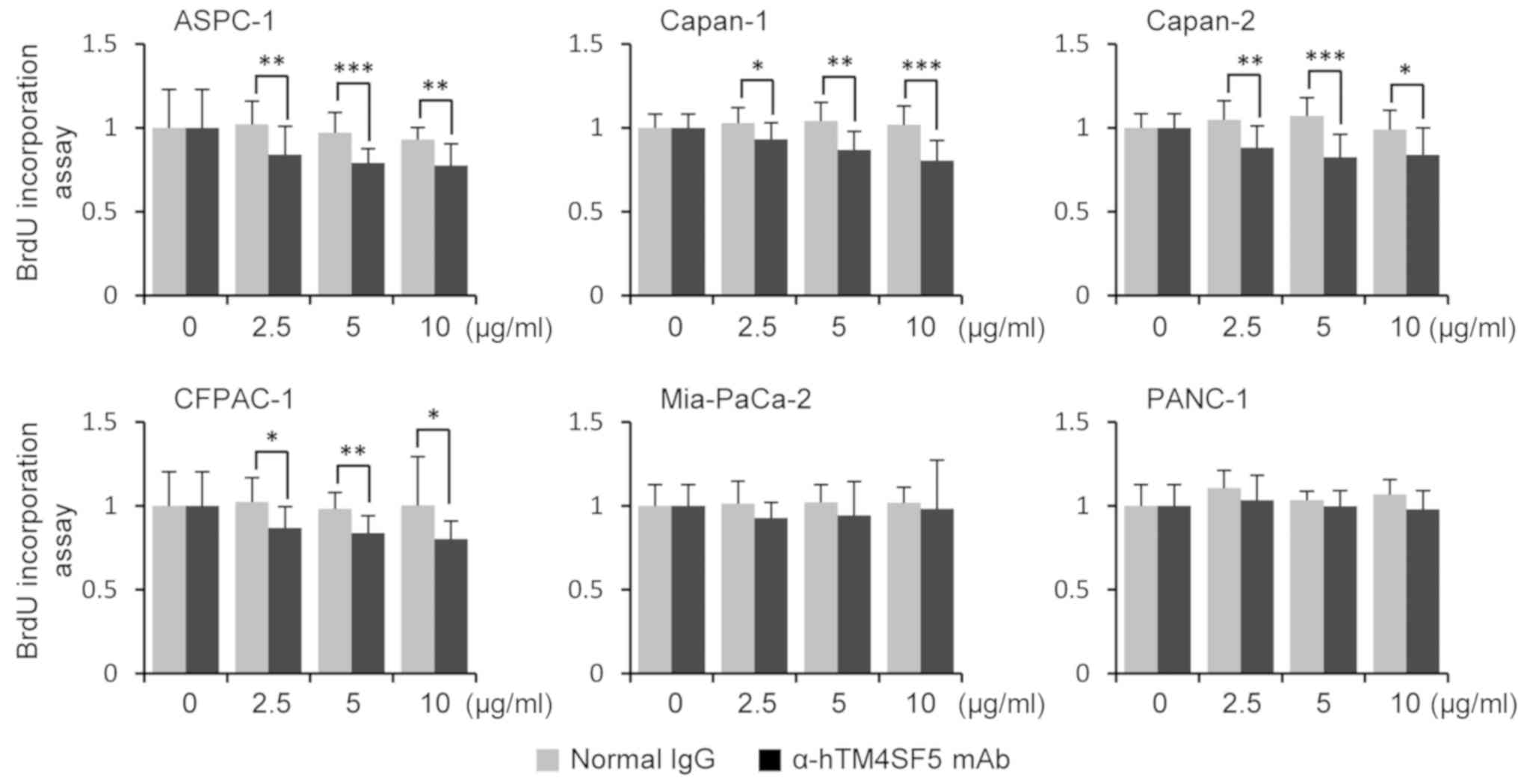
interrupting the function of TM4SF5. To check the cell growth, we
measured the proliferation rate of human pancreatic cancer cells
using the BrdU incorporation assay. The proliferation rates were
significantly decreased by the treatment with anti-hTM4SF5 antibody
compared to normal IgG treatment in TM4SF5-positive cell lines
(ASPC-1, Capan-1, Capan-2, and CFPAC-1). However, there was no
difference between the treatment groups in TM4SF5-negative cell
lines (Mia-PaCa-2 and PANC-1) (Fig.
2). These data revealed that anti-hTM4SF5 antibody suppresses
growth of TM4SF5-expressing human pancreatic cells.
Suppression of human pancreatic cancer
cell motility by treatment with the anti-hTM4SF5 antibody
Previously, we reported that targeting of TM4SF5
inhibits motility of HCC and colon cancer cells in vitro and
in vivo (10,14,15).
