Introduction
Breast cancer, the most common type of cancer in
women, presents a challenge for global research (1). Molecular research on breast cancer was
at a bottleneck, until the identification of a correlation between
BRCA1/2 DNA repair associated and breast cancer was reported and
marked great progress in the research, treatment and prognosis of
breast cancer (2,3). Following this, genetic susceptibility
has become the focus of breast cancer research (4). However, in the context of big data,
with the development of high-throughput sequencing technology, it
is not difficult to find susceptibility genes.
The Homeobox (HOX) genes is a large class of
transcription factors that play an important role in embryogenesis
and oncogenesis, as well as the distribution of fat and hair in
body (5–7). In humans, the HOX gene family contains
39 HOX genes located on 4 different chromosomes (7p15, 17q21.2,
12q13 and 2q31) (8). The 39 HOX
genes are divided into 4 clusters (HOXA, HOXB, HOXC and HOXD)
(9). Each HOX gene contains a
well-conserved DNA sequence, known as the homeobox (10). The unique expression pattern,
including mutation, and dependent mechanism of the HOX genes
regulates, to some extent, the embryonic development of vertebrates
(11,12). When HOX protein expression is
upregulated, it may lead to cancer (5). It has also been reported that the HOXC
gene family is highly expressed in certain solid tumors, including
lung, prostate and colon cancer (13,14). The
HOXA and HOXB gene families have a similar expression in breast
cancer, which is derived from the ectoderm. Whether the expression
level of the HOX gene follows the origin of the germ layer in
cancer requires further investigation. A study has reported that
HOXC13, a member of the HOXC gene family, is highly
expressed in the MCF-7 cell line (15). Thus, the present study aimed to
explore the expression and significance of HOXC13 in breast
cancer.
In the present study, the Oncomine and tumor public
databases (bc-GenExMinerv 4.2; GOBO database; CCLE) were used to
analyze the expression level of HOXC13 in different types of cancer
including breast cancer. HOXC13 was then further investigated in
breast cancer. The expression and co-expression of HOXC13 in
breast cancer was re-analyzed using the University of California,
Santa Cruz (UCSC) cancer genomics browser. Finally, the clinical
significance of HOXC13 in breast cancer was further
explored.
Materials and methods
Oncomine database verification
The Oncomine (www.oncomine.org) database is a public bioinformatics
database containing gene expression data set that has become an
industry-standard tool cited in >1,100 peer-reviewed journal
articles (16,17). The Oncomine platform has been used as
a foundation for ground-breaking discoveries with unique features
that include scalability, high quality, consistency and
standardized analysis (18). In
order to screen out the most meaningful RNA probes, the paired
Student's t-test was used to generate P-values to compare
expression differences between cancer and healthy adjacent
non-cancerous tissues. Relevant statistical indicators were used as
follows: P<1×10−4, fold change >4 and gene ranking
in the top 10%. Moreover, the Oncomine database was used to explore
the co-expression analysis of HOXC13 in breast cancer. The
search term ‘HOXC13’ was used, followed by coexpression analysis
and selecting the cancer type as breast cancer. Lastly, the
database of TCGA breast was chosen. Furthermore, the same cut-off
values used as aforementioned.
Cancer cell line encyclopedia (CCLE)
verification
The CCLE (portals.broadinstitute.org) provides public access to
genomic data, analysis and visualization for >1,100 cell lines
from various tumors, such as gastric cancer cell line AGS and
intestinal cancer cell line SW480 (19,20).
Each gene of the human genome has multiple datasets and data
identifiers, obtained by high-throughput sequencing. The 5 major
dataset types are copy number, mRNA expression (Affymetrix),
reverse phase protein array, reduced representation bisulfite
sequencing, and mRNA expression (RNA sequencing). The expression
and methylation level of HOXC13 was analyzed in each tumor
cell line using CCLE, using the search term ‘HOXC13’.
Gene expression-based Outcome for
Breast cancer Online (GOBO) analysis
The GOBO database (version 1.0.3; co.bmc.lu.se/gobo/gsa_cellines.pl) is a
user-friendly public database. It allows for rapid assessment of
gene expression levels, identification of co-expressed genes and
association with outcome for single genes, gene sets or gene
signatures in an 1,881-sample breast cancer dataset (21). The most important functionality of
the GOBO database is the possibility of investigating gene
expression levels in breast cancer subgroups and cell lines for
gene sets (22). Breast cancer
subtypes are classified into basal A, basal B and luminal in the
GOBO database. A correlation map is a square table where each line
and column represent a gene. Each cell represents an interaction
between two genes and is colored according to the value of the
Pearson's correlation coefficient between these two genes, from
dark blue (coefficient=−1) to dark red (coefficient=1). Cells from
the diagonal of the correlation map represent the interaction of a
gene with itself and are colored black.
UCSC cancer genomics browser
analysis
UCSC (xena.ucsc.edu)
is an online exploration tool for public and private multi-omics
functional genomics and clinical/phenotype data (23). The Cancer Genome Atlas (TCGA;
http://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga)
were used in the present study. Using the UCSC database, a heat map
of HOXC13 expression in various subtypes of breast cancer,
such as LuminalA, luminalB, normal-like, Basal-like and Her2-riched
was generated. At the same time, the co-expression of HOXC13
in breast cancer was analyzed.
cBioPortal database analysis
The public cBioPortal site (www.cbioportal.org; last entered, 28th March 2019) is
hosted by the Center for Molecular Oncology at Memorial Sloan
Kettering Cancer Center. The cBioportal database has been
recognized as a way to verify gene mutations (24–26).
This database can also analyze gene expression in different tumors
and different studies (23,27). This database has been recognized by
multiple studies (26,28). The online cBioPortal for Cancer
Genomics was used to provide mutations of HOXC13 (including
nonstart, missense, truncation and missense) and its expression in
different studies (28). The cancer
types and databases used were breast (TCGA2015), breast (TCGA),
breast invasive carcinoma breast (TCGA PanCan) (29–34).
Breast cancer gene-expression miner
(bc-GenExMiner) analysis
bc-GenExMinerv (version 4.2) is a statistical mining
tool of published annotated genomic data (35,36). The
statistical analyses are grouped in three modules: Expression,
prognosis and correlation. The co-expression of HOXC13 in
breast cancer was explored using this database; each study is
validated across multiple databases to avoid discrepancies in
individual databases. At the same time, the effects of
HOXC13 and HOX transcript antisense RNA (HOTAIR) on
the survival prognosis in breast cancer were also analyzed.
Statistical analysis
Pearson test and Spearman's rank test were used to
evaluate coexpression. The analysis criteria selected were as
follows: Gene, nodal and estrogen receptor status of the cohorts to
be explored, event on which survival analysis will be based and
splitting criterion of median HOXC13 expression. To analyze the
prognostic value of HOXC13 and HOTAIR, the patient
samples are split into two groups according to the median
expressions of HOXC13 and HOTAIR. The two patient
cohorts were compared by a Kaplan-Meier survival plot, and the
hazard ratio with 95% confidence intervals and logrank P-value are
calculated. Significance was determined by the P-value provided by
each database.
Results
HOXC13 mRNA expression in human
cancer
HOXC13 has been proven to be highly expressed
in digestive tract-derived tumors (37–39).
However, to the best of our knowledge, there have been no reports
on the expression of HOXC13 in breast cancer. Therefore the
expression levels of HOXC13 in various human tumors from the
Oncomine database and CCLE were determined. A simultaneous fold
change of >4, gene rank of >10% and P<1×10−4
was set as the threshold. To our surprise, HOXC13 was found
by high-throughput sequencing and biological gene chip technology
to be abnormally highly expressed in breast cancer (Fig. 1A). Only breast cancer revealed
significant unique analysis in the Oncomine database when the fold
change was >4.
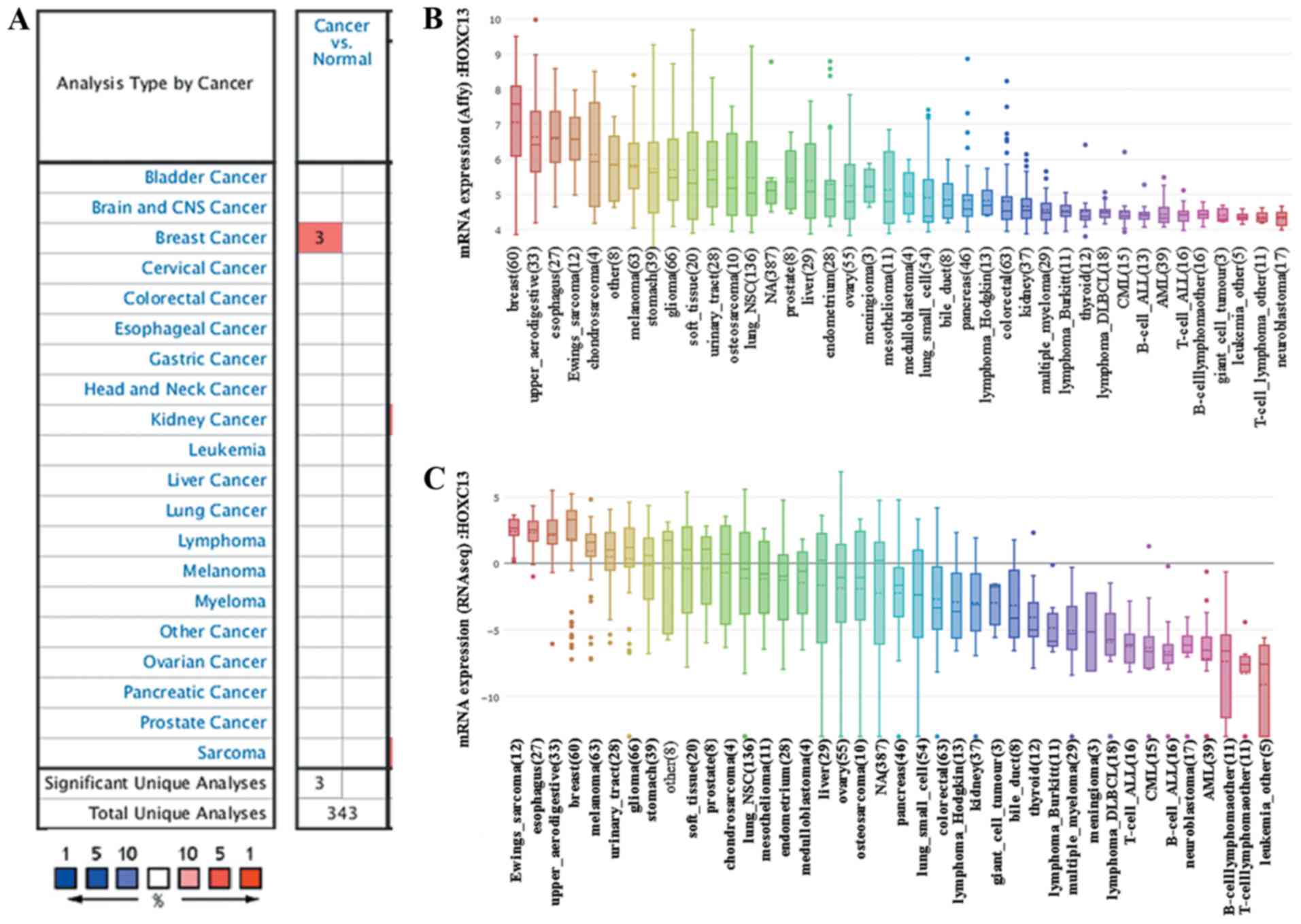 | Figure 1.HOXC13 mRNA expression level
in different types of human cancer. This image shows the expression
of HOXC13 in human tumors. (A) Graph showing the number of datasets
with a statistically significant mRNA HOXC13 overexpression, based
on the cut-off value of P<1×10−4 and fold change
>4 in the Oncomine database. The cell color is determined by the
best gene rank percentile for the analyses within the cell. Blue
represents low expression in tumors, red represents high expression
in tumors and white represents no difference in tumor tissues and
normal tissues. As shown in the figure, breast cancer has three
data sets showing high expression, based on the cut-off value of
P<1×10−4, fold change >4 and gene ranking in the
top 10%. (B) mRNA expression of HOXC13 in different cancer
cell lines. The expression of HOXC13 ranks highest in breast
cancer using Affy gene chip data in the Cancer Cell Line
Encyclopedia. (C) The mRNA expression of HOXC13 ranks fourth
highest in different tumor cell lines RNA-seq data, behind that of