Therefore, we checked the motility of human pancreatic cancer cells
using wound healing assay and transwell migration/invasion assay
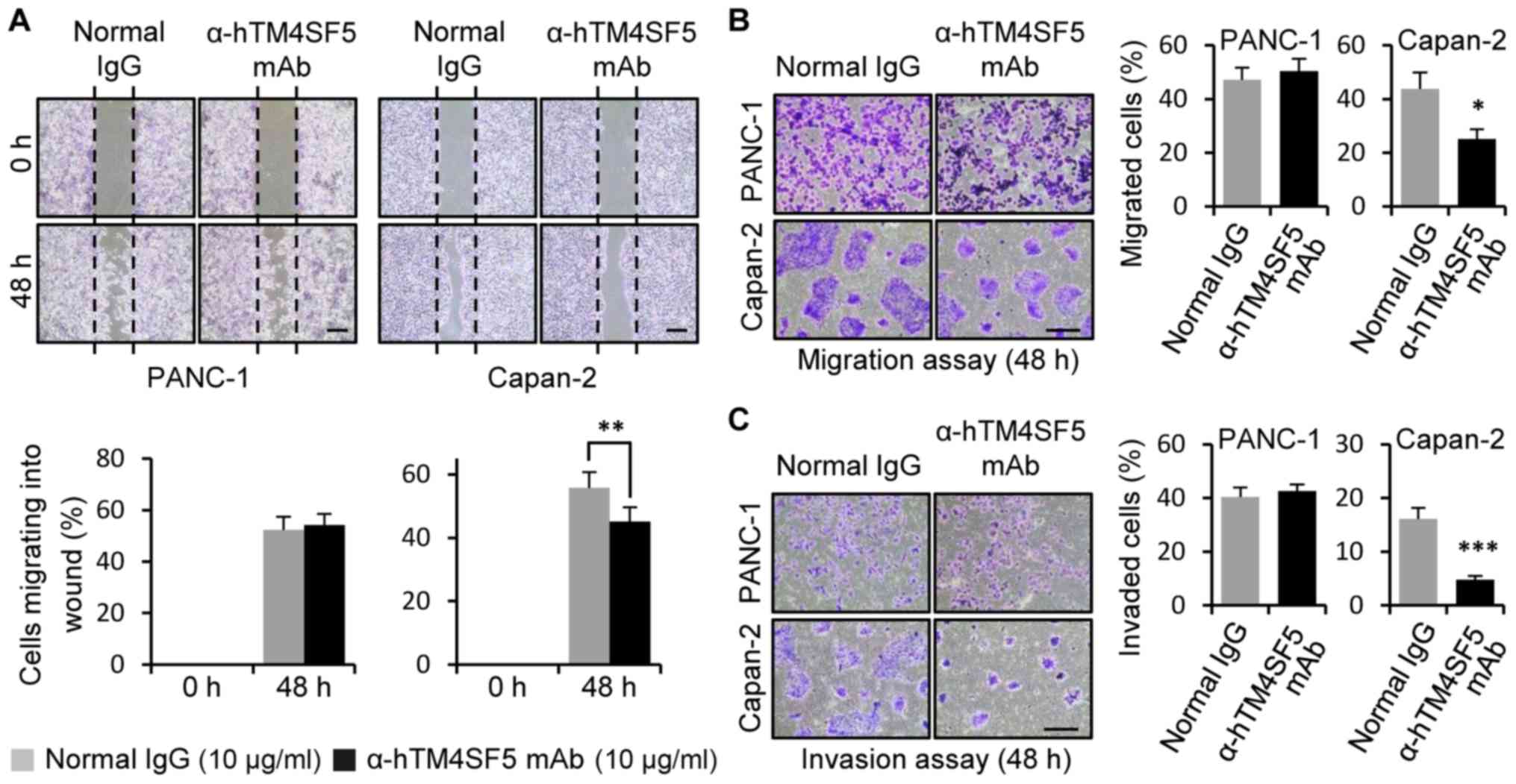
after treatment with the anti-hTM4SF5 antibody. As shown in
Fig. 3A, the wound healing activity
was significantly decreased by the treatment with the anti-hTM4SF5
antibody compared to normal IgG in the TM4SF5-positive cell line
Capan-2. In contrast, the anti-hTM4SF5 antibody treatment had no
effect in the TM4SF5-negative cell line PANC-1. The transwell
migration and invasion activities were reduced by the anti-hTM4SF5
antibody treatment, but not by the normal IgG treatment, in
Capan-2. However, anti-hTM4SF5 antibody had no effect in PANC-1
(Fig. 3B and C). Similar results
were obtained in other TM4SF5-positive cell lines (ASPC-1 and
CFPAC-1) and TM4SF5-negative cell line Mia-PaCa-2 (Fig. S1). Therefore, these results have
shown that the anti-hTM4SF5 antibody inhibits the motility of
TM4SF5-expressing pancreatic cancer cells in vitro.
Molecular change of EMT markers by
anti-hTM4SF5 antibody treatment
In our previous studies, molecular levels of the EMT
markers were changed by targeting TM4SF5 with antibody in HCC and
colon cancer cells (10,15). Therefore, we checked the expression
of EMT markers after anti-hTM4SF5 antibody treatment in human
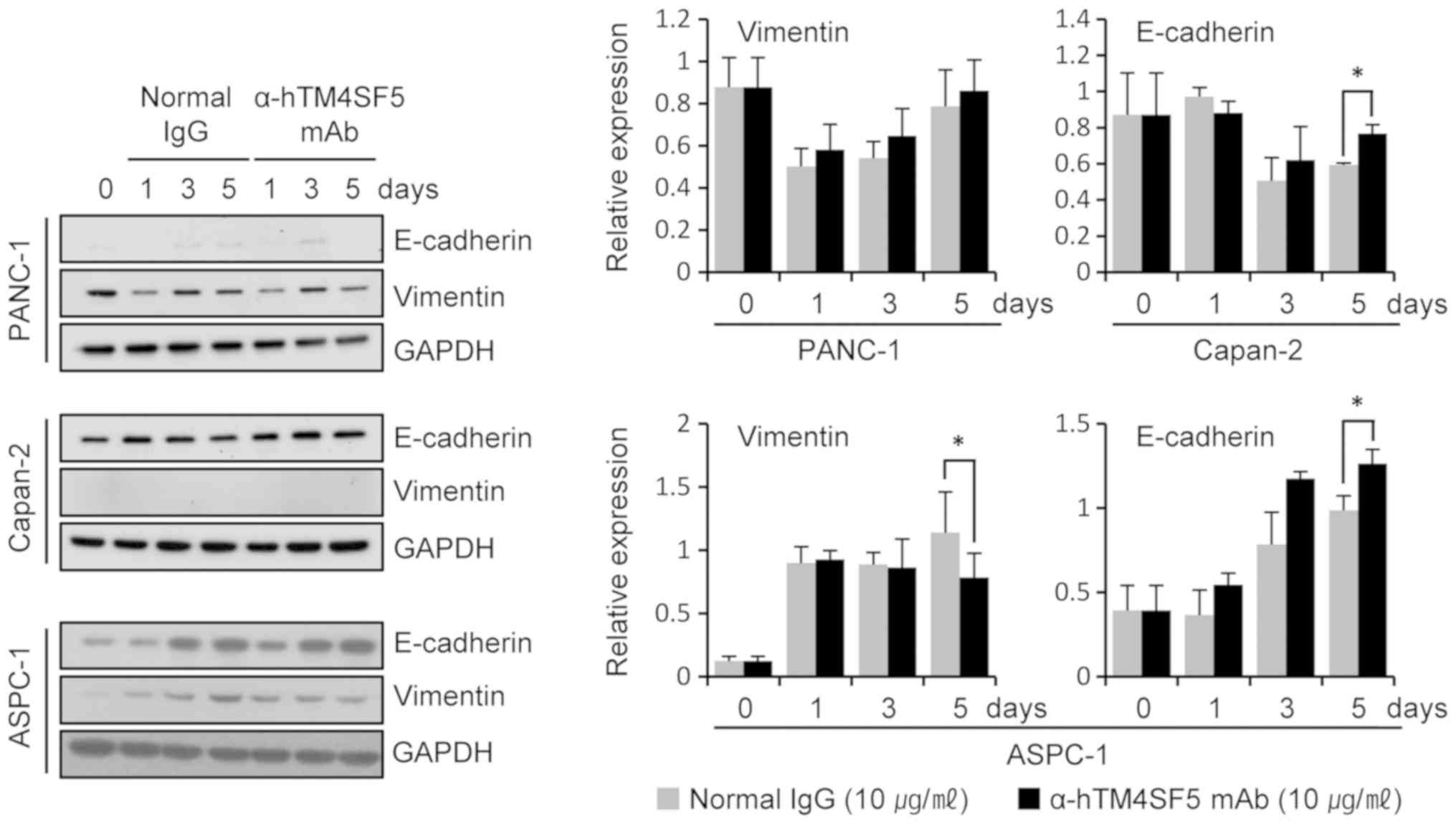
pancreatic cancer cells. Vimentin and E-cadherin are mesenchymal
and epithelial markers, respectively, and their levels of
expression change during EMT (27,28). In
the TM4SF5-positive cell lines Capan-2 and ASPC-1, the expression
level of E-cadherin was increased at 5 days after treatment with
anti-hTM4SF5 antibody compared to the normal IgG (Fig. 4). Similar results were obtained in
another TM4SF5-positive cell line CFPAC-1 (Fig. S2). In ASPC-1 cells, the expression
level of Vimentin was decreased by the treatment with anti-hTM4SF5
antibody. There was no expression of Vimentin in Capan-2 (Fig. 4) and CFPAC-1 (Fig. S2) cells irrespective of antibody
treatment. TM4SF5-negative cell lines PANC-1 (Fig. 4) and Mia-PaCa-2 (Fig. S2) did not express or only slightly
expressed E-cadherin and commonly expressed Vimentin. The
expression levels of E-cadherin and Vimentin were not changed by
treatment with the anti-hTM4SF5 antibody or the normal IgG in the
cells. These data suggest that the anti-hTM4SF5 antibody can
inhibit EMT associated with tumor progression in TM4SF5-positive
pancreatic cancer cells.
Establishment of the
TM4SF5-overexpressing human pancreatic cancer cells
To validate the effects of TM4SF5 expression in
human pancreatic cancer cells directly, we established a human
pancreatic cancer cell line stably expressing TM4SF5 using
TM4SF5-negative Mia-PaCa-2 cells. For exogenous expression of
TM4SF5 in TM4SF5-negative pancreatic cancer cells, we used a
retroviral system. We established control Mia-PaCa-2 cells
transduced with the parental pLXSN vector (Mia-PaCa-2-mock) and
TM4SF5-expressing Mia-PaCa-2 cells transduced with the recombinant
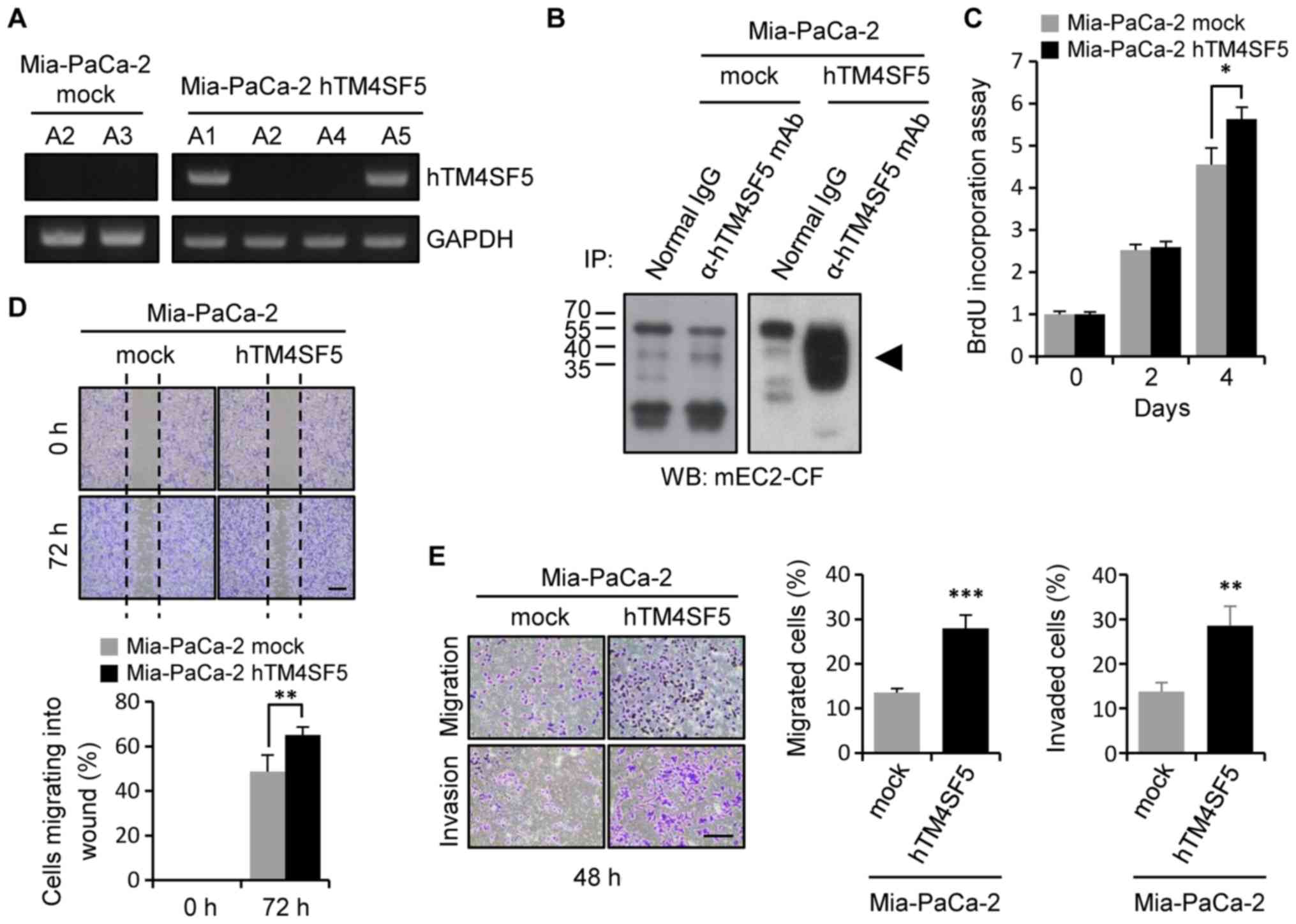
vector pLXSN-hTM4SF5 (Mia-PaCa-2-hTM4SF5). To validate expression
of TM4SF5, we detected TM4SF5 mRNA levels by RT-PCR (Fig. 5A). The TM4SF5 mRNA was detected in
the clones A1 and A5 of Mia-PaCa-2-hTM4SF5 cells, but not in the
clones of Mia-PaCa-2-mock cells. As we obtained similar results
from the two clones, we further analyzed the clone A5 of
Mia-PaCa-2-hTM4SF5 for the remaining investigation. The expression
level of TM4SF5 protein was also checked by immunoprecipitation
followed by western blot analysis (Fig.