Ewings sarcoma, esophagus and upper aerodigestive tract. Affy,
Affymetrix; RNA seq, RNA sequencing; CNS, central nervous system;
HOXC13, homeobox C13. |
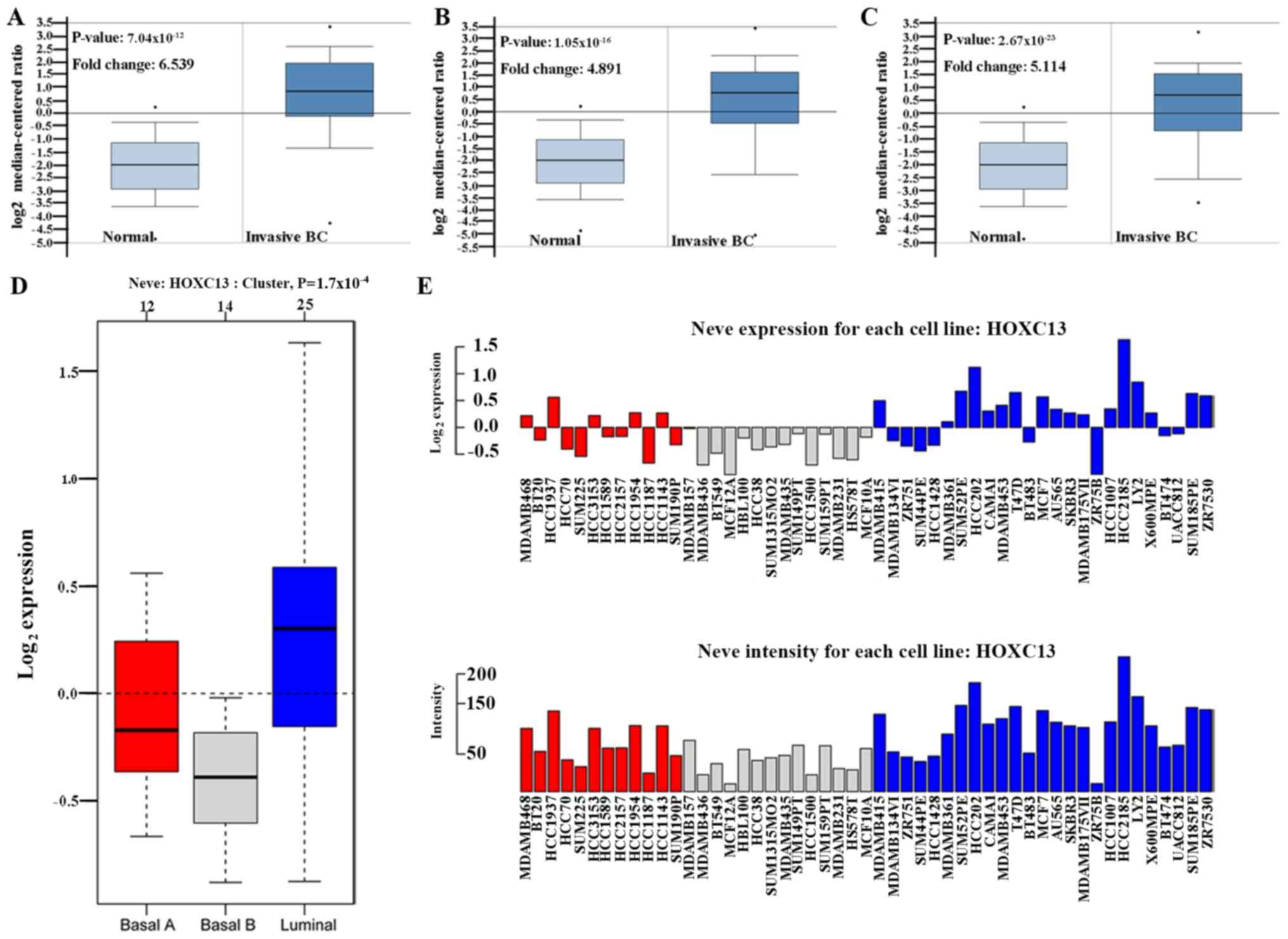
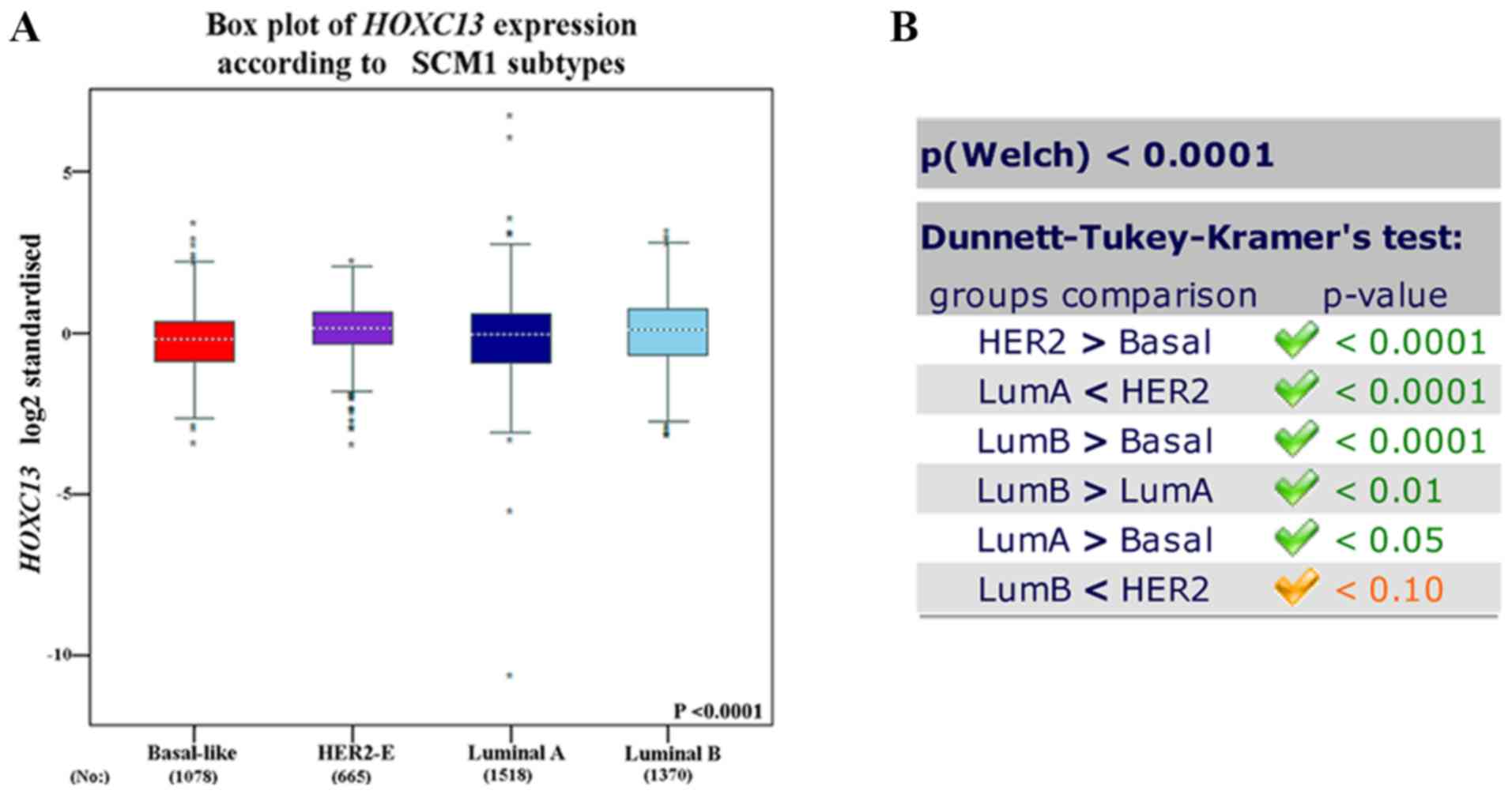
HOXC13 mRNA expression in breast
cancer
The high expression of HOXC13 in breast
cancer has been preliminary confirmed in the Oncomine database,
GOBO database and CCLE. From the CCLE database, it was found that
at the RNAseq level, the expression level of HOXC13 in
breast cancer ranked fourth and ranked first in the Affy level.
However, there is no report on the specific expression of
HOXC13 in breast cancer. Therefore, its specific expression
in various subtypes of breast cancer was further explored. HOXC13
was analyzed in various tumor types via Oncomine database and GOBO
database and explored HOXC13 in various breast cancer cells via
GOBO database and CCLE. HOXC13 was found to be highly expressed in
invasive and luminal-like breast cancer than in any other subtype
(Figs. 2 and 3). Such expression characteristics were
consistent with breast cancer cell lines and tissues (Figs. 2D and 3). Using the UCSC cancer genomics browser
analysis, the heat map of the gene and exon expression of HOXC13 in
various subtypes of breast cancer was obtained (Fig. 4A). Furthermore, the expression of
HOXC13 in the different data sets, including breast (TCGA
2015), breast (TCGA), breast invasive carcinoma breast (TCGA
PanCan), was explored using the cBioPortal database. The expression
characteristics of breast cancer in multiple data sets such as
amplification and missense mutations, were demonstrated (Fig. 4B).