5B). TM4SF5 protein was detected in the Mia-PaCa-2-hTM4SF5
cells, but not in the Mia-PaCa-2-mock cells.
To analyze the cellular change induced by TM4SF5
expression, we checked cell growth and motility of the
Mia-PaCa-2-hTM4SF5 and Mia-PaCa-2-mock cells. First, we measured
the change of cell growth using a BrdU incorporation assay. The
cell growth was increased in Mia-PaCa-2-hTM4SF5 cells compared to
Mia-PaCa-2-mock cells (Fig. 5C).
Next, we measured the change of cell motility using a wound healing
assay and a transwell migration/invasion chamber. The cell
migration into the wound area was increased in Mia-PaCa-2-hTM4SF5
cells compared to Mia-PaCa-2-mock cells (Fig. 5D), and the rate of cell migration and
invasion through the transwell chamber also increased in
Mia-PaCa-2-hTM4SF5 cells (Fig. 5E).
Therefore, we conclude that expression of TM4SF5 enhanced cell
growth and motility in human pancreatic cancer cells.
Suppression of cell growth and
motility by anti-hTM4SF5 antibody treatment in
TM4SF5-overexpressing pancreatic cancer cells
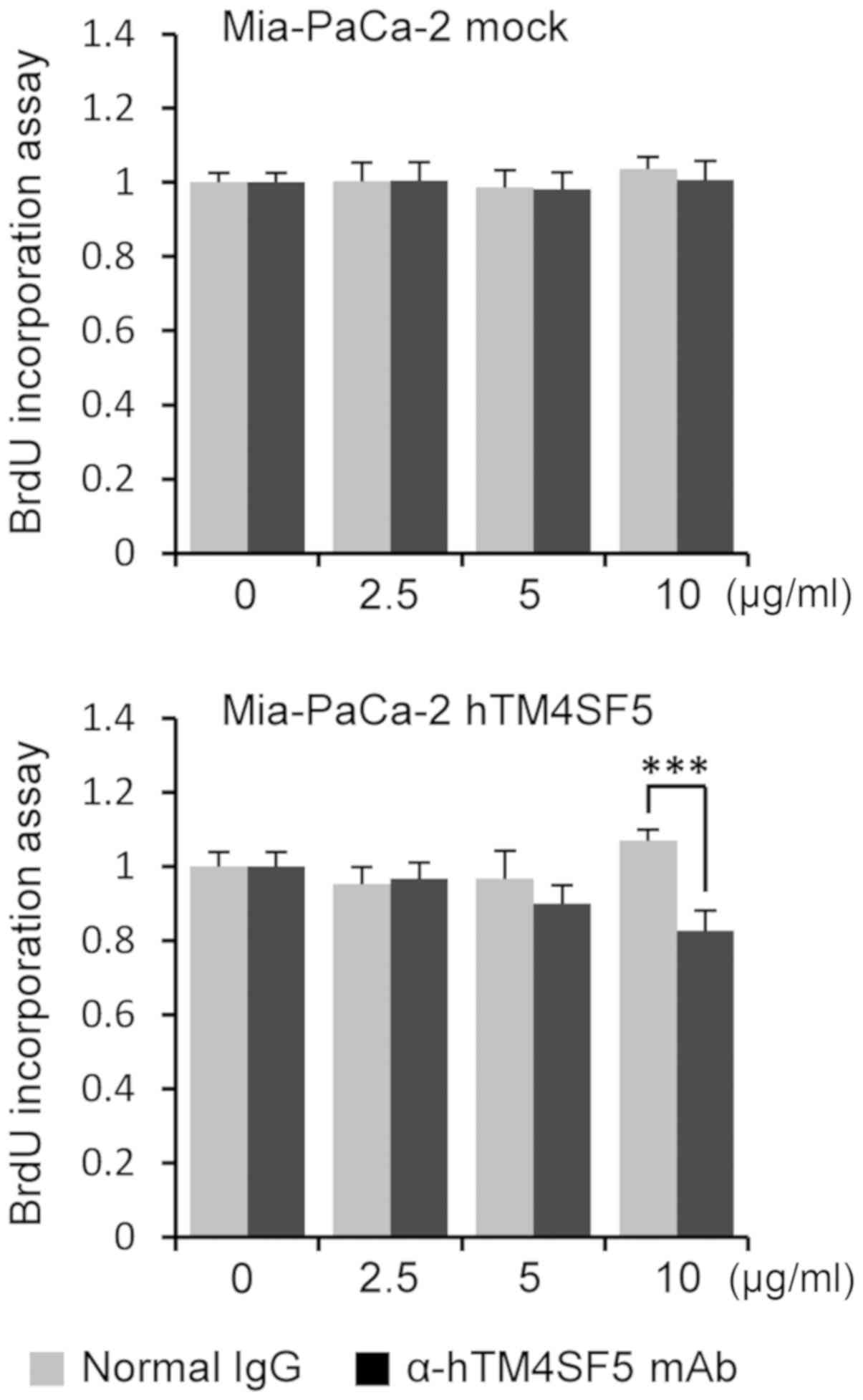
To check whether the down-regulation of cell
proliferation and motility by the treatment with anti-hTM4SF5
antibody observed in human pancreatic cancer cells naturally
expressing TM4SF5 (Figs. 2–4) also occurs in TM4SF5-overexpressing
pancreatic calls, we measured cell proliferation and motility after
treatment with anti-hTM4SF5 antibody in the Mia-PaCa-2-hTM4SF5
cells. First, we measured the cell proliferation using a BrdU
incorporation assay. The cell proliferation was significantly
reduced in the Mia-PaCa-2-hTM4SF5 cells by treatment with
anti-hTM4SF5 antibody compared to normal IgG but was not changed in
the Mia-PaCa-2-mock cells (Fig. 6).
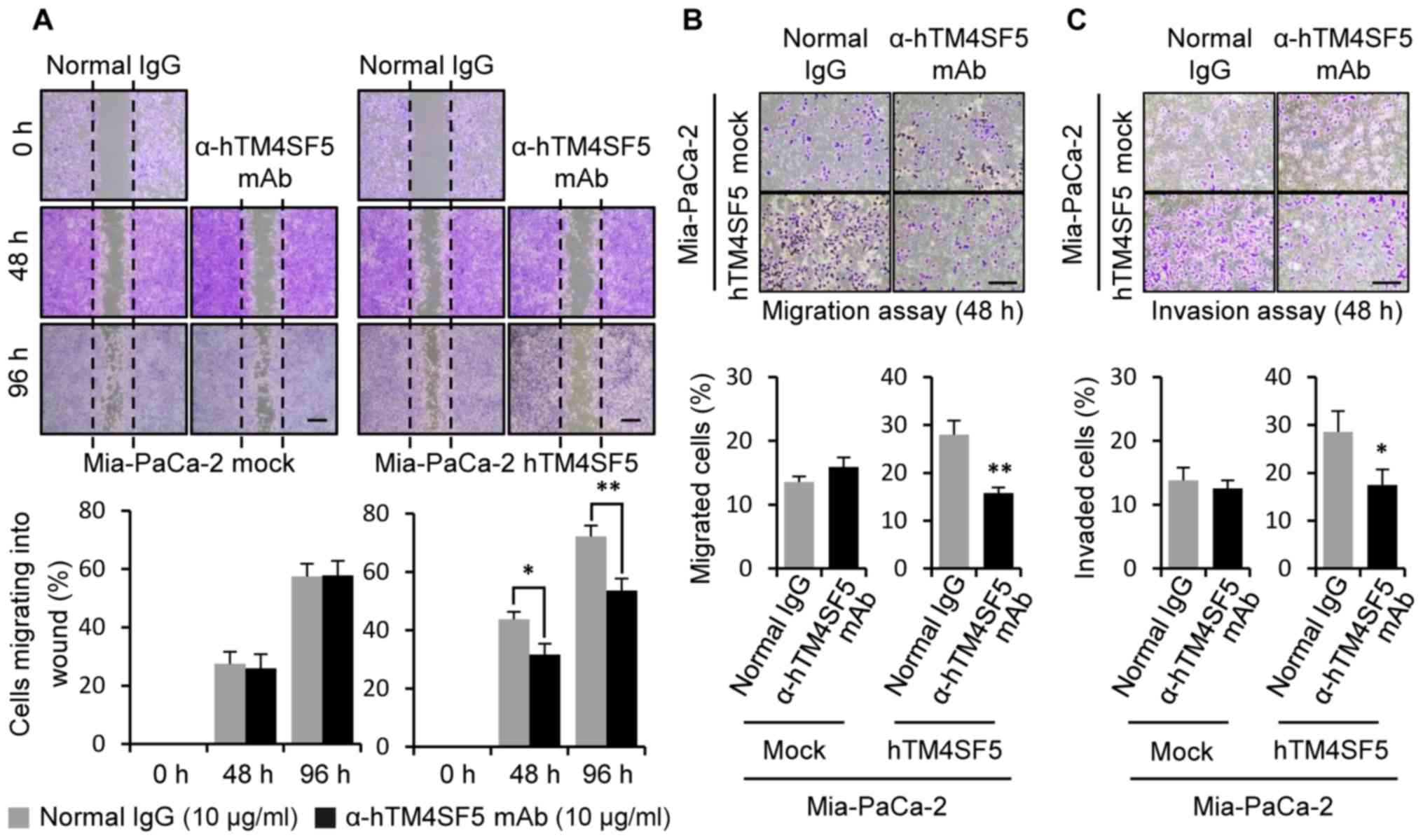
Next, we measured migration activity. As shown in Fig. 7A, the wound healing activity was
decreased by the anti-hTM4SF5 antibody treatment compared to the
normal IgG treatment in the Mia-PaCa-2-TM4SF5 cells. The rate of
migration/invasion was also decreased by anti-hTM4SF5 antibody
treatment compared to the normal IgG control in the
Mia-PaCa-2-hTM4SF5 cells (Fig. 7B and
C). However, there was no difference induced by the antibody
treatment in Mia-PaCa-2-mock cells. These results show that
anti-hTM4SF5 antibody reduced cell proliferation and motility of
the TM4SF5-overexpressing pancreatic cancer cells as it reduced
these parameters of the TM4SF5-positive pancreatic cancer
cells.
Modified expression of EMT markers
after anti-hTM4SF5 antibody treatment in TM4SF5-overexpressing
pancreatic cancer cells
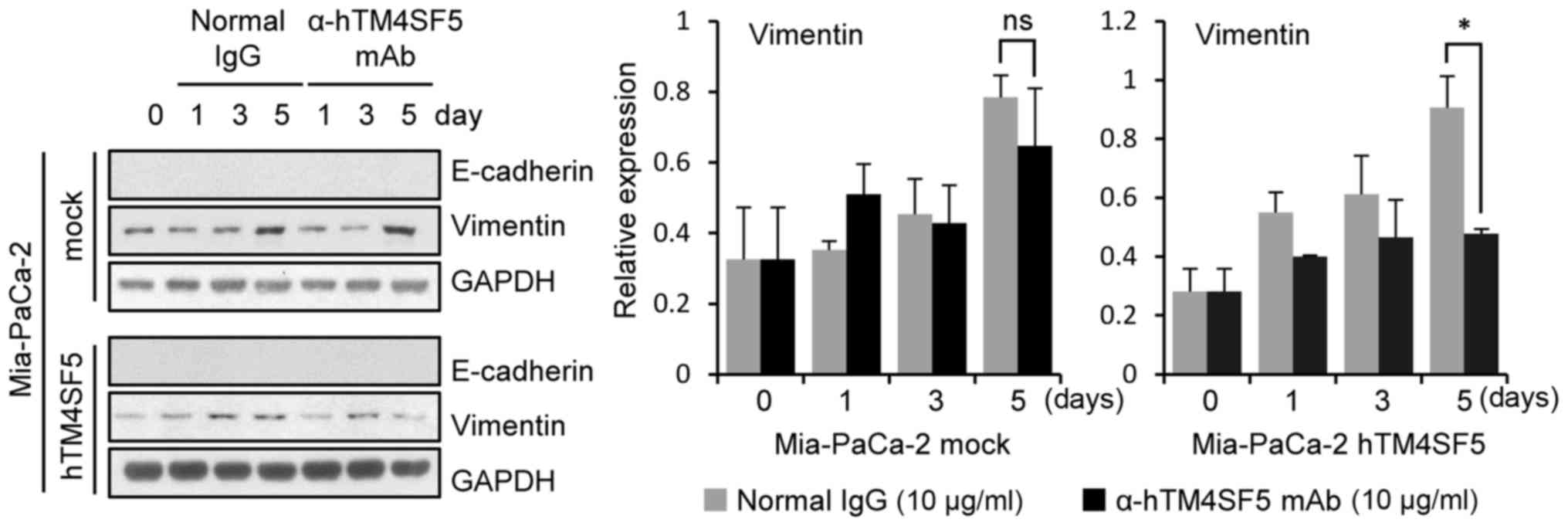
Because the cell motility was increased by TM4SF5
transduction (Fig. 5D and E) and
decreased by anti-hTM4SF5 antibody treatment in the
TM4SF5-overexpressing pancreatic cancer cells (Fig. 7), we next checked whether expression
of EMT markers (E-cadherin and Vimentin) in the
TM4SF5-overexpressing pancreatic cancer cells was changed after
anti-hTM4SF5 antibody treatment. The expression level of Vimentin
was decreased by anti-hTM4SF5 antibody treatment in the
Mia-PaCa-2-hTM4SF5 cells compared to normal IgG treatment. In
contrast, the Vimentin expression level was not changed in
Mia-PaCa-2-mock cells by anti-hTM4SF5 antibody treatment. The
E-cadherin was not detected in Mia-PaCa-2-mock and
Mia-PaCa-2-hTM4SF5 cells (Fig. 8).