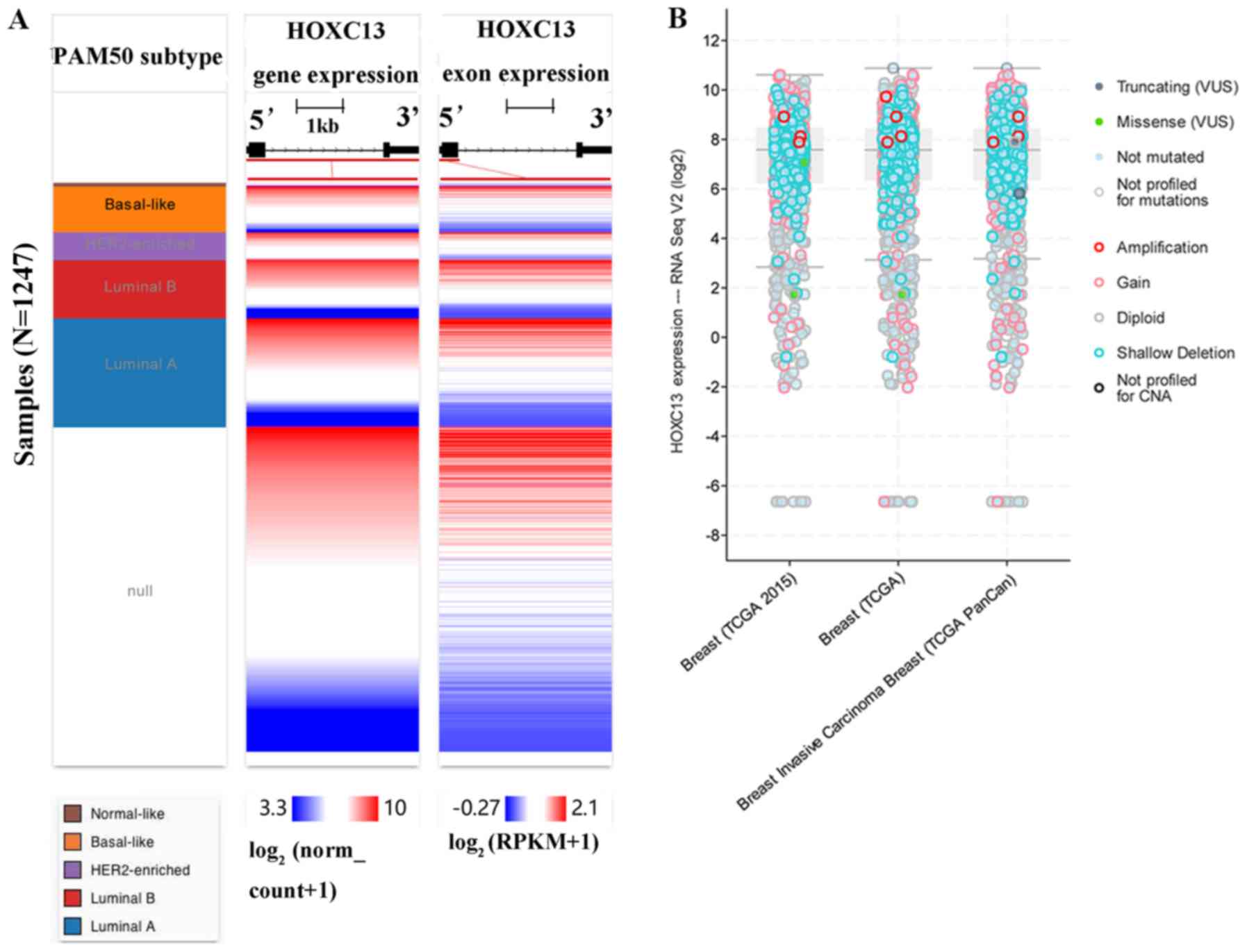 | Figure 4.Analysis of the expression of
HOXC13 in breast cancer subtypes. (A) HOXC13
expression heatmap of each breast cancer subtype in TCGA from the
University of California, Santa Cruz Xena browser. Null data
indicates samples that have no gene expression data. (B)
HOXC13 expression analysis and mutation status in breast
cancer using cBioPortal. HOXC13 was significantly amplified
in breast cancer subtypes, including breast invasive carcinoma. The
present study included datasets on breast (TCGA2015), breast (TCGA)
and breast invasive carcinoma breast (TCGA PanCan) datasets. TCGA,
The Cancer Genome Atlas; RNA seq, RNA sequencing; HER2, human
epidermal growth factor receptor 2; RPKM, reads per kilobase of
transcript per million mapped reads; HOXC13, homeobox C13;
VUS, variants of uncertain significance; CNA, copy number
alteration. |
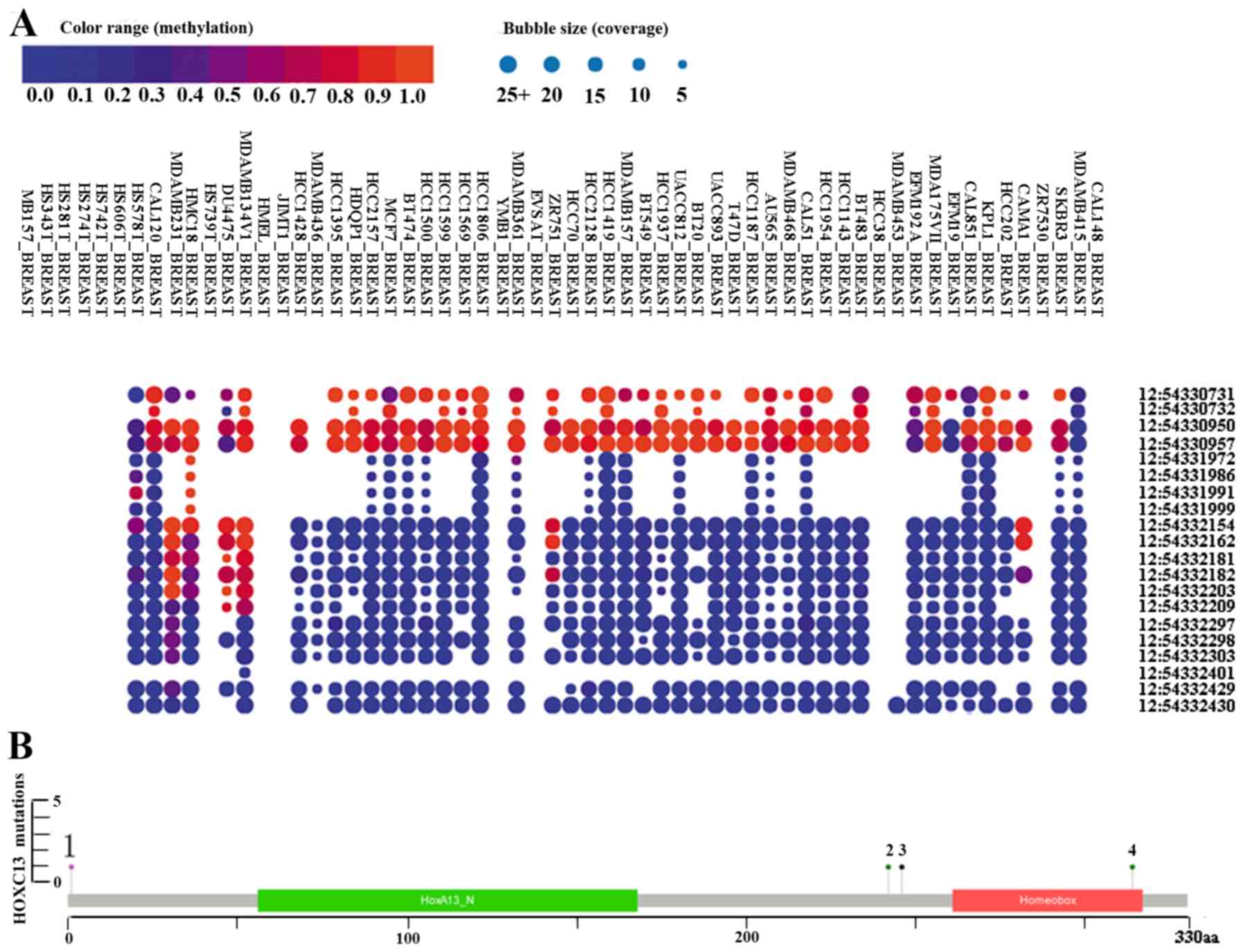
HOXC13 methylation and mutation in
human breast cancer
The bubble chart shows the methylation level of
HOXC13 in breast cancer cell lines from the CCLE (Fig. 5A). HOXC13 is highly methylated at
three sites (positions 54,330,731, 54,330,950 and 54,330,957) on
chromosome 12 from methylation and coverage (Fig. 5A). The discovery of cpG island
methylation further supported the high expression of HOXC13
in breast cancer. cBioPortal was used to assess the frequency of
HOXC13 mutations in breast cancer (Figs. 4B and 5). HOXC13 contains multiple mutations in
breast cancer such as amplification, gain, missense and truncation
(Fig. 4B). Missense and truncation
are two major forms of mutation (Fig.