These data suggest that anti-hTM4SF5 antibody can influence
mesenchymal-epithelial transition (MET) in a TM4SF5-overexpressing
human pancreatic cancer cell model.
Discussion
Previously, we found that TM4SF5 can be a target for
therapy and prevention of HCC and colon cancer (10,14,15,20,21). Ahn
et al also produced TM4SF5-targeted chimeric antibodies
using phage display method and showed that TM4SF5-targeting
antibodies had anti-cancer activity in TM4SF5-expressing HCC and
colon cancer (29). Because
expression of TM4SF5 in pancreatic cancer was previously reported
(8,10), here we investigated expression and
function of TM4SF5 in human pancreatic cancer cell lines and
confirmed anti-cancer effects of the antibody targeting TM4SF5 on
TM4SF5-expressing cells to evaluate its possible application to
pancreatic cancer.
Treatment of TM4SF5-expressing human pancreatic
cancer cells with anti-hTM4SF5 antibody significantly suppressed
cell growth (Figs. 2 and 6) and motility (Figs. 3, 7
and S1). Furthermore, the
expression of EMT markers was changed by treatment of anti-hTM4SF5
antibody (Figs. 4, 8 and S2).
Taken together, these results show that high expression of TM4SF5
can endow the human pancreatic cells with oncogenic properties and
that anti-hTM4SF5 antibody has therapeutic effects in pancreatic
cancer cells, suggesting possible application of the anti-hTM4SF5
antibody in treating pancreatic cancer. From a practical
perspective, the anti-hTM4SF5 antibody can be applied to
antibody-drug conjugates (ADC). The use of ADCs is an emerging
strategy for anticancer therapy that combines antibody-mediated
targeted treatment with cytotoxic chemotherapy drugs (30). The ADCs induce specific targeting and
therapeutic effects through antibody-dependent cellular
cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC)
(31).
E-cadherin and Vimentin are typical EMT markers.
Loss of E-cadherin expression induced or contributed to drug
resistance of colon cancer and breast cancer (32,33). In
addition, Vimentin expression was shown to be involved in the drug
resistance of colon cancer (34).
EMT marker expression is correlated with conventional drug
resistance also in pancreatic cancer cells, and suppression of
mesenchymal marker ZEB-1 induces an increase of E-cadherin and
overcoming of drug resistance (35,36).
Based on our results, E-cadherin was not or very weakly detected in
PANC-1 and Mia-PaCa-2, and Vimentin was not or very weakly detected
in Capan-2 and CFPAC-1. These expression patterns in these cell
lines have been reported by many groups and are associated with
cellular phenomenon (37–41). In terms of anti-cancer drug
resistance, anti-cancer drug sensitive cells (BxPC-3, HPAC, ASPC-1,
and CFPAC-1) expressed E-cadherin, whereas the less sensitive cells
(PANC-1 and Mia-PaCa-2) expressed Vimentin (39). In terms of invasive properties,
E-cadherin was expressed in low invasive cells such as BxPC-3,
CFPAC-1, and SW1990, and Vimentin was expressed in highly-invasive
cells such as PaTu8988 (37). In
addition, E-cadherin was expressed in cells showing epithelial
characteristics (Capan-2 and BxPC-3), and Vimentin was expressed in
mesenchymal-like cells (Mia-PaCa-2) in terms of cell shape
(41). Based on our investigation,
all the TM4SF5-expressing cells we examined in detail (ASPC-1,
Capan-2, and CFPAC-1) expressed E-cadherin. Furthermore, the
TM4SF5-positive cells were responsive to the suppressive effects of
anti-TM4SF5 antibody. Considering regulation of EMT marker
expression by the anti-hTM4SF5 antibody (Figs. 4 and 8) and correlation between drug resistance
and EMT properties, the anti-hTM4SF5 antibody treatment may enhance
the efficacy of anti-cancer reagent in chemotherapy of pancreatic
cancer patients.
For the treatment of pancreatic ductal
adenocarcinoma (PDAC), Gemcitabine has been considered a first-line
therapy. However, gemcitabine treatment provides only a slight
effect and consequently the overall survival of patients is
approximately 6 months (42).
Therefore, investigators have explored various therapeutic
strategies including the use of therapeutic antibodies (43–45).
Various therapeutic antibody candidates bind to different targets:
Cetucimab, anti-EGFR chimeric antibody (46); trastuzumab, humanized anti-ErbB2/HER2
antibody (47); tigatuzumab,
humanized anti-death receptor 5 antibody (48); cixutumumab, anti-IGF-1R antibody
(49); bevacizumab, humanized
anti-VEGF-A antibody (50), and so
on. The antibodies have been studied and used in clinical trials to
treat pancreatic cancer (43–45,51).
Despite these attempts, the clinical trials did not show adequate
clinical outcomes, and pancreatic cancer remains a lethal disease.
Therefore, continuous investigation and new target discovery are
needed for the treatment of pancreatic cancer. Previously, we
suggested TM4SF5 as an anti-cancer target of pancreatic cancer
because vaccination with TM4SF5 peptide vaccine suppressed growth
of TM4SF5-expressing tumors in a mouse pancreatic cancer model
(22). However, the effect of
peptide vaccine may have limitations because of low antigenicity
and tumor heterogeneity (52).
Therefore, therapeutic antibodies, which can be evaluated in detail
and applied promptly in necessity, may have advantages in the
aspect of practical application. Therefore, it was required to
investigate the efficacy of the anti-hTM4SF5 antibody using human
pancreatic cancer cells. In this study, we found that treatment of
the anti-hTM4SF5 antibody suppressed the growth and motility of
TM4SF5-expressing pancreatic cancer cells. In addition, TM4SF5
expression induced growth and motility of pancreatic tumor cells.