5).
Co-expression of HOXC13 mRNA in breast
cancer
To investigate the reason for the high expression of
HOXC13 in breast cancer, bc-GenExMiner version 4.2 was used
to analyze the potential co-expression of HOXC13 in breast
cancer. It was found that HOTAIR and HOXC13 are
highly co-expressed in breast cancer (Fig. 6A). Furthermore, to verify the
co-expression of HOXC13 and HOTAIR in breast cancer,
their co-expression heat maps were obtained and mined (Fig. 6B) and correlation analysis was
performed using the UCSC Xena browser (Fig. 6C).
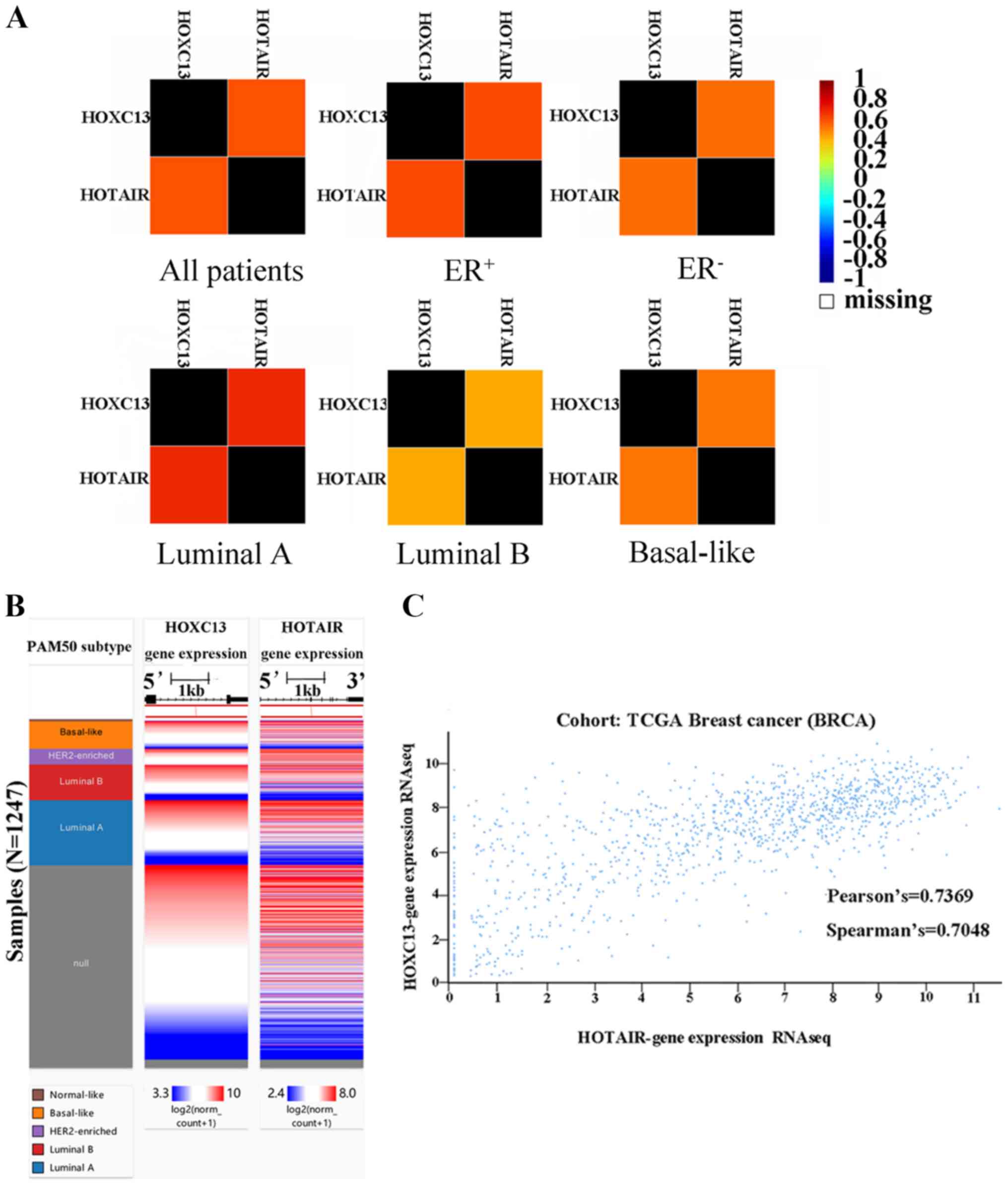 | Figure 6.Co-expression analysis of
HOXC13 and HOTAIR. (A) A correlation map illustrates
pairwise correlations between HOXC13 and HOTAIR. They
are all patients, ER+, ER-, luminal A, luminal B and basal-like
respectively according to the group. (B) The co-expression heat map
of HOXC13 and HOTAIR in TCGA derived from the UCSC
Xena browser. (C) Association between HOXC13 and
HOTAIR gene expression in TCGA breast cancer derived from
the UCSC Xena browser. The Pearson's value was 0.7369 and the
Spearman's value was 0.7048. HOXC13, homeobox C13; HOTAIR, HOX
transcript antisense RNA; UCSC, University of California Santa
Cruz; ER, estrogen receptor; -, negative; +, positive; TCGA, The
Cancer Genome Atlas; RNA seq, RNA sequencing; HER2, human epidermal
growth factor receptor 2; PAM, prediction analysis of
microarray. |
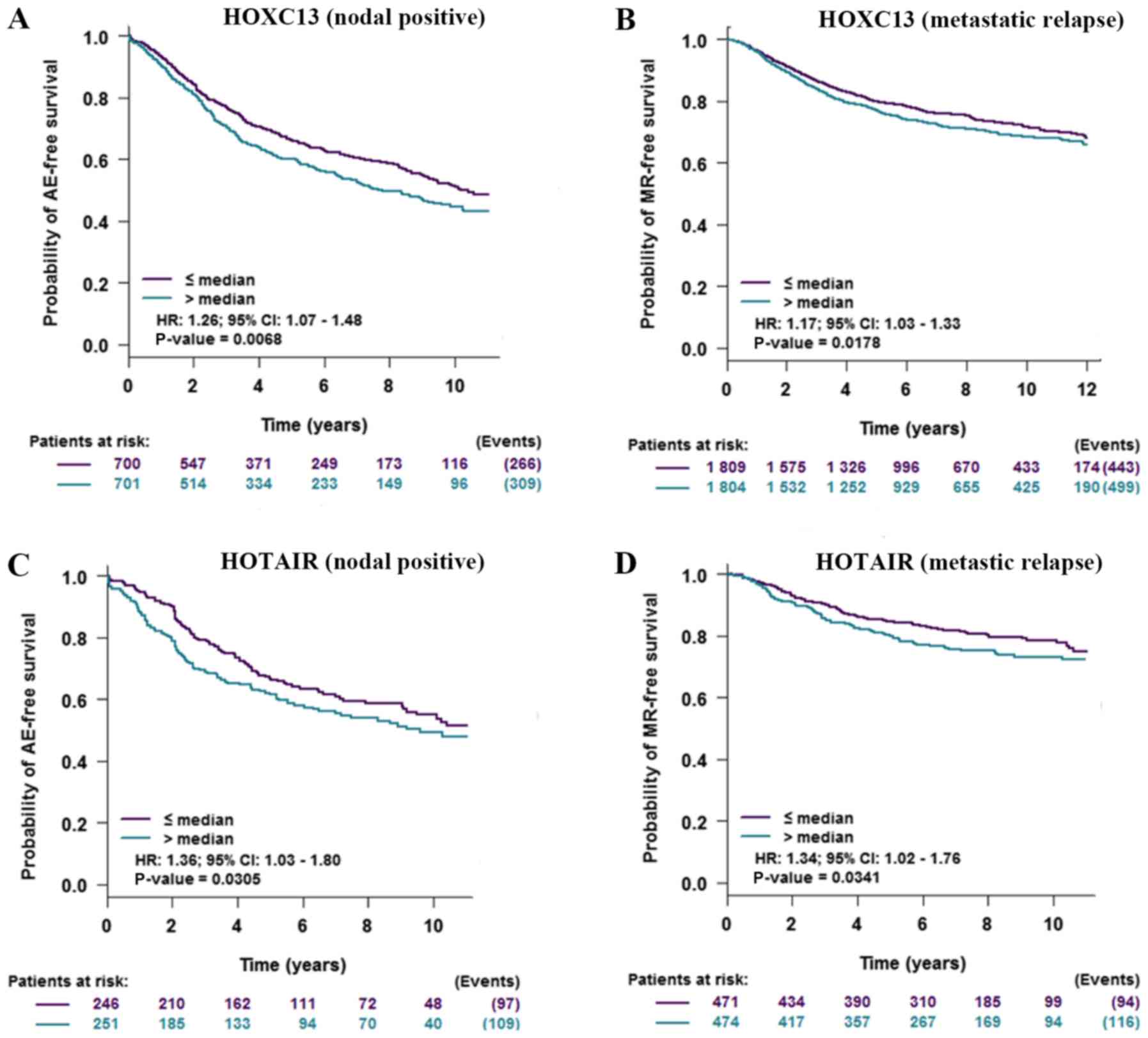
Impact of HOXC13 and HOTAIR on the
prognosis of patients with breast cancer
To verify the impact of the high expression of
HOXC13 and HOTAIR on patients with breast cancer,
prognostic analysis of HOXC13 and HOTAIR in breast
cancer was performed and HOXC13 and HOTAIR were found
to have a negative impact on the prognosis of patients with tumor
and lymph node metastasis (Fig.
7).
Discussion
The mammary gland is an appendage of the ectoderm,
whose formation begins during embryonic development (40). Moreover, breast cancer cells remain
highly associated with ectoderm cells (41). Studies have shown that the HOX gene
plays an important role in embryonic development and tumor
formation (9,42–44).
However, to the best of our knowledge, no research has reported the
exact role of HOXC13 in breast cancer so far.
The HOXC13 gene is considered an element of
hair morphogenesis, and the HOXC13 protein is a member of the human
replication complex in growing cells (45,46).
This id the same for breast adenocarcinoma MCF7 lines (46). HOXC13 can promote the
expression of a series of proto-oncogenes, including topoisomerase
I and II, and allow these expression products to form a replication
complex (47,48). Therefore, HOXC13 is involved
in the development of tumors. In addition, HOXC13 increases
the metastasis of ectodermal-derived melanoma (49). HOTAIR is the product of the
transcription of the HOXC gene cluster antisense strand (50). HOTAIR is transcribed from the
mammalian HOXC gene cluster on chromosome 12q13.13 (51,52).
HOTAIR predicts poor prognosis in tumor cell cycle and metastasis
(53). It has been reported that
HOTAIR upregulates HOX in colon cancer (39). A strong co-expression of
HOTAIR and HOXC13 was confirmed in proximal and
distal colon cancer, suggesting that HOTAIR and
HOXC13 could promote tumor and lymph node metastasis
(39,54–56).
In the present study, it was first identified that
HOXC13 is highly expressed in breast cancer both at the
cellular and tissue levels. This was a finding from the
co-verification of the data from TCGA's Oncomine or GSE's
bc-GenExMiner. It was found for the first time that HOXC13
is most highly expressed in luminal-like subtype of breast cancer.
This laid the foundation for the future study of the relationship
between HOXC13 and breast cancer surface hormone receptors
(estrogen receptor and progesterone receptor). In order to explore
the high expression of HOXC13 in breast cancer, its
methylation and mutation status were investigated. To our surprise,
three sites on chromosome 12 were found to be consistently highly
methylated in different breast cancer cell lines. Certain studies
have reported that HOX gene methylation regulates hereditary breast
cancer (57–59). In addition, the methylation level of
HOXA11 is significantly higher in breast cancer compared
with that in normal tissues, and is positively associated with
family history and lymph node metastasis in breast cancer (59). Furthermore, the methylation level of
HOXD13 in breast cancer is almost identical to that of
HOXA11, and leads to shorter survival time (58). Therefore, the present findings and
the HOX gene family have consistent trends in methylation levels
and similar prognostic effects in breast cancer. However, further
studies on the specific regulation mechanism of HOXC13
methylation in HOXC13 transcription is required. The present
study is the first to report the mutation of HOXC13 in
breast cancer. Missense may be an indispensable cause of the high
expression of HOXC13 in breast cancer.
The lncRNA HOTAIR is derived from the region
between HOXC11 and HOXC12 (51). It has been shown that HOXC10,
HOXC11, HOXC12 and HOXC13 are adjacent to each other in
the HOXC gene cluster (52).