Although effectiveness and safety of anti-TM4SF5 antibody in
vivo have to be tested using animal model in the future, we
believe that our approach and results may respectively provide a
novel strategy and useful information to treat pancreatic
cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The current study was supported by grants from the
National Research Foundation (grant nos. NRF-2015R1A2A2A01007209
and NRF-2018R1A2B6002504) funded by the Ministry of Science and ICT
in the Republic of Korea.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SP, HJK and YL conceived the study and its design,
and wrote the manuscript. SP and JAP performed experiments
including PCR, cell line establishment, proliferation assays,
migration assays and western blot analysis. DK performed
immunostaining and confocal analysis. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
HCC
|
hepatocellular carcinoma
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
TAA
|
tumor-associated antigen
|
|
TM4SF5
|
transmembrane 4 superfamily member 5
protein
|
References
|
1
|
Negoita S, Feuer EJ, Mariotto A, Cronin
KA, Petkov VI, Hussey SK, Benard V, Henley SJ, Anderson RN, Fedewa
S, et al: Annual report to the nation on the status of cancer, part
II: Recent changes in prostate cancer trends and disease
characteristics. Cancer. 124:2801–2814. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ansari D, Tingstedt B, Andersson B,
Holmquist F, Sturesson C, Williamsson C, Sasor A, Borg D, Bauden M
and Andersson R: Pancreatic cancer: Yesterday, today and tomorrow.
Future Oncol. 12:1929–1946. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:2140–2141. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yáñez-Mó M, Barreiro O, Gordon-Alonso M,
Sala-Valdés M and Sánchez-Madrid F: Tetraspanin-enriched
microdomains: A functional unit in cell plasma membranes. Trends
Cell Biol. 19:434–446. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anderson KR, Singer RA, Balderes DA,
Hernandez-Lagunas L, Johnson CW, Artinger KB and Sussel L: The L6
domain tetraspanin Tm4sf4 regulates endocrine pancreas
differentiation and directed cell migration. Development.
138:3213–3224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Richardson MM, Jennings LK and Zhang XA:
Tetraspanins and tumor progression. Clin Exp Metastasis.
28:261–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Müller-Pillasch F, Wallrapp C, Lacher U,
Friess H, Büchler M, Adler G and Gress TM: Identification of a new
tumour-associated antigen TM4SF5 and its expression in human
cancer. Gene. 208:25–30. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SA, Lee SY, Cho IH, Oh MA, Kang ES,
Kim YB, Seo WD, Choi S, Nam JO, Tamamori-Adachi M, et al:
Tetraspanin TM4SF5 mediates loss of contact inhibition through
epithelial-mesenchymal transition in human hepatocarcinoma. J Clin
Invest. 118:1354–1366. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwon S, Choi KC, Kim YE, Ha YW, Kim D,
Park BK, Wu G, Kim DS, Lee Y and Kwon HJ: Monoclonal antibody
targeting of the cell surface molecule TM4SF5 inhibits the growth
of hepatocellular carcinoma. Cancer Res. 74:3844–3856. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JW: TM4SF5-mediated protein-protein
networks and tumorigenic roles. BMB Rep. 47:483–487. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim H, Kang M, Lee SA, Kwak TK, Jung O,
Lee HJ, Kim SH and Lee JW: TM4SF5 accelerates G1/S phase
progression via cytosolic p27Kip1 expression and RhoA activity.
Biochim Biophys Acta. 1803:975–982. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi S, Lee SA, Kwak TK, Kim HJ, Lee MJ,
Ye SK, Kim SH, Kim S and Lee JW: Cooperation between integrin
alpha5 and tetraspan TM4SF5 regulates VEGF-mediated angiogenic
activity. Blood. 113:1845–1855. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu G, Kim D, Park BK, Park S, Ha JH, Kim
TH, Gautam A, Kim JN, Lee SI, Park HB, et al: Anti-metastatic
effect of the TM4SF5-specific peptide vaccine and humanized
monoclonal antibody on colon cancer in a mouse lung metastasis
model. Oncotarget. 7:79170–79186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim YE, Kwon S, Wu G, Kim D, Park BK, Park
JA, Choi KC, Kim DS, Kwon HJ and Lee Y: Therapeutic effect of a
TM4SF5-specific monoclonal antibody against colon cancer in a mouse
model. Oncotarget. 5:8402–8415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Köhler G and Milstein C: Continuous
cultures of fused cells secreting antibody of predefined
specificity. Nature. 256:495–497. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Winter G and Milstein C: Man-made
antibodies. Nature. 349:293–299. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chames P, Van Regenmortel M, Weiss E and
Baty D: Therapeutic antibodies: Successes, limitations and hopes
for the future. Br J Pharmacol. 157:220–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weiner LM, Murray JC and Shuptrine CW:
Antibody-based immunotherapy of cancer. Cell. 148:1081–1084. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kwon S, Kim D, Park BK, Cho S, Kim KD, Kim
YE, Park CS, Ahn HJ, Seo JN, Choi KC, et al: Prevention and therapy
of hepatocellular carcinoma by vaccination with TM4SF5
epitope-CpG-DNA-liposome complex without carriers. PLoS One.
7:e331212012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kwon S, Kim YE, Kim D, Park BK, Wu G, Kim
TH, Choi SH, Kim DS, Kwon HJ and Lee Y: Prophylactic effect of a
peptide vaccine targeting TM4SF5 against colon cancer in a mouse
model. Biochem Biophys Res Commun. 435:134–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park S, Kim D, Wu G, Jung H, Park JA, Kwon
HJ and Lee Y: A peptide-CpG-DNA-liposome complex vaccine targeting
TM4SF5 suppresses growth of pancreatic cancer in a mouse allograft
model. OncoTargets Ther. 11:8655–8672. 2018. View Article : Google Scholar
|
|
23
|
Park BK, Park JY, Kim TH, Kim D, Wu G,
Gautam A, Maharjan S, Lee SI, Lee Y, Kwon HJ and Choi KC:
Production of an anti-TM4SF5 monoclonal antibody and its
application in the detection of TM4SF5 as a possible marker of a
poor prognosis in colorectal cancer. Int J Oncol. 53:275–285.
2018.PubMed/NCBI
|
|
24
|
Detchokul S, Williams ED, Parker MW and
Frauman AG: Tetraspanins as regulators of the tumour
microenvironment: Implications for metastasis and therapeutic
strategies. Br J Pharmacol. 171:5462–5490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vences-Catalán F, Rajapaksa R, Srivastava
MK, Marabelle A, Kuo CC, Levy R and Levy S: Tetraspanin CD81
promotes tumor growth and metastasis by modulating the functions of
T regulatory and myeloid-derived suppressor cells. Cancer Res.
75:4517–4526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zijlstra A, Lewis J, Degryse B, Stuhlmann
H and Quigley JP: The inhibition of tumor cell intravasation and
subsequent metastasis via regulation of in vivo tumor cell motility
by the tetraspanin CD151. Cancer Cell. 13:221–234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu CY, Lin HH, Tang MJ and Wang YK:
Vimentin contributes to epithelial-mesenchymal transition cancer
cell mechanics by mediating cytoskeletal organization and focal
adhesion maturation. Oncotarget. 6:15966–15983. 2015.PubMed/NCBI
|
|
28
|
Tian X, Liu Z, Niu B, Zhang J, Tan TK, Lee
SR, Zhao Y, Harris DC and Zheng G: E-cadherin/β-catenin complex and
the epithelial barrier. J Biomed Biotechnol. 2011:5673052011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahn HM, Ryu J, Song JM, Lee Y, Kim HJ, Ko
D, Choi I, Kim SJ, Lee JW and Kim S: Anti-cancer activity of novel
TM4SF5-targeting antibodies through TM4SF5 neutralization and
immune cell-mediated cytotoxicity. Theranostics. 7:594–613. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Diamantis N and Banerji U: Antibody-drug
conjugates-an emerging class of cancer treatment. Br J Cancer.
114:362–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perez HL, Cardarelli PM, Deshpande S,
Gangwar S, Schroeder GM, Vite GD and Borzilleri RM: Antibody-drug
conjugates: Current status and future directions. Drug Discov
Today. 19:869–881. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Berezhnaya NM, Belova OB, Vinnichuk YD and
Tarutinov VI: Expression of E-cadherin in drug resistant human
breast cancer cells and their sensitivity to lymphokine-activated
lymphocytes action. Exp Oncol. 31:242–245. 2009.PubMed/NCBI
|
|
33
|
Chen X, Wang Y, Xia H, Wang Q, Jiang X,
Lin Z, Ma Y, Yang Y and Hu M: Loss of E-cadherin promotes the
growth, invasion and drug resistance of colorectal cancer cells and
is associated with liver metastasis. Mol Biol Rep. 39:6707–6714.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lazarova DL and Bordonaro M: Vimentin,
colon cancer progression and resistance to butyrate and other
HDACis. J Cell Mol Med. 20:989–993. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arumugam T, Ramachandran V, Fournier KF,
Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey
DJ and Choi W: Epithelial to mesenchymal transition contributes to
drug resistance in pancreatic cancer. Cancer Res. 69:5820–5828.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, VandenBoom TG II, Kong D, Wang Z,
Ali S, Philip PA and Sarkar FH: Up-regulation of miR-200 and let-7
by natural agents leads to the reversal of
epithelial-to-mesenchymal transition in gemcitabine-resistant
pancreatic cancer cells. Cancer Res. 69:6704–6712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xie CG, Wei SM, Chen JM, Xu XF, Cai JT,
Chen QY and Jia LT: Down-regulation of GEP100 causes increase in
E-cadherin levels and inhibits pancreatic cancer cell invasion.
PLoS One. 7:e378542012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gradiz R, Silva HC, Carvalho L, Botelho MF
and Mota-Pinto A: MIA PaCa-2 and PANC-1-pancreas ductal
adenocarcinoma cell lines with neuroendocrine differentiation and
somatostatin receptors. Sci Rep. 6:216482016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Teixidó C, Marés R, Aracil M, Ramón y
Cajal S and Hernández-Losa J: Epithelial-mesenchymal transition
markers and HER3 expression are predictors of elisidepsin treatment
response in breast and pancreatic cancer cell lines. PLoS One.
8:e536452013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nishino H, Takano S, Yoshitomi H, Suzuki
K, Kagawa S, Shimazaki R, Shimizu H, Furukawa K, Miyazaki M and
Ohtsuka M: Grainyhead-like 2 (GRHL2) regulates epithelial
plasticity in pancreatic cancer progression. Cancer Med.
6:2686–2696. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lafitte M, Moranvillier I, Garcia S,
Peuchant E, Iovanna J, Rousseau B, Dubus P, Guyonnet-Dupérat V,
Belleannée G, Ramos J, et al: FGFR3 has tumor suppressor properties
in cells with epithelial phenotype. Mol Cancer. 12:832013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Teague A, Lim KH and Wang-Gillam A:
Advanced pancreatic adenocarcinoma: A review of current treatment
strategies and developing therapies. Ther Adv Med Oncol. 7:68–84.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huang ZQ and Buchsbaum DJ: Monoclonal
antibodies in the treatment of pancreatic cancer. Immunotherapy.
1:223–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chames P, Kerfelec B and Baty D:
Therapeutic antibodies for the treatment of pancreatic cancer.
ScientificWorldJournal. 10:1107–1120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Luedke E, Jaime-Ramirez AC, Bhave N and
Carson WE III: Monoclonal antibody therapy of pancreatic cancer
with cetuximab: Potential for immune modulation. J Immunother.
35:367–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Harder J, Ihorst G, Heinemann V, Hofheinz
R, Moehler M, Buechler P, Kloeppel G, Röcken C, Bitzer M, Boeck S,
et al: Multicentre phase II trial of trastuzumab and capecitabine
in patients with HER2 overexpressing metastatic pancreatic cancer.
Br J Cancer. 106:1033–1038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Forero-Torres A, Infante JR, Waterhouse D,
Wong L, Vickers S, Arrowsmith E, He AR, Hart L, Trent D, Wade J, et
al: Phase 2, multicenter, open-label study of tigatuzumab
(CS-1008), a humanized monoclonal antibody targeting death receptor
5, in combination with gemcitabine in chemotherapy-naive patients
with unresectable or metastatic pancreatic cancer. Cancer Med.
2:925–932. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Philip PA, Goldman B, Ramanathan RK, Lenz
HJ, Lowy AM, Whitehead RP, Wakatsuki T, Iqbal S, Gaur R, Benedetti
JK and Blanke CD: Dual blockade of epidermal growth factor receptor
and insulin-like growth factor receptor-1 signaling in metastatic
pancreatic cancer: Phase Ib and randomized phase II trial of
gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus
erlotinib (SWOG S0727). Cancer. 120:2980–2985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Van Cutsem E, Vervenne WL, Bennouna J,
Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang
A, Cosaert J and Moore MJ: Phase III trial of bevacizumab in
combination with gemcitabine and erlotinib in patients with
metastatic pancreatic cancer. J Clin Oncol. 27:2231–2237. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Karanikas M, Esempidis A, Chasan ZT,
Deftereou T, Antonopoulou M, Bozali F, Amarantidis K and Man YG:
Pancreatic cancer from molecular pathways to treatment opinion. J
Cancer. 7:1328–1339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Slingluff CL Jr: The present and future of
peptide vaccines for cancer: Single or multiple, long or short,
alone or in combination? Cancer J. 17:343–350. 2011. View Article : Google Scholar : PubMed/NCBI
|