Simultaneously, the HOXC distal enhancer has non-specific
enhancement of HOXC10 and HOTAIR enhancer activity,
promoting the HOXC10 and HOTAIR expression (60). On the other hand, specific intergenic
non-coding RNAs (including HOTAIR) in the HOX loci can directly
modulate the expression of the HOX gene in normal and cancer status
(61). This has been confirmed in
colon cancer (39,55). Therefore the mechanism between
HOXC13 and HOTAIR will be explored further in this
respect. In the present study, the co-expression of HOTAIR
and HOXC13 provided a new direction for studying the
function of HOTAIR in breast cancer. In addition, a study
has identified through meta-analysis that HOTAIR has a
statistically significant effect on lymph node and distant
metastasis in various types of cancer, including breast cancer,
gastric cancer and colorectal cancer, which was consistent with our
conclusion (62). The present data
showed that HOTAIR and HOXC13 were significantly
associated with lymph node metastasis and distant metastasis
recurrence. Furthermore, they were shown to have a significant
impact on survival period.
In conclusion, the present study was performed using
public databases and revealed the expression and clinical
significance of HOXC13 in breast cancer. However, the
specific interactions and mechanisms involved require further
experimental verification, which will be performed in future
studies.
Acknowledgements
Not applicable.
Funding
This study was supported by the Shenzhen Science and
Research Innovation Foundation (grant no.
JCYJ20170815090309586).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW contributed to the experimental design and
fundraising. JWC and LZ contributed to the acquisition of data. LZZ
assisted in the data processing. CL processed the data and wrote
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barrios CH, Reinert T and Werutsky G:
Global breast cancer research: Moving forward. Am Soc Clin Oncol
Educ Book. 38:441–450. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harbeck N and Gnant M: Breast cancer.
Lancet. 389:1134–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nathanson KL and Domchek SM: Therapeutic
approaches for women predisposed to breast cancer. Annu Rev Med.
62:295–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rousset-Jablonski C and Gompel A:
Screening for familial cancer risk: Focus on breast cancer.
Maturitas. 105:69–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo Z, Rhie SK and Farnham PJ: The
enigmatic HOX genes: Can we crack their code? Cancers (Basel).
11(pii): E3232019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Turcotte M, Abadi A, Peralta-Romero J,
Suarez F, Reddon H, Gomez-Zamudio J, Burguete-Garcia AI, Cruz M and
Meyre D: Genetic contribution to waist-to-hip ratio in Mexican
children and adolescents based on 12 loci validated in European
adults. Int J Obes (Lond). 43:13–22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu F, Chen Y, Zhu G, Hysi PG, Wu S,
Adhikari K, Breslin K, Pospiech E, Hamer MA, Peng F, et al:
Meta-analysis of genome-wide association studies identifies 8 novel
loci involved in shape variation of human head hair. Hum Mol Genet.
27:559–575. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li L, Wang Y, Song G, Zhang X, Gao S and
Liu H: HOX cluster-embedded antisense long non-coding RNAs in lung
cancer. Cancer Lett. 450:14–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhatlekar S, Fields JZ and Boman BM: Role
of HOX genes in stem cell differentiation and cancer. Stem Cells
Int. 2018:35694932018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vaquerizas JM, Kummerfeld SK, Teichmann SA
and Luscombe NM: A census of human transcription factors: Function,
expression and evolution. Nat Rev Genet. 10:252–263. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gordon J: Hox genes in the pharyngeal
region: How Hoxa3 controls early embryonic development of the
pharyngeal organs. Int J Dev Biol. 62:775–783. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mallo M: The vertebrate tail: A gene
playground for evolution. Cell Mol Life Sci. Sep 26–2019.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhatlekar S, Fields JZ and Boman BM: HOX
genes and their role in the development of human cancers. J Mol Med
(Berl). 92:811–823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yao Y, Luo J, Sun Q, Xu T, Sun S, Chen M,
Lin X, Qian Q, Zhang Y, Cao L, et al: HOXC13 promotes proliferation
of lung adenocarcinoma via modulation of CCND1 and CCNE1. Am J
Cancer Res. 7:1820–1834. 2017.PubMed/NCBI
|
|
15
|
Svingen T and Tonissen KF: Altered HOX
gene expression in human skin and breast cancer cells. Cancer Biol
Ther. 2:518–523. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi S and Zhang ZG: Role of Sp1 expression
in gastric cancer: A meta-analysis and bioinformatics analysis.
Oncol Lett. 18:4126–4135. 2019.PubMed/NCBI
|
|
17
|
Cheng L, Shi L and Dai H: Bioinformatics
prognostic biomarkers among Krüppel-like transcription factors
(KLFs) in breast cancer. Cancer Biomark. Oct 12–2019.(Epub ahead of
print). View Article : Google Scholar
|
|
18
|
Yang K, Gao J and Luo M: Identification of
key pathways and hub genes in basal-like breast cancer using
bioinformatics analysis. Onco Targets Ther. 12:1319–1331. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng L, Pandya PH, Liu E, Chandra P, Wang
L, Murray ME, Carter J, Ferguson M, Saadatzadeh MR,
Bijangi-Visheshsaraei K, et al: Integration of genomic copy number
variations and chemotherapy-response biomarkers in pediatric
sarcoma. BMC Med Genomics. 12 (Suppl 1):S232019. View Article : Google Scholar
|
|
20
|
Streit M, Gratzl S, Stitz H, Wernitznig A,
Zichner T and Haslinger C: Ordino: A visual cancer analysis tool
for ranking and exploring genes, cell lines and tissue samples.
Bioinformatics. 35:3140–3142. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fernández-Nogueira P, Bragado P, Almendro
V, Ametller E, Rios J, Choudhury S, Mancino M and Gascón P:
Differential expression of neurogenes among breast cancer subtypes
identifies high risk patients. Oncotarget. 7:5313–5326. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zen g, Xiao Y, Zhu J, Peng C, Liang W and
Lin H: Knockdown of nucleophosmin 1 suppresses proliferation of
triple-negative breast cancer cells through activating
CDH1/Skp2/p27kip1 pathway. Cancer Manag Res. 11:143–156. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klonowska K, Czubak K, Wojciechowska M,
Handschuh L, Zmienko A, Figlerowicz M, Dams-Kozlowska H and
Kozlowski P: Oncogenomic portals for the visualization and analysis
of genome-wide cancer data. Oncotarget. 7:176–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wen W, Gong J, Wu P, Zhao M, Wang M, Chen
H and Sun J: Mutations in gliclazide-associated genes may predict
poor bladder cancer prognosis. FEBS Open Bio. 9:457–467.
2019.PubMed/NCBI
|
|
25
|
Chen E, Qin X, Peng K, Xu X, Li W, Cheng
X, Tang C, Cui Y, Wang Z and Liu T: Identification of potential
therapeutic targets among CXC chemokines in breast tumor
microenvironment using integrative bioinformatics analysis. Cell
Physiol Biochem. 45:1731–1746. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: an open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kline CLB, Ralff MD, Lulla AR, Wagner JM,
Abbosh PH, Dicker DT, Allen JE and El-Deiry WS: Role of dopamine
receptors in the anticancer activity of ONC201. Neoplasia.
20:80–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Banerji S, Cibulskis K, Rangel-Escareno C,
Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY,
Sougnez C, Zou L, et al: Sequence analysis of mutations and
translocations across breast cancer subtypes. Nature. 486:405–409.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eirew P, Steif A, Khattra J, Ha G, Yap D,
Farahani H, Gelmon K, Chia S, Mar C, Wan A, et al: Dynamics of
genomic clones in breast cancer patient xenografts at single-cell
resolution. Nature. 518:422–426. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martelotto LG, De Filippo MR, Ng CK,
Natrajan R, Fuhrmann L, Cyrta J, Piscuoglio S, Wen HC, Lim RS, Shen
R, et al: Genomic landscape of adenoid cystic carcinoma of the
breast. J Pathol. 237:179–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Razavi P, Chang MT, Xu G, Bandlamudi C,
Ross DS, Vasan N, Cai Y, Bielski CM, Donoghue MTA, Jonsson P, et
al: The genomic landscape of endocrine-resistant advanced breast
cancers. Cancer Cell. 34:427–438.e6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shah SP, Roth A, Goya R, Oloumi A, Ha G,
Zhao Y, Turashvili G, Ding J, Tse K, Haffari G, et al: The clonal
and mutational evolution spectrum of primary triple-negative breast
cancers. Nature. 486:395–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tan J, Ong CK, Lim WK, Ng CC, Thike AA, Ng
LM, Rajasegaran V, Myint SS, Nagarajan S, Thangaraju S, et al:
Genomic landscapes of breast fibroepithelial tumors. Nat Genet.
47:1341–1345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jézéquel P, Campone M, Gouraud W,
Guérin-Charbonnel C, Leux C, Ricolleau G and Campion L:
bc-GenExMiner: An easy-to-use online platform for gene prognostic
analyses in breast cancer. Breast Cancer Res Treat. 131:765–775.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jézéquel P, Frénel JS, Campion L,
Guérin-Charbonnel C, Gouraud W, Ricolleau G and Campone M:
bc-GenExMiner 3.0: New mining module computes breast cancer gene
expression correlation analyses. Database (Oxford).
2013:bas0602013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luo J, Wang Z, Huang J, Yao Y, Sun Q, Wang
J, Shen Y, Xu L and Ren B: HOXC13 promotes proliferation of
esophageal squamous cell carcinoma via repressing transcription of
CASP3. Cancer Sci. 109:317–329. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marcinkiewicz KM and Gudas LJ: Altered
histone mark deposition and DNA methylation at homeobox genes in
human oral squamous cell carcinoma. J Cell Physiol. 229:1405–1416.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tatangelo F, Di Mauro A, Scognamiglio G,
Aquino G, Lettiero A, Delrio P, Avallone A, Cantile M and Botti G:
Posterior HOX genes and HOTAIR expression in the proximal and
distal colon cancer pathogenesis. J Transl Med. 16:3502018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Robinson GW: Identification of signaling
pathways in early mammary gland development by mouse genetics.
Breast Cancer Res. 6:105–108. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tickle C, Crawley A and Goodman M:
Mechanisms of invasiveness of epithelial tumours: Ultrastructure of
the interactions of carcinoma cells with embryonic mesenchyme and
epithelium. J Cell Sci. 33:133–155. 1978.PubMed/NCBI
|
|
42
|
Morgan R and El-Tanani M: HOX genes as
potential markers of circulating tumour cells. Curr Mol Med.
16:322–327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kamkar F, Xaymardan M and Asli NS:
Hox-mediated spatial and temporal coding of stem cells in
homeostasis and neoplasia. Stem Cells Dev. 25:1282–1289. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nunes FD, de Almeida FC, Tucci R and de
Sousa SC: Homeobox genes: A molecular link between development and
cancer. Pesqui Odontol Bras. 17:94–98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Godwin AR and Capecchi MR: Hoxc13 mutant
mice lack external hair. Genes Dev. 12:11–20. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Comelli L, Marchetti L, Arosio D, Riva S,
Abdurashidova G, Beltram F and Falaschi A: The homeotic protein
HOXC13 is a member of human DNA replication complexes. Cell Cycle.
8:454–459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
La Starza R, Trubia M, Crescenzi B,
Matteucci C, Negrini M, Martelli MF, Pelicci PG and Mecucci C:
Human homeobox gene HOXC13 is the partner of NUP98 in adult acute
myeloid leukemia with t(11;12)(p15;q13). Genes Chromosomes Cancer.
36:420–423. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gurevich RM, Aplan PD and Humphries RK:
NUP98-topoisomerase I acute myeloid leukemia-associated fusion gene
has potent leukemogenic activities independent of an engineered
catalytic site mutation. Blood. 104:1127–1136. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cantile M, Scognamiglio G, Anniciello A,
Farina M, Gentilcore G, Santonastaso C, Fulciniti F, Cillo C,
Franco R, Ascierto PA and Botti G: Increased HOX C13 expression in
metastatic melanoma progression. J Transl Med. 10:912012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Woo CJ and Kingston RE: HOTAIR lifts
noncoding RNAs to new levels. Cell. 129:1257–1259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E and
Chang HY: Functional demarcation of active and silent chromatin
domains in human HOX loci by noncoding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li J, Wang J, Zhong Y, Guo R, Chu D, Qiu H
and Yuan Z: HOTAIR: A key regulator in gynecologic cancers. Cancer
Cell Int. 17:652017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang JX, Han L, Bao ZS, Wang YY, Chen LY,
Yan W, Yu SZ, Pu PY, Liu N, You YP, et al: HOTAIR, a cell
cycle-associated long noncoding RNA and a strong predictor of
survival, is preferentially expressed in classical and mesenchymal
glioma. Neuro Oncol. 15:1595–1603. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhao W, An Y, Liang Y and Xie XW: Role of
HOTAIR long noncoding RNA in metastatic progression of lung cancer.
Eur Rev Med Pharmacol Sci. 18:1930–1936. 2014.PubMed/NCBI
|
|
55
|
Weidle UH, Birzele F, Kollmorgen G and
Ruger R: Long non-coding RNAs and their role in metastasis. Cancer
Genomics Proteomics. 14:143–160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhou Y, Zang Y, Yang Y, Xiang J and Chen
Z: Candidate genes involved in metastasis of colon cancer
identified by integrated analysis. Cancer Med. 8:2338–2347. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pilato B, Pinto R, De Summa S, Lambo R,
Paradiso A and Tommasi S: HOX gene methylation status analysis in
patients with hereditary breast cancer. J Hum Genet. 58:51–53.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhong Z, Shan M, Wang J, Liu T, Xia B, Niu
M, Ren Y and Pang D: HOXD13 methylation status is a prognostic
indicator in breast cancer. Int J Clin Exp Pathol. 8:10716–10724.
2015.PubMed/NCBI
|
|
59
|
Xia B, Shan M, Wang J, Zhong Z, Geng J, He
X, Vu T, Zhang D and Pang D: Homeobox A11 hypermethylation
indicates unfavorable prognosis in breast cancer. Oncotarget.
8:9794–9805. 2017.PubMed/NCBI
|
|
60
|
Milevskiy MJ, Al-Ejeh F, Saunus JM,
Northwood KS, Bailey PJ, Betts JA, McCart Reed AE, Nephew KP, Stone
A, Gee JM, et al: Long-range regulators of the lncRNA HOTAIR
enhance its prognostic potential in breast cancer. Hum Mol Genet.
25:3269–3283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Botti G, De Chiara A, Di Bonito M, Cerrone
M, Malzone MG, Collina F and Cantile M: Noncoding RNAs within the
HOX gene network in tumor pathogenesis and progression. J Cell
Physiol. 234:395–413. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sun Z, Wu XY and Wu CL: The association
between LncRNA HOTAIR and cancer lymph node metastasis and distant
metastasis: A meta-analysis. Neoplasma. 65:178–184. 2018.
View Article : Google Scholar : PubMed/NCBI
